Abstract
Objectives
To critically examine the methods used for full economic evaluations of preventive interventions for dental caries and periodontitis.
Methods
Published literature post-2000 was searched to April 2021. Based on a developed intervention classification framework for dental caries and periodontitis, only universal, selective or indicated interventions were included in this review. The Drummond 10-point checklist was used for quality appraisal.
Results
Of 3,007 unique records screened for relevance, 73 studies were reviewed. Most model-based studies (61/73) used cost-effectiveness analysis (49%) or cost-benefit analysis (28%). Trial-based studies (16/73) commonly used cost-effectiveness analysis (59%). Four studies used both economic evaluation methods. Sixty-four papers (88%) were on dental caries, eight papers (11%) focused on periodontitis, and one paper (1%) included both oral diseases; 72% of model-based and 82% of trial-based studies were of good quality. The most frequently investigated dental caries preventive interventions were water fluoridation (universal intervention; cost-saving or cost-effective), fissure sealant and fluoride varnish (selective and indicated interventions; cost-effectiveness outcomes were inconsistent). Supportive periodontal therapy with oral health education (indicated intervention; cost-effective) was the most frequently evaluated preventive intervention for periodontitis. Thirty percent of studies with a time horizon > 1 year did not apply an appropriate discount rate and 26% did not comprehensively discuss other important considerations beyond the technical analysis.
Conclusions
Generic health outcome measures should be incorporated for economic evaluations on preventive interventions for dental caries and periodontitis, and an increased focus to prevent periodontitis using economic evaluation methods is needed to inform resource allocation and policy decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40258-022-00758-5.
Key Points for Decision Makers
| Methods used for economic evaluations on preventive interventions for dental caries and periodontitis were diverse, including the cost components, the consequences, and health outcomes. |
| These differences make it difficult to enable comparability between interventions to inform health investment decisions. |
| Future research in the field should consider how to incorporate generic health outcome measures, increase focus on performing economic evaluations to prevent periodontitis, and consider health equity impacts. |
Introduction
The major contributors impacting quality of life due to oral disease are predominantly caused by advanced dental caries, periodontitis (gum disease) and advanced periodontitis. Advanced dental caries and advanced periodontitis affecting multiple teeth can lead to edentulism (total tooth loss). The 2017 Global Burden of Disease (GBD) study estimated 2.3 billion people had dental caries in permanent (adult) teeth, 532 million children had dental caries in deciduous (baby) teeth, 796 million people have advanced periodontitis, and 267 million people have edentulism [1]. These statistics correspond to 1.6 million years lived with disability (YLD) for permanent teeth, 0.1 million YLD for deciduous teeth, 5.2 million YLD for severe periodontitis and 7.3 million YLD for edentulism [1].
Dental caries and periodontitis are experienced disproportionately by different populations in society [2–4], and share common risk factors with other noncommunicable diseases (NCDs) [5, 6]. Additionally, periodontitis can have a direct association with other NCDs including cardiovascular disease [7, 8], dementia and cognitive impairment [9, 10], diabetes [11, 12] and obesity [13, 14]. The 2017 GBD study reports that more economically developed countries had the lowest burden of dental caries and advanced periodontitis [1]. The burden of oral diseases is significant, yet oral health is often excluded from evidence-based public health policy [15–17].
Despite a large body of evidence demonstrating the clinical effectiveness of a broad range of preventive interventions for dental caries and periodontitis, few interventions are translated into routine clinical dental practice or public health programs [18–22]. Dentistry has remained treatment-dominated and a highly specialised area of healthcare, which, as a result, has not successfully tackled the global burden of oral diseases [5].
Evidence for the cost-effectiveness of interventions is also an important consideration to assist decision makers in determining investment decisions. In the past 10 years, seven systematic reviews of economic evaluations were focused on preventive interventions for dental caries and/or periodontitis [23–29]. Six systematic reviews were exclusively focused on dental caries, of which four reviews were on child populations [25–28]. One systematic review assessed interventions for dental caries, periodontitis and oral cancer [29].
When considering the focus of these reviews, five reviews were primarily concerned with investigating the quality of the economic evaluation studies [23–26, 29]. Four reviews provided an overall economic evaluation quality score using various checklists [23, 25, 27, 29], although determining study quality using a scoring system is not a best practice recommendation [30, 31]. One review did avoid reporting an overall score but developed and used a risk of bias grading assessment [24].
The most common preventive interventions that have been economically evaluated include water fluoridation [23, 29], fissure sealant applications [23, 26, 29], and the use of topical fluorides such as fluoride varnish applications and fluoride mouth rinses [24, 26, 28]. Only three out of the six systematic reviews that assessed these preventive interventions reported whether the preventive interventions were cost-effective [24–26]. This is due, in part, to the reviews’ primary aim being to determine the quality of economic evaluation studies. In general, preventive interventions found to be cost-effective were water fluoridation, taxation of sugar sweetened beverages (SSBs), and anticipatory guidance inclusive of oral health education. Fissure sealant applications and interventions (with or without dental treatment) incorporating the use of topical fluorides (mouth rinses, gels and fluoride varnish applications) were shown to have mixed cost-effectiveness outcomes (either cost-effective or more costly and more effective). The lack of primary studies on economic evaluations for the prevention of periodontitis continues to be a gap in the literature. In addition, the economic evaluation methodology on prevention interventions for dental caries and periodontitis have not yet been fully explored. Therefore, the aim of this study was to conduct a systematic literature review to critically examine the methods used for full economic evaluations of preventive interventions for dental caries and periodontitis.
Methods
The study protocol was registered in May 2020 in PROSPERO (CRD42020186409) and revised in October 2020 to ensure there was contemporary relevance.
Literature Search
Published literature was searched in May 2020 and updated in April 2021 for publications post-2000 using the following electronic databases: The CRD databases (DARE, NHS EED, HTA), EBSCO databases (CINAHL, ERIC, Global Health, OpenDissertations), EMBASE, Medline and Web of Science. Efforts were made to identify relevant unpublished ‘grey’ literature and conference proceedings through appropriate websites and databases such as Proquest Dissertations and Theses Global, OpenGrey, and EThOS databases for potentially relevant studies. Google Scholar and government websites were not searched due to the limitations of search replicability. Bibliographic information from identified relevant systematic reviews were examined for potentially applicable studies. Medical Subject Headings (MeSH) and free-text search terms were combined with key concepts relating to dental caries, periodontitis and economic evaluation. The adopted search terms and strategy are specified in Online Supplementary Material (OSM) Appendix 1.
Study Selection and Eligibility
The study eligibility criteria were based on the population, intervention, comparator and outcome (PICO) framework: P—all ages, I—Preventive interventions for dental caries and periodontitis, C—at least one strategy (the intervention or comparator) must be a preventive intervention or prevention is a primary focus (dental treatment can be a consequence), and O—dental caries or periodontitis and economic evaluation.
In this review, we adapted the intervention framework that has been commonly used in mental health prevention [32, 33] to develop the Intervention Classification Framework for Dental Caries and Periodontitis. The spectrum of interventions illustrated in Fig. 1 classifies six categories according to their descriptors as explained in OSM Appendix 2. Only universal, selective or indicated interventions were included in this review. Universal interventions target the whole population and not people identified based on individual risk for dental caries or periodontitis. Selective interventions target community population subgroups whose risk of developing dental caries or periodontitis is higher than average. Indicated interventions target high-risk individuals or those with initial stages of dental caries or periodontitis.
Fig. 1.
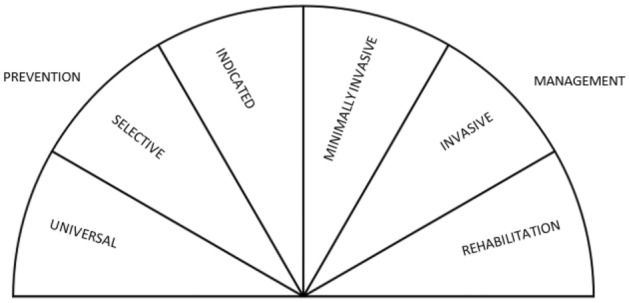
Interventions classification framework for dental caries and periodontitis, adapted from Mrazek and Haggerty [32]
Other inclusion parameters included full-texts, English language publication, model-based or trial-based full economic evaluations (primary or secondary data), original empirical research, peer-reviewed and non-peer-reviewed publications (e.g., theses, government reports). Full economic evaluation is a systematic framework that allows the incremental analysis of alternative interventions in terms of both costs (resource use) and consequences (outcomes, effects) [34].
Other specific exclusion criteria include:
Studies published earlier than 2000.
Partial economic evaluations, where the incremental analysis of alternative interventions was not performed.
Studies that made comparisons of different dental materials or clinical techniques, investigated the different use of the dental workforce or payment schemes.
Studies that did not have a primary focus on preventive interventions.
Literature/systematic reviews, letters, editorials, conference abstracts and commentaries.
The screening process and quality appraisal were evaluated for their level of agreement according to Cohen’s Kappa values whereby a score < 0.40 corresponds to poor, 0.40–0.74 is considered fair to good, and 0.75–1 is defined as perfect agreement [35]. It was agreed that studies pre-2000 would be excluded considering that economic evaluations in oral health research have considerably increased from the year 2000 onwards [36]. Potential papers identified using the literature search strategy were imported and duplicate records removed using Endnote X9.2 (Clarivate Analytics). The remaining papers for screening were imported and managed using Covidence. Two reviewers (TMN and UT) independently screened title/abstract and full-text screening. Any differences in agreement were discussed and a consensus reached.
Data Extraction
TMN completed the data extraction for studies into Covidence using the following parameters:
-
A.
Publication year, author, classification of preventive intervention, country context, data source (primary/secondary data), economic evaluation study type (model/trial/combination)
-
B.
Type of economic evaluation (e.g., cost-benefit analysis (CBA), cost-consequence analysis (CCA), cost-minimisation analysis (CMA), cost-effectiveness analysis (CEA), cost-utility analysis (CUA) or combination and return on investment (ROI) studies.
-
C.
Economic evaluation characteristics including perspective, reference year, time horizon, discount rate for costs and outcomes, cost categories.
-
D.
Intervention and comparator description.
-
E.
Population characteristics including age of target population, and number of participants.
-
F.Reported outcomes including:
- Dental outcomes (e.g., caries free, decayed teeth surfaces (ds/DS), decayed teeth (dt/DT), decayed, missing, and filled surfaces (dmfs/DFMS), decayed, missing, and filled teeth (dmft/DMFT), proportion of bleeding sites, retained-tooth per year, quality-adjusted tooth years (QATYs),
- Health outcomes (e.g., disability-adjusted life years (DALYs) and quality-adjusted life years (QALYs), and
- Economic evaluation outcomes (e.g., incremental cost-effectiveness ratio (ICER), cost-benefit ratio (CBR), and ROI.
