Abstract
Helicobacter pylori infection of the gastric mucosa can be found in approximately 50% of the world's population and is associated with a range of pathology, including peptic ulcer, atrophic gastritis, and gastric cancer. To explore immunization as a strategy for preventing and treating H. pylori-associated disease, we assessed the safety and immunogenicity in healthy adults of a formalin-inactivated, oral H. pylori whole-cell (HWC) vaccine, administered with or without mutant Escherichia coli heat-labile toxin (LTR192G) as a mucosal adjuvant. In a dose-response study, 23 subjects with or without H. pylori infection were vaccinated with either 2.5 × 106 HWC, 2.5 × 108 HWC, or 2.5 × 1010 HWC, plus 25 μg of LTR192G. Thereafter, a randomized study was conducted in which 18 H. pylori-infected subjects were assigned, in a double-blind fashion, to receive either 2.5 × 1010 HWC plus placebo-adjuvant, placebo-vaccine plus 25 μg of LTR192G, placebo-vaccine plus placebo-adjuvant, or 2.5 × 1010 HWC plus 25 μg of LTR192G. Diarrhea (six subjects), low-grade fever (five subjects), and vomiting (two subjects) were observed, usually after the first dose. Significant rises in geometric mean mucosal (fecal and salivary) anti-HWC immunoglobulin A antibodies occurred among H. pylori-infected and uninfected subjects following inoculation with 2.5 × 1010 HWC plus 25 μg of LTR192G. Moreover, among H. pylori-negative volunteers, this regimen induced significant lymphoproliferative responses in 5 of 10 subjects and gamma interferon production responses to H. pylori sonicate in 7 of 10 subjects. There was no evidence that vaccination eradicated H. pylori in infected volunteers. These results suggest that it is possible to stimulate mucosal and systemic immune responses in humans to H. pylori antigens by using an HWC vaccine.
Helicobacter pylori infects nearly half of the world's population, resulting in chronic active gastritis, which persists throughout life unless the organism is eradicated (16, 22). Although most individuals experience no symptoms, 10 to 20% develop peptic ulcer disease (25, 55). Furthermore, chronic H. pylori infection confers a 3- to 12-fold increased risk of developing gastric cancers such as adenocarcinoma and low-grade B-cell lymphoma (6, 23, 28, 41, 51, 52).
Randomized, placebo-controlled trials have demonstrated that eradication of H. pylori infection from the stomach with antimicrobial therapy heals chronic gastritis and peptic ulcers, prevents ulcers from recurring (25, 31, 47, 55) and may lead to regression of gastric lymphoma (50, 65). However, there are impediments to identifying a simple, inexpensive, safe, and effective treatment, including the high cost and side effects associated with standard multidrug regimens (57), the appearance of antibiotic-resistant H. pylori strains (3), and the measurable risk of reinfection following antibiotic-induced eradication (36, 56). For these reasons, the use of vaccines for treatment and prevention of H. pylori infection has been explored.
Preclinical studies have identified a number of promising Helicobacter antigens, including urease (20, 44, 48), VacA (45), CagA (46), heat shock protein (64), neutrophil-activating protein (59), and outer membrane lipoprotein (34). Mucosal administration of inactivated Helicobacter whole-cell (HWC) preparations is another approach that has been extensively explored. A series of independent experiments in animal models has demonstrated that mucosal vaccination with whole-cell preparations of H. pylori confers protection against challenge with wild-type H. pylori or H. felis organisms (11, 21, 44–46; M. Chen, A. Lee, and S. Hazell, Letter, Lancet 339:1120–1121, 1992). Coadministration of a mucosal adjuvant, such as cholera toxin (CT) (11; Chen et al., Letter), CT B subunit (42), the heat-labile enterotoxin (LT) of Escherichia coli (45), and mutant LT K63 (46), has been essential to elicit these protective responses. HWC vaccination has also been explored as a therapeutic strategy (24, 32). For example, administration of either H. felis or H. pylori sonicate plus CT eradicated H. felis infection in mice; 94% of the animals remained cured of their infection for 3 months after vaccination, as detected by histology and local urease activity (15).
Despite the growing body of preclinical data, there have been few clinical trials to determine whether Helicobacter vaccines can achieve similar success in humans, and these have thus far involved either recombinant urease (rUrease) or urease expressed by Salmonella spp. (1, 14, 49; T. Buclin, M. Cosma, I. Corthesy-Theulaz, and P. Michetti, Letter, Lancet 347:1630–1631, 1996). We report here the clinical acceptability and immunogenicity of formalin-inactivated HWC vaccine administered to healthy adults with or without natural subclinical H. pylori infection and the effect of coadministered mucosal adjuvant on these responses.
MATERIALS AND METHODS
Vaccine.
The formalin-inactivated HWC vaccine used in this study (lot 0290, under commercial development by a subsidiary of Antex Biologics, Inc.) was derived from a frozen stock of a clinical strain (ATCC 55713) that was originally isolated from a human duodenal ulcer biopsy. The parent strain, designated G1-4, is highly motile and expresses CagA, VacA, urease, and catalase. In addition, G1-4 binds to asialo-GM1 (39) but not to other gangliosides (GB4, GD1-B, and GM3) (40). The vaccine was prepared at the Walter Reed Army Institute of Research (WRAIR) Forest Glen Annex Facility using Good Manufacturing Practice. In brief, G1-4 was grown to a concentration of 5 × 108 bacterial cells per ml in 320 liters of brain heart infusion broth supplemented with bovine calf serum. At the time of harvest, the culture medium was centrifuged, and the bacteria were resuspended in phosphate-buffered saline (PBS), to which formalin was added to a concentration of 0.025 M for 18 h at room temperature. Inactivated cells were then separated by centrifugation and suspended in sterile PBS to achieve a final optical density at 625 nm (OD625) of 30 ± 2. Vaccine was packaged in 20-dose (20 ml) vials each containing 2.5 × 1010 to 5.0 × 1010 bacterial cells and 0.1 mg of sodium thimerisol per ml of PBS (formaldehyde content, <0.01 M) and stored at 4°C. When it was necessary to administer an inoculum of either 2.5 × 108 or 2.5 × 106, the vaccine was diluted with PBS immediately prior to use.
The formalin-inactivated cells contain lipopolysaccharide (as shown by Limulus lysate assay), do not produce urease, but nonetheless induce antibodies to urease, catalase, and flagellin in mice as shown by enzyme-linked immunosorbent assay (ELISA) and retain the overlaying binding characteristics of live cells. The vaccine strain also induces mouse antibodies by Western blot to immunodominant proteins in the 30 to 60-kDa range from homologous and heterologous H. pylori strains.
Adjuvant.
The adjuvant is a modified form of the heat-labile enterotoxin of E. coli, designated LTR192G, having a glycine residue substituted for the arginine at position 192 from the amino terminus of the A1 subunit of the molecule (12). Removal of this arginine residue renders the molecule trypsin-insensitive, thereby interfering with its activation to an enterotoxic form. LTR192G was produced to specifications by the Swiss Serum and Vaccine Institute, Berne, Switzerland. Each 1-mg portion was lyophilized in 3.29 mg of Tris, 0.146 mg of EDTA, and 5.84 mg of NaCl per ml and 5% (wt/vol) lactose and then stored at 4°C until use. The adjuvant (lot mHT419) was rehydrated with 1 ml of sterile water. The dose administered was 25 μl (25 μg).
