Abstract
We have located a locus, pgl, in Neisseria meningitidis strain NMB required for the glycosylation of class II pili. Between five and eight open reading frames (ORFs) (pglF, pglB, pglC, pglB2, orf2, orf3, orf8, and avtA) were present in the pgl clusters of different meningococcal isolates. The Class I pilus-expressing strains Neisseria gonorrhoeae MS11 and N. meningitidis MC58 each contain a pgl cluster in which orf2 and orf3 have been deleted. Strain NMB and other meningococcal isolates which express class II type IV pili contained pgl clusters in which pglB had been replaced by pglB2 and an additional novel ORF, orf8, had been inserted between pglB2 and pglC. Insertional inactivation of the eight ORFs of the pgl cluster of strain NMB showed that pglF, pglB2, pglC, and pglD, but not orf2, orf3, orf8, and avtA, were necessary for pilin glycosylation. Pilin glycosylation was not essential for resistance to normal human serum, as pglF and pglD mutants retained wild-type levels of serum resistance. Although pglB2 and pglC mutants were significantly sensitive to normal human serum under the experimental conditions used, subsequent examination of the encapsulation phenotypes revealed that pglB2 and pglC mutants expressed almost 50% less capsule than wild-type NMB. A mutation in orf3, which did not affect pilin glycosylation, also resulted in a 10% reduction in capsule expression and a moderately serum sensitive phenotype. On the basis of these results we suggest that pilin glycosylation may proceed via a lipid-linked oligosaccharide intermediate and that blockages in this pathway may interfere with capsular transport or assembly.
Both Neisseria meningitidis and Neisseria gonorrhoeae express Type IV pili that are composed of multimers of the PilE protein subunit (17 to 20 kDa) and a possible tip-associated adhesin, PilC (27). Type IV pili are necessary for initial attachment of these pathogens to epithelial and endothelial cell monolayers (27, 37), twitching motility (47), and natural competence (7).
Gonococci and some meningococci express a type IV class I pilus that is identified by reactivity with monoclonal antibody (MAb) SM1 (45). Class I type IV pili are notable for high-frequency antigenic variation, which is the result of primary nucleotide sequence changes that occur through a recombination mechanism involving several silent pilin loci (pilS) and the expressed pilin locus (pilE) (8). Meningococci express either class I type IV pili or class II pili, which are distinguished by nonreactivity with MAb SM1. The class II pilE locus, which is not found in the same location of the genome as class I pilE, was recently identified in strain FAM18 (1). A comparison of class I and II PilE subunits revealed structural similarities between the two proteins including a conserved N-terminal domain and hypervariable central and C-terminal domains. Class II pilE loci have also been identified in the commensal Neisseria spp. N. lactamica and N. cinerea, which may act as reservoirs for allelic exchange (2).
The complete crystallographic structure of class I pilin from N. gonorrhoeae strain MS11 has been determined, and three posttranslational modifications have been identified (6). These attachments include an α-glycerophosphate moiety on Ser94, a phosphate group on Ser68, and a disaccharide consisting of Gal(α1-3)GlcNAc attached to Ser63. Class I pilin from N. meningitidis isolates is also substituted in this way (9, 21), but in some instances the disaccharide attachment is replaced with a trisaccharide group consisting of Gal(β1-4)Gal(α1-3)-2,4-diacetamido-2,4,6-trideoxyhexose (Gal-Gal-DATDH) (38, 39).
Various proposals have been made regarding the role of glycosylation in pilus function. Marceau et al. (21) found that changing the primary amino acid sequence to prevent glycosylation of pili resulted in the loss of soluble pilin production in meningococci but not in gonococci (22). Glycosylation of pili may also play a significant role in the resistance of meningococci to complement-mediated lysis by normal human serum (NHS). One model suggests that nonbactericidal anti-Gal immunoglobulin A (IgA) antibodies bind to glycosylated pili, thereby effectively blocking the access of bactericidal antibodies to the bacterial-cell surface (9).
Examination of the amino acid sequence of the class II type IV pilin reveals that the sites for posttranslational processing are conserved (1). We have examined the role of the pgl locus in the glycosylation of class II pilin expressed by N. meningitidis and determined whether removal of the glycan moieties on pili effect resistance to complement-mediated lysis by human sera.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Meningococcal and gonococcal strains were cultured under aerobic conditions with 3.5% CO2 at 37°C on GC agar (Difco) supplemented with 0.4% glucose, 0.01% glutamine, 0.2 mg of cocarboxylase/liter, and 5 mg of Fe(NO3)3/liter. The wild-type strains and constructed mutants used in this study are shown in Table 1. Antibiotic selection for meningococcal mutants was performed in brain heart infusion medium (BHI) supplemented with 2.5% fetal calf serum and 80 μg of kanamycin (sulfate salt)/ml or in GC medium containing 60 μg of spectinomycin/ml. Escherichia coli JM109 was used as a host for all DNA manipulations. It was routinely grown on Luria-Bertani medium which, where appropriate, was supplemented with antibiotics at the following concentrations: ampicillin and spectinomycin at 50 μg/ml, and kanamycin and chloramphenicol at 25 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| N. meningitidis | ||
| NMB | Serogroup B (CDC8201085) | 36 |
| H44/76 | Serogroup B | 5 |
| M981 | Serogroup B | 49 |
| MC58 | Serogroup B | 44 |
| FAM18 | Serogroup C | 1 |
| F8229 | Serogroup A | 41 |
| GA0929 | Serogroup Y | This study |
| 6083 | Serogroup W-15 | 25 |
| M7 | NMBsynX::Tn916 | 36 |
| CMK20 | NMB pglF::aphA-3 | This study |
| CMK21 | NMB orf2::aphA-3 | This study |
| CMK22 | NMB orf3::aphA-3 | This study |
| CMK23 | NMB pglB2::aphA-3 | This study |
| CMK24 | NMB orf8::aphA-3 | This study |
| CMK25 | NMB pglC::aphA-3 | This study |
| CMK26 | NMB pglD::aphA-3 | This study |
| CMK27 | NMB avtA::aphA-3 | This study |
| CMK28 | NMB pilEII::Ω | This study |
| N. gonorrhoeae | ||
| FA1090 | 4 | |
| MS11 | 6 | |
| Plasmids | ||
| pUC18K | Apr vector + aphA-3 (Kmr) cassette | 26 |
| pHP45 | Apr vector + Ω(Spr) | 30 |
| pHSG576 | Cmr vector | 43 |
| pHSG298 | Kmr vector | 43 |
| pJKD2407 | pHSG576 + pglF::aphA-3 | This study |
| pJKD2410 | pHSG298 + orf2::aphA-3 | This study |
| pJKD2412 | pHSG76 + orf3::aphA-3 | This study |
| pJKD2414 | pHSG576 + pglB2::aphA-3 | This study |
| pJKD2416 | pHSG576 + orf8::aphA-3 | This study |
| pJKD2419 | pHSG298 + pglC::aphA-3 | This study |
| pJKD2422 | pHSG298 + pglD::aphA-3 | This study |
| pJKD2424 | pHSG576 + avtA::aphA-3 | This study |
| pJKD2426 | pHSG298 + pilEII::Ω | This study |
Detection of polymorphisms in the pgl locus.
