Abstract
Acute lymphoblastic leukemia (ALL) is the most common type of cancer in children (age 0–14 years); however, the etiology remains incompletely understood. Several environmental exposures have been linked to risk of childhood ALL, including air pollution. Closely related to air pollution and human development is artificial light at night (ALAN), which is believed to disrupt circadian rhythm and impact health. We sought to evaluate outdoor ALAN and air pollution on risk of childhood ALL. The California Linkage Study of Early-Onset Cancers is a large population-based case–control in California that identifies and links cancer diagnoses from the California Cancer Registry to birth records. For each case, 50 controls with the same year of birth were obtained from birth records. A total of 2,782 ALL cases and 139,100 controls were identified during 2000–2015. ALAN was assessed with the New World Atlas of Artificial Night Sky Brightness and air pollution with an ensemble-based air pollution model of particulate matter smaller than 2.5 microns (PM2.5). After adjusting for known and suspected risk factors, the highest tertile of ALAN was associated with an increased risk of ALL in Hispanic children (odds ratio [OR] = 1.15, 95% confidence interval [CI] 1.01–1.32). There also appeared to be a borderline association between PM2.5 level and risk of ALL among non-Hispanic White children (OR per 10 µg/m3 = 1.24, 95% CI 0.98–1.56). We observed elevated risk of ALL in Hispanic children residing in areas of greater ALAN. Further work is needed to understand the role of ALAN and air pollution in the etiology of childhood ALL in different racial/ethnic groups.
Subject terms: Cancer epidemiology, Acute lymphocytic leukaemia, Environmental impact
Leukemia is the most common type of cancer in children (age 0–14 years), accounting for approximately one third of all cancers in this age group1. The most common subtype (80%) of childhood leukemia is acute lymphoblastic leukemia (ALL), which is a disease of the immune system caused in part by mutations that occur during the division of blood cells2. While several genetic germline alleles and somatic alterations have been linked to the development of childhood ALL (e.g. ETV6-RUNX1, high hyperdiploidy), a large majority of cases do not have a dominant genetic component and may be attributable, at least in part, to environmental exposures. Several environmental exposures such as air pollution, radiation, and pesticide use have been previously associated with increased risk of childhood ALL3. Outdoor artificial light at night (ALAN), another environmental risk factor, has been linked to adult hematologic malignancies and circadian disruption4–6, but has yet to be extensively studied in childhood cancers.
ALAN contributes to circadian disruption by desynchronizing the sleep–wake cycle7. External light stimuli to the retina triggers a response in the suprachiasmatic nucleus in the hypothalamus. This response in turn modulates body temperature, cortisol, melatonin, and other hormones that are major pathways that bring about sleepiness. Light stimuli prior to sleep can delay the release of melatonin, increasing sleep latency and disrupting circadian rhythm8. The increase in exposure to outdoor ALAN over the past several years has been well documented and may be a contributing factor to poor sleep and overall health9,10. There is also evidence suggesting ALAN may play a stronger role in sleep disruption in children than in adults11.
Outdoor ALAN is, by nature, higher in urban environments, and therefore other exposures associated with urban environments need to be considered, a major one being air pollution. Childhood ALL risk has been associated with pollution from industrial sources12 and traffic, with a recent meta-analysis reporting a 9% increased risk for those residing in areas of higher traffic density13. Exposure to fine particulates, particulate matter less than 2.5 microns in aerodynamic diameter (PM2.5), has been linked to increased overall morbidity and mortality14, and a 2016 report by the World Health Organization International Agency for Research on Cancer classified the evidence for an association between PM2.5 and childhood ALL risk as suggestive15. To date, outdoor ALAN has not been examined in epidemiological studies of childhood ALL, and most existing studies on air population have small or moderate sample sizes16,17.
The aim of this study was to evaluate the association between ALAN, air pollution and risk of childhood ALL in a population-based case–control study in California.
Methods
Study population
The California Linkage Study of Early-Onset Cancers is a statewide linkage of birth records maintained by the California Department of Public Health and cancer diagnoses reported to the California Cancer Registry. The study was performed in accordance with relevant guidelines and regulations and the protocol was approved by the institutional review boards at the University of California, Berkeley, University of Southern California, and Yale University. This study was reviewed and approved by the Vital Statistics Advisory Committee and the institutional review board of the California Health and Human Services Agency and exempt from informed consent as data used was de-identified in accordance with California Code Sect. 102430 for the protection of human subjects that is approved by the federal Department of Health and Human Services and has a general assurance pursuant to Part 46 of Title 45 of the Code of Federal Regulations.
For the current analysis, eligible cases included all children who were born in California from 2000 through 2015 and were diagnosed with incident primary ALL between 2000 to 2015. ALL was defined as a diagnosis with an International Classification of Diseases for Oncology, 3rd edition, code of 9820, 9823, 9826, 9827, 9831–9837, 9940, or 9948. There were 2,819 cases initially identified, from which we excluded missing data on maternal residential address at the time of delivery (n = 6), maternal residence outside of California (n = 4), and missing data on congenital anomality or unknown information on congenital anomality (n = 27). For each case, we randomly selected 50 controls born in the same year from birth records. The final study population consisted of 2,782 childhood ALL cases and 139,100 controls.
Exposure assessment
We assessed ALAN using the New World Atlas of Artificial Night Sky Brightness18, which provides a 750-m gridded spatial resolution global measure of luminance at the zenith (directly overhead) in millicandela per meter squared (mcd/m2). In contrast to using observations only captured by a satellite, which represents light that escapes upwards into the atmosphere, the World Atlas produces a better estimate of ground level light exposure because in includes measurements from handheld sky meters19. The New World Atlas was developed with observations from the National Oceanic and Atmospheric Administration Visible Infrared Imaging Radiometer Suite observations from May-December 2014. While this only provides a single 6-month average that was assigned retrospectively, the improved resolution of the sensor technology greatly reduce misclassification, and multiple studies have demonstrated stability in ALAN over time and high correlation between years in satellite estimates (R2 0.92–0.98)20–24. ALAN was assigned to each case and control child by their geocoded maternal residential addresses at the time of delivery.
