Abstract
Background
Follitropin delta, a novel recombinant follicle-stimulating hormone (rFSH) preparation derived from a human cell line, has different pharmacokinetic and pharmacodynamic properties compared with existing rFSH preparations expressed by Chinese hamster ovary cells (CHO).
Objectives
The objective of this study was to assess the pharmacokinetic characteristics, dose proportionality, and safety of follitropin delta in healthy Chinese women.
Methods
This was a phase I, randomized, open-label study. Twenty-four healthy Chinese women were randomized (1:1:1) to receive a single subcutaneous administration of follitropin delta 12, 18, or 24 μg. The pharmacokinetic parameters (maximum observed serum concentration [Cmax], time to reach Cmax [tmax], area under the serum concentration–time curve from dosing to infinity [AUC∞], and elimination phase half-life [t½]) of follitropin delta were derived using noncompartmental analysis.
Results
Following a single subcutaneous administration of follitropin delta 12, 18, or 24 μg, mean Cmax (0.388, 0.677, and 0.825 ng/mL, respectively) and AUC∞ (41.3, 62.9, and 83.1 h·ng/mL, respectively) increased in a dose-proportional manner. The median tmax was 24 h, and the mean t½ was in the range of 50.5–60.9 h. All treatment-related adverse events were categorized as mild, except for one case of urticaria from the follitropin delta 18-μg dose group which was considered moderate. Only one woman presented with elevation of alanine transaminase and aspartate aminotransferase at the follow-up visit, which was reported as a treatment-emergent adverse event. There were no injection-site reactions and none of the participants showed any confirmed presence of treatment-induced anti-FSH antibodies.
Conclusions
The administration of single doses of follitropin delta to healthy Chinese women demonstrated dose-proportional pharmacokinetics over the dose range of 12–24 μg, and these doses were well tolerated.
Clinical Trial Registration
Clinicaltrials.gov registration no. NCT04150861.
Key Points
| The present study evaluated the pharmacokinetic properties of follitropin delta following single subcutaneous administrations in healthy Chinese women. |
| Dose proportionality of AUC∞ and Cmax of FSH was demonstrated over the dose range 12–24 μg of follitropin delta, supporting the predictability of the pharmacokinetics of follitropin delta at the tested doses. |
| Single-dose administration of follitropin delta was safe and well tolerated, and no anti-FSH antibodies were formed. |
Introduction
Follicle-stimulating hormone (FSH) is one of the gonadotropins that has a central role in the treatment of female infertility [1]. FSH is responsible for the development and maturation of follicles and is used to stimulate the development of multiple follicles in women undergoing assisted reproductive technology (ART). The currently available FSH-containing products are either urinary-derived or manufactured through recombinant technology [2]. Human menopausal gonadotropin (hMG) is derived from the urine of postmenopausal women and contains the three gonadotropins FSH, human chorionic gonadotropin (hCG), and luteinizing hormone (LH). Highly purified urinary FSH is manufactured using monoclonal antibodies specific to FSH. Among the recombinant FSH (rFSH) products, follitropin alfa, follitropin beta, and corifollitropin alfa are expressed in Chinese hamster ovary (CHO) cell lines [3]. In contrast, the latest rFSH to receive regulatory approval, follitropin delta (Rekovelle®), is derived from a cell line of human fetal retinal origin (PER.C6®). This difference results in follitropin delta having a distinct glycosylation profile and a higher overall sialic acid content in comparison with the other rFSH products [4]. A high degree of sialylation reduces the clearance of FSH and increases the circulating half-life by blocking binding to the asialoglycoprotein receptor (ASGPR) in the liver [5]. While follitropin delta and follitropin alfa were shown to have the same in vitro potency at the FSH receptor, the compounds differ in their pharmacokinetic properties [4]. Follitropin delta clearance, but not follitropin alfa clearance, was reduced by ASGPR inhibition in rats and by genetic ablation in mice [4]. In healthy women, the systemic clearance of follitropin delta was lower compared with that of follitropin alfa, leading to higher serum FSH concentrations [6]. Accordingly, the number of follicles, increases in serum inhibin B, and increases in estradiol were greater for follitropin delta compared with follitropin alfa after daily administration of 225 IU of each compound in this study. The dose of 225 IU was based on the biological activity of follitropin delta in the Steelman-Pohley assay, which does not appear to accurately reflect the potency of follitropin delta in humans. The dose of follitropin delta is for this reason expressed in micrograms instead of IU, and a dose-finding study was conducted to identify therapeutically appropriate doses of follitropin delta [7], leading to the development of a unique algorithm-based dosing regimen based on each woman’s body weight and serum anti-Müllerian hormone (AMH) concentration [8, 9]. This algorithmic treatment approach was subsequently shown to minimize the risk of ovarian hyperstimulation syndrome (OHSS) without compromising pregnancy outcomes [10–12]. Body weight is an integral part of the dosing regimen to account for an inverse relationship between body weight and serum FSH concentrations, underlining the importance of the pharmacokinetics of follitropin delta being predictable. To support the dose rationale in Chinese women, the present study was conducted assessing the pharmacokinetic characteristics and dose proportionality of follitropin delta in healthy Chinese women following a single subcutaneous administration.
