Abstract
Gamma interferon (IFN-γ), a pleiotropic cytokine, is now known to be produced by macrophages as well as by NK cells, γδ cells, and activated T cells. The autocrine biological functions of IFN-γ on the macrophage include the upregulation of major histocompatibility complex MHC class II and the activation to an antiviral state. In this study, the production of IFN-γ by macrophages was demonstrated to correspond to antibacterial activity. Legionella pneumophila replicates intracellularly in thioglycolate (TG)-elicited macrophages (TG-macrophages) from A/J mice, while TG-macrophages from BALB/c mice restrict bacterial growth after an initial period of growth. BALB/c TG-macrophages were shown to express IFN-γ mRNA at 24 and 28 h, which corresponded to the initiation of anti-L. pneumophila activity. Moreover, IFN-γneutralization by antibody treatment of the cultures resulted in increased L. pneumophila growth in the macrophages. In contrast, no IFN-γ mRNA was expressed in TG-macrophages from A/J mice, where L. pneumophila grew unrestricted. As would be expected, IFN-γ treatment decreased bacterial growth. An IFN-γ-mediated antibacterial activity was, however, inducible in A/J macrophages by the addition of interleukin-12 following L. pneumophila infection. Thus, autocrine IFN-γ is involved in anti-L. pneumophila activity associated with different growth patterns and appears to be important during intracellular infection.
Macrophages have long been recognized as important in the production of proinflammatory cytokines and chemokines involved in innate responses and in coordination of adaptive immunity (1, 27). They are therefore important components of host immunity to infection with intracellular pathogens such as Mycobacterium spp. and Legionella spp. (27). Legionella pneumophila replicates in specialized vacuoles within human macrophages and monocytes (17, 20, 24) and certain types of murine macrophages (5, 35). Gamma interferon (IFN-γ) (4, 14, 23, 30, 31, 34), lipopolysaccharide (LPS) (2, 8, 21), and tumor necrosis factor alpha (TNF-α) (21, 31) have been demonstrated to activate host cells to control the growth of L. pneumophila. Interleukin-12 (IL-12) is also important in regulating L. pneumophila growth, apparently through TNF-α, during in vivo infection in A/J mice (6).
While macrophages are known to produce TNF-α and IL-12, the source of IFN-γ was generally considered to be NK cells, γδ cells, and activated T cells (18). However, recently IL-12 and other factors have been reported to induce macrophages to produce IFN-γ (7, 9, 11, 22, 26, 33). Murine resident peritoneal macrophages constitutively produce IFN-γ (7, 26), and this production has been suggested to be important during the early immune response to infection (15). Resident macrophages do not support the growth of L. pneumophila (36), which is only partly due to reduced intracellular iron, an important growth nutrient, in these cells (12). The presence of IFN-γ in the resident macrophages suggests that they may also have a basal antimicrobial activation state, which, in combination with the poor nutrient environment, inhibits the growth of L. pneumophila.
We have previously reported that thioglycolate (TG)-elicited macrophages (TG-macrophages) from A/J mice are permissive for L. pneumophila growth while TG-macrophages from BALB/c mice are not (36). The availability of iron or the productions of nitric oxide (13) or of TNF-α (3) were found not to differ between these macrophage cultures. Others have observed variation in IFN-γ production by TG-macrophages (11) and bone marrow-derived macrophages (22) from different mouse strains. Moreover, in vitro infection of alveolar macrophages with Mycobacterium tuberculosis results in IFN-γ production (9). Therefore, we examined the production of IFN-γ by TG-macrophages from BALB/c and A/J mice. These data demonstrate that L. pneumophila-infected BALB/c macrophages produce IFN-γ, which appears to be responsible, at least in part, for the ability of BALB/c macrophages to restrict L. pneumophila growth. Macrophages from A/J mice did not produce IFN-γ unless they were stimulated by IL-12 treatment.
MATERIALS AND METHODS
Mice.
Female A/J and BALB/c mice, 6 to 8 weeks old (Jackson Laboratory Bar Harbor, Maine), were used in these studies. They were housed and cared for in the USF Health Sciences Center animal facility, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Bacteria.
L. pneumophila M124, a serogroup 1 strain originally isolated at Tampa General Hospital (Tampa, Fla.), was grown on buffered charcoal yeast extract agar (BCYE; Difco, Detroit, Mich.) for 48 h from a passage 3 stock maintained at −80°C. The avirulent strain used was isolated by serial passage of M124 on BCYE and modified Mueller-Hinton agar (3). The bacteria were suspended in pyrogen-free saline, and the concentration was adjusted spectrophotometrically.