Air pollution was assessed with a validated United States (US) national model developed by Di et al25. Briefly, the model combines outputs from a chemical transport model with variables characterizing land use, population density, weather patterns, and satellite derived aerosol optical depth in an ensemble of machine learning models to estimate daily PM2.5 concentrations at a 1-km resolution. Validation of this model against air monitoring stations resulted in an R2 of 0.802 for the Pacific region. Cases were assigned the average PM2.5 concentration from birth until diagnosis. The exposure window for matched controls began at birth and ended when the corresponding case was diagnosed (e.g., if a case was diagnosed at 30 months, the matched controls were assigned the average PM2.5 from birth until 30 months of age).
Other variables of interest
We abstracted characteristics from birth records, including birthweight, gestational age (22–36, 37–41, 42–44 weeks, or unknown), plurality (singleton or multiple), birth order (1st, 2nd, 3rd or higher), mode of delivery (cesarean section [C-section] or vaginal), complications during pregnancy, maternal history of miscarriage or stillbirth (yes/no), or history of previous cesarean section (no/yes/unknown). We also retrieved demographic and parental characteristics including sex, race/ethnicity, maternal age at delivery, mother’s place of birth, maternal education, and paternal age at delivery. We also linked maternal residential address to 2000 Census block group data to obtain the percentage of the population in the block group living below 150% of the federal poverty level. Of these factors, Hispanic ethnicity1, higher birthweight26,27, lower birth order28,29, caesarean delivery30,31, and older parental ages32,33, have been linked to an increased risk of childhood ALL, and the others are potential confounders for our analysis of ALAN and air pollution in relation to ALL risk.
Statistical methods
Spearman’s correlation was performed to evaluate correlation between ALAN and PM2.5 , with the former being the primary exposure of interest in this study and the latter being a potential confounder that has been linked to both ALAN and ALL risk in previous studies13. The associations between outdoor ALAN, PM2.5 and risk of childhood ALL were assessed using logistic regression models, and odds ratios (OR) and 95% confidence intervals (95% CI) were calculated. Examining the variables listed in Table 1, univariate ORs and their 95% CIs were obtained from bivariate logistic regression models with one independent variable at a time. A multivariable logistic regression model that included all variables was then fit, retaining those with P < 0.05 for the final model with the exception of outdoor ALAN and PM2.5, which were always included. The final model adjusted for percentage of population living below 150% of the poverty level in the census block group (tertiles), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander, other), birthweight (249–2499, 2500–2999, 3000–3499, 3500–3999, and ≥ 4000 g), mode of delivery (vaginal vs C-section), maternal age (< 20, 20–24, 25–29, 30–34, ≥ 35 years), maternal education (up to 8 years, 9–11 years, 13–15 years, ≥ 16 years, unknown), mother’s place of birth (US vs foreign countries), complications during pregnancy (yes/no), and paternal age (< 25, 25–29, 30–34, 35–39, ≥ 40 years, or unknown). Adjusted ORs and their 95% CIs were derived from the final model that simultaneously included these variables, outdoor ALAN, and PM2.5 (Tables 2, Figs. 1, 2). In sensitivity analyses, we further stratified cases based on their age of diagnosis (0–2, 3–7, 8–14 years).
Table 1.
Distribution of outdoor artificial light at night, air pollution, and birth characteristics in the California Linkage Study of Early-Onset Cancers.
| Case | Control | |||
|---|---|---|---|---|
| n = 2782 | %* | n = 139,100 | %* | |
| Light at night (mcd/m2) | ||||
| 1st tertile | 910 | 32.7 | 46,321 | 33.3 |
| 2nd tertile | 900 | 32.4 | 46,478 | 33.4 |
| 3rd tertile | 972 | 34.9 | 46,301 | 33.3 |