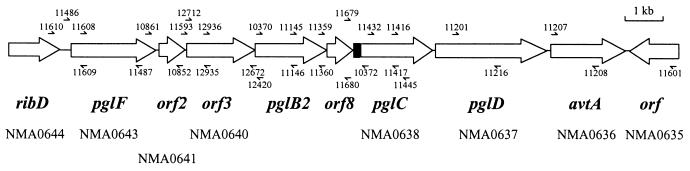
For colony PCR, a single colony was resuspended in 20 μl of sterile water and 1 μl of this preparation was used as a template in the PCRs. The following primer pairs were used to detect polymorphisms in the pgl locus: 11610 and 11487, 11486 and 12420, 10861 and 12420, 11593 and 12420, 10370 and 10372, 10370 and 11445, 10370 and 11216, 10370 and 11601, 11610 and 12420 (Table 2). PCR amplification of this locus from N. gonorrhoeae strain FA1090 using the same primers was used as a control for the PCR conditions. The locations of primers in the pgl locus of N. meningitidis strain NMB are shown in Fig. 1.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequencea |
|---|---|
| 10370 | 5′-CGTCTTCTTCTTTCAGGAACGC-3′ |
| 10372 | 5′-CTTTGTTGGACAGCAGGACTTTGG-3′ |
| 10852 | 5′-CTTCGTCATCGCCGAAAGGAATGC-3′ |
| 10861 | 5′-CAAACGCCTGCCGCTTTATATGC-3′ |
| 11145b | 5′-CTTGCTTCGCCAAGCCTTATGACG-3′ |
| 11146b | 5′-CGTCATAAGGCTTGGCGAAGCAAG-3′ |
| 11201 | 5′-CTACGACCTTGCCGTTTACCAGC-3′ |
| 11207 | 5′-CATCCTCCAACTGATGGACGACC-3′ |
| 11208 | 5′-GAGCATTTCGTACAGGGTTTGCG-3′ |
| 11216 | 5′-GGTAATGAAGATTTCGATGTCGC-3′ |
| 11360 | 5′-GGATTTCTTCGTCGCCGAGGTATT-3′ |
| 11359b | 5′-GAATACCTCGGCGACGAAGAAATC-3′ |
| 11417 | 5′-GTTCGATGTAGTCGACGACTTCAACC-3′ |
| 11416 | 5′-GAAGTCGTCGACTACATCGAACACG-3′ |
| 11432 | 5′-GATTGTTACTTCGCGCACCTTCC-3′ |
| 11445 | 5′-GCTTCGGTCAATACGGCTTCG-3′ |
| 11486 | 5′-GGCCTGAAAGCCGTCAAATTCCG-3′ |
| 11487 | 5′-GCATACACGATACGACGATAAACCG-3′ |
| 11593b | 5′-GAAATCTACCGCACCACCATGG-3′ |
| 11601 | 5′-CTCGGTTTGCGCCTGATTAACG-3′ |
| 11608 | 5′-GGTGCTGTTAACACTGAGCTTCC-3′ |
| 11609 | 5′-GGAAGCTCAGTGTTAACAGCACC-3′ |
| 11610 | 5′-CGAAATCATGGTCGAAGCAGGC-3′ |
| 11679b | 5′-CGAACACTTTGGGCTGGATAACC-3′ |
| 11680b | 5′-GGTTATCCAGCCCAAAGTGTTCG-3′ |
| 12420b | 5′-CGTTCCTGAAAGAAGAAGACG-3′ |
| 12672b | 5′-GACGATTTCGTCGGACTGAACG-3′ |
| 12712b | 5′-GCTTTTGGTATCTGGCAGAACG-3′ |
| 12935b | 5′-GCTTCTTTGGCGCGCTCGAGGTAG-3′ |
| 12936b | 5′-CCTACCTCGAGCGCGCCAAAGAAGC-3′ |
| 13411c | 5′-GTCAAACCCGGTCATTGTCC-3′ |
| 13412c | 5′-CAGGAGTCATCCAAATGAAAGC-3′ |
| 13511b | 5′-GTTTTCACGACCGGGTCAAACC-3′ |
| 13512b | 5′-GTGAGTTCTGTTGACGTTACAGC-3′ |
| 13513b | 5′-GGTCAAATACATTACGGGTTTACG-3′ |
| 13537b | 5′-CGTCAACAGAACTCACATATTTACC-3′ |
| KANCd | 5′-GTGGTATGACATTGCCTTCTGCG-3′ |
Unless otherwise specified, these primers were generated from an alignment of the genome sequences of N. gonorrhoeae strain FA1090 and N. meningitidis strain Z2491 (28).
Designed based on nucleotide sequence generated from strain NMB.
Designed from an alignment of the pilEII loci from N. meningitidis strain FAM18 (1) and commensal neisseria isolates (2).
Generated from the sequence of pUC18K (14).
FIG. 1.
Locations of primers in the pgl locus of N. meningitidis strain NMB. Small half arrows and primer designations show the relative positions and directions of the primers. Below the ORF names are the location identifiers from the complete genome of N. meningitidis strain Z2491 (28).
Construction of mutants in the pgl locus.
The methods used for the preparation and manipulation of DNA have been described previously (14). Internal fragments of each open reading frame (ORF) in the pgl cluster were individually amplified by PCR from strain NMB genomic DNA (Table 3). Where no convenient restriction sites were present in the gene, the splice overlap extension (SOE) technique (46) was used to introduce restriction sites into the appropriate positions within the PCR product (Table 3). The PCR products were purified using QIAquick spin columns, treated with T4 DNA polymerase, and cloned into the pHSG576 vector (43). The aphA-3 cassette from pUC18K (26) was released by restriction enzyme cleavage or amplified by PCR, and the resultant fragments inserted into unique restriction sites of each ORF cloned into pHSG576. The orientation of the aphA-3 cassette in each construct was determined by directional PCR using the KANC primer (14) and the reverse primer for each ORF. Every construct was sequenced to confirm an in-frame fusion of the aphA-3 cassette with the ORF into which it had been inserted. In some cases, the ORF::aphA-3 cassette was subcloned into pHSG298 (43) in an attempt to increase the frequency of transformation into strain NMB (Table 3). Transformation of strain NMB was performed as described previously (14), and mutants were checked by colony PCR using the oligonucleotide primer pairs listed in Table 3. A negative stain was performed on each mutant, and results were examined by electron microscopy (JEOL 100) to confirm piliation.