Cost-Effectiveness
Value for money judgments were made based on whether the preventive intervention was cost-saving, dominant, or was below a willingness-to-pay (WTP) threshold. For CEA studies that calculated the ICER, where the intervention was more costly and more effective, cost-effectiveness was ‘not stated’ due to the absence of WTP thresholds for dental outcomes. If a WTP threshold was used to judge cost-effectiveness for CUA studies, as these can differ between countries, this was included in the review.
Quality Appraisal
The methodological quality of eligible model-based and trial-based studies was appraised using the Drummond 10-point checklist, including the 32 sub-questions, which informed the response of the overarching question (answer options being ‘yes’, ‘no’ or ‘unsure’) [34]. The Drummond 10-point checklist has previously been applied for systematic reviews of economic evaluations on oral health interventions [24, 27, 36, 37]. Quality appraisal was also used to assist in identifying the methodological characteristics of the economic evaluations included in this review. All studies were appraised by one reviewer (TMN), and a second appraisal was provided by two other independent reviewers (50:50; UT and LL). Any differences in the quality appraisal scoring were discussed between the reviewers to reach a consensus, with unresolved issues mediated by the third reviewer. Individual study and overall study quality according to the preventive interventions were classified using Method 1 from the Gonzalez-Perez (2002) criteria [38] for the Drummond 10-point checklist [34]: low quality (0–0.69), moderate quality (0.7–0.89) and good quality (0.9–1.0).
Results
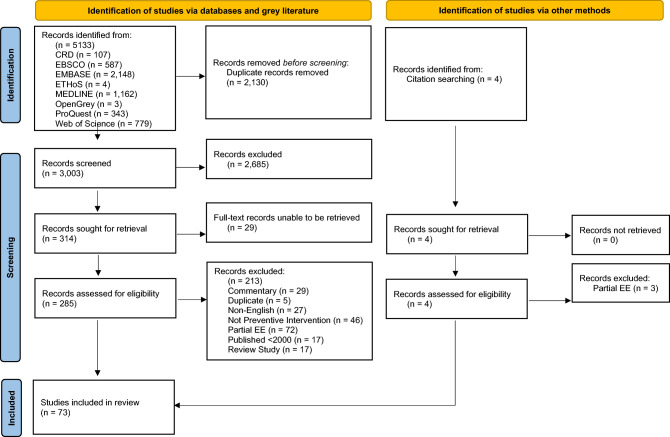
The literature search process is summarised using the PRISMA guidelines (Fig. 2) [39]. Of the 5,133 records identified, the duplicate study removal process resulted in 3,007 unique records, with 2,685 records excluded at the title/abstract screening stage (TMN/UT; Cohen’s Kappa: 0.605). Four records were identified via bibliographic citation search. Of the remaining 322 records subjected to full-text screening (TMN/UT; Cohen’s kappa: 0.430), 29 records were automatically removed since they reported abstracts only or the full-texts could not be retrieved. The final number of studies meeting the inclusion eligibility criteria was 73 full-text articles. Both title/abstract and full-text screening had fair to good agreement between reviewers.
Fig. 2.
Results of the systematic review search strategy using the PRISMA guidelines [39]
Findings Summary
A summary of the study characteristics is reported in Table 1, with the individual details presented in OSM Appendix 3. Based on the full-text analysis, 64 studies (88%) were related to dental caries, eight (11%) to periodontitis, and one study (1%) investigated impacts on both dental caries and periodontitis. Most studies (90%) were in high-income countries including the USA (n = 17), Australia (n = 15), and the UK (n = 11). Of the 73 full-text papers, there were 34 categories of preventive interventions for dental caries and/or periodontitis; ten were universal interventions, nine were selective interventions, and 15 were indicated interventions (Table 2).
Table 1.
A summary of the study characteristics included in the systematic review
| Summary | Characteristic | No. of studies N = 73 (%) |
|
|---|---|---|---|
| Country context* | High income | ||
| Australia [52, 57, 63–65, 67, 68, 70, 87, 92–94, 99, 103, 108] | 15 | (19%) | |
| Canada [96, 100] | 2 | (3%) | |
| Chile [44, 59, 61, 82, 85] | 5 | (6%) | |
| Finland [60] | 1 | (1%) | |
| Germany [49, 50, 62, 89, 90, 108, 110] | 7 | (9%) | |
| Ireland [71, 108] | 2 | (3%) | |
| Japan [97, 108] | 2 | (3%) | |
| Netherlands [86, 105] | 2 | (3%) | |
| New Zealand [55, 73, 79] | 3 | (4%) | |
| Norway [109] | 1 | (1%) | |
| Spain [108] | 1 | (1%) | |
| Sweden [48, 106, 107] | 3 | (4%) | |
| UK [40, 54, 58, 84, 95, 102, 104, 108, 111–113] | 12 | (15%) | |
| USA [41, 43, 45–47, 53, 69, 74, 76, 88, 91, 98, 101, 102, 108, 123, 124] | 17 | (21%) | |
| Subtotal | 72 | (90%) | |
| Upper-middle income | |||
| Brazil [42] | 1 | (1%) | |
| Iran [72] | 1 | (1%) | |
| Peru [80] | 1 | (1%) | |
| South Africa [75, 78] | 2 | (3% | |
| Thailand [81] | 1 | (1%) | |
| Subtotal | 6 | (8%) | |
| Low-middle income | |||
| Sri Lanka [108] | 1 | (1%) | |
| Low income | |||
| Nepal [83] | 1 | (1%) | |
| Target oral disease | Dental caries [40–50, 52, 54, 55, 57–65, 67–69, 71–91, 93–101, 103–107, 111, 112, 123, 124] | 65 | (88%) |
| Periodontitis [53, 92, 102, 108–110, 113] | 7 | (11%) | |
| Caries and periodontitis [70] | 1 | (1%) | |
| Target population | Adults [50, 53, 58, 64, 88, 92, 102, 108–110] | 10 | (14%) |
| Children^ [40–49, 52, 54, 57, 59–62, 67–69, 71, 74, 80–85, 90, 91, 93–101, 103–107, 112, 123, 124] | 47 | (64%) | |
| Adults + children^ [55, 63, 65, 70, 72, 73, 75–79, 86, 87, 89, 111, 113] | 16 | (22%) | |
| Economic evaluation study type | Model-based [41, 43–47, 49, 50, 52–55, 57, 59, 61–63, 67–91, 93, 95, 96, 98–101, 103, 105, 108–111, 123, 124] | 57 | (78%) |
| Trial-based [48, 58, 60, 92, 94, 97, 102, 104, 106, 107, 112, 113] | 12 | (16%) | |
| Model + trial [40, 42, 64, 65] | 4 | (5%) | |
| Data source | Primary [40, 42, 48, 58, 60, 63, 64, 92, 94, 97, 102, 104–107, 112, 113] | 17 | (23%) |
| Secondary [41, 43–47, 49, 50, 52–55, 57, 59, 61, 62, 65, 67–91, 93, 95, 96, 98–101, 103, 108–111, 123, 124] | 58 | (77%) | |
N total number of studies
*One study reported economic evaluations for eight different country contexts [108]
^Children were defined as < 18 years old
Table 2.
The categories of preventive interventions that were economically evaluated for dental caries and periodontitis
| Preventive intervention description | Number of economic evaluations N = 100 (%) |
Target oral disease | Economic evaluation outcome | Overall quality of studies* | |
|---|---|---|---|---|---|
| Universal interventions (N = 28) | |||||
| 20% sugar-sweetened beverages tax vs. no intervention [86, 87] | 2 | (2%) | Dental caries | Cost-effective or Cost-saving | Good |
| Bacterial screening saliva testing vs. standard care [91] | 1 | (1%) | Dental caries | N/S | Moderate |
| Ban on sugar-sweetened beverages sales vs. no intervention [88] | 1 | (1%) | Dental caries | Cost-saving | Good |
| Fluoridation of toothpastes vs. no intervention [83] | 1 | (1%) | Dental caries | Cost-saving | Moderate |
| Front-of-package food labelling vs. no intervention [89] | 1 | (1%) | Dental caries | Cost-effective | Good |
| Increasing the use of chewing gum vs. no intervention [90] | 1 | (1%) | Dental caries | Cost-saving | Good |
| Interleukin-1 genetic testing vs. standard care [53] | 1 | (1%) | Periodontitis | N/S | Good |
| Milk fluoridation vs. no intervention [81, 82, 85] | 3 | (2%) | Dental caries | Cost-effective | Good |
| Salt fluoridation vs. no intervention [80, 85] | 2 | (2%) | Dental caries | Cost-effective | Good |
| Water fluoridation vs. no intervention [55, 67, 68, 70–79, 84, 85] | 15 | (12%) | Dental caries and periodontitis# | Cost-effective or Cost-saving | Moderate |
| Selective interventions (N = 29) | |||||
| Targeted postage of oral hygiene products vs. no intervention [95] | 1 | (3%) | Dental caries | N/S | Low |
| Targeted pre/primary school-based dental check-up vs. standard care [94] | 1 | (3%) | Dental caries | Cost-effective | Good |
| Targeted pre/primary school-based fluoride varnish by dental/non-dental primary care providers with/without dental screening [44, 54, 61, 96] | Dental caries | ||||
| vs. counselling [44] | 2 | (7%) | Not cost-effective or N/S | Good | |
| vs. no intervention [54, 61] | 2 | (7%) | Cost-effective or N/S | Moderate | |
| vs. standard care [96] | 1 | (3%) | Cost-effective | Moderate | |
| Targeted primary school-based fissure sealant program with/without fluoride mouthrinse (intervention) | Dental caries | ||||
| vs. no intervention [59, 84, 85, 97, 98] | 5 | (17%) | Cost-effective or Cost-saving or N/S | Moderate | |
| vs. standard care [69, 99, 100] | 4 | (14%) | Cost-effective or N/S | Good | |
| vs. targeted school-based toothbrushing program [42] | 1 | (3%) | N/S | Good | |
| Targeted primary school-based fissure sealant program (comparator) | Dental caries | ||||
| All seal vs. risk-based [98, 101] | 2 | (7%) | N/S | Moderate | |
| No intervention vs. risk-based [98, 101] | 2 | (7%) | Not cost-effective | Moderate | |
| Targeted primary school-based fluoride gel vs. no intervention [85] | 1 | (3%) | Dental caries | N/S | Good |
| Targeted primary school-based fluoride mouth rinse vs. no intervention [85] | 1 | (3%) | Dental caries | Cost-effective | Good |
| Targeted supervised toothbrushing program vs. no intervention [42, 54, 85] | 2 | (7%) | Dental caries | N/S | Moderate |
| Targeted telehealth or home visit oral health education vs. standard care [57, 84, 93] | 3 | (10%) | Dental caries | Cost-saving or Cost-effective | Moderate |
| Targeted community-based group oral health education vs. chairside oral health education [92] | 1 | (3%) | Periodontitis | Cost-saving | Good |
| Indicated interventions (N = 42) | |||||
| Artificial intelligence for intra-oral radiographic detection of dental caries vs. no intervention [49] | 1 | (2%) | Dental caries | Cost-effective | Good |
| Oral prophylaxis and oral health education vs. fissure sealant and fluoride varnish [103] | 1 | (2%) | Dental caries | Not cost-effective | Moderate |
| Fluoride and xylitol supplement, oral prophylaxis and fluoride/chlorhexidine varnish vs. standard care [60, 107] | 2 | (5%) | Dental caries | N/S | Good |
| Fissure sealant vs. no intervention [41, 45–47] | 5 | (12%) | Dental caries | N/S | Moderate |
| Fluoride varnish vs. fissure sealant [40, 41] | 2 | (5%) | Dental caries | Cost-effective or Not cost-effective | Moderate |