Placebo for vaccine and adjuvant.
The placebo for the vaccine (designated placebo-vaccine) and for the adjuvant (designated placebo-adjuvant) consisted of sterile buffer mixed with powered skimmed milk to match the turbidity of the vaccine and adjuvant formulations.
Subjects.
Healthy volunteers 18 to 55 years of age were recruited from the Baltimore-Washington metropolitan area. They were determined to be in good health on the basis of medical history, physical examination, and a battery of clinical laboratory tests. Prospective volunteers were excluded if they gave a history of major gastrointestinal surgery or illness, current gastrointestinal symptoms such as dyspepsia requiring daily therapy, regular use of aspirin or nonsteroidal anti-inflammatory drugs, allergy to a study medication, or receipt of a vaccine or investigational drug during the 30 days prior to enrollment. Women received a serum pregnancy test before each vaccination to ensure that pregnant women were not vaccinated. Volunteers completed a written examination to test their comprehension of the purpose, procedures, and risks of the trial and were required to answer at least 70% of the questions correctly in order to participate. All enrolled subjects provided informed, written consent according to the guidelines of the University of Maryland, Baltimore, Institutional Review Board.
Screening for H. pylori infection.
A two-stage process was used to determine whether prospective volunteers were infected with H. pylori. First, subjects were screened for the presence of serum antibody to H. pylori using a commercial ELISA manufactured by BioWhittaker, Inc. (Walkersville, Md.) in the dose-response study and by Wampole Laboratories, Dist., Carter-Wallace, Inc. [Cranbury, N.J.]) in the randomized safety and immunogenicity study. Next, a 13C urea breath test (13C UBT; Meretek, Inc., Houston, Tex.) was used to confirm the presence or absence of active infection. Volunteers who were positive by both the ELISA and breath test were considered H. pylori-infected and those negative by both assays were considered uninfected. Seropositive subjects who had negative breath tests were excluded from participation.
Study design. (i) Dose-response study among H. pylori-infected and uninfected subjects.
An initial dose-response study was conducted among 23 volunteers to determine whether increasing inocula of HWC, coadministered with 25 μg of LTR192G, were well tolerated and to evaluate whether increasing HWC inocula enhanced the immune response. It was anticipated that the optimal dose would contain 2.5 × 1010 HWC plus 25 μg of LTR192G. Groups of 3 to 10 H. pylori-infected or H. pylori-uninfected subjects were assigned in an unblinded fashion to receive three oral doses of vaccine on days 0, 14, and 28 at an inoculum of either 2.5 × 106, 2.5 × 108, or 2.5 × 1010 HWC plus 25 μg of LTR192G (Table 1). Safety was established at each dose level before a new group of volunteers received a higher inoculum of vaccine. For the purpose of characterizing the dose response, the eight H. pylori-infected subjects who received 2.5 × 1010 HWC plus 25 μg of LTR192G as part of the randomized safety and immunogenicity study, described below (Table 2), are also included in this analysis.
TABLE 1.
Open-label, dose-response study designa
| HWC vaccine inoculum | LTR192G dose (μg) | H. pylori status | No. of subjects |
|---|---|---|---|
| 2.5 × 106 | 25 | Uninfected | 3 |
| 2.5 × 106 | 25 | Infected | 3 |
| 2.5 × 108 | 25 | Uninfected | 4 |
| 2.5 × 108 | 25 | Infected | 3 |
| 2.5 × 1010 | 25 | Uninfected | 10 |
For the purpose of characterizing the dose response, the eight H. pylori-infected subjects who received 2.5 × 1010 inactivated HWC vaccine plus 25 μg of LTR192G adjuvant as part of the randomized safety and immunogenicity study (Table 2) are also included in the analysis of this portion of the study.
TABLE 2.
Randomized, double-blind, placebo-controlled safety and immunogenicity study design among subjects with subclinical H. pylori infection
| No. of subjects | Randomized assignment
|
|
|---|---|---|
| Vaccine | Adjuvant | |
| 2 | 2.5 × 1010 HWC | Placebo-adjuvant |
| 3 | Placebo-vaccine | LTR192G |
| 5 | Placebo-vaccine | Placebo-adjuvant |
| 8 | 2.5 × 1010 HWC | LTR192G |
(ii) Randomized safety and immunogenicity study among H. pylori-infected subjects.
After determining that subjects tolerated the target inoculum of 2.5 × 1010 HWC plus 25 μg of LTR192G, we conducted a randomized study among H. pylori-infected subjects to investigate in a preliminary fashion the safety and immunogenicity of the oral HWC vaccine administered with or without mucosal adjuvant. Twenty H. pylori-infected subjects were randomly assigned, in a double-blind, placebo-controlled fashion, to receive, on days 0, 14, and 28, either 2.5 × 1010 HWC plus placebo-adjuvant, placebo-vaccine plus 25 μg of LTR192G, placebo-vaccine plus placebo-adjuvant, or 2.5 × 1010 HWC plus 25 μg of LTR192G (Table 2). Two subjects were withdrawn after a single inoculation because of scheduling conflicts (one subject had received HWC with placebo-adjuvant, and the other subject had received placebo-vaccine plus LTR192G), leaving 18 analyzable subjects.
(iii) Inoculation.
Volunteers fasted for 90 min before and after inoculation. Immediately before inoculation a buffer solution was prepared by dissolving 2 g of NaHCO3 in 150 ml of sterile water. Volunteers drank 120 ml of buffer solution followed 1 min later by the test inoculum suspended in the remaining 30 ml of buffer solution.
(iv) Clinical evaluation.
Volunteers were observed at the study site for at least 1 h before and after inoculation to ensure that fasting was maintained and to monitor for immediate reactions. For 7 days following each inoculation, volunteers completed a standardized diary form to assess their clinical response. They recorded their evening oral temperature, the presence of symptoms (epigastric pain, heartburn, malaise, nausea, or bloating), vomiting, and the consistency (loose or formed) and presence of gross blood in each stool passed. Symptoms were graded as follows: 0, absent; 1, mild (hardly noticed); 2, moderate (bothersome, but continued the same activities); and 3, severe (interrupted activities or sleep). Fever was defined as an oral temperature of 100°F or higher, and diarrhea was defined as three or more loose stools within a 24-h period.
H. pylori-uninfected subjects underwent a repeat 13C UBT on day 56 to exclude the possibility of interim H. pylori infection. Infected subjects in the dose-response study completed follow-up breath tests on day 56, and those in the randomized study completed follow-up breath tests on days 56, 180, and 210 to determine whether H. pylori had been eradicated following vaccination.
Immunology. (i) Serum antibody.