| Median (IQR) | 3.27 (1.83–5.92) | 3.28 (1.85–5.70) | ||
| Average PM2.5 (µg/m3) | ||||
| Mean (SD) | 12.81 (3.88) | 12.65 (3.88) | ||
| Census block group below 150% poverty | ||||
| 1st tertile | 919 | 33.0 | 46,297 | 33.3 |
| 2nd tertile | 904 | 32.5 | 46,435 | 33.4 |
| 3rd tertile | 958 | 34.4 | 46,300 | 33.3 |
| Unknown | 1 | 0.04 | 68 | 0.05 |
| Sex | ||||
| Female | 1244 | 44.7 | 68,108 | 49.0 |
| Male | 1538 | 55.3 | 70,992 | 51.0 |
| Race/ethnicity | ||||
| Non-Hispanic White | 705 | 25.3 | 36,417 | 26.2 |
| Non-Hispanic Black | 89 | 3.2 | 7610 | 5.5 |
| Hispanic | 1656 | 59.5 | 76,196 | 54.8 |
| Non-Hispanic Asian/Pacific Islander | 280 | 10.1 | 16,643 | 12.0 |
| Other | 52 | 1.9 | 2234 | 1.6 |
| Birth weight (grams) | ||||
| 250–2499 | 140 | 5.0 | 9042 | 6.5 |
| 2500–2999 | 420 | 15.1 | 23,112 | 16.6 |
| 3000–3499 | 1063 | 38.2 | 54,466 | 39.2 |
| 3500–3999 | 855 | 30.7 | 39,866 | 28.7 |
| 4000 + | 304 | 10.9 | 12,614 | 9.1 |
| Gestational age (weeks) | ||||
| 37–41 | 286 | 10.3 | 13,790 | 9.9 |
| 22–36 | 2200 | 79.1 | 110,499 | 79.4 |
| 42–44 | 153 | 5.5 | 7270 | 5.2 |
| Unknown | 143 | 5.1 | 7541 | 5.4 |
| Birth plurality | ||||
| Singleton | 2695 | 96.9 | 134,946 | 97.0 |
| Multiple | 87 | 3.1 | 4154 | 3.0 |
| Birth order | ||||
| 1st | 1063 | 38.2 | 54,201 | 39.0 |
| 2nd | 890 | 32.0 | 44,104 | 31.7 |
| 3rd and higher | 829 | 29.8 | 40,795 | 29.3 |
| Mode of delivery | ||||
| Vaginal | 1867 | 67.1 | 98,178 | 70.6 |
| C-section | 915 | 32.9 | 40,922 | 29.4 |
| Year of birth | ||||
| 2000–2003 | 1025 | 36.8 | 51,250 | 36.8 |
| 2004–2007 | 892 | 32.1 | 44,600 | 32.1 |
| 2008–2015 | 865 | 31.1 | 43,250 | 31.1 |
| Maternal age (years) | ||||
| < 20 | 234 | 8.4 | 12,966 | 9.3 |
| 20–24 | 563 | 20.2 | 31,316 | 22.5 |
| 25–29 | 737 | 26.5 | 36,553 | 26.3 |
| 30–34 | 713 | 25.6 | 34,376 | 24.7 |
| ≥ 35 | 535 | 19.2 | 23,889 | 17.2 |
| Maternal education | ||||
| Up to 8 years | 234 | 8.4 | 13,485 | 9.7 |
| 9–11 years | 460 | 16.5 | 24,000 | 17.3 |
| 12 years | 753 | 27.1 | 36,400 | 26.2 |
| 13–15 years | 604 | 21.7 | 29,008 | 20.9 |
| 16 or more years | 650 | 23.4 | 32,452 | 23.3 |
| Unknown | 81 | 2.9 | 3755 | 2.7 |
| Mother's place of birth | ||||
| US | 1290 | 46.4 | 62,090 | 44.6 |
| Foreign | 1492 | 53.6 | 77,010 | 55.4 |
| Miscarriage/stillbirth | ||||
| Never | 2269 | 81.6 | 114,918 | 82.6 |
| Ever | 513 | 18.4 | 24,139 | 17.4 |
| Unknown | 0 | 43 | 0.03 | |
| Maternal complication during pregnancy | ||||
| Never | 2180 | 78.4 | 109,098 | 78.4 |
| Ever | 602 | 21.6 | 29,993 | 21.6 |
| Unknown | 0 | 9 | 0.01 | |
| Previous C-section | ||||
| Never | 2390 | 85.9 | 120,489 | 86.6 |
| Ever | 392 | 14.1 | 18,611 | 13.4 |
| Paternal age (years) | ||||
| < 25 | 455 | 16.4 | 26,200 | 18.8 |
| 25–29 | 620 | 22.3 | 30,717 | 22.1 |
| 30–34 | 727 | 26.1 | 33,702 | 24.2 |
| 35–39 | 491 | 17.6 | 23,278 | 16.7 |
| ≥ 40 | 313 | 11.3 | 15,356 | 11.0 |
| Unknown | 176 | 6.3 | 9847 | 7.1 |
* Percentages may not add up to 100 due to rounding.
Table 2.
Association between outdoor artificial light at night, air pollution, birth characteristics, and risk of childhood acute lymphoblastic leukemia in the California Linkage Study of Early-Onset Cancers.
| Unadjusted* | Adjusted* | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Light at night (mcd/m2) | ||||||
| 1st tertile | 1.00 | 1.00 | ||||
| 2nd tertile | 0.99 | 0.90–1.08 | 0.76 | 0.99 | 0.90–1.10 | 0.91 |
| 3rd tertile | 1.07 | 0.98–1.17 | 0.15 | 1.05 | 0.95–1.17 | 0.33 |
| Average PM2.5 | ||||||
| (Per 10 µg/m3) | 1.11 | 1.01–1.22 | 0.04 | 1.08 | 0.96–1.20 | 0.20 |
| Census block group below 150% poverty | ||||||
| 1st tertile | 1.00 | 1.00 | ||||
| 2nd tertile | 0.98 | 0.89–1.08 | 0.68 | 0.97 | 0.88–1.07 | 0.58 |
| 3rd tertile | 1.04 | 0.95–1.14 | 0.37 | 1.03 | 0.93–1.15 | 0.57 |
| Unknown | 0.74 | 0.10–5.34 | 0.77 | 0.73 | 0.10–5.30 | 0.76 |
| Sex | ||||||
| Female | 1.00 | 1.00 | ||||
| Male | 1.19 | 1.10–1.28 | < 0.01 | 1.17 | 1.08–1.26 | < 0.01 |
| Race/ethnicity | ||||||
| Non-hispanic White | 1.00 | 1.00 | ||||
| Black | 0.60 | 0.48–0.75 | < 0.01 | 0.65 | 0.52–0.82 | < 0.01 |
