Abstract
Nontypeable Haemophilus influenzae (NTHi) causes repeated respiratory infections in patients with chronic lung diseases. These infections are characterized by a brisk inflammatory response which results in the accumulation of polymorphonucleated cells in the lungs and is dependent on the expression and secretion of proinflammatory cytokines. We hypothesize that multiple NTHi molecules, including lipooligosaccharide (LOS), mediate cellular interactions with respiratory epithelial cells, leading to the production of proinflammatory cytokines. To address this hypothesis, we exposed 9HTEo− human tracheal epithelial cells to NTHi and compared the resulting profiles of cytokine gene expression and secretion using multiprobe RNase protection assays and enzyme-linked immunosorbent assays (ELISA), respectively. Dose-response experiments demonstrated a maximum stimulation of most cytokines tested, using a ratio of 100 NTHi bacterial cells to 1 9HTEo− tracheal epithelial cell. Compared with purified LOS, NTHi bacterial cells stimulated 3.6- and 4.5-fold increases in epithelial cell expression of interleukin-8 (IL-8) and IL-6 genes, respectively. Similar results were seen with epithelial cell macrophage chemotactic protein 1, IL-1α, IL-1β, and tumor necrosis factor alpha expression. Polymyxin B completely inhibited LOS stimulation but only partially reduced NTHi whole cell stimulation. Taken together, these results suggest that multiple bacterial molecules including LOS contribute to the NTHi stimulation of respiratory epithelial cell cytokine production. Moreover, no correlation was seen between NTHi adherence to epithelial cells mediated by hemagglutinating pili, Hia, HMW1, HMW2, and Hap and epithelial cytokine secretion. These data suggest that bacterial molecules beyond previously described NTHi cell surface adhesins and LOS play a role in the induction of proinflammatory cytokines from respiratory epithelial cells.
Nontypeable Haemophilus influenzae (NTHi) strains are fastidious gram-negative bacteria that exist as commensal organisms in the human nasopharynx (42, 48). In addition, NTHi strains cause otitis media, sinusitis, and conjunctivitis in otherwise healthy individuals (65). NTHi has also been associated with chronic bronchitis and pneumonia in patients with chronic pulmonary diseases such as cystic fibrosis and chronic obstructive pulmonary disease (13, 49, 65). An acute inflammatory response and the accumulation of polymorphonuclear leukocytes characterize NTHi respiratory infections. The initial events leading to respiratory inflammation are thought to involve cellular interactions between infecting bacteria and respiratory epithelial cells; these bacterial-epithelial cell interactions stimulate the release of inflammatory mediators (i.e., cytokines and chemokines) (26, 35).
Several respiratory pathogens such as Pseudomonas aeruginosa, Burkholderia cepacia, Streptococcus pneumoniae, and Bordetella pertussis produce a variety of molecules that interact with epithelial cells and cause a secretion of proinflammatory cytokines (10, 19, 25, 26, 29, 39, 54, 58, 63, 74, 76). These interactions involve bacterial cell-associated and extracellular products that include cell wall fragments, outer membrane proteins, autoinducers, pili and flagella, and secreted molecules. The bacterial molecules that are capable of perturbing cytokine networks are called modulins (26, 74). In response to bacterial modulins, airway epithelial cells produce inflammatory mediators such as interleukin-6 (IL-6), IL-1α, IL-1β, and tumor necrosis factor alpha (TNF-α), along with the chemokines IL-8 and macrophage chemotactic protein 1 (MCP-1), which stimulate the activation and influx of neutrophils, monocytes, and macrophages (2, 5–7, 10, 19, 39–41, 50, 53, 57, 62, 64).
Preliminary studies have begun to characterize the modulin activity of NTHi and its ability to perturb the inflammatory response. These studies have focused on H. influenzae lipooligosaccharide (LOS) as a major stimulator of proinflammatory cytokines. Recent in vitro studies showed that H. influenzae LOS stimulated the release of IL-6, IL-8, and TNF-α from cultured, primary human bronchial epithelial cells and TNF-α, IL-1β, and IL-6 from human monocytes (18, 37, 38, 51). Adherent, intact H. influenzae bacterial cells have also been shown to stimulate IL-6 secretion from human macrophages and IL-6 and IL-8 from respiratory syncytial virus-infected human respiratory epithelial cells (31; C. Maguire, and G. Noel, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B16, p. 24. 1996). These studies, however, have not comprehensively analyzed all of the proinflammatory mediators involved in H. influenzae epithelial cell infection or characterized completely the bacterial factors that influence this host response. The aim of the present study was to examine the relative ability of intact H. influenzae, purified H. influenzae LOS, and specific adhesin-deficient H. influenzae to stimulate respiratory epithelial cytokine production in vitro.
MATERIALS AND METHODS
Bacteria.
Table 1 describes the bacterial strains used in this study. All bacteria were cultured on chocolate agar (Becton Dickinson, Cockeysville, Md.) in 5% CO2 at 37°C overnight and transferred to 10 ml of Levinthal broth (brain heart infusion broth [Difco Laboratories, Detroit, Mich.] supplemented with hemin [100 μg/ml] and NAD [20 μg/ml]) and grown in 5% CO2 at 37°C overnight to stationary phase. Adhesin-deficient NTHi strains carrying the kanamycin resistance (Kanr) gene were cultured in medium containing 25 μg of kanamycin (Sigma-Aldrich Co., St. Louis, Mo.) per ml of medium. Long-term storage of bacteria was in sterile skim milk at −70°C.
TABLE 1.
Strains used in this study
| Strain | Capsular type | Clinical origin | Surface adhesins | Reference(s) |
|---|---|---|---|---|
| AAr176p+ | Nontypeable | Nasopharynx | Pili+ | 14, 16 |
| AAr176p− | Nontypeable | Pili− | 14, 16 | |
| Rd | Acapsular type d | Pili−, Hap−, Hia−, HMW 1−, HMW2− | 12 | |
| ST11hia+ | Nontypeable | Middle ear | Pili−, Hap+, Hia+, HMW1−, HMW2− | 1 |
| ST11hia− | Nontypeable | Pili−, Hap+, Hia−, HMW1−, HMW2− | 1 | |
| ST12hmw 1+/2+ | Nontypeable | Middle ear | Pili−, Hap+, Hia−, HMW1+, HMW2+ | 67 |
| ST12hmw 1−/2+ | Nontypeable | Pili−, Hap+, Hia−, HMW1−, HMW2+ | 67 | |
| ST12hmw 1+/2− | Nontypeable | Pili−, Hap+, Hia−, HMW1+, HMW2− | 67 | |
| ST12hmw 1−/2− | Nontypeable | Pili−, Hap+, Hia−, HMW1−, HMW2− | 67 | |
| N187hap+ | Nontypeable | Middle ear | Pili−, Hap+, Hia−, HMW1+, HMW2+ | 66 |
| N187hap− | Nontypeable | Pili−, Hap−, Hia−, HMW1+, HMW2+ | This study |
NTHi strain AAr176p− used is this study is a nonpiliated phase variant and has been described previously (14).
H. influenzae strain N187hap− was constructed using plasmid pJS104, a pT7-7 derivative that contains a 6.5-kb PstI fragment harboring a full-length hap gene (66). pJS104 was partially digested with BglII, and linearized DNA was purified and then ligated with the 1.3-kb BamHI fragment harboring the Kanr cassette from pUC4K (72). Recombinant plasmids were analyzed by restriction mapping, and a plasmid with the Kanr cassette inserted at the BglII site in the middle of the hap coding sequence was designated pJS107. Subsequently pJS107 was linearized with BglII and transformed into NTHi strain N187 made competent by the M-IV method (27). Transformants were selected by growth on agar containing kanamycin. Southern analysis and Western immunoblotting were used to confirm that the mutants contained an interrupted hap locus and failed to produce Hap. One mutant was saved for further study and was designated N187hap−.
Cell culture.