Blood was collected on days 0, 14, 28, 35, 56, and 180 to measure immunoglobulin A (IgA) and IgG responses to LTR129G and HWC by ELISA; published assays were used for LTR129G and adapted for use for the HWC antigen (62). Briefly, microtiter plates were coated in rows alternating with specific antigen (LTR129G at 1 μg/ml or HCW at 1:1,000 in PBS [pH 7.2] or with PBS alone, as the negative control. A single 1:100 dilution of each test serum sample was used for the IgG LT assay; twofold dilutions of serum were used for all other antigen and isotype assays starting at 1:100 for the IgA LT and IgG HWC assays and 1:25 for IgA HWC assay. Endpoints for the titers were determined using an H. pylori-seronegative population in standardization studies; 0.1 for IgA LT and HWC and 0.3 for IgG HWC.
(ii) Mucosal antibody.
Samples of stool (prepared as a 10% stool supernatant) and saliva (after a 1-h fast) were collected for measurement of total and antigen-specific IgA antibody production on days 0, 7, 14, 21, 28, 56, and 180. Protease inhibitors were added, and the samples were stored at −70°C until testing.
Total IgA was measured using a quantitative ELISA (limit of detection, 6 ng/ml) according to previously described methods (62). Specific IgA antibody responses to HWC and LTR192G were determined, as described above, on samples which contained at least 2 mg of total IgA per ml (using twofold dilutions of the test sample); IgA levels were determined by extrapolating to the linear part of the quantitative ELISA curve, which was run in parallel with each ELISA and then standardizing the value to 20 mg of total IgA per ml.
(iii) ASCs.
As a measure of intestinal priming induced by inoculation, circulating specific antibody-secreting cells (ASCs) were quantified on days 0, 7, 10, 14, 21, 28, and 35 using ELISPOT, as previously described (64). Microdilution plates were coated with either 1 μg of LTR129G per ml or 1:000 dilution of HWC (WRAIR, BPR 144-01).
(iv) CMI.
Cell-mediated immunity (CMI) responses were assessed in subjects who received 2.5 × 1010 HWC plus 25 μg of LTR192G using peripheral blood mononuclear cells (PBMC) isolated before and 56 days after the first inoculation and frozen in liquid nitrogen until use, as previously described (61).
(a) Lymphoproliferative responses.
Lymphoproliferative responses to H. pylori sonicate and purified recombinant catalase (a putative immunoprotective antigen (54) expressed by the vaccine) were measured. H. pylori sonicate was prepared from an overnight broth culture, which was pelleted by centrifugation, washed, resuspended in deionized water, disrupted by sonication, and then stored at −20°C. To derive recombinant catalase, the Fe2+ catalase gene from H. pylori ATCC 49503 was PCR cloned into plasmid pKK223-3 (Pharmacia Biotech, Inc., Piscataway, N.J.) as a −1.1-kbp EcoRI fragment and transformed into E. coli JM105. Derivatives of JM105 expressing high levels of catalase were identified by examining crude whole-cell lysates on sodium dodecyl sulfate (SDS)-gels for the presence of a novel ∼55-kDa IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible protein. Soluble catalase was purified by DEAE-Sepharose chromatography to ∼90% homogeneity as previously described (53). Purity was assessed on SDS-gels, and the material was tested for activity using H2O2.
Phytohemagglutinin (HA-17; Wellcome Diagnostics, Beckenham, United Kingdom) and tetanus toxoid (Wyeth, Marietta, Pa.) were used to confirm the ability of PBMC to proliferate to mitogenic stimulation and antigenic stimulation, respectively (61). Antigens were added in the following final concentrations: H. pylori sonicate, 0.2, 2, and 20 μg/ml; recombinant catalase, 0.2, 2, and 20 μg/ml; bovine serum albumin (BSA), 20 μg/ml; tetanus toxoid, 2 μg/ml; and phytohemagglutinin, 2 μg/ml. Net counts per minute (cpm) represented the difference in 3H-labeled thymidine incorporation by antigen-exposed versus unstimulated cells, for that same day and subject. A positive response was defined as a difference (P < 0.05, two-tailed t test) in mean net cpm between triplicate pre- and postvaccination samples stimulated with each individual antigen (i.e., H. pylori sonicate, recombinant catalase or BSA).
(b) IFN-γ and IL-5 production.
Cytokines were measured by chemiluminescence ELISA (the limits of detection were 4 pg/ml for gamma interferon[IFN-γ] and 2 pg/ml for interleukin-5 [IL-5]) as previously described (53, 66). Net cytokine production levels (in picograms/milliter) were calculated by subtracting cytokine levels in negative control wells (no antigen exposure) from the levels in test sample wells for that same day and subject. A positive IFN-γ or IL-5 response was defined as a difference (P < 0.05, two-tailed t test) in mean chemiluminescence units between duplicate pre- and postvaccination samples stimulated with each individual antigen (i.e., H. pylori sonicate, recombinant catalase, or BSA).
Statistical analysis.
Serum and local immunoglobulin titers were converted to natural logarithms prior to analysis. ASC counts were converted to logarithms after coding (one was added to remove values of zero). Standard errors presented in this report represent the back-transformed standard error of the log-transformed observations.
In the dose-response study, pre-versus peak postvaccination geometric means were compared using Wilcoxon's signed-rank test. Significant differences in mean lymphoproliferative and cytokine responses pre-versus postvaccination were detected with the paired Student's t test.
In the randomized safety and immunogenicity study, two immunologic comparisons were of interest: (i) responses to HWC antigen following administration of HWC plus placebo-adjuvant versus placebo-vaccine plus placebo-adjuvant to assess the immunogenicity of the vaccine when given without mucosal adjuvant and (ii) responses to HWC antigen following administration of HWC plus LTR192G versus HWC plus placebo-adjuvant to determine whether addition of mucosal adjuvant enhances the immune response to the HWC vaccine. Geometric mean values (peak postvaccination) were compared between the randomized vaccine groups using analysis of covariance, adjusting for prevaccination values.
Two-tailed hypotheses were evaluated throughout, with statistical significance determined at the 5% level. Corrections for multiple comparisons were not made.
RESULTS
Clinical tolerance.
The clinical response to vaccination among the 41 subjects who participated in the trial is shown in Table 3. Six subjects experienced diarrhea (three of whom had baseline H. pylori infection); one subject received placebo-vaccine plus LTR192G, and the remaining five received 2.5 × 1010 HWC plus LTR192G (Table 3). Thus, diarrhea was seen only among subjects who received LTR192G (with or without vaccine) and only following the highest (2.5 × 1010) HWC dose. Diarrhea followed the first inoculation in all but one subject. The episodes lasted for 1 to 3 days, during which time these subjects passed a total of 3 to 17 loose stools. Five subjects met the definition of fever (including one who received only placebo) but experienced only a single temperature elevation of 100 to 101°F 2 to 5 days after the first inoculation (Table 3). Two recipients of 2.5 × 1010 HWC plus LTR192G vomited once after the first inoculation; one also had diarrhea, and the other also had a fever.
TABLE 3.