| Hispanic | 1.12 | 1.03–1.23 | 0.01 | 1.29 | 1.16–1.43 | < 0.01 |
| Asian | 0.87 | 0.76–1.00 | 0.05 | 0.93 | 0.80–1.08 | 0.34 |
| Other | 1.20 | 0.90–1.60 | 0.20 | 1.26 | 0.90–1.77 | 0.17 |
| Birth weight (grams) | ||||||
| 250–2499 | 0.79 | 0.66–0.95 | 0.01 | 0.77 | 0.64–0.92 | < 0.01 |
| 2500–2999 | 0.93 | 0.83–1.04 | 0.22 | 0.95 | 0.85–1.06 | 0.37 |
| 3000–3499 | 1.00 | 1.00 | ||||
| 3500–3999 | 1.10 | 1.00–1.20 | 0.04 | 1.07 | 0.97–1.17 | 0.17 |
| 4000 + | 1.24 | 1.09–1.40 | < 0.01 | 1.15 | 1.01–1.31 | 0.03 |
| Mode of delivery | ||||||
| Vaginal | 1.00 | 1.00 | ||||
| C-section | 1.18 | 1.09–1.27 | < 0.01 | 1.16 | 1.07–1.26 | < 0.01 |
| Maternal age (years) | ||||||
| < 20 | 0.90 | 0.77–1.04 | 0.14 | 0.99 | 0.83–1.19 | 0.95 |
| 20–24 | 0.89 | 0.80–1.00 | 0.04 | 0.93 | 0.83–1.06 | 0.27 |
| 25–29 | 1.00 | 1.00 | ||||
| 30–34 | 1.03 | 0.93–1.14 | 0.59 | 1.04 | 0.93–1.16 | 0.51 |
| ≥ 35 | 1.11 | 0.99–1.24 | 0.07 | 1.16 | 1.01–1.32 | 0.04 |
| Maternal education | ||||||
| Up to 8 years | 0.84 | 0.72–0.97 | 0.02 | 0.76 | 0.65–0.89 | < 0.01 |
| 9–11 years | 0.93 | 0.82–1.04 | 0.20 | 0.90 | 0.79–1.01 | 0.08 |
| 12 years | 1.00 | 1.00 | ||||
| 13–15 years | 1.01 | 0.90–1.12 | 0.91 | 1.01 | 0.90–1.13 | 0.87 |
| 16 or more years | 0.97 | 0.87–1.08 | 0.55 | 0.96 | 0.85–1.09 | 0.52 |
| Unknown | 1.04 | 0.83–1.31 | 0.72 | 0.99 | 0.75–1.30 | 0.93 |
| Mother's place of birth | ||||||
| US | 1.00 | US | 1.00 | |||
| Foreign | 0.93 | 0.86–1.01 | 0.07 | 0.90 | 0.82–0.98 | 0.02 |
| Maternal complication during pregnancy | ||||||
| Never | 1.00 | 1.00 | ||||
| Ever | 1.00 | 0.92–1.10 | 0.92 | 1.03 | 0.93–1.13 | 0.60 |
| Paternal age (years) | ||||||
| < 25 | 0.81 | 0.72–0.91 | < 0.01 | 0.82 | 0.71–0.95 | < 0.01 |
| 25–29 | 0.94 | 0.84–1.04 | 0.23 | 0.94 | 0.84–1.06 | 0.30 |
| 30–34 | 1.00 | 1.00 | ||||
| 35–39 | 0.98 | 0.87–1.10 | 0.70 | 0.95 | 0.84–1.07 | 0.43 |
| ≥ 40 | 0.94 | 0.83–1.08 | 0.41 | 0.91 | 0.79–1.06 | 0.23 |
| Unknown | 0.83 | 0.70–0.98 | 0.03 | 0.88 | 0.74–1.06 | 0.17 |
*Unadjusted odds ratios and 95% confident intervals were obtained from bivariate logistic regression models that included one independent variable at a time. Adjusted odds ratios and 95% confidence intervals were derived from a multivariable logistic regression model that simultaneously included all variables listed in this table.
Significant values are in [bold].
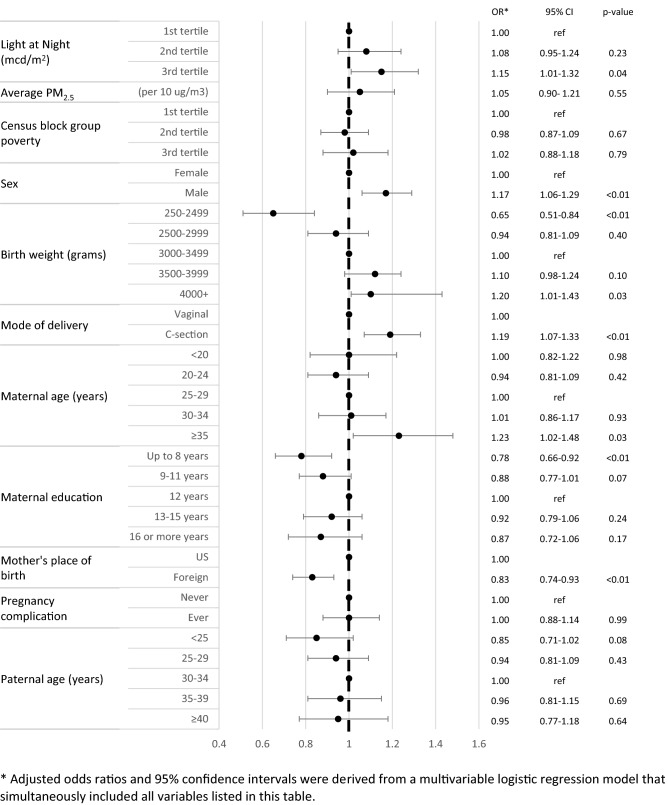
Figure 1.
Association between outdoor artificial light at night, air pollution, birth characteristics, and risk of childhood acute lymphoblastic leukemia among Hispanic children in the California Linkage Study of Early-Onset Cancers. *Adjusted odds ratios and 95% confidence intervals were derived from a multivariable logistic regression model that simultaneously included all variables listed in this figure.
Figure 2.
Association between outdoor artificial light at night, air pollution, birth characteristics, and risk of childhood acute lymphoblastic leukemia among Non-Hispanic White children in the California Linkage Study of Early-Onset Cancers. *Adjusted odds ratios and 95% confidence intervals were derived from a multivariable logistic regression model that simultaneously included all variables listed in this figure.
All analyses were performed using SAS (version 9.4, SAS Institute, Inc., Cary, North Carolina) and all tests were two-sided with an alpha value of 0.05.
Results
The median ALAN level was 3.27 mcd/m2 (inter-quartile range, IQR 1.83–5.92) in cases and 3.28 mcd/m2 (IQR 1.85–5.70) in controls (Table 1). The mean PM2.5 level was 12.81 µg/m3 (standard deviation, SD 3.88) in cases and 12.65 µg/m3 (SD 3.88) in controls. ALAN and air pollution were moderately correlated with a Spearman coefficient of 0.52. There were more male cases (55.3%) than females. A majority of the study population was Hispanic (59.5% cases and 54.8% controls). ALAN and PM2.5 were significantly higher in non-Hispanic White children (median ALAN 2.38 mcd/m2, mean PM2.5 11.47 µg/m3) than in Hispanic children (median ALAN 2.03 mcd/m2, mean PM2.5 13.34 µg/m3, Supplemental Table 1).