Methods
Study Design and Procedures
The study had an open-label, randomized, parallel group design and was conducted at Jiangsu Province Hospital, China, from June through December 2019. The study (ClinicalTrials.gov identifier: NCT04150861) was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation (ICH) guidelines for Good Clinical Practice (GCP). The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. All subjects gave their written informed consent before enrolment in the study.
The investigational medicinal product (IMP) was follitropin delta (Rekovelle®, Ferring Pharmaceuticals, Switzerland). The participating women received two administrations of a 1-month depot formulation of the gonadotropin-releasing hormone agonist decapeptyl depot 3.75 mg (triptorelin) in order to suppress endogenous release of FSH. The first dose of decapeptyl was given 28 days before administration of the first dose of IMP and the second dose was given 10 days prior to the first IMP administration.
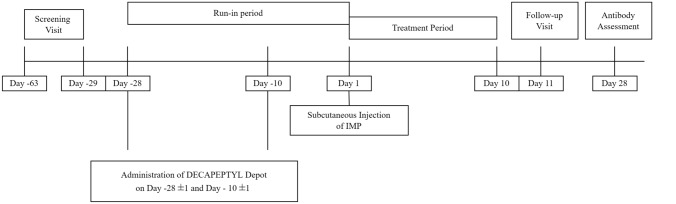
On the morning of the IMP administration day, women were randomized to receive a single dose of follitropin delta (12, 18, or 24 μg) administered subcutaneously in the abdominal region. A follow-up visit was scheduled 10 days after IMP administration and an anti-FSH antibody assessment after 27 days (Fig. 1).
Fig. 1.
Study design. IMP investigational medicinal product
Subjects
Healthy women aged 21–40 years, with a body mass index (BMI) of 18.5–25 kg/m2, and a normal menstrual cycle with a range of 24–35 days in the absence of oral contraceptives were enrolled. All women were in good physical health, as assessed by a full physical examination, electrocardiogram (ECG), vital signs, and clinical laboratory assays. Pituitary suppression (serum FSH ≤ 5 IU/L) was confirmed at 3 days prior to the IMP administration day and on the day prior to IMP administration.
Exclusion criteria included history/presence of any disease, in particular cardiovascular, musculoskeletal, immunological, endocrine, or metabolic disease. Presence or history of severe allergy or anaphylactic reactions to any non-registered investigational drug were also ruled out. Women taking gonadotropin preparations within the 6 months prior to screening were excluded. Women were also not enrolled if they had participated in other clinical trials or donated blood in the past 4 weeks.