| Fluoride varnish vs. standard care [48, 52, 62, 105, 106, 112] | 6 | (14%) | Dental caries | N/S | Good |
| Minimally invasive dentistry (intervention) | Dental caries | ||||
| vs. no intervention [104] | 1 | (2%) | N/S | Good | |
| vs. standard care [63–65, 104, 105] | 5 | (12%) | Not cost-effective or N/S | Good | |
| Standard care vs. minimally invasive dentistry [104] | 1 | (2%) | Dental caries | Not cost-effective | Good |
| Slow releasing fluoride glass devices program [84] | 1 | (2%) | Dental caries | Cost-saving | Low |
| Resin infiltration vs. fluoride varnish and/or oral health education [50] | 1 | (2%) | Dental caries | N/S | Moderate |
| Varied intervals of dental check-ups e.g., 3-, 9-, 12-, 24-, 36-monthly, risk-based | Dental caries | ||||
| vs. standard care [58, 111] | 7 | (17%) | N/S | Good | |
| 24-monthly vs. risk-based [58] | 1 | (2%) | Cost-effective | Good | |
| Regular supportive periodontal therapy | Periodontitis | ||||
| vs. irregular supportive periodontal therapy [109, 110] | 2 | (5%) | N/S | Moderate | |
| vs. tooth removal and implant crown replacement [110] | 1 | (2%) | N/S | Good | |
|
Supportive periodontal therapy by dental specialist vs. general dental practitioner [108] |
1 | (2%) | Periodontitis | N/S | Good |
| Varied intervals of supportive periodontal therapy and standard oral health education (e.g., 12-monthly) | Periodontitis | ||||
| vs. standard care [102] | 1 | (2%) | Cost-saving | Good | |
| Varied intervals of supportive periodontal therapy and tailored oral health education (e.g., 6-monthly, 12-monthly, risk-based) | Periodontitis | ||||
| 6-monthly vs. standard care [102] | 1 | (2%) | N/S | Good | |
| 12-monthly vs. standard care [102] | 1 | (2%) | N/S | Good | |
| Risk-based vs. standard care [102] | 1 | (2%) | N/S | Good | |
N = total number of economic evaluations, N/S not stated, Standard care refers to treatment as usual with 6-monthly dental check-ups
#Only one study on water fluoridation economically evaluated dental caries and periodontitis [70]
*Overall quality of studies using the Gonzalez-Perez (2002) criteria [38] for the Drummond 10-point checklist [34]
The most frequently economically evaluated preventive interventions for dental caries were water fluoridation (universal), fissure sealant and fluoride varnish (selective and indicated). For periodontitis, the most common preventive intervention economically evaluated was supportive periodontal therapy with oral health education. The target population for these interventions were mainly only for children (64%) followed by both adults and children (22%).
A summary of the characteristics of included studies is reported in Table 3. The most common economic evaluation approaches used were CEA and CBA (47% and 25%, respectively). Model-based studies were the most common approach for economic evaluation (78%), with a few studies using both model-based and trial-based studies (5%).
Table 3.
A summary of characteristics for model-based and trial-based economic evaluation studies
| Characteristics | Model-based Economic evaluations N = 61* (%) |
|
|---|---|---|
| Economic evaluation approach | ||
| CBA [55, 70, 72, 74–78, 83, 84, 86–88, 90, 91, 123, 124] | 17 | (28%) |
| CEA [41–47, 49, 59, 61–65, 73, 79–82, 85, 93, 95, 96, 98, 100, 101, 105, 108, 109] | 30 | (49%) |
| CUA [53, 54, 57, 67–69] | 6 | (10%) |
| CBA and CEA [50, 71, 89, 99, 111] | 5 | (8%) |
| CEA and CUA [40, 52] | 2 | (3%) |
| Perspective | ||
| Healthcare [41, 43, 45–47, 49, 50, 52–54, 59, 61–65, 84, 85, 87, 90, 91, 101, 109–111, 123, 124] | 27 | (44%) |
| Societal [42, 44, 55, 57, 67–83, 86, 89, 93, 95, 96, 98–100, 108] | 30 | (49%) |
| Healthcare and societal [40, 88, 105] | 3 | (5%) |
| Reference year | ||
| N/R [54, 75, 84, 91, 95, 96, 123] | 7 | (11%) |
| < 2000 [53, 74, 78, 79, 82, 98, 99] | 7 | (11%) |
| 2000–2005 [43, 45, 46, 67, 68, 70, 77, 81, 83, 101, 111] | 11 | (18%) |
| 2006–2010 [64, 65, 80, 85, 100, 108] | 6 | (10%) |
| 2011–2015 [40–42, 44, 47, 49, 50, 52, 55, 57, 63, 69, 72, 73, 76, 87, 90, 93, 105, 109, 110] | 21 | (34%) |
| 2016–2020 [59, 61, 62, 71, 86, 88, 89, 124] | 8 | (13%) |
| Time horizon | ||
| N/R [73] | 1 | (2%) |
| < 5 years [125] | 14 | (23%) |
| 5–10 years [40, 41, 45, 47, 57, 59, 63, 75, 80, 81, 83, 85, 87, 89, 93, 96, 98–101, 123] | 21 | (34%) |
| > 10 years [49, 50, 52, 53, 55, 62, 67, 68, 70, 71, 74, 76, 77, 79, 86, 90, 108–110] | 19 | (31%) |
| Mixed (< 5 and 5–10 years) [124] | 1 | (2%) |
| Mixed (< 5 and > 10 years) [63, 64] | 2 | (3%) |
| Mixed (5–10 and > 10 years) [88, 111] | 2 | (3%) |
| Cost categories—healthcare and societal perspective# | ||
| Direct treatment costs—N/R [41, 44, 95] | 3 | (5%) |
| Direct treatment costs—restorations only [42, 61, 67–69, 72–75, 78, 79, 83, 87, 89, 98–101, 105, 111] | 20 | (33%) |
| Direct treatment costs—restorations and extractions only [40, 47, 59, 71, 76, 80–82, 85] | 9 | (15%) |
| Direct treatment costs—restorations, extractions and other direct costs for dental caries [43, 45, 46, 49, 50, 52, 54, 55, 57, 62–65, 77, 84, 86, 90, 91, 93, 96, 123, 124] | 22 | (36%) |
| Direct treatment costs—restorations, extractions and other direct costs for dental caries and periodontitis [70, 88, 110] | 3 | (5%) |
| Direct treatment costs for periodontitis only [53, 108, 109] | 3 | (5%) |
| Cost categories—societal perspective | ||
| Direct societal costs—N/R [87] | 1 | (2%) |
| Direct societal costs (intervention only) [42, 55, 67, 68, 71, 72, 75, 76, 78, 79, 83, 86, 89, 95, 96, 99] | 15 | (25%) |
| Direct and indirect societal costs (productivity costs lost, travel costs, etc.) [40, 44, 57, 69, 70, 73, 74, 77, 80–82, 85, 88, 93, 98, 100, 105, 108] | 17 | (28%) |
| Dental outcomes measures | ||
| Decayed teeth surfaces/teeth averted (ds/DS)ˆ [41, 42, 50, 57, 70, 76–79, 89, 91, 93, 96, 98] | 13 | (21%) |
| Decayed, missing and filled teeth surfaces/teeth averted (dmfs/DMFT, dmft/DMFT) [52, 55, 62, 64, 65, 71–75, 80–85, 87, 90, 95, 99, 105, 111, 124] | 23 | (38%) |
| Caries free (%/months/person) [40, 43, 44, 61, 86, 95, 100, 101] | 8 | (13%) |
| Clinical attachment loss averted [108] | 1 | (2%) |
| QATY [40, 46, 59] | 3 | (5%) |
| Restorative/extraction/episode of pain/hospital/event averted [43, 47, 63, 95, 123] | 5 | (8%) |
| Retained-tooth per year [49, 62, 109, 110] | 4 | (7%) |
| Tooth loss averted [108] | 1 | (2%) |
| Generic health outcome measures | ||
| DALY [67–69] | 3 | (5%) |
| QALY [40, 52–55, 57, 88] | 7 | (11%) |
| Characteristics | Trial-based economic evaluations N = 17* (%) |
|
|---|---|---|
| Economic evaluation approach | ||
| CBA [102] | 1 | (6% |
| CEA [42, 48, 60, 64, 65, 104, 106, 107, 112, 113] | 10 | (59%) |
| CMA [92, 103] | 2 | (12%) |
| CBA and CEA [97] | 1 | (6%) |
| CBA and CUA [58] | 1 | (6%) |
| CEA and CUA [40, 94] | 2 | (12%) |
| Perspective | ||
| Healthcare [60, 64, 65, 92, 103, 104] | 6 | (35%) |
| Societal [40, 58, 102, 106, 112, 113] | 6 | (35%) |
| Healthcare and Societal [42, 48, 94, 97, 107] | 5 | (29%) |
| Reference year | ||
| < 2000 [103, 107] | 2 | (12%) |
| 2000–2005 [60, 97] | 2 | (12%) |
| 2006–2010 [64, 65, 92, 113] | 4 | (24%) |
| 2011–2015 [40, 42, 94, 102, 106, 112] | 6 | (35%) |
| 2016–2020 [48, 58, 104] | 3 | (18%) |
| Time horizon | ||
| < 5 years [40, 42, 48, 58, 60, 64, 65, 92, 94, 102–104, 106, 112, 113] | 15 | (88%) |
| 5–10 years [107] | 1 | (6%) |
| Mixed (< 5 and 5–10 years) [97] | 1 | (6%) |
| Cost categories—healthcare and societal perspective | ||
| Direct treatment costs—N/R [103, 106] | 2 | (12%) |
| Direct treatment costs—restorations only [42, 48, 107] | 3 | (18%) |
| Direct treatment costs—restorations and extractions only [40] | 1 | (6%) |
| Direct treatment costs—restorations, extractions and other direct costs for dental caries [60, 64, 65, 94, 97, 104, 112] | 7 | (41%) |
| Direct treatment costs—restorations, extractions and other direct costs for dental caries and periodontitis [58] | 1 | (6%) |
| Direct treatment costs for periodontitis only [92, 102, 113] | 3 | (18%) |
| Cost categories—societal perspective | ||
| Direct societal costs (intervention only) [42, 48, 97] | 3 | (18%) |
| Direct and indirect societal costs (productivity costs lost, travel costs, etc.) [40, 58, 94, 102, 106, 107, 112, 113] | 8 | (47%) |
| Dental outcomes measures | ||
| Decayed teeth surfaces/teeth averted (ds/DS)ˆ [42, 103, 112] | 3 | (18%) |
|
Decayed, missing and filled teeth surfaces/teeth averted |
8 | (47%) |
| Caries free (%/months/person) [40, 112] | 2 | (12%) |
| Bleeding sites (%) [92, 102] | 2 | (12%) |
| QATY [40, 94] | 2 | (12%) |
| Restorative/extraction/episode of pain/hospital/event averted [104, 112] | 2 | (12%) |
| Successful case managed for periodontitis [113] | 1 | (6%) |
| Generic health outcome measures | ||
| QALY [40] | 1 | (12%) |
| Other outcome measures | ||
| Per 1% card-holder reached [94] | 1 | (6%) |
N total number of studies/interventions, N/R not reported, CBA cost-benefit analysis, CMA cost-minimisation analysis, CEA cost-effectiveness analysis, CUA cost-utility analysis, dmfs/DMFS+ number of decayed, missing, and filled teeth surfaces for the deciduous or permanent dentition, dmft/DMFT+ number of decayed, missing, and filled teeth for the deciduous or permanent dentition, ds/DS+ number of decayed teeth surfaces for the deciduous or permanent dentition, dt/DT+ number of decayed teeth for the deciduous or permanent dentition, + lower-case letters refer to the deciduous dentition and upper-case letters refer to the permanent dentition, DALY disability-adjusted life years, QATY quality-adjusted tooth years, QALY quality-adjusted life years
*Four studies performed model-based and trial-based economic evaluations [40, 42, 64, 65]
#Four studies did not report the intervention costs [54, 87, 88, 90]
