Abstract
Background
In the present study, we examined the association of immunosuppressant drug prescriptions with the growth of small abdominal aortic aneurysms (AAAs).
Methods
Participants with an AAA measuring between 30 and 50 mm were recruited from four Australian centers. AAA growth was monitored by ultrasound. The immunosuppressant drugs included conventional disease-modifying antirheumatic drugs (eg, methotrexate, sulfasalazine, leflunomide), steroids, hydroxychloroquine, other immunosuppressant drugs (eg, cyclosporine, azacitidine), or a combination of these drugs. Linear mixed effects modeling was performed to examine the independent association of an immunosuppressant prescription with AAA growth. A subanalysis examined the association of steroids with AAA growth.
Results
Of the 621 patients, 34 (5.3%) had been prescribed at least one (n = 26) or more (n = 8) immunosuppressant drug and had been followed up for a median period of 2.1 years (interquartile range, 1.1-3.5 years), with a median of three ultrasound scans (interquartile range, two to five ultrasound scans). No significant difference was found in AAA growth when stratified by a prescription of immunosuppressant drugs on either unadjusted (mean difference, 0.2 mm/y; 95% confidence interval [CI], −0.4 to 0.7; P = .589) or risk factor-adjusted (mean difference, 0.2 mm/y; 95% CI, −0.3 to 0.7; P = .369) analyses. The findings were similar for the unadjusted (mean difference, 0.0 mm/y; 95% CI, −0.7 to 0.7; P = .980) and risk factor-adjusted (mean difference, 0.1 mm/y; 95% CI, −0.6 to 0.7; P = .886) subanalyses focused on steroid use.
Conclusions
The results from this study suggest that AAA growth is not affected by immunosuppressant drug prescription. Studies with larger sample sizes are needed before reliable conclusions can be drawn.
Keywords: AAA growth, Abdominal aortic aneurysm, Immunosuppression, Linear mixed effects modeling
Clinical relevance
At present, it is unclear whether immunosuppression promotes or inhibits abdominal aortic aneurysm (AAA) progression. This has important implications for the management of AAA. With the increasing range of indications for which immunosuppressant medications are being prescribed, it is likely that more patients with AAAs will be prescribed these drugs for the treatment of comorbid diseases, such as rheumatoid arthritis, other autoimmune diseases, after organ transplantation, or coronary heart disease. The results from the present study suggest that patients prescribed a range of different immunosuppressant drugs for treating a variety of different inflammatory diseases did not experience a significantly different AAA growth compared with patients not receiving these medications. Our findings suggest that no strong case exists to either start or stop immunosuppressant drugs for AAA patients
Article Highlights.
-
•
Type of Research: A multicenter, prospective cohort study
-
•
Key Findings: In the present study of 621 participants with small abdominal aortic aneurysms, no association of immunosuppressant drug prescription with aneurysm growth was found.
-
•
Take Home Message: We found no strong case to either start or stop immunosuppressant drugs for patients with abdominal aortic aneurysms.
After positive findings from the CANTOS trial [cardiovascular risk reduction study (reduction in recurrent major cardiovascular disease events)], which found interleukin-1β blocking antibody significantly reduced the rate of recurrent cardiovascular events after myocardial infarction, interest in developing anti-inflammatory drugs for other cardiovascular diseases has been growing.1 At present, no drug therapy for abdominal aortic aneurysms (AAAs) is available.2 Inflammation has been strongly implicated in AAA pathogenesis, with human AAA biopsies demonstrating dense infiltration from a range of innate and adaptive immune cells and cytokines.3 The results from animal studies have suggested that inhibiting elements of both the innate and the adaptive immune system will inhibit aortic expansion.4,5 In contrast to these findings, however, two small human observational studies have reported that patients receiving immunosuppression had experienced increased AAA progression.6,7 In one of these studies, a patient with a small AAA who had started immunosuppression therapy was reported to have experienced rapid AAA growth and rupture, suggesting important safety concerns with this treatment.6
Whether immunosuppression promotes or inhibits AAA progression has important implications for the management of AAAs. With the increasing range of indications for which immunosuppressant medications are being prescribed, it is likely that more patients with AAAs will be prescribed these drugs for the treatment of comorbid diseases, such as rheumatoid arthritis, other autoimmune diseases, after organ transplantation, or coronary heart disease. In the present study, we examined the association of the prescription of immunosuppressant drugs with the growth of small AAAs.
