Abstract
Background
A large volume of evidence and reviews about COVID-19 recommendations have been published; however, only a few provide recommendations on maternal health services ready for their adoption in Indonesia. This review aims to identify a set of potential recommendations for improving maternal care in the Indonesian primary care setting under the COVID-19 pandemic.
Methods
A literature search to identify articles that cover a set of recommendations, or maternal guideline under the SARS-COV-1 outbreak or COVID-19 pandemic that were published from 2020 to 1 November 2022 was applied in six academic databases. The search used various keywords and phrases of ‘maternal’, ‘model’ and ‘coronavirus’, and excluded reviews and those evaluating interventions or medicine prescription. The eligible guidelines were appraised using AGREE II instrument, coded, and thematically analysed for their potential adoption to Indonesian settings.
Findings
Fourteen guidelines were fully reviewed, and most of them had high AGREE II scores. Two main themes emerged from the analysis: clinical and supporting arrangements for maternal health services. Potential challenges for the implementation of these recommendations in Indonesia were also discussed.
Conclusion and implication for practice
Potential recommendations for improving maternal health for women in Indonesia under the COVID-19 pandemic both on the clinical and supporting services arrangements have been identified. Available clinical resources, different context of providers’ practice authority and patients’ literacy may challenge their implementation in practice. Further research is needed to seek consensus on the recommendation adoption in practice and to desirably redesign maternal health service in Indonesia.
Keywords: Primary care, Maternal health, Antenatal care, Indonesia, Systematic review, COVID-19
Introduction
Indonesia and other low-and-middle-income countries (LMICs) suffer most from the COVID-19 pandemic. Many of the countries already have high maternal mortality ratios and now have to also hold massive burdens of the pandemics, such as an increased number of pregnant women, limited maternal services, and more women with serious COVID-19 complications [1], [2]. Moreover, it has been predicted that the pandemic may increase the maternal mortality up to 38·6% in 118 countries, such as India, Indonesia, and Pakistan [3], and potentially risks pregnant women for developing anxiety, depression, domestic violence and the reduced visits to health providers [2], [4], [5].
To prepare providers providing their care for the women, there are two main guidelines currently available in Indonesian primary care: Petunjuk teknis pelayanan Puskesmas pada masa COVID-19 (Guidance for primary care service under COVID-19 pandemic) [6] and the Pedoman bagi ibu hamil, bersalin, nifas, dan bayi baru lahir di era pandemik COVID-19 Revisi 1 and 2 (Guideline for pregnancy, postpartum and newborn care under COVID 19 pandemic Revision 1 and 2) [7], [8]. Unfortunately, much misinformation still occurs in practice, such as confusion on distancing measures and number of the needed face-to-face antenatal visits to the clinics. These confusions and ineffective management then may harm the safety of providers in primary care as well as the women, who are in the high risk of getting severe COVID- 19 complication or deaths, which may subsequently increase the maternal mortality [3].
While many publications were available to provide recommendations for improving maternity care at the international level, very limited evidence is available to assist Indonesian primary care providers, particularly when Indonesia became the epicentre of COVID-19 pandemic from June-August 2021. Usually, management about COVID-19 care are from developed countries with complete facilities and strong health infrastructure for the recommendation implementation, while different context, limited facilities and low quality of practice of the providers may challenge those recommendations adoption in limited resource settings [9]. Nonetheless, maintaining the good-quality provision of maternity service in Indonesian primary care is essential in the context of the COVID-19 pandemic.
This systematic review is part of a more extensive study aiming to improve the design of maternal health services in Indonesia under the COVID- 19 pandemic. The review aims to identify any clinical and supporting recommendations for improving primary maternal health services and appraise them for their potential adoption in Indonesian primary care practice.
Methods
Search strategy and eligibility criteria
We systematically searched PubMed, EMBASE, MEDLINE, CINAHL, Web of Science, and Cochrane Library for any guideline, model, a set of recommendations or similar form on maternal health service under the COVID-19 pandemic. The systematic search was conducted on 29 January 2021 and was repeated again on 1 November 2022 using MeSH terms and phrases, including ‘maternal care’, ‘primary care’. ‘model’ and ‘coronavirus’ for article that was published between 2000 and 1 November 2022. The publication from 2000 was included to look for any practice recommendation arising from the SARS-Coronavirus 1 outbreak in 2002–2004.
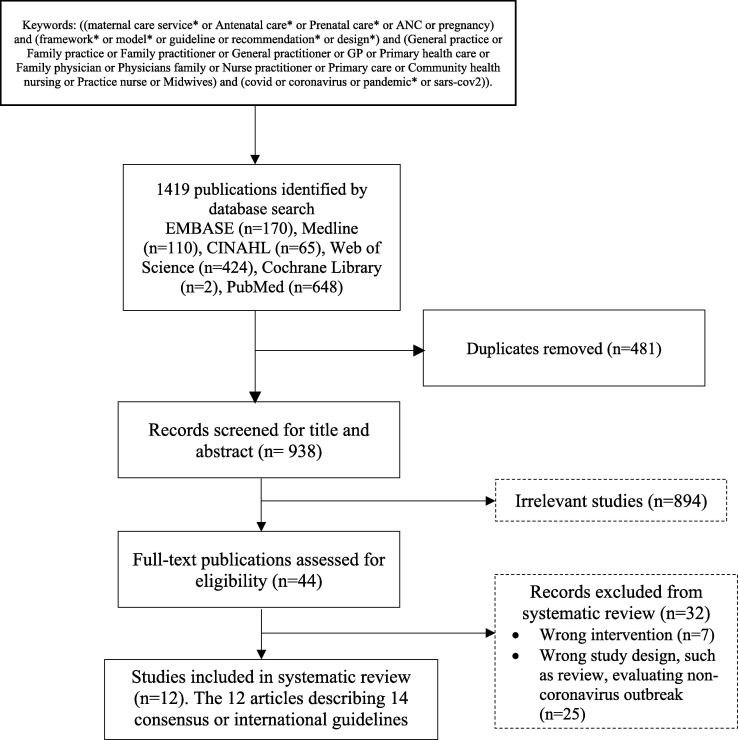
Our inclusion criteria were any published guidelines or models or consensus or set of recommendations or design of maternal care potentially adopted for primary care during COVID-19 pandemics, and were published in Spanish or English due to the authors' fluency in the languages. We excluded reviews, sole opinion articles or commentaries without discussing any referred guidelines, perspectives, non-peer-reviewed papers; and articles which evaluated specific interventions or single medication for COVID-19. If the articles or commentaries discussing a guideline, then the referred guideline would be appraised and fully reviewed. The Bahasa Indonesia related guidelines and recommendations were also searched and were included as the comparison context for those international guidelines to analyse the suitability of the recommendation to Indonesian settings. The detailed of the search terms used along with the flowchart of paper selection are reported in Fig. 1 .
Fig. 1.
Flowchart of selection of eligible publications.