ˆIncludes dental outcomes exclusively for occlusal or proximal surfaces
Almost half (49%) of model-based studies used the societal perspective and 44% used the healthcare perspective, compared with 35% for trial-based studies that used either the healthcare or the societal perspective. A greater proportion of trial-based studies (29%) compared with model-based studies (5%) performed economic evaluation from both the healthcare and societal perspectives. The most common cost categories were the cost of direct restorations only (33%) and direct treatment costs inclusive of restorations, extractions and other direct costs for dental caries (36%) for model-based studies, where the latter cost category was the most used for trial-based studies. Other direct costs for dental caries include the cost of root canal treatments, crowns, bridges and implant crowns, noting that these dental treatment procedures may be caused by trauma, cracked tooth syndrome, and severe stages of periodontitis rather than because of dental caries.
The most common dental outcome measures for dental caries were dmfs/DMFS, dmft/DMFT averted, ds/DS averted and caries-free proportion of the population, months or persons. The most common dental outcomes for periodontitis were retained-tooth per year and the proportion of teeth with bleeding sites. CUA model-based and trial-based studies (14%) used either DALYs for dental caries and QALYs for dental caries and periodontitis as the health outcome measures. One study quantitatively used a health equity outcome measure, which evaluated whether the intervention increased access to dental services for children from low-income households [94].
Derivation of Disability and Utility Weights for General Health Outcome Measures
The use of disability and utility weights in the selected studies to quantify DALY averted and QALY gained was variable. Two studies measured DALY using the disability weight of 0.057 for symptomatic dental caries according to the 2003 Australian Burden of Disease study. It includes two time periods spent with disability, an initial phase where the individual experiences intermittent pain and the terminal phase where the individual experiences constant pain. This approach has later been adopted by the 2017 GBD study [1]. One study used the disability weight of 0.012 for dental caries derived from the 2010 GBD study [51] but did not state if the initial and terminal phase experience of pain was considered.
Except for two studies [52, 53], there were generally two approaches to quantify QALYs: (1) one minus the disability weight used in GBD studies or (2) measured using quality-of-life survey instruments for trial-based studies. One study used the disutility weight of 0.720 for otitis media (middle ear infection) [54], and one study used the disutility weight for different severity of dental caries experience (dmft/DMFT) [55], derived from the New Zealand Burden of Disease study [56]. For trial-based studies, one study used the Child Health Utility Nine-Dimension (CHU-9D) instrument, either completed by the parent/primary caregiver as a proxy measure [57] or via the children directly [40], and another study used the EQ-5D-3L instrument to derive QALY utility weights for adults [58]. One study used ‘expert opinion’ for the utility weights for periodontitis, and another study represented QALY as the aggregate preference tooth utility weight of eight permanent molar teeth to represent the individual [52].
Willingness-To-Pay Thresholds
Various hypothetical WTP thresholds for dental outcomes were considered by seven studies [48–50, 59–62]. Two Chilean studies have suggested a WTP threshold of 20,000 Chilean pesos per QATY gained or caries-free person, based on the cost of a restoration from the healthcare perspective [59, 61], and three studies have referenced AU$3,300 per restoration averted [63–65], derived from a study estimating the average lifetime average costs of probable avoided restoration per person [66]. Two other studies used various WTP thresholds using proposed dental outcomes [48, 60]. The WTP threshold of AU$50,000 per DALY averted [67, 68], £20,000 per QALY gained [40, 58], and the 2014 US gross domestic gross product per capital (US$54,639 per DALY averted) [69] were used for CUA studies in Australia, the UK and the USA, respectively.
Universal Interventions
The majority of universal interventions were reported to be cost-effective or cost-saving. These universal interventions included various forms of topical fluorides [55, 67, 68, 70–85], interventions targeting the reduction of sugar consumption such as SSBs taxation or ban on selling SSBs at the workplace [86–89], increasing use of chewing sugar-free gum [90], and bacterial screening saliva test for virulent levels of mutans streptococci [91]. For interleukin-1 genetic screening to prevent periodontitis for persons aged 35 years, the ICER was calculated but the cost-effectiveness was not stated [53].
Selective Interventions
For selective interventions, targeted community-based group oral health education for adults [92], targeted primary school-based fluoride mouth-rinse program [85], targeted telehealth or home visit oral health education for young children [57, 84, 93], and targeted pre/primary school-based dental check-up programs [94], were cost-saving or cost-effective.
The cost-effectiveness was not stated for targeted postage of oral hygiene products [95], targeted primary school-based fluoride gel [85], and targeted supervised toothbrushing program [42, 54, 85]. There were mixed cost-effectiveness results for targeted pre/primary school-based fluoride varnish by dental/non-dental primary care providers with/without dental screening [44, 54, 61, 96] and targeted primary school-based fissure sealant program with/without fluoride mouth rinse [42, 59, 69, 84, 85, 97–101]. In the context of targeted primary school-based fissure sealant programs, no intervention versus risk-based (fissure sealant application was based on the patient’s dental caries risk) interventions was not cost-effective [98, 101].
Indicated Interventions
Under indicated interventions, the use of artificial intelligence for the detection of dental caries on dental intra-oral radiographs was cost-effective [49]. Supportive periodontal therapy and standard oral health education provided 12-monthly versus 6-monthly [102], and the use of slow-releasing fluoride glass devices (glass ionomer cements) for children at high risk for dental caries risk [84] were cost-saving. The economic evaluation outcomes were variable for the remaining studies, either cost-effective, not cost-effective or not stated [40, 41, 45–48, 50, 52, 58, 62–65, 92, 98, 100–113]. Oral prophylaxis and oral hygiene education versus fissure sealant and fluoride varnish application was not cost-saving according to the CMA [103], and standard care versus minimally invasive dentistry was not cost-effective [104].
Quality Appraisal
The results of the quality appraisal using the Drummond 10-point checklist are reported in Table 4 regarding the sub-questions and Cohen’s Kappa values. The study quality according to model-based and trial-based studies is shown in Table 5. Generally, the study quality for economic evaluations improved for studies published after 2010. For all 73 studies, some criteria of the Drummond 10-point checklist were not met, including 30% of studies not applying an appropriate discount rate (question 7) when the time horizon is greater than 1 year and 26% of the studies did not comprehensively discuss other important considerations beyond the technical analysis (question 10). Most model-based (72%) and trial-based studies (82%) were of good quality. In terms of quality appraisal assessment consistency, there was poor inter-rater agreement using the Drummond 10-point checklist (TMN/UT Overall Cohen’s kappa: 0.164; TMN/LL Overall Cohen’s kappa: 0.148; individual question Cohen’s Kappa scores is reported in OSM Appendix 4).
Table 4.
The quality appraisal results using the Drummond 10-point checklist [34]
| Drummond 10-point checklist question | Publication year | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2000–2010 N = 24 |
2011–2021 N = 49 |
2000–2021 N = 73 |
|||||||
| Yes | No | Can't tell | Yes | No | Can't tell | Yes | No | Can't tell | |
| Q1. Was a well-defined question posed in answerable form? | 92% | 4% | 0% | 98% | 2% | 0% | 96% | 3% | 1% |
| Q2. Was a comprehensive description of the competing alternatives given? (i.e., can you tell who did what to whom, where and how often?) | 92% | 4% | 4% | 100% | 0% | 0% | 97% | 1% | 1% |
| Q3. Was the effectiveness of the programmes or services established? | 96% | 0% | 4% | 100% | 0% | 0% | 99% | 0% | 1% |
| Q4. Were all the important and relevant costs and consequences for each alternative identified? | 83% | 0% | 13% | 94% | 0% | 6% | 90% | 0% | 10% |
| Q5. Were costs and consequences measured accurately in appropriate physical units prior to valuation (e.g., hours of nursing time, number of physician visits, lost work days, gained life-years)? | 96% | 0% | 4% | 96% | 2% | 2% | 96% | 1% | 3% |
| Q6. Were costs and consequences valued credibly? | 96% | 0% | 0% | 86% | 4% | 10% | 89% | 3% | 7% |
| Q7. Were costs and consequences adjusted for differential timing? | 67% | 25% | 8% | 71% | 20% | 8% | 70% | 22% | 8% |
| Q8. Was an incremental analysis of costs and consequences of alternatives performed? | 92% | 4% | 0% | 96% | 4% | 0% | 95% | 5% | 0% |
| Q9. Was uncertainty in the estimates of costs and consequences adequately characterised? | 79% | 21% | 0% | 90% | 10% | 0% | 86% | 14% | 0% |
| Q10. Did the presentation and discussion of study results include all issues of concern to users? | 58% | 38% | 4% | 82% | 16% | 2% | 74% | 23% | 3% |
N number of studies
Table 5.