The human tracheal epithelial cell line 9HTEo− was obtained from Dieter C. Gruenert (University of California, San Francisco) (17). Cells were cultured at 37°C and 5% CO2 in 75-cm2 tissue culture flasks (Costar Corp., Cambridge, Mass.) in 20 ml of Eagle's minimum essential medium supplemented with 10% fetal bovine serum, 1% l-glutamine, 1% penicillin, and 1% streptomycin (all from Gibco BRL, Gaithersburg, Md.). The confluent, adherent monolayers were released from the plastic surface after treatment with trypsin-EDTA (Gibco BRL) and split 1:4 three times a week.
Stimulation of 9HTEo− cells.
Six-well tissue culture plates (Costar) were seeded with 106 9HTEo− cells per well in 3 ml of fresh supplemented Eagle's minimal essential medium and allowed to grow overnight at 37°C in 5% CO2. On the second day, the medium was replaced with 3 ml of fresh serum-free medium (SAGM from Clonetics, San Diego, Calif.) and allowed to grow overnight. On the third day, cells were 95 to 100% confluent. The growth medium was replaced with 6 ml of fresh serum-free medium. All experiments included an unstimulated negative control well(s), a positive control well(s) containing IL-1β (20 ng/ml; Pharmingen, San Diego, Calif.) (7), and experimental wells containing various stimuli. Cell culture supernatant fluid and host cell RNA were isolated at various times after the start of the experiment.
Results of dose-response experiments of cytokine expression using purified AAr176p+ whole cells and AAr176p+ LOS with 9HTEo− epithelial cells were analyzed using regression analysis. Correlation coefficients were used to determine the linearity of epithelial cytokine responses to increasing amounts of AAr176p+ whole cells and AAr176p+ LOS.
Control experiments using the lipopolysaccharide (LPS) inhibitor polymyxin B (Sigma) were performed to determine the contribution of NTHi LOS to the induction of epithelial cytokines. In these experiments, IL-1β (20 ng/ml), purified LOS from NTHi strain AAr176p+ (0.21 μg/ml), NTHi strain AAr176p+ whole cells (5 × 107 CFU/ml), and sterile tissue culture medium were incubated with increasing amounts of polymyxin B (0.05, 0.5, 5.0, and 50 μg/ml) at room temperature for 30 min. The levels of polymyxin B used for these experiments were based on studies by Hedlund et al. (23). Confluent layers of 9HTEo− cells were then exposed to these stimuli for 16 h at 37°C in 5% CO2. After exposure, the tissue culture fluid from the 9HTEo− cells was removed and assayed for the presence of chemokine IL-8, using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, Minn.).
LOS purification and analysis.
LOS was isolated from NTHi strain AAr176p+ after growth overnight at 37°C in 5% CO2 on 150-mm-diameter Levinthal agar plates (18, 20). The bacteria were harvested from 18 plates into 180 ml of buffer containing 10 mM Tris-HCl (pH 8.0)–5 mM EDTA buffer. S1 nuclease (20,000 U; Gibco BRL), micrococcal nuclease (50 U; Sigma) and RNase T1 (40,000 U; Gibco BRL) were added, and the digestion mixture was incubated at 37°C for 1 h. Proteinase K (5 mg; Gibco BRL) was added, and the mixture was incubated at 65°C for 2 h. The sample was incubated on ice for 1 h, sonicated briefly on ice to reduce viscosity, and centrifuged at 3,000 × g for 30 min at 4°C. The supernatant fluid was transferred to dialysis tubing (Spectrum Medical, Laguna Hills, Calif.) and electrodialyzed against Milli-Q water (Millipore, Bedford, Mass.) at 500 V for 2 h with frequent changes of water and then overnight at 100 V. The precipitated LOS was removed from the dialysis bags, transferred to centrifuge tubes, and centrifuged at 23,000 × g for 30 min at 4°C. The pellet was washed once with HPLC (high-pressure liquid chromatography)-grade water, resuspended in HPLC-grade water, and further purified by the classical phenol-water extraction method (73). LOS was precipitated with sodium acetate (0.3 M, final concentration) and 2 volumes of ice-cold 95% ethanol. Pellets were washed twice with 70% ethanol and then resuspended in HPLC-grade water. The LOS was lyophilized, weighed, and resuspended to a final concentration of 1 mg/ml in 0.85% saline and stored at 4°C.
Purified and cell-bound LOS from AAr176p+ was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 16% Tris-glycine polyacrylamide gels (NOVEX, San Diego, Calif.) according to the procedure of Gu et al. (18). Purified LOS (0.375, 0.75, 1.5, and 3.0 μg) was mixed with SDS-PAGE digestion buffer, boiled for 10 min, and then loaded on an SDS-polyacrylamide gel. For cell-bound LOS, 3 × 108 CFU of strain AAr176p+ cells was resuspended and boiled in SDS-PAGE digestion buffer and incubated with 100 mg of proteinase K (final concentration, 0.5 mg/ml; Gibco BRL) at 60°C for 2 h before being loaded (28). After electrophoresis, the gels were silver stained and scanned with a Quickscan R&D laser densitometer (Helena Laboratories, Beaumont, Tex.). The amount of cell-bound LOS was compared to a standard curve generated with increasing amounts of the purified LOS (18). LOS data are means of four different experiments. Benchmark (Gibco BRL) prestained molecular weight markers were run to estimate size of bands.
Total RNA isolation.
Total RNA was isolated from the 9HTEo− tracheal epithelial cells using Trizol Reagent (Gibco BRL) according to the manufacturer's directions.
RNase protection assay.
The expression of cytokine and chemokine mRNA was determined and quantified by the RiboQuant RNase protection assay system (Pharmingen) according to the manufacturer's directions. The 32P-labeled antisense HL-14 probe set simultaneously detected expression of the human cytokine IL-6, IL-1α, IL-1β, and TNF-α genes (61). The 32P-labeled antisense hCK-5 multiprobe (Pharmingen) detected simultaneous expression of IL-8 and MCP-1 genes. Both probe sets contain the housekeeping gene L32 as an internal standard for normalization of cytokine and chemokine gene expression. A PhosphorImager (Storm 860; Molecular Dynamics, Sunnyvale, Calif.) with ImageQuaNT version 4.2 software was used to measure and analyze the expression intensity of the cytokine and chemokine mRNA species; the data were expressed as fold increase over the unstimulated control (61).
ELISA.
Secreted cytokines IL-1α, IL-6, and chemokine MCP-1 were determined by ELISA kits (CYTImmune Sciences, Inc. College Park, Md.). Biosource International (Camarillo, Calif.) ELISA kits were used to assay for TNF-α and IL-1β. R&D Systems ELISA kits were used to assay IL-8. Comparisons of cytokine secretion were made using Student's two-tailed paired t tests and were calculated using Microsoft Excel 97 software.
Adherence assay.
Adherence assays were performed according to the procedure of Gilsdorf et al. (16). Briefly, epithelial monolayers in 96-well tissue culture plates were fixed with 1% glutaraldehyde, washed with 0.2% Triton X-100, and blocked with phosphate-buffered saline containing 2% bovine serum albumin. The epithelial monolayers were then incubated with 107 CFU of biotinylated bacteria (Sulfo-NHS-Biotin; Pierce Chemical Company, Rockford, Ill.) for 1 h, washed, and incubated further with ExtrAvidin-peroxidase conjugate (Sigma). The wells were developed with the enzyme substrate o-phenylenediamine dihydrochloride (Sigma), and the intensities of the reactions were determined at A490 on a Dynatech microplate reader (Dynatech Laboratories, Inc., Alexandria, Va.). Control wells contained epithelial cells with no bacteria. Comparisons of adherence, as measured by A490 of the immunoassays, were made using Student's two-tailed paired t test, calculated using Microsoft Excel 97 software.
RESULTS
Time course of epithelial cell cytokine production using different stimuli in the normal tracheal cell line 9HTEo−.