Among the overall study population, we did not observe a clear association between outdoor ALAN and childhood ALL (OR3rd tertile 1.07, 95% CI 0.98–1.17) (Table 2). We did observe an association between PM2.5 and ALL risk (OR 1.11, 95% CI 1.01–1.22) that was no longer significant after adjusting for other covariates (adjusted OR 1.08, 95% CI 0.96–1.20). There was increased risk of childhood ALL in male children compared to female (adjusted OR 1.17, 95% CI 1.08–1.26). Compared to non-Hispanic White children, Hispanic ethnicity was associated with increased ALL risk (adjusted OR 1.29, 95% CI 1.16–1.43) while non-Hispanic Black (adjusted OR 0.65, 95% CI 0.52–0.82) children had a lower risk. Compared to children born at a normal birthweight (3000–3499 g), low birthweight (< 2500 g) was associated with decreased ALL risk (adjusted OR<2500 g 0.77, 95% CI 0.64–0.92) while high birthweight (> 4000 g) was associated with increased ALL risk (adjusted OR>4000 g 1.15, 95% CI 1.01–1.31). Delivery by C-section was associated with a 1.16 (95% CI 1.07–1.26) increased risk of ALL. Mothers older than 35 years were more likely to have children who developed ALL compared to mothers between 25–29 years (adjusted OR 1.16, 95% CI 1.01–1.32). Children with fathers under age 25 years were less likely to have ALL compared to those with fathers between the age of 30–34 years (adjusted OR 0.82, 95% CI 0.71–0.95). Compared to mothers who completed high school, those with less than 8 years of school were less likely to have children who developed ALL (adjusted OR 0.76, 95% CI 0.65–0.89). Children of foreign-born mothers were less likely to develop ALL compared to US born mothers (adjusted OR 0.90, 95% CI 0.82–0.98).
Among Hispanic children, there was a 15% increased risk of childhood ALL (95% CI 1.01–1.32) for those residing in the highest tertile of ALAN compared to the lowest (Fig. 1). In non-Hispanic White children, there did not appear to be an association between exposure to outdoor ALAN and ALL risk (adjusted OR3rd tertile 0.84, 95% CI 0.66–1.06, Fig. 2). The association between PM2.5 and ALL appeared to be stronger in non-Hispanic White children (adjusted OR 1.24, 95% CI 0.98–1.56) than Hispanic children (adjusted OR 1.05, 95% CI 0.90–1.21). Delivery by C-section was the only covariate still significantly associated with increased ALL risk in non-Hispanic White children (adjusted OR 1.28, 95% CI 1.08–1.51).
When stratified by age, the risk association of ALAN and ALL was highest for the oldest age group with evidence of an increasing trend from 1.00 (95% CI 0.84–1.19, Supplemental Table 2 and Supplemental Fig. 1) in the 0–2 years age group to 1.22 (95% CI 0.88–1.69) in the 8–14 years age group. Foreign maternal place of birth was only protective in the 0–2 years age group (adjusted OR0-2 years 0.84, 95% CI 0.73–0.97). Compared to non-Hispanic White children, the risk among non-Hispanic Black children was lower in the 0–2 years age group (adjusted OR0-2 years 0.60, 95% CI 0.41–0.87) and 3–7 years age group (adjusted OR3-7 years 0.61, 95% CI 0.44–0.85) and null in the oldest age group. In contrast, the risk in Hispanic children was not significant in the 0–2 years age group (adjusted OR0-2 years 1.17, 95% CI 0.98–1.38) but increased in successive older age groups (adjusted OR3-7 years 1.33, 95% CI 1.14–1.54, adjusted OR8-14 years 1.60, 95% CI 1.16–2.22). Birthweight was also only significantly associated with ALL risk in the 0–2 years age group (adjusted OR<2500 g 0.52, 95% CI 0.37–0.74).
Discussion
In this large, population-based case–control study, exposure to outdoor ALAN was associated with increased childhood ALL risk, but only among Hispanic children. These results are similar to those reported in adult leukemia5. Compared to many ecological studies on the association between ALAN and cancer risk, we were able to adjust for important confounders, including birth characteristics, parental factors, markers of socioeconomic status, and air pollution. Since air pollution is highly correlated with ALAN and has been previously linked to the etiology of childhood ALL13, we accounted for it as a potential confounder. The magnitude of association between PM2.5 and ALL risk in our study (OR 1.08; 95% CI 0.96–1.20) was consistent with what was reported in a meta-analysis by Filippini et al. (OR 1.11; 95% CI 0.95–1.31)13. PM2.5 did not appear to be a confounder of ALAN in multivariable analyses, suggesting that the association between ALAN exposure and childhood ALL risk was independent of air pollution.
Exposure to light prior to sleep leads to suppression of melatonin and delayed onset of sleep34–37. Melatonin is involved in the nuclear transcription factor kappa beta pathway38, which modulates levels of many immune cytokines, such as interleukin-8 and interleukin-10, that have been previously implicated in the etiology of childhood ALL39,40. Disruptions to sleep can also alter inflammatory response from Toll-like receptors41, which are involved in hematopoiesis and leukeogenesis42. We observed the strongest association between ALAN and ALL in the older 8–14 years age group (Supplemental Fig. 1), so it may be the continued disruption to these pathways over time that contribute to development of leukemia. Additional studies have also linked ALAN exposure to circadian disruption43 and tumor progression through potential epigenetic pathways44,45. Several genes involved in circadian rhythm have been found to be differentially expressed in ALL patients as well46. As our study is the first to report a novel association between exposure to outdoor ALAN and childhood ALL risk, it would be helpful to further examine the role of ALAN in other epidemiological studies of childhood ALL and conduct additional mechanistic evaluations.