Blood Sampling
Blood samples for serum FSH concentration measurements were collected at 1 and 0.5 h prior to IMP administration, immediately before administration, and at 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, and 48 h, and 3, 4, 5, 6, 7, 8, and 9 days after administration. At each time point, 5 mL was collected in a gold top serum separation tube and was thoroughly mixed with a clotting activation agent by inverting the tube a minimum of five times. The samples were centrifuged at a minimum of 1500–2000 × g for 15 min, and serum samples were transferred into appropriately labeled tubes and stored frozen at − 20 °C until shipping. All primary samples were received frozen with dry ice and stored at − 60 to − 80 °C, except during analysis of the sample.
Bioanalytical Assay
Serum FSH concentrations were determined at Covance Pharmaceutical R&D (Shanghai) Co., Ltd., using Meso Scale Discovery (MSD) assay. Intra-assay and inter-assay accuracy and precision at upper limit of quantification (ULOQ), high quality control sample (HQC), medium quality control sample (MQC), low quality control sample (LQC), and lower limit of quantification (LLOQ) met the acceptance criteria. No significant matrix effect was observed on normal human serum, 10% hemolyzed human serum, and lipemic human serum. When LH and hCG concentrations were up to 50.0 mIU/mL, no interference for follitropin delta was detected.
When thyroid-stimulating hormone concentration was up to 4.00 mIU/L and triptorelin pamoate concentration was up to 10.0 ng/mL, no interference for follitropin delta was detected.
Each sample analysis plate included calibration standards, quality control samples, and blank matrix, if no other specification. Each sample was analyzed in duplicate and the mean value of the two replicates was reported. The inter-assay precision of the back-calculated concentrations from reported batches was ≤ 1.5% for the usable range of the curve (0.0750–5.00 ng/mL). The inter-assay bias of the back-calculated concentrations within this range varied between − 0.9 and 0.9%. The inter-assay precision in the determination of the QC samples was 4.8% at the LQC, 4.1% at the MQC, and 3.4% at the HQC. The mean accuracy bias at these levels was 1.1, 0.6, and − 0.3%, respectively. The acceptable calibration and quality control sample data indicated that the method performed satisfactorily for all reported batches.
Pharmacokinetic Parameters
Serum FSH pharmacokinetic parameters were determined using noncompartmental methods implemented in the software Phoenix WinNonlin® 8.2, Certara, Inc. The area under the serum concentration–time curve from dosing to infinity (AUC∞), area under the serum concentration–time curve from dosing up to time t (AUCt), where t was the last time point at which the concentration is above the lower limit of quantification, apparent systemic clearance (CL/F), maximum concentration observed (Cmax), elimination (phase) half-life (t½), time to reach Cmax (tmax), and apparent volume of distribution associated with the terminal phase (Vz/F) baseline were determined. All pharmacokinetic parameters were derived using actual sampling time points and using baseline-adjusted FSH concentrations. The baseline value was the mean of the three values obtained prior to administration of IMP (− 1, − 0.5, and 0 h). Baseline-adjusted values below the LLQ were set to zero.
Safety Assessments
The safety and tolerability of follitropin delta was assessed by physical examination, vital sign check, ECG, and routine laboratory tests performed before study enrolment and at the follow-up visit. Blood samples (8.5 mL) for assessment of serum anti-FSH antibodies were collected 1 day before administration of IMP, and 6 and 27 days after. Confirmed positive samples were subsequently analyzed for neutralizing activity by a validated cell-based neutralizing antibody assay. Adverse events (AEs) were recorded throughout the study. In addition, injection-site reactions were assessed immediately, 30 min, and 24 h after administration of the IMP.
Statistical Analysis
Pharmacokinetic parameters were summarized by dose group using descriptive statistics. Dose-proportionality was investigated for each of AUC∞ and Cmax using a multiplicative analysis of variance model (i.e., AUC∞ and Cmax were log-transformed before analysis) with log (dose) as covariate. In this model, a covariate coefficient of 1 indicated dose proportionality.
Results
Demographic Characteristics
In total, 133 women were screened, and of these, 109 were screening failures (reasons were as follows: not fulfilling the inclusion/exclusion criteria [n = 90], withdrawal of consent [n = 7], and other reasons [n = 12]). Finally, 24 healthy women were randomized with eight women assigned to each follitropin delta dose group (12, 18, and 24 µg). All 24 women completed the trial. Their baseline demographic characteristics are summarized by dose group in Table 1. The mean (SD) age at screening was 27.8 (5.8) years. The mean BMI was 21.6 kg/m2, and individual values ranged from 18.5 to 22.9 kg/m2.
