Abstract
Background
The incidence of acute pancreatitis (AP) is increasing over years, which brings enormous economy and health burden. However, the aetiologies of AP and underlying mechanisms are still unclear. Here, we performed a two‐sample Mendelian randomization (MR) analysis to investigate the associations between all reported possible risk factors and AP using publicly available genome‐wide association study summary statistics.
Methods
A series of quality control steps were taken in our analysis to select eligible instrumental single nucleotide polymorphisms which were strongly associated with exposures. To make the conclusions more robust and reliable, we utilized several analytical methods (inverse‐variance weighting, MR‐PRESSO method, weighted median, MR‐Egger regression) that are based on different assumptions of two‐sample MR analysis. The MR‐Egger intercept test, radial regression and leave‐one‐out sensitivity analysis were performed to evaluate the horizontal pleiotropy, heterogeneities, and stability of these genetic variants on each exposure. A two‐step MR method was applied to explore mediators in significant associations.
Results
Genetic predisposition to cholelithiasis (effect estimate: 17.30, 95% CI: 12.25–22.36, p = 1.95 E‐11), body mass index (0.32, 95% CI: 0.13–0.51, p < 0.001), body fat percentage (0.57, 95% CI: 0.31–0.83, p = 1.31 E‐05), trunk fat percentage (0.36, 95% CI: 0.14–0.59, p < 0.005), ever smoked (1.61, 95% CI: 0.45–2.77, p = 0.007), and limbs fat percentage (0.55, 95% CI: 0.41–0.69, p < 0.001) were associated with an increased risk of AP. In addition, whole‐body fat‐free mass (−0.32, 95% CI: −0.55 to −0.10, p = 0.004) was associated with a decrease risk of AP.
Conclusion
Genetic predisposition to cholelithiasis, obesity and smoking could be causally associated with an increased risk of AP, and whole body fat‐free mass could be associated with a decreased risk of AP.
The incidence of acute pancreatitis is increasing over years especially in western countries, but the etiologies of acute pancreatitis have not been well understood. And it is unclear whether the reported possible risk factors are real causes of acute pancreatitis. To our knowledge, our research is the first to explore the associations of all reported possible risk factors and acute pancreatitis using public data from the UK biobank and the FinnGen consortium.
1. INTRODUCTION
Acute pancreatitis (AP) is a common emergency in clinical practice, which accounts for almost 300,000 hospitalizations per year in the United States (Gardner, 2021) and lead to a huge burden on health system. In the past five decades, the incidence of AP has been rising in most countries especially the western countries, which brings enormous health and economic burdens (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). The severity of AP varies a lot in different patients; while most cases are mild, approximately 20% of them are moderate/severe AP with pancreatic necrosis or organ failure.
Though gallstones, alcohol intake, and hypertriglyceridemia (HTG) are well‐known risk factors of AP, however, those three have only accounted up to 50% and others are listed in Table 1 (Mederos et al., 2021). The available evidence on possible risk factors for AP is mostly based on observational studies that are prone to be challenged by biases and residual confounding. Moreover, reverse causality could be of concern in such studies because levels of certain biomarkers and behaviors may be influenced by the pathophysiological change of AP itself or other underlying diseases. Therefore, it remains unclear whether previously reported risk factors are causally related to AP.
TABLE 1.
Reported risk factors of acute pancreatitis
| Diseases |
| Gallstone |
| Obesity |
| HIV‐positive status |
| Hyperparathyroidism |
| IBD |
| Hypercalcemia |
| Hypertriglyceridemia |
| Diabetic ketoacidosis |
| Pancreas divisum |
| Pancreas tumor |
| Pancreas cystic lesions |
| Traits |
| Alcohol |
| Older age |
| Smoking |
| Medication: Acetaminophen |
| Medication:5‐animosalicylate |
| Medication: Amiodarone |
| Medication: Androgenic anabolic steroids |
| Medication: Azathioprine |
| Medication: Cannabis |
| Medication: Carbamazepine |
| Medication: Carbimazole |
| Medication: Cimetidine |
| Medication: Cisplatin |
| Medication: Clomiphene |
| Medication: Didanosine |
| Medication: Enalapril |
| Medication: Estrogen |
| Medication: Furosemide |
| Medication: Isoniazid |
| Medication: Lamivudine |
| Medication: Losartan |
| Medication: Methyldopa |
| Medication: Metronidazole |
| Medication: Nadolol |
| Medication: Pravastatin |
| Medication: Perindopril |
| Medication: Procainamide |
| Medication: Pyritinol |
| Medication: Ranitidine |
| Medication: Rosuvastatin |
| Medication: Saxagliptin |
| Medication: Simvastatin |
| Medication: Sulindac |
| Medication: Tamoxifen |
| Medication: Telaprevir |
| Medication: Tetracycline |
| Medication: Trimethoprim |
| Medication: Valproic acid |
Mendelian randomization (MR) is an approach that can help deal with those problems through the use of genetic variants as instrumental variables to infer causality among correlated traits (Davey Smith & Hemani, 2014; Davies et al., 2018; Smith & Ebrahim, 2003). As an individual's genotype is established at zygote formation then genetic variation is robust to reverse causation and confounding is considerably less evident than in conventional observational studies (Smith et al., 2007). Furthermore, a method named two‐step MR have been developed to determine whether the causal relationship between exposure and outcome is mediated by other factors (Relton & Davey Smith, 2012). MR study in the field of AP was scarce, recently, Yuan et al. (2021) conducted a MR study to determine the potential associations of cholelithiasis, diabetes, serum calcium, triglyceride levels, smoking, and alcohol consumption with acute and chronic pancreatitis, which provides evidence in supporting that cholelithiasis, type 2 diabetes, and smoking initiation was associated with an increased risk of AP. However, the rest of possible aetiologies and related mechanisms of AP are still poorly understood. Hence, we sought to conduct an exposure‐wide MR study to determine the causal associations between all reported risk factors and AP.