Selecting studies and collecting data
All retrieved studies were managed and screened using Covidence (https://www.covidence.org/) by three authors, who independently screened publications for titles and abstracts. All publications that met eligibility criteria were then retrieved in the full-text format, followed by a full-text review. Data extraction, i.e., author’s name, year of study, expected audience, potential recommendations, was performed by the first and the third author; and if any discrepancies occurred, the second and forth authors would be involved. This study design is registered in PROSPERO (CRD42021257030).
Risk of bias assessment
The reviewed guidelines were assessed and analysed based on the Appraisal of Guidelines for Research & Evaluation (AGREE) II instrument [10]-an appraisal tool to evaluate the quality of a guideline or recommendations against six domains: scope and purposes, stakeholder involvement, rigour of development, clarity of presentation, applicability, editorial independence, and overall guideline assessment. Appraisals of the guidelines were performed by two authors independently, and the results were compared and counted for the final scores. Each guideline was also rated on its potential adoption to Indonesian practice: ‘Yes’ if they were likely applicable for the settings, ‘Yes with modification’ if the quality of the guideline were scored well, but its potential implementation in Indonesia was undetermined or need to be modified, and ‘No’ if the quality of the guideline was scored low and unlikely to be implemented in Indonesian contexts. Our consideration on the recommendation applicability was based on the general facility availability in Indonesia, particularly in Yogyakarta and South-East Sulawesi Province, that represent condition in the urban and rural primary care practice in Indonesia; and where the authors are also familiar with the context of practice in the settings. For instance, the available facilities and the context that prescription and care for severe ill pregnant women are usually under the obstetrician care and the task of midwives and general practitioners are to manage uncomplicated pregnancy care [11], [12], [13]. Any disagreement on the AGREE II scores and appraisal of the guidelines were also discussed with all investigators until the authors achieved a final decision.
Comparison to the Indonesian context
The first and second authors, who are consisted of two general practitioners and two midwives with more than ten years-experience of practicing and conducting research in maternal health in Indonesian primary care, compared and analysed content of the reviewed guidelines or recommendations to the Indonesian context. The comparison guided by the authors’ clinical experience and consideration based on seven Indonesian regulations and guidelines for maternal health and primary care service under COVID-19 pandemic ( Table 1 ).
Table 1.
National guidelines and regulations used to provide context of maternal health services in Indonesia.
|
|
|
|
|
Extraction and analysis of the recommendations
Recommendations of each guideline was analysed and synthesised using thematic analysis [14], aided by NVivo-12 Software [15]. The guidelines were uploaded into the software and were coded using inductive thematic analysis principles as follows: any significant recommendations for improving maternal health service in primary care, such as breastfeeding, triage, and the use of protective measurements, prereferral procedures, etc, were coded and grouped into themes of procedures, and the similar themes were then also grouped into overarching themes of procedures. During the coding process, any advanced procedures that may not be suitable for Indonesian primary care, such as the use of advanced medicines or procedures in the hospital were excluded, and any potential barriers to their implementation were also noted. This coding process were conducted by the first and the third author and the results were also discussed and validated by all authors in a series of meeting until consensus was achieved. Results of the analysis are presented in the headings below informed by the The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist (Supplementary file 1) [16].
Results:
A total of 1419 articles were identified, and 481 of them were removed for duplications. Among the 938 papers, 894 were excluded from the title screening, and another 32 papers were excluded from the abstract review as or suitable for the inclusion criteria for this study. Finally, twelve articles fit for a complete review in this study (Fig. 1). The twelve final reviewed papers described fourteen consensus or international guidelines or a set of recommendations for maternal care (Table 2 ). There are four articles that referred another guidelines reviewed in this review i.e: Smith [17], that indicates the Centers of Disease Control and Prevention (CDC) guidelines on maternal and breastfeeding care for women in the context of COVID-19, [18], [19], [20]; Larki, Sharifi et al. [21] and the three CDC guidelines also indicate a complementary guideline from United Nations Population Fund (UNFPA) COVID-19 Technical Brief for Maternity Services [22]. Meanwhile, the article from Cortes [23] indicates Technical document: Management of the pregnant woman and the newborn born with COVID-19 (Documento técnico: Manejo de la mujer embarazada y el recién nacido con COVID-19 [24] , and article from Giusti, Zambri et al. [25] referred the Interim guidance on pregnancy, childbirth, breastfeeding and the care of infants (0–2 years) in response to the COVID-19 emergency Version 2021 [26] . These fourteen guidelines then were fully reviewed in this study (Fig. 1 , Table 2).
Table 2.
Characteristic of selected papers and guidelines for improving maternal services in primary care under the COVID-19 pandemic in low-and middle-income countries (n = 12).
| Author of eligible articles | Year published | Context and country of origin | Expected Audience | |
|---|---|---|---|---|
| Afshar, Silverman et al. [29] | As per the publication | 2020 | United States | Maternal fetal medicine specialists |
| Smith [17] | CDC [20] Maternal, neonatal and child service | 2020 | United States | Not specified |
| CDC [18] Care for Breastfeeding people interim guidance | 2020 | United States | Not specified | |
| CDC [19] Considerations for Inpatient Obstetric - Healthcare Settings | 2020 | United States | Staff at hospital/inpatient obstetric settings | |
| Chawla, Chirla et al. [27] | Same as the publication | 2020 | India | Not specified |
| Cortes [23] | Management of the pregnant woman and and newborn born with COVID-19 (Documento técnico: Manejo de la mujer embarazada y el recién nacido con COVID-19[24] | 2020 | Spain | Not specified |
| Okunade, Makwe et al. [30] | As per the publication | 2020 | Nigeria | Low-resource countries |
| Larki, Sharifi et al. [21] | UNFPA [22] COVID-19 Technical Brief for Maternity Services | 2020 | United States | Not specified, but include considerations for low resource settings. |
| Vogel, Tendal et al. [28] | As per the publication, reporting living guidelines from The National COVID-19 Clinical Evidence Taskforce, Australia on clinical care for pregnant and postpartum women. | 2020 | Australia | Australian maternity services |
| Giusti, Zambri et al. [25] | Interim guidance on pregnancy, childbirth, breastfeeding and the care of infants (0–2 years) in response to the COVID-19 emergency Version 2021 [26] | 2021 | Italy | Not specified, but covering many aspects of maternal and perinatal care during COVID-19 pandemic |
| Vivanti, Deruelle et al. [32] | As per the publication, answering the request from French Ministry of Solidarity and Healthcare associations. | 2020 | France | French healthcare professionals and/or health system users |
| Vivanti, Deruelle et al. [54] | As per the publication, answering the request from French Ministry of Solidarity and Healthcare associations. | 2020 | France | French healthcare professionals and/or health system users |
| Homer, Roach et al. [33] | As per the publication, reporting update recommendations for pregnancy care from The National COVID-19 Clinical Evidence Taskforce, Australia | 2022 | Australia | Australian maternity care providers |
| Maxwell, McGeer et al. [31] | As per the publication, summarizing the guideline for pregnancy care during SARS-COV 1, sponsored by The Society of Obstetricians and Gynaecologists of Canada | 2009 | Canada | Not specified, aiming to support health care providers caring for pregnant women in Canada |
AGREE II Scores for the reviewed guidelines.