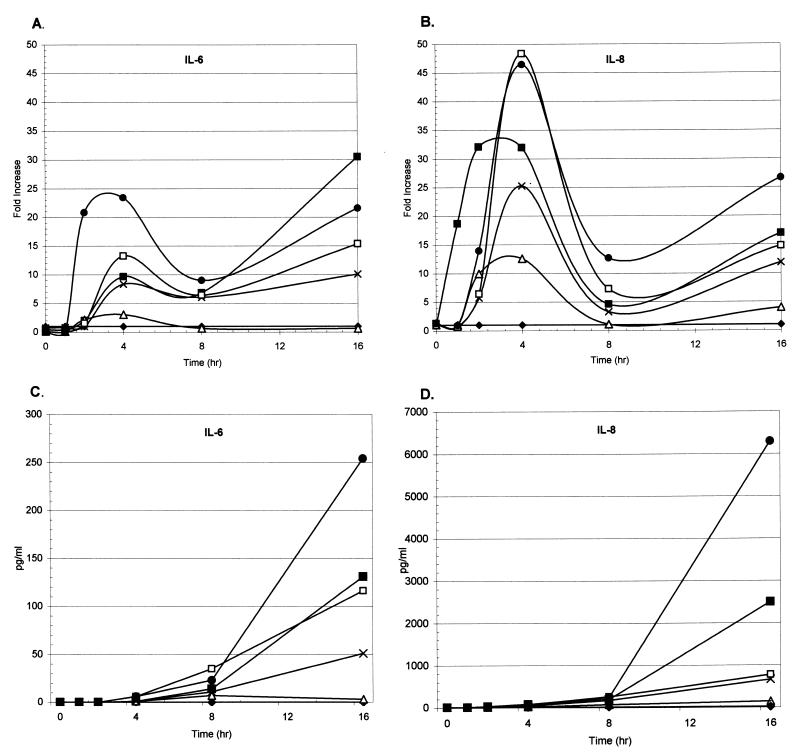
To determine the kinetics of cytokine gene expression and secretion, extended time courses were performed using the normal human tracheal epithelial cell line 9HTEo− coincubated with different H. influenzae stimuli. Representative time courses for IL-6 and IL-8 gene expression and secretion are shown in Fig. 1. Stimulation of 9HTEo− cells with IL-1β (the positive control), purified LOS, and NTHi bacterial stimuli showed an initial peak mRNA response with cytokines IL-6 and IL-8 between 2 and 4 h of coincubation. A second peak of IL-6 and IL-8 gene expression was observed after 16 h of costimulation with the various stimuli (Fig. 1A and B). The stimulation of epithelial cells with the stimuli showed a peak mRNA response with cytokines IL-1α, IL-1β, MCP-1, and TNF-α between 2 and 4 h of coincubation that decreased over the rest of the time course with no secondary peak (data not shown). The stimulation of epithelial cytokine gene expression with intact NTHi cells generated a response greater than that seen with purified LOS.
FIG. 1.
Time course of 9HTEo− cytokine IL-6 and IL-8 gene expression and secretion in response to different stimuli. Cultured human tracheal epithelial cells (9HTEo−) were coincubated with tissue culture medium alone (⧫), 20 ng of IL-1β per ml (■), 1 μg of LOS per ml (▵), and 5 × 107 CFU of NTHi strains AAr176p+ (×), 11 (□), and AA238 (●) per ml. An NTHi cell density of 5 × 107 CFU/ml corresponds to a 100::1 ratio of bacteria to epithelial cells. Based on the calculations of Gu et al. (18), 5 × 107 CFU of NTHi per ml contains approximately 1 μg of LOS per ml. Total RNA and cell culture fluid were isolated from epithelial cells after 0, 1, 2, 4, 8, and 16 h of coincubation with the stimulus. Levels of IL-6 (A) and IL-8 (B) gene expression were subsequently tested by multiprobe RNase protection assays. Representative IL-6 and IL-8 time courses are shown as fold increase over the unstimulated control. Secreted cytokines IL-6 (C) and IL-8 (D) were assayed by ELISA using commercially available kits. The results are shown as picograms of IL-6 and IL-8 per milliliter of culture fluid.
Secretion of cytokines from 9HTEo− cells coincubated with different stimuli were assayed from culture supernatant fluids using commercially available ELISAs (Fig. 1C and D). Peak secretion of IL-6 and IL-8 was seen between 16 and 24 h after coincubation with different stimuli. Similar secretion was seen for cytokine MCP-1; no IL-1α or IL-1β secretion was seen under any stimulation conditions (data not shown). TNF-α secretion, however, rose faster than that of IL-6 and IL-8, showing a range of 39 to 75% maximal response, compared to a range of 1 to 17% for both IL-6 and IL-8 after 4 h of coincubation of 9HTEo− cells with various stimuli. Similar to the mRNA expression data, intact bacterial cells stimulated greater cytokine secretion than the purified LOS.
As a negative control, monolayers of unstimulated control 9HTEo− cells demonstrated low levels of cytokine gene expression and secretion of IL-6 and IL-8 (Fig. 1), as well as with cytokines IL-1α, IL-1β, TNF-α, and MCP-1 (data not shown). Control experiments with epithelial cells coincubated with sterile bacterial growth medium or 0.85% saline demonstrated no cytokine expression or secretion (data not shown).
Dose response of 9HTEo− tracheal epithelial cytokine production with NTHi strain AAr176p+ whole cells and purified LOS.
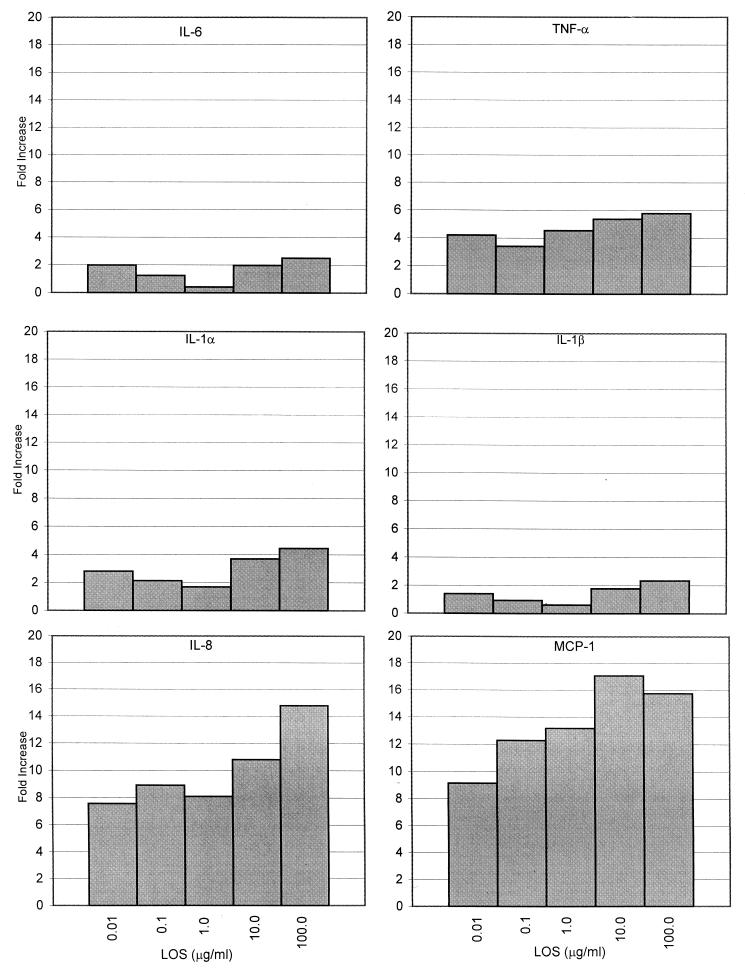
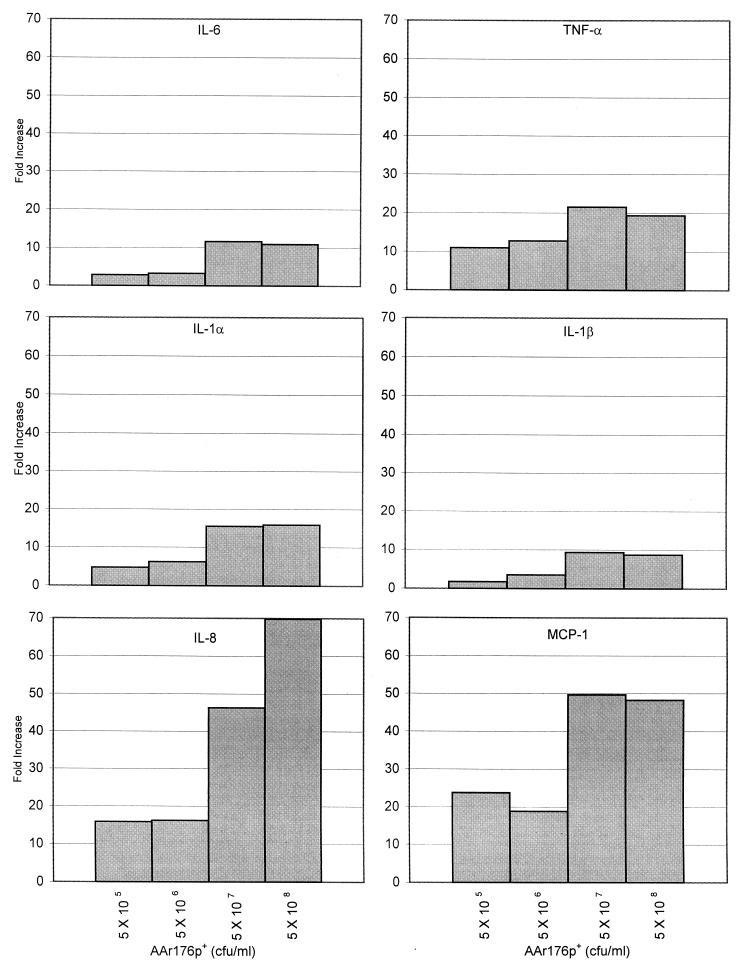