Table 1.
Demographic characteristics
| Parameters | Follitropin delta | |||
|---|---|---|---|---|
| 12 µg (n = 8) | 18 µg (n = 8) | 24 µg (n = 8) | Total (n = 24) | |
| Age (years) | 28.6 (6.0) | 29.5 (6.7) | 25.4 (4.6) | 27.8 (5.8) |
| Weight (kg) | 55.8 (6.2) | 55.4 (7.1) | 57.6 (7.3) | 56.3 (6.6) |
| Height (m) | 1.58 (0.05) | 1.63 (0.07) | 1.62 (0.06) | 1.61 (0.06) |
| BMI (kg/m2) | 22.2 (1.6) | 20.7 (1.9) | 22.0 (2.3) | 21.6 (2.0) |
Data are baseline values presented as mean (SD)
BMI body mass index, SD standard deviation
Pharmacokinetics
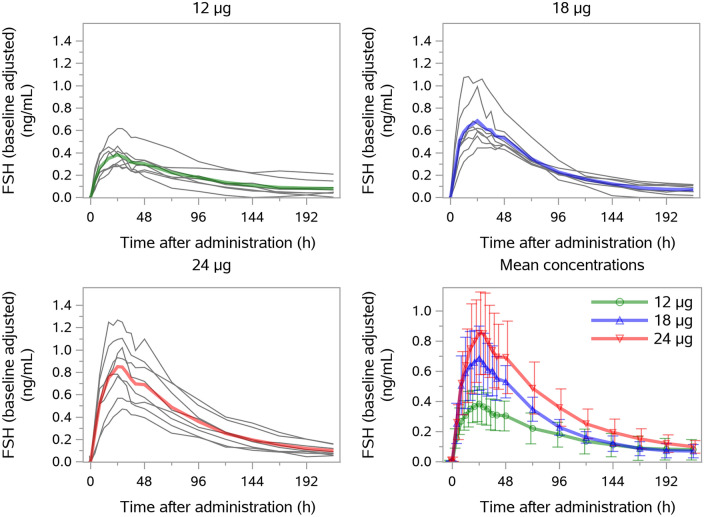
All 24 randomized women contributed to the pharmacokinetic evaluation. The individual and mean baseline-adjusted serum FSH concentrations are presented in Fig. 2. Following a single subcutaneous administration of follitropin delta 12, 18, and 24 μg, serum FSH concentrations increased in a dose-orderly manner. The mean AUC∞ values were 41.8, 62.9, and 83.1 h·ng/mL, respectively, and the mean Cmax values were 0.388, 0.677, and 0.825 ng/mL, respectively (Table 2). The median tmax value of FSH was 24 h in each dose group, and the harmonic mean values of t½ ranged from 46.9 to 60.4 h across dose groups.
Fig. 2.
Individual and mean baseline-adjusted serum FSH concentrations. Bars represent standard deviation. FSH follicle-stimulating hormone
Table 2.