The AGREE II scores of the guidelines are varied. Most of the guidelines or the set of recommendations are scored well, such as Breastfeeding guideline in the context of COVID-19 by CDC [18], Chawla, Chirla et al. [27], UNFPA [22], and Vogel, Tendal et al. [28] for their comprehensiveness of maternal recommendations and potential adoption in Indonesian settings. The other guidelines are scored low due to their lack of clarity across stakeholder involvement, rigour of development, and applicability domains of AGREE (Table 3 ).
Table 3.
Guideline’s AGREE II scores.
| Guidelines | Domain 1. Scope and purposes | Domain 2. Stakeholder involvement | Domain 3. Rigor of development | Domain 4. Clarity of the presentation | Domain 5. Applicability | Domain 6. Editorial independence | Overall | Use of the Guidelines | Comments for the recommendation potential implementation in primary care in LMICs |
|---|---|---|---|---|---|---|---|---|---|
| Afshar, Silverman et al. [29] | 72 | 64 | 29 | 47 | 44 | 92 | 42 | Yes, with modification | Recommendations in this guideline are aimed primarily for maternal foetal medicine specialists at the hospital, and not primarily in primary care. Only some of the recommendations from this paper can be adopted to primary care practice, such as on the triage, and maintaining routine ANC. Other recommendation needs further considerations due to lack of facilities and patients’ literacy level for telemedicine, and level of competence compared to specialists, such as prescription for ill pregnant women. |
| CDC [20] Maternal, neonatal and child service | 72 | 47 | 48 | 97 | 58 | 50 | 67 | Yes | This guideline is succinct, and clear, most of the recommendations potentially be adopted to Indonesian context. |
| CDC [18] Care for Breastfeeding people interim guidance | 86 | 58 | 50 | 89 | 56 | 58 | 75 | Yes | Most of the recommendations in the guideline can be implemented in Indonesian primary care. |
| CDC [19] Considerations for Inpatient Obstetric - Healthcare Settings | 86 | 69 | 35 | 89 | 63 | 67 | 67 | Yes, with modification | The guidelines aimed for patient management in inpatient obstetric settings (hospital). There are inpatient services for women in primary care in Indonesia, however, with obvious limited facilities compared to hospitals. |
| Chawla, Chirla et al. [27] | 92 | 86 | 76 | 89 | 71 | 85 | 92 | Yes | This article covers consensus recommendations from Federation of Obstetric and Gynaecological Societies of India (FOGSI), National Neonatology Forum of India (NNF), and Indian Academy of Pediatrics (IAP). However, the recommendations are potential for their adoption in Indonesian primary care due to facility similarities. |
| España [24] | 18 | 17 | 10 | 16 | 13 | 8 | 5 | Yes, with modification | This guideline provides recommendations for management of pregnant women and newborns with COVID-19 mainly at hospital care level, which obviously has different facilities compared to primary care. However, some can be adopted to Indonesian primary care settings, such as: Isolation at home for eligible women with COVID-19 and maintaining routine ANC service for women during the pandemic. |
| Okunade, Makweet al. [30] | 84 | 81 | 51 | 81 | 56 | 83 | 67 | Yes | The article covers recommendations on maternal care under COVID-19 pandemic in Nigeria, however, most of the recommendations, that are not hospital based, are able to be adopted to the context of primary care in Indonesia. |
| UNFPA [22] | 92 | 81 | 48 | 89 | 60 | 67 | 83 | Yes | Recommendations covered in this guideline can be implemented in Indonesia. |
| Vogel, Tendal et al. [28] | 92 | 89 | 85 | 92 | 94 | 92 | 92 | Yes, with modification | This article provides comprehensive living guideline for management of women with COVID-19, that can be accessed through https://COVID19evidence.net.au/. Most of the recommendations can be adopted in Indonesian context, however, recommendations on prescription for severe ill pregnant women may be difficult to adopt as it usually be provided by obstetricians. |
| Giusti, Zambriet al. [25] | 94 | 89 | 71 | 94 | 75 | 92 | 83 | Yes | This guidance provides a clear and practical set of recommendations that could be applied to Indonesian settings. |
| Vivanti, Deruelle et al. [32] | 94 | 94 | 90 | 94 | 79 | 92 | 83 | Yes | Recommendations covered in this guideline can be implemented in Indonesia, eventhough there may be challenges on the use of telemedicine. |
| Vivanti, Deruelle et al. [54] | 94 | 94 | 90 | 94 | 79 | 92 | 83 | Yes | Recommendations covered in this guideline can be implemented in Indonesia. |
| Homer, Roach et al. [33] | 94 | 94 | 94 | 100 | 79 | 92 | 100 | Yes, with modification | The guideline is well developed guidance made by The National COVID-19 Clinical Evidence Taskforce (Australia), covering a clear and succinct set of recommendations that can be adopted to Indonesian setting, except for some antiviral drugs that are not readily available in the country. There is also a living guidelines approach that can be accessed anytime. |
| Maxwell, McGeeret al. [31] | 94 | 78 | 94 | 94 | 83 | 83 | 83 | Yes, with modification | This guideline provides recommendations for management of pregnant women during the Coronavirus (SARS-COV) 1 outbreak at hospital care level, however the some of the recommendations, such as isolation requirement and triage could be adopted in the Indonesian primary care settings during this COVID-19 pandemic. |
Identified recommendations for improving primary maternity care in Indonesia.
Most of the guidelines provide recommendations on clinical care of pregnant women with COVID-19 or pregnancy-related care during the pandemic. Nonetheless, the analysis raised two main groups of recommendations for pregnancy care during the pandemic: clinical care and supporting arrangements for maternal health services.
Clinical care
This theme presents our findings on recommendations that can be used to improve the services from direct clinical maternity care. It consists of several subthemes: the importance of attention to COVID-19 in pregnancy and maintaining routine antenatal care (ANC) for women in primary care. They were then followed by the direct clinical recommendations on triage, laboratory and imaging arrangement for women, medication used in pregnancy, care for labour and delivery, breastfeeding and well-baby checks in primary care. The analysis of the recommendations' suitability for Indonesian context is also provided at the end of this theme (Table 4 ).
Table 4.