Macrophage infections and stimulations.
Elicited macrophages were collected 4 days after intraperitoneal injection of TG medium (Difco) by peritoneal lavage with phosphate-buffered saline (Sigma, St. Louis, Mo.). The peritoneal cells (106/ml) were cultured in RPMI 1640 medium (Sigma) supplemented with 10% heat-inactivated fetal calf serum (HyClone Laboratories, Logan, Utah) and 2-mercaptoethanol (5 × 10−6 M; Sigma) in either 6-well (mRNA assays; 3 × 106 cells/well), 24-well (enzyme-linked immunosorbent assays [ELISAs]; 1 × 106 cells/well), or 96-well (CFU determination; 1 × 105 cells/well) plates (Corning Costar Corp., Cambridge, Mass.) for 2 h. The nonadherent cells (less than 5% lymphocytes) were removed by washing three times. The adherent cells were then cultured in penicillin- and streptomycin-supplemented medium overnight. The cells were washed three times, cultured in penicillin- and streptomycin-free medium for an additional hour, and washed two more times. The resultant adherent population was more than 99% macrophages as determined by F4/80 staining and fluorescence microscopy. TG-macrophage monolayers were infected with bacteria at a 10:1 ratio of bacteria (107/ml) to cells (106/ml) for 30 min. Nonphagocytized bacteria were removed by washing the macrophages three times. The infected TG-macrophages were cultured for up to an additional 48 h. Alternately, formalin-killed L. pneumophila (108/ml) or Escherichia coli O111:B4 LPS (5 μg/ml; Sigma) was added to the 24-well plates for IL-12 ELISAs.
Cytokine addition or neutralization.
In some cultures, recombinant IL-12 p70 (40 ng/ml), anti-IFN-γ antibodies (10 μg/ml), recombinant IFN-γ (rIFN-γ) (20 ng/ml), or anti-IL-12 p40/p70 antibodies (10 μg/ml) were added to the cultures following infection. All cytokines and antibodies were obtained from PharMingen (San Diego, Calif.).
CFU determinations.
At the indicated times, macrophage cultures in the 96-well plates were lysed with 0.1% saponin (Sigma). The lysates were diluted in Hanks balanced salt solution, plated on BCYE plates, and incubated at 37°C for 72 h. CFU counts were determined on an AutoCount apparatus (Dynatech Labs, Chantilly, Va.).
ELISAs.
Supernatants were collected from 24-well plates for cytokine determinations. Cytokine levels of IFN-γ and IL-12 p40/p70 were determined by sandwich ELISAs using antibody pairs from PharMingen, following protocols previously described (25). Briefly, the ELISAs were performed in Medium-Bind EIA plates (Costar) with capture antibodies (IFN-γ, 4μg/ml; IL-12, 10 μg/ml), biotinylated detection antibodies (2 μg/ml), and streptavidin-horseradish peroxidase (1:1,000) (Southern Biotechnology, Birmingham, Ala.). 3,3′,5,5′-Tetramethylbenzidine liquid substrate system (TMB) (Sigma) was added for 5 to 30 min, and the horseradish peroxidase reaction was stopped with 1 N sulfuric acid. The plates were read at 450 nm on an Emax Microplate Reader (Molecular Devices, Mento Park, Calif.). Concentrations were calculated from a standard curve generated for each plate. The low-end sensitivity was 200 pg/ml for both ELISAs.
RT-PCR.
RNA was extracted by standard methods with 1 ml of TriReagent (Sigma) per 3 × 106 macrophages. RNA was quantitated using RiboGreen (Molecular Probes, Eugene, Oreg.) and an fmax Fluorometer (Molecular Devices). Reverse transcription (RT) of RNA (2 μg) was performed with avian myeloblastosis virus reverse transcriptase, and the RT products (2 μl) were PCR amplified as previously described (37). The primer sequences were as follows: 5′CATTGAAAGCCTAGAAAGTCT and 3′CTCATGGAATGCATCCTTTTTCG for IFN-γ and 5′ATGGATGACGATATCGCT and 3′ATGAGGTAGTCTGTCAGGT for β-actin. Amplification cycles and annealing temperatures were 40 cycles at 55°C for IFN-γ and 26 cycles at 60°C for β-actin. The PCR was performed in a Minicycler (MJ Research, Watertown, Miss.). The products were visualized with ethidium bromide in a 2% agarose gel.
Statistical analysis.
