Abstract
Background:
Psilocybin is a serotonin type 2A (5-HT2A) receptor agonist and naturally occurring psychedelic. 5-HT2A receptor density is known to be associated with body mass index (BMI), however, the impact of this on psilocybin therapy has not been explored. While body weight-adjusted dosing is widely used, this imposes a practical and financial strain on the scalability of psychedelic therapy. This gap between evidence and practice is caused by the absence of studies clarifying the relationship between BMI, the acute psychedelic experience and long-term psychological outcomes.
Method:
Data were pooled across three studies using a fixed 25 mg dose of psilocybin delivered in a therapeutic context to assess whether BMI predicts characteristics of the acute experience and changes in well-being 2 weeks later. Supplementing frequentist analysis with Bayes Factors has enabled for conclusions to be drawn regarding the null hypothesis.
Results:
Results support the null hypothesis that BMI does not predict overall intensity of the altered state, mystical experiences, perceptual changes or emotional breakthroughs during the acute experience. There was weak evidence for greater ‘dread of ego dissolution’ in participants with lower BMI, however, further analysis suggested BMI did not meaningfully add to the combination of the other covariates (age, sex and study). While mystical-type experiences and emotional breakthroughs were strong predictors of improvements in well-being, BMI was not.
Conclusions:
These findings have important implications for our understanding of pharmacological and extra-pharmacological contributors to psychedelic-assisted therapy and for the standardization of a fixed therapeutic dose in psychedelic-assisted therapy.
Keywords: Psychedelic therapy, psychedelic-assisted therapy, body mass index, body weight, classic psychedelic, hallucinogen, Bayes Factor, 5-HT2A
Psilocybin-assisted therapy is making a resurgence in psychiatry (Aday et al., 2020; Nutt et al., 2020). Following ingestion, psilocybin (one of the active constituents of ‘magic mushrooms’) is rapidly dephosphorylated to psilocin (4-hydroxy-N,N-dimethytryptamine), which acts (non-selectively) as a serotonin type 2A (5-HT2A) receptor agonist (Nichols, 2016). Serotonin 2A receptor agonism is responsible for the signature acute psychological effects of psychedelics (Carter et al., 2005; Vollenweider et al., 1998), with greater plasma psilocin and 5-HT2A receptor occupancy being associated with a greater intensity of subjective effects (Madsen et al., 2019). There is growing interest in aspects of the acute experience that are important for facilitating long-term positive outcomes following administration within a therapeutic setting, with both the ‘mystical-type’ experience (Bogenschutz et al., 2015; Griffiths et al., 2011, 2016; MacLean et al., 2011; Roseman et al., 2018; Ross et al., 2016) and acute emotional-breakthroughs (Roseman et al., 2019; Spriggs et al., 2020) being identified as robust and reliable predictors of therapeutic change. Stenbæk et al. (2020) recently demonstrated that the relationship between individual differences in 5-HT2A receptor binding and the mystical-type experience is not straightforward; lower pre-drug 5-HT2A receptor binding was associated with greater mystical-type experience, longer peak duration and a steeper return to normal waking consciousness. There is currently an absence of data on how the relationship between 5-HT2A receptor expression and acute experience translates into long-term outcomes of psychedelic therapy.
5-HT2A receptor density in the brain varies across individuals based on clinical and demographic factors (Adams et al., 2004), one of which is body mass index (BMI). BMI is defined as a person’s weight (in kilograms) divided by the square of their height (in m). It is commonly used as a health classifier for four weight categories (underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (BMI > 29.9 kg/m2)) (World Health Organization, 1995, 2006) and is used diagnostically in feeding and eating disorders such as anorexia nervosa (American Psychiatric Association, 2013). Prior studies have demonstrated a relationship between expression of 5-HT2A receptors in the brain, and diet and body mass. A high-fat diet is associated with a greater density of 5-HT2A receptors (Huang et al., 2004), while a reduction in 5-HT2A receptor density has been demonstrated in patients who are currently ill and recovered from anorexia nervosa (Frank et al., 2002; Kaye, 2008). The -1438 G/A polymorphism of the 5-HT2A receptor gene has been linked to differences in body mass (Rosmond et al., 2002) and the pathogenesis of eating disorders (Ricca et al., 2002; Rosmond et al., 2002). Furthermore, BMI has been shown to correlate positively with 5-HT2A receptor binding in superior temporal cortex, medial inferior temporal cortex, right dorsolateral prefrontal cortex (DLPFC) and right sensory motor cortex (Adams et al., 2004; Erritzoe et al., 2009).
Body weight-adjusted dosing is a ‘gold standard’ in pharmacological research, and has been widely used in research on psilocybin (Bogenschutz et al., 2015; Brown et al., 2017; Carbonaro et al., 2016; Carter et al., 2005, 2007; Grob et al., 2011; Nicholas et al., 2018; Ross et al., 2016; Schmidt et al., 2012; Umbricht et al., 2002; Vollenweider et al., 2007). Given the PET findings above (Stenbæk et al., 2020), and an increased receptor density in those with a higher BMI, it is unclear what impact weight-adjusted dosing would have on the acute experience. More broadly, it has been suggested that body weight may be insufficient as an adjustment metric as it does not account for body composition, which is particularly important for those within the obese BMI range (Green and Duffull, 2004; Morrish et al., 2011). In a pooled analysis of studies using weight-adjusted doses of psilocybin, Studerus et al. (2011) found that BMI was not a significant predictor of the intensity of the acute psilocybin experience across a dose range of 115–315 mg/kg. A more recent analysis across trials using doses of 20 mg/70 kg and 30 mg/70 kg also found no evidence for a relationship between body weight and acute experience (Garcia-Romeu et al., 2021). While these authors did mimic a ‘fixed dose’ group analysis (dose range 23–27 mg), all these studies employed body weight-adjusted dosing, largely limiting any conclusions that can be made about BMI. Additionally, neither of these previous analyses looked at the long-term psychological outcomes of the psychedelic experience.