Baseline-adjusted pharmacokinetic parameters of FSH
| Parameters | Follitropin delta | ||
|---|---|---|---|
| 12 µg (n = 8)a | 18 µg (n = 8) | 24 µg (n = 8) | |
| AUC∞ (h·ng/mL) | |||
| Geometric mean | 41.3b | 62.9 | 83.1 |
| CV% | 44.3 | 19.7 | 36.6 |
| Min; max | 22.0; 91.1 | 52.7; 94.7 | 50.2; 134.4 |
| AUCt (h·ng/mL) | |||
| Geometric mean | 36.4 | 56.6 | 74.6 |
| CV% | 38.0 | 18.7 | 35.9 |
| Min; max | 21.9; 69.1 | 47.3; 87.6 | 43.4; 118.9 |
| CL/F (L/h) | n = 7 | ||
| Geometric mean | 0.301 | 0.286 | 0.289 |
| CV% | 46.6 | 19.7 | 36.6 |
| Min; max | 0.132; 0.545 | 0.190; 0.342 | 0.179; 0.478 |
| Cmax (ng/mL) | |||
| Geometric mean | 0.388 | 0.677 | 0.825 |
| CV% | 26.5 | 29.1 | 34.6 |
| Min; max | 0.294; 0.617 | 0.473; 1.081 | 0.472; 1.270 |
| t½ (h) | n = 7 | ||
| Geometric mean | 58.6 | 50.5 | 60.9 |
| Harmonic mean | 53.6 | 46.9 | 60.4 |
| CV% | 47.5 | 43.5 | 13.6 |
| Min; max | 28.1; 104.0 | 27.6; 92.8 | 50.8; 72.3 |
| Tmax (h) | |||
| Median | 24.0 | 24.0 | 24.0 |
| Min; max | 16.0; 36.0 | 16.0; 48.0 | 24.0; 28.0 |
| Vz/F (L) | n = 7 | ||
| Geometric mean | 25.5 | 20.8 | 25.4 |
| CV% | 19.5 | 43.5 | 31.5 |
| Min; max | 19.8; 34.9 | 11.7; 34.7 | 17.5; 44.7 |
AUC∞ area under the serum concentration–time curve from dosing to infinity, AUCt area under the serum concentration–time curve from dosing up to time t, where t is the last time point at which the concentration is above the lower limit of quantification, CL/F apparent systemic clearance, Cmax maximum concentration observed, FSH follicle-stimulating hormone, Tmax time to reach Cmax, t½ elimination (phase) half-life, Vz/F apparent volume of distribution associated with the terminal phase
an = 8, unless otherwise specified
bIn a single subject, the slope of the terminal phase (λz) after administration of 12 µg FE 999049 could not be estimated. In this subject, AUC∞ was imputed with AUCt, and other pharmacokinetic parameters depending on λz were set to missing
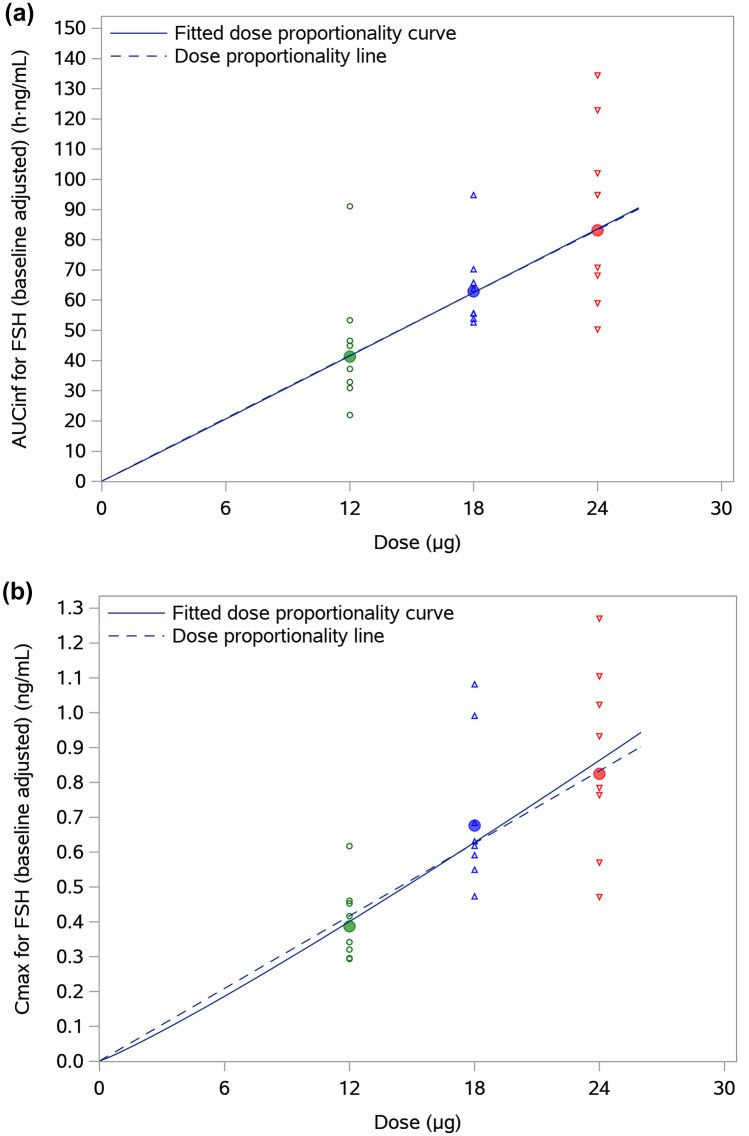
Analysis of dose-proportionality for AUC∞ and Cmax of FSH indicated a linear or very close to linear dose-exposure relationship of follitropin delta in the dose range of 12–24 μg. The estimates for the log (dose) coefficients in the dose-proportionality model were 1.01 [0.52, 1.50] (h·ng/mL) for AUC∞ and 1.11 [0.67, 1.55] (ng/mL) for Cmax. Figure 3a, b show the dose dependence of AUC∞ and Cmax of serum FSH concentrations, respectively. The fitted dose-proportionality curves were almost identical to the linear fit, illustrating the consistency with dose proportionality for AUC∞ and Cmax of FSH across the studied dose range of 12–24 μg of follitropin delta.
Fig. 3.
a Individual and geometric mean AUC∞ of serum FSH concentrations versus dose and b individual and geometric mean Cmax of serum FSH concentrations versus dose. AUC∞ area under the concentration–time curve from dosing to infinity, Cmax maximum concentration observed, FSH follicle-stimulating hormone
Safety and Tolerability
