Abstract
Introduction
Brolucizumab, a low-molecular weight anti-vascular endothelial growth factor, was approved in the USA in October 2019 for the treatment of neovascular age-related macular degeneration. Following post-marketing reports of vasculitis, including retinal occlusive vasculitis, a safety signal of retinal vasculitis (RV) and/or retinal vascular occlusion (RO) that may result in severe vision loss was confirmed. This brief communication reviews the trends in the cumulative reporting rates of RV and/or RO and associated vision loss from May 2020 to September 2022.
Methods
This is a descriptive analysis of the cumulative post-marketing reporting rates of RV and/or RO cases, and associated vision loss included in the Novartis safety database between May 2020 and September 2022, utilizing an enhanced pharmacovigilance program.
Results
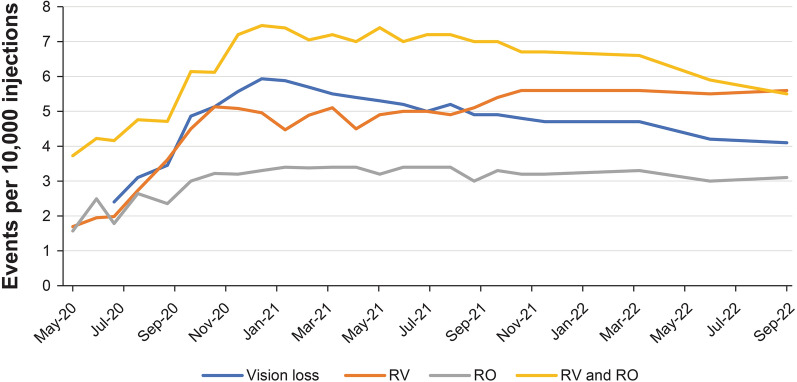
The RV-alone rates demonstrated an upward trend, rising to 5.1 events per 10,000 injections by October 2020 and subsequently remained stable until July 2021. Thereafter, the rate for RV-alone events increased modestly until October 2021 and then remained stable until September 2022. The RO-alone rates increased to 3.4 events per 10,000 injections by January 2021 and subsequently remained stable until September 2022. The combined reports of RV and RO showed an upward trend until December 2020 (7.5 events per 10,000 injections), followed by a plateau until September 2021 and then a downward trend until September 2022. Vision loss associated with RV and/or RO progressively increased until December 2020 (5.9 events per 10,000 injections) followed by a declining trend until September 2022 to the most recent reporting rate of 4.1 events per 10,000 injections.
Conclusion
The cumulative post-marketing reporting rates of vision loss associated with RV and/or RO, following brolucizumab treatment, have shown a declining trend after an initial rise in the reporting rates immediately after identification of the safety signal.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-022-00617-5.
Keywords: Adverse event rates, Brolucizumab, Intraocular inflammation, Pharmacovigilance, Post-marketing rates, Retinal vascular occlusion, Retinal vasculitis, Safety, Vision loss
Key Summary Points
| Why carry out this study? |
| Following post-marketing reports of vasculitis, including retinal occlusive vasculitis, a safety signal of retinal vasculitis (RV) and/or retinal vascular occlusion (RO) that may result in severe vision loss was confirmed. |
| An enhanced pharmacovigilance program was designed by Novartis to capture, analyze, and report these data to better understand the nature and outcomes associated with RV and/or RO and intraocular inflammation with brolucizumab treatment. |
| This analysis presents the cumulative post-marketing reporting rates of RV-alone, RO-alone, combined reports of RV and RO, and vision loss associated with RV and/or RO over time from May 2020 to September 2022. |
| What was learned from the study? |
| The reporting rate of vision loss associated with adverse events of RV and/or RO progressively increased until December 2020 (5.9 events per 10,000 injections), which was followed by a declining trend until September 2022 to the most recent reporting rate of 4.1 events per 10,000 injections. |
| The observed trends in the reporting rate demonstrate how these adverse events and the associated vision loss have evolved since the safety signal was first identified. |
Introduction
Brolucizumab gained US Food and Drug Administration (FDA) approval for the treatment of neovascular age-related macular degeneration in October 2019, on the basis of the results from the phase III HAWK and HARRIER trials [1–3]. Following FDA approval there were post-marketing reports of retinal vasculitis (RV), including retinal occlusive vasculitis [4]. Novartis initiated an internal review of these post-marketing safety case reports, which included the establishment of an external safety review committee to provide an independent, objective review of these cases along with a comparison with events seen in HAWK and HARRIER. Subsequently, a safety signal of RV and/or retinal vascular occlusion (RO) that may result in severe vision loss was confirmed. Typically, these events occur in the presence of intraocular inflammation (IOI) [5].