2. METHODS
2.1. Ethical compliance
This study involved analysis of publicly available, de‐identified data. Specific ethical review and informed consent had been obtained in all the original studies.
2.2. Study design
The MR approach builds upon three principal assumptions; (1) The genetic variants proposed as instrumental variables should be robustly associated with the risk factors of interest; (2) The used genetic variants should not be associated with potential confounders; (3) The selected genetic variants should affect the risk of outcome merely through the risk factor, not via alternative pathways (Figure 1).
FIGURE 1.
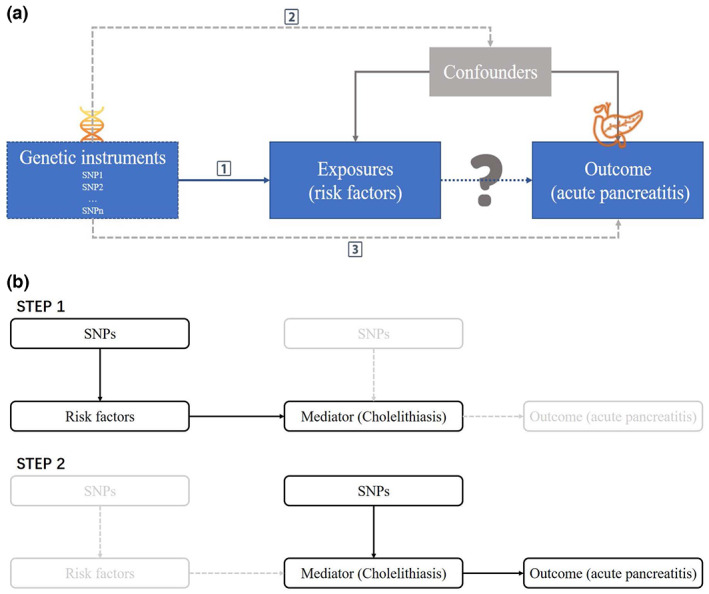
Study design and assumptions: (1) the genetic variants proposed as instrumental variables should be robustly associated with the risk factors of interest; (2) the used genetic variants should not be associated with potential confounders; (3) the selected genetic variants should affect the risk of outcome merely through the risk factor, not via alternative pathways.
2.3. Outcome and exposure data resource
Summary‐level data of GWAS for AP as outcome was obtained from the FinnGen consortium. FinnGen study launched in Finland from 2017 combines genome information with digital health care data. We used the R5 release of the FinnGen consortium data with 3022 AP cases and 195,144 controls of Finnish ancestry. In this dataset, associations had been adjusted for age, sex, 10 genetic principal components, and genotyping batch.
Detailed information on used studies and genetic consortia (de Lange et al., 2017; Jiang et al., 2019; Klimentidis et al., 2018; Liu et al., 2021; Locke et al., 2019; Onengut‐Gumuscu et al., 2015; Sinnott‐Armstrong et al., 2021; Wang et al., 2019) as exposure data was shown in Table S1, 55 different phenotypes involved and 42 datasets of them were obtained from the GCTA data resource consisting of 2173 traits on 456,422 array‐genotyped as well as 49,960 whole‐exome‐sequenced individuals of European ancestry in the UK Biobank based on a resource‐efficient tool (i.e., fastGWA) mixed‐model association analysis (Jiang et al., 2019). Four genome‐wide association study (GWAS) summary data of cholelithiasis, ketoacidosis, human immunodeficiency virus infection and hyperparathyroidism were obtained from OpenGWAS project (Elsworth et al., 2020; Lyon et al., 2020), which is a manually curated collection of complete GWAS summary datasets.