To assess the contribution of H. influenzae LOS to cytokine gene expression by H. influenzae whole cells, subsequent experiments assayed RNA expression at the 4-h peak expression time. Dose-response experiments were performed with 9HTEo− cells coincubated with increasing levels of LOS purified from strain AAr176p+ and with increasing amounts of AAr176p+ bacterial cells. The levels of purified LOS used in these experiments were chosen based on the levels used by Khair et al. (38) (10 and 100 μg/ml) with human bronchial epithelial cells and the levels used by Gu et al. (18) (0.01 ng/ml to 10 μg/ml) with human monocytes. The NTHi levels used in these experiments represent bacterium-to-epithelial cell ratios of 1:1 to 10,000:1 and were chosen based on those used by Bresser et al. (4). Representative graphs for cytokine gene expression are shown in Fig. 2 and 3.
FIG. 2.
Dose response of 9HTEo− cytokine gene expression to increasing amounts of NTHi AAr176p+ LOS. Epithelial cells were coincubated with 0.01, 0.1, 1.0, 10, and 100 μg of purified LOS per ml from strain AAr176p+ for 4 h. Cytokine gene expression was analyzed as for Fig. 1. Cytokine IL-6, IL-1α, IL-1β, TNF-α, IL-8, and MCP-1 expression is shown as fold increase over the unstimulated control. The data shown are from one representative experiment of four performed.
FIG. 3.
Dose response of 9HTEo− cytokine gene expression to increasing amounts of NTHi AAr176p+ whole cells. Epithelial cells were coincubated with 5 × 105, 5 × 106, 5 × 107, and 5 × 108 CFU of strain AAr176p+ per ml. Cytokine IL-6, IL-1α, IL-1β, TNF-α, IL-8, and MCP-1 expression is shown as fold increase over the unstimulated control. The data shown are from one representative experiment of four performed.
Cytokine expression stimulated by purified LOS showed a dose response when coincubated with the epithelial cells (Fig. 2). Regression analysis of the dose-response data using purified AAr176p+ LOS resulted in correlation coefficients of 0.91 (IL-1α), 0.61 (IL-1β), 0.78 (IL-6), 0.93 (TNF-α), 0.88 (IL-8), and 0.92 (MCP-1). The magnitude of incremental steps over the 5-log range of doses of LOS, however, was small. The difference in fold increase of cytokine expression between the lowest and highest levels of LOS used (0.01 and 100 μg/ml, respectively) ranged between 1.3 and 12.
All levels of LOS stimulated a 0.4- to 2.5-fold increase in IL-1β and IL-6 gene expression over that of unstimulated controls. In addition, a 1.7- to 5.8-fold increase was observed with TNF-α and IL-1α, and a 7.6- to 17.1-fold increase was seen with IL-8 and MCP-1 over unstimulated controls (Fig. 2).
Strain AAr176p+ bacterial cells also showed a dose response with respect to cytokine gene expression (Fig. 3); regression analysis of this response showed correlation coefficients of 0.78 (IL-1α), 0.89 (IL-1β), 0.61 (IL-6), 0.71 (TNF-α), 0.88 (IL-8), and 0.84 (MCP-1). The peak response of all cytokines was seen with either 5 × 107 CFU/ml (3 × 108 CFU/well) or 5 × 108 CFU/ml (3 × 109 CFU/well) bacteria, which represented a approximate 100:1 and 1,000:1, respectively, ratios of bacteria to epithelial cells.
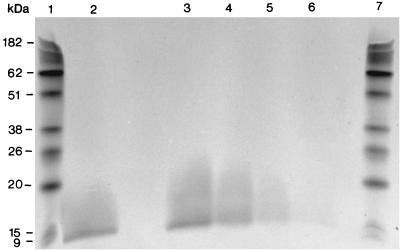
Quantitation of cell-bound LOS on NTHi strain AAr176p+.
The dose-response studies presented above indicated that peak cytokine expression was seen with a bacterium/epithelial cell ratio of approximately 100:1, or 3 × 108 CFU of strain AAr176p+ per well (5 × 107 CFU/ml). To better assess the relative contributions of NTHi whole cells and LOS to epithelial cytokine gene expression, the amount of cell-bound LOS on 3 × 108 CFU of bacterial whole cells (the peak stimulating inoculum) was determined. For this, the LOS of 3 × 108 bacterial cells and increasing amounts of purified LOS were isolated, fractionated by SDS-PAGE, and quantitated by scanning laser densitometry (Fig. 4). The results showed that 3 × 108 CFU of AAr176p+ bacterial cells contained 1.25 μg of LOS. Standardizing for the amount of LOS in the inoculum, AAr176p+ bacterial cells showed significant levels of cytokine gene expression over that of the purified LOS (Table 2).
FIG. 4.
Quantitation of cell bound LOS on NTHi strain AAr176p+ after SDS-PAGE and silver staining. Lanes 1 and 7, molecular weight standards; lane 2, 3 × 108 CFU of strain AAr176p+; lanes 3 through 6, 3, 1.5, 0.75, and 0.375 μg, respectively, of purified LOS from strain AAr176p+.
TABLE 2.