Prior studies on ALL have demonstrated differential risk patterns between Hispanic and non-Hispanic White children – including risk factors such as daycare attendance, infections, diet, and genetics47–50. Hispanic children have the highest risk of ALL among all racial/ethnic groups in California and the US51, supporting a possibly different etiological profile compared to non-Hispanic Whites. For instance, compared to non-Hispanic White children in our study, Hispanic children resided in areas of greater PM2.5 and ALAN (Supplemental Table 1). Differences in genetics may also explain how the environment influences the way certain individuals experience ALAN and disruptions in circadian rhythm. Studies have reported shorter sleep durations in minority populations compared to non-Hispanic Whites52. It could be a combination of both environment and genetics as interactions have been observed for air pollution and other conditions such as cardiovascular disease53 and Parkinson’s disease54. There may also be interactions with other attributes of the built environment that we did not evaluate, such as green space, which has been shown to improve health55 and attenuate the risks observed with air pollution exposure56,57, especially in communities of lower socioeconomic status58.
Strengths of our study include the large, population-based sample that we were able to assemble by linking statewide cancer diagnosis information to the statewide birth records in California, the most populous state in the US. The California Cancer Registry is a part of the Surveillance, Epidemiology, and End Results program and provides high quality and comprehensive ascertainment of cancer cases diagnosed in the state. Linkage to the birth records provides information on many important covariates encompassing known/suspected risk factors for childhood ALL and other potential confounders for our analysis of ALAN and air pollution. Notably, no cases or controls had to be contacted for participation in the study, minimizing selection bias. In addition, we derived high resolution estimates of ALAN and PM2.5 based on maternal residential address at the time of delivery, which was documented in birth records prior to the diagnosis of childhood ALL, therefore reducing recall bias.
A primary limitation of this study is the lack of data on residential history. While we do have additional address information for cases at time of ALL diagnosis, the corresponding information for the reference date (age at which the matched case was diagnosed) is not available for our controls. Residential mobility appears to be greater in early childhood, though most moves tend to be to similar neighborhoods59,60. In addition, we have no reason to believe that residential mobility, in regards to ALAN or PM2.5 exposure, would differ systematically between cases and controls. Another limitation is that the measure of outdoor ALAN that we used was based on observations from 2014 and consisted of an estimate of luminance at the zenith. It did not characterize light at various wavelengths, which may be an important distinction. Animal studies show that shorter, blue spectrum wavelength light (450–500 nm) was more detrimental to sleep8,61, which could be an issue as cities are moving away from amber lights towards more shorter wavelength LED (light emitting diode) streetlights44. While our use of the New World Atlas provides a better estimate of ALAN exposure at a more precise 750-m grid (compared to the 2.7-km Defense Meteorological Satellite Program Operational Linescan System) , McIsaac et al. recently demonstrated the misclassification that is still present in such large grids and how recent advances may further improve the accuracy of exposure estimate20. Another important constraint is that we do not have any data on indoor exposure to light at night, which could be influenced by curtains or other light blocking accessories, type of windows and glasses, and type of indoor light sources. A comparison of outdoor ALAN and personal exposure in children in the Netherlands only found a weak correlation of 0.3162. However, disruptions to levels of melatonin have been shown to persist hours after exposure to light stimuli34,35. The primary exposure of interest in this study is outdoor ALAN, not indoor ALAN or all ALAN. Hopefully, our work can inspire investigators of future studies to expand the scope of exposure assessment to include all types of ALAN.
With regard to air pollution, we recognize that a limitation of our study is the inability to evaluate the composition of particulate matter, e.g., air pollution from farming may contain pesticides as opposed to carbon and brake dust from road traffic. Elucidation of the components of air pollution is needed to better understand the constituents behind the risk association between air pollution and childhood ALL. A recent review found levels of outdoor PM2.5 to be correlated with indoor PM2.5, though it varied depending upon the composition63. Lastly, despite our ability to adjust for many known/suspected risk factors of childhood ALL, residual confounding due to imprecise measurements or unmeasured factors (e.g., exposure to smoking) remains a possibility.
In summary, exposure to outdoor ALAN was associated with an increased risk of ALL among Hispanic children. Future studies of ALAN and childhood cancers will require additional assessment of indoor ALAN and the relationship between ALAN and sleep. Inclusion of genetics may identify potential gene-environment interactions that cause individuals to differentially respond to their environment. This is especially important in Hispanic children, a subgroup of the population in which childhood ALL rates have been increasing in recent years1.
Supplementary Information
Acknowledgements
Research reported in this publication was supported by the National Institute of Environmental Health Sciences under award number T32 ES013678. We thank the Center for Health Statistics and Informatics within the California Department of Public Health for the data used in this analysis. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Author contributions
C.Z., M.F., C.M., J.L.W., and X.M., conceived of the study; C.Z., R.W., L.M.M., T.L., M.F., C.M., J.L.W., and X.M., N.C. conducted data collection and data analysis; all contributed to data interpretation and manuscript preparation. All authors have read and approved the submitted manuscript.
Funding
The project is funded by National Institute of Environmental Health Sciences, Grant No. T32ES013678.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to data sharing policies set by the state of California. We welcome questions from other investigators or requests for additional analyses that are pertinent to the data presented in this manuscript, and potential data sharing when permitted by the California Health and Human Services Agency Committee for the Protection of Human Subjects.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-23682-z.