2.4. Genetic instrumental variables selection
Genetic instruments for each exposure trait or disease were selected at the genome‐wide significance threshold (p < 5 × 10−8) from corresponding GWASs. Independent single nucleotide polymorphisms (SNPs) were defined by r 2 < 0.001 and clump window >10 kb and correlated SNPs (e.g., linkage disequilibrium) with the lowest p‐value was retained. Linkage disequilibrium among SNPs for each risk factors was calculated based on 1000 genomes LD reference panel (European population) (Abecasis et al., 2012) using the PLINK clumping approach (Chang et al., 2015). We estimated the variance of each SNP and total variance of selected SNPs explained to the corresponding phenotype by using “2β2MAF(1‐MAF)”, where β was the effect size of the genetic variation and MAF represented the minor allele frequency.
2.5. Statistical analysis
The univariate fixed‐effects inverse‐variance weighted model was used as the main analysis, which estimated the causal effect of an exposure on an outcome by combining ratio estimates using each variant in a fixed effect meta‐analysis model (Burgess et al., 2013). The weighted median approach and MR‐Egger regression were used as a sensitivity analysis (Bowden et al., 2015, 2016). The p‐value for the MR‐Egger intercept was used to indicate directional pleiotropy and the p‐value <0.05 indicated possible pleiotropy. The MR pleiotropy residual sum and outlier (MR‐PRESSO) test was also applied to identify horizontal pleiotropic outliers (Verbanck et al., 2018). Radial regression was used for the inverse‐variance weighted model to detect outlier variables with heterogenicity (Bowden et al., 2018). The association with two‐sided false discovery rate corrected p values <0.05 were deemed statistically significant.
From previous studies, cholelithiasis is known as the most important risk factor of AP (Vege et al., 2018; Yuan et al., 2021). In this MR study, we postulated that if some risk factors have causal effects on AP, then the effects could be direct or indirect that are likely mediated by cholelithiasis. This scenario is possible to investigate using two‐step MR. To assess whether cholelithiasis is a mediator in the pathway from possible risk factors to AP, a two‐step MR analysis (Relton & Davey Smith, 2012) was performed. In step 1, causal effects of risk factors detected from two‐sample MR on mediator (i.e., cholelithiasis) were tested, and then the causal effect of cholelithiasis on AP as step 2.
All analysis was performed using the TwoSampleMR (Hemani et al., 2018), RadialMR (Bowden et al., 2018), and MR‐PRESSO (Verbanck et al., 2018) packages in R Software 4.1.0 (R Core Team. R Foundation for Statistical Computing. Vienna, Austria. 2021. https://www.R‐project.org). Plots were created using the packages ggplot2 (Wickham, 2016) and pheatmap (Kolde, 2019).
3. RESULTS
Summary data for the genetic instruments were available for 17 binary traits or diseases, corresponding to 846,202 cases (median, 7682 per trait or disease), 3,260,307 controls (median, 96,367 per trait or disease), and 38 quantitative traits or diseases corresponding to 10,904,652 participants (median, 141,119 per trait or disease). After genetic instrumental variables selection in the GWAS summary datasets of corresponding risk factors, 18 presented no available instruments, a total of 37 traits or diseases were included in the MR analysis (Table S2). The median number of SNPs available as instruments across traits or diseases was 90 ranging from 1 to 530 (Figure 2).
FIGURE 2.
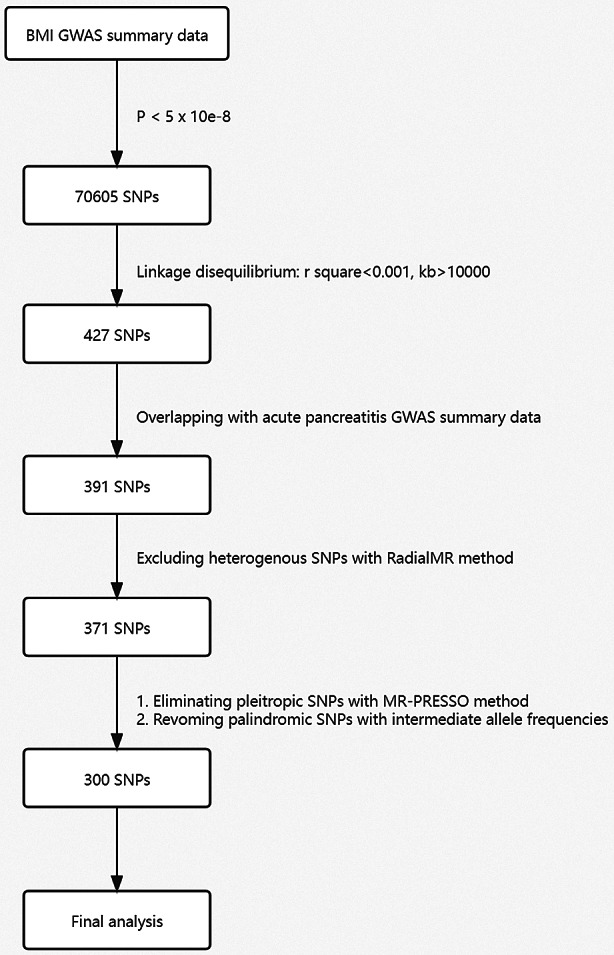
Flow chart of the instrumental variant screening.