Data were analyzed by one-way analysis of variance with Dunnett's test for comparing individuals, using SigmaStat (Jandel Scientific, San Rafael, Calif.).
RESULTS
L. pneumophila growth is unrestricted in A/J macrophages and restricted in BALB/c macrophages.
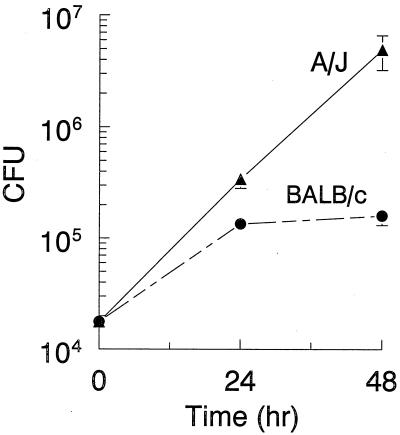
TG-elicited macrophages from A/J and BALB/c mice were infected with L. pneumophila, and CFU counts determined at 24 and 48 h postinfection. L. pneumophila displayed continuous growth in A/J macrophages throughout the 48-h period, as indicated by the increase in CFU counts (Fig. 1). In contrast, L. pneumophila initially grew in BALB/c macrophages but was restricted after 24 h (Fig. 1).
FIG. 1.
L. pneumophila growth patterns vary within TG-macrophage cultures from A/J and BALB/c mice. TG-macrophages were infected with L. pneumophila (10:1), and the cells were washed and incubated for 24 or 48 h. The cells were lysed, and CFU counts were determined. Data are means and standard errors of the means (SEM) of four experiments.
Infected BALB/c macrophages express IFN-γ mRNA.
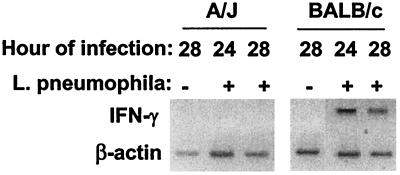
The CFU data above suggested that BALB/c macrophages, after the initial infection, were activated in some way to suppress L. pneumophila growth. We speculated that IFN-γ production might be involved, and so the level of cellular mRNA was examined. A/J and BALB/c TG-macrophage cultures were infected and at 24 and 28 h postinfection, and RNA was collected and analyzed by RT-PCR. IFN-γ mRNA was not detected in uninfected cultures or in infected A/J macrophages (Fig. 2). However, IFN-γ mRNA was detected in infected BALB/c macrophages at both 24 and 28 h (Fig. 2). These time points corresponded to the times when CFU counts had leveled off in BALB/c cultures (Fig. 1). We performed ELISA for IFN-γ protein in supernatants and freeze-thawed samples of BALB/c macrophage cultures at 24 and 48 h postinfection. The freeze-thawed samples were examined because others had detected IFN-γ only by intracellular immunofluorescence in TG-macrophages (11). The detected levels were minimal (200 to 300 pg/ml) and just above the sensitivity end point for our ELISA system, with no significant difference being observed between uninfected and L. pneumophila-infected cultures (data not shown). IFN-γ was detectable in resident peritoneal BALB/c macrophages (578 ± 166 pg/ml [total mean ± standard deviation]), with the majority being found in the frozen-thawed samples. Interestingly, L. pneumophila infection did not increase the amount of IFN-γ, although the presence of killed L. pneumophila did (1,026 ± 72 pg/ml).
FIG. 2.
L. pneumophila induces IFN-γ mRNA in TG-macrophage cultures from BALB/c mice. TG-macrophages were infected with L. pneumophila (10:1) or left uninfected. The cells were washed and incubated for 24 or 28 h. RNA was extracted, subjected to RT-PCR, and then visualized on 2% agarose gel with ethidium bromide. The gels are from a representative of three experiments.
Anti-IFN-γ antibodies increase L. pneumophila growth in BALB/c macrophages while rIFN-γ decreases growth in A/J macrophages.