Clinical tolerance of oral inactivated whole-cell vaccine plus adjuvant by study group
| Symptom | No. of subjects
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
H. pylori uninfected (unblinded dose response at:)
|
H. pylori infected
|
||||||||
| Unblinded dose response at:
|
Randomized assignment at:
|
||||||||
| 106, 25 μg (n = 3)a | 108, 25 μg (n = 4) | 1010, 25 μg (n = 10) | 106, 25 μg (n = 3) | 108, 25 μg (n = 3) | 1010, 0 (n = 2) | 0, 25 μg (n = 3) | 0, 0 (n = 5) | 1010, 25 μg (n = 8) | |
| Nausea | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 2 | 3 |
| Vomiting | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Anorexia | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Malaise | 2 | 0 | 3 | 1 | 0 | 1 | 1 | 2 | 3 |
| Heartburn | 1 | 2 | 1 | 2 | 1 | 0 | 1 | 1 | 2 |
| Stomachache | 1 | 1 | 3 | 2 | 0 | 0 | 1 | 3 | 3 |
| Abdominal pain | 0 | 1 | 4 | 3 | 0 | 0 | 1 | 2 | 2 |
| Fever of >100°F | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Diarrheab | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 2 |
| Blood in stool | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Each study design is presented as follows: HWC dose, LTR192G dose (n = number of subjects). The actual doses of HWC were 2.5 × 106, 2.5 × 108, or 2.5 × 1010.
Defined as three or more loose or watery stools within a 24-h period.
One or more gastrointestinal complaints (nausea, anorexia, malaise, heartburn, stomachache, and abdominal pain) was reported by subjects in all study groups (Table 3). There was no apparent effect of H. pylori infection status on the occurrence of these symptoms following vaccination. Affected subjects rated their symptoms as mild or moderate in severity with the exception of two recipients of 2.5 × 1010 HWC vaccine plus LTR192G who experienced abdominal pain that made them sufficiently uncomfortable to alter their normal activity. Two H. pylori-infected subjects observed blood streaks in a formed stool; one subject had received 2.5 × 1010 HWC plus LTR192G, and the other had received placebo-adjuvant plus placebo-vaccine.
Effect of vaccination on H. pylori infection, as measured by 13C UBT.
H. pylori-infected subjects had repeat 13C UBT after vaccination. In the dose-response study, all six subjects remained positive when the test was repeated 2 months after vaccination. In the randomized study, the 13C UBT was repeated 2, 6, and 7.5 months after vaccination, and 17 of 18 remained positive. One recipient of placebo-vaccine plus LTR192G reverted to negative at 6 months; she had received a 1-week course of metronidazole to treat an upper respiratory infection approximately 1 month earlier. All 17 H. pylori-uninfected subjects had negative 13C UBT results when the test was repeated 2 months after vaccination.
Dose-dependent immune responses to HWC vaccine plus LTR192G among H. pylori-infected and uninfected subjects. (i) Anti-HWC responses.
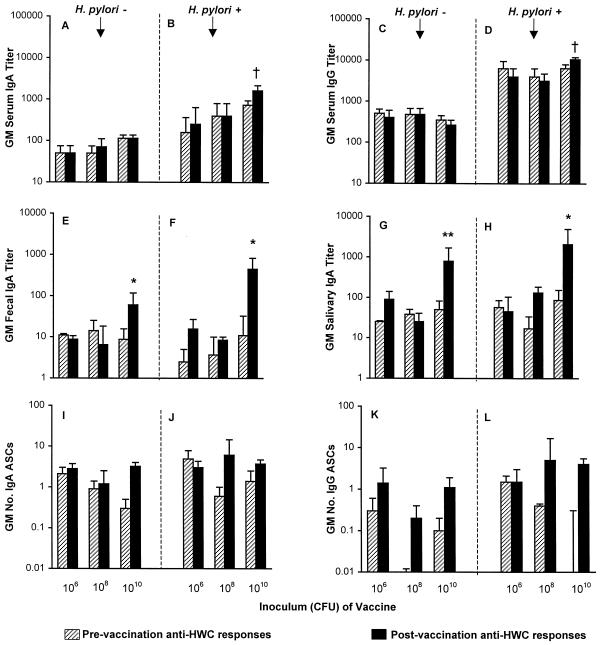
Immunization elicited rises in the geometric mean serum and mucosal anti-HWC antibodies only among subjects who received the highest (2.5 × 1010 HWC) vaccine dose (Fig. 1). Whereas postvaccination increases in geometric mean peak serum IgA and IgG titers were marginal (P = 0.06) and were seen only among H. pylori-infected subjects, the fecal and salivary IgA responses were statistically significant and occurred in both H. pylori-infected and uninfected volunteers. Anti-HWC ASC responses were meager (none exceeded 10 cells per 106 PBMC) and so were not subjected to statistical analysis (Fig. 1).
FIG. 1.
Immune responses to HWC antigen according to H. pylori infection status and vaccine dose. Volunteers received an oral dose of either 2.5 × 106, 2.5 × 108, or 2.5 × 1010 inactivated HWC vaccine plus 25 μg of LTR192G adjuvant on days 0, 14, and 28. Responses are expressed as the geometric mean (GM) titer or geometric mean number of ASCs per 106 PBMC ± the back-transformed standard error measured prevaccination and postvaccination. Panels A, C, E, G, I, and K represent volunteers with no evidence of H. pylori infection (there were 3, 4, and 10 recipients of the 106, 108, and 1010 doses of HWC, respectively). Panels B, D, F, H, J, and L represent volunteers with subclinical H. pylori infection at baseline (there were 3, 3, and 8 recipients of the 106, 108, and 1010 doses of HWC, respectively). Comparisons of pre- and postvaccination titers: ∗, P < 0.05; ∗∗, P < 0.01; †, P = 0.06.
(ii) Anti-LTR192G responses.
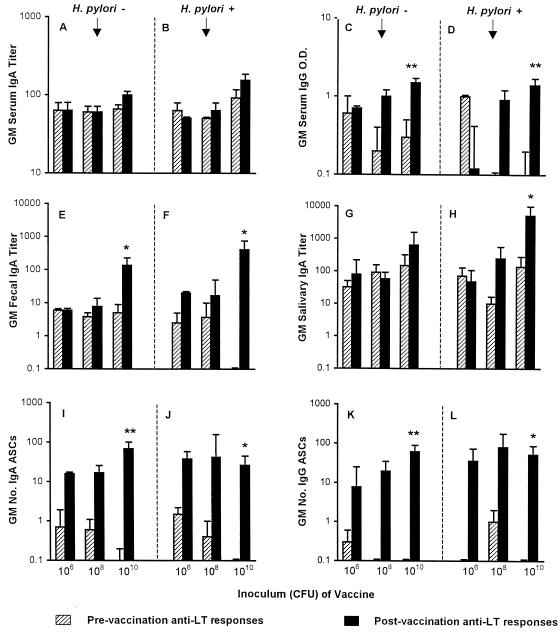
As shown in Fig. 2, rises in serum and mucosal anti-LTR192G antibodies were observed following vaccination. Interestingly, postvaccination anti-LTR192G levels appeared to rise as the dose of HWC vaccine increased, despite a constant dose of adjuvant. Statistically significant anti-LTR192G antibody increases occurred only in the groups (both H. pylori-infected and uninfected) receiving the highest (2.5 × 1010 HWC) vaccine dose, for serum IgG (but not IgA), fecal IgA, and salivary IgA. In contrast to the minimal ASC responses to HWC, nearly half of the anti-LTR192G ASC responses exceeded 100 cells per 106 PBMC. Significant increases in the geometric mean number of LTR192G-specific IgA- and IgG-producing ASCs were observed following vaccination among H. pylori-infected and uninfected subjects who received 2.5 × 1010 HWC (Fig. 2).