Effect estimates from the current MR study reflected the change of risk of AP for each unit change of our derived exposures. The results showed that genetic predisposition to cholelithiasis (effect estimate: 17.30, 95% CI: 12.25–22.36, p < 0.001), body mass index (0.32, 95% CI: 0.13–0.51, p < 0.001), body fat percentage (0.57, 95% CI: 0.31–0.83, p < 0.001), trunk fat percentage (0.36, 95% CI: 0.14–0.59, p < 0.005), ever smoked (1.61, 95% CI: 0.45–2.77, p = 0.007), and limbs fat percentage (0.55, 95% CI: 0.41–0.69, p < 0.001) was associated with an increased risk of AP. And whole‐body fat‐free mass (−0.32, 95% CI: −0.55 to −0.10, p = 0.004) were associated with a decrease risk of AP (Figures 3 and 4). The associations were, however, highly variable across risk factors, varying from an effect estimate of 0.32 for body mass index and 17.30 for cholelithiasis. Except for factors of ever smoked and cholelithiasis, the rest of statistically significant traits are all quantitative indicators of obesity, which presented similar strength of association with risk of AP. A suggestive association of triglyceride and serum calcium with AP was also observed at non‐FDR p < 0.05. Bidirectional MR analysis was performed to detect the potential reverse causal relationship from AP to cholelithiasis, body mass index, body fat percentage, trunk fat percentage, ever smoked, and limbs fat percentage, showing that AP had no significantly causal effects on those traits or diseases (Table S3).
FIGURE 3.
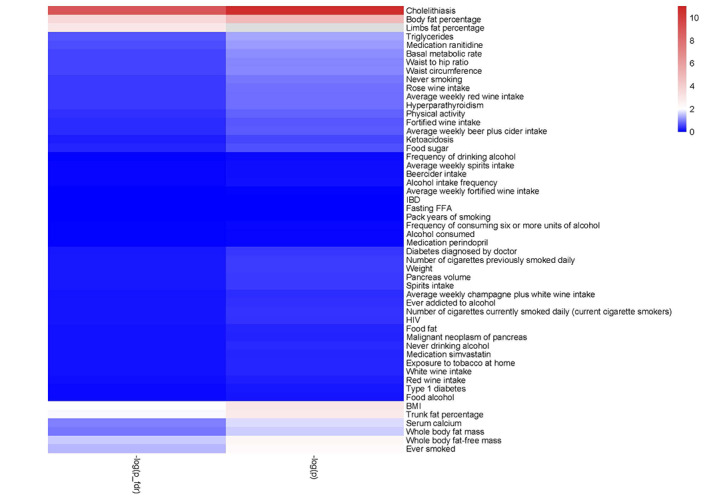
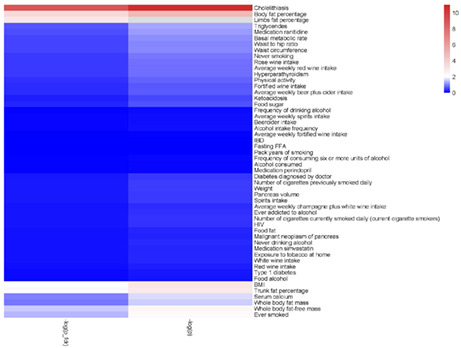
Heatmap included every exposure according to −lg(p‐value).
FIGURE 4.
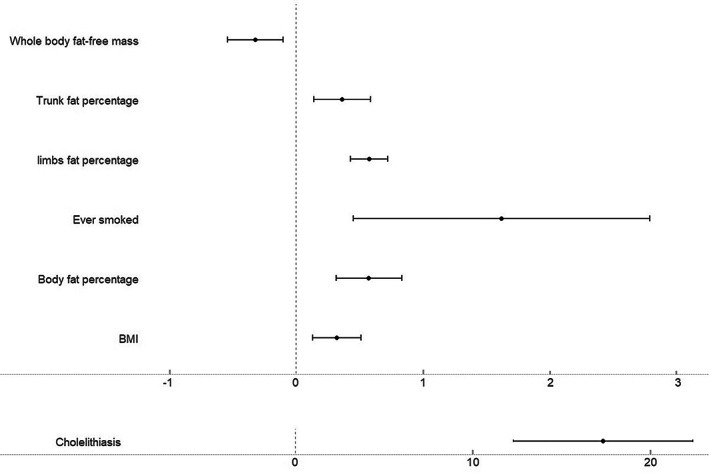
Forest plot of the effect estimates of statistically significant exposures in two‐sample Mendelian randomization.