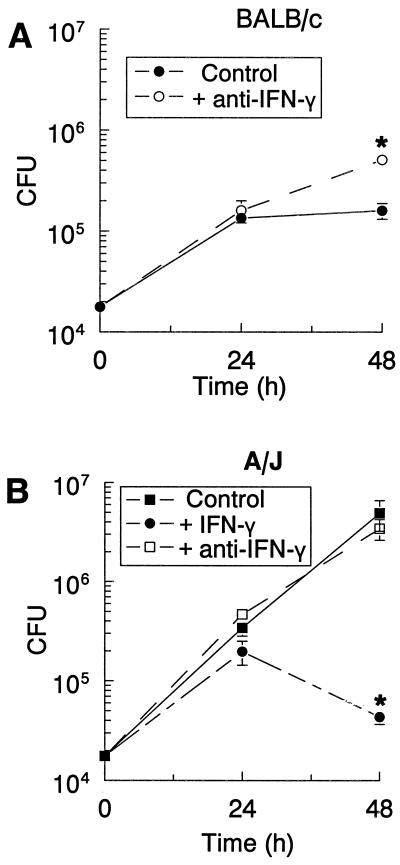
The RT-PCR data suggested that the induced IFN-γ in BALB/c macrophages was involved in the growth restriction of L. pneumophila. To examine this issue further, anti-IFN-γ antibodies were added to the BALB/c macrophage cultures to neutralize endogenous IFN-γ. Antibody treatment increased the growth of L. pneumophila, as indicated by the higher CFU counts at 48 h (Fig. 3A) compared to the control. The addition of anti-IFN-γ had no effect on L. pneumophila growth in A/J macrophages (Fig. 3B), although rIFN-γ treatment of these cultures restricted the growth of L. pneumophila at 48 h (Fig. 3B).
FIG. 3.
Neutralizing IFN-γ increases L. pneumophila growth in TG-macrophages from BALB/c mice (A) but IFN-γ decreases growth in cultures from A/J mice (B). TG-macrophages were infected with L. pneumophila (10:1). The cells were washed, anti-IFN-γ antibodies were added, and the cells were incubated for 24 or 48 h. The cells were lysed, and CFU counts were determined. Data are means and SEM of three experiments. ∗, P < 0.05.
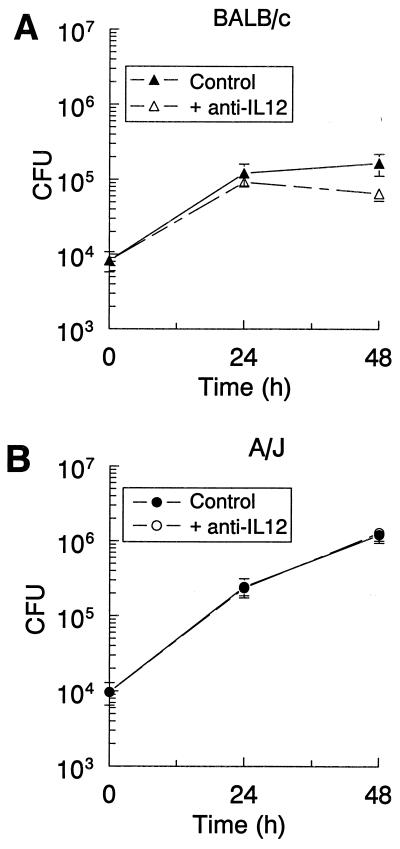
Growth restriction of L. pneumophila in A/J and BALB/c TG-macrophages in response to treatment with IL-12 p70 is mediated through IFN-γ.
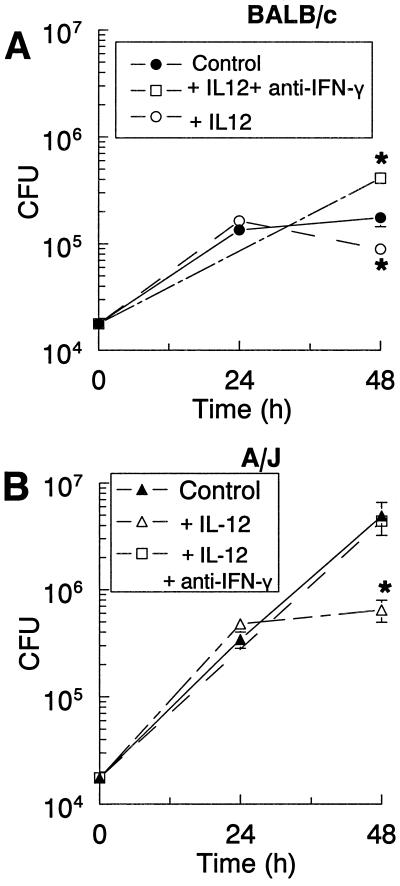
Because IFN-γ appeared to be involved in restricting the growth of L. pneumophila and IL-12 activated cells to produce IFN-γ, bacterial growth was examined in macrophage cultures treated with IL-12 p70. The addition of IL-12 after infection significantly reduced the CFU counts in A/J macrophages (Fig. 4B); however, it caused only a slight decrease in counts in BALB/c TG-macrophages, (Fig. 4A). The IL-12 restriction appeared to require at least 24 h to become effective. To determine if the antimicrobial activity of IL-12 occurred via IFN-γ induction, IL-12 p70 and anti-IFN-γ antibodies were added to the infected cultures and CFU counts were determined at 48 h. In BALB/c cultures, addition of anti-IFN-γ antibodies increased the CFU counts above those in the IL-12-treated cultures and control cultures (Fig. 4A). This latter effect was seen previously in Fig. 3A and is most probably due to neutralizing endogenous IFN-γ. In A/J cultures, the addition of anti-IFN-γ antibodies also increased the CFU counts to a level similar to that in the infected controls (Fig. 4B). Thus, the restriction of bacterial growth by IL-12 p70 appeared to be mediated through the induction of IFN-γ.