An attractive alternative to body weight-adjusted dosing is to use a fixed dose. Such a strategy has the important benefit of simplifying the logistics of large-scale clinical roll-out. Moreover, this approach has been supported by pharmacokinetic simulations conducted by Brown et al. (2017) who demonstrated that 25 mg would be a sufficient fixed dose to induce the therapeutically meaningful acute experiences of a dose of 0.3 mg/kg (Griffiths et al., 2011; Hirschfeld and Schmidt, 2020; Nicholas et al., 2018). No studies to date have directly assessed the relationship between BMI and the acute experience after a fixed high (i.e. therapeutically meaningful) dose of psilocybin, and whether this has any impact on the long-term psychological outcomes.
To address this, we conducted a pooled analysis across three recent studies from the Centre for Psychedelic Research, Imperial College London where a fixed high dose of 25 mg psilocybin was administered within a supportive environment. The aim of this analysis was to ascertain the relationship between BMI and acute psilocybin experiences, as well as BMI and long-term psychological outcomes. Given that no strong hypotheses could be made based on current literature, we employed a combination of frequentist and Bayesian hypothesis testing for all analyses to enable conclusions to be drawn regarding both the presence and absence of effect (i.e. support for or against the null) (Mulder and Wagenmakers, 2016).
Materials and methods
Studies
Three studies were included in the current analysis. Two of the studies were clinical studies: an open-label study in treatment-resistant unipolar depression (depression open-label (D-OL)) and a randomized control trial (RCT) in moderate-severe unipolar depression (depression RCT; D-RCT). The third study was in psychedelic-naïve (i.e. had never taken a psychedelic drug) healthy volunteers (HVs). All trials received favourable opinion from the National Research Ethics Service and were sponsored by Imperial College London’s Joint Research and Compliance Office. The two clinical studies were adopted by the National Institute of Health Research (NIHR) Clinical Research Network and reviewed and approved by the Medicines and Healthcare products Regulatory Agency. All study sessions for all studies took place at the NIHR-funded Imperial College Research Facility.
Participants
All participants provided written informed consent after being provided with a complete description of the relevant study and before taking part. For all studies, inclusion criteria included an age range of 18–85 years and physically healthy (determined via physical examination). Primary exclusion criteria included past or present diagnosis of a psychotic disorder, immediate family member with a psychotic disorder, MRI contraindications, positive pregnancy test at screening or during the study and excessive alcohol or drug use (for further study-specific criteria, refer to relevant publications). With the exception of one participant in D-OL, all participants in the two depression trials withdrew from serotonergic medication prior to starting the trial.
Procedure
Dose
For consistency, the current analysis focuses on the 25 mg dose in all studies. Where more than one 25 mg dose was delivered, the first of these is used. This dose is generally well tolerated and is thought to maximize therapeutic outcomes while having minimal side effects. Across all three studies, no serious adverse events occurred at this dose.
D-OL trial
D-OL was an open-label feasibility trial of psilocybin-assisted psychotherapy for treatment-resistant depression (ISRCTN registration number ISRCTN14426797). Twenty participants (six females) received two oral doses of psilocybin (10 mg followed by 25 mg) 1 week apart. Psilocybin was obtained from THC Pharm (Frankfurt, Germany) and formulated into the investigational medicinal product (5 mg psilocybin in size 0 capsules) by Guy’s and St. Thomas’ Hospital’s Pharmacy Manufacturing Unit (London, UK). For details on full trial procedures, refer to Carhart-Harris et al., (2016).
D-RCT
D-RCT compared psilocybin to the selective serotonin reuptake inhibitor escitalopram (NCT03429075). Fifty-nine participants received two oral doses of psilocybin separated by 3 weeks. Thirty participants (11 females) received two 25 mg doses of psilocybin and a 6-week course of placebo, while the remaining 29 (9 females) received two 1 mg doses of psilocybin coupled with a 6-week course of escitalopram. Only participants receiving the 25 mg dose of psilocybin are included in the current analysis as the coupling of the 1 mg dose with escitalopram would confound the analysis. Psilocybin (COMP360) was supplied by Compass Pathways. Manufacture was performed by Onyx and encapsulation was performed by Catalent (formerly Juniper) pharmaceuticals. Bottling and labelling were performed by Fisher pharmaceuticals. For full trial details, refer to Carhart-Harris et al., (2021).
HV trial
The HV study assessed long-term psychological and brain changes following a 25 mg dose of psilocybin in psychedelic-naïve individuals (Lyons, 2020). One month prior, all participants also received a low dose (1 mg) but were blind to the dosing schedule. A total of 28 participants (13 females) completed this study. Psilocybin supply was identical to D-RCT.
Psychological support
Given the role of extra-pharmacological factors in shaping the acute experience (and our psychological indices thereof) (Carhart-Harris et al., 2018), and important emphasis was placed upon the ‘set and setting’ of all dosing sessions. Across all studies, each participant was paired with two experienced guides/sitters who provided psychological support. Dosing days took place in a therapeutic environment and were enveloped by psychological preparation (becoming acquainted with the participant, building trust and discussing what to expect from the experience) and integration (non-judgemental, compassionate listening to the participants’ experience, aiding their ability to contextualize and assimilate it).
During dosing, participants were encouraged to recline in a semi-supine position and were provided with an eye mask and headphones. The music playlist was specially designed to map onto the psychedelic experience and participants were encouraged to go on an inward journey while they were supported by the presence of the two guides.
Measures
Demographics and BMI
Demographic information was collected at the screening visit for each study including age, sex, height and weight. BMI was calculated for all participants using the formula weight (kg)/height (m)2.
ASC questionnaire
The altered states of consciousness (ASC) questionnaire is the most widely used measure of altered states of consciousness (particularly in the psychedelic state (Dittrich, 1998; Studerus et al., 2010)) and was included in all three studies discussed here. The ASC can be parcellated into either 5 (5D-ASC, from 94 questions) or 11 (11D-ASC, from 42 questions) factors (Dittrich, 1998; Studerus, Gamma, & Vollenweider, 2010). Each item is measured on visual analogue scales from 0 (‘not more than usual’) to 100 (‘much more than usual’). Averages are calculated for total and subscale scores.