Summary of selected studies for improving maternal services in primary care under the COVID-19 pandemic in low-and middle-income countries (n = 12).
| Recommendations | Authors/Name of the consensus or guideline | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Giusti, Zambriet.al. [50] | Vivanti, Deruelleet.al. [32] | Afshar, Silvermanet.al. [29] | Vivanti, Deruelleet.al. [54] | CDC [20] Maternal, neonatal and child service | CDC [18] Care for Breastfeeding people interim guidance | CDC [19] Considerations for Inpatient Obstetric - Healthcare Settings | Chawla, Chirla etal. [27] | España [24] | Homer, Roachet.al. [33] | Okunade, Makwe et al. [30] | UNFPA [22] | Vogel, Tendal et al. [28] | Maxwell, McGeer et al. [31] | |
| Care for pregnant women in primary care | ||||||||||||||
|
√ | – | √ | √ | – | √ | √ | √ | √ | √ | √ | – | – | √ |
|
√ | √ | √ | – | – | – | – | √ | – | – | – | √ | – | – |
|
√ | √ | √ | – | √ | √ | √ | – | √ | – | – | √ | – | √ |
|
√ | √ | √ | – | – | – | – | – | √ | – | √ | – | – | – |
|
√ | – | – | – | – | – | – | – | – | √ | √ | √ | √ | √ |
|
√ | – | – | – | – | √ | – | √ | √ | √ | – | √ | √ | √ |
|
√ | √ | √ | √ | √ | – | – | √ | √ | √ | – | √ | √ | √ |
|
√ | √ | – | √ | √ | √ | √ | – | – | – | – | √ | – | – |
| Supporting arrangements in maternal health services | ||||||||||||||
|
√ | – | √ | – | – | – | √ | √ | – | – | – | √ | – | – |
|
√ | √ | – | √ | √ | √ | – | – | √ | – | √ | √ | – | √ |
|
√ | √ | √ | √ | – | – | √ | √ | √ | √ | – | – | – | √ |
|
√ | √ | √ | √ | – | – | – | – | – | – | √ | √ | – | – |
Impacts of COVID-19 in pregnancy and the importance of maintaining routine ANC
Understanding of the impacts of COVID-19 and the importance of maintaining routing ANC is essential for pregnant women. COVID-19 could have severe complications for pregnant women, and that they are more susceptible to the risk of preeclampsia like symptoms, venous thromboembolism to the severe respiratory manifestation of COVID-19 symptoms [19], [20], [27], [29], [30]. The women, infants, and family may also experience anxiety, depression symptoms or domestic violence caused restriction and isolation during the pandemic [24].
Therefore, it is important to keep maintaining the routine ANC despite the restrictions applied in COVID-19 pandemic. Women are advised to have the first ANC booking and be determined their pregnancy status either directly from the health care facility or through surveillance provided by community health workers (cadres) [20], [22]. The ANC shall cover important information about routine pregnancy care as well as information on COVID-19, hygiene and distancing measures [22], [29]. In the case where the women have the COVID- 19, their condition should be carefully monitored. Asymptomatic women with no comorbidities can isolate at home; however, those with morbidities and incapability to take care of themselves should be managed closely and be considered for hospital monitoring [27]. They can continue to have their visit to the clinic again after the symptoms resolved, have negative tests, or based on the end of isolation decision by the health providers [22].
Triage
All women and the accompanying persons visiting to health care service should be triaged for any COVID-19-related symptoms, or whether are in close contact with suspected or confirmed cases or are currently in isolation [26], [31], [32]. If they have these conditions, they should be directed to have a COVID-19 test and stay at home as per the isolation requirement [20]. These triage procedures are also recommended for women during their referral, well-baby service, or lactation consultation during postpartum periods [18], [22].
Necessary laboratory and imaging arrangement for women
Any examination for pregnant women, whether to determine the COVID-19 severity or to follow up the pregnancy, should be conducted with appropriate precaution. Chest imaging (X-ray or CT) to determine the severity of COVID-19 should be conducted with abdominal shielding and may not be delayed due to the foetal concern [24], [30]. Additional examinations for COVID-19, such as electrocardiogram, CT-scan and echocardiogram, can also be conducted to rule out differential diagnoses [24], [30].
Examinations to assess foetal growth and well-being should not also be delayed. When possible, the examinations can be conducted without any accompanying person, conducted by limited staff and precautionary measures. Staff is recommended to wear masks, shield, and goggles and perform routine cleaning for the exam table and USG machine to minimise any potential virus spread [29]. While women without risks of COVID-19 can continue to have their scheduled scan, USG scan for confirmed COVID-19 women, should be performed after the isolation.
Medication for COVID-19 in pregnancy
Despite most COVID-19 patients having mild symptoms and no medication needed, the severity of the disease in pregnant women can be more serious [22], [30]. The women may need to deliver the babies earlier, considering whether delivery can improve their respiratory system. If they have to deliver in less than 34 weeks, prenatal corticosteroids should be given when indicated as per the usual guideline (Homer et al., 2022). There is no evidence to suggest that steroids to help foetal lung maturation cause any harm to women that any urgent consideration may not prevent its administration [22], [30]. Women with COVID-19 may also experience coagulation syndrome and can be suitable for low molecular weight heparins once daily based on the consultations with specialists [28].
End of pregnancy and caring for birth
There were limited recommendations about the timing and mode of delivery for women with COVID-19 in the reviewed guidelines. However, the birth mode needs to be decided based on individual basis and whether there is any obstetric indication. Unless there is a maternal or foetal emergency, the labour should proceed as usual practice. Special consideration for early delivery of their babies may be given to women with moderate-severe COVID-19 who may have respiratory distress syndrome or dehydration [26], [28].
When women with COVID-19 require a caesarean section, they should be referred to a hospital with advanced life support and adequate protective measurements [22], [27]. If rooms to separate women and babies are not available, the women and babies need to stay in isolated rooms away from other patients. HEPA (high-efficiency particulate air) filters should be used to prevent infection. Elective caesarean delivery for women with COVID-19 without any medical emergency may be postponed to optimize the women’s respiratory status prior to surgery under the care of obstetricians. There was also no direct evidence on the delayed cord clamping in women with COVID-19 that may increase potential vertical transmission between women and babies; therefore, the recommendation remains similar to the usual care [28].
While healthy women without any complications during the birth may be discharged after six hours of monitoring from the delivery unit, women with COVID-19 should remain in the facility until their symptoms are clear. Separating COVID-19-positive women with their babies may reduce the infection transmission, however, keeping them together provides benefits of better breastfeeding initiation, thermal regulation, and reduce the risks of sepsis and death in infants, particularly low birthweight babies, and that COVID-19 infection for babies generally mild [20], [33]. Parents with COVID-19 should use masks, practice appropriate distancing measures and hand hygiene to minimise the risk of infection spread [28].
Newborns exposed to COVID-19 from their mothers or households can be in the same isolation as their mothers and breastfeed as usual [24], [27]. Ideally, a negative air-borne isolation room is preferred to minimise aerosolization; otherwise, a room with exhaust fans or wide ventilation. If same isolation place for newborns with their mothers is not possible due to their infection severity, the newborn can be discharged within 48-hours and cared for by another family member. This early discharge should be followed up with a telephone or home visit monitoring, adequate education to the family about any red-flags in newborns and when to report them to the health providers [24], [27].
Breastfeeding and neonatal vaccination
It has been recommended in most of the reviewed papers that COVID-19 status in women should not prevent breastfeeding activities for their babies as benefits of breastfeeding outweigh the transmission risk, and it is unlikely to transmit the virus [22], [28], [34]. Women with COVID-19 can breastfeed their babies using proper hand-hygiene measures, wearing masks, or practicing expressed breastmilk [29]. Healthy adults who are not at higher risk of developing severe COVID-19 disease may also help feed the babies with the expressed breastmilk. If the adults were the close contact with the positive women, they should wear a mask during their quarantine time while feeding the babies [18], [27]. In the situation related to COVID-19, women cannot donate their breastmilk, while the breast pump used to express the milk has to be also cleaned regularly [24], [27].