A summary of the study quality using the Gonzalez-Perez [38] criteria for the Drummond 10-point checklist for model-based and trial-based studies [34]
| Study quality | Model-based studies N = 61 |
Trial-based studies N = 17 |
||
|---|---|---|---|---|
| Good | 44 | (72%) | 14 | (82%) |
| Moderate | 14 | (23%) | 3 | (18%) |
| Poor | 3 | (5%) | – | |
N number of studies
Discussion
This study found that there was a significant number of peer-reviewed publications on the economic evaluation of preventive interventions for dental caries, but there were limited publications on economic evaluation for periodontitis. Most of the studies (90%) were conducted in high-income countries. The choice in the selection of parameters used for economic evaluation was variable throughout the studies, including the time horizon, cost categories, and the chosen dental and health outcome measure(s). In general, universal interventions such as water fluoridation were cost-effective and cost-saving. Selective interventions were either cost-effective or more costly and more effective, and indicated interventions had mixed cost-effectiveness.
Generally, the studies included in the review were demonstrated to be of moderate or good quality. There was generally poor inter-rater reliability using the Drummond 10-point checklist, but this is likely to be influenced by the assessor’s interpretation of the question rather than the quality appraisal instrument itself [35]. Two of the three assessors had previous experience using the Drummond 10-point checklist, and two of three assessors have a background in dentistry. The poor level of inter-rater agreement highlights the importance of using at least two assessors, which may have influenced the quality appraisal of each study in either direction.
Our updated review noted there was a diversity of dental and generic health outcomes used in the studies included in this review and identified that there was sparse evidence for cost-effective interventions to prevent periodontitis [29, 114–116], which is a consistent finding from previous reviews [23–29]. Different outcome measures do not allow comparison between interventions or diseases to guide resource allocation. Our review found very few economic evaluations were conducted in low- and low-middle-income countries. Although quantitative outcome measures that capture health equity were not specifically investigated in our review, we found one study [94, 117] that incorporated it in their analysis. The issue of health equity is missing from previous reviews.
Interpretation and Implication of Outcome Measures
The use of dental outcome measures for economic evaluation has considerable limitations. For example, some authors compared preventive interventions using dental outcomes exclusively for occlusal or proximal surfaces [41, 48, 50, 98, 103], and described them as cavities [42, 96] or caries lesions [49, 57, 86, 89]. There were many studies that used the term caries increment with dental outcomes reported as either ds/DS, dt/DT, dmfs/DMFS and dmft/DMFT [43, 44, 48, 62, 64, 68, 71, 74–76, 85, 86, 89, 98, 105–107]. The nuance of terminology in dentistry to describe dental caries as numbers of tooth surfaces or teeth is distinct, as opposed to dental caries incidence in epidemiology, where an individual develops at least one tooth with dental caries. These measurement differences have implications for intervention comparability. With regards to periodontitis, there were variations in using tooth-specific or individual person measures such as retained-tooth per year, percentage of bleeding sites, and successful case managed.
In addition, the dmfs/DMFS and dmft/DMFT are only composite measures for dental caries since decayed teeth (d/D) is only one component of these indices [118]. Despite the limitations of both indices, the dmft/DMFT index is incorporated in the methodology used by the 2017 Global Burden of Disease study to calculate dental caries incidence given it is the most widely used instrument to measure for dental caries experience [1]. In brief, based on longitudinal studies, the population incremental difference in the dmft/DMFT index for the initial and subsequent dental check-up is accepted as the number of dental caries incidence. These differences in outcomes are a limitation in quantifying the true measure of dental caries incidence. Adding to the complexity, many studies using CEA approaches could not be assessed for cost-effectiveness due to an absent WTP threshold using dental outcomes. This makes it difficult to interpret the cost effectiveness credentials of interventions that have been economically assessed using CEA frameworks. However, if studies use the same outcome measures, then their cost-effectiveness ratios can be compared.
Regarding CUA studies, there are important differences in using general health outcome measures such as DALY and QALY, whereby their results need to be interpreted with caution. This means that preventive interventions for dental caries and periodontitis cannot be directly compared or ranked against each other, nor can they be compared with other health interventions for appraisal and make health policy investment decisions. To date, there is still ongoing research to validate the use of quality-of-life survey instruments for children with dental caries, although the CHU-9D and the EQ-5D-Y instruments show promise, but these have not been tested for face validity [119]. The application of quality-of-life survey instruments for adults have had more extensive research generally [120], but they have not been widely applied in economic evaluations for preventive interventions for dental caries and periodontitis. To date, research using QALY has shown they is not sensitive to oral diseases [125], but since DALY is independent of quality-of-life survey instruments. It may be the only outcome now that can directly allow comparisons between other health interventions.
Cost-Effectiveness Analysis Methods
There were concerns regarding how the benefits were defined for CEA studies. For example, three studies (model-based and trial-based studies) performed an economic evaluation for the cost-effectiveness of fissure sealants and fluoride varnish [40, 41], or fissure sealant and a school-based toothbrushing program [42]. In this context, fissure sealants have a clinical benefit for the occlusal surfaces of posterior molar teeth, where they are applied, whereas the clinical benefit of fluoride varnish and regular toothbrushing with fluoride toothpaste can have a spill-over benefit to other surfaces and numbers of teeth for an individual. i.e., the clinical effectiveness of each strategy was not fully incorporated and consistent.
Other studies used effectiveness data based on older studies (>20 years ago), perhaps as no recent studies were applicable [43, 44]. Other model-based studies have made assumptions for the consequences of an intervention using panel datasets of dental services, such as for the effectiveness of fissure sealants [45–47], which may or may not have a preventive effect as smooth surfaces of teeth are not protected by the fissure sealant. Similarly, three studies (model-based and trial-based studies) investigated the cost-effectiveness of fluoride varnish interventions against standard care, where the effectiveness was evaluated for surfaces in between teeth (proximal) and not smooth surfaces [48–50]. These methodological issues on effectiveness indicate potential bias and limitation of the results.
Strengths and Limitations
One of the key strengths of the present systematic review is the breadth of the study, which includes populations of all age groups and periodontitis as one of the target oral diseases. We articulated an intervention framework to classify prevention interventions for dental caries and periodontitis according to the existing classifications for the oral disease severity. i.e., the Intervention Classification Framework for Dental Caries and Periodontitis. The framework can be useful for future systematic reviews of economic evaluations in oral health and dentistry. Given most model-based and trial-based studies were of moderate or good quality, the decision to not exclude lower quality studies is unlikely to influence the results. Method 1 from the Gonzalez-Perez (2002) study quality criteria [38] was used in preference to Methods 2 and 3 given the Drummond 10-point checklist does not include the 11th criteria question ‘Is the paper relevant for the NHS and are the results sufficiently transparent for them to be replicated in an NHS setting?’
Our review expands on previous reviews by clearly articulating whether the prevention interventions were cost-effective and discussed in detail the interpretation and limitation of dental and generic health outcomes. Although dental caries is highly prevalent, their burden of disease measured by YLDs is considerably less than for periodontitis and signifies the importance for prevention efforts needed beyond dental caries.
We are aware that some publications excluded at the full-text screening stage were of partial economic evaluations, such as the 20% sugar-sweetened beverages tax intervention [121, 122], which could be informative in the present analysis. Our review may be biased since it was restricted to publications after 2000 (inclusive), papers had to be reported in English, a limited search of databases for relevant grey literature was conducted, and relevant full-text may be excluded given the lower inter-rater reliability value of ‘fair to good’ agreement according to the Cohen’s Kappa assessment. It is apparent that many of the studies included in our review referred to dental caries using other descriptions such as cavities and caries lesions, which could potentially result in relevant publications being excluded from our systematic review search strategy. These concerns were not observed for economic evaluations of preventive interventions for periodontitis. Finally, it is important to consider how the economic evaluation studies were validated. We did not explicitly review this as part of the a priori review protocol, and, therefore, this is essential to capture and discuss in future reviews in the field according to the ISPOR-SMDM Modeling Good Research Practices Taskforce [126].
Recommendations
We recommend three key essential considerations for future research, which can accelerate oral health policy investment: (1) use generic health outcomes to facilitate the assessment for cost-effectiveness, (2) conduct economic evaluation research to prevent periodontitis, and (3) make considerations for health equity.
Conclusion
Universal interventions, in particular water fluoridation, were generally demonstrated to be cost-effective, while the cost-effectiveness for selective and indicated interventions were mixed. Other universal interventions that had at least more than one study demonstrating cost-effectiveness include taxation on sugar-sweetened beverages, milk and salt fluoridation. The disparate selection of the cost components, the consequences, and the choice for measuring health outcomes, meant that it was difficult to make investment decisions for preventive interventions for dental caries and periodontitis. We propose future research on economic evaluation for the oral health context using generic health outcome measures to determine cost-effectiveness outcomes. In addition, there is an increasing need to conduct research on the cost-effectiveness of preventive interventions related to periodontitis and to reflect on health equity considerations. These recommendations will enable comparability with mainstream economic evaluations of health interventions to inform policy decision making.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was supported by the National Health and Medical Research Council Postgraduate Scholarship Scheme APP1189802 and the Australian Government Research Training Program (RTP) Scholarship.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Availability of data and material
Available on request.
Code availability
Not applicable.
Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Tan Minh Nguyen, Utsana Tonmukayakul and Long Khanh-Dao Le. The first draft of the manuscript was written by Tan Minh Nguyen and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1.GBD 2017 Oral Disorders Collaborators, Bernabe E, Marcenes W, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J Dent Res. 2020;99(4):362–73. 10.1177/0022034520908533. [DOI] [PMC free article] [PubMed]
- 2.Singh A, Peres MA, Watt RG. The relationship between income and oral health: a critical review. J Dent Res. 2019;98(8):853–860. doi: 10.1177/0022034519849557. [DOI] [PubMed] [Google Scholar]
- 3.Northridge ME, Kumar A, Kaur R. Disparities in access to oral health care. Annu Rev Public Health. 2020;41:513–535. doi: 10.1146/annurev-publhealth-040119-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleitas Alfonzo L, Bentley R, Singh A. Home ownership, income and oral health of children in Australia—a population-based study. Community Dent Oral Epidemiol. 2021;00:1–8. doi: 10.1111/cdoe.12646. [DOI] [PubMed] [Google Scholar]
- 5.Watt RG, Daly B, Allison P, et al. The lancet oral health series: implications for oral and dental research. J Dent Res. 2020;99(1):8–10. doi: 10.1177/0022034519889050. [DOI] [PubMed] [Google Scholar]
- 6.Moynihan P, Makino Y, Petersen PE, Ogawa H. Implications of WHO guideline on sugars for dental health professionals. Community Dent Oral Epidemiol. 2018;46(1):1–7. doi: 10.1111/cdoe.12353. [DOI] [PubMed] [Google Scholar]
- 7.Sanz M, Del Castillo AM, Jepsen S, et al. Periodontitis and cardiovascular diseases. Consensus report. Glob Heart. 2020;15(1):1. doi: 10.5334/gh.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zardawi F, Gul S, Abdulkareem A, Sha A, Yates J. Association between periodontal disease and atherosclerotic cardiovascular diseases: revisited. Front Cardiovasc Med. 2021;7:625579. doi: 10.3389/fcvm.2020.625579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapellas K, Ju X, Wang X, Muller N, Jamieson L. The association between periodontal disease and dementia: a systematic review and meta-analysis. Dent Oral Biol Craniofacial Res. 2019;2(1):1–11. 10.31487/j.DOBCR.2019.01.005.
- 10.Nascimento PC, Castro MML, Magno MB, et al. Association between periodontitis and cognitive impairment in adults: a systematic review. Front Neurol. 2019;10:323. doi: 10.3389/fneur.2019.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nascimento GG, Leite FRM, Vestergaard P, Scheutz F, López R. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol. 2018;55(7):653–667. doi: 10.1007/s00592-018-1120-4. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2013;40(Suppl 14):S113–134. doi: 10.1111/jcpe.12059. [DOI] [PubMed] [Google Scholar]
- 13.Keller A, Rohde JF, Raymond K, Heitmann BL. Association between periodontal disease and overweight and obesity: a systematic review. J Periodontol. 2015;86(6):766–776. doi: 10.1902/jop.2015.140589. [DOI] [PubMed] [Google Scholar]
- 14.Khan S, Barrington G, Bettiol S, Barnett T, Crocombe L. Is overweight/obesity a risk factor for periodontitis in young adults and adolescents? A systematic review. Obes Rev. 2018;19(6):852–883. doi: 10.1111/obr.12668. [DOI] [PubMed] [Google Scholar]
- 15.Jin LJ, Lamster IB, Greenspan JS, Pitts NB, Scully C, Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 2016;22(7):609–619. doi: 10.1111/odi.12428. [DOI] [PubMed] [Google Scholar]
- 16.Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394(10194):249–260. doi: 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 17.Watt RG, Daly B, Allison P, et al. Ending the neglect of global oral health: time for radical action. Lancet. 2019;394(10194):261–272. doi: 10.1016/S0140-6736(19)31133-X. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TM, Tonmukayakul U, Calache H. Evaluation of an intervention to promote minimally invasive dentistry (MID) in an Australian community dental agency—a pilot study. Int J Dent Hyg. 2021;00:1–8. doi: 10.1111/idh.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McPhee C, Nguyen T, Trezona A, Tonmukayakul U, Calache H. Understanding the barriers and enablers to Minimal Intervention Dentistry in an Australian community dental agency. ANZJDOHT. 2021;9(1):8–15. [Google Scholar]
- 20.Calache H, Hopcraft MS, Martin JM. Minimum intervention dentistry–a new horizon in public oral health care. Aust Dent J. 2013;58(Suppl 1):17–25. doi: 10.1111/adj.12046. [DOI] [PubMed] [Google Scholar]
- 21.Innes NPT, Schwendicke F. Restorative thresholds for carious lesions: systematic review and meta-analysis. J Dent Res. 2017;96(5):501–508. doi: 10.1177/0022034517693605. [DOI] [PubMed] [Google Scholar]
- 22.Vernazza CR, Birch S, Pitts NB. Reorienting oral health services to prevention: economic perspectives. J Dent Res. 2021;100(6):576–582. doi: 10.1177/0022034520986794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariño RJ, Khan AR, Morgan M. Systematic review of publications on economic evaluations of caries prevention programs. Caries Res. 2013;47(4):265–272. doi: 10.1159/000346917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson T, Blomma C, Bågesund M, et al. Cost-effectiveness of caries preventive interventions—a systematic review. Acta Odontol Scand. 2021;79(4):309–320. doi: 10.1080/00016357.2020.1862293. [DOI] [PubMed] [Google Scholar]
- 25.Anopa Y, Macpherson L, McIntosh E. Systematic review of economic evaluations of primary caries prevention in 2- to 5-year-old preschool children. Value Health. 2020;23(8):1109–1118. doi: 10.1016/j.jval.2020.04.1823. [DOI] [PubMed] [Google Scholar]
- 26.Murthy AK, Fareed N. Economic evaluation of school-based caries preventive programs: a systematic review. Community Dent Health. 2020;37(3):205–215. doi: 10.1922/CDH_00010Murthy11. [DOI] [PubMed] [Google Scholar]
- 27.Fraihat N, Madae'en S, Bencze Z, Herczeg A, Varga O. Clinical effectiveness and cost-effectiveness of oral-health promotion in dental caries prevention among children: systematic review and meta-analysis. Int J Environ Res Public Health. 2019;16(15):2668. doi: 10.3390/ijerph16152668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonmukayakul U, Sia KL, Gold L, Hegde S, de Silva AM, Moodie M. Economic models of preventive dentistry for australian children and adolescents: a systematic review. Oral Health Prev Dent. 2015;13(6):481–494. doi: 10.3290/j.ohpd.a35005. [DOI] [PubMed] [Google Scholar]
- 29.Mariño R, Ravisankar G, Zaror C. Quality appraisal of economic evaluations done on oral health preventive programs—a systematic review. J Public Health Dent. 2020;80(3):194–207. doi: 10.1111/jphd.12368. [DOI] [PubMed] [Google Scholar]
- 30.Thurston SJ, Craig D, Wilson P, Drummond MF. Increasing decision-makers' access to economic evaluations: alternative methods of communicating the information. Int J Technol Assess Health Care. 2008;24(2):151–157. doi: 10.1017/S0266462308080215. [DOI] [PubMed] [Google Scholar]
- 31.Centre for Reviews and Dissemination. Systematic Reviews. CRD’s Guidance for Undertaking Reviews in Health Care. York (UK): York Publishing Services; 2008.
- 32.Institute of Medicine (US) Committee on Prevention of Mental Disorders. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. Mrazek PJ, Haggerty RJ, (eds). Washington (DC): National Academies Press (US); 1994. [PubMed]
- 33.Le LK, Esturas AC, Mihalopoulos C, et al. Cost-effectiveness evidence of mental health prevention and promotion interventions: a systematic review of economic evaluations. PLoS Med. 2021;18(5):e1003606. doi: 10.1371/journal.pmed.1003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the economic evaluation of health care programmes. 4. Oxford: Oxford University Press; 2015. [Google Scholar]
- 35.Cohen DJ, Reynolds MR. Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol. 2008;16;52(25):2119–26. 10.1016/j.jacc.2008.09.018. [DOI] [PMC free article] [PubMed]
- 36.Rogers HJ, Rodd HD, Vermaire JH, et al. A systematic review of the quality and scope of economic evaluations in child oral health research. BMC Oral Health. 2019;19(1):132. doi: 10.1186/s12903-019-0825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tonmukayakul U, Calache H, Clark R, Wasiak J, Faggion CM., Jr Systematic review and quality appraisal of economic evaluation publications in dentistry. J Dent Res. 2015;94(10):1348–1354. doi: 10.1177/0022034515589958. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Perez JG. Developing a scoring system to quality assess economic evaluations. Eur J Health Econ. 2002;3(2):131–136. doi: 10.1007/s10198-002-0100-2. [DOI] [PubMed] [Google Scholar]
- 39.Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Chestnutt IG, Hutchings S, Playle R, et al. Seal or Varnish? A randomised controlled trial to determine the relative cost and effectiveness of pit and fissure sealant and fluoride varnish in preventing dental decay. Health Technol Assess. 2017;21(21):1–256. doi: 10.3310/hta21210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khouja T, Smith KJ. Cost-effectiveness analysis of two caries prevention methods in the first permanent molar in children. J Public Health Dent. 2018;78(2):118–126. doi: 10.1111/jphd.12246. [DOI] [PubMed] [Google Scholar]
- 42.Goldman A, Leal SC, de Amorim RG, Frencken JE. Treating high-caries risk occlusal surfaces in first permanent molars through sealants and supervised toothbrushing: a 3-year cost-effective analysis. Caries Res. 2017;51(5):489–499. doi: 10.1159/000477822. [DOI] [PubMed] [Google Scholar]
- 43.Quiñonez RB, Stearns SC, Talekar BS, Rozier RG, Downs SM. Simulating cost-effectiveness of fluoride varnish during well-child visits for Medicaid-enrolled children. Arch Pediatr Adolesc Med. 2006;160(2):164–170. doi: 10.1001/archpedi.160.2.164. [DOI] [PubMed] [Google Scholar]
- 44.Palacio Rodriguez RA. Caries prevention in Chile : an epidemiological, econometric, and economic evaluation. PhD thesis, Newcastle University, Newcastle (2017). http://hdl.handle.net/10443/3957. Accessed 24 Apr 2022.
- 45.Ouyang W. Cost-effectiveness analysis of dental sealant using econometric modeling. Ph.D. thesis, University of Minnesota, Minneapolis (2009). https://hdl.handle.net/11299/52377. Accessed 24 Apr 2022.
- 46.Bhuridej P. Treatment outcomes of sealants on first permanent molars: Natural history, survivorship, and cost-utility analysis. PhD thesis, University of Iowa, Iowa City (2009). 2003;(3097519):188. Available from ProQuest One Academic. (305337400). http://ezproxy.deakin.edu.au/login?url=). https://www.proquest.com/dissertations-theses/treatment-outcomes-sealants-on-first-permanent/docview/305337400/se-2?accountid=10445. Accessed 24 Apr 2022.