FIG. 4.
IL-12 treatment of L. pneumophila-infected cultures decreased bacterial growth in TG-macrophages from BALB/c (A) and A/J (B) mice, which was attenuated by neutralization of IFN-γ. TG-macrophages were infected with L. pneumophila (10:1). The cells were washed, and IL-12 or a combination of IL-12 and anti-IFN-γ antibodies was added. The cells were lysed at 24 or 48 h postinfection, and CFU counts were determined. Data are means and SEM of three experiments. ∗, P < 0.05.
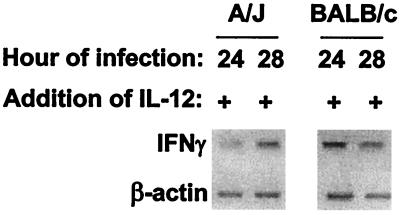
Addition of IL-12 induces IFN-γ mRNA expression in infected TG-macrophage.
To determine if IL-12 was inducing IFN-γ mRNA, as the antibody neutralization studies implied, RT-PCR was performed on IL-12-treated, infected macrophage cultures at 24 and 28 h postinfection. As seen in Fig. 5, the addition of IL-12 to A/J macrophage cultures induced a weak but detectable IFN-γ band at 24 h and a stronger band at 28 h. These results are in contrast to those in Fig. 2, wherein no IFN-γ expression was detected. In BALB/c cultures, the IL-12 had little effect on IFN-γ induction. These results supported the CFU data observed in Fig. 4, in that IL-12 treatment had a much stronger effect on macrophages from A/J mice than on those from BALB/c mice.
FIG. 5.
IL-12 treatment of L. pneumophila-infected cultures induces IFN-γ mRNA. TG-macrophages were infected with L. pneumophila (10:1). The cells were washed, and IL-12 p70 was added. After 24 or 28 h of incubation, RNA was extracted, subjected to RT-PCR, and then visualized on 2% agarose gel with ethidium bromide. The gels are from a representative of three experiments.
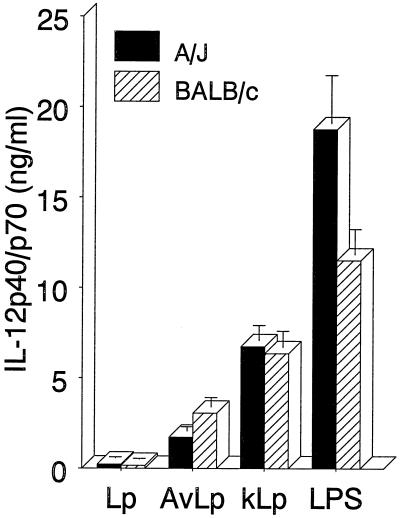
L. pneumophila infection has little effect on IL-12 p40/p70 production.
Because it is thought that bacterial infections induce IL-12 and the current data demonstrate that IL-12 inhibited L. pneumophila growth by inducing IFN-γ, the production of IL-12 following infection was examined. TG-macrophage cultures were exposed to virulent or avirulent L. pneumophila or stimulated with killed L. pneumophila or E. coli LPS. The culture supernatants were harvested, and the IL-12 protein levels were measured. As shown in Fig. 6, small but equivalent amounts of IL-12 p40/p70 were detected following infection of macrophage cultures from A/J and BALB/c mice. Avirulent L. pneumophila, as well as killed L. pneumophila, induced more IL-12 p40/p70 protein. E. coli LPS was included as a positive control and induced even more IL-12. The low induction of IL-12 observed following L. pneumophila infection was supported by studies examining the effect of anti-IL-12 antibody treatment on L. pneumophila growth. Macrophage cultures from A/J and BALB/c mice were either infected only or infected and treated with anti-IL-12 p70. The results showed that antibody treatment had little effect on the number of CFU counts in either A/J or BALB/c macrophage cultures (Fig. 7). Slight decreases in CFU counts were observed in the BALB/c macrophages (Fig. 7A), which contrast with the increased CFU counts that one would predict if endogenous IL-12 was inducing the IFN-γ. These results suggested that L. pneumophila infection of these cells induced small amounts of IL-12, which had little effect on the subsequent capacity of the cultures to support the growth of the bacteria.