Methods
Study design and participants
Data from an ongoing prospective cohort study designed to identify risk factors for the outcome of vascular disease were used to retrospectively examine the association of the prescription of immunosuppressant drugs with AAA growth. For inclusion in the present study, the patients were required to have a diagnosis of a small AAA (maximum AAA diameter at recruitment, 30-50 mm) and have been followed up with a minimum of two ultrasound scans performed 6 months apart. The imaging data collected during routine AAA surveillance between 2003 and 2018 from four outpatient vascular services in Australia (Townsville University Hospital, Mater Hospital Townsville, Gosford Vascular Services, and Royal Brisbane and Women's Hospital) were used in the present study. The institutional ethics committee at each institution approved the present study (approval no. HREC/14/QTHS/203). All included patients provided written informed consent, and the study was performed in accordance with the principles of the Declaration of Helsinki. The collected data were stored in a centralized database accessible only to the approved personnel.
Prescription of immunosuppressive agents
The immunosuppressant agents administered either orally or parentally were recorded at recruitment. Immunosuppressant therapy was defined as the prescription of conventional disease-modifying antirheumatic drugs (eg, methotrexate, sulfasalazine, leflunomide), steroids, hydroxychloroquine, other immunosuppressant drugs (eg, cyclosporine and azacitidine), or a combination of these drugs.8
Risk factors and medications
The patients eligible for inclusion underwent a clinical interview and physical examination, during which the risk factors and medication history were collected. The risk factors collected at recruitment included age, sex, history of hypertension, stroke, diabetes, ischemic heart disease (IHD), and smoking. Hypertension, diabetes, and stroke were defined as either a prior diagnosis or receipt of treatment.9, 10, 11 IHD was defined as a history of myocardial infarction, angina, or treatment of IHD.9, 10, 11 Smoking was defined as a former smoker (no smoking within the previous month), current smoker (smoking in the previous month) or never smoker (never smoked regularly).9, 10, 11 The medication history collected at recruitment included prescriptions of aspirin, metformin, and statins.
AAA imaging
Experienced sonographers who were unaware of the medication history of the participants measured the maximum infrarenal aortic diameters in the anteroposterior and transverse orthogonal planes, as previously described.12, 13, 14 The aortic diameter was measured from the outer to outer walls of the infrarenal aorta. The reproducibility of the aortic diameter measurements was assessed at each vascular laboratory, with an interobserver reproducibility coefficient <4 mm, as previously reported.12, 13, 14, 15
Statistical analysis
Continuous data were tested for a normal distribution using the Shapiro-Wilk test and are reported as the mean ± standard deviation or median and interquartile range (IQR), depending on the distribution. Differences in the normally distributed data between groups were tested using the t test. Non-normally distributed data were examined using the Mann-Whitney U test or Wilcoxon test. Categorical data are reported as percentages. Differences in the nominal variables were tested using the χ2 test.
The association between a prescription of immunosuppressant drugs and AAA growth was analyzed using linear mixed effects (LME) modeling. A subanalysis was performed to examine the association between a steroid prescription and AAA growth. A maximum of 6 years of follow-up data were included from each participant, and the LME models were adjusted for recognized risk factors and those covariates that were unequally distributed between groups at entry based on P values < .10. Further details about the analysis methods are provided in the Supplementary Methods.
Results
Participants
Of the 621 participants, 34 (5.3%) had been prescribed at least one (n = 26) or more (n = 8) immunosuppressant drugs (Table I). The immunosuppressant drugs included steroids (prednisolone; n = 22), conventional disease-modifying antirheumatic drugs (methotrexate, n = 7; sulfasalazine, n = 1; hydroxychloroquine, n = 1; leflunomide, n = 2), and other immunosuppressant drugs (colchicine, n = 7; cyclosporine, n = 1; azacitidine, n = 1). These drugs had been prescribed for the treatment of a variety of comorbidities, including rheumatoid arthritis (n = 15), chronic obstructive airway disease (n = 7), gout (n = 7), polymyalgia rheumatica (n = 3), Sjögren syndrome (n = 1), and myeloid leukemia (n = 1).