Over the last few years, there has been a substantial amount of research in this area and medical experts from across the world have offered recommendations on how to mitigate and manage the risk of these adverse events [5–13]. Novartis has continued to collect reports of RV and/or RO, and vision loss due to these adverse events via its pharmacovigilance system and has regularly updated the cumulative post-marketing reporting rates for these events per 10,000 injections on the brolucizumab.info website [4]. To better understand the nature and outcomes associated with RV and/or RO and IOI with brolucizumab treatment in patients, an enhanced pharmacovigilance (EPV) program was designed by Novartis to capture, analyze, and report these data. EPV is a set of pharmacovigilance activities intended to collect further information on post-marketing reports. For brolucizumab, the Novartis EPV program covered the collection of information using targeted follow-up checklists.
Here, we present the cumulative post-marketing reporting rates of RV-alone, RO-alone, combined reports of RV and RO (RV + RO combined), and vision loss associated with RV and/or RO over time from May 2020 to September 2022.
Methods
This is a descriptive analysis of the cumulative post-marketing reporting rates of RV-alone, RO-alone, RV + RO combined events and vision loss associated with RV and/or RO included in the Novartis safety database between May 2020 and September 2022, utilizing an EPV program established for the collection of post-marketing reports of such events. The post-marketing reporting rates for each event category are discrete; there is no duplication between categories. The term “RO” includes physician reports of ocular and retinal infarction and ischemia, retinal vascular (artery and vein) occlusion, stenosis, thrombosis, and embolism. The term “vision loss” includes physician reports of blindness, blindness unilateral, blindness transient or central vision loss. Of note, the search strategy for the category of “retinal vasculitis” evolved during this period, primarily with the inclusion of the term “retinal occlusive vasculitis” on the basis of changes in the Medical Dictionary for Regulatory Activities coding dictionary.
The cumulative reporting rates for RV and/or RO and associated vision loss are based on the number of post-marketing reports for the respective adverse events against the estimated post-marketing exposure to brolucizumab in terms of number of injections administered up to a given time point.
Reporting rate was calculated using the following formula:
As all data used in this descriptive analysis were derived from the Novartis Global Safety Database, were fully anonymized, and no new data on human participants was collected as part of the analysis, this study was exempt from ethics committee approval.
Results
The cumulative post-marketing reporting rates of RV and/or RO and associated vision loss per 10,000 injections are shown in Fig. 1 and are available in Table S1 in the electronic supplementary material. The RV-alone rates demonstrated an upward trend from May 2020 rising to 5.1 events per 10,000 injections by October 2020, and then remained relatively stable until July 2021. Following that, the rate for RV-alone events increased modestly until October 2021 to 5.6 events per 10,000 injections and then was stable until September 2022. The RO-alone rates increased to 3.4 events per 10,000 injections by January 2021 and remained stable until September 2022. The rates for RV + RO combined events showed an upward trend until December 2020 (7.5 events per 10,000 injections), followed by a plateau until September 2021. From September 2021 onwards, a declining trend in the reporting rates of RV + RO combined events was observed.
Fig. 1.
Cumulative post-marketing reporting rates of RV and/or RO and associated vision loss over time. RO retinal vascular occlusion, RV retinal vasculitis
Vision loss associated with adverse events of RV and/or RO progressively increased from June 2020 to December 2020 (5.9 events per 10,000 injections) followed by a declining trend until September 2022 to the most recent reporting rate of 4.1 events per 10,000 injections.
Discussion
In this analysis of the Novartis safety database, since December 2020, the cumulative post-marketing reporting rates of RO-alone events appears to be relatively stable, and the rates of RV-alone events have modestly increased until October 2021 and then remained stable, whereas the rates of RV + RO combined events and vision loss associated with RV and/or RO have declined over the same duration.