FIG. 6.
L. pneumophila infection induces little IL-12 p40/p70, although avirulent or killed L. pneumophila stimulates higher levels. TG-macrophages were infected with virulent L. pneumophila (Lp) or avirulent L. pneumophila (AvLp) as described in Materials and Methods or stimulated with formalin-killed L. pneumophila (kLp) or E. coli LPS. At 24 h, supernatants were collected and analyzed by ELISA for IL-12 p40/p70. Data are the means and SEM of three experiments.
FIG. 7.
Neutralizing IL-12 has no effect on the growth of L. pneumophila in TG-macrophages from BALB/c (A) or A/J (B) mice. TG-macrophages were infected with L. pneumophila (10:1). The cells were washed, and anti-IL-12 antibodies were added. After 24 of 48 h of incubation, the cells were lysed and CFU counts were determined. Data are means and SEM of three experiments.
DISCUSSION
TG-elicited macrophages from BALB/c and A/J mice in culture have very different capacities to support the intracellular growth of L. pneumophila, with BALB/c macrophages displaying a restrictive phenotype and A/J macrophages displaying a permissive phenotype. Our results show that infected BALB/c macrophages produce IFN-γ, as demonstrated by RT-PCR and antibody neutralization studies, at a time when the cells were initiating anti-L. pneumophila activity. Furthermore, permissive A/J macrophages did not express IFN-γ mRNA, nor did anti-IFN-γ treatment affect bacterial growth. Thus, a correlation between IFN-γ production and the induction of antibacterial activity was suggested. This IFN-γ-mediated anti-Legionella activity was further demonstrated by IL-12-induced antimicrobial activity. We observed that IL-12 treatment increased anti-L. pneumophila activity in TG-macrophages from both mouse strains by a mechanism involving IFN-γ. A similar IL-12-induced antiviral activation of macrophages has been reported to also involve IFN-γ (26). Therefore, although L. pneumophila-infected macrophages from A/J and BALB/c mice differ in their initial potential for IFN-γ production, the addition of IL-12 could induce IFN-γ and a corresponding antibacterial state in both strains of macrophages.
The mechanisms involved in IFN-γ induction by BALB/c TG-macrophages, as well as an explanation of the difference between cells from the mouse strains, are unclear. The literature suggests that IL-12 is involved in IFN-γ production by macrophages. The induction of IFN-γ by M. tuberculosis in alveolar macrophages is partially blocked by anti-IL-12 antibodies (9). Furthermore, Wang et al. observed that lung macrophages from IL-12−/− mice did not produce IFN-γ when stimulated with LPS or purified protein derivative, while wild-type cells did (33). However, when IL-12 (20 pg/ml) was added to stimulated IL-12−/− cells, they produced IFN-γ. In our studies, only BALB/c macrophages produced IFN-γ in response to L. pneumophila infection even though IL-12 production by both cell types was equivalent. Although the small amount of IL-12 (∼200 pg/ml) produced might have been sufficient to induce IFN-γ, as previously demonstrated in alveolar macrophages (33), IL-12 neutralization studies (Fig. 7) suggested that endogenous IL-12 was not affecting L. pneumophila growth. Additional studies are required to determine if endogenous IL-12 is inducing IFN-γ or if an IL-12-independent mechanism of induction is involved.
The relatively low level of IL-12 p40/p70 detected in the macrophage cultures surprised us. We had previously observed that IL-12 in the nanogram-per-milliliter range was detected in the serum of mice within 3 h of a systemic infection (19). The reason for this discrepancy is unclear, but it may be due to IL-12 production by other antigen-presenting cells such as dendritic cells, as has been observed in Toxoplasma gondii (28, 29) and Leishmania donovani (16) infections. Alternately, cofactors such as cytokines or CD40/CD40L interactions that are present in vivo may be required for optimal IL-12 production. Bone marrow-derived macrophages from C57BL/6 mice require priming with IFN-γ to produce IL-12 when infected with Mycobacterium bovis BCG (10). Furthermore, macrophages from IFN regulatory factor 1 (IRF-1)-deficient mice have impaired IL-12 production that is associated with a failed Th1 development (32). These observations suggest that the autocrine IFN-γ could prime the cells for IL-12 production. However, that does not appear to have occurred in our study, because the two types of infected macrophages produced equivalent IL-12 levels while only BALB/c macrophages expressed IFN-γ mRNA.