Table I.
Risk factors for included participants
| Variables at baseline | Participants prescribed immunosuppressant therapy |
P value | |
|---|---|---|---|
| Yes (n = 34) | No (n = 587) | ||
| Age, years | 73.6 (69.3-80.9) | 74.7 (69.6-79.8) | .894 |
| Initial AAA diameter | 40.0 (36.5-43.1) | 39.0 (35.0-43.0) | .669 |
| Male sex | 31 (91.2) | 479 (81.6) | .235 |
| Smoking history | .464 | ||
| Never smoker | 3 (8.8) | 63 (10.7) | |
| Ex-smoker | 25 (73.5) | 371 (63.2) | |
| Current smoker | 6 (17.7) | 153 (26.1) | |
| Comorbidity | |||
| Hypertension | 21 (61.8) | 441 (75.1) | .082 |
| Diabetes mellitus | 10 (29.4) | 133 (22.7) | .484 |
| History of stroke | 5 (14.7) | 59 (10.0) | .571 |
| Ischemic heart disease | 13 (38.2) | 285 (48.6) | .320 |
| Preoperative medication | |||
| Aspirin | 15 (44.1) | 371 (63.2) | .040 |
| Statins | 21 (61.8) | 401 (67.2) | .544 |
| Metformin | 5 (14.7) | 72 (12.3) | .879 |
AAA, Abdominal aortic aneurysm.
Data presented as median (interquartile range) for continuous variables, which were compared using the Mann-Whitney U test, or number (%) for nominal variables, which were compared using the χ2 test.
Most risk factors, including age, sex, smoking, initial AAA diameter, hypertension, stroke, diabetes, IHD, and prescriptions of statins and metformin, were not significantly different statistically when stratified by a prescription of immunosuppressant drugs. The prescription of aspirin (P = .040) was significantly less common for those prescribed immunosuppressant drugs than for those not receiving these medications (Table I).
Association between immunosuppressant drug prescription and AAA growth
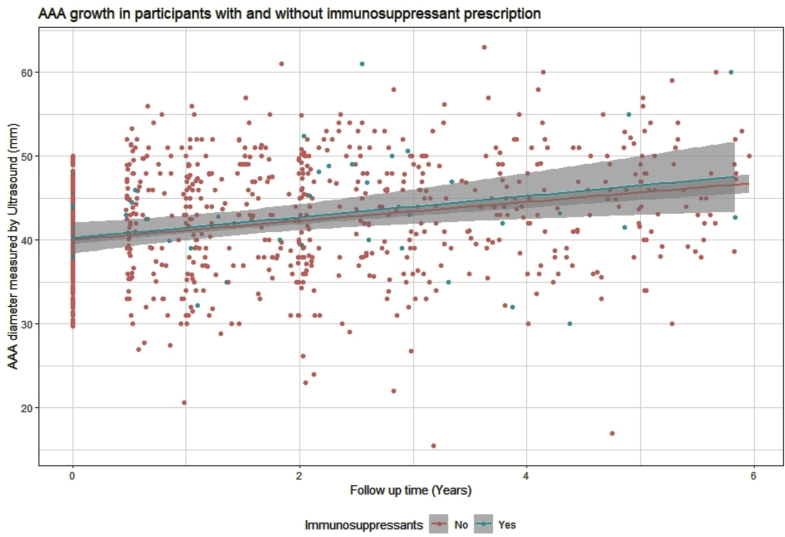
The patients had been followed up for a median of 2.1 years (IQR, 1.1-3.5 years) with a median of three ultrasound scans (IQR, two to five ultrasound scans). On unadjusted analysis, no significant differences were found in the annual AAA growth rate (mean difference, 0.2 mm/y; 95% confidence interval [CI], −0.4 to 0.7; P = .589) for those prescribed immunosuppressant drugs compared with those not receiving these medications (Table II; Fig). The findings were similar in an analysis adjusted for initial AAA size, current smoking, hypertension, and aspirin (mean difference, 0.2 mm/y; 95% CI, −0.3 to 0.7; P = .369; Table III). The quantile–quantile plot suggested the fitness of the model, because no outliers were noted in plotting the standardized residuals (Supplementary Fig). The predicted AAA diameter at the end of follow-up was 41.4 mm (95% CI, 38.4-43.7) for those prescribed one or more immunosuppressant drugs compared with 41.1 mm (95% CI, 39.0-43.3) for those not prescribed these medications. The most common immunosuppressant medication prescribed was steroids. A subanalysis excluding participants prescribed other immunosuppressant drugs found that AAA growth for those prescribed steroids was not significantly different from that for those not prescribed an immunosuppressant in both unadjusted (mean difference, 0.0 mm/y; 95% CI, −0.7 to 0.7; P = .980) and adjusted (mean difference, 0.1 mm/y; 95% CI, −0.6 to 0.7; P = .886) models (Table IV).