- 47.Chi DL, van der Goes DN, Ney JP. Cost-effectiveness of pit-and-fissure sealants on primary molars in Medicaid-enrolled children. Am J Public Health. 2014;104(3):555–561. doi: 10.2105/AJPH.2013.301588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergström EK, Davidson T, Moberg SU. Cost-effectiveness through the dental-health FRAMM guideline for caries prevention among 12- to 15-year-olds in Sweden. Caries Res. 2019;53(3):339–346. doi: 10.1159/000495360. [DOI] [PubMed] [Google Scholar]
- 49.Schwendicke F, Rossi JG, Göstemeyer G, et al. Cost-effectiveness of artificial intelligence for proximal caries detection. J Dent Res. 2021;100(4):369–376. doi: 10.1177/0022034520972335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwendicke F, Meyer-Lueckel H, Stolpe M, Dörfer CE, Paris S. Costs and effectiveness of treatment alternatives for proximal caries lesions. PLoS ONE. 2014;9(1):e86992. doi: 10.1371/journal.pone.0086992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marcenes W, Kassebaum NJ, Bernabé E, et al. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. 2013;92(7):592–597. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen TM, Tonmukayakul U, Warren E, Cartwright S, Liew D. A Markov cost-effective analysis of biannual fluoride varnish for preventing dental caries in permanent teeth over a 70-year time horizon. Health Promot J Austr. 2020;31(2):177–183. doi: 10.1002/hpja.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higashi MK, Veenstra DL, del Aguila M, Hujoel P. The cost-effectiveness of interleukin-1 genetic testing for periodontal disease. J Periodontol. 2002;73(12):1474–1484. doi: 10.1902/jop.2002.73.12.1474. [DOI] [PubMed] [Google Scholar]
- 54.Kay E, Owen L, Taylor M, Claxton L, Sheppard L. The use of cost-utility analysis for the evaluation of caries prevention: an exploratory case study of two community-based public health interventions in a high-risk population in the UK. Community Dent Health. 2018;35(1):30–36. doi: 10.1922/CDH_4115Owen07. [DOI] [PubMed] [Google Scholar]
- 55.Moore D, Poynton M, Broadbent JM, Thomson WM. The costs and benefits of water fluoridation in NZ. BMC Oral Health. 2017;17(1):134. doi: 10.1186/s12903-017-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ministry of Health. Health loss in New Zealand: a report from the New Zealand burden of diseases, injuries and risk factors study, 2006–2016. Wellington (NZ): Ministry of Health (2013).
- 57.Koh R, Pukallus M, Kularatna S, et al. Relative cost-effectiveness of home visits and telephone contacts in preventing early childhood caries. Community Dent Oral Epidemiol. 2015;43(6):560–568. doi: 10.1111/cdoe.12181. [DOI] [PubMed] [Google Scholar]
- 58.Clarkson JE, Pitts NB, Goulao B, et al. Risk-based, 6-monthly and 24-monthly dental check-ups for adults: the INTERVAL three-arm RCT. Health Technol Assess. 2020;24(60):1–138. doi: 10.3310/hta24600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Espinoza-Espinoza G, Corsini G, Rojas R, Mariño R, Zaror C. The cost-utility of school-based first permanent molar sealants programs: a Markov model. BMC Oral Health. 2019;19(1):293. doi: 10.1186/s12903-019-0990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hietasalo P, Seppä L, Lahti S, et al. Cost-effectiveness of an experimental caries-control regimen in a 3.4-yr randomized clinical trial among 11-12-yr-old Finnish schoolchildren. Eur J Oral Sci. 2009;117(6):728–33. 10.1111/j.1600-0722.2009.00687.x. [DOI] [PubMed]
- 61.Zaror C, Muñoz-Millán P, Espinoza-Espinoza G, Vergara-González C, Martínez-Zapata MJ. Cost-effectiveness of adding fluoride varnish to a preventive protocol for early childhood caries in rural children with no access to fluoridated drinking water. J Dent. 2020;98:103374. doi: 10.1016/j.jdent.2020.103374. [DOI] [PubMed] [Google Scholar]
- 62.Schwendicke F, Splieth CH, Thomson WM, Reda S, Stolpe M, Foster PL. Cost-effectiveness of caries-preventive fluoride varnish applications in clinic settings among patients of low, moderate and high risk. Community Dent Oral Epidemiol. 2018;46(1):8–16. doi: 10.1111/cdoe.12320. [DOI] [PubMed] [Google Scholar]
- 63.Warren E, Curtis BH, Jia N, Evans RW. The caries management system: updating cost-effectiveness with 4-year posttrial data. Int J Technol Assess Health Care. 2016;32(3):107–115. doi: 10.1017/S0266462316000246. [DOI] [PubMed] [Google Scholar]
- 64.Curtis B, Warren E, Pollicino C, Evans RW, Schwarz E, Sbaraini A. The Monitor Practice Programme: is non-invasive management of dental caries in private practice cost-effective? Aust Dent J. 2011;56(1):48–55. doi: 10.1111/j.1834-7819.2010.01286.x. [DOI] [PubMed] [Google Scholar]
- 65.Warren E, Pollicino C, Curtis B, Evans W, Sbaraini A, Schwarz E. Modeling the long-term cost-effectiveness of the caries management system in an Australian population. Value Health. 2010;13(6):750–760. doi: 10.1111/j.1524-4733.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 66.Anderson M. Current concepts of dental caries and its prevention. Oper Dent. 2001;26(Suppl 6):11–18. [Google Scholar]
- 67.Ciketic S, Hayatbakhsh MR, Doran CM. Drinking water fluoridation in South East Queensland: a cost-effectiveness evaluation. Health Promot J Austr. 2010;21(1):51–56. doi: 10.1071/he10051. [DOI] [PubMed] [Google Scholar]
- 68.Cobiac LJ, Vos T. Cost-effectiveness of extending the coverage of water supply fluoridation for the prevention of dental caries in Australia. Community Dent Oral Epidemiol. 2012;40(4):369–376. doi: 10.1111/j.1600-0528.2012.00684.x. [DOI] [PubMed] [Google Scholar]
- 69.Griffin S, Naavaal S, Scherrer C, Griffin PM, Harris K, Chattopadhyay S. School-based dental sealant programs prevent cavities and are cost-effective. Health Aff (Millwood). 2016;35(12):2233–2240. doi: 10.1377/hlthaff.2016.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campain AC, Mariño RJ, Wright FA, Harrison D, Bailey DL, Morgan MV. The impact of changing dental needs on cost savings from fluoridation. Aust Dent J. 2010;55(1):37–44. doi: 10.1111/j.1834-7819.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- 71.Cronin J, Moore S, Harding M, Whelton H, Woods N. A cost-effectiveness analysis of community water fluoridation for schoolchildren. BMC Oral Health. 2021;21(1):158. doi: 10.1186/s12903-021-01490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eskandarizadeh A, Jalilian H, Vali L, Nekoie-Moghadam M, Barouni M, Malek-Mohammadi T. Cost-savings of community water fluoridation program; Kerman, Iran, 2016. JOHOE. 2017;6(2):85–91. [Google Scholar]
- 73.Fyfe C, Borman B, Scott G, Birks S. A cost effectiveness analysis of community water fluoridation in New Zealand. N Z Med J. 2015;128(1427):38–46. [PubMed] [Google Scholar]
- 74.Griffin SO, Jones K, Tomar SL. An economic evaluation of community water fluoridation. J Public Health Dent. 2001;61(2):78–86. doi: 10.1111/j.1752-7325.2001.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 75.Kroon J, Van Wyk PJ. A retrospective view on the viability of water fluoridation in South Africa to prevent dental caries. Community Dent Oral Epidemiol. 2012;40(5):441–450. doi: 10.1111/j.1600-0528.2012.00681.x. [DOI] [PubMed] [Google Scholar]
- 76.O'Connell J, Rockell J, Ouellet J, Tomar SL, Maas W. Costs and savings associated with community water fluoridation in the United States. Health Aff (Millwood). 2016;35(12):2224–2232. doi: 10.1377/hlthaff.2016.0881. [DOI] [PubMed] [Google Scholar]
- 77.O'Connell JM, Brunson D, Anselmo T, Sullivan PW. Costs and savings associated with community water fluoridation programs in Colorado. Prev Chronic Dis. 2005;2 Spec no (Spec No):A06. [PMC free article] [PubMed]
- 78.van Wyk PJ, Kroon J, Holtshousen WS. Cost evaluation for the implementation of water fluoridation in Gauteng. SADJ. 2001;56(2):71–76. [PubMed] [Google Scholar]
- 79.Wright JC, Bates MN, Cutress T, Lee M. The cost-effectiveness of fluoridating water supplies in New Zealand. Aust N Z J Public Health. 2001;25(2):170–178. doi: 10.1111/j.1753-6405.2001.tb01841.x. [DOI] [PubMed] [Google Scholar]
- 80.Mariño RJ, Fajardo J, Arana A, Garcia C, Pachas F. Modeling an economic evaluation of a salt fluoridation program in Peru. J Public Health Dent. 2011;71(2):125–130. doi: 10.1111/j.1752-7325.2010.00209.x. [DOI] [PubMed] [Google Scholar]
- 81.Mariño R, Traub F, Lekfuangfu P, Niyomsilp K. Cost-effectiveness analysis of a school-based dental caries prevention program using fluoridated milk in Bangkok, Thailand. BMC Oral Health. 2018;18(1):24. doi: 10.1186/s12903-018-0485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mariño R, Villa A, Guerrero S. A community trial of fluoridated powdered milk in Chile. Community Dent Oral Epidemiol. 2001;29(6):435–442. doi: 10.1034/j.1600-0528.2001.290604.x. [DOI] [PubMed] [Google Scholar]
- 83.Yee R, McDonald N, Walker D. A cost-benefit analysis of an advocacy project to fluoridate toothpastes in Nepal. Community Dent Health. 2004;21(4):265–270. [PubMed] [Google Scholar]
- 84.Kowash MB, Toumba KJ, Curzon ME. Cost-effectiveness of a long-term dental health education program for the prevention of early childhood caries. Eur Arch Paediatr Dent. 2006;7(3):130–135. doi: 10.1007/BF03262553. [DOI] [PubMed] [Google Scholar]
- 85.Mariño R, Fajardo J, Morgan M. Cost-effectiveness models for dental caries prevention programmes among Chilean schoolchildren. Community Dent Health. 2012;29(4):302–08. 10.1922/CDH_2893Mariño07. [PubMed]
- 86.Jevdjevic M, Trescher AL, Rovers M, Listl S. The caries-related cost and effects of a tax on sugar-sweetened beverages. Public Health. 2019;169:125–132. doi: 10.1016/j.puhe.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 87.Sowa PM, Keller E, Stormon N, Lalloo R, Ford PJ. The impact of a sugar-sweetened beverages tax on oral health and costs of dental care in Australia. Eur J Public Health. 2019;29(1):173–177. doi: 10.1093/eurpub/cky087. [DOI] [PubMed] [Google Scholar]