Consistent with Roseman et al. (2018), we utilized the 42-item version of the ASC which only indexes three of the five dimensions defined by the 5D-ASC: oceanic boundlessness (OBN), dread of ego dissolution (DED) and visionary restructuralization (VRS). The OBN subscale indexes the ‘mystical type’ experience – that has been deemed important in predicting long-term psychological gains following a psychedelic experience (Erritzoe et al., 2018; Griffiths et al., 2008; Roseman et al., 2018; Ross et al., 2016). Within the OBN factor are five of the 11D-ASC sub-factors: ‘insightfulness’, ‘blissful state’, ‘experience of unity’, ‘spiritual experience’ and ‘disembodiment’. DED assesses the negative aspects of the experience and encompasses the 11D subscales of ‘anxiety’ and ‘impaired control or cognition’. VRS refers to perceptual distortions and encompasses the 11D subscales of ‘complex imagery’, ‘elementary imagery’, ‘audio/visual synaesthesia’ and ‘changed meaning of percepts’. By focusing on these three factors, we explored the association between BMI and mystical, challenging and perceptual experiences (the pillars of a psychedelic experience), while avoiding the additional computational load of the final two sub-factors of the 5D-ASC (‘auditory alterations’ and ‘reduction of vigilance’) which are not central to the altered states induced via classic psychedelics (Studerus et al., 2010).
Emotional breakthrough inventory
The emotional breakthrough inventory (EBI) is a recently validated measure of emotional release/breakthrough experienced during the acute psychedelic state (Roseman et al., 2019; Spriggs et al., 2020). The EBI consists of six statements: ‘I faced emotionally difficult feelings that I usually push aside’, ‘I experienced a resolution of personal conflict/trauma’, ‘I felt able to explore challenging emotions and memories’, ‘I had an emotional breakthrough’, ‘I was able to get a sense of closure on an emotional problem’ and ‘I achieved an emotional release followed by a sense of relief’. Each statement is designed to reflect aspects of overcoming emotions through the psychedelic experience. The EBI was only included in the D-RCT and HV studies and was run at the end of the dosing day (i.e. alongside the ASC) in reference to the acute experience.
Warwick-Edinburgh Mental Well-being Scale
The Warwick-Edinburgh Mental Well-being Scale (WEMWBS) is a validated 14-item scale designed to measure both the hedonic (i.e. subjective experience of happiness) and the eudaemonic (i.e. psychological functioning) aspects of positive mental health, or well-being (Tennant et al., 2007). Each item is a positive statement that participants rate on a 5-point Likert-type scale from ‘none of the time’ to ‘all of the time’ in reference to the previous 2 weeks. Higher scores represent greater well-being, with a maximum score of 70. The WEMWBS was included within the 10 days before and exactly 2 weeks after the dosing day in question in the D-RCT and HV studies.
Analysis
The analyses performed here were conceived of post hoc and were not part of an a priori statistical analysis plan for any of the studies and neither were they specifically approved a priori by an ethics committee. However, such post hoc analyses are commonplace in clinical research where new questions naturally arise that warrant addressing. As is standard, ethics approval does not adjudicate on such post hoc analyses, but simply ask that good practice be maintained in data management and protection and all other relevant aspects of clinical research. In accordance with Imperial College guidelines, all participants across studies consented to their research data being stored and analysed by researchers at Imperial College London.
Frequentist analyses
Preliminary analyses were conducted to assess for differences in age, sex and BMI between the studies, and for a relationship between demographics and BMI. Data were then pooled across studies for subsequent analysis. Linear regressions were used to assess BMI as a predictor of both the acute psychedelic experience (ASC and EBI) and psychological outcomes (WEMWBS). Age (mean-centred), sex and study were included as control variables in all analyses, with baseline WEMWBS also added as a control variable in the WEMWBS analysis (a = 0.05; two tailed).
Bayesian analyses
To complement these frequentist analyses, Bayes Factors (BFs) were also computed for linear regressions and, where appropriate, follow-up t-tests. BFs represent the relative evidence for one model over another, conditioned on the observed data. Bayesian linear regressions were performed, including the covariates age (mean-centred), sex and study. For prediction of the ASC and EBI, two alternative models were defined and compared to the null (M0): M1 did not include BMI (M1: Age, Sex, Study), while M2 did (M2: Age, Sex, Study, BMI). Two BFs are therefore presented for each analysis: the first assesses the relative evidence for all covariates of the full model as compared to the null model (BF02). The second directly compares the two alternative models and therefore establishes the relative evidence for the single covariate BMI (BF12).
To assess BMI as a predictor of well-being, three models were compared, all of which included age, sex, study and baseline WEMWBS as covariates: M1 additionally included BMI, M2 additionally included EBI or ASC-OBN and M3 included both BMI and either EBI or ASC-OBN. BFs were computed to assess the relative evidence of M3 versus models that did not include either BMI (BF32) or EBI/ASC-OBN (BF31).
We provide the following guidelines for the interpretation of BF values. The further a BF value is from 1, the stronger the evidence for either the model of interest (BF > 1) or the competing model (BF < 1). Typically, a BF value that is >3 is regarded as strong evidence when comparing an alternative to null; however, lower thresholds are accepted when comparing alternative models (Mulder et al., 2009). As decimal-place BFs are more challenging to interpret, we present BFs as >1 where possible, for example, when model evidence most consistently supports the null, we present BF01 > 1, rather than BF10 > 1. This does not change the statistical value, and this is purely for ease of interpretation (Rouder and Morey, 2012). All analyses were performed using default JSZ priors (Rouder et al., 2009) in R Studio (https://rstudio.com/) using the BayesFactor package (Morey et al., 2015). Plots were generated using ggplot2 (Wickham, 2009).
Results
Demographics and BMI
Demographic data for the three studies are presented in Table 1. There were no significant differences between studies in age (F(2,74) = 0.95, p = 0.393) and sex (χ2(2,N=77) = 1.15, p = 0.560), and there was no significant differences in BMI between the studies (F(2,74) = 2.386, p = 0.099; Figure 1). Across the three studies, there was no significant relationship between age and BMI (r75 = 0.19, p = 0.104); however, females had significantly lower BMIs than males (female M (SD) = 24.1 kg/m2 (4.8 kg/m2); male M (SD) = 26.5 kg/m2 (4.36 kg/m2); t(57.58) = 2.241, p = 0.03). Age, sex and study were used as covariates in all analyses.
Table 1.