Table II.
Association of immunosuppressant drug prescription with abdominal aortic aneurysm (AAA) growth assessed by linear mixed effects models: summary of fixed effects
| Covariate | Estimate | 95% CI | P value |
|---|---|---|---|
| Immunosuppressant drug prescription | 0.2 | −2.0 to 2.3 | .890 |
| Follow-up | 1.4 | 1.3 to 1.5 | <.001 |
| Immunosuppressant drug prescription × follow-up | 0.2 | −0.4 to 0.7 | .589 |
CI, Confidence interval.
Fig.
Graph showing abdominal aortic aneurysm (AAA) growth stratified by prescription of immunosuppression drugs during 6 years of follow-up.
Table III.
Linear regression model assessing the association of immunosuppressant drug prescription with abdominal aortic aneurysm (AAA) growth after adjustment for other risk factorsa
| Predictor | Estimate | 95% CI | P value |
|---|---|---|---|
| Immunosuppressant drug prescription | −0.3 | −1.3 to 0.7 | .561 |
| Follow-up | 1.4 | 1.3 to 1.5 | <.001 |
| Initial AAA size | 1.0 | 1.0 to 1.1 | <.001 |
| HTN | −0.2 | −0.7 to 0.2 | .289 |
| Current smoking | −0.2 | −0.7 to 0.2 | .273 |
| Aspirin | 0.1 | −0.3 to 0.5 | .569 |
| Immunosuppressant prescription × follow-up | 0.2 | −0.3 to 0.7 | .369 |
CI, Confidence interval; HTN, hypertension.
Boldface P values represent statistical significance.
Random effects: residual variance, 10.82; number of included patients, 621; marginal correlation coefficient, 0.74; conditional correlation coefficient, 0.74.
Supplementary Fig.
Quantile–quantile (Q-Q) plot showing sample and theoretical quantiles.
Table IV.
Subanalysis assessing the association of steroid drug prescription with abdominal aortic aneurysm (AAA) growtha
| Model | Estimate | 95% CI | P value |
|---|---|---|---|
| Unadjusted | |||
| Covariate | |||
| Steroid prescription | −0.3 | −2.9 to 2.4 | .843 |
| Follow-up | 1.4 | 1.3 to 1.5 | <.001 |
| Steroid prescription × follow-up | 0.0 | −0.7 to 0.7 | .980 |
| Adjusted | |||
| Predictor | |||
| Steroid prescription | −0.1 | −1.4 to 1.2 | .875 |
| Follow-up | 1.4 | 1.3 to 1.5 | <.001 |
| Initial AAA size | 1.0 | 1.0 to 1.1 | <.001 |
| HTN | −0.3 | −0.7 to 0.2 | .243 |
| Current smoking | −0.2 | −0.64 to 0.2 | .305 |
| Aspirin | 0.2 | −0.2 to 0.6 | .407 |
| Steroid prescription × follow-up | 0.1 | −0.6 to 0.7 | .886 |
CI, Confidence interval; HTN, hypertension.
Boldface P values represent statistical significance.
Random effects: residual variance, 10.79; number of included patients, 609; marginal correlation coefficient, 0.74; conditional correlation coefficient, 0.74.