Epithelial cytokine gene expression
| Stimulant | Mean (range)a fold increase over unstimulated control
|
|||||
|---|---|---|---|---|---|---|
| IL-1α | IL-1β | IL-6 | TNF-α | IL-8 | MCP-1 | |
| IL-1β (20 ng/ml) | 6.4 (4.9–9.3) | 2.7 (2.4–3.5) | 3.4 (2.5–4.2) | 2.4 (2.1–2.9) | 29.1 (12.5–44.8) | 9.4 (5.2–25.8) |
| LOS (0.21 μg/ml) | 0.3 (0.1–0.5) | 1.5 (1.3–1.8) | 1.4 (1.1–1.7) | 2.8 (1.7–3.6) | 11.0 (7.5–14.4) | 10.1 (7.4–14.8) |
| AAr176p+ (5 × 107 cfu/ml) | 5.3 (4.1–6.3) | 2.8 (2.3–3.9) | 6.3 (4.6–7.0) | 7.1 (4.2–9.3) | 39.9 (27.7–48.9) | 18.2 (15.9–23.8) |
| AAr176p+/LOSb | 17.7* | 1.9 | 4.5* | 2.5* | 3.6* | 1.8 |
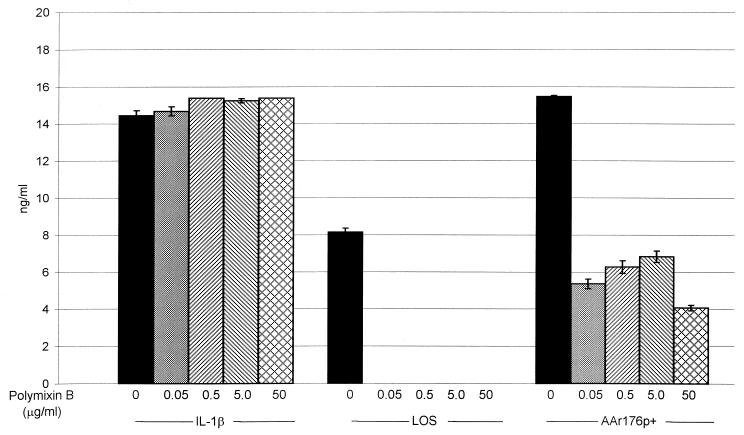
To determine the contribution of LOS in the total 9HTEo− cellular response to AAr176p+ cells, polymyxin B, which binds to LOS and neutralizes the endotoxic activity, was used as a control (Fig. 5) (23, 37). Polymyxin B at all levels (0.05 to 50 μg/ml) did not affect IL-8 secretion when added to 9HTEo− cells with and without IL-1β. All levels of polymyxin B blocked the secretion of IL-8 from 9HTEo− cells in the presence of purified LOS from NTHi strain AAr176p+. Incubation of AAr176p+ cells with increasing amounts of polymyxin B (0.05 μg/ml to 50 μg/ml) showed a 55.8 to 73.7% reduction in 9HTEo− IL-8 secretion compared to AAr176p+ cells without polymyxin B (Fig. 5).
FIG. 5.
Inhibition of NTHi LOS stimulation of 9HTEo− IL-8 secretion. Monolayers of cultured human tracheal epithelial cells (3 × 106 cells/well in six-well tissue culture plates; serum-free culture medium) were coincubated with tissue culture medium alone, IL-1β (20 ng/ml), LOS (0.21 μg/ml), and NTHi strain AAr176p+ cells (5 × 107 CFU/ml) with or without polymyxin B (0.05, 0.5, 5.0, and 50.0 μg/ml). Culture fluid was harvested after 16 h of coincubation with various stimuli, and secreted IL-8 was measured using commercially available ELISAs. The results are shown as nanograms of IL-8 per milliliter of culture fluid. Error bars each represent the standard error of the mean of three different wells each analyzed in triplicate by ELISA. Unstimulated control wells showed no IL-8 secretion.
Stimulation of epithelial cell cytokine production by adherent NTHi.
Adhesive interactions between bacterial pathogens and mucosal epithelial cells have been shown to play a key role in the stimulation of epithelial cytokine production (9, 10, 26, 35, 74). Several NTHi adhesins that mediate adherence to epithelial cells have been described (15, 59). To assess the role of NTHi adherence in the stimulation of respiratory epithelial cytokine production, sets of wild-type and adhesin-deficient NTHi strains, representing five different adhesins, were first tested for their adherence to respiratory epithelial cells. H. influenzae strain Rd, which lacks the genes for pili, HMW1, and HMW2 and contains a truncated, nonfunctional gene for Hia and a mutated, nonfunctional gene for Hap, was used as a minimal adherence control (Table 1) (12). In this assay, adhesin-proficient strains adhered significantly better to 9HTEo− cells than adhesin-deficient strains for all adhesins tested (P < 0.0001) (data not shown).
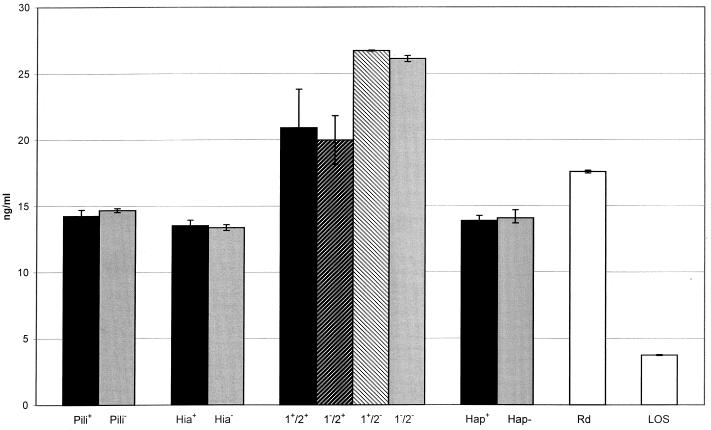
To assess the role of adhesin-epithelial cell interactions to stimulate cytokine production, representative NTHi wild-type and adhesin-deficient strains were coincubated with 9HTEo− cells for 16 h before the culture supernatant fluids were harvested and assayed for secreted cytokines. Secreted IL-8 and IL-6 were chosen as models for cytokine secretion and were assayed from the epithelial culture supernatant fluid; the data for IL-8 secretion are presented in Fig. 6.
FIG. 6.
Screening of H. influenzae clinical isolates with 9HTEo− cells for epithelial IL-8 secretion. Monolayers of cultured human tracheal epithelial cells (3 × 106 cells/well in six-well tissue culture plates; serum-free culture media) were coincubated with tissue culture medium alone, IL-1β (20 ng/ml), LOS (0.21 μg/ml), and H. influenzae clinical isolates (5 × 107 CFU/ml). Culture fluid was harvested after 16 h of coincubation with various stimuli, and secreted IL-8 was measured using commercially available ELISAs. The results are shown as nanograms of IL-8 per milliliter of culture fluid. Error bars each represent the standard error of the mean of three different wells each analyzed in triplicate by ELISA. IL-8 concentrations were 0.317 ± 0.006 ng/ml in unstimulated control wells and 14.25 ± 4.87 ng/ml in IL-1β-stimulated control wells. NTHi strains used in this study were derived from wild-type strains AAr176p+ (Pili), 11 (Hia), 12 (HMW1/2), N187 (Hap), and Rd. See Table 1 for strain details.
No significant differences in IL-6 or IL-8 secretion were observed between NTHi wild-type and adhesin-deficient strains. Both NTHi strains lacking HMW2 showed increases in the stimulation of epithelial IL-8 secretion compared to NTHi strains containing HMW2. H. influenzae strain Rd, which lacks all of the adhesins tested, showed significantly higher levels of IL-8 secretion compared to NTHi strains containing hemagglutinating pili, Hia, or Hap (P < 0.05).
Taken together, these results suggest that the presence of the NTHi cell surface adhesins HMW1, HMW2, Hia, Hap, and hemagglutinating pili did not stimulate epithelial cytokine secretion under the conditions tested. Further, these data suggest that unidentified NTHi modulins interact with respiratory epithelial cells to stimulate cytokine production.
DISCUSSION
Mucosal epithelial cells play an active role in the host response to microbial pathogens and act not only as a mechanical barrier to infection but also as environmental sensors that modulate the host immune response through the secretion of cytokines (35, 39, 69, 74). These cytokine responses occur through microbial attachment to specific receptors and transmembrane signaling, microbial invasion of epithelial cells followed by intracellular activation, or microbial activation of nonepithelial cytokines (69, 74). This study sought to identify those interactions between respiratory epithelial cells and NTHi that resulted in epithelial cytokine production and focused on epithelial cell interactions mediated by intact NTHi bacterial cells, purified NTHi LOS, and known NTHi adhesins.
Our results showed that epithelial cell interactions with intact NTHi bacterial cells and purified NTHi LOS stimulated the early expression of the proinflammatory cytokines IL-1α, IL-1β, TNF-α, IL-6, IL-8, and MCP-1. This early gene expression led to the secretion of the cytokines TNF-α, IL-6, IL-8, and MCP-1; no secretion of cytokines IL-1α and IL-1β was seen. This result is not surprising since IL-1α tends to be cell associated, and IL-1β transcription does not always correlate with translation (11).