TABLE 3.
Construction of mutations in each ORF of the pgl cluster in N. meningitidis strain NMB
| ORF | Oligonucleotide primers to amplify ORF | Insertion site for aphA-3 cassette | Final plasmid construct | Strain NMB mutant derivative | Oligonucleotide primers used to confirm mutation in strain NMB |
|---|---|---|---|---|---|
| pglF | 11610/11609 and 11608/10852; joined by 11486/11487 | HincIIa | pJKD207 | CMK20 | 11610/11487 |
| orf2 | 10861/10852 | BssHII | pJKD2410 | CMK21 | 10861/12420 |
| orf3 | 12936/12420 and 12935/11593; joined by 12672/12712 | BssHIIa | pJKD2412 | CMK22 | 11593/12420 |
| pglB2 | 10370/11146 | HincII | pJKD2414 | CMK23 | 10370/11360 |
| orf8 | 11359/11680 | BssHII | pJKD2416 | CMK24 | 11145/10372 |
| pglC | 10370/11417 and 11216/11416; joined by 11432/11445 | HincIIa | pJKD2419 | CMK25 | 11679/11216 |
| pglD | 11201/11216 | BssHII | pJKD2422 | CMK26 | 11201/11601 |
| avtA | 11207/11208 | BssHII | pJKD2424 | CMK27 | 1201/11601 |
Restriction enzyme site introduced by SOE (46).
Construction of an insertionally inactivated mutant in the class II pilE locus in N. meningitidis strain NMB.
Primers for the class II pilE locus (pilEII) were designed from an alignment of the pilEII loci from N. meningitidis strain FAM18 (1) and commensal neisseria isolates (2). The pilEII gene from strain NMB was amplified using the primer pair 13412 and 13411 and was completely sequenced (GenBank accession no. AF320321). To construct a pilEII::Ω cassette, a unique HincII site was incorporated into pilEII using the primer pair 13513 and 13537 and the primer pair 13511 and 13512 to generate two products which were subsequently joined together using the primer pair 13412 and 13411. This product was cloned into the HincII site of pHSG298. The Ω spectinomycin cassette was released from pHP45 on a SmaI fragment and was ligated into the introduced HincII site to form pJKD2426. pJKD2426 was used to transform NMB, and spectinomycin-resistant mutants were screened for acquisition of the cassette using the primer pair 13412 and 13411.
Conditions for SDS-PAGE and Western immunoblotting.
MAb SM1 (45) was used for the detection of class I pili, and class II pili were detected using MAb AD211 (34). For the detection of pili, 10 μg of total protein from each strain was loaded per lane on a 12.5% (wt/vol) Laemmli sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (20). The proteins were transferred to a carboxymethylcellulose membrane in standard transfer buffer (25 mM Tris [pH 8.3], 192 mM glycine, and 20% [vol/vol] methanol) at 30 V overnight using a Bio-Rad Protean transfer apparatus. After the transfer was completed, the membranes were blocked for 1 h with bovine serum albumin (2% BSA) in TBS (50 mM Tris–150 mM NaCl [pH 7.4]). This was followed by two 10-min washes in TBS before incubation overnight with a 1:200 dilution of MAb SM1 or a 1:3,000 dilution of MAb AD211 in TBS. The membrane was washed five times (10 min each time) before incubation for 2 h with a 1:2,000 dilution of anti-mouse IgG-horseradish perioxidase (HRP) conjugate. Again the membrane was thoroughly washed as before, and the assay was developed with the addition of the color substrate solution for HRP (20).
Detection of glycosylated pilin.
Glycosylation of pilin was detected using the digoxigenin (DIG) glycan detection kit (Boehringer Mannheim) according to the manufacturer's instructions (9). In brief, the immunoblots of whole-cell lysate proteins were incubated with periodate, which oxidizes adjacent hydroxyl groups of sugars to aldehyde groups. After removal of the periodate solution and extensive washing, the derivatized sugars were labeled with a spacer-linked steroid hapten DIG covalently linked to a hydrazide group. In the final steps, DIG was detected in a standard immunoblot format using an anti-DIG-alkaline phosphatase conjugate.
Serum bactericidal assays.
The serum bactericidal assays were performed as previously described (15).
Quantitative ELISA for the detection of capsular polysaccharide on whole cells.
A whole-cell enzyme-linked immunosorbent assay (ELISA) was performed according to the published procedure (41) with minor modifications using the serogroup B capsule-specific MAb 2-2-B. One hundred microliters of a 1:3 dilution of the cell suspension at an optical density at 650 nm (OD650) of 0.1 was added to the microtiter plates, and the incubation was carried out at 37°C instead of 33°C.
Nucleotide sequence accession number.
The sequence of the pilEII gene from strain NMB has been assigned GenBank accession no. AF320321. The orf3-pglB2-orf8 region from this strain has been assigned accession no. AF320320.
RESULTS
Glycosylation of class II pili but not of class I pili is detected by periodate oxidation.
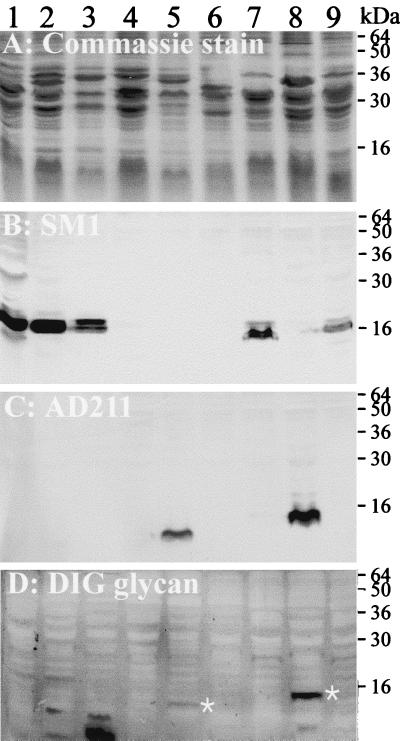
We examined the glycosylation phenotypes of pili from a limited selection of meningococcal strains and included N. gonorrhoeae strain MS11 as a control for the expression of glycosylated class I pilin (Fig. 2). Colony PCR of the eight meningococcal isolates in this panel using the pilEII primer pair 13412 and 13411 indicated that three strains, NMB, FAM18, and F8229, contained the class II pilin locus (Materials and Methods). Western immunoblotting using MAb AD211 confirmed that strains NMB and FAM18, but not F8229, expressed class II pilin (Fig. 2C). Conversely, all of the remaining strains, except M981, expressed MAb SM1-reactive class I pili. The glycosylation immunoblot showed that the pilin of strain NMB reacted strongly with the glycan detection reagents, whereas a much fainter band corresponding in size to the pilin of FAM18 was detected by this assay. In all instances, the glycosylation assay did not detect pilin-linked glycans in class I pilin-expressing isolates. Even though reactive bands were present in some strains, they did not correspond in size to the pilins and were variable in appearance when the assay was repeated (data not shown). Based upon this survey, we decided to investigate the genetics for the glycosylation of class II pili in strain NMB.