If the infant is breastfed by a suspected or confirmed COVID-19 mother, they should be considered as close contact with a person with COVID-19 and quarantined as recommended. Mask is not recommended for children under two years for the risk of suffocation; therefore, the infection control is conducted by the breastfeeding mothers and the household by wearing masks, staying at a distance from the infant, and regular hand washing [18]. Routine vaccination for healthy babies born to mothers with suspected/proven COVID-19 infection can follow usual practice; even babies with suspected COVID-19 can complete their first Hepatitis B (0) vaccination before hospital discharge as per existing policy or based on consultation with paediatrician [27].
Comparisons from the Indonesian guidelines regarding the identified clinical aspect of maternity care under COVID-19.
The impact of COVID-19 infection in pregnant women and the importance of maintaining monitored ANC visits are mentioned superficially in Indonesian regulations and guidelines (Table 1). Most of the guidelines focus on the triage and referring women with COVID-19 to hospitals with limited practice guideline on escalation procedures to hospitals, i.e., only using the Modified Early Obstetric Warning Score (MEOWS) to assess the women before referrals [35] . The guideline also has limited recommendations on the importance of maintaining ANC visits in primary care, even though most of them recommend alternatives to telemedicine using phone calls or WhatsApp messaging if women develop any COVID-19 symptoms [35], [36], [37], [38].
Triage for pregnant women in Indonesian guidelines is conducted using questions on the infection history and rapid antigen tests [7], [35], [36]. However, recommendations about imaging or scanning for women in primary care are superficially covered. The use of antiviral therapy (Remdesivir and Lopinavir) in Indonesian guidelines has limited evidence for its benefits in pregnant women; however, recommendation for dexamethasone and low-molecular-weight heparins are mentioned in the POGI guidelines for hospital obstetricians [36].
Regarding the labour and delivery, women are only advised to self-isolate at home for 14 days close to their labour and tested with COVID antigen tests before their hospital admission without any specific recommendation on the time and mode of delivery [8], [35]. There was a trend of sectiocaesarean for women with COVID-19 in Indonesia during the early pandemic as recommendation for routine delivery had not yet been available [8], [36]. At that time, the Indonesian guidelines also recommend the women deliveries take place in isolation ward hospitals to minimise the spread of COVID-19 infection [8], [35], [36], [39]. However, in the case of emergency cases, Puskesmas or private midwifery services may prepare the baby delivery using COVID-19 safety measures [8], [35]. Some of the Indonesian guidelines also previously recommended the use of delivery chambers in primary care. Delivery chamber is a humble designated room covered with curtains or wide clothes to assist delivery of baby from women with COVID positive [8], [35]; however, this recommendation is no longer advised in POGI guideline [36].
There is no recommendation for a specific method to manage infants of COVID-19 positive women in Indonesian guidelines. Delayed umbilical cord is not recommended for newborns of women with COVID-19, and babies have to be bathed immediately to reduce the risk of infection [36], [39]. Limited recommendations are also available for rooming-in for babies and women with COVID-19 in Indonesian guidelines, or any room requirement for postnatal care for the women and babies [8], [36].
Recommendations for early breastfeeding initiation are also not clearly stated. Instead, the women are recommended to be counselled about the risks and benefits of rooming-in and breastfeeding or they can breastfeed from expressed breast milk with a proper hand-hygiene and equipment [8], [36]. Regular vaccination for newborns from confirmed COVID-19 women is recommended as routine procedure based on specialists’ recommendation [8], [36], [39].
Supporting arrangements in maternal health service
Limitation to visitors
Some of the reviewed guidelines mentioned only one accompanying person to provide essential support for the women, and they need to be triage as above recommendation [19], [27]. Suspected COVID-19 company should not visit health centres, and all visitors should practice adequate hand hygiene and infection control procedures. They may not be allowed to enter other rooms in the clinic or inpatient ward [19], [22], [29]. If the visit cannot be made, patients are recommended using teleconference or mobile video calls and family members may participate in the consultation [22], [29].
Multidisciplinary team
A multidisciplinary team approach in maternal health is also essential under COVID-19 pandemic. The UNFPA and CDC guidelines highlight the roles of cadres/community health workers as the first contact of the women, help triage the women, and to provide the necessary support for them in the community [18], [20], [22]. A multidisciplinary team is also required to assist women with pregnancy complications, which may include general practitioners, nurses, midwives in primary care, and obstetricians and neonatologists in the hospital [24], [30].
Protective equipment, cleaning and other precautions
Health workers should always wear adequate personal protective equipment when providing care for women and babies. In the setting where the status of the women can hardly be determined, a high-level precaution such as using a face shield or goggles is advised to protect the worker and other women from the virus transmission [27]. Besides those precautions, routine surface cleaning using disinfectant and handwashing between patients encounters is also strongly recommended, including maintaining 1.5 m from another person as much as possible [22], [24]. Health workers should also report to their supervisors if they develop COVID-19 symptoms, isolate until the symptoms resolved and they have a negative COVID-19 test. All healthcare facilities should always remind their staff of these precautions [19], [27].
Telemedicine
Since face-to-face encounters with patients are limited due to the COVID-19 safety requirements, telemedicine in maternal health has been increasingly used. Telemedicine is safer and can be used to monitor high-risk patients based on the physician's consideration [22], [29], [30]. Teleconference can also be used by inpatient women to communicate with the rest of their families impacted by limiting visitor regulation [22]. However, successful telemedicine usually depends on the quality of the device, internet networks and the user familiarity with the apps or webpages. It would also require a safe and private environment in the women’s residences, i.e., in the case of domestic violence that may prevent honest consultation from the patients [22], [29], [30].
Comparison from the Indonesian guidelines related to supporting arrangements in maternal health services under COVID-19
In Indonesian guidelines (Table 1), supporting arrangements of women’s care, such as limitations to visitors, cleaning and the importance of multidisciplinary team are not mentioned. The guidelines; however, are very detailed mentioning procedures for PPE use [8], [36], the use of telemedicine as an alternative to usual care, and financing for the services [35], [36], [37], [38]. They also mention activities that can be facilitated through telemedicine, such as health promotion, consultation, and maternal class, and mentioning many telemedicine apps, such as SEHATI tele-CTG, Halodoc, Alodoc, Teman Bumil, and SMSBunda [39] . Challenges for the supporting arrangements for maternal care under COVID-19 in Indonesia remain on whether the health service has adequate supply of PPE and whether the workers have a good practice of wearing it [40]. There is also unclear evidence on whether women can use telemedicine optimally, considering the available technology and their health literacy level [41].
Discussion:
This review has identified recommendations for improving maternal services that are potential for their adoption in Indonesian primary care settings. The guidelines appraisals using AGREE II scores showed high quality of most of the reviewed guidelines particularly for Chawla, Chirla et al. [27], UNFPA [22], [33] and Vogel, Tendal et al. [28] that increase confidence of the recommendation adoption in practice.