In the framework of two step MR study, the causal effects of cholelithiasis, body mass index, body fat percentage, trunk fat percentage, ever smoked, right or left leg fat percentage, and right or left arm fat percentage on AP were maintained. In addition, all aforementioned factors except for ever smoked showed positive causal effects on cholelithiasis (Table S4), which indicated the obesity related effects to the increased risk of AP are mediated by cholelithiasis.
In the sensitivity analysis, we appraised the potential impact of confounding by pleiotropic pathways on our results. Associations estimated by the weighted median and MR‐Egger were similar to the main results (Table S2).
4. DISCUSSION
This exposure‐wide MR study found cholelithiasis, body mass index, body fat percentage, trunk fat percentage, ever smoked, limbs fat percentage were associated with an increased risk of AP. And whole‐body fat‐free mass were associated with a decrease risk of AP.
Cholelithiasis, HTG, and alcohol intake have been reported as the three main risk factors of AP based on observational and animal experimental studies (Roberts et al., 2017; Vege et al., 2018; Yang & McNabb‐Baltar, 2020). However, the underlying mechanisms of how these factors cause AP are still unclear. In addition, there are diverse less common risk factors of AP reported which are almost based on observational studies with small sample size and case reports.
Hypothesis about cholelithiasis‐relating AP, accounting for approximately 35%–40% of AP cases, vary from block of pancreatic fluid to reflux of bile into pancreatic duct (Gardner, 2021). The present MR study found evidence that genetic predisposition to cholelithiasis is consistently associated with an increased risk of AP. It strengthened the causality from cholelithiasis to AP based on human genetic data, in line with the study by Yuan et al. (2021). However, the associations between AP and HTG or alcohol failed to reach statistical significance in our study. Given the published studies about alcohol‐related AP, long‐term and heavy alcohol drinking posed great risk to AP especially in Western countries (Roberts et al., 2017). A meta‐analysis suggested that a dose–response relationship between alcohol consumption and risk of AP existed, which is monotonic in men and no‐linear in women, and alcohol consumption below 40 g/day was even associated with reduced risk of AP in women (Samokhvalov et al., 2015). In our study, we explored different traits including alcohol type, amount of alcohol intake and frequency of alcohol intake. However, the results showed no association of the alcohol‐related exposures with the increased risk of AP. Similar result happened to HTG which only showed suggestive association with AP (non‐FDR p < 0.05). The discrepancies might be explained by which there are only a small portion of drinkers with a long‐term and heavy alcohol intake in our study, and the median triglyceride level in our study population is 1.5 mmol/L (Interquartile Range 1.11 mmol/L) which is far lower than the diagnostic criteria of HTG‐related AP (11.3 mmol/L). Therefore, future studies with larger sample size are needed.
Obesity is defined as an excess accumulation of body fat which can be assessed with BMI and fat percentage, which we also included in our study as exposures. Most of the epidemiological data on obesity were based on BMI (in kg/m2) and used the ranges of 18.5–24.9 for normality, 25–29.9 for overweight, and ≥30 for obesity (Caballero, 2019). A meta‐analysis included 12 clinical studies with a total of 1483 patients showed that obese patients (BMI ≥ 25 kg/m2 in Asian countries and BMI ≥ 30 kg/m2 in non‐Asian countries) had a significantly increases risk of severe AP (risk ratio = 2.20, 95% CI 1.82–2.66, p < 0.05) (Chen et al., 2012). Our findings based on several relevant traits further confirmed the causality of this obesity, whereof BMI, body fat percentage, and limbs fat percentage were shown to be significantly associated with increased risk of AP, and whole‐body fat‐free mass was significantly associated with a decreased risk of AP. Previous studies (Khatua et al., 2017) explained the possible underlying mechanisms of how obesity causes AP, mainly focused on HTG, diabetes, therapeutic interventions for obesity. Obesity could also increase AP severity through the effects of cytokines, adipokines, damage‐associated molecular patterns and unsaturated fatty acid‐mediated lipotoxicity. The two‐step MR analysis in our study revealed that cholelithiasis act as a mediator in the causality association of body mass index, body fat percentage, trunk fat percentage, right or left leg fat percentage and right or left arm fat percentage with AP. The 2020 Global Burden of Disease report noted a global year‐over‐year increase in BMI, blood pressure, and fasting plasma glucose (i.e., metabolic syndrome) (GBD 2019 Risk Factors Collaborators, 2020), which explained partially the increase of incidence of AP over the past 5 decades (Iannuzzi et al., 2021). Prevention of cholelithiasis should be strongly recommended to lower the risk of AP for those individuals with a high risk of cholelithiasis.