FIG. 2.
Glycosylation status of class I and class II pilin from eight meningococcal isolates. Whole-cell lysates were electrophoresed on an SDS-PAGE gel and stained with Coomassie blue (A). Duplicate gels were transferred to nitrocellulose membranes and either incubated with MAb SM1 (B) to detect class I pilin or with MAb AD211 (C) to detect class II pilin or assayed for detection of glycoproteins using the DIG-glycan kit (D). White asterisks indicate the positions of glycosylated pilin in panel D. Lanes: 1, N. gonorrhoeae strain MS11; 2, N. meningitidis strain 6083; 3, N. meningitidis strain GA0929; 4, N. meningitidis strain F8229; 5, N. meningitidis strain FAM18; 6, N. meningitidis strain M981; 7, N. meninigitidis strain H44/76; 8, N. meningitidis strain NMB; 9, N. meningitidis strain MC58.
Identification of the pgl cluster in the class II pilin-expressing N. meningitidis strain NMB.
Previous studies of glycoproteins in archaebacteria (10, 17, 18, 40, 48) and Bacillus alvei (11) have shown that glycans destined for attachment to proteins are synthesized as lipid-linked intermediates. These studies identified the lipid carrier as dolichol phosphate and undecaprenol phosphate (UdP) (10), thereby suggesting that synthesis and transport of these glycans are similar to those of O antigens. Based on this premise, we hypothesized that the synthesis of lipid-linked glycans for pilin glycosylation in Neisseria spp. would require a UdP transferase that would have significant amino acid sequence similarity to either the UdP-galactose-phosphotransferase, RfbP, or the UdP-N-acetylglucosamine transferase, RfbU. A BLAST search of all six translated frames of the meningococcal Z2491 genome using the amino acid sequence of Salmonella enterica serovar Typhimurium RfbP (accession no. S15314) identified one strong candidate that was 37% identical to this protein and was encoded by a single ORF, termed pglB (NMA0639). Analysis of the surrounding nucleotide sequence identified a further six ORFs in this cluster (Fig. 3). The subsequent genome annotation of Z2491 identified all of the ORFs in this cluster as potentially encoding enzymes necessary for lipopolysaccharide or capsule biosynthesis (28). The cluster of seven ORFs was delineated upstream by an ORF encoding an enzyme necessary for ribose synthesis, ribD (NMA0644), and downstream by an ORF (NMA0636) encoding a protein with sequence similarity to a hypothetical protein from Haemophilus spp. (Fig. 1 and 3). The potential involvement of this locus in pilin glycosylation was further supported by the discovery of a flagellin glycosylation locus in Campylobacter jejuni (42) in which four ORFs were homologous to those found in the meningococcal locus (data not shown). Further, Power et al. (29) described the involvement of three of these ORFs, pglB, pglC, and pglD, in the glycosylation of class I pilin from N. meningitidis strain C311.
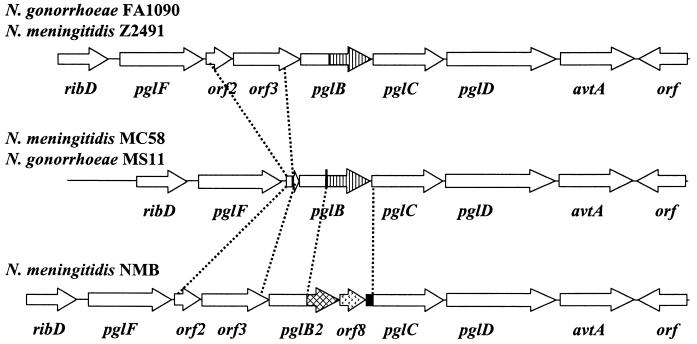
FIG. 3.
Polymorphisms in the pgl locus of N. gonorrhoeae and N. meningitidis. The sequences of the pgl clusters in strain FA1090 (available at the Gonococcal Genome Sequencing Project website, http://www.genome.ou.edu), N. meningitidis strain Z2491 (28), and N. meningitidis strain MC58 (44) can be found in the genome databases. The organization of the pgl locus in strain NMB and strain MS11 was determined by PCR, and the region corresponding to orf3-pglC in strain NMB has been sequenced (GenBank accession no. AF320320). The C-terminal domains of pglB (striped) and pglB2 (crosshatched) are indicated. The unique orf8 (stippled arrow) and the Correia element (solid box) are indicated in the pgl locus of strain NMB.
We PCR amplified this locus, using a variety of primer combinations, in N. gonorrhoeae strain MS11, and the class II pilin-expressing meningococcal isolate, strain NMB. As a result of this screen and the data from the genomic databases for meningococcal strains MC58 and Z2491, as well as gonococcal strain FA1090, it was apparent that there were two major polymorphisms present in this locus (Fig. 3). First, the amplification product using primer pair 10861 and 12420 (Fig. 1) was reduced by 1.7 kb for strain MS11 compared to those for the control strain FA1090 and strain NMB. Sequencing of this product (data not shown) revealed that orf2 and orf3 have been deleted from strain MS11, leaving the first 40 bp of orf2 separated from the last 100 bp of orf3 by a 30-bp sequence of unknown origin (data not shown). The genome database of N. meningitidis strain MC58 also confirmed that this strain has an arrangement similar to that of gonococcal strain MS11 (data not shown).