The core of the findings in this review consists of clinical care and supporting service arrangements for the women, from the structure, clinical procedures, and model of care to well-baby visits. The findings have stressed the importance of pregnancy and care for birth that may be adjusted from face-to-face to telemedicine and considering the local context and COVID-19 regulations. The recommendations from this review have also been analysed to complement the previous review on maternal health guidelines [42], as well as the potential of the use of the recommendations for improving the current Indonesian practice. Such as, more detailed recommendation on stressing the importance of continuing routine ANC, end of pregnancy, breastfeeding, infant vaccination, limiting visitors, and multidisciplinary team in primary care.
However, there are also potential challenges to the recommendation adoption in the Indonesian settings implied from our appraisals. First, even though they are potential for their adoption for improving maternal service in Indonesia, the speed of knowledge transfer and evidence spread in Indonesia is slow due to the dependency on the higher-level providers. This challenge particularly occurs in any procedures involving medicine prescriptions, or tests for women with morbidities or pregnancy complications, that are usually ordered by obstetricians, while midwives and general practitioners’ are claimed to take care normal pregnant women without complicated diseases in primary care [13], [43]. Second, the challenges of facilities and clinical resources in Indonesia are prominent. Even though some primary care clinics are equipped with essential medicines and 2D ultrasound scans, not all of the scans are functional due to the available access to electricity or skilled providers. Meanwhile, challenges to the women’s health literacy and social culture may also affect the acceptance of some recommendations, such as limited accompanying persons and the use of telemedicine [41]. For instance, limiting visitors during the ANC may challenge the ability of the women to decide on treatment as they are likely depending the decision the husbands or respected men in the family [44], [45]. Not all women are also familiar and able to use phones and apps for telemedicine. Therefore, some of the recommendations may need to be further contextualised for their adoption in primary care practice.
This review also has some limitations. The results of this review may require careful consideration and periodic review of care to response the fast-changing recommendation for the COVID-19 pandemic. This review also only included six academic databases with a limited snowballing search of grey recommendations, and the context of our review is Indonesia, which may represent some but not all conditions of LMICs. Context of comparison and appraisal of the guidelines were also only based on the identified Indonesian regulations, guidelines and healthcare situations in two regions in Indonesia (Yogyakarta and South-East Sulawesi Province). Even though Yogyakarta and Sulawesi are in the western and eastern part of Indonesia, it is worth noting that results of this review may not be applicable in other Indonesian provinces due to different practice situation.
Authors of this review are also aware that research about COVID-19 is continuing, and it is expected that more recommendations will be available in the literature, while the initial literature search for this review was conducted before recommendations for vaccination in pregnant women were published. Pregnant and lactating women were previously not included in the COVID vaccine development and trial, even though COVID-19 severe complications in pregnant women were already known [46]. The recommendations of vaccination pregnant and breastfeeding women has been recommended after the spread of Delta variant, and now the benefits of the produced COVID-19 antibody from vaccination now have been more understood [47], [48], [49], [50].
Nowadays, with the extensive uptake of the vaccination, many countries have been able to relax the restriction. However, challenges remain on the equity of the vaccine distribution in some LMICs, such as Indonesia where some regions still apply the restrictions, including the care for pregnant women [51]. Therefore, these recommendations for improving maternity care may still benefit health providers to better prepare for emergency conditions [52]. Further research is still also needed to seek consensus on the recommendation adoption in the practice, and perhaps to also improve the design the maternal health service in Indonesia.
Ethical clearance.
Not applicable.
Funding.
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Available upon request.
References
- 1.Townsend R., Chmielewska B., Barratt I., Kalafat E., van der Meulen J., Gurol-Urganci I., et al. Global changes in maternity care provision during the COVID-19 pandemic: a systematic review and meta-analysis. EClinicalMedicine. 2021;37:100947. doi: 10.1016/j.eclinm.2021.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald C.R., Weckman A.M., Wright J.K., Conroy A.L., Kain K.C. Pregnant women in low-and middle-income countries require a special focus during the COVID-19 pandemic. Front Global Womens Health. 2020;1 doi: 10.3389/fgwh.2020.564560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menendez C., Gonzalez R., Donnay F., Leke R.G.F. Avoiding indirect effects of COVID-19 on maternal and child health. Lancet Global Health. 2020;8(7):e863–e864. doi: 10.1016/S2214-109X(20)30239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kingsley J.P., Vijay P.K., Kumaresan J., Sathiakumar N. The changing aspects of motherhood in face of the COVID-19 pandemic in low-and middle-income countries. Matern Child Health J. 2021;25(1):15–21. doi: 10.1007/s10995-020-03044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarker B.K., Rahman M., Rahman T., Rahman T., Khalil J.J., Hasan M., et al. Status of the WHO recommended timing and frequency of antenatal care visits in Northern Bangladesh. PLoS One. 2020;15(11):e0241185. doi: 10.1371/journal.pone.0241185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemekes R.i. Kemenkes RI; 2020. Petunjuk teknis pelayanan Puskesmas pada masa pandemi Covid-19. [Google Scholar]
- 7.Republik Indonesia Kementerian Kesehatan. 2020. Pedoman bagi ibu hamil, bersalin, nifas dan bayi baru lahir di Era Pandemi COVID-19 Revision 1. https://www.ibi.or.id/media/Webinar%20IDM%202020/Pedoman_bagi_ibu_hamil_ibu_nifas_dan_BBL_selama_social_distancing.pdf.
- 8.Indonesia Ministry of Health. Guidelines for Antenatal, Childbirth, Postpartum, and Newborn Services Revision 2 (Pedoman Pelayanan Antenatal, Persalinan, Nifas, Dan Bayi Baru Lahir Revisi 2). 2020 Date [cited 31 April 2021]. In: Book Guidelines for Antenatal, Childbirth, Postpartum, and Newborn Services Revision 2 (Pedoman Pelayanan Antenatal, Persalinan, Nifas, Dan Bayi Baru Lahir Revisi 2) [Internet]. [cited 31 April 2021]. Available from: https://covid19.go.id/storage/app/media/Materi%20Edukasi/2020/Oktober/revisi-2-a5-pedoman-pelayanan-antenatal-persalinan-nifas-dan-bbl-di-era-adaptasi-kebiasaan-baru.pdf.
- 9.Ahmed T., Rahman A.E., et al. The effect of COVID-19 on maternal newborn and child health (MNCH) services in Bangladesh, Nigeria and South Africa: call for a contextualised pandemic response in LMICs. Int J Equity Health. 2021;20(1):1–6. doi: 10.1186/s12939-021-01414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouwers M.C., Kho M.E., et al. AGREE II: advancing guideline development, reporting and evaluation in health care. Can Med Assoc J. 2010;182(18):E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health Republic indonesia. Keputusan Menteri Kesehatan Republik Indonesia Nomor HK.01.07/MENKES/813/2019 Tentang Formularium Nasional (Decree of the Minister of Health of the Republic of Indonesia Number HK.01.07 / MENKES / 813/2019 about the Indonesian National Formulary). 2019 Date [cited 22 January 2020]. In: Book Keputusan Menteri Kesehatan Republik Indonesia Nomor HK.01.07/MENKES/813/2019 Tentang Formularium Nasional (Decree of the Minister of Health of the Republic of Indonesia Number HK.01.07 / MENKES / 813/2019 about the Indonesian National Formulary) [Internet]. [cited 22 January 2020]; [154]. Available from: http://hukor.kemkes.go.id/uploads/produk_hukum/KMK_No__HK_01_07-MENKES-813-2019_ttg_Formularium_Nasional.pdf.