FIG. 2.
Immune responses to the mucosal adjuvant LTR192G according to H. pylori infection status and vaccine dose. Volunteers received an oral dose of either 2.5 × 106, 2.5 × 108, or 2.5 × 1010 inactivated HWC vaccine plus 25 μg of LTR192G adjuvant on days 0, 14, and 28. Responses are expressed as the geometric mean (GM) titer, the geometric mean OD, or the geometric mean number of ASCs per 106 PBMC ± the back-transformed standard error measured prevaccination and postvaccination. Panels A, C, E, G, I, and K represent volunteers with no evidence of H. pylori infection (there were 3, 4, and 10 recipients of the 106, 108, and 1010 doses of HWC, respectively). Panels B, D, F, H, J, and L represent volunteers with subclinical H. pylori infection at baseline (there were 3, 3, and 8 recipients of the 106, 108, and 1010 doses of HWC, respectively). Comparisons of pre- and postvaccination titers: ∗, P < 0.05; ∗∗, P < 0.01.
CMI responses among H. pylori-infected subjects to immunization with 2.5 × 1010 HWC plus LTR192G. (i) Lymphoproliferative responses.
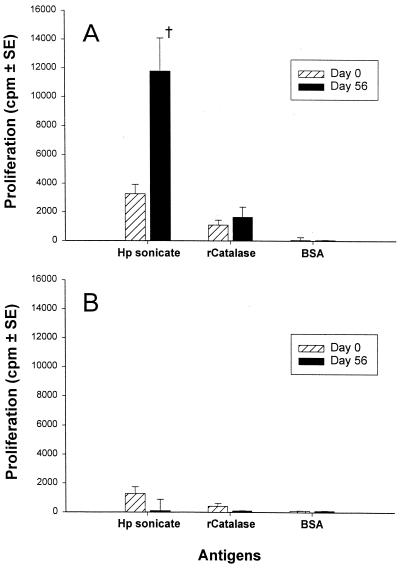
Immunization with 2.5 × 1010 HWC plus LTR192G resulted in increased, albeit statistically insignificant (P = 0.22) mean group proliferative responses to the H. pylori sonicate among H. pylori uninfected volunteers (Fig. 3A). Significant rises in proliferative responses to 2 μg of H. pylori sonicate per ml were observed in 5 of the 10 volunteers evaluated, while no significant increases in proliferative responses were observed when PBMC were incubated with either recombinant catalase or BSA (Fig. 3A). In contrast, no significant increases in mean lymphoproliferative responses to the H. pylori sonicate were observed following immunization of H. pylori-infected volunteers (Fig. 3B).
FIG. 3.
Proliferative responses by PBMC from eight H. pylori uninfected (A) and 10 H. pylori-infected (B) volunteers following ingestion of 2.5 × 1010 inactivated HWC vaccine plus 25 μg of LTR192G adjuvant. PBMC obtained from volunteers before or 56 days after immunization were evaluated for lymphoproliferative responses to an H. pylori sonicate or purified recombinant catalase. The results are expressed as the mean net cpm ± the standard error for all volunteers in each group †, P = 0.22 (paired t test) of mean postimmunization versus preimmunization values.
(ii) Production of IFN-γ and IL-5.
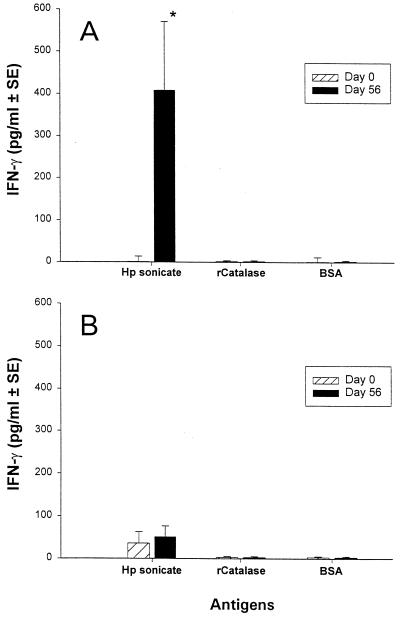
Immunization with 2.5 × 1010 HWC plus LTR192G resulted in significant (P < 0.05) increases among H. pylori uninfected volunteers in mean group IFN-γ production to the H. pylori sonicate at 2 μg/ml (Fig. 4A); significant rises were observed in 7 of the 10 volunteers studied. Postvaccination responses, albeit not statistically significant, were also observed when cultures contained 0.2 and 20 μg of the H. pylori sonicate per ml (data not shown). No significant increases in mean IFN-γ production were observed when PBMC were incubated with either recombinant catalase or BSA (Fig. 4A). In contrast, significant increases in mean IFN-γ production to the H. pylori sonicate were not observed following the immunization of H. pylori-infected volunteers (Fig. 4B). Undetectable or minimal levels of IL-5 were observed in culture supernatants from PBMC obtained before and after immunization of H. pylori-infected and uninfected volunteers (data not shown).
FIG. 4.
IFN-γ production by PBMC from eight H. pylori-uninfected (A) and 10 H. pylori-infected (B) volunteers following ingestion of 2.5 × 1010 inactivated HWC vaccine plus 25 μg of LTR192G adjuvant. PBMC obtained from the volunteers before or 56 days after immunization were evaluated for IFN-γ production to an H. pylori sonicate or purified recombinant catalase. The results are expressed as the mean net pg/ml ± the standard error for all volunteers in each group. ∗, P < 0.05 (paired t test) of mean postimmunization versus preimmunization measurements.
Immune response of H. pylori-infected subjects to HWC vaccine with or without coadministered LTR192G.
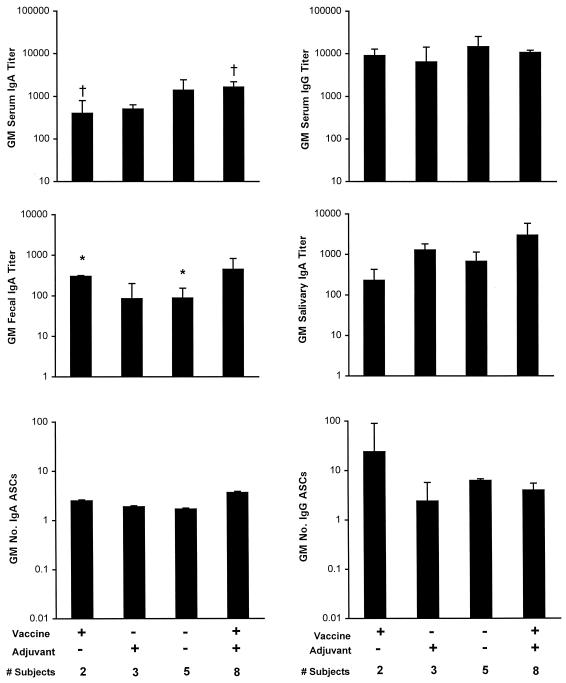
In the first analysis, the anti-HWC responses among recipients of 2.5 × 1010 HWC plus placebo-adjuvant were compared with the responses among recipients of placebo-vaccine plus placebo-adjuvant to assess the immunogenicity of the vaccine alone in H. pylori-infected subjects. It was observed that HWC recipients achieved significantly higher geometric mean fecal IgA titers than those receiving placebo (301 versus 88, P < 0.001; Fig. 5).