Since the recognition that macrophages produce IFN-γ, the biological significance of this production has been questioned (15). A variety of macrophage populations have been demonstrated to produce IFN-γ, from human (9) and murine (33) alveolar macrophages to resting (7, 26) and elicited (11) murine peritoneal macrophages. The IFN-γ induction has been demonstrated to upregulate nitric oxide synthase, CD40 (22), and major histocompatibility complex class II expression (33), in addition to activating an antiviral state (26). In this study we demonstrated that IFN-γ induction caused the activation of antibacterial activity that inhibited the growth of L. pneumophila in TG-macrophage cultures. This induction explains, in part, the variation in growth patterns of L. pneumophila within macrophages from the two strains of mice and demonstrates a definite biological function for the IFN-γ. The autocrine activity of IFN-γ in macrophages therefore appears to be important during infection at least with intracellular bacteria.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant AI45169 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Aderem A, Underhill D M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Arata S, Newton C, Klein T W, Friedman H. Legionella pneumophila growth restriction in permissive macrophages cocultured with nonpermissive lipopolysaccharide-activated macrophages. Infect Immun. 1993;61:5056–5061. doi: 10.1128/iai.61.12.5056-5061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arata S, Newton C, Klein T W, Yamamoto Y, Friedman H. Legionella pneumophila induced tumor necrosis factor production in permissive versus nonpermissive macrophages. Proc Soc Exp Biol Med. 1993;203:26–29. doi: 10.3181/00379727-203-43568. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaj N, Nash T W, Horwitz M A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986;137:2662–2669. [PubMed] [Google Scholar]
- 5.Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg C. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires' disease. Am J Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 6.Brieland J K, Remick D G, LeGendre M L, Engleberg N C, Fantone J C. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous interleukin-12. Infect Immun. 1998;66:65–69. doi: 10.1128/iai.66.1.65-69.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Marzio P, Puddu P, Conti L, Belardelli F, Gessani S. Interferon gamma upregulates its own gene expression in mouse peritoneal macrophages. J Exp Med. 1994;179:1731–1736. doi: 10.1084/jem.179.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egawa K, Klein T W, Yamamoto Y, Newton C A, Friedman H. Enhanced growth restriction of Legionella pneumophila in endotoxin-treated macrophages. Proc Soc Exp Biol Med. 1992;200:338–342. doi: 10.3181/00379727-200-43439. [DOI] [PubMed] [Google Scholar]
- 9.Fenton M J, Vermeulen M W, Kim S, Burdick M, Strieter R M, Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997;65:5149–5156. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flesch I E, Hess J H, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann S H. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fultz M J, Barber S A, Dieffenbach C W, Vogel S N. Induction of IFN-gamma in macrophages by lipopolysaccharide. Int Immunol. 1993;5:1383–1392. doi: 10.1093/intimm/5.11.1383. [DOI] [PubMed] [Google Scholar]
- 12.Gebran S J, Newton C, Yamamoto Y, Widen R, Klein T W, Friedman H. Macrophage permissiveness for Legionella pneumophila growth modulated by iron. Infect Immun. 1994;62:564–568. doi: 10.1128/iai.62.2.564-568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebran S J, Yamamoto Y, McHugh S, Newton C, Klein T W, Friedman H. Differences and similarities in permissive A/J versus non-permissive BALB/c murine macrophages infected with Legionella pneumophila: the role of iron. FEMS Immunol Med Microbiol. 1994;9:7–14. doi: 10.1111/j.1574-695X.1994.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 14.Gebran S J, Yamamoto Y, Newton C, Klein T W, Friedman H. Inhibition of Legionella pneumophila growth by gamma interferon in permissive A/J mouse macrophages: role of reactive oxygen species, nitric oxide, tryptophan, and iron(III) Infect Immun. 1994;62:3197–3205. doi: 10.1128/iai.62.8.3197-3205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gessani S, Belardelli F. IFN-gamma expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998;9:117–123. doi: 10.1016/s1359-6101(98)00007-0. [DOI] [PubMed] [Google Scholar]