Discussion
The results from the present study suggest that patients prescribed a range of different immunosuppressant drugs for treating a variety of different inflammatory diseases do not experience significantly different AAA growth compared with patients not receiving these medications. These findings were consistent in the unadjusted and risk factor-adjusted analyses and a subanalysis of only those who had been prescribed steroids.
The findings from the present study are in contrast to a number of previous observational studies and case reports. A previous case study reported that a small AAA had rapidly expanded and ruptured after the patient had begun immunosuppression therapy required after a kidney transplant.6 The patient had undergone emergency AAA repair, and the samples obtained from the aorta suggested minimal aortic inflammation. Another patient, who had undergone simultaneous liver and kidney transplantation and was prescribed an immunosuppression regimen, was reported to have had AAA growth of >10 mm within 1 month and, therefore, underwent endovascular repair.16 In addition, a study of 18 patients with AAAs who had undergone a variety of organ transplantations, reported that 7 (23%) had experienced AAA rupture at a mean diameter of 6 cm.17 The mean AAA growth was reported to have increased from 4.6 mm/y before transplantation to 10.0 mm/y after transplantation (P = .08).17 Furthermore, a previous retrospective observational study of 176 Japanese patients reported that the prescription of oral corticosteroids was independently associated with a greater likelihood of rapid AAA growth (odds ratio, 4.12; 95% CI, 1.15-17.0; P = .029).7 Most of these case series had included patients who had undergone organ transplantation, which might be a unique situation owing to the abdominal surgery needed and the potency of the immunosuppression required. The Japanese study had a number of limitations, including a small sample size (only 15 patients had received corticosteroids), sampling bias (only patients who had undergone AAA repair were included), one immunosuppressant drug type was investigated, and limited follow-up data (only two scans were included from each participant).7 The present study included more than four times as many participants, including more than twice as many who had been prescribed immunosuppressant drugs. Another possible reason for the different findings from these two studies is the racial variation in the different populations studied. Also, the participants had received a variety of different immunosuppressant drugs in the present study, but the previous study had included only those who had been prescribed steroids. It is unlikely, however, that this difference could explain the contrasting findings because our subanalysis showed no association of steroids with AAA growth. The prior study also had a number of other differences compared with the present study, including using axial measurements from computed tomography images rather than orthogonal diameters from ultrasound to monitor AAA growth, using only two images to assess growth, and limiting the analyses to bivariate analyses comparing slow and rapid growth, rather than examining the full range of AAA growth using continuous LME analyses. Also, the present study was a larger and more detailed analysis of the association of immunosuppressant drugs with AAA growth. Our results suggest immunosuppressant drugs do not affect AAA growth. However, given the small number of patients prescribed immunosuppressant drugs, a moderate or small effect could not be ruled out. Patients who have undergone organ transplantation were not included in the current study. Based on the previous studies outlined above, these patients may require careful monitoring of any AAA.
The present study had a number of strengths and weaknesses. A major limitation was the small number of participants who had been prescribed immunosuppressant drugs, although, to the best of our knowledge, this is the largest study to examine the association of immunosuppressant therapy and AAA growth. Because this was a retrospective study, no sample size calculation was performed. In addition, the indications for immunosuppressant drug prescription varied among the included patients, which could have confounded the findings. Furthermore, the treatment period, drug adherence, and drug dosage were not collected. Therefore, it was impossible to completely exclude the presence of a residual bias. The observational design of our study also meant that any casual inferences must be interpreted very cautiously. It might not be ethically feasible to perform a randomized controlled trial of the use of immunosuppressant drugs for patients with small AAAs owing to safety concerns, such as an increased risk of infection. Thus, observational studies remain important for investigating the pathogenesis of AAAs. Larger observational studies of the association of distinct immunosuppressant drugs with AAA growth are ideally needed to more fully examine the effect of the immune response on AAA progression. Ideally, such studies would also consider the dose of medications administered. Given the relatively small population of patients with AAAs requiring long-term immunosuppression, these studies would be challenging to design.
Conclusions
The findings from this study suggest that patients prescribed immunosuppressant medications to treat comorbid conditions do not experience significantly different AAA growth compared with patients not receiving these medications. Therefore, at present, no strong case exists to stop or start immunosuppressant drugs for patients with a small AAA. However, studies with a larger sample size are warranted before reliable conclusions can be drawn.