FIG. 5.
Postvaccination peak immune responses of H. pylori-infected subjects to HWC antigen according to a randomized immunizing regimen. Volunteers received an oral dose of either 2.5 × 1010 HWC vaccine (vaccine+) or placebo-vaccine (vaccine−), plus either 25 μg of LTR192G (adjuvant+) or placebo-adjuvant (adjuvant−). The responses are expressed as the geometric mean (GM) titer or the geometric mean number of ASCs per 106 PBMC ± the back-transformed standard error. ∗, P < 0.001, comparing recipients vaccine+ plus adjuvant− with recipients of vaccine− plus adjuvant−; †, P = 0.06, comparing recipients of vaccine+ plus adjuvant− with recipients of vaccine+ plus adjuvant+. Note that the responses in the group that received vaccine+ and adjuvant+ are also shown in Fig. 1.
In the second analysis, comparisons were performed to determine whether coadministered adjuvant increased the immunogenicity of the high dose of HWC. The only comparison of anti-HWC responses that approached statistical significance was the geometric mean serum IgA titer among subjects who received HWC with adjuvant versus those who received HWC alone (1,646 versus 400, P = 0.06; Fig. 5).
DISCUSSION
These results demonstrate that vaccination with inactivated HWC vaccine is immunogenic when given to volunteers with or without subclinical H. pylori infection. Furthermore, it provides the first indication in humans that an orally administered vaccine against H. pylori can induce mucosal IgA responses, as measured in stool and saliva, and elicit both IFN-γ and the appearance of circulating sensitized lymphocytes that proliferate.
Despite extensive investigation in animals demonstrating that mucosal adjuvants are essential to produce protective immunity to Helicobacter, comparable human experience is lacking. Although the sample sizes were small in our study and the results must be considered preliminary, one response (serum anti-HWC IgA) approached statistical significance when subjects who received HWC with adjuvant were compared to those who received HWC alone (1,646 versus 400, P = 0.06). A previous series of clinical trials suggested that native LT adjuvanted the immune responses to rUrease, although direct comparisons of rUrease with or without adjuvant were not made (49). In these trials, native LT coadministered with rUrease vaccine induced serum and ASC IgA responses but not local (salivary and gastric) responses to the vaccine antigen (49), whereas in a previous trial, rUrease vaccine alone failed to induce an immune response (Kreiss et al., Letter). Although no recipients of LT plus rUrease were cured of their H. pylori infection, a significant decrease in gastric H. pylori bacterial density (but not inflammation) was observed in biopsy tissue. We were unable to determine whether vaccination similarly reduced the bacterial burden in our trial because biopsies were not taken. Growing evidence in animal models suggests that both prophylactic and theraupeutic Helicobacter vaccines do not achieve sterilizing immunity but rather reduce levels of bacterial colonization (13, 17, 35, 43). It remains to be determined whether sterilizing immunity can be achieved and, if not, whether suppression alone can prevent the pathological consequences of H. pylori infection.
It has been hypothesized that a balance of Th1 and Th2 responses is necessary to invoke protective immunity against H. pylori. Initial H. pylori vaccines were designed to target Th2-type responses, reasoning that activation of antigen-specific IgA at the mucosal surface would facilitate the clearance of bacteria from the stomach (10, 24). In mice, the enhanced efficacy of vaccine antigens conferred by coadministered native or nontoxic mutants of LT and CT in preventing and eradicating H. pylori infection has been attributed to the ability of these mucosal adjuvants to drive preferential activation of Th2-type CD4+ responses (7, 15, 24, 58, 63, 68). This view is supported by observations that mice given monoclonal anti-H. felis (11) or anti-urease (4) IgA at the time of wild-type challenge were significantly protected against infection. Furthermore, the presence of antigen-specific secretory IgA in mucosal secretions (44) and not serum IgG (21) has been associated with protection in mice against acquisition of H. felis infection following challenge. In contrast, natural infection induces a more proinflammatory Th1-type response in the mouse H. felis model (21) and also in humans with H. pylori-associated peptic disease (30). However, the optimal type of immune response to be induced by vaccination requires further investigation. Recent observations showed that protection induced by mucosal immunization with rUrease plus LT in B-cell knockout mice was equivalent to that observed in the wild-type mouse strain, suggesting that antibody responses to urease are not required for protection (18). Furthermore, rUrease vaccine injected with adjuvants that induce strong Th1- and Th2-type responses (e.g., saponin and glycol-lipopeptide) elicits better protection of mice against H. pylori challenge than rUrease mixed with adjuvants that induced a predominant Th2-type response (e.g., LT) (29).
Interestingly, vaccination with 2.5 × 1010 HWC plus 25 μg of LTR192G elicited significant increases in sensitized lymphocytes that proliferated and produced IFN-γ, but not IL-5, in response to an H. pylori antigenic preparation. However, these responses were observed only in volunteers who were H. pylori negative. These results suggest that as an immunoprophylactic agent in H. pylori-negative individuals, the HWC vaccine can induce both type 1 and type 2 responses. Recent data suggest a marked predominance of a type 1 pattern of cytokine production, characterized by a prevalence of IFN-γ over IL-4 and IL-5 production by cells isolated from gastric biopsies of H. pylori-infected volunteers (2, 33). Our observations that immunization of H. pylori-uninfected volunteers with a whole-cell H. pylori vaccine elicits the appearance in peripheral blood of sensitized cells that proliferate and produce predominantly type 1 cytokines (i.e., IFN-γ but not IL-5 production) to H. pylori antigens suggest that immunization with whole-cell vaccine may mimic to some extent the responses observed during natural infection. However, the fact that immunization of volunteers also produces increases in anti-H. pylori fecal and salivary IgA suggests that type 2 cytokine responses are also elicited. In contrast, the responses observed following immunization in H. pylori-infected volunteers, characterized by increases in serum, fecal, and salivary IgA in the absence of proliferation or IFN-γ production, suggest a predominance of type 2 responses. Alternatively, our inability to detect IFN-γ and proliferative responses in H. pylori-infected volunteers might be related to the previously observed phenomenon that exposure of PBMC and lamina propria lymphocytes from H. pylori-infected volunteers to H. pylori antigens resulted in lower proliferative responses and IFN-γ production than that observed with cells isolated from noninfected volunteers, suggesting that H. pylori antigens might suppress specific immune responses (19).
Vaccination was generally well tolerated, although self-limited diarrhea occurred (generally only after the first dose) in 28% of subjects who received 2.5 × 1010 HWC plus LTR192G and in one additional subject who received LTR192G alone. Some subjects also experienced vomiting and low-grade fever. In comparison, diarrhea occurred in 66% of subjects participating in another study who received native LT (49). The self-limited diarrheal illnesses in our study may have resulted from residual enterotoxigenicity of LTR192G, which retains some activity in the mouse Y-1 adrenal tumor cell assay (37). The substituted arginine residue at position 192 on the LT molecule is a trypsin cleavage site of A subunit to A1 and A2 components. LT has been reported to be activated by proteolytic cleavage at this site (5); however, cleavage at this site is not essential for the expression of enzymatic activity (26). Given the relationship between diarrhea and increasing doses of vaccine, it is also possible that the enterotoxic activity of Helicobacter's vacuolating toxin (VacA) was not completely eliminated with formalin processing (27).