Demographic information for the three studies.
| Study | N | Age (years) | BMI (kg/m2) | |||
|---|---|---|---|---|---|---|
| Total | Females | M (SD) | Range | M (SD) | Range | |
| HV | 28 | 13 | 40.6 (8.7) | 28–59 | 24.0 (5.0) | 16.4–41.3 |
| D-RCT | 30 | 11 | 43.3 (11.7) | 21–64 | 26.4 (4.6) | 17.7–37.1 |
| D-OL | 19 | 6 | 44.7 (10.9) | 27–64 | 26.4 (4.3) | 18.3–36.4 |
| Total | 77 | 30 | 42.7 (10.5) | 21–64 | 25.6 (4.7) | 16.4–41.3 |
BMI: body mass index; D-OL: depression open-label; D-RCT: depression randomized control trial; HV: healthy volunteer.
Figure 1.
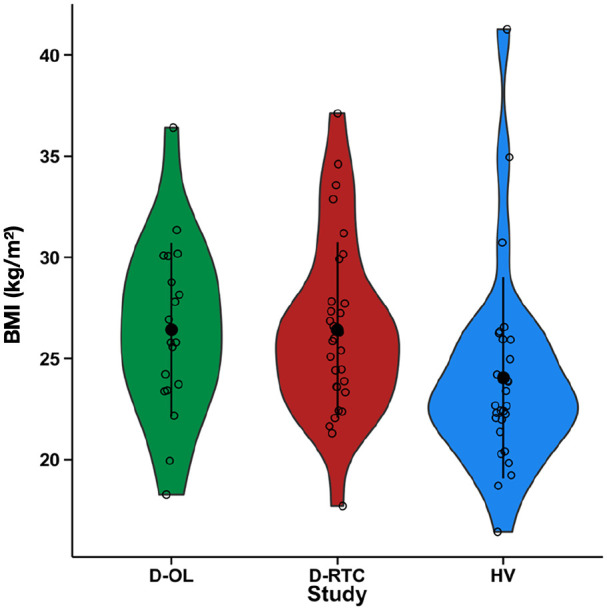
BMI in the three studies separately. Violin plots represent the distribution of scores. Black dots and bars represent the mean and standard deviation, respectively, while open circles represent individual participants. BMI: body mass index; D-OL: depression open-label; D-RCT: depression randomized control trial; HV: healthy volunteer.
All three studies were included in analyses on ASC (total N = 77); however, EBI and WEMWBS were only included in D-RCT and HV with some drop-out seen in the collection of WEMWBS at follow-up (EBI analysis N = 56; WEMWBS analysis N = 51).
BMI as a predictor of acute experience
Altered states of consciousness
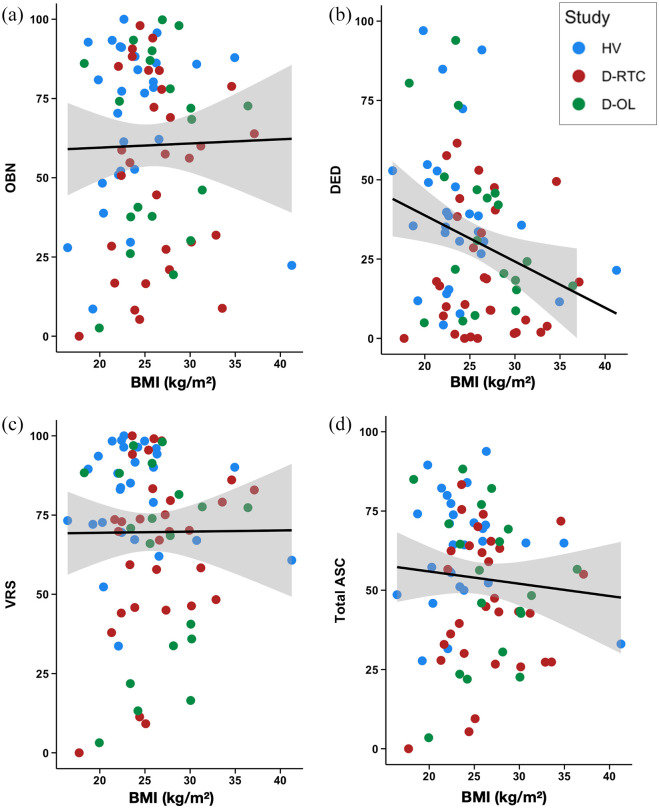
The results of the linear regressions used to assess whether BMI predicts the intensity of the acute experience (indexed with the ASC), while controlling for age, sex and study, are presented in Table 2 and Figure 2. The results of frequentist analyses were non-significant for the total ASC and the three subscales (OBN, DED and VRS), with the DED subscale showing a marginal effect (p = 0.076). The BFs comparing the full alternative M2 to the null M0 all provided strong evidence towards the null, with the exception of DED where the evidence was low/anecdotal towards the alternative. When comparing the two alternative models, the model without BMI as a predictor (M1) was favoured in all cases except for DED, where the evidence was inconclusive.
Table 2.
Results of linear regressions assessing BMI as a predictor of ASC and EBI controlling for age, sex and study.
| Acute scale | Frequentist | Bayesian* | |||
|---|---|---|---|---|---|
| B | 95% CI | p | BF02 | BF12 | |
| ASC-OBN | 0.30 | −1.20, 1.81 | 0.689 | 39.63 | 2.82 |
| ASC-DED | −1.07 | −2.25, 0.12 | 0.076 | 0.38 | 0.69 |
| ASC-VRS | 0.40 | −0.90, 1.70 | 0.538 | 3.63 | 2.35 |
| ASC total | −0.09 | −1.20, 1.01 | 0.87 | 5.83 | 2.66 |
| EBI | −0.11 | −1.79, 2.02 | 0.904 | 30.91 | 2.60 |
ASC, N = 77; EBI, N = 56.
ASC: altered states of consciousness; BF: Bayes Factor; BMI: body mass index; DED: dread of ego dissolution; OBN: oceanic boundlessness; VRS: visionary restructuralization; EBI: emotional breakthrough inventory.
M0: null model.
M1: Age + Sex + Study.
M2: Age + Sex + Study + BMI.
While BFs are commonly presented as evidence for the alternative relative to the null, here we presented evidence for the null relative to the alternative (BF01 and BF12) because BF > 1 are easier to interpret than BF < 1 (Rouder and Morey, 2012).
Figure 2.