References
- 1.Barrington-Trimis JL, et al. Trends in childhood leukemia incidence over two decades from 1992 to 2013. Int. J. Cancer. 2017;140:1000–1008. doi: 10.1002/ijc.30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiemels J. Perspectives on the causes of childhood leukemia. Chem. Biol. Interact. 2012;196:59–67. doi: 10.1016/j.cbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schüz J, Erdmann F. Environmental exposure and risk of childhood leukemia: An overview. Arch. Med. Res. 2016;47:607–614. doi: 10.1016/j.arcmed.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Saenz A, et al. Evaluating the association between artificial light-at-night exposure and breast and prostate cancer risk in spain (MCC-Spain Study) Environ. Health Perspect. 2018;126:047011. doi: 10.1289/EHP1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu F, et al. Longitude position in a time zone and cancer risk in the United States. Cancer Epidemiol. Biomarkers Prev. 2017;26:1306–1311. doi: 10.1158/1055-9965.EPI-16-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong C, et al. Outdoor artificial light at night and risk of non-Hodgkin lymphoma among women in the California Teachers Study cohort. Cancer Epidemiol. 2020;69:101811. doi: 10.1016/j.canep.2020.101811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aulsebrook AE, Jones TM, Mulder RA, Lesku JA. Impacts of artificial light at night on sleep: A review and prospectus. J. Exp. Zool. Part A: Ecol. Integr. Physiol. 2018;329:409–418. doi: 10.1002/jez.2189. [DOI] [PubMed] [Google Scholar]
- 8.Tähkämö L, Partonen T, Pesonen A-K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019;36:151–170. doi: 10.1080/07420528.2018.1527773. [DOI] [PubMed] [Google Scholar]
- 9.Kyba CCM, et al. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2017 doi: 10.1126/sciadv.1701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hölker F, et al. The dark side of light: a transdisciplinary research agenda for light pollution policy. Ecol. Soc. 2010 doi: 10.5751/ES-03685-150413. [DOI] [Google Scholar]
- 11.Higuchi S, Nagafuchi Y, Lee SI, Harada T. Influence of light at night on melatonin suppression in children. J. Clin. Endocrinol. Metab. 2014;99:3298–3303. doi: 10.1210/jc.2014-1629. [DOI] [PubMed] [Google Scholar]
- 12.Parodi S, et al. Risk of leukaemia and residential exposure to air pollution in an industrial area in Northern Italy: a case-control study. Int. J. Environ. Health Res. 2015;25:393–404. doi: 10.1080/09603123.2014.958136. [DOI] [PubMed] [Google Scholar]
- 13.Filippini T, et al. Association between outdoor air pollution and childhood leukemia: A systematic review and dose–response meta-analysis. Environ. Health Perspect. 2019;127:046002. doi: 10.1289/EHP4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostro B, et al. Assessing the recent estimates of the global burden of disease for ambient air pollution: Methodological changes and implications for low- and middle-income countries. Environ. Res. 2018;166:713–725. doi: 10.1016/j.envres.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Outdoor Air Pollution IARC Monogr Eval Carcinog Risks Hum. 2016;109:9–444. [PMC free article] [PubMed] [Google Scholar]
- 16.Heck JE, et al. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ. Health Perspect. 2013;121:1385–1391. doi: 10.1289/ehp.1306761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badaloni C, et al. Air pollution and childhood leukaemia: a nationwide case-control study in Italy. Occup. Environ. Med. 2013;70:876. doi: 10.1136/oemed-2013-101604. [DOI] [PubMed] [Google Scholar]
- 18.Falchi F, et al. The new world atlas of artificial night sky brightness. Sci. Adv. 2016;2:e1600377. doi: 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simons AL, Yin X, Longcore T. High correlation but high scale-dependent variance between satellite measured night lights and terrestrial exposure. Environ. Res. Commun. 2020 doi: 10.1088/2515-7620/ab7501. [DOI] [Google Scholar]
- 20.McIsaac MA, Sanders E, Kuester T, Aronson KJ, Kyba CCM. The impact of image resolution on power, bias, and confounding: A simulation study of ambient light at night exposure. Environ. Epidemiol. 2021;5:e145. doi: 10.1097/ee9.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rybnikova NA, Portnov BA. Outdoor light and breast cancer incidence: A comparative analysis of DMSP and VIIRS-DNB satellite data. Int. J. Remote Sens. 2016;38:5952–5961. doi: 10.1080/01431161.2016.1246778. [DOI] [Google Scholar]
- 22.Elvidge CD, Baugh K, Zhizhin M, Hsu FC, Ghosh T. VIIRS night-time lights. Int. J. Remote Sens. 2017;38:5860–5879. doi: 10.1080/01431161.2017.1342050. [DOI] [Google Scholar]
- 23.Kyba CCM, Aronson KJ. Assessing exposure to outdoor lighting and health risks. Epidemiology. 2015;26:e50. doi: 10.1097/ede.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 24.Zhong, C. The role of inflammation in non-Hodgkin lymphoma etiology PhD thesis, University of Southern California, (2019).
- 25.Di Q, et al. An ensemble-based model of PM25 concentration across the contiguous United States with high spatiotemporal resolution. Environ. Int. 2019;130:104909. doi: 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caughey RW, Michels KB. Birth weight and childhood leukemia: A meta-analysis and review of the current evidence. Int. J. Cancer. 2009;124:2658–2670. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 27.Milne E, Laurvick CL, Blair E, Bower C, de Klerk N. Fetal growth and acute childhood leukemia: Looking beyond birth weight. Am. J. Epidemiol. 2007;166:151–159. doi: 10.1093/aje/kwm065. [DOI] [PubMed] [Google Scholar]
- 28.Rudant J, et al. Childhood acute lymphoblastic leukemia and indicators of early immune stimulation: A Childhood Leukemia International Consortium study. Am. J. Epidemiol. 2015;181:549–562. doi: 10.1093/aje/kwu298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Behren J, et al. Birth order and risk of childhood cancer: a pooled analysis from five US States. Int. J. Cancer. 2011;128:2709–2716. doi: 10.1002/ijc.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcotte EL, et al. Caesarean delivery and risk of childhood leukaemia: a pooled analysis from the Childhood Leukemia International Consortium (CLIC) Lancet Haematol. 2016;3:e176–185. doi: 10.1016/S2352-3026(16)00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, et al. Cesarean section and risk of childhood acute lymphoblastic leukemia in a population-based, record-linkage study in California. Am. J. Epidemiol. 2017;185:96–105. doi: 10.1093/aje/kww153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petridou ET, et al. Advanced parental age as risk factor for childhood acute lymphoblastic leukemia: Results from studies of the Childhood Leukemia International Consortium. Eur. J. Epidemiol. 2018;33:965–976. doi: 10.1007/s10654-018-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R, et al. Parental age and risk of pediatric cancer in the offspring: A population-based record-linkage study in California. Am. J. Epidemiol. 