Author Contributions
Conception and design: ST, JG
Analysis and interpretation: ST, JP, JG
Data collection: FQ, MB, BB, RV, JJ, JG
Writing the article: ST, JG
Critical revision of the article: ST, JP, FQ, MB, BB, RV, JJ, JG
Final approval of the article: ST, JP, FQ, MB, BB, RV, JJ, JG
Statistical analysis: ST, JG
Obtained funding: JG
Overall responsibility: JG
Footnotes
The present study was supported by the National Health and Medical Research Council (grants 1180736 and 1022752), the Queensland Government, and James Cook University. JG holds a Practitioner Fellowship (grant number 1117601) from the National Health and Medical Research Council and a Senior Clinical Research Fellowship from the Queensland Government and received a secondary prevention strategic grant from the Heart Foundation.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Supplementary Methods: Linear mixed effects data analysis
An unadjusted model of random intercept with slope was developed with individual patients as the random effects. The adjusted model included variables that were unequally distributed between groups based on P values < .10. Both the individual patients and the follow-up period were used as random effects in the final multivariate adjusted model. In both unadjusted and adjusted linear mixed effects models, an interaction of follow-up period and patient group was used as the test statistic. Because the initial abdominal aortic aneurysm (AAA) diameter varied between patients, the presence of any potential two-way or three-way interaction of the included covariates with follow-up and the initial AAA diameter was assessed. The initial AAA diameter was assessed as tertiles for the covariate interaction assessment to agree with the model linearity assumption.
The standardized residual distribution was plotted using normal quantile–quantile plots to identify the presence of any influential outliers and, thereby, confirm model fitness. Once the model fitness had been confirmed, the AAA diameter was predicted using the predict function by developing a new dataset containing all combinations of the included covariates within the model. This was followed by the development of a matrix using the “model.matrix” function to extract the diagonals. The diagonals were then used to derive the mean AAA diameter (95% confidence intervals) at the mean follow-up period.
Supplementary Table I.
Association of steroid drug prescription with abdominal aortic aneurysm (AAA) growth assessed by linear mixed effects models: summary of fixed effects
| Covariate | Estimate | 95% CI | P value |
|---|---|---|---|
| Steroid drug prescription | −0.3 | −2.9 to 2.4 | .843 |
| Follow-up | 1.4 | 1.3 to 1.5 | <.001 |
| Steroid drug prescription × follow-up | 0.0 | −0.7 to 0.7 | .980 |
CI, Confidence interval.
Bolded P values represent statistical significant findings.
Supplementary Table II.
Linear regression model assessing association of steroid drug prescription with abdominal aortic aneurysm (AAA) growth after adjustment for other risk factorsa
| Predictor | Estimate | 95% CI | P value |
|---|---|---|---|
| Steroid drug prescription | −0.1 | −1.4 to 1.2 | .875 |
| Follow-up | 1.4 | 1.3 to 1.5 | <.001 |
| Initial AAA size | 1.0 | 1.0 to 1.1 | <.001 |
| HTN | −0.3 | −0.7 to 0.2 | .243 |
| Current smoking | −0.2 | −0.6 to 0.2 | .305 |
| Aspirin | 0.2 | −0.2 to 0.6 | .407 |
| Steroid drug prescription × follow-up | 0.1 | −0.6 to 0.7 | .886 |
CI, Confidence interval; HTN, hypertension.
Boldface P values represent statistical significance.
Random effects: residual variance, 10.79; number of included patients, 609; marginal correlation coefficient, 0.74; conditional correlation coefficient, 0.74.