Scatter plots of the relationship between BMI and the ASC subscales of (a) OBN, (b) DED, and (c) VRS as well as (d) total ASC score. DED: dread of ego dissolution; OBN: oceanic boundlessness; VRS: visual restructuralization; BMI: body mass index; ASC: altered states of consciousness.
To further explore the low/anecdotal evidence for M2 predicting DED, three additional models were defined to determine the contribution of the three other predictors to the full model. Specifically, these three models removed each of the predictors one-by-one for comparison with the full model (M3 = Sex, Study, BMI; M4 = Age, Study, BMI; M5 = Age, Sex, BMI). Evidence was inconclusive for the predictor age (BF23 = 1.08), was strong against sex as a predictor (BF24 = 3.83) and was strong for study as a predictor (BF52 = 3.902). Additional Bayesian t-tests (default JSZ priors) were performed to determine evidence for differences between the studies. Here, there was strong support for a difference in DED between D-RCT and HV (BF = 22.07), moderate evidence against a difference between HV and D-OL (BF = 0.36) and inconclusive evidence for a difference between the two depression trials (BF = 1.76) (Figure 3). These differences are driven by overall lower DED in D-RCT. To summarize, these analyses indicate that study is the strongest predictor driving the evidence for M2 over the null in predicting DED.
Figure 3.
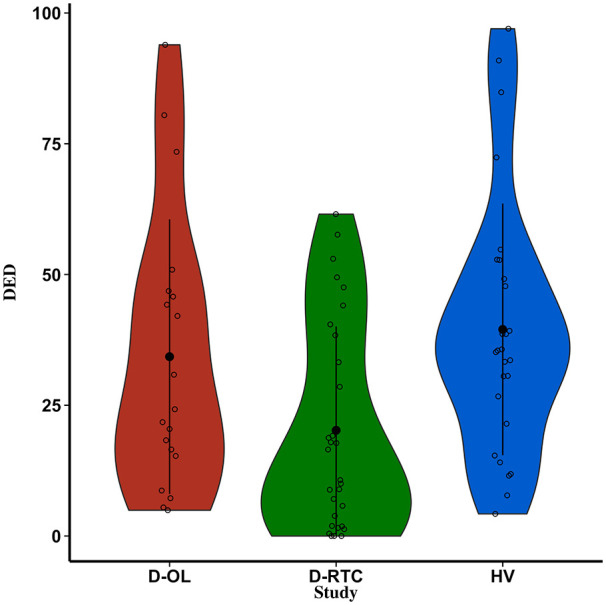
DED across the three studies separately. Violin plots represent the distribution of scores. Black dots and bars represent the mean and standard deviation, respectively, while open circles represent individual participants. DED: dread of ego dissolution.
Emotional breakthrough inventory
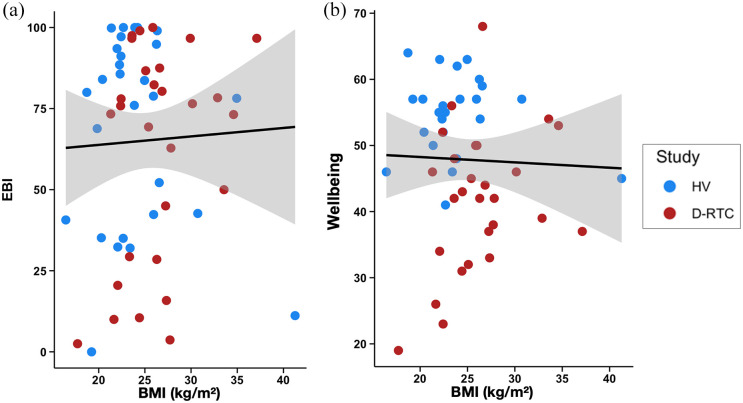
The results of the linear regressions used to assess whether BMI predicts emotional breakthrough (EBI) are presented in Table 2 and Figure 4(a). The frequentist linear regression assessing BMI as a predictor of EBI and controlling for age, sex and study was not significant (p = 0.904). Additionally, the Bayesian regression provided very strong evidence towards the null model (BF02 = 30.91), and moderate evidence against the inclusion of BMI (BF12 = 2.60).
Figure 4.
Scatter plots of the relationship between BMI and (a) EBI and (b) WEMWBS 2-weeks post-high dose. BMI: body mass index; EBI: emotional breakthrough inventory
BMI as a predictor of psychological outcome
BMI was found to be a non-significant predictor of WEMWBS in a linear regression controlling for age, sex, study and baseline WEMWBS (b = 0.27 95%CI [−0.28, 0.82], p = 0.325; Figure 4(b)). Furthermore, when comparing models without (M1) and with (M2) BMI as a predictor, the BF analysis revealed moderate evidence against BMI as a predictor (BF12 = 2.051).
A final set of regressions were computed on WEMWBS scores that also included EBI or ASC-OBN as predictors. A frequentist regression including BMI and ASC-OBN as predictors demonstrated that the ASC-OBN significantly predicted improvements in WEMWBS (b = 0.10 95%CI [0.03, 0.18], p = 0.009), while BMI did not (b = 0.29 95%CI [−0.22, 0.81], p = 0.258). Similarly, a regression including BMI and EBI as predictors demonstrated that EBI predicted improved WEMWBS (b = 0.10 95%CI [0.03, 0.18], p = 0.005), while BMI did not (b = −0.31 95%CI [−0.2, 0.82], p = 0.221). For each regression, BFs were computed to assess the relative evidence of each predictor of interest against a full model including all covariates (M3). This demonstrated strong evidence for ASC-OBN as a predictor of WEMWBS (BF31 = 6.50) and weak evidence against BMI (BF23 = 1.85). Similarly, strong evidence was demonstrated for EBI (BF31 = 8.95) while weak evidence was provided against BMI (BF23 = 1.86).