Second, PCR products amplified using primer pairs spanning the pglB gene were consistently larger by approximately 1.4 kb in strain NMB than in strain FA1090. The nucleotide sequence of this region from strain NMB revealed that a new ORF, termed orf8, as well as a 105-bp Correia element, was inserted into the intergenic space between a pglB-like gene, termed pglB2, and pglC (GenBank accession no. AF320320). The nucleotide sequence of pglB2 was highly conserved at the 5′ end of the gene but diverged from the database sequences of pglB at the 3′ end. PglB has two domains, an N-terminal domain of 200 amino acids which contains significant identity to a large family of RfbP-like enzymes from many sources, and a 213-amino-acid C-terminal domain containing four hexapeptide motifs which are commonly found in acyltransferases (32). In contrast, although the N-terminal domain remains conserved (99% identity) in PglB2, a 326-amino-acid C-terminal domain with no significant homology to the corresponding section of PglB is present at this site. The C-terminal domain of PglB2 had 24% identity with the entire amino acid sequence of the hypothetical protein MTH736 (338 amino acids) from Methanobacterium thermoautotrophicum (35) and 23% identity with the conserved protein slr1616 (341 amino acids) from Synechocystis sp. (16). Neither of these proteins has been assigned a function.
The novel ORF in strain NMB located between pglB2 and pglC, orf8, encodes a protein with 29% identity with the l-2-haloalkanoic acid dehalogenase isologue from Methanococcus jannaschii (3) and belongs to the Pfam group of haloacid dehalogenase-like hydrolases, which contains dehalogenases, epoxide hydrolases, and phosphatases (accession no. PF00702).
Glycosylation phenotype of mutants in the pgl cluster.
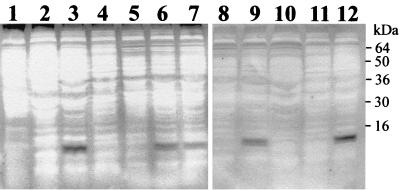
To determine the roles of the eight ORFs found in the pgl cluster of strain NMB, each ORF was disrupted by the insertion of a nonpolar kanamycin resistance cassette and whole-cell lysates of each mutant were assayed for the presence of glycosylated pili (See Materials and Methods; also Table 1). Electron microscopy confirmed that all the mutants in the pgl cluster had normal piliation phenotypes (data not shown). As expected, strain NMB expressed glycosylated pilin, whereas the negative control strain CMK28, in which the class II pilin locus was disrupted, did not react with the DIG-glycan reagents (Fig. 4). This assay revealed that pglF, pglB2, pglC, and pglD mutants produced pili with a modified glycosylation pattern (partly or completely removed), whereas orf2, orf3, orf8, and avtA were normal for this phenotype.
FIG. 4.
Pilin glycosylation phenotypes of pgl mutants in strain NMB. Whole-cell lysates of the pgl mutants and control strains were separated on an SDS-PAGE gel and transferred to a nitrocellulose membrane. Glycoproteins were detected using the DIG-glycan kit. Lanes: 1, N. gonorrhoeae strain MS11; 2, N. meningitidis strain MC58; 3, N. meningitidis strain NMB; 4, CMK28 (pilEII); 5, CMK20 (pglF); 6, CMK21 (orf2); 7, CMK22 (orf3); 8, CMK23 (pglB2); 9, CMK24 (orf8); 10, CMK25 (pglC); 11, CMK26 (pglD); 12, CMK27 (avtA).
Glycosylated pili are not required for resistance to NHS.
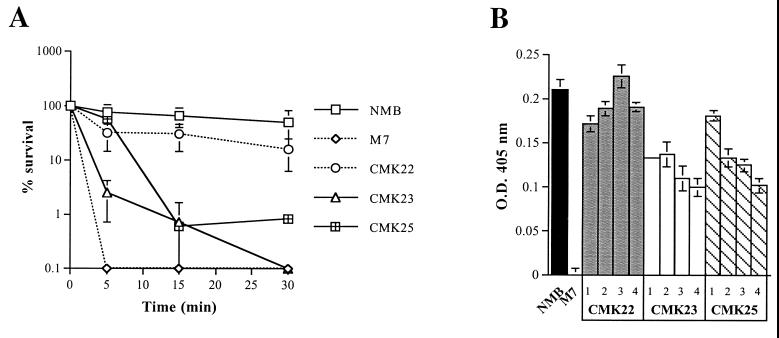
Hamadeh et al. (9) have proposed that elevated levels of anti-Gal IgA antibody in human serum may protect N. meningitidis from killing by binding to glycosylated pili. We therefore examined whether removal of the glycans attached to pili would result in increased sensitivity to NHS. Mutant M7, in which capsule biosynthesis has been abolished (36), was used as a positive control for sensitivity to NHS (15). After 30 min in 50% NHS, wild-type NMB and strains carrying mutations in pglF, orf2, orf8, pglD, and avtA remained resistant to killing (Fig. 5). In contrast, mutations in pglB2 and pglC significantly decreased the resistance of strain NMB to NHS (>2-log kill; P < 0.05). Interestingly, inactivation of orf3, which does not affect pilin glycosylation, also resulted in a modest decrease in serum resistance (∼1-log kill; P = 0.018) (Fig. 5). Therefore, the loss of pilin glycosylation did not necessarily result in serum sensitivity, suggesting that other factors were responsible for this phenotype in orf3, pglB2, and pglC mutants.
FIG. 5.
(A) Serum resistance profiles of N. meningitidis strain NMB pgl mutants CMK22 (orf3), CMK23 (pglB2), and CMK25 (pglC). The graph represents a time course assay over 30 min using 50% pooled NHS. Each point on the graph represents the mean percent survival in duplicate experiments, and the error bars are standard errors of the mean for each data set. The acapsulate mutant derivative of strain NMB, M7, was used as a serum-sensitive control. (B) Capsule expression profiles of CMK22 (orf3), CMK23 (pglB2), and CMK25 (pglC) nonsibling mutants. Each column represents the mean optical density at 405 nm (OD405) from five capsule ELISA readings, while the error bars represent the standard deviation of each data set. Percentage values were calculated on the assumption that the mean value for the parental strain NMB was equivalent to 100%. Similarly, two-tailed Student t tests were performed using these data.
Since encapsulation and lipooligosaccharide (LOS) structure have been shown to modify the serum resistance phenotype of strain NMB (15), we examined whether these factors had been affected by mutations in the pgl cluster. The LOS profiles of the pgl mutants of strain NMB were examined by Tricine SDS-PAGE and could not be distinguished from the parental LOS profile (data not shown). A quantitative serogroup B capsule ELISA revealed that the orf3, pglB2, and pglC mutants expressed less capsule than wild-type NMB (P = 0.046, 0.0061, and 0.076, respectively), whereas all other mutants expressed wild-type levels of serogroup B capsular polysaccharide (P > 0.2). To determine whether the decrease in capsule expression was genetically linked to the pgl cluster mutations, three nonsiblings of each mutant were examined for capsule expression using the quantitative serogroup B ELISA. All three of the nonsibling mutants of pglB2 and pglC expressed less capsule than the parent (52 to 65% [P < 0.0001] and 49 to 86% [P < 0.0001] of wild-type capsule expression, respectively) (Fig. 5B). In contrast, two of three nonsibling orf3 mutants expressed approximately 71 to 90% (P < 0.0098) of wild-type capsular levels (Fig. 5B). These results indicate that decreased capsule expression is associated with the serum-sensitive phenotype of the pglB2 and pglC mutants. As well, a modest reduction of capsular expression in orf3 mutants has also led to a detectable decrease in serum resistance.