RV and RO are established adverse drug reactions of brolucizumab and categorized as an important identified risk in the brolucizumab European Risk Management Plan [14]. The results from the BASICHR0049 mechanistic study indicate that the underlying mechanism is immune-mediated [15, 16]. These results suggest a mature, high-affinity, and diverse immunoglobulin G-driven anti-drug antibody (ADA) response with ADAs recognizing multiple different B cell epitopes on the brolucizumab molecule. The presence of a polyclonal and diverse immune response against multiple epitopes to brolucizumab, along with a possible increased potential for platelet aggregation, could be consistent with an increased risk of the formation of immune complexes resulting in IOI and vascular occlusion. In addition, the in vitro activation of T cell by brolucizumab in case subjects shows that these master regulators of the immune response carried a memory of previous activation to brolucizumab. This provides evidence that the subjects with brolucizumab-associated RV and/or RO had a coordinated and specific immunity to brolucizumab. Typically, these events are seen in the presence of IOI [15].
Evidenced-based recommendations that have been developed by different groups of ophthalmologists, since the safety signal has been confirmed, suggest that monitoring and vigilance for signs of IOI throughout treatment plays a key role in the management of patients treated with brolucizumab [5–13]. This includes a thorough examination of the eye for inflammation prior to injection and instructing patients to report any change in vision or symptoms of IOI (including RV) and/or RO without delay, to ensure timely intervention [6–13]. Additionally, ophthalmologists recommend that if an IOI-related adverse event occurs, it should be addressed with prompt and intensive treatment as per standard of care [7, 9–13]. Real-world evidence suggests that such treatment can help prevent or recover vision loss [17–20].
For this analysis, it is important to note that spontaneous post-marketing reporting systems have several limitations, which can include under-reporting, incompletely documented cases, and cases without imaging information. The information collected as part of the post-marketing surveillance is not as robust as controlled clinical trials or solicited sources of information, and relevant information on risk characterization, including event severity, may be missing from the reports. Accumulated (“spontaneous”) case reports from the post-marketing surveillance cannot be used to reliably calculate incidences or estimate the frequency of drug risk as the number of spontaneous reports received may not be a reliable indicator of the actual number of cases that have occurred. Furthermore, the precise number of patients that have received the drug up to a certain time point is not known precisely and can only be estimated. Despite the possibility of under-reporting for all adverse events and associated vision loss, it is improbable that this is the primary cause of the declining trend in reporting rates of RV + RO combined events and vision loss associated with RV and/or RO given that reporting rates of RV-alone events have increased and RO-alone events have stabilized during the same timeframe.
Conclusion
This analysis presents for the first time the trends in the cumulative post-marketing reporting rates for RV and/or RO and associated vision loss following brolucizumab treatment in the real world. The cumulative post-marketing reporting rates of vision loss associated with RV and/or RO, following brolucizumab treatment, have shown a declining trend after an initial rise in the reporting rates immediately after identification of the safety signal. The trends observed may inform healthcare professionals in how these adverse events and associated vision loss have evolved over time.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This post-marketing activity, and the journal’s Rapid Service Fees, were funded by Novartis Pharma A.G., Basel, Switzerland.
Medical Writing, Editorial, and Other Assistance
The authors thank Divya Sharma (Novartis Healthcare Pvt. Ltd., Hyderabad, India) and Susan Simpson (Novartis Ireland Ltd., Dublin, Ireland) for medical writing and editorial assistance towards the development of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Franklin Igwe, Amit Lodha, and Arun Ravindran contributed to the material preparation, data collection, and analysis.
Disclosures
Franklin Igwe is an employee and shareholder of Novartis. Amit Lodha is an employee and shareholder of Novartis. Arun Ravindran was an employee of Novartis at the time of analysis. Arun Ravindran is an employee of UCB Pharmaceuticals.
Compliance with Ethics Guidelines
This article is based on a safety database analysis and does not contain any new studies with human participants performed by any of the authors; therefore, ethics committee approval was not required. All data collected for post-marketing reports in the Novartis Global Safety Database were anonymized and analyzed in aggregated form without individual data becoming available.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- 1.Beovu® [Summary of Product Characteristics], Basel, Switzerland, Novartis Pharma A.G. Sep 2020. www.ema.europa.eu/en/documents/product-information/beovu-epar-product-information_en.pdf. Accessed 18 Jul 2022.