Discussion
The aim of the current study was to investigate whether BMI predicts acute and long-term effects of a fixed dose of psilocybin administered in a supportive setting. Data were pooled across three trials conducted at the Centre for Psychedelic Research, Imperial College London, totalling 77 individuals receiving a 25 mg dose of psilocybin. When controlling for age, sex, and study, evidence supported the null hypothesis that BMI does not predict overall intensity of the altered state (total ASC), mystical-type experiences (ASC-OBN), perceptual changes (ASC-VRS) or emotional breakthroughs (EBI) during the acute experience. There was weak, non-significant evidence for greater dread of ego dissolution (ASC-DED) in participants with lower BMI, however, further analysis provided no evidence for BMI meaningfully contributing to the combination of the other covariates (age, sex and study) in predicting ASC-DED. Finally, while both greater mystical-type experiences and emotional breakthroughs were strong predictors of greater improvements in well-being, BMI was not. Overall, this is – to the best of our knowledge – the first demonstration that BMI does not predict long-term, as well as acute effects of a fixed dose of psilocybin (but see Studerus et al., (2011) and Garcia-Romeu et al. (2021) for an exploration using weight-adjusted doses). By employing Bayesian hypothesis testing, we were able to conclude that this largely reflects evidence of absence, rather than an absence of evidence.
The present study also expands upon the results of previous studies (Garcia-Romeu et al., 2021; Studerus et al., 2011) by providing the first pooled analysis across studies employing a fixed dose of psilocybin. While body weight-adjusted dosing is currently regarded as a ‘gold-standard’ in research, there has been increasing recognition that body weight may not provide a sufficient measure of body composition when adjusting dosing (Green and Duffull, 2004; Morrish et al., 2011). Additionally, it adds practical and financial complexity to standardization, validation and large-scale distribution (Brown et al., 2017), which in turn significantly impacts the feasibility of large-scale roll-out of psychedelic-assisted therapy. Having a fixed dose could significantly simplify the design and logistics of numerous future clinical studies, particularly in clinical trials where BMI is a feature of the target population, such as psilocybin-assisted psychotherapy in anorexia nervosa. Such trials are currently underway with more planned for the future (NCT04505189; NCT04052568; NCT04661514) (Foldi et al., 2020; Spriggs et al., 2021).
To assess the quality of the acute experience, this study used the ASC (the most widely used subjective index of altered states), with a focus on three of its subscales that are central to the psychedelic experience (Studerus et al., 2010), namely: mystical experiences (ASC-OBN), negative experiences (ASC-DED) and perceptual changes (ASC-VRN). The OBN subscale has been a particular focus of psychedelic research as greater mystical-type experiences have been associated with greater long-term mental health outcomes from psychedelic therapy (Erritzoe et al., 2018; Griffiths et al., 2008; Roseman et al., 2018; Ross et al., 2016). Recent evidence also points towards a distinct component of the acute experience, namely emotional breakthrough, as an additional key mediator of long-term outcome (Roseman et al., 2019; Spriggs et al., 2020). Across our pooled dataset, scores of mystical-type experience and emotional breakthrough within the trial setting predicted long-term increases in well-being. Importantly however, BMI did not predict scores of mystical-type experience, emotion breakthrough or long-term changes in well-being when using a fixed dose within a therapeutic environment.
While the evidence supported the full model over the null in predicting DED, the contribution of BMI to the combination of the other covariates (age, sex and study) was equivocal. To further explore this trend, the independent contribution of each covariate was assessed, and study was the only covariate with strong evidence towards inclusion in the model. There was strong evidence for lower DED in D-RCT than HV and moderate evidence for no difference between D-OL and HV. One possibility for this effect of study is the setting, where the setting for HV was more scientifically focused (e.g., with electroencephalography recording in-session) than therapeutic in nature. Previous work has found brain imaging to be a predictor of more challenging experiences under psychedelics (Studerus et al., 2011). Another possibility is differential therapeutic alliance across studies. Challenging experiences have previously been associated with a weaker therapeutic relationship (Haijen et al., 2018; Kettner et al., 2021; Stauffer et al., 2020) and a separate analysis performed on data from the D-RCT supports therapeutic alliance as a predictor of acute experience and long-term outcomes (Murphy et al., 2022). Interestingly, although females had significantly lower BMI on average than males, there was strong evidence against sex as a predictor of DED. One recent study found a trend towards females having greater challenging experiences than males with approximately 25 mg psilocybin (Garcia-Romeu et al., 2021). From a constructionist perspective, this may reflect a greater likelihood for women versus men to report challenging experiences – for example, due to cultural gender norms (McLean and Anderson, 2009).
In positron emission tomography (PET) studies, BMI has been found to positively correlate with 5-HT2A receptor binding in the superior temporal cortex, medial inferior temporal, right DLPFC and right sensory motor cortex (Adams et al., 2004; Erritzoe et al., 2009). One PET study using a displacement paradigm with psilocybin as the ‘cold’ displacing ligand – recently found a positive relationship between the subjective intensity and the corresponding percentage occupancy of 5-HT2A receptors achieved by a range of psilocybin dosages – that is, greater intensity was associated with greater 5-HT2A receptor occupancy (Madsen et al., 2019). Pooling across this and one additional PET study (N = 16), Stenbæk et al. (2020) further demonstrated how lower pre-drug 5-HT2A receptor binding was associated with greater mystical-type experience, longer peak duration and a more rapid offset of subjective drug effects post-peak. As well as these pharmacological factors, one should also consider how non-pharmacological, contextual factors could impinge on the experience felt and reported (Carhart-Harris et al., 2018; Haijen et al., 2018; Hartogsohn, 2016, 2017; Kettner et al., 2021; Mediano et al., 2020; Studerus et al., 2011). While the current results provide strong evidence against BMI as a predictor of acute psychedelic experience or long-term outcome, it may be important for future PET studies to elaborate on this further.
It is worth highlighting here the insights offered by Bayesian statistics that would not be provided by frequentist approaches alone (Mulder and Wagenmakers, 2016; Wagenmakers et al., 2018). A BF is a quantification of the relative evidence that the data provide for two competing hypotheses, thus, it allows for conclusions to be made about the relative evidence for or against the null hypothesis. As such, we are able to draw conclusions on the evidence of absence, rather than mere absence of evidence – as would be the case using purely frequentists approaches. Additionally, the BF is a meaningful index of relative evidence, where, for example, BF01 = 4, means that the data are four times more likely under the null hypothesis. Frequentist statistics, in contrast, provide a dichotomous outcome of ‘significance’ based on a (somewhat arbitrary) p value of 0.05. Finally, as opposed to frequentist statistics, a BF does not depend on the sampling plan, or on a hypothetical data set that has not been observed. This is relevant for the post hoc pooling that was employed here.