References
- 1.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 2.Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. 2019;16:225–242. doi: 10.1038/s41569-018-0114-9. [DOI] [PubMed] [Google Scholar]
- 3.Lindeman J.H.N., Abdul-Hussien H., van Bockel J.H., Wolterbeek R., Kleemann R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm. Circulation. 2009;119:2209–2216. doi: 10.1161/CIRCULATIONAHA.108.806505. [DOI] [PubMed] [Google Scholar]
- 4.Liu B., Kong J., An G., Zhang K., Qin W., Meng X. Regulatory T cells protected against abdominal aortic aneurysm by suppression of the COX-2 expression. J Cell Mol Med. 2019;23:6766–6774. doi: 10.1111/jcmm.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yodoi K., Yamashita T., Sasaki N., Kasahara K., Emoto T., Matsumoto T., et al. Foxp3+ regulatory T cells play a protective role in angiotensin II-induced aortic aneurysm formation in mice. Hypertension. 2015;65:889–895. doi: 10.1161/HYPERTENSIONAHA.114.04934. [DOI] [PubMed] [Google Scholar]
- 6.Lindeman J.H., Rabelink T.J., van Bockel J.H. Immunosuppression and the abdominal aortic aneurysm: Doctor Jekyll or Mister Hyde? Circulation. 2011;124:e463–e465. doi: 10.1161/CIRCULATIONAHA.110.008573. [DOI] [PubMed] [Google Scholar]
- 7.Tajima Y., Goto H., Ohara M., Hashimoto M., Akamatsu D., Shimizu T., et al. Oral steroid use and abdominal aortic aneurysm expansion – positive association. Circ J. 2017;81:1774–1782. doi: 10.1253/circj.CJ-16-0902. [DOI] [PubMed] [Google Scholar]
- 8.Thanigaimani S., Phie J., Krishna S., Moxon J., Golledge J. Effect of disease modifying anti-rheumatic drugs on major cardiovascular events: a meta-analysis of randomized controlled trials. Sci Rep. 2021;11:6627. doi: 10.1038/s41598-021-86128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golledge J., Jayalath R., Oliver L., Parr A., Schurgers L., Clancy P. Relationship between CT anthropometric measurements, adipokines and abdominal aortic calcification. Atherosclerosis. 2008;197:428–434. doi: 10.1016/j.atherosclerosis.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parr A., McCann M., Bradshaw B., Shahzad A., Buttner P., Golledge J. Thrombus volume is associated with cardiovascular events and aneurysm growth in patients who have abdominal aortic aneurysms. J Vasc Surg. 2011;53:28–35. doi: 10.1016/j.jvs.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parr A., Buttner P., Shahzad A., Golledge J. Relation of infra-renal abdominal aortic calcific deposits and cardiovascular events in patients with peripheral artery disease. Am J Cardiol. 2010;105:895–899. doi: 10.1016/j.amjcard.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 12.Golledge J., Karan M., Moran C.S., Muller J., Clancy P., Dear A.E., et al. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte–matrix interactions. Eur Heart J. 2008;29:665–672. doi: 10.1093/eurheartj/ehm557. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson C.D., Clancy P., Bourke B., Walker P.J., Dear A., Buckenham T., et al. Association of statin prescription with small abdominal aortic aneurysm progression. Am Heart J. 2010;159:307–313. doi: 10.1016/j.ahj.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golledge J., Moxon J., Pinchbeck J., Anderson G., Rowbotham S., Jenkins J., et al. Association between metformin prescription and growth rates of abdominal aortic aneurysms. Br J Surg. 2017;104:1486–1493. doi: 10.1002/bjs.10587. [DOI] [PubMed] [Google Scholar]
- 15.Norman P., Spencer C.A., Lawrence-Brown M.M., Jamrozik K. C-reactive protein levels and the expansion of screen-detected abdominal aortic aneurysms in men. Circulation. 2004;110:862–866. doi: 10.1161/01.CIR.0000138746.14425.00. [DOI] [PubMed] [Google Scholar]
- 16.Salimi J., Jafarian A., Behzadi M., Nejat A., Fakhar N. Endovascular abdominal aortic aneurysm repair in a patient with previous history of simultaneous orthotopic liver kidney transplantation. J Surg Case Rep. 2021;2021:rjab332. doi: 10.1093/jscr/rjab332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Englesbe M.J., Wu A.H., Clowes A.W., Zierler R.E. The prevalence and natural history of aortic aneurysms in heart and abdominal organ transplant patients. J Vasc Surg. 2003;37:27–31. doi: 10.1067/mva.2003.57. [DOI] [PubMed] [Google Scholar]