In sum, the encouraging results of this study suggest that it is possible to stimulate an immune response to H. pylori antigens using an inactivated whole-cell vaccine. However, there is controversy regarding which immune responses are necessary to prevent or cure infection, particularly in light of the fact that chronic H. pylori infection occurs in the face of measurable systemic and local (gastric and salivary) antibody responses (8, 9, 38) and individuals who have been cured of their H. pylori infection are occasionally reinfected (67), even with a homologous strain (60). The success of any H. pylori vaccine to prevent and/or cure infection in humans hinges on the ability to identify antigens and delivery systems which stimulate active immunity without inducing undesirable inflammatory processes and to target these responses to the stomach.
ACKNOWLEDGMENTS
We thank the volunteers who participated in this trial, Kathy Palmer for help with recruitment and clinical evaluation of volunteers, James Nataro and Sofie Livio for inoculum preparation, Mardi Reyman for laboratory assistance, and Robert Edelman, George Fantry, and Steven James for helpful suggestions.
This study was supported by Antex Biologics, Inc.
REFERENCES
- 1.Angelakopoulos H, Hohmann E L. Pilot study of phoPlphoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect Immun. 2000;68:2135–2141. doi: 10.1128/iai.68.4.2135-2141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamford K B, Fan X, Crowe S E, Leary J F, Gourley W K, Luthra G K, Brooks E G, Graham D Y, Reyes V E, Ernst P B. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 3.Banatvala N, Davies G R, Abdi Y, Clements L, Rampton D S, Hardie J M, Feldman R A. High prevalence of Helicobacter pylori metronidazole resistance in migrants to east London: relation with previous nitroimidazole exposure and gastroduodenal disease. Gut. 1994;35:1562–1566. doi: 10.1136/gut.35.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanchard T G, Czinn S J, Maurer R, Thomas W D, Soman G, Nedrud J G. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect Immun. 1995;63:1394–1399. doi: 10.1128/iai.63.4.1394-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements J D, Finkelstein R A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979;24:760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa P, Fox J, Fontham E, Ruiz B, Lin Y P, Zavala D, Taylor N, Mackinley D, de Lima E, Portilla H. Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer. 1990;66:2569–2574. doi: 10.1002/1097-0142(19901215)66:12<2569::aid-cncr2820661220>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Corthesy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A C, Haas R, Kraehenbuhl J P, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 8.Cover T L, Cao P, Murthy U K, Sipple M S, Blaser M J. Serum neutralizing antibody response to the vacuolating cytotoxin of Helicobacter pylori. J Clin Investig. 1992;90:913–918. doi: 10.1172/JCI115967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crabtree J E, Taylor J D, Wyatt J I, Heatley R V, Shallcross T M, Tompkins D S, Rathbone B J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 10.Czinn S J. What is the role for vaccination in Helicobacter pylori? Gastroenterology. 1997;113:S149–S153. doi: 10.1016/s0016-5085(97)80028-5. [DOI] [PubMed] [Google Scholar]
- 11.Czinn S J, Cai A, Nedrud J G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieterich C, Bouzourene H, Blum A L, Corthesy-Theulaz I E. Urease-based mucosal immunization against Helicobacter heilmannii infection induces corpus atrophy in mice. Infect Immun. 1999;67:6206–6209. doi: 10.1128/iai.67.11.6206-6209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiPetrillo M D, Tibbetts T, Kleanthous H, Killeen K P, Hohmann E L. Safety and immunogenicity of phoPlphoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine. 1999;18:449–459. doi: 10.1016/s0264-410x(99)00246-7. [DOI] [PubMed] [Google Scholar]
- 15.Doidge C, Crust I, Lee A, Buck F, Hazell S, Manne U. Therapeutic immunisation against Helicobacter infection. Lancet. 1994;343:914–915. doi: 10.1016/s0140-6736(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 16.Drumm B. Helicobacter pylori in the pediatric patient. Gastroenterol Clin N Am. 1993;22:169–182. [PubMed] [Google Scholar]
- 17.Eaton K A, Ringler S S, Krakowka S. Vaccination of gnotobiotic piglets against Helicobacter pylori. J Infect Dis. 1998;178:1399–1405. doi: 10.1086/314463. [DOI] [PubMed] [Google Scholar]
- 18.Ermak T H, Glannasca P J, Nichols R, Myers G A, Nedrud J, Weltzin R, Lee C K, Kleanthous H, Monath T P. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan X J, Chua A, Shahi C N, McDevitt J, Keeling P W, Kelleher D. Gastric T lymphocyte responses to Helicobacter pylori in patients with H. pylori colonisation. Gut. 1994;35:1379–1384. doi: 10.1136/gut.35.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrero R L, Thiberge J M, Huerre M, Labigne A. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrero R L, Thiberge J M, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiocca R, Villani L, Turpini F, Turpini R, Solcia E. High incidence of Campylobacter-like organisms in endoscopic biopsies from patients with gastritis, with or without peptic ulcer. Digestion. 1987;38:234–244. doi: 10.1159/000199597. [DOI] [PubMed] [Google Scholar]
- 23.Forman D, Newell D G, Fullerton F, Yarnell J W, Stacey A R, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. Br Med J. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghiara P, Rossi M, Marchetti M, Di Tommaso A, Vindigni C, Ciampolini F, Covacci A, Telford J L, De Magistris M T, Pizza M, Rappuoli R, Del Giudice G. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham D Y, Lew G M, Klein P D, Evans D G, Evans D J, Jr, Saeed Z A, Malaty H M. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992;116:705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- 26.Grant C C, Messer R J, Cieplak W., Jr Role of trypsin-like cleavage at arginine 192 in the enzymatic and cytotonic activities of Escherichia coli heat-labile enterotoxin. Infect Immun. 1994;62:4270–4278. doi: 10.1128/iai.62.10.4270-4278.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guarino A, Bisceglia M, Canani R B, Boccia M C, Mallardo G, Bruzzese E, Massari P, Rappuoli R, Telford J. Enterotoxic effect of the vacuolating toxin produced by Helicobacter pylori in Caco-2 cells. J Infect Dis. 1998;178:1373–1378. doi: 10.1086/314427. [DOI] [PubMed] [Google Scholar]
- 28.Guarner J, Mohar A, Parsonnet J, Halperin D. The association of Helicobacter pylori with gastric cancer and preneoplastic gastric lesions in Chiapas, Mexico. Cancer. 1993;71:297–301. doi: 10.1002/1097-0142(19930115)71:2<297::aid-cncr2820710205>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Guy B, Hessler C, Fourage S, Haensler J, Vialon-Lafay E, Rokbi B, Millet M J. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850–856. doi: 10.1016/s0264-410x(97)00258-2. [DOI] [PubMed] [Google Scholar]