There are a few notable limitations of the current study. Firstly, 15 participants in the current analysis fell within the obese weight range (BMI > 29.9 kg/m2) but only three participants were classified as underweight (BMI < 18.5 kg/m2), precluding any comparison of the extreme tails of the cohort. Secondly, the absence of pharmacokinetic measures in this study precluded the measurement of drug concentration changes or the relationship between pharmacokinetic processes and the observed effects. Third, we only analysed data from 25 mg dosing sessions and so it is unclear how these findings would translate to other doses of psilocybin, or indeed other psychedelic drugs or mixtures (such as ayahuasca). Finally, while all data correspond to a participant’s first 25 mg dose, the three studies had slightly different designs, meaning that there is some variation in prior experience between participants. We did, however, control for ‘study’ as a covariate.
To conclude, this paper provides the clearest demonstration to-date that the acute psychedelic experience and long-term psychological outcome from a fixed 25 mg dose of psilocybin is not predicted by BMI. This discovery has important implications, not only for our understanding of pharmacological and extra-pharmacological contributors to the psychedelic experience, but also to the large-scale roll-out of psychedelic-assisted therapy. Future studies are required to explore this matter further in more wide-ranging populations, and with different doses and psychedelics.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Professor Carhart-Harris reports receiving consulting fees from Entheon Biomedical and Beckley Psytech; Dr Erritzoe, receiving consulting fees from Field Trip, Entheon Biomedical, Clerkenwell Health and Mydecine; and Dr Nutt, receiving consulting fees from Awakn, H. Lundbeck, and Psyched Wellness, advisory board fees from COMPASS Pathways, and lecture fees from Takeda Medical Research Foundation and owning stock in Alcarelle. Dr Timmermann reports receiving consulting fees from Entheon Biomedical. Bruna Giribaldi reports receiving consulting fees from SmallPharma. No other potential conflict of interest relevant to this article was reported.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the funders of Imperial College London’s Centre for Psychedelic Research (https://www.imperial.ac.uk/psychedelic-research-centre/funding-partners/) and D-OL was funded by the UK Medical Research Council (MR/J00460X/1). HMD is funded by the Imperial College London’s President’s PhD Scholarships. RCH is support by the Ralph Metzner Professorship. This research was supported by the NIHR CRF at Imperial College Healthcare NHS Trust. The views expressed are those of the author(s) and not necessarily those of the funders, the NHS, the NIHR or the Department of Health.
ORCID iDs: Tobias Buchborn  https://orcid.org/0000-0003-0538-5184
https://orcid.org/0000-0003-0538-5184
Hannah M Douglass  https://orcid.org/0000-0002-4033-385X
https://orcid.org/0000-0002-4033-385X
Leor Roseman  https://orcid.org/0000-0001-9990-6029
https://orcid.org/0000-0001-9990-6029
David Erritzoe  https://orcid.org/0000-0002-7022-6211
https://orcid.org/0000-0002-7022-6211
Robin L Carhart-Harris  https://orcid.org/0000-0002-6062-7150
https://orcid.org/0000-0002-6062-7150
References
- Adams KH, Pinborg LH, Svarer C, et al. (2004) A database of [18F]-altanserin binding to 5-HT2A receptors in normal volunteers: Normative data and relationship to physiological and demographic variables. NeuroImage 21: 1105–1113. [DOI] [PubMed] [Google Scholar]
- Aday JS, Bloesch EK, Davoli CC. (2020). 2019: A year of expansion in psychedelic research, industry, and deregulation. Drug Sci Policy Law 6: 2050324520974484. [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Pub. [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, et al. (2015) Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J Psychopharmacol (Oxf) 29: 289–299. [DOI] [PubMed] [Google Scholar]
- Brown RT, Nicholas CR, Cozzi NV, et al. (2017) Pharmacokinetics of escalating doses of oral psilocybin in healthy adults. Clin Pharmacokinet 56: 1543–1554. [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Bradstreet MP, Barrett FS, et al. (2016) Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J Psychopharmacol 30: 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R, Giribaldi B, Watts R, et al. (2021) Trial of psilocybin versus escitalopram for depression. N Engl J Med 384: 1402–1411. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. (2016) Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiat 3: 619–627. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Haijen E, et al. (2018) Psychedelics and the essential importance of context. J Psychopharmacol (Oxf) 32: 725–731. [DOI] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, et al. (2005) Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci 17: 1497–1508. [DOI] [PubMed] [Google Scholar]
- Carter OL, Hasler F, Pettigrew JD, et al. (2007) Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (Berl) 195: 415–424. [DOI] [PubMed] [Google Scholar]
- Dittrich A. (1998) The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31: 80–84. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Frokjaer VG, Haugbol S, et al. (2009). Brain serotonin 2A receptor binding: Relations to body mass index, tobacco and alcohol use. Neuroimage 46: 23–30. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Roseman L, Nour MM, et al. (2018) Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand 138: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldi CJ, Liknaitzky P, Williams M, et al. (2020). Rethinking therapeutic strategies for anorexia nervosa: Insights from psychedelic medicine and animal models. Front Neurosci 14: 43. DOI: 10.3389/fnins.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Kaye WH, Meltzer CC, et al. (2002) Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biol Psychiatry 52: 896–906. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Barrett FS, Carbonaro TM, et al. (2021) Optimal dosing for psilocybin pharmacotherapy: Considering weight-adjusted and fixed dosing approaches. J Psychopharmacol (Oxf) 35: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B, Duffull SB. (2004) What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 58: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol (Oxf) 30: 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2011). Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (Berl) 218: 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, Johnson MW, et al. (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol (Oxf) 22: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, et al. (2011). Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68: 71–78. [DOI] [PubMed] [Google Scholar]
- Haijen EC, Kaelen M, Roseman L, et al. (2018) Predicting responses to psychedelics: A prospective study. Front Pharmacol 9: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartogsohn I. (2016) Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J Psychopharmacol (Oxf) 30: 1259–1267. [DOI] [PubMed] [Google Scholar]
- Hartogsohn I. (2017) Constructing drug effects: A history of set and setting. Drug Sci Policy Law 3: 2050324516683325. [Google Scholar]