- 16.Gorak P M, Engwerda C R, Kaye P M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz M A, Silverstein S C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann S H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 19.Klein T W, Newton C A, Nakachi N, Friedman H. Δ9-tetrahydrocannabinol treatment suppresses immunity and early IFN-gamma, IL-12, and IL-12 receptor β2 responses to Legionella pneumophila infection. J Immunol. 2000;164:6461–6466. doi: 10.4049/jimmunol.164.12.6461. [DOI] [PubMed] [Google Scholar]
- 20.Marra A, Horwitz M A, Shuman H A. The HL-60 model for the interaction of human macrophages with the Legionnaires' disease bacterium. J Immunol. 1990;144:2738–2744. [PubMed] [Google Scholar]
- 21.Matsiota-Bernard P, Lefebre C, Sedqui M, Cornillet P, Guenounou M. Involvement of tumor necrosis factor alpha in intracellular multiplication of Legionella pneumophila in human monocytes. Infect Immun. 1993;61:4980–4983. doi: 10.1128/iai.61.12.4980-4983.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nash T W, Libby D M, Horwitz M A. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1988;140:3978–3981. [PubMed] [Google Scholar]
- 24.Nash T W, Libby D M, Horwitz M A. Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Investig. 1984;74:771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newton C, Klein T, Friedman H. The role of macrophages in THC-induced alteration of the cytokine network. Adv Exp Med Biol. 1998;437:207–214. doi: 10.1007/978-1-4615-5347-2_23. [DOI] [PubMed] [Google Scholar]
- 26.Puddu P, Fantuzzi L, Borghi P, Varano B, Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf S F, Belardelli F, Gessani S. IL-12 induces IFN-gamma expression and secretion in mouse peritoneal macrophages. J Immunol. 1997;159:3490–3497. [PubMed] [Google Scholar]
- 27.Schaible U E, Collins H L, Kaufmann S H. Confrontation between intracellular bacteria and the immune system. Adv Immunol. 1999;71:267–377. doi: 10.1016/s0065-2776(08)60405-8. [DOI] [PubMed] [Google Scholar]
- 28.Sher A, Hieny S, Charest H, Scharton-Kersten T, Collazo C, Germain R N, Reis e Sousa C. The role of dendritic cells in the initiation of host resistance to Toxoplasma gondii. Adv Exp Med Biol. 1998;452:103–110. doi: 10.1007/978-1-4615-5355-7_12. [DOI] [PubMed] [Google Scholar]
- 29.Sher A, Reis e Sousa C. Ignition of the type 1 response to intracellular infection by dendritic cell-derived interleukin-12. Eur Cytokine Netw. 1998;9:65–68. [PubMed] [Google Scholar]
- 30.Skerrett S J, Martin T R. Recombinant murine interferon-gamma reversibly activates rat alveolar macrophages to kill Legionella pneumophila. J Infect Dis. 1992;166:1354–1361. doi: 10.1093/infdis/166.6.1354. [DOI] [PubMed] [Google Scholar]
- 31.Skerrett S J, Martin T R. Roles for tumor necrosis factor alpha and nitric oxide in resistance of rat alveolar macrophages to Legionella pneumophila. Infect Immun. 1996;64:3236–3243. doi: 10.1128/iai.64.8.3236-3243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taki S, Sato T, Ogasawara K, Fukuda T, Sato M, Hida S, Suzuki G, Mitsuyama M, Shin E H, Kojima S, Taniguchi T, Asano Y. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–679. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Wakeham J, Harkness R, Xing Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J Clin Investig. 1999;103:1023–1029. doi: 10.1172/JCI6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe M, Shimamoto Y, Yoshida S, Suga K, Mizuguchi Y, Kohashi O, Yamaguchi M. Intracellular multiplication of Legionella pneumophila in HL-60 cells differentiated by 1,25-dihydroxyvitamin D3 and the effect of interferon gamma. J Leukoc Biol. 1993;54:40–46. doi: 10.1002/jlb.54.1.40. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Klein T W, Newton C A, Friedman H. Interaction of Legionella pneumophila with peritoneal macrophages from various mouse strains. Adv Exp Med Biol. 1988;239:89–98. doi: 10.1007/978-1-4757-5421-6_10. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Klein T W, Newton C A, Widen R, Friedman H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun. 1988;56:370–375. doi: 10.1128/iai.56.2.370-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Retzlaff C, He P, Klein T W, Friedman H. Quantitative reverse transcription-PCR analysis of Legionella pneumophila-induced cytokine mRNA in different macrophage populations by high-performance liquid chromatography. Clin Diagn Lab Immunol. 1995;2:18–24. doi: 10.1128/cdli.2.1.18-24.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]