Follitropin delta administered by subcutaneous injection was well tolerated: no deaths, serious adverse events, or discontinuations due to an adverse event were reported during the study. A total of three adverse drug reactions were reported by two out of 24 women having reasonable possible causality to follitropin delta. One woman reported urticaria 3 days after administration of 18 µg of follitropin delta. She recovered fully from the adverse event one day after the onset and the event was categorized as moderate. In the second subject, alanine transaminase (ALT) and aspartate aminotransferase (AST) were elevated at 2 days after administration of 12 µg of follitropin delta and these values had returned to normal at the follow-up visit. These two events as well as all remaining adverse events in the study were categorized as mild. In one additional subject, ALT and AST were elevated at the follow-up visit, which was reported as a treatment-emergent adverse event.
No clinically relevant changes were observed in ECGs, vital sign parameters, or laboratory test results. There were no injection-site reactions at any time point and none of the subjects showed any confirmed presence of treatment-induced anti-FSH antibodies.
Discussion
The present study evaluated the pharmacokinetic properties of follitropin delta following single subcutaneous administrations in healthy Chinese women. Dose proportionality of AUC∞ and Cmax of FSH was demonstrated over the dose range of 12–24 μg of follitropin delta, supporting the predictability of the pharmacokinetics of follitropin delta at the tested doses.
Follitropin delta was absorbed slowly after subcutaneous administration with a median tmax of 24 h. This value is similar to the tmax values reported previously in studies with follitropin delta [6, 13]. Given the previously reported difference in serum FSH exposure after administration of equal IU doses of follitropin delta and follitropin alfa [6], the CL/F is of particular interest. In the present study, CL/F was approximately 0.3 L/h. This value is in the same range as the values previously reported in studies with follitropin delta in healthy Caucasian and Japanese women [6, 13]. The similarity in exposure across these studies supports use of the same follitropin delta dosing regimen across ethnicities and geographic regions. CL/F has also been reported for CHO-derived rFSH. In a study with follitropin alfa, CL/F after a single subcutaneous dose was reported to be 0.74–0.77 L/h [14], and in a study with follitropin beta a similar value of 0.81 L/h was found [15]. The finding of a comparatively low CL/F of approximately 0.3 L/h in the present study is in line with a previous study directly comparing the pharmacokinetics of follitropin delta and follitropin alfa [6]. A mechanistic explanation for the difference is the higher overall sialic acid content in follitropin delta in comparison with CHO-derived rFSH products, resulting in a lower clearance by the ASGPR in the liver [4]. Consequently, follitropin delta cannot be dosed based on bioactivity or specific bioactivity like other follitropins, and it is instead dosed by mass (μg) [16].