Smoking has been established as an independent risk factor of AP (Majumder et al., 2015). Our study found that the factor ever smoked increased the risk of AP, but the number of cigarettes smoked daily for both former and current smoker was not significantly associated with the increased risk of AP. The results of two‐step MR showed no mediation effect of cholelithiasis in the causal relationship of ever smoked with AP. Further research is warrant to demonstrating the underlying mechanism.
The major strength of the present study is the MR design which can minimize residual confounding and diminish reverse causality. Another strength is the two‐step MR design which can explain to some extent the mechanism of causality relationship. A limitation of our study is possible pleiotropy, where a genetic variant has more than one direct correlate that would invalidate conclusions based on the assumption of a single pathway. However, our results remained quite stable across all MR methods and MR‐PRESSO method was applied to detect possible pleiotropy. Population stratification bias is less likely to affect our results since the used genome‐wide association studies were based on individuals of solely European ancestry. In addition, adjustment was made for population structure through genetic principal components in these genome‐wide association studies. However, this population restriction might confine the generalizability of our findings to other populations. Another limitation of our study is weak instrument bias. We observed the variance of each SNP and total variance of selected SNPs explained to the corresponding phenotypes or traits was relatively small. In 2‐sample MR, this bias would tend to make estimates closer to the null. However, we have followed a strict screening process of instrumental SNPs and the results showed stability through all the sensitivity analysis, we therefore, admit that weak instrument bias is a notable issue in our results but not critical to the main findings. Nevertheless, further research is required to clarify our finds especially those contradictories between MR results and observational epidemiology.
5. CONCLUSION
The present MR study suggested that genetic predisposition to cholelithiasis, obesity and smoking could be causally associated with an increased risk of AP, whole body fat‐free mass could be associated with a decreased risk of AP. And the association between obesity and AP could be mediated by cholelithiasis. The suggestive associations of triglyceride and serum calcium with AP need further investigations.
AUTHOR CONTRIBUTIONS
Conceived and designed the study: Evan Yi‐Wen Yu, Qiu‐Yi Tang; supervision: Wei‐Qin Li, Evan Yi‐Wen Yu; conducted data analyses and interpretation and drafted the manuscript: Qiu‐Yi Tang; data curation: Qiu‐Yi Tang; Critical revision of the manuscript: Qi Yang, Xian‐Qiang Yu, Yu‐Xiu Liu, Zhi‐Hui Tong, Bai‐Qiang Li, Ya‐Ting Chen, Evan Yi‐Wen Yu, Wei‐Qin Li. All authors read and approved the final manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This study was supported by National Natural Science Foundation of China (No. 82070669).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
CONSENT FOR PUBLICATION
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research did not involve patients or the public as it used data from UK biobank, FinnGen consortium, and other published GWAS datasets that were previously obtained from a cohort of people who had already been recruited. As such, no patients or member of the public were involved in the design or implementation of this study, or the research questions addressed, and no ethical committee approval was required. Each study included was approved by their institutional ethics review committees, and all participants provided written informed consent.
Supporting information
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
We want to acknowledge the participants and investigators of FinnGen study.
Tang, Q.‐Y. , Yang, Q. , Yu, X.‐Q. , Liu, Y.‐X. , Tong, Z.‐H. , Li, B.‐Q. , Chen, Y.‐T. , Yu, E.‐W. , & Li, W.‐Q. (2023). Association of demographic and clinical factors with risk of acute pancreatitis: An exposure‐wide Mendelian randomization study. Molecular Genetics & Genomic Medicine, 11, e2091. 10.1002/mgg3.2091
Contributor Information
Evan Yi‐Wen Yu, Email: evan.y.w.yu@gmail.com.
Wei‐Qin Li, Email: njzy_pancrea@163.com.