DISCUSSION
The glycosylation of proteins in procaryotes has been most extensively studied in relation to archaebacterial S-layer proteins and pilin (10, 17, 18, 40, 48). Together these studies indicate that the sugar groups destined to be attached to glycoproteins are first synthesized on a lipid anchor, either C60-dolichol or C55-dolichol, and then transferred to the protein. Many aspects of this process resemble the steps in the assembly of lipid-linked oligosaccharide intermediates for capsular polysaccharide (33), O-antigen (31), and peptidoglycan (12) biosynthesis. Until recently, the genes encoding the enzymes necessary for the synthesis of these lipid-linked sugars and the transfer of these groups to a glycoprotein were unknown. Investigations into the genetic basis for glycosylation of flagella from C. jejuni (42) and Caulobacter crescentus (19) revealed that many of the genes necessary for this process encode proteins that are highly related to transferases involved in O-antigen and capsule biosynthesis. In particular, the genetic cluster required for glycosylation of flagella in C. jejuni encodes a protein with significant sequence identity to undecaprenol galactose phosphotransferase (RfbP), which is necessary for transfer of galactose 1-phosphate from UDP-galactose to UdP (31). These studies suggested to us that an RfbP homologue might be necessary for pilin glycosylation in Neisseria spp., and this has resulted in the identification of a cluster of eight ORFs that were subsequently investigated for involvement in pilin glycosylation.
We detected glycosylation of the class II pili from strain NMB after periodate oxidation of the glycoproteins. Interestingly, this assay was unable to detect glycosylated class I pili from N. gonorrhoeae strain MS11, which has the disaccharide substitution (6). Power et al. (29) also noted a similar phenomenon with the trisaccharide glycosylated class I pilin from N. meningitidis strain C311 but were able to overcome this lack of sensitivity by pretreating samples with galactose oxidase. The basis for this difference in sensitivity to periodate oxidation is unclear, although it may indicate that the class II pilin from strains NMB and FAM18 have different or modified glycan attachments.
The pgl cluster in strain NMB contained a modified version of the pglB found in the database sequences and was designated pglB2. In strain NMB, a novel ORF, orf8, was also inserted between pglB2 and pglC. Although PglB and PglB2 have different C-terminal domains, insertional inactivation of pglB2 in strain NMB confirmed that the protein encoded by this gene is required for pilin glycosylation. Power et al. (29) have previously shown that pglB is necessary for the addition of the trisaccharide glycan to class I pili in N. meningitidis strain C311. They proposed that PglB may operate as a bifunctional enzyme, with the N-terminal domain acting as a potential undecaprenol transferase and the C-terminal domain functioning as an acetylase required for the biosynthesis of the 2,4-diacetamido sugar residue of the glycan (DATDH). PglB2 has likely retained the undecaprenol transferase activity, since the N-terminal domain is intact. The C-terminal domain of PglB2, however, may be functionally different and may result in a different modification pattern of the acetamido sugar. This hypothesis could also potentially explain why the glycosylated class II pilin of strain NMB is more sensitive to periodate oxidation.
Serial inactivation of the genes in the pgl cluster in strain NMB confirmed that, in addition to pglB2, pglF, pglC, and pglD were involved in pilin glycosylation. Power et al. (29) have also recently shown that pglC and pglD are necessary for the addition of the trisaccharide glycan to class I pili in N. meningitidis strain C311. They have tentatively proposed that PglC and PglD are enzymes necessary for the biosynthesis of DATDH. PglF, which was not included in the study by Power et al. (29), contains a 70-amino-acid domain that has significant identity with a corresponding region of a potential repeat unit transporter, Cps2J, required for capsule transport in Streptococcus pneumoniae (13). Repeat unit transporters, or “flippases,” are proposed to transport lipid-linked oligosaccharide intermediates from the cytoplasm to the external surface of the inner membrane prior to polymerization and transfer to the final anchor (in the case of capsule and O antigen, phospholipids and lipid A core, respectively) (31, 33). If indeed pilin glycosylation proceeds through a lipid-linked intermediate, then PglF would be necessary to transfer the trisaccharide-undecaprenol intermediate to a position where the glycan could be transferred to the pilin subunit before polymerization into pili. Future experiments will explore whether this model is possible.
Hamadeh et al. (9) have suggested that glycosylation of pili may increase resistance to complement-mediated lysis by NHS. They proposed that anti-Gal IgA antibody, which is normally found in human serum and recognizes terminally linked α-galactosyl residues, could bind to glycosylated pili and block activation of the alternative complement pathway. At the time these experiments were conducted, mutants expressing nonglycosylated pili were not available. Therefore, we decided to test the NHS sensitivity of the glycosylation mutants of the serum-resistant strain NMB (15). These experiments revealed that glycosylation of pili in strain NMB had no direct effect on serum sensitivity. Although pglB2 and pglC mutants were significantly more serum sensitive than wild-type NMB, pglF and pglD mutants that were also defective in pilin glycosylation retained a normal serum resistance profile. In addition, the mutation of orf3, which did not affect pilin glycosylation, also resulted in a modest reduction in resistance to NHS. We subsequently found that orf3, pglB2, and pglC mutants, but not pglF and pglD mutants, expressed less capsular polysaccharide. Moreover, the reduction in capsule expression in the orf3, pglB2, and pglC mutants appeared to correlate with the serum sensitivity profiles of these mutants. In other words, a 10% reduction in capsule expression resulted in a 1-log kill of orf3 mutants, whereas a reduction of approximately 50% resulted in a 2-log kill of pglB2 and pglC mutants. Therefore, these experiments indicate that the expression of capsular polysaccharide is the predominant serum resistance determinant in meningococci irrespective of pilin glycosylation. Masson and Holbein have also previously observed that decreased levels of capsular polysaccharide, apart from complete abolition of capsule expression, have significant effects on the serum resistance of meningococci (23, 24). On the basis of these results, we hypothesize that lipid-linked oligosaccharide intermediates may accumulate in the pglB2 and pglC mutants and therefore interfere in capsular assembly or transport. The basis for the capsule deficiency of orf3 mutants is less clear but may also have a similar explanation if this gene is proven to encode an enzyme necessary for UdP processing.