Single-dose administration of follitropin delta was safe and well tolerated as assessed by adverse events, vital signs, ECG, clinical laboratory measurements, and physical examination. There were no signs of anti-FSH antibody formation or allergic reactions. However, it should be noted that this was a single-dose study of limited size not enabling full assessment of the immunogenicity of follitropin delta, which has been addressed extensively in previous trials [10, 12, 17, 18]. A total of three adverse events were assessed as possibly related to follitropin delta (urticaria, increased ALT and AST).
Conclusion
The administration of single doses of follitropin delta to healthy Chinese women demonstrated dose-proportional pharmacokinetics over the dose range of 12–24 μg, and was safe and well tolerated as assessed by adverse events, vital signs, ECG, laboratory parameters, injection-site reactions, and presence of anti-FSH antibodies.
Acknowledgements
The authors would like to acknowledge the investigators and staff at the study center and the women who participated in the study. They would also like to thank Christine Tsou, MBBS, MSc, for her valuable contribution to the manuscript development and writing. Medical writing assistance was also provided by Swapnil Bhowate.
Declarations
Funding
The study was funded by Ferring Pharmaceuticals.
Conflict of interest
DMJ, PL, and PP are employees of Ferring Pharmaceuticals. FS, YJ and SD have nothing to disclose.
Availability of data and material
Data are available from the author (DMJ) upon reasonable request.
Ethics approval
The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University and conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation (ICH) guidelines for Good Clinical Practice (GCP).
Consent to participate
All subjects gave their written informed consent before enrolment in the study.
Author contributions
SF, YJ, and SD collected the study data; PL performed the statistical analysis and contributed to the data interpretation; PP contributed to the data interpretation and to the manuscript writing; DMJ performed the pharmacokinetic analysis and contributed to the data interpretation and to the manuscript writing. All authors approved the submitted version of the manuscript.
Consent for publication
Not applicable.
Code availability
Not applicable.
References
- 1.Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 2.Bergandi L, Canosa S, Carosso AR, Paschero C, Gennarelli G, Silvagno F, et al. Human recombinant FSH and its biosimilars: clinical efficacy, safety, and cost-effectiveness in controlled ovarian stimulation for in vitro fertilization. Pharmaceuticals (Basel). 2020;13(7):136. [DOI] [PMC free article] [PubMed]
- 3.Howles CM. Genetic engineering of human FSH (Gonal-F) Hum Reprod Update. 1996;2(2):172–191. doi: 10.1093/humupd/2.2.172. [DOI] [PubMed] [Google Scholar]
- 4.Koechling W, Plaksin D, Croston GE, Jeppesen JV, Macklon KT, Andersen CY. Comparative pharmacology of a new recombinant FSH expressed by a human cell line. Endocr Connect. 2017;6(5):297–305. doi: 10.1530/EC-17-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dias JA, Ulloa-Aguirre A. New human follitropin preparations: how glycan structural differences may affect biochemical and biological function and clinical effect. Front Endocrinol (Lausanne). 2021;12:636038. doi: 10.3389/fendo.2021.636038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsson H, Sandstrom R, Grundemar L. Different pharmacokinetic and pharmacodynamic properties of recombinant follicle-stimulating hormone (rFSH) derived from a human cell line compared with rFSH from a non-human cell line. J Clin Pharmacol. 2014;54(11):1299–1307. doi: 10.1002/jcph.328. [DOI] [PubMed] [Google Scholar]