- 12.Ministry of Health Republic Indonesia. Peraturan Kementerian Kesehatan Republik Indonesia Nomor 37 tahun 2012 tentang Penyelenggaraan Laboratorium Pusat Kesehatan Masyarakat (Regulation of the Ministry of Health of the Republic of Indonesia Number 37 of 2012 concerning the Implementation of a Public Health Center Laboratory). 2012 Date [cited 16 September 2019]. In: Book Peraturan Kementerian Kesehatan Republik Indonesia Nomor 37 tahun 2012 tentang Penyelenggaraan Laboratorium Pusat Kesehatan Masyarakat (Regulation of the Ministry of Health of the Republic of Indonesia Number 37 of 2012 concerning the Implementation of a Public Health Center Laboratory) [Internet]. Jakarta: Kementerian Kesehatan Republik Indonesia, [cited 16 September 2019]. Available from: https://dpmpt.gunungkidulkab.go.id/upload/download/6818b91c11e544425531c9ddcbd6ff13_lab%20puskesmas.pdf.
- 13.Fitriana Murriya Ekawati, Emilia, Ova, et al. Challenging the status quo: results of an acceptability and feasibility study of hypertensive disorders of pregnancy (HDP) management pathways in Indonesian primary care. BMC Pregnancy and Childbirth. 2021;21(1):507.10.1186/s12884-021-03970-8. [DOI] [PMC free article] [PubMed]
- 14.Michael Quinn Patton. Qualitative research & evaluation methods : integrating theory and practice. Fourth edition. ed: SAGE Publications, Inc.; 2015.9781412972123 1412972124 9781506303215 1506303218.
- 15.QSR International. 2019. Nvivo (Software) version 12.2.0 (3262). https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/support-services/nvivo-downloads.
- 16.Page M.J., McKenzie J.E., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):1–11. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith C.K. Midwifery Today; 2020. Pregnancy, Birth, and Breastfeeding with Covid-19. [Google Scholar]
- 18.CDC. 2020. Care for Breastfeeding People. https://www.cdc.gov/coronavirus/2019-ncov/hcp/care-for-breastfeeding-women.html#PreviousUpdates.
- 19.CDC. 2020. Considerations for Inpatient Obstetric Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html#print.
- 20.CDC. 20Maternal, neonatal and child health services during Covid-19. https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/pregnancy-services.html.
- 21.Larki, M., Sharifi, F., et al., 2020. Models of maternity care for pregnant women during the COVID-19 pandemic. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 26(9), 994-8.10.26719/emhj.20.097. [DOI] [PubMed]
- 22.UNFPA. 2020. COVID-19 Technical Brief for Maternity Services. https://www.unfpa.org/resources/covid-19-technical-brief-maternity-services.
- 23.Cortes J. Pregnancy, newborn and COVID-19. Medicina Balear. 2020;35(3):35–38. doi: 10.3306/medicinabalear.35.03.35. [DOI] [Google Scholar]
- 24.Ministerio de Sanidad España. Documento técnico: Manejo de la mujer embarazada y el recién nacido con COVID-19, Versión de 17 junio de 2020. 2020 Date [cited 17 February 2021]. In: Book Documento técnico: Manejo de la mujer embarazada y el recién nacido con COVID-19, Versión de 17 junio de 2020 [Internet]. [cited 17 February 2021]. Available from: https://www.COVID-19.seth.es/wp-content/uploads/2020/06/2020-06-17_Documento-manejo-embarazo-y-recien-nacido-COVID19.pdf.
- 25.Giusti A., Zambri F., et al. COVID-19 and pregnancy, childbirth, and breastfeeding: the interim guidance of the Italian National Institute of Health. Epidemiol Prev. 2021;45(1–2):14–26. doi: 10.19191/ep21.1-2.P014.030. [DOI] [PubMed] [Google Scholar]
- 26.Giusti A, Zambri F et al. Interim guidance on pregnancy, childbirth, breastfeeding and the care of infants (0-2 years) in response to the COVID-19 emergency Version 2021. Updating of the Rapporto ISS COVID-19 n. 2021;45.
- 27.Chawla D, Chirla D, et al. Perinatal-Neonatal Management of COVID-19 Infection - Guidelines of the Federation of Obstetric and Gynaecological Societies of India (FOGSI), National Neonatology Forum of India (NNF), and Indian Academy of Pediatrics (IAP). Indian Pediatr 2020;57(6):536–48.10.1007/s13312-020-1852-4. [DOI] [PMC free article] [PubMed]
- 28.Vogel J.P., Tendal B., et al. Clinical care of pregnant and postpartum women with COVID-19: Living recommendations from the National COVID-19 Clinical Evidence Taskforce. Aust N Z J Obstet Gynaecol. 2020 doi: 10.1111/ajo.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afshar Y., Silverman N.S., et al. Clinical guidance and perinatal care in the era of coronavirus disease 2019 (COVID-19) J Perinat Med. 2020;48(9):925–930. doi: 10.1515/jpm-2020-0400. [DOI] [PubMed] [Google Scholar]
- 30.Okunade K.S., Makwe C.C., et al. Good clinical practice advice for the management of pregnant women with suspected or confirmed COVID-19 in Nigeria. Int J Gynecol Obstet. 2020;150(3):278–284. doi: 10.1002/ijgo.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maxwell C., McGeer A., et al. Management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS) J Obstet Gynaecol Can. 2009;31(4):358–364. doi: 10.1016/S1701-2163(16)34155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vivanti A.J., Deruelle P., et al. Follow-up for pregnant women during the COVID-19 pandemic: French national authority for health recommendations. J Gynecol Obstetr Human Reproduct. 2020;49(7):101804. doi: 10.1016/j.jogoh.2020.101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homer C.S., Roach V., et al. The National COVID-19 Clinical Evidence Taskforce: pregnancy and perinatal guidelines. Med J Aust. 2022 doi: 10.5694/mja2.51729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vivanti A.J., Deruelle P., et al. Post-natal follow-up for women and neonates during the COVID-19 pandemic: French National Authority for Health recommendations. J Gynecol Obstetr Human Reproduct. 2020;49(7):101805. doi: 10.1016/j.jogoh.2020.101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Republik Indonesia Kementerian Kesehatan. Petunjuk teknis pelayanan kesehatan di klinik pada masa adaptasi kebiasaan baru 2020 Date [cited 28 April 2021]. In: Book Petunjuk teknis pelayanan kesehatan di klinik pada masa adaptasi kebiasaan baru [Internet]. Jakarta, [cited 28 April 2021]. Available from: https://www.pkfi.net/file/media/source/Buku%20Juknis%20Pelayanan%20Kesehatan%20di%20Klinik%20Pada%20Masa%20Adaptasi%20Kebiasaan%20Baru.pdf.