- 30.Haeberle H A, Kubin M, Bamford K B, Garofalo R, Graham D Y, El-Zaatari F, Karttunen R, Crowe S E, Reyes V E, Ernst P B. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229–4235. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosking S W, Ling T K, Chung S C, Yung M Y, Cheng A F, Sung J J, Li A K. Duodenal ulcer healing by eradication of Helicobacter pylori without anti-acid treatment: randomised controlled trial. Lancet. 1994;343:508–510. doi: 10.1016/s0140-6736(94)91460-5. [DOI] [PubMed] [Google Scholar]
- 32.Ikewaki J, Nishizono A, Goto T, Fujioka T, Mifune K. Therapeutic oral vaccination induces mucosal immune response sufficient to eliminate long-term Helicobacter pylori infection. Microbiol Immunol. 2000;44:29–39. doi: 10.1111/j.1348-0421.2000.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 33.Karttunen R, Karttunen T, Ekre H P, MacDonald T T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keenan J, Oliaro J, Domigan N, Potter H, Aitken G, Allardyce R, Roake J. Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect Immun. 2000;68:3337–3343. doi: 10.1128/iai.68.6.3337-3343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleanthous H, Myers G A, Georgakopoulos K M, Tibbitts T J, Ingrassia J W, Gray H L, Ding R, Zhang Z Z, Lei W, Nichols R, Lee C K, Ermak T H, Monath T P. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect Immun. 1998;66:2879–2886. doi: 10.1128/iai.66.6.2879-2886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein P D, Gilman R H, Leon-Barua R, Diaz F, Smith E O, Graham D Y. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am J Gastroenterol. 1994;89:2196–2200. [PubMed] [Google Scholar]
- 37.Komase K, Tamura S, Matsuo K, Watanabe K, Hattori N, Odaka A, Suzuki Y, Kurata T, Aizawa C. Mutants of Escherichia coli heat-labile enterotoxin as an adjuvant for nasal influenza vaccine. Vaccine. 1998;16:248–254. doi: 10.1016/s0264-410x(97)00176-x. [DOI] [PubMed] [Google Scholar]
- 38.Kosunen T U, Seppala K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori. Lancet. 1992;339:893–895. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 39.Krivan H C, Ginsburg V, Roberts D D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2) Arch Biochem Biophys. 1988;260:493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- 40.Krivan H C, Roberts D D, Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambert J R, Lin S K. Prevalence/disease correlates of H. pylori. In: Hunt R H, Tygat G N J, editors. Helicobacter pylori: basic mechanisms to clinical cure. Amsterdam, The Netherlands: Kluwer Academic Publishers; 1994. pp. 95–112. [Google Scholar]
- 42.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee C K, Soike K, Hill J, Georgakopoulos K, Tibbitts T, Ingrassia J, Gray H, Boden J, Kleanthous H, Giannasca P, Ermak T, Weltzin R, Blanchard J, Monath T P. Immunization with recombinant Helicobacter pylori urease decreases colonization levels following experimental infection of rhesus monkeys. Vaccine. 1999;17:1493–1505. doi: 10.1016/s0264-410x(98)00365-x. [DOI] [PubMed] [Google Scholar]
- 44.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 45.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 46.Marchetti M, Rossi M, Giannelli V, Gluliani M M, Pizza M, Censini S, Covacci A, Massari P, Pagliaccia C, Manetti R, Telford J L, Douce G, Dougan G, Rappuoli R, Ghiara P. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 47.Marshall B J, Goodwin C S, Warren J R, Murray R, Blincow E D, Blackbourn S J, Phillips M, Waters T E, Sanderson C R. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988;2:1437–1442. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- 48.Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney A C, Heltz M, Bille J, Kraehenbuhl J P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 49.Michetti P, Kreiss C, Kotloff K L, Porta N, Blanco J L, Bachmann D, Herranz M, Saldinger P F, Corthesy-Theulaz I, Losonsky G, Nichols R, Simon J, Stolte M, Ackerman S, Monath T P, Blum A L. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116:804–812. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 50.NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. NIH Consensus Conference: Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 51.Nomura A, Stemmermann G N, Chyou P H, Kato I, Perez-Perez G I, Blaser M J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 52.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 53.Pasetti M F, Anderson R J, Noriega F R, Levine M M, Sztein M B. Attenuated ΔguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin Immunol. 1999;92:76–89. doi: 10.1006/clim.1999.4733. [DOI] [PubMed] [Google Scholar]
- 54.Radcliff F J, Hazell S L, Kolesnikow T, Doidge C, Lee A. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect Immun. 1997;65:4668–4674. doi: 10.1128/iai.65.11.4668-4674.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauws E A, Tytgat G N. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 56.Rowland M, Kumar D, Daly L, O'Connor P, Vaughan D, Drumm B. Low rates of Helicobacter pylori reinfection in children. Gastroenterology. 1999;117:336–341. doi: 10.1053/gast.1999.0029900336. [DOI] [PubMed] [Google Scholar]
- 57.Rupnow M F, Owens D K, Shachter R, Parsonnet J. Helicobacter pylori vaccine development and use: a cost-effectiveness analysis using the Institute of Medicine methodology. Helicobacter. 1999;4:272–280. doi: 10.1046/j.1523-5378.1999.99311.x. [DOI] [PubMed] [Google Scholar]
- 58.Saldinger P F, Porta N, Launois P, Louis J A, Waanders G A, Bouzourene H, Michetti P, Blum A L, Corthesy-Theulaz I E. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter Infection. Gastroenterology. 1998;115:891–897. doi: 10.1016/s0016-5085(98)70261-6. [DOI] [PubMed] [Google Scholar]
- 59.Satin B, Del Giudice G, Della B, Dusi V S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467–1476. doi: 10.1084/jem.191.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schutze K, Hentschel E, Dragosics B, Hirschl A M. Helicobacter pylori reinfection with identical organisms: transmission by the patients' spouses. Gut. 1995;36:831–833. doi: 10.1136/gut.36.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sztein M B, Wasserman S S, Tacket C O, Edelman R, Hone D, Lindberg A A, Levine M M. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 62.Tacket C O, Mason H S, Losonsky G, Clements J D, Levine M M, Arntzen C J. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat Med. 1998;4:607–609. doi: 10.1038/nm0598-607. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 64.Todoroki I, Joh T, Watanabe K, Miyashita M, Seno K, Nomura T, Ohara H, Yokoyama Y, Tochikubo K, Itoh M. Suppressive effects of DNA vaccines encoding heat shock protein on Helicobacter pylori-induced gastritis in mice. Biochem Biophys Res Commun. 2000;277:159–163. doi: 10.1006/bbrc.2000.3632. [DOI] [PubMed] [Google Scholar]
- 65.Wotherspoon A C, Doglioni C, Diss T C, Pan L, Moschini A, de Boni M, Isaacson P G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 66.Wyant T L, Tanner M K, Sztein M B. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun. 1999;67:3619–3624. doi: 10.1128/iai.67.7.3619-3624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia H X, Talley N J, Keane C T, O'Morain C A. Recurrence of Helicobacter pylori infection after successful eradication: nature and possible causes. Dig Dis Sci. 1997;42:1821–1834. doi: 10.1023/a:1018827322470. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]