- 7.Arce JC, Andersen AN, Fernandez-Sanchez M, Visnova H, Bosch E, Garcia-Velasco JA, et al. Ovarian response to recombinant human follicle-stimulating hormone: a randomized, antimullerian hormone-stratified, dose-response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2014;102(6):1633–40 e5. doi: 10.1016/j.fertnstert.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Bosch E, Nyboe Andersen A, Barri P, Garcia-Velasco JA, de Sutter P, Fernandez-Sanchez M, et al. Follicular and endocrine dose responses according to anti-Mullerian hormone levels in IVF patients treated with a novel human recombinant FSH (FE 999049) Clin Endocrinol (Oxf) 2015;83(6):902–912. doi: 10.1111/cen.12864. [DOI] [PubMed] [Google Scholar]
- 9.Rose TH, Roshammar D, Erichsen L, Grundemar L, Ottesen JT. Characterisation of population pharmacokinetics and endogenous follicle-stimulating hormone (FSH) levels after multiple dosing of a recombinant human FSH (FE 999049) in healthy women. Drugs RD. 2016;16(2):165–172. doi: 10.1007/s40268-016-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyboe Andersen A, Nelson SM, Fauser BC, Garcia-Velasco JA, Klein BM, Arce JC, et al. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017;107(2):387–96 e4. doi: 10.1016/j.fertnstert.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara O, Klein BM, Arce JC, Japanese Follitropin Delta Phase 2 Trial G Randomized, assessor-blind, antimullerian hormone-stratified, dose-response trial in Japanese in vitro fertilization/intracytoplasmic sperm injection patients undergoing controlled ovarian stimulation with follitropin delta. Fertil Steril. 2021;115(6):1478–1486. doi: 10.1016/j.fertnstert.2020.10.059. [DOI] [PubMed] [Google Scholar]
- 12.Qiao J, Zhang Y, Liang X, Ho T, Huang HY, Kim SH, et al. A randomised controlled trial to clinically validate follitropin delta in its individualised dosing regimen for ovarian stimulation in Asian IVF/ICSI patients. Hum Reprod. 2021;36(9):2452–2462. doi: 10.1093/humrep/deab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson H, Sandstrom R, Bagger Y. Dose-exposure proportionality of a novel recombinant follicle-stimulating hormone (rFSH), FE 999049, derived from a human cell line, with comparison between Caucasian and Japanese women after subcutaneous administration. Clin Drug Investig. 2015;35(4):247–253. doi: 10.1007/s40261-015-0276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voortman G, Mannaerts BM, Huisman JA. A dose proportionality study of subcutaneously and intramuscularly administered recombinant human follicle-stimulating hormone (Follistim*/Puregon) in healthy female volunteers. Fertil Steril. 2000;73(6):1187–1193. doi: 10.1016/S0015-0282(00)00542-2. [DOI] [PubMed] [Google Scholar]
- 15.le Cotonnec JY, Porchet HC, Beltrami V, Khan A, Toon S, Rowland M. Clinical pharmacology of recombinant human follicle-stimulating hormone. II. Single doses and steady state pharmacokinetics. Fertil Steril. 1994;61(4):679–686. doi: 10.1016/S0015-0282(16)56645-X. [DOI] [PubMed] [Google Scholar]
- 16.Arce JC, Larsson P, García-Velasco JA. Establishing the follitropin delta dose that provides a comparable ovarian response to 150 IU/day follitropin alfa. Reprod Biomed Online. 2020;41(4):616–622. doi: 10.1016/j.rbmo.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Bosch E, Havelock J, Martin FS, Rasmussen BB, Klein BM, Mannaerts B, Arce JC, ESTHER-2 Study Group Follitropin delta in repeated ovarian stimulation for IVF: a controlled, assessor-blind phase 3 safety trial. Reprod Biomed Online. 2019;38(2):195–205. doi: 10.1016/j.rbmo.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Ishihara O, Arce JC, Japanese Follitropin Delta Phase 3 Trial (STORK) Group Individualized follitropin delta dosing reduces OHSS risk in Japanese IVF/ICSI patients: a randomized controlled trial. Reprod Biomed Online. 2021;42(5):909–918. doi: 10.1016/j.rbmo.2021.01.023. [DOI] [PubMed] [Google Scholar]