In conclusion, we have explored the pgl cluster necessary for pilin glycosylation of a class II isolate, strain NMB, and shown that the genetic organization of this cluster differs from that in class I meningococci and N. gonorrhoeae. Our preliminary data may also indicate that a modified glycan is attached to class II pili. Since pglF, pglC, and pglD are conserved in gonococcal and meningococcal isolates expressing glycosylated (class I) pilin with a disaccharide (strain MS11) or a trisaccharide (strain C311) addition, the functions of the proteins encoded by these genes are likely to be necessary for common steps in pilin glycosylation. However, pglB is a polymorphic gene, and different forms of the encoded protein may be involved in specific modifications of the acetamide sugar. Further, blockage of pilin glycosylation at certain points in this pathway has unexpected pleiotropic effects on other cell surface structures, particularly the expression of capsular polysaccharide.
ACKNOWLEDGMENTS
We thank Mumtaz Virji of the University of Bristol (Bristol, United Kingdom), Mark Achtman of the Max-Planck Institut für Molekulare Genetik (Berlin, Germany), and W. Zollinger (Walter Reed Army Institute of Research) for their kind gifts of MAbs used in this study.
This work was supported by NIH grant AI 40247.
REFERENCES
- 1.Aho E L, Botten J W, Hall R J, Larson M K, Ness J K. Characterization of a class II pilin expression locus from Neisseria meningitidis: evidence for increased diversity among pilin genes in pathogenic Neisseria species. Infect Immun. 1997;65:2613–2620. doi: 10.1128/iai.65.7.2613-2620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aho E L, Keating A M, McGillivray S M. A comparative analysis of pilin genes from pathogenic and nonpathogenic Neisseria species. Microb Pathog. 2000;28:81–88. doi: 10.1006/mpat.1999.0325. [DOI] [PubMed] [Google Scholar]
- 3.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, FitzGerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 4.Dempsey J F, Cannon J G. Locations of genetic markers on the physical map of the chromosome of Neisseria gonorrhoeae FA1090. J Bacteriol. 1994;176:2055–2060. doi: 10.1128/jb.176.7.2055-2060.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vries F P, van der Ende A, van Putten J P M, Dankert J. Invasion of primary nasopharyngeal epithelial cells by Neisseira meningitidis is controlled by phase variation of multiple surface antigens. Infect Immun. 1996;64:2998–3006. doi: 10.1128/iai.64.8.2998-3006.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forest K T, Dunham S A, Koomey M, Tainer J A. Crystallographic structure reveals phosphorylated pilin from Neisseria: phosphoserine sites modify type IV pilus surface chemistry and fibre morphology. Mol Microbiol. 1999;31:743–752. doi: 10.1046/j.1365-2958.1999.01184.x. [DOI] [PubMed] [Google Scholar]
- 7.Fussenegger M, Rudel T, Barten R, Ryll R, Meyer T F. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae—a review. Gene. 1997;192:125–134. doi: 10.1016/s0378-1119(97)00038-3. [DOI] [PubMed] [Google Scholar]
- 8.Haas R, Meyer T F. Molecular principles of antigenic variation in Neisseria gonorrhoeae. Antonie Leeuwenhoek. 1987;53:431–434. doi: 10.1007/BF00415498. [DOI] [PubMed] [Google Scholar]
- 9.Hamadeh R M, Estabrook M M, Zhou P, Jarvis G A, Griffiss J M. Anti-Gal binds to pili of Neisseria meningitidis: the immunoglobulin A isotype blocks complement-mediated killing. Infect Immun. 1995;63:4900–4906. doi: 10.1128/iai.63.12.4900-4906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann E, Konig H. Uridine and dolichyl diphosphate activated oligosaccharides are intermediates in the biosynthesis of the S-layer glycoprotein of Methanothermus fervidus. Arch Microbiol. 1989;151:274–281. [Google Scholar]
- 11.Hartmann E, Messner P, Allmeier G, Konig H. Proposed pathway for biosynthesis of the S-layer glycoprotein of Bacillus alvei. J Bacteriol. 1993;175:4515–4519. doi: 10.1128/jb.175.14.4515-4519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heijenoort V. Murein biosynthesis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1025–1034. [Google Scholar]
- 13.Iannelli F, Pearce B J, Pozzi G. The type 2 capsule locus of Streptococcus pneumoniae. J Bacteriol. 1999;181:2652–2654. doi: 10.1128/jb.181.8.2652-2654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahler C M, Carlson R W, Rahman M M, Martin L E, Stephens D S. Inner core biosynthesis of lipooligosaccharide (LOS) in Neisseria meningitidis serogroup B: identification and role in LOS assembly of the α1,2-N-acetylglucosamine transferase (rfaK) J Bacteriol. 1996;178:1265–1273. doi: 10.1128/jb.178.5.1265-1273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahler C M, Martin L E, Shih G, Carlson R W, Stephens D S. The (α2-8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect Immun. 1998;66:5939–5947. doi: 10.1128/iai.66.12.5939-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 17.Lechner J, Wieland F, Sumper M. Biosynthesis of sulfated saccharides N-glycosidically linked to the protein via glucose. Purification and identification of sulfated dolichyl monophosphoryl tetrasaccharides from halobacteria. J Biol Chem. 1985;260:860–866. [PubMed] [Google Scholar]
- 18.Lechner J, Wieland F, Sumper M. Transient methylation of dolichyl oligosaccharides is an obligatory step in halobacterial sulfated glycoprotein biosynthesis. J Biol Chem. 1985;260:8984–8989. [PubMed] [Google Scholar]
- 19.Leclerc G, Wang S P, Ely B. A new class of Caulobacter crescentus flagellar genes. J Bacteriol. 1998;180:5010–5019. doi: 10.1128/jb.180.19.5010-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 21.Marceau M, Forest K, Beretti J L, Tainer J, Nassif X. Consequences of the loss of O-linked glycosylation of meningococcal type IV pilin on piliation and pilus-mediated adhesion. Mol Microbiol. 1998;27:705–715. doi: 10.1046/j.1365-2958.1998.00706.x. [DOI] [PubMed] [Google Scholar]
- 22.Marceau M, Nassif X. Role of glycosylation at Ser63 in production of soluble pilin in pathogenic Neisseria. J Bacteriol. 1999;181:656–661. doi: 10.1128/jb.181.2.656-661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masson L, Holbein B E. Influence of environmental conditions on serogroup B Neisseria meningitidis capsular polysaccharide levels. In: Schoolnik G K, et al., editors. The pathogenic Neisseria. Washington, D.C.: American Society for Microbiology; 1985. pp. 571–578. [Google Scholar]