- 2.Beovu® [Prescribing Information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; May 2022. https://www.novartis.us/sites/www.novartis.us/files/beovu.pdf. Accessed 18 Jul 2022.
- 3.Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Safety of Beovu® (brolucizumab). https://www.brolucizumab.info/. Accessed 07 Oct 2022.
- 5.Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050–1059. doi: 10.1016/j.ophtha.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127(10):1345–1359. doi: 10.1016/j.ophtha.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Baumal CR, Bodaghi B, Singer M, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2021;5(6):519–527. doi: 10.1016/j.oret.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Kilmartin DJ. Literature review and proposal of best practice for ophthalmologists: monitoring of patients following intravitreal brolucizumab therapy. Ir J Med Sci. 2022 doi: 10.1007/s11845-022-02929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holz FG, Heinz C, Wolf A, Hoerauf H, Pleyer U. Intraocular inflammation with brolucizumab use: patient management-diagnosis-therapy. Ophthalmologe. 2021;118(3):248–256. doi: 10.1007/s00347-021-01321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce I, Amoaku W, Bailey C, et al. The changing landscape for the management of patients with neovascular AMD: brolucizumab in clinical practice. Eye (Lond) 2022;36(9):1725–1734. doi: 10.1038/s41433-022-02008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonollosa A, Gallego-Pinazo R, Sararols L, Adán A, López-Gálvez M, Figueroa MS. Guidance on brolucizumab management recommendations. Arch Soc Esp Oftalmol (Engl Ed) 2022 doi: 10.1016/j.oftale.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Sadda SR, Guymer R, Holz FG, et al. The importance of imaging to identify early signs of intraocular inflammation: expert opinion for brolucizumab. Ophthalmologica. 2022. 10.1159/000526703. [DOI] [PMC free article] [PubMed]
- 13.Holz FG, Iida T, Maruko I, Sadda SR. A consensus on risk mitigation for brolucizumab in neovascular age-related macular degeneration: patient selection, evaluation, and treatment. Retina. 2022;42(9):1629–1637. doi: 10.1097/IAE.0000000000003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.BEOVU®. EPAR: risk management plan summary. April 2022. https://www.ema.europa.eu/en/documents/rmp-summary/beovu-epar-risk-management-plan-summary_en.pdf. Accessed 07 Oct 2022.
- 15.BEOVU® (brolucizumab): updated recommendations to minimize the known risk of intraocular inflammation, including retinal vasculitis and/or retinal vascular occlusion. October 2021. https://www.ema.europa.eu/en/documents/dhpc/direct-healthcare-professional-communication-dhpc-beovu-r-brolucizumab-updated-recommendations/retinal-vascular-occlusion_en.pdf. Accessed 07 Oct 2022.
- 16.Schmouder R, Maciejewski B, Karle A, et al. Immunologic features of beovu-associated retinal vasculitis/retinal vascular occlusion. Presented at: EURETINA 2021 Virtual Congress; September 9–12, 2021.
- 17.Kurup SK, Tabbaa T, Echegaray JJ, Oliver AL. Intraocular inflammation secondary to intravitreal brolucizumab treated successfully with sub-tenon triamcinolone: a case report. Am J Ophthalmol Case Rep. 2022;25:101289. doi: 10.1016/j.ajoc.2022.101289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto H, Hoshino J, Mukai R, Nakamura K, Akiyama H. One-year results of treat-and-extend regimen with intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 macular neovascularization. Sci Rep. 2022;12(1):8195. doi: 10.1038/s41598-022-10578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kataoka K, Horiguchi E, Kawano K, et al. Three cases of brolucizumab-associated retinal vasculitis treated with systemic and local steroid therapy. Jpn J Ophthalmol. 2021;65(2):199–207. doi: 10.1007/s10384-021-00818-8. [DOI] [PubMed] [Google Scholar]
- 20.Haensli C, Pfister IB, Garweg JG. Switching to brolucizumab in neovascular age-related macular degeneration incompletely responsive to ranibizumab or aflibercept: real-life 6 month outcomes. J Clin Med. 2021;10(12):2666. doi: 10.3390/jcm10122666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.