The secretion of cytokines TNF-α, IL-6, IL-8, and MCP-1 in response to NTHi stimulation correlates well with the cytokines found in the lungs of patients with cystic fibrosis and chronic obstructive pulmonary disease and in the middle ear effusions of patients with otitis media (2, 34, 45, 47, 49, 52, 53, 55, 57, 68). Further, these data correlate with studies showing the secretion of cytokines from primary bronchial epithelial cells in response to stimulation with NTHi LOS (38, 51).
The expression of IL-6 and IL-8 declined after the 4-h peak to a low level of expression by 8 h followed by a secondary peak at 16 h (Fig. 1A and B). This secondary peak of IL-6 and IL-8 gene expression was more pronounced with 9HTEo− cells stimulated with intact NTHi bacterial cells; LOS-stimulated gene expression fell off by 8 h and showed either no increase or a small increase at 16 h. The secondary peak of IL-6 and IL-8 expression is probably due to the autocrine effects elicited by the initial secretion of TNF-α (7, 35, 39, 43, 64). A recent study by Khair et al. (39) demonstrated a similar biphasic IL-8 gene expression pattern with human bronchial epithelial cells stimulated with H. influenzae LOS. They also showed that antibodies directed against IL-1β and TNF-α abrogated the secondary peak of IL-8 expression (39). This secondary peak of IL-6 and IL-8 expression may play a role in sustaining the bacterium-induced inflammation and recruitment of polymorphonuclear leukocytes into the respiratory tract (7, 35, 39, 43, 64).
TNF-α secretion by 9HTEo− cells in response to NTHi whole cell and LOS stimulation rose faster than that of IL-6 and IL-8 secretion (data not shown). This, in part, may represent a selective early response by epithelial cells to bacterial insult resulting in sustained production of other cytokines like IL-8 and IL-6 and activation of other cells of the immune system (7, 35, 39, 43, 64, 69). Hence, epithelial cells may act as sensors and play a key role in the modulation of the immune response to microbial infections (22, 35, 39). Further, IL-1 and TNF-α have been shown to damage respiratory epithelial cells (25, 36), and H. influenzae has been shown to preferentially adhere to damaged epithelial cells (60, 75). Thus, cytokine-induced respiratory epithelial cell damage may provide a means for H. influenzae to adhere to and invade host cells.
The time courses of epithelial cytokine production suggested that NTHi bacterial cells stimulated a response greater than that of purified LOS. The dose-response (Fig. 2 and 3), LOS quantitation (Table 2), and polymyxin B inhibition (Fig. 5) experiments corroborated that observation, demonstrating that NTHi cells elicited an epithelial cytokine response greater than that of purified LOS. These experiments suggest that NTHi factors, beyond LOS, contribute to the stimulation of respiratory epithelial cytokine production. Henderson et al. (26) have suggested that bacterial factors (i.e., modulins) beyond LPS play a role in the induction of cytokine synthesis by host cells. For example, cell surface adhesins (pilin and flagellin), secreted products (autoinducer and pyocyanin), and periplasmic components (nitrite reductase) of P. aeruginosa stimulated IL-8 production from a variety of human respiratory epithelial cells (8, 10, 54, 63). P. aeruginosa LPS, however, minimally stimulated IL-8 production from the respiratory epithelial cells (10, 54). Similar results have been described for cytokine production from human epithelial cells stimulated with B. cepacia, uropathogenic Escherichia coli, and Helicobacter pylori (21, 24, 30, 56). The present study demonstrated that NTHi bacterial adherence mediated by hemagglutinating pili, HMW1, HMW2, Hap, or Hia to the tracheal epithelial cells did not correlate with IL-8 and IL-6 secretion (Fig. 6). In fact, the least adherent H. influenzae strain (Rd) elicited strong IL-6 and IL-8 responses from the epithelial cells, suggesting that other bacterial modulins contribute to epithelial cytokine stimulation. Jiang and Patel (31) showed that P5 fimbriae enhanced the IL-6 and IL-8 secretion from respiratory syncytial virus-infected and uninfected type II alveolar epithelial cells (A549). None of the H. influenzae strains tested in this study were known P5 fimbriae mutants. The strong epithelial cytokine response stimulated by strain H. influenzae strain Rd could in part be due to adherence by P5 fimbriae. Bresser et al. (4) recently identified a heat-stable soluble factor from NTHi culture fluid that stimulated IL-6 and IL-8 production from H292 human lung epithelial cells. Approximately 70% of this cytokine stimulating activity, however, was due to LOS (4).
The role of NTHi LOS in respiratory epithelial cell cytokine production is unclear. Khair et al. (38) and Nichols et al. (51) have shown that purified NTHi LOS stimulate the production of proinflammatory cytokines from primary bronchial epithelial cells. No comparative analyses, however, between NTHi bacterial cells and LOS were performed in these studies to determine the relative contribution of each to epithelial cytokine production (38, 51). The present study suggests that NTHi molecules, beyond LOS, play a role in respiratory epithelial cytokine synthesis.
The stimulation of respiratory epithelial cell cytokine production by NTHi LOS appears to be somewhat of a paradox. Several reports have suggested that epithelial cells lack the membrane bound CD14 receptor for LPS (22, 24, 39, 44). Preliminary fluorescence-activated cell sorting analysis in our laboratory of a variety of human respiratory epithelial cells (e.g., A549, HEp-2, 16HBE14o−, and CFTE29o−) demonstrated no cell-associated CD14 (data not shown). LPS-binding protein (LBP), a serum-derived protein that binds LPS and delivers it to CD14, is not present in this system since serum-free culture media was used for all epithelial cytokine stimulation assays. Further, LBP alone cannot mediate cellular responses to LPS independent of CD14 (71). Levels of respiratory epithelial cytokine stimulation by LPS/LOS vary greatly; this variation may depend on the bacterial source and structure of the LPS/LOS (10, 18, 51, 54, 59, 63, 64, 70).
The actual signaling pathway for H. influenzae LOS-mediated stimulation of respiratory epithelial cytokine production remains to be determined. Three separate mechanisms, however, have been proposed in the literature. First, Li et al. (44) suggested that as yet unidentified receptors are responsible for LPS-dependent expression in human epithelial cells. Khair et al. (39) postulated that the alternate receptors could include the platelet-activating factor (PAF) receptors since PAF receptor antagonists block LPS-induced Ca2+ increase in platelets. Alternatively, NTHi LOS may act as an epithelial cell cytotoxin and stimulate a stress response resulting in epithelial cytokine synthesis. LOS has been shown to act as an epithelial cytotoxin in organ cultures of rat trachea (32). Further cytokine production by the epithelial cells could then lead to further epithelial cell damage (25, 36). Finally, Joseph et al. (33) suggested that LPS could act as a structural analog of ceramide and hence could stimulate host cells by mimicking the second messenger function of ceramide.
In summary, the data presented show that NTHi possess other modulins, beyond LOS, that stimulate the expression of proinflammatory cytokines from respiratory epithelial cells. Further, our studies showed that NTHi adherence to epithelial cells mediated by hemagglutinating pili, Hia, HMW1, HMW2, or Hap did not play a role in epithelial cytokine stimulation. Epithelial cells derived from a variety of locations in the human respiratory tract have been shown to respond differentially to bacterial stimuli (4, 7, 8, 10, 31, 39, 51, 54, 63, 64). Here we have presented results using only one human respiratory epithelial cell line (9HTEo−). Additional studies incorporating different cell lines need to be done to more fully understand the cytokine response to NTHi of the entire human respiratory tract. Future studies will focus on the identification and characterization of the modulins from NTHi responsible for the stimulation of a variety of respiratory epithelial cells to secrete cytokines.
ACKNOWLEDGMENTS
We are very grateful to R. Rochford for providing the human riboprobe template set HL-14 and to Mayurika Patel for excellent technical assistance.
This work was supported in part by grants from the American Lung Association of Michigan (to D.L.C.), the University of Michigan Office of the Vice-President for Research and Biomedical Research Council (to D.L.C.), and the National Institutes of Health (RO1-AI25630 to J.R.G.).