- 24.Masson L, Holbein B E. Influence of nutrient limitation and low pH on serogroup B Neisseria meningitidis capsular polysaccharide levels: correlation with virulence for mice. Infect Immun. 1985;47:465–471. doi: 10.1128/iai.47.2.465-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAllister C F, Stephens D S. Analysis in Neisseria meningitidis and other Neisseria species of genes homologous to the FKBP immunophilin family. Mol Microbiol. 1993;10:13–23. doi: 10.1111/j.1365-2958.1993.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 26.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nassif X, Marceau M, Pujol C, Pron B, Beretti J L, Taha M K. Type-4 pili and meningococcal adhesiveness. Gene. 1997;192:149–153. doi: 10.1016/s0378-1119(96)00802-5. [DOI] [PubMed] [Google Scholar]
- 28.Parkhill J, Achtman M, James K D, Bentley S D, Churcher C, Klee S R, Morelli G, Basham D, Brown D, Chillingworth T, Davies R M, Davis P, Devlin K, Feltwell T, Hamlin N, Holroyd S, Jagels K, Leather S, Moule S, Mungall K, Quail M A, Rajandream M-A, Rutherford K M, Simmonds M, Skelton J, Whitehead S, Spratt B G, Barrell B G. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- 29.Power P M, Roddam L F, Dieckelmann M, Srikhanta Y N, Tan Y C, Berrington A W, Jennings M P. Genetic characterization of pilin glycosylation in Neisseria meningitidis. Microbiology. 2000;146:967–979. doi: 10.1099/00221287-146-4-967. [DOI] [PubMed] [Google Scholar]
- 30.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 31.Raetz C R. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular microbiology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1035–1063. [Google Scholar]
- 32.Raetz C R H, Roderick S L. A left-handed parallel β helix in the structure of UDP-N-acetylglucosamine acyltransferase. Science. 1995;270:997–1000. doi: 10.1126/science.270.5238.997. [DOI] [PubMed] [Google Scholar]
- 33.Rick P D, Silver R P. Enterobacterial common antigen and capsular polysaccharides. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 104–122. [Google Scholar]
- 34.Serino L, Virji M. Phosphorylcholine decoration of lipopolysaccharide differentiates commensal neisseriae from pathogenic strains: identification of licA-type genes in commensal neisseriae. Mol Microbiol. 2000;35:1550–1559. doi: 10.1046/j.1365-2958.2000.01825.x. [DOI] [PubMed] [Google Scholar]
- 35.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Reeve J N, et al. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens D S, Swartley J S, Kathariou S, Morse S A. Insertion of Tn916 in Neisseria meningitidis resulting in loss of group B capsular polysaccharide. Infect Immun. 1991;59:4097–4102. doi: 10.1128/iai.59.11.4097-4102.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens D S, Whitney A M, Rothbard J, Schoolnik G K. Pili of Neisseria meningitidis. Analysis of structure and investigation of structural and antigenic relationships to gonococcal pili. J Exp Med. 1985;161:1539–1553. doi: 10.1084/jem.161.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stimson E, Virji M, Barker S, Panico M, Blench I, Saunders J, Payne G, Moxon E R, Dell A, Morris H R. Discovery of a novel protein modification: α-glycerophosphate is a substituent of meningococcal pilin. Biochem J. 1996;316:29–33. doi: 10.1042/bj3160029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stimson E, Virji M, Makepeace K, Dell A, Morris H R, Payne G, Saunders J R, Jennings M P, Barker S, Panico M, et al. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- 40.Sumper M. Halobacterial glycoprotein biosynthesis. Biochim Biophys Acta. 1987;906:69–79. doi: 10.1016/0304-4157(87)90005-0. [DOI] [PubMed] [Google Scholar]
- 41.Swartley J S, Liu L-J, Miller Y K, Martin L E, Edupuganti S, Stephens D S. Characterization of the gene cassette required for biosynthesis of the (α1-6)-linked N-acetyl-d-mannosamine-1-phosphate capsule of serogroup A Neisseria meningitidis. J Bacteriol. 1998;180:1533–1539. doi: 10.1128/jb.180.6.1533-1539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szymanski C M, Yao R, Ewing C P, Trust T J, Guerry P. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol Microbiol. 1999;32:1022–1030. doi: 10.1046/j.1365-2958.1999.01415.x. [DOI] [PubMed] [Google Scholar]
- 43.Takeshita S, Sato M, Toba M, Masahashii W, Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZα-complementation and chloramphencol- or kanamycin-resistance selection. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 44.Tettelin H, Saunders N J, Heidelberg J, Jeffries A C, Nelson K E, Eisen J A, Ketchum K A, Hood D W, Peden J F, Dodson R J, Nelson W C, Gwinn M L, DeBoy R, Peterson J D, Hickey E K, Haft D H, Salzberg S L, White O, Fleischmann R D, Dougherty B A, Mason T, Cieko A, Parksey D S, Blair E, Cittone H, Clark E B, Cotton M D, Utterback T R, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith H O, Fraser C M, Moxon E R, Rappuoli R, Venter J C. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 45.Virji M, Heckels J E. Antigenic cross-reactivity of Neisseria pili: investigations with type- and species-specific monoclonal antibodies. J Gen Microbiol. 1983;129:2761–2768. doi: 10.1099/00221287-129-9-2761. [DOI] [PubMed] [Google Scholar]
- 46.Warrens A N, Jones M D, Lechler R I. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene. 1997;186:29–35. doi: 10.1016/s0378-1119(96)00674-9. [DOI] [PubMed] [Google Scholar]
- 47.Wolfgang M, Lauer P, Park H S, Brossay L, Hebert J, Koomey M. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol Microbiol. 1998;29:321–330. doi: 10.1046/j.1365-2958.1998.00935.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhu B C, Drake R R, Schweingruber H, Laine R A. Inhibition of glycosylation by amphomycin and sugar nucleotide analogs PP36 and PP55 indicates that Haloferax volcanii β-glucosylates both glycoproteins and glycolipids through lipid-linked sugar intermediates: evidence for three novel glycoproteins and a novel sulfated dihexosyl-archaeol glycolipid. Arch Biochem Biophys. 1995;319:355–364. doi: 10.1006/abbi.1995.1305. [DOI] [PubMed] [Google Scholar]
- 49.Zollinger W D, Mandrell R E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat modifiable outer membrane proteins. Infect Immun. 1980;28:451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]