2017;186:843–856. doi: 10.1093/aje/kwx160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gooley JJ, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011;96:E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akacem LD, Wright KP, Jr, LeBourgeois MK. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol. Rep. 2018;6:e13617. doi: 10.14814/phy2.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017;173:94–106. doi: 10.1016/j.lfs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Ohayon MM, Milesi C. Artificial outdoor nighttime lights associate with altered sleep behavior in the american general population. Sleep. 2016;39:1311–1320. doi: 10.5665/sleep.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markus RP, Cecon E, Pires-Lapa MA. Immune-pineal axis: Nuclear factor kappaB (NF-kB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int. J. Mol. Sci. 2013;14:10979–10997. doi: 10.3390/ijms140610979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang JS, et al. Profound deficit of IL10 at birth in children who develop childhood acute lymphoblastic leukemia. Cancer Epidemiol. Biomark. Prev. 2011;20:1736–1740. doi: 10.1158/1055-9965.Epi-11-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitehead TP, et al. Cytokine levels at birth in children who developed acute lymphoblastic leukemia. Cancer Epidemiol. Biomarkers Prev. 2021 doi: 10.1158/1055-9965.Epi-20-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisor JP, Clegern WC, Schmidt MA. Toll-like receptor 4 is a regulator of monocyte and electroencephalographic responses to sleep loss. Sleep. 2011;34:1335–1345. doi: 10.5665/sleep.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monlish DA, Bhatt ST, Schuettpelz LG. The role of toll-like receptors in hematopoietic malignancies. Front. Immunol. 2016;7:390. doi: 10.3389/fimmu.2016.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aiello I, et al. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 2020;6:eaaz4530. doi: 10.1126/sciadv.aaz4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zubidat AE, Fares B, Fares F, Haim A. Artificial light at night of different spectral compositions differentially affects tumor growth in mice: interaction with melatonin and epigenetic pathways. Cancer Control. 2018;25:1073274818812908. doi: 10.1177/1073274818812908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blask DE, et al. Light exposure at night disrupts host/cancer circadian regulatory dynamics: impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS ONE. 2014;9:e102776. doi: 10.1371/journal.pone.0102776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lagunas-Rangel FA, Kudłak B, Liu W, Williams MJ, Schiöth HB. The potential interaction of environmental pollutants and circadian rhythm regulations that may cause leukemia. Crit. Rev. Environ. Sci. Technol. 2021 doi: 10.1080/10643389.2021.1985882. [DOI] [Google Scholar]
- 47.Urayama KY, et al. Early life exposure to infections and risk of childhood acute lymphoblastic leukemia. Int. J. Cancer. 2011;128:1632–1643. doi: 10.1002/ijc.25752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singer AW, et al. Maternal prenatal intake of one-carbon metabolism nutrients and risk of childhood leukemia. Cancer Causes Control. 2016;27:929–940. doi: 10.1007/s10552-016-0773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh KM, et al. Associations between genome-wide Native American ancestry, known risk alleles and B-cell ALL risk in Hispanic children. Leukemia. 2013;27:2416–2419. doi: 10.1038/leu.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma X, et al. Ethnic difference in daycare attendance, early infections, and risk of childhood acute lymphoblastic leukemia. Cancer Epidemiol. Biomarkers Prev. 2005;14:1928–1934. doi: 10.1158/1055-9965.Epi-05-0115. [DOI] [PubMed] [Google Scholar]
- 51.Feng Q, et al. Trends in acute lymphoblastic leukemia incidence in the US from 2000–2016: An increased risk in Latinos across all age groups. Am. J. Epidemiol. 2020 doi: 10.1093/aje/kwaa215. [DOI] [PubMed] [Google Scholar]
- 52.Egan KJ, Knutson KL, Pereira AC, von Schantz M. The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Med. Rev. 2017;33:70–78. doi: 10.1016/j.smrv.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanobetti A, Baccarelli A, Schwartz J. Gene-air pollution interaction and cardiovascular disease: A review. Prog. Cardiovasc. Dis. 2011;53:344–352. doi: 10.1016/j.pcad.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee P-C, et al. Gene-environment interactions linking air pollution and inflammation in Parkinson's disease. Environ. Res. 2016;151:713–720. doi: 10.1016/j.envres.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Twohig-Bennett C, Jones A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018;166:628–637. doi: 10.1016/j.envres.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Y, et al. Associations between green space and preterm birth: Windows of susceptibility and interaction with air pollution. Environ. Int. 2020;142:105804. doi: 10.1016/j.envint.2020.105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji JS, Zhu A, Lv Y, Shi X. Interaction between residential greenness and air pollution mortality: Analysis of the Chinese Longitudinal Healthy Longevity Survey. Lancet Planet. Health. 2020;4:e107–e115. doi: 10.1016/S2542-5196(20)30027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rigolon A, Browning MHEM, McAnirlin O, Yoon H. Green space and health equity: A systematic review on the potential of green space to reduce health disparities. Int. J. Environ. Res. Public Health. 2021;18:2563. doi: 10.3390/ijerph18052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark WAV. Moving and staying in Los Aangeles neighborhoods: Money matters, but so does family composition. Cityscape. 2012;14:115–135. [Google Scholar]
- 60.Mollborn S, Lawrence E, Root ED. Residential mobility across early childhood and children’s kindergarten readiness. Demography. 2018;55:485–510. doi: 10.1007/s13524-018-0652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mortazavi SAR, et al. Blocking short-wavelength component of the visible light emitted by smartphones' screens improves human sleep quality. J. Biomed. Phys. Eng. 2018;8:375–380. doi: 10.31661/jbpe.v8i4Dec.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huss A, et al. Shedding some light in the dark-a comparison of personal measurements with satellite-based estimates of exposure to light at night among children in the Netherlands. Environ. Health Perspect. 2019;127:67001. doi: 10.1289/EHP3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohammed MOA, et al. Trends in indoor–outdoor PM2.5 research: A systematic review of studies conducted during the last decade (2003–2013) Atmosph. Pollut. Res. 2015;6:893–903. doi: 10.5094/APR.2015.099. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to data sharing policies set by the state of California. We welcome questions from other investigators or requests for additional analyses that are pertinent to the data presented in this manuscript, and potential data sharing when permitted by the California Health and Human Services Agency Committee for the Protection of Human Subjects.