- Hirschfeld T, Schmidt TT. (2020) How does it feel to be on psilocybin? Dose-response relationships of subjective experiences in humans. BioRxiv. DOI: 10.1101/2020.06.09.142802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X-F, Huang X, Han M, et al. (2004) 5-HT2A/2C receptor and 5-HT transporter densities in mice prone or resistant to chronic high-fat diet-induced obesity: A quantitative autoradiography study. Brain Res 1018: 227–235. [DOI] [PubMed] [Google Scholar]
- Kaye W. (2008) Neurobiology of anorexia and bulimia nervosa. Physiol Behav 94: 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner H, Rosas FE, Timmermann C, et al. (2021) Psychedelic communitas: Intersubjective experience during psychedelic group sessions predicts enduring changes in psychological wellbeing and social connectedness. Front Pharmacol 12: 623985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons T. (2020) The acute and long-term effects of psilocybin in healthy volunteers. Presented at the Interdisciplinary Conference on Psychedelic Research, Amsterdam, Netherlands. [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR. (2011) Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol (Oxf) 25: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen MK, Fisher PM, Burmester D, et al. (2019) Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44: 1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Anderson ER. (2009) Brave men and timid women? A review of the gender differences in fear and anxiety. Clin Psychol Rev 29: 496–505. [DOI] [PubMed] [Google Scholar]
- Mediano PAM, Rosas FE, Timmermann C, et al. (2020) Effects of external stimulation on psychedelic state neurodynamics. BioRxiv. DOI: 10.1101/2020.11.01.356071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RD, Rouder JN, Jamil T. (2015) BayesFactor: Computation of Bayes factors for common designs. R Package Version 09 9. [Google Scholar]
- Morrish GA, Pai MP, Green B. (2011) The effects of obesity on drug pharmacokinetics in humans. Expert Opin Drug Metab Toxicol 7: 697–706. [DOI] [PubMed] [Google Scholar]
- Mulder J, Klugkist I, van de Schoot R, et al. (2009) Bayesian model selection of informative hypotheses for repeated measurements. J Math Psychol 53: 530–546. [Google Scholar]
- Mulder J, Wagenmakers E-J. (2016) Editors’ introduction to the special issue “Bayes factors for testing hypotheses in psychological research: Practical relevance and new developments.” J Math Psychol 72: 1–5. [Google Scholar]
- Murphy R, Kettner H, Zeifman R, et al. (2022) Therapeutic alliance and rapport modulate responses to psilocybin assisted therapy for depression. Front Pharmacol 12: 788155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CR, Henriquez KM, Gassman MC, et al. (2018) High dose psilocybin is associated with positive subjective effects in healthy volunteers. J Psychopharmacol (Oxf) 32: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. (2016). Psychedelics. Pharmacol Rev 68: 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Erritzoe D, Carhart-Harris R. (2020) Psychedelic psychiatry’s brave new world. Cell 181: 24–28. [DOI] [PubMed] [Google Scholar]
- Ricca V, Nacmias B, Cellini E, et al. (2002) 5-HT2A receptor gene polymorphism and eating disorders. Neurosci Lett 323: 105–108. [DOI] [PubMed] [Google Scholar]
- Roseman L, Haijen E, Idialu-Ikato K, et al. (2019) Emotional breakthrough and psychedelics: Validation of the Emotional Breakthrough Inventory. J Psychopharmacol (Oxf) 33: 1076–1087. [DOI] [PubMed] [Google Scholar]
- Roseman L, Nutt D, Carhart-Harris R. (2018) Quality of Acute Psychedelic Experience Predicts Therapeutic Efficacy of Psilocybin for Treatment-Resistant Depression [WWW Document]. Available at: https://www.frontiersin.org/articles/10.3389/fphar2017.00974/full (accessed 7 August 2020). [DOI] [PMC free article] [PubMed]
- Rosmond R, Bouchard C, Björntorp P. (2002) 5-HT2A receptor gene promoter polymorphism in relation to abdominal obesity and cortisol. Obes Res 10: 585–589. [DOI] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J Psychopharmacol (Oxf) 30: 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, Morey RD. (2012) Default Bayes factors for model selection in regression. Multivar Behav Res 47: 877–903. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, et al. (2009) Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev 16: 225–237. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Bachmann R, Kometer M, et al. (2012) Mismatch negativity encoding of prediction errors predicts S-ketamine-induced cognitive impairments. Neuropsychopharmacology 37: 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs MJ, Douglass HM, Park RJ, et al. (2021) Study protocol for “Psilocybin as a Treatment for Anorexia Nervosa: A Pilot Study.” Front Psychiatry 12: 1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs MJ, Kettner H, Carhart-Harris RL. (2020) Positive effects of psychedelics on depression and wellbeing scores in individuals reporting an eating disorder. J Eat Weight Disord 26: 1265–1270. [DOI] [PubMed] [Google Scholar]
- Stauffer CS, Anderson BT, Ortigo KM, et al. (2020). Psilocybin-assisted group therapy and attachment: Observed reduction in attachment anxiety and influences of attachment insecurity on the psilocybin experience. ACS Pharmacol Transl Sci 4(2): 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbæk DS, Madsen MK, Ozenne B, et al. (2020). Brain serotonin 2A receptor binding predicts subjective temporal and mystical effects of psilocybin in healthy humans. J Psychopharmacol (Oxf) 35(4): 459–468. [DOI] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX. (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PloS One 5: e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Kometer M, Hasler F, et al. (2011) Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J Psychopharmacol (Oxf) 25: 1434–1452. [DOI] [PubMed] [Google Scholar]
- Tennant R, Hiller L, Fishwick R, et al. (2007). The Warwick-Edinburgh mental well-being scale (WEMWBS): Development and UK validation. Health Qual. Life Outcomes 5: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Koller R, Vollenweider FX, et al. (2002) Mismatch negativity predicts psychotic experiences induced by nmda receptor antagonist in healthy volunteers. Biol Psychiatry 51: 400–406. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, et al. (2007). The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 32: 1876–1887. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MFI, Bäbler A, et al. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport 9: 3897–3902. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E-J, Marsman M, Jamil T, et al. (2018) Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychon Bull Rev 25: 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2009) ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag. [Google Scholar]
- World Health Organization (1995) Physical Status: The Use and Interpretation of Anthropometry: Report of a World Health Organization (WHO) Expert Committee. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- World Health Organization ( 2006) Global Database on Body Mass Index [WWW Document]. Available at: http://www.assessmentpsychology.com/icbmi.htm (accessed 20 March 2021).