REFERENCES
- 1.Barenkamp S J, St. Geme J W., III Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol. 1996;19:1215–1223. doi: 10.1111/j.1365-2958.1996.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonfield T, Panuska J, Konstan M, Hilliard K, Hilliard J, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 3.Bonfield T, Konstan M, Burfeind P, Panuska J, Hilliard J, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–261. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 4.Bresser P, van Alphen L, Habets F J M, Hart A A M, Dankert J, Jansen H M, Lutter R. Persisting Haemophilus influenzae strains induce lower levels of interleukin-6 and interleukin-8 in H292 lung epithelial cells than nonpersisting strains. Eur Respir J. 1997;10:2319–2326. doi: 10.1183/09031936.97.10102319. [DOI] [PubMed] [Google Scholar]
- 5.Broug-Holub E, Toews G B, Van Iwaarden J F, Strieter R M, Kunkel S L, Paine III R, Standiford T J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crestani B, Cornillet P, Dehoux M, Rolland C, Guenounou M, Aubier M. Alveolar type II epithelial cells produce interleukin-6 in vitro and in vivo. J Clin Investig. 1994;94:731–740. doi: 10.1172/JCI117392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cromwell O, Hamid Q, Corrigan C J, Barkans J, Meng Q, Collins P. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1β and tumour necrosis factor-α. Immunology. 1992;77:330–337. [PMC free article] [PubMed] [Google Scholar]
- 8.Denning G M, Wollenweber L A, Railsback M A, Cox C D, Stoll L L, Britigan B E. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect Immun. 1998;66:5777–5784. doi: 10.1128/iai.66.12.5777-5784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMango E, Ratner A, Bryan R, Tabibi S, Prince A. Activation of NF-κB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Investig. 1998;101:2598–2606. doi: 10.1172/JCI2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMango E, Zar H J, Bryan A P R. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Investig. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarello C A. Interleukin-1. In: Thompson A W, editor. The cytokine handbook. 3rd ed. San Diego, Calif: Academic Press; 1998. pp. 35–72. [Google Scholar]
- 12.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritch J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 13.Foxwell A, Kyd J, Cripps A. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol Mol Biol Rev. 1998;62:294–308. doi: 10.1128/mmbr.62.2.294-308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilsdorf J R, Chang H Y, McCrea K W, Bakaletz L O. Comparison of hemagglutinating pili of Haemophilus influenzae type b with similar structures of nontypeable H. influenzae. Infect Immun. 1992;60:374–379. doi: 10.1128/iai.60.2.374-379.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilsdorf J R, McCrea K W, Marrs C F. Role of pili in Haemophilus influenzae adherence and colonization. Infect Immun. 1997;65:2997–3002. doi: 10.1128/iai.65.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilsdorf J R, Tucci M, Marrs C F. Role of pili in Haemophilus influenzae adherence to, and internalization by, respiratory cells. Pediatr Res. 1996;39:343–348. doi: 10.1203/00006450-199602000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Gruenert D C, Basbaum C B, Welsh M J, Li M, Finkbeiner W E, Nadel J A. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci USA. 1988;85:5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X-X, Tsai C M, Apicella M A, Lim D J. Quantitation and biological properties of released and cell-bound lipooligosaccharides from nontypeable Haemophilus influenzae. Infect Immun. 1995;63:4115–4120. doi: 10.1128/iai.63.10.4115-4120.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hakansson A, Carlstedt I, Davies J, Mossberg A-K, Sabharwal H, Svanborg C. Aspects on the interaction of Streptococcus pneumoniae and Haemophilus influenzae with human respiratory tract mucosa. Am J Respir Crit Care Med. 1996;154:S187–S191. doi: 10.1164/ajrccm/154.4_Pt_2.S187. [DOI] [PubMed] [Google Scholar]
- 20.Hancock I, Poxton I. Preparation of lipopolysaccharide and enterobacterial common antigen. In: Hancock I, Poxton I, editors. Bacterial cell surface techniques. Chichester, England: John Wiley & Sons Ltd.; 1988. pp. 93–95. [Google Scholar]
- 21.Hedges S R, Svensson M, Svanborg C. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect Immun. 1992;60:1295–1301. doi: 10.1128/iai.60.4.1295-1301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedges S R, Agace W W, Svanborg C. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 1995;3:266–270. doi: 10.1016/s0966-842x(00)88941-6. [DOI] [PubMed] [Google Scholar]
- 23.Hedlund M, Wachtler C, Johansson E, Hang L, Somerville J E, Darveau R P, Svanborg C. P fimbriae-dependent, lipopolysaccharide-independent activation of epithelial cytokine responses. Mol Microbiol. 1999;33:693–703. doi: 10.1046/j.1365-2958.1999.01513.x. [DOI] [PubMed] [Google Scholar]
- 24.Hedlund M, Agace W, Godaly G, Haraoka M, Svanborg C. Fimbriae-mediated attachment, transmembrane signaling and epithelial cytokine responses. Bacterial Protein Toxins. 1998;29:200–207. [Google Scholar]
- 25.Heiss L N, Moser S A, Unanue E R, Goldman W E. Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infect Immun. 1993;61:3123–3128. doi: 10.1128/iai.61.8.3123-3128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herriott R M, Meyer E M, Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970;101:517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houldsworth S, Andrew P, Mitchell T. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1B by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, O'Toole W, Doig P, Trust T. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Z, Patel J. Fimbriae-mediated attachment of and cytokine potentiation by nontypable Haemophilus influenzae (NTHI) in respiratory syncytial virus (RSV)-infected epithelial cell cultures. Pediatr Res. 1998;43:148A. [Google Scholar]
- 32.Johnson A P, Inzana T J. Loss of ciliary activity in organ cultures of rat trachea treated with lipo-oligosaccharide from Haemophilus influenzae. J Med Microbiol. 1986;22:265–268. doi: 10.1099/00222615-22-3-265. [DOI] [PubMed] [Google Scholar]
- 33.Joseph C K, Wright S D, Bornmann W G, Randolph J T, Kumar E R, Bittman R, Liu J, Kolesnick R N. Bacterial lipopolysaccharide has structural similarity to ceramide and stimulates ceramide-activated protein kinase in myeloid cells. J Biol Chem. 1994;269:17606–17610. [PubMed] [Google Scholar]
- 34.Juhn S K, Lees C, Amesara R, Kim Y, Lee C T, Giebink G S. Role of cytokines in the pathogenesis of otitis media. In: Lim D, Bluestone C, Klein J, Nelson J, Ogra P, editors. Recent advances in otitis media. B. C. Philadelphia, Pa: Decker; 1993. pp. 431–434. [Google Scholar]
- 35.Kagnoff M F, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Investig. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kampf C, Relova A J, Sandler S, Roomans G M. Effects of TNF-α, IFN-γ, and IL-1β on normal human bronchial epithelial cells. Eur Respir J. 1999;14:84–91. doi: 10.1034/j.1399-3003.1999.14a15.x. [DOI] [PubMed] [Google Scholar]
- 37.Khair O A, Devalia J L, Abdelaziz M M, Sapsford R J, Davies R J. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur Respir J. 1995;8:1451–1457. [PubMed] [Google Scholar]
- 38.Khair O A, Devalia J L, Abdelaziz M M, Sapsford R J, Tarraf H, Davies R J. Effect of Haemophilus influenzae endotoxin on the synthesis of IL-6, IL-8, TNF-α, and expression of ICAM-1 in cultured human bronchial epithelial cells. Eur Respir J. 1994;7:2109–2116. doi: 10.1183/09031936.94.07122109. [DOI] [PubMed] [Google Scholar]