- 88.Basu S, Jacobs LM, Epel E, Schillinger D, Schmidt L. Cost-effectiveness of a workplace ban on sugar-sweetened beverage sales: a microsimulation model. Health Aff (Millwood). 2020;39(7):1140–1148. doi: 10.1377/hlthaff.2019.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jevdjevic M, Wijn SRW, Trescher AL, Nair R, Rovers M, Listl S. Front-of-package food labeling to reduce caries: economic evaluation. J Dent Res. 2021;100(5):472–478. doi: 10.1177/0022034520979147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimmer S, Spyra A, Kreimendahl F, Blaich C, Rychlik R. Elevating the use of sugar-free chewing gum in Germany: cost saving and caries prevention. Acta Odontol Scand. 2018;76(6):407–414. doi: 10.1080/00016357.2018.1487994. [DOI] [PubMed] [Google Scholar]
- 91.Zavras AI, Edelstein BL, Vamvakidis A. Health care savings from microbiological caries risk screening of toddlers: a cost estimation model. J Public Health Dent. 2000;60(3):182–188. doi: 10.1111/j.1752-7325.2000.tb03325.x. [DOI] [PubMed] [Google Scholar]
- 92.Mariño RJ, Fajardo J, Calache H, Morgan M. Cost-minimization analysis of a tailored oral health intervention designed for immigrant older adults. Geriatr Gerontol Int. 2014;14(2):336–340. doi: 10.1111/ggi.12103. [DOI] [PubMed] [Google Scholar]
- 93.Pukallus M, Plonka K, Kularatna S, et al. Cost-effectiveness of a telephone-delivered education programme to prevent early childhood caries in a disadvantaged area: a cohort study. BMJ Open. 2013;3(5):e002579. doi: 10.1136/bmjopen-2013-002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nguyen TM, Hsueh YS, Morgan MV, Mariño RJ, Koshy S. Economic evaluation of a pilot school-based dental checkup program. JDR Clin Trans Res. 2017;2(3):214–222. doi: 10.1177/2380084417708549. [DOI] [PubMed] [Google Scholar]
- 95.Davies GM, Worthington HV, Ellwood RP, et al. An assessment of the cost effectiveness of a postal toothpaste programme to prevent caries among five-year-old children in the North West of England. Community Dent Health. 2003;20(4):207–210. [PubMed] [Google Scholar]
- 96.Norrie O, Pharand L. Cost effectiveness of a fluoride varnish daycare program versus usual care in central Winnipeg, Canada. Can J Dent Hyg. 2020;54(2):68–74. [PMC free article] [PubMed] [Google Scholar]
- 97.Sakuma S, Yoshihara A, Miyazaki H, Kobayashi S. Economic evaluation of a school-based combined program with a targeted pit and fissure sealant and fluoride mouth rinse in Japan. Open Dent J. 2010;4:230–236. doi: 10.2174/1874210601004010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Griffin SO, Griffin PM, Gooch BF, Barker LK. Comparing the costs of three sealant delivery strategies. J Dent Res. 2002;81(9):641–645. doi: 10.1177/154405910208100913. [DOI] [PubMed] [Google Scholar]
- 99.Crowley SJ, Campain AC, Morgan MV. An economic evaluation of a publicly funded dental prevention programme in regional and rural Victoria: an extrapolated analysis. Community Dent Health. 2000;17(3):145–151. [PubMed] [Google Scholar]
- 100.Bertrand E, Mallis M, Bui NM, Reinharz D. Cost-effectiveness simulation of a universal publicly funded sealants application program. J Public Health Dent. 2011;71(1):38–45. doi: 10.1111/j.1752-7325.2010.00200.x. [DOI] [PubMed] [Google Scholar]
- 101.Quiñonez RB, Downs SM, Shugars D, Christensen J, Vann WF., Jr Assessing cost-effectiveness of sealant placement in children. J Public Health Dent. 2005;65(2):82–89. doi: 10.1111/j.1752-7325.2005.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 102.Ramsay CR, Clarkson JE, Duncan A, et al. Improving the Quality of Dentistry (IQuaD): a cluster factorial randomised controlled trial comparing the effectiveness and cost-benefit of oral hygiene advice and/or periodontal instrumentation with routine care for the prevention and management of periodontal disease in dentate adults attending dental primary care. Health Technol Assess. 2018;22(38):1–144. doi: 10.3310/hta22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arrow P. Cost minimisation analysis of two occlusal caries preventive programmes. Community Dent Health. 2000;17(2):85–91. [PubMed] [Google Scholar]
- 104.Maguire A, Clarkson JE, Douglas GV, et al. Best-practice prevention alone or with conventional or biological caries management for 3- to 7-year-olds: the FiCTION three-arm RCT. Health Technol Assess. 2020;24(1):1–174. doi: 10.3310/hta24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vermaire JH, van Loveren C, Brouwer WB, Krol M. Value for money: economic evaluation of two different caries prevention programmes compared with standard care in a randomized controlled trial. Caries Res. 2014;48(3):244–253. doi: 10.1159/000356859. [DOI] [PubMed] [Google Scholar]
- 106.Anderson M, Davidson T, Dahllöf G, Grindefjord M. Economic evaluation of an expanded caries-preventive program targeting toddlers in high-risk areas in Sweden. Acta Odontol Scand. 2019;77(4):303–309. doi: 10.1080/00016357.2018.1548709. [DOI] [PubMed] [Google Scholar]
- 107.Jönsson B, Ohrn K, Oscarson N, Lindberg P. The effectiveness of an individually tailored oral health educational programme on oral hygiene behaviour in patients with periodontal disease: a blinded randomized-controlled clinical trial (one-year follow-up) J Clin Periodontol. 2009;36(12):1025–1034. doi: 10.1111/j.1600-051X.2009.01453.x. [DOI] [PubMed] [Google Scholar]
- 108.Oscarson N, Källestål C, Fjelddahl A, Lindholm L. Cost-effectiveness of different caries preventive measures in a high-risk population of Swedish adolescents. Community Dent Oral Epidemiol. 2003;31(3):169–178. doi: 10.1034/j.1600-0528.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 109.Pennington M, Heasman P, Gaunt F, et al. The cost-effectiveness of supportive periodontal care: a global perspective. J Clin Periodontol. 2011;38(6):553–561. doi: 10.1111/j.1600-051X.2011.01722.x. [DOI] [PubMed] [Google Scholar]
- 110.Fardal Ø, Grytten J. Applying quality assurance in real time to compliant long-term periodontal maintenance patients utilizing cost-effectiveness and cost utility. J Clin Periodontol. 2014;41(6):604–611. doi: 10.1111/jcpe.12252. [DOI] [PubMed] [Google Scholar]
- 111.Schwendicke F, Stolpe M, Plaumann A, Graetz C. Cost-effectiveness of regular versus irregular supportive periodontal therapy or tooth removal. J Clin Periodontol. 2016;43(11):940–947. doi: 10.1111/jcpe.12595. [DOI] [PubMed] [Google Scholar]
- 112.Davenport C, Elley K, Salas C, et al. The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation. Health Technol Assess. 2003;7(7):iii-v, 1–127. 10.3310/hta7070 [DOI] [PubMed]
- 113.Tickle M, O'Neill C, Donaldson M, et al. A randomised controlled trial to measure the effects and costs of a dental caries prevention regime for young children attending primary care dental services: the Northern Ireland Caries Prevention In Practice (NIC-PIP) trial. Health Technol Assess. 2016;20(71):1–96. doi: 10.3310/hta20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fransson H, Davidson T, Rohlin M; Foresight Research Consortium, Christell H. There is a paucity of economic evaluations of prediction methods of caries and periodontitis—a systematic review. Clin Exp Dent Res. 2021;7(3):385–98. 10.1002/cre2.405. [DOI] [PMC free article] [PubMed]
- 115.Hettiarachchi RM, Kularatna S, Downes MJ, et al. The cost-effectiveness of oral health interventions: a systematic review of cost-utility analyses. Community Dent Oral Epidemiol. 2018;46(2):118–124. doi: 10.1111/cdoe.12336. [DOI] [PubMed] [Google Scholar]
- 116.Tay JRH, Ng E, Nair R, Tan ZS, Tan SHX. Economic evaluations in the treatment and evaluation of patients with periodontal disease: a critical review. J Clin Periodontol. 2021;48(5):679–694. doi: 10.1111/jcpe.13456. [DOI] [PubMed] [Google Scholar]
- 117.Nguyen TM, Morgan MV, Koshy S, Mathew S, Lew S. Revisiting the value of school dental check-up programs. ANZJDOHT. 2015;2:6–9. [Google Scholar]
- 118.Lewis JM. Improving dental health status indicators for evaluation. Community Dent Oral Epidemiol. 1996;24(1):32–36. doi: 10.1111/j.1600-0528.1996.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 119.Hettiarachchi RM, Kularatna S, Byrnes J, Scuffham PA. Pediatric quality of life instruments in oral health research: a systematic review. Value Health. 2019;22(1):129–135. doi: 10.1016/j.jval.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 120.Pequeno NPF, Cabral NLA, Marchioni DM, Lima SCVC, Lyra CO. Quality of life assessment instruments for adults: a systematic review of population-based studies. Health Qual Life Outcomes. 2020;18(1):208. doi: 10.1186/s12955-020-01347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schwendicke F, Thomson WM, Broadbent JM, Stolpe M. Effects of taxing sugar-sweetened beverages on caries and treatment costs. J Dent Res. 2016;95(12):1327–1332. doi: 10.1177/0022034516660278. [DOI] [PubMed] [Google Scholar]
- 122.Briggs ADM, Mytton OT, Kehlbacher A, et al. Health impact assessment of the UK soft drinks industry levy: a comparative risk assessment modelling study. Lancet Public Health. 2016;2(1):e15–e22. doi: 10.1016/S2468-2667(16)30037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stearns SC, Rozier RG, Kranz AM, Pahel BT, Quiñonez RB. Cost-effectiveness of preventive oral health care in medical offices for young Medicaid enrollees. Arch Pediatr Adolesc Med. 2012;166(10):945–951. doi: 10.1001/archpediatrics.2012.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scherrer CR, Naavaal S. Cost-savings of fluoride varnish application in primary care for Medicaid-enrolled children in Virginia. J Pediatr. 2019;212:201–207.e1. doi: 10.1016/j.jpeds.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 125.Kastenbom L, Falsen A, Larsson P, et al. Costs and health-related quality of life in relation to caries. BMC Oral Health. 2019;19:187. doi: 10.1186/s12903-019-0874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Mak. 2012;32(5):733–743. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.