REFERENCES
- Abecasis, G. R. , Auton, A. , Brooks, L. D. , DePristo, M. A. , Durbin, R. M. , Handsaker, R. E. , Kang, H. M. , Marth, G. T. , & McVean, G. A. (2012). An integrated map of genetic variation from 1,092 human genomes. Nature, 491(7422), 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden, J. , Davey Smith, G. , & Burgess, S. (2015). Mendelian randomization with invalid instruments: Effect estimation and bias detection through egger regression. International Journal of Epidemiology, 44(2), 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden, J. , Davey Smith, G. , Haycock, P. C. , & Burgess, S. (2016). Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genetic Epidemiology, 40(4), 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden, J. , Spiller, W. , Del Greco, M. F. , Sheehan, N. , Thompson, J. , Minelli, C. , & Davey Smith, G. (2018). Improving the visualization, interpretation and analysis of two‐sample summary data Mendelian randomization via the radial plot and radial regression. International Journal of Epidemiology, 47(6), 2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, S. , Butterworth, A. , & Thompson, S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genetic Epidemiology, 37(7), 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero, B. (2019). Humans against obesity: Who will win? Advances in Nutrition, 10(suppl_1), S4–s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. C. , Chow, C. C. , Tellier, L. C. , Vattikuti, S. , Purcell, S. M. , & Lee, J. J. (2015). Second‐generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience, 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. M. , Xiong, G. S. , & Wu, S. M. (2012). Is obesity an indicator of complications and mortality in acute pancreatitis? An updated meta‐analysis. Journal of Digestive Diseases, 13(5), 244–251. [DOI] [PubMed] [Google Scholar]
- Davey Smith, G. , & Hemani, G. (2014). Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Human Molecular Genetics, 23(R1), R89–R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, N. M. , Holmes, M. V. , & Davey Smith, G. (2018). Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ, 362, k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange, K. M. , Moutsianas, L. , Lee, J. C. , Lamb, C. A. , Luo, Y. , Kennedy, N. A. , Jostins, L. , Rice, D. L. , Gutierrez‐Achury, J. , Ji, S. G. , Heap, G. , Nimmo, E. R. , Edwards, C. , Henderson, P. , Mowat, C. , Sanderson, J. , Satsangi, J. , Simmons, A. , Wilson, D. C. , … Barrett, J. C. (2017). Genome‐wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nature Genetics, 49(2), 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth, B. , Lyon, M. , Alexander, T. , Liu, Y. , Matthews, P. , Hallett, J. , Bates, P. , Palmer, T. , Haberland, V. , Smith, G. D. , Zheng, J. , Haycock, P. , Gaunt, T. R. , & Hemani, G. (2020). The MRC IEU OpenGWAS data infrastructure. bioRxiv. 10.1101/2020.08.10.244293 [DOI] [Google Scholar]
- Gardner, T. B. (2021). Acute Pancreatitis. Annals of Internal Medicine, 174(2), Itc17–itc32. [DOI] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: A systematic analysis for the global burden of disease study 2017. Lancet, 392(10159), 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Risk Factors Collaborators . (2020). Global burden of 87 risk factors in 204 countries and territories, 1990‐2019: A systematic analysis for the global burden of disease study 2019. Lancet, 396(10258), 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani, G. , Zheng, J. , Elsworth, B. , Wade, K. H. , Haberland, V. , Baird, D. , Laurin, C. , Burgess, S. , Bowden, J. , Langdon, R. , Tan, V. Y. , Yarmolinsky, J. , Shihab, H. A. , Timpson, N. J. , Evans, D. M. , Relton, C. , Martin, R. M. , Davey Smith, G. , Gaunt, T. R. , & Haycock, P. C. (2018. The MR‐base platform supports systematic causal inference across the human phenome. eLife, 7, e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannuzzi, J. P. , King, J. A. , Leong, J. H. , Quan, J. , Windsor, J. W. , Tanyingoh, D. , Coward, S. , Forbes, N. , Heitman, S. J. , Shaheen, A. A. , Swain, M. , Buie, M. , Underwood, F. E. , & Kaplan, G. G. (2021). Global incidence of acute pancreatitis is increasing over time: A systematic review and meta‐analysis. Gastroenterology, 160, S‐466–S‐467. [DOI] [PubMed] [Google Scholar]
- Jiang, L. , Zheng, Z. , Qi, T. , Kemper, K. E. , Wray, N. R. , Visscher, P. M. , & Yang, J. (2019). A resource‐efficient tool for mixed model association analysis of large‐scale data. Nature Genetics, 51(12), 1749–1755. [DOI] [PubMed] [Google Scholar]
- Khatua, B. , El‐Kurdi, B. , & Singh, V. P. (2017). Obesity and pancreatitis. Current Opinion in Gastroenterology, 33(5), 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimentidis, Y. C. , Raichlen, D. A. , Bea, J. , Garcia, D. O. , Wineinger, N. E. , Mandarino, L. J. , Alexander, G. E. , Chen, Z. , & Going, S. B. (2018). Genome‐wide association study of habitual physical activity in over 377,000 UK biobank participants identifies multiple variants including CADM2 and APOE. International Journal of Obesity, 42(6), 1161–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolde, R. (2019). Pheatmap: Pretty heatmaps.