- 36.dan Ginekologi Indonesia Perhimpunan Obstetri. 2020. Rekomendasi Penanganan Infeksi Virus Corona (Covid-19) Pada Maternal (Hamil, Bersalin Dan Nifas). https://pogi.or.id/publish/wp-content/uploads/2020/10/Rekomendasi-Covid-Maternal-POGI.pdf.
- 37.Indonesian Minister of Health. Decree of the Minister of Health of the Republic of Indonesia Number Hk.01.07/Menkes/4829/2021 concerning Guidelines for Health Services Through Telemedicine During the Corona Virus Disease 2019 (Covid-19) Pandemic (Keputusan Menteri Kesehatan Republik Indonesia Nomor Hk.01.07/Menkes/4829/2021 Tentang Pedoman Pelayanan Kesehatan Melalui Telemedicine Pada Masa Pandemi Corona Virus Disease 2019 (Covid-19)). 2020 Date [cited 31 October 2021]. In: Book Decree of the Minister of Health of the Republic of Indonesia Number Hk.01.07/Menkes/4829/2021 concerning Guidelines for Health Services Through Telemedicine During the Corona Virus Disease 2019 (Covid-19) Pandemic (Keputusan Menteri Kesehatan Republik Indonesia Nomor Hk.01.07/Menkes/4829/2021 Tentang Pedoman Pelayanan Kesehatan Melalui Telemedicine Pada Masa Pandemi Corona Virus Disease 2019 (Covid-19)) [Internet]. Jakarta, [cited 31 October 2021]. Available from: https://covid19.hukumonline.com/wp-content/uploads/2021/07/keputusan_menteri_kesehatan_nomor_hk_01_07_menkes_4829_2021_tahun_2021.pdf.
- 38.Kementerian Kesehatan Republik Indonesia. Peraturan menteri kesehatan republik indonesia nomor 20 tahun 2019 tentang penyelenggaraan pelayanan telemedicine antar fasilitas pelayanan kesehatan 2019 Date [cited 29 April 2021]. In: Book Peraturan menteri kesehatan republik indonesia nomor 20 tahun 2019 tentang penyelenggaraan pelayanan telemedicine antar fasilitas pelayanan kesehatan [Internet]. [cited 29 April 2021]. Available from: https://persi.or.id/wp-content/uploads/2020/11/pmk202019.pdf.
- 39.Satgas Covid-19 Indonesia. Protokol Petunjuk Praktis Layanan Kesehatan Ibu Dan Bayi Baru Lahir Selama Pandemi Covid-19. 2020 Date [cited 19 December 2020]. In: Book Protokol Petunjuk Praktis Layanan Kesehatan Ibu Dan Bayi Baru Lahir Selama Pandemi Covid-19 [Internet]. Jakarta, [cited 19 December 2020]. Available from: https://covid19.go.id/p/protokol/protokol-b-4-petunjuk-praktis-layanan-kesehatan-ibu-dan-bbl-pada-masa-pandemi-covid-19.
- 40.Nurbeti M., Prabowo E.A., et al. Hubungan Antara Tingkat Pengetahuan Dengan Kepatuhan Staf Rumah Sakit Dalam Penggunaan Alat Pelindung Diri Secara Rasional Di Masa Pandemi Covid-19. J Hospit Accreditation. 2021;3(2):96–100. [Google Scholar]
- 41.Bindra V. Telemedicine for Women's health during COVID-19 pandemic in India: a short commentary and important practice points for obstetricians and gynaecologists. J Obstetr Gynecol India. 2020;70(4):279–282. doi: 10.1007/s13224-020-01346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavlidis P., Eddy K., et al. Clinical guidelines for caring for women with COVID-19 during pregnancy, childbirth and the immediate postpartum period. Women Birth. 2020 doi: 10.1016/j.wombi.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitriana Murriya Ekawati, Emilia, Ova, et al. Opportunities for improving hypertensive disorders of pregnancy (HDP) management in primary care settings: A review of international published guidelines in the context of pregnancy care in Indonesia. Pregnancy Hypertension. 2020;19:195-204.https://doi.org/10.1016/j.preghy.2020.01.012. [DOI] [PubMed]
- 44.Diba F., Ichsan I., et al. Healthcare providers’ perception of the referral system in maternal care facilities in Aceh, Indonesia: a cross-sectional study. BMJ Open. 2019;9(12) doi: 10.1136/bmjopen-2019-031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Ambruoso L., Martha E., et al. Maternal mortality and severe morbidity in rural Indonesia Part 1: the community perspective. Soc Med. 2013;7(2):47–67. [Google Scholar]
- 46.Adhikari E.H., Spong C.Y. COVID-19 vaccination in pregnant and lactating women. JAMA. 2021;325(11):1039–1040. doi: 10.1001/jama.2021.1658. [DOI] [PubMed] [Google Scholar]
- 47.Gray K.J., Bordt E.A., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perl S.H., Uzan-Yulzari A., et al. SARS-CoV-2–specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325(19):2013–2014. doi: 10.1001/jama.2021.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldshtein I., Nevo D., et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326(8):728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. The Pfizer BioNTech (BNT162b2) COVID-19 vaccine: What you need to know 2021 [updated 21 April 2021; cited 2021 29 October 2021]. Available from: https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine.
- 51.Russell F.M., Greenwood B. Who should be prioritised for COVID-19 vaccination? Hum Vaccin Immunother. 2021;17(5):1317–1321. doi: 10.1080/21645515.2020.1827882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwan J.N., Loh H.C., et al. COVID-19 vaccination during pregnancy in Southeast Asia. Progress Microb Mol Biol. 2021;4(1) [Google Scholar]
- 53.Indonesian Minister of Health. Decree Of The Minister Of Health Of The Republic Of Indonesia Number Hk.01.07/Menkes/413/2020 About Prevention And Control Guidelines Coronavirus Disease 2019 (Covid-19) (Keputusan Menteri Kesehatan Republik Indonesia Nomor Hk.01.07/Menkes/413/2020 Tentang Pedoman Pencegahan Dan Pengendalian Coronavirus Disease 2019 (Covid-19)). 2020 Date [cited 31 October 2021]. In: Book Decree Of The Minister Of Health Of The Republic Of Indonesia Number Hk.01.07/Menkes/413/2020 About Prevention And Control Guidelines Coronavirus Disease 2019 (Covid-19) (Keputusan Menteri Kesehatan Republik Indonesia Nomor Hk.01.07/Menkes/413/2020 Tentang Pedoman Pencegahan Dan Pengendalian Coronavirus Disease 2019 (Covid-19)) [Internet]. [cited 31 October 2021]. Available from: https://covid19.kemkes.go.id/download/KMK_No._HK.01.07-MENKES-413-2020_ttg_Pedoman_Pencegahan_dan_Pengendalian_COVID-19.pdf.
- 54.Alexandre J. Vivanti, Deruelle, Philippe, et al. Post-natal follow-up for women and neonates during the COVID-19 pandemic: French National Authority for Health recommendations. J Gynecol Obstetr Human Reproduct. 2020;49(7):101805.https://doi.org/10.1016/j.jogoh.2020.101805. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request.