- 39.Khair O A, Davies R J, Devalia J L. Bacterial-induced release of inflammatory mediators by bronchial epithelial cells. Eur Respir J. 1996;9:1913–1922. doi: 10.1183/09031936.96.09091913. [DOI] [PubMed] [Google Scholar]
- 40.Khan T Z, Wagener J S, Bost T, Martinez J, Accurso F J, Riches W. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 41.Konstan M, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24:137–142. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Kuklinska D, Kilian M. Relative proportions of Haemophilus species in the throat of healthy children and adults. Eur J Clin Microbiol. 1984;3:249–252. doi: 10.1007/BF02014895. [DOI] [PubMed] [Google Scholar]
- 43.LaFleur R L, Abrahamsen M S, Maheswaran S K. The biphasic mRNA expression pattern of bovine interleukin-8 in Pasteurella haemolytica lipopolysaccharide-stimulated alveolar macrophages is primarily due to tumor necrosis factor alpha. Infect Immun. 1998;66:4087–4092. doi: 10.1128/iai.66.9.4087-4092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J-D, Feng W, Gallup M, Kim J-H, Gum J, Kim Y, Basbaum C. Activation of NF-κB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc Natl Acad Sci USA. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindberg K, Rynnel-Dagoo B, Sundqvist K-G. Cytokines in nasopharyngeal secretions; evidence for defective IL-1β production in children with recurrent episodes of acute otitis media. Clin Exp Immunol. 1994;97:396–402. doi: 10.1111/j.1365-2249.1994.tb06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malefyt R W, Moore K W. Interleukin-10. In: Thomson A W, editor. The cytokine handbook. 3rd ed. San Diego, Calif: Academic Press; 1998. pp. 333–364. [Google Scholar]
- 47.Mills P R, Davies R J, Devalia J L. Airway epithelial cells, cytokines, and pollutants. Am J Respir Crit Care Med. 1999;160:S38–S43. doi: 10.1164/ajrccm.160.supplement_1.11. [DOI] [PubMed] [Google Scholar]
- 48.Moxon E. The carrier state: Haemophilus influenzae. J Antimicrob Chemother. 1986;18(Suppl. A):17–24. doi: 10.1093/jac/18.supplement_a.17. [DOI] [PubMed] [Google Scholar]
- 49.Murphy T, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura H, Yoshimura K, McElvaney N G, Crystal R G. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Investig. 1992;89:1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nichols W, Raetz C R H, Clementz T, Smith A L, Hanson J A, Ketterer M R, Sunshine M, Apicella M A. htrB of Haemophilus influenzae: determination of biochemical activity and effects on virulence and lipooligosaccharide toxicity. J Endotoxin Res. 1997;4:163–172. [Google Scholar]
- 52.Nixon L S, Yung B, Bell S C, Elborn J S, Shale D J. Circulating immunoreactive interleukin-6 in cystic fibrosis. Am J Respir Crit Care Med. 1998;157:1764–1769. doi: 10.1164/ajrccm.157.6.9704086. [DOI] [PubMed] [Google Scholar]
- 53.Noah T L, Black H R, Cheng P-W, Wood R E, Leigh M W. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis. 1997;175:638–647. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 54.Oishi K, Sar B, Wada A, Hidaka Y, Matsumoto S, Amano H, Sonoda F, Kobayashi S, Hirayama T, Nagatake T, Matsushima K. Nitrite reductase from Pseudomonas aeruginosa induces inflammatory cytokines in cultured respiratory cells. Infect Immun. 1997;65:2648–2655. doi: 10.1128/iai.65.7.2648-2655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ophir D, Hahn T, Schattner A, Wallach D, Aviel A. Tumor necrosis factor in middle ear effusions. Arch Otolaryngol Head Neck Surg. 1988;114:1256–1258. doi: 10.1001/archotol.1988.01860230050021. [DOI] [PubMed] [Google Scholar]
- 56.Palfreyman R W, Watson M L, Eden C, Smith A W. Induction of biologically active interleukin-8 from lung epithelial cells by Burkholderia (Pseudomonas) cepacia products. Infect Immun. 1997;65:617–622. doi: 10.1128/iai.65.2.617-622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pastore F D, Schlegel-Haueter S E, Belli D C, Rochat T, Dudez T S, Suter S. Chemotactic factors in bronchial secretions of cystic fibrosis patients. J Infect Dis. 1998;177:1413–1417. doi: 10.1086/517827. [DOI] [PubMed] [Google Scholar]
- 58.Pier G. Pseudomonas aeruginosa: a key problem in cystic fibrosis. ASM News. 1998;64:339–347. [Google Scholar]
- 59.Rao V K, Krasan G P, Hendrixson D R, Dawid S, St. Geme J W., III Molecular determinants of the pathogenesis of disease due to non-typable Haemophilus influenzae. FEMS Microbiol Rev. 1999;23:99–129. doi: 10.1111/j.1574-6976.1999.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 60.Read R, Wilson R, Rutman A, Lund V, Todd H, Brain P. Interaction of nontypable Haemophilus influenzae with human respiratory mucosa in vitro. J Infect Dis. 1991;163:549–558. doi: 10.1093/infdis/163.3.549. [DOI] [PubMed] [Google Scholar]
- 61.Rochford R, Cannon M J, Sabbe R E, Adusumilli K, Picchio G, Glynn J M, Noonan D J, Mosier D E, Hobbs M V. Common and idiosyncratic patterns of cytokine gene expression by Epstein-Barr virus transformed human B cell lines. Viral Immunol. 1997;4:183–195. doi: 10.1089/vim.1997.10.183. [DOI] [PubMed] [Google Scholar]
- 62.Ruef C, Jefferson D, Schlegel-Haueter S, Suter S. Regulation of cytokine secretion by cystic fibrosis airway epithelial cells. Eur Respir J. 1993;6:1429–1436. [PubMed] [Google Scholar]
- 63.Sar B, Oishi K, Matsushima K, Nagatake T. Induction of interleukin 8 (IL-8) production by Pseudomonas nitrite reductase in human alveolar macrophages and epithelial cells. Microbiol Immunol. 1999;43:409–417. doi: 10.1111/j.1348-0421.1999.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 64.Standiford T J, Kunkel S L, Basha M A, Chensue S W, Lynch III J P, Toews G B, Westwick J, Strieter R M. Interleukin-8 gene expression by a pulmonary epithelial cell line. J Clin Investig. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.St. Geme J W., III Nontypeable Haemophilus influenzae disease: epidemiology, pathogenesis, and prospects for prevention. Infect Agents Dis. 1993;2:1–16. [PubMed] [Google Scholar]
- 66.St. Geme J W, III, de la Morena M L, Falkow S. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14:217–233. doi: 10.1111/j.1365-2958.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 67.St. Geme J W, III, Falkow S, Barenkamp S J. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci USA. 1993;90:2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Storgaard M, Larsen K, Blegvad S, Nodgaard H, Ovesen T, Andersen P L, Obel N. Interleukin-8 and chemotactic activity of middle ear effusions. J Infect Dis. 1997;175:474–477. doi: 10.1093/infdis/175.2.474. [DOI] [PubMed] [Google Scholar]
- 69.Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr Opin Microbiol. 1999;2:99–105. doi: 10.1016/s1369-5274(99)80017-4. [DOI] [PubMed] [Google Scholar]
- 70.Tang H B, DiMango E, Bryan R, Gambello M, Iglewski B H, Goldberg J B, Prince A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tapping R I, Orr S L, Lawson E M, Soldau K, Tobias P S. Membrane-anchored forms of lipopolysaccharide (LPS)-binding protein do not mediate cellular responses to LPS independently of CD14. J Immunol. 1999;162:5483–5489. [PubMed] [Google Scholar]
- 72.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 73.Westphal O, Jann K. Bacterial lipopolysaccharide extraction with phenol:water and further application of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 74.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson R, Dowling R B, Jackson A D. The biology of bacterial colonization and invasion of the respiratory mucosa. Eur Respir J. 1996;9:1523–1530. doi: 10.1183/09031936.96.09071523. [DOI] [PubMed] [Google Scholar]
- 76.Wilson R, Dowling R B, Jackson A D. The effects of bacterial products on airway cells and their function. Am J Respir Crit Care Med. 1996;154:S197–S201. doi: 10.1164/ajrccm/154.4_Pt_2.S197. [DOI] [PubMed] [Google Scholar]