- Liu, Y. , Basty, N. , Whitcher, B. , Bell, J. D. , Sorokin, E. P. , van Bruggen, N. , Thomas, E. L. , & Cule, M. (2021). Genetic architecture of 11 organ traits derived from abdominal MRI using deep learning. eLife, 10, e65554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, A. E. , Steinberg, K. M. , Chiang, C. W. K. , Service, S. K. , Havulinna, A. S. , Stell, L. , Pirinen, M. , Abel, H. J. , Chiang, C. C. , Fulton, R. S. , Jackson, A. U. , Kang, C. J. , Kanchi, K. L. , Koboldt, D. C. , Larson, D. E. , Nelson, J. , Nicholas, T. J. , Pietilä, A. , Ramensky, V. , … Freimer, N. B. (2019). Exome sequencing of Finnish isolates enhances rare‐variant association power. Nature, 572(7769), 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon, M. , Andrews, S. J. , Elsworth, B. , Gaunt, T. R. , Hemani, G. , & Marcora, E. (2020). The variant call format provides efficient and robust storage of GWAS summary statistics. bioRxiv. 10.1101/2020.05.29.115824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder, S. , Gierisch, J. M. , & Bastian, L. A. (2015). The association of smoking and acute pancreatitis: A systematic review and meta‐analysis. Pancreas, 44(4), 540–546. [DOI] [PubMed] [Google Scholar]
- Mederos, M. A. , Reber, H. A. , & Girgis, M. D. (2021). Acute pancreatitis: A review. JAMA, 325(4), 382–390. [DOI] [PubMed] [Google Scholar]
- Onengut‐Gumuscu, S. , Chen, W. M. , Burren, O. , Cooper, N. J. , Quinlan, A. R. , Mychaleckyj, J. C. , Farber, E. , Bonnie, J. K. , Szpak, M. , Schofield, E. , Achuthan, P. , Guo, H. , Fortune, M. D. , Stevens, H. , Walker, N. M. , Ward, L. D. , Kundaje, A. , Kellis, M. , Daly, M. J. , … Rich, S. S. (2015). Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nature Genetics, 47(4), 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton, C. L. , & Davey Smith, G. (2012). Two‐step epigenetic Mendelian randomization: A strategy for establishing the causal role of epigenetic processes in pathways to disease. International Journal of Epidemiology, 41(1), 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, S. E. , Morrison‐Rees, S. , John, A. , Williams, J. G. , Brown, T. H. , & Samuel, D. G. (2017). The incidence and aetiology of acute pancreatitis across Europe. Pancreatology, 17(2), 155–165. [DOI] [PubMed] [Google Scholar]
- Samokhvalov, A. V. , Rehm, J. , & Roerecke, M. (2015). Alcohol consumption as a risk factor for acute and chronic pancreatitis: A systematic review and a series of meta‐analyses. eBioMedicine, 2(12), 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott‐Armstrong, N. , Tanigawa, Y. , Amar, D. , Mars, N. , Benner, C. , Aguirre, M. , Venkataraman, G. R. , Wainberg, M. , Ollila, H. M. , Kiiskinen, T. , Havulinna, A. S. , Pirruccello, J. P. , Qian, J. , Shcherbina, A. , FinnGen , Rodriguez, F. , Assimes, T. L. , Agarwala, V. , Tibshirani, R. , … Rivas, M. A. (2021). Genetics of 35 blood and urine biomarkers in the UK biobank. Nature Genetics, 53(2), 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. D. , & Ebrahim, S. (2003). ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? International Journal of Epidemiology, 32(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Smith, G. D. , Lawlor, D. A. , Harbord, R. , Timpson, N. , Day, I. , & Ebrahim, S. (2007). Clustered environments and randomized genes: A fundamental distinction between conventional and genetic epidemiology. PLoS Medicine, 4(12), e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vege, S. S. , DiMagno, M. J. , Forsmark, C. E. , Martel, M. , & Barkun, A. N. (2018). Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology, 154(4), 1103–1139. [DOI] [PubMed] [Google Scholar]
- Verbanck, M. , Chen, C. Y. , Neale, B. , & Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genetics, 50(5), 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Zhang, F. , Zeng, J. , Wu, Y. , Kemper, K. E. , Xue, A. , Zhang, M. , Powell, J. E. , Goddard, M. E. , Wray, N. R. , Visscher, P. M. , McRae, A. F. , & Yang, J. (2019). Genotype‐by‐environment interactions inferred from genetic effects on phenotypic variability in the UK biobank. Science Advances, 5(8), eaaw3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant graphics for data analysis. Springer‐Verlag. [Google Scholar]
- Yang, A. L. , & McNabb‐Baltar, J. (2020). Hypertriglyceridemia and acute pancreatitis. Pancreatology, 20(5), 795–800. [DOI] [PubMed] [Google Scholar]
- Yuan, S. , Giovannucci, E. L. , & Larsson, S. C. (2021). Gallstone disease, diabetes, calcium, triglycerides, smoking and alcohol consumption and pancreatitis risk: Mendelian randomization study. NPJ Genomic Medicine, 6(1), 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4


