Abstract
The enduring view of eosinophils, as immune effector cells whose primary function is host defence against infection by helminths and other microbial pathogens, sets the stage for a fundamental question regarding the safety of therapeutic eosinophil depletion. If eosinophils are significantly reduced or altogether depleted in an effort to alleviate the negative effects of tissue eosinophilia and eosinophilic inflammation in conditions such as asthma, COPD, chronic rhinosinusitis with nasal polyps, eosinophilic granulomatosis with polyangiitis and hypereosinophilic syndrome, would these patients become susceptible to infection or another illness? Development of mouse models in which the eosinophil lineage has been ablated, observations in patients naturally lacking eosinophils and data from studies of eosinophil-depleting medical therapies indicate that the absence of eosinophils is not detrimental to health. The evidence available to date, as presented in this review, supports the conclusion that even if certain homeostatic roles for the eosinophil may be demonstrable in controlled animal models and human in vitro settings, the evolution of the human species appears to have provided sufficient immune redundancy such that one may be hale and hearty without eosinophils.
Short abstract
This review of accumulated evidence for eosinophil depletion in mouse models and human clinical trials affirms that targeting eosinophils to treat eosinophil-associated diseases has therapeutic value without detrimental effects on health https://bit.ly/3biObDI
Introduction
The eosinophil is enjoying a period of renaissance as scientific discoveries, aided by model systems and pharmacological interventions, uncover a world of heretofore unknown functions in health and disease. In simplest terms, eosinophils are granulocytes of the myeloid lineage that develop from interleukin (IL)-5 receptor α (IL-5Rα)-expressing progenitor cells (figure 1) [1–3]. The process of differentiation, maturation and migration from their site of origin in bone marrow involves a specific constellation of transcription factors and cytokines, with IL-5 playing a prominent role. Once in circulation, where they have finite residency (half-life 8–18 h), eosinophils are recruited by chemokines such as eotaxin-1, -2 and -3 (chemokine (CC motif) ligand CCL11, CCL24 and CCL26, respectively) to peripheral tissue, where they survive for an estimated 2–5 days [4]. Whereas much is known regarding basic eosinophil biology and the processes surrounding eosinophil development, the actions of eosinophils residing in peripheral tissue have been an open question and subject of debate.
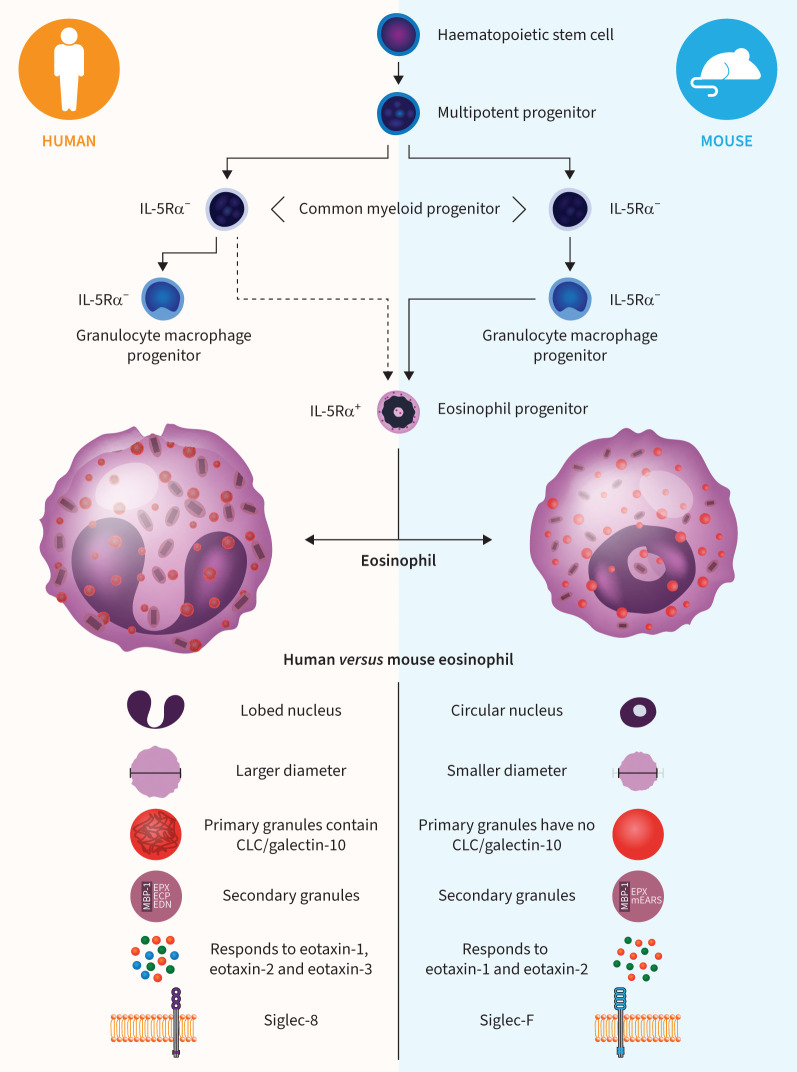
FIGURE 1.
Development and characteristics of human and mouse eosinophils [1–3]. Several differences have been noted between mature eosinophils from humans versus mice. Human eosinophils are larger than mouse eosinophils, stain more intensely with eosin and have a lobed rather than a circular nucleus. Primary granules in human (but not mouse) eosinophils contain Charcot–Leyden crystal (CLC) protein/galectin-10. The dense core of secondary granules in both human and mouse eosinophils is predominantly composed of major basic protein-1 (MBP-1). Eosinophil-associated RNases in human secondary granules include eosinophil protein X (EPX), eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN). Eosinophil peroxidase is stored in secondary granules in both human and mouse eosinophils. Human eosinophils respond to eotaxin-1 (CCL11), -2 (CCL24) and -3 (CCL26), whereas mouse eosinophils respond to eotaxin-1 and -2. Among cell surface molecule differences, human eosinophils express Siglec-8 and mouse eosinophils express Siglec-F. CCL: chemokine (CC motif) ligand; IL-5Rα: interleukin-5 receptor α; mEARS: mouse eosinophil associated RNases. Development schematic adapted from Lee et al. [1] with permssion.
Conventional wisdom ascribes the primary role of eosinophils as host defence against helminth infection [5]. Eosinophils are believed to migrate to the site of infection in reaction to the signalling cascade induced by an antigen-specific type 2 (T2) immune response. Eosinophils directly interact with the parasite via an antibody-mediated process, resulting in the release of cytotoxic substances, including granule proteins, that damage the cell membrane and increase its permeability [6]. Eosinophils have also been implicated in host defence against viral, bacterial and fungal pathogens through mechanisms distinct from that of the response to helminths (reviewed in [7–9]). As we are beginning to understand, however, combatting infection is only one aspect of the eosinophil story.
The routine finding of eosinophils throughout the body, predominantly in the mucosal lining of the digestive tract, but also in adipose tissue, airways, mammary glands, thymus and uterus [10], in the absence of infective challenge, raises the potential for involvement of eosinophils in activities other than host defence, i.e. maintenance of homeostasis (figure 2) [11]. Support for the functional significance of eosinophils residing in peripheral tissue comes from eosinophil-deficient mice in which defects in the intestinal mucus shield, alterations in the microbiome and differences in size of Peyer's patches have been observed [12–14]. Studies using eosinophil-deficient mice also suggest a role for eosinophils in the regulation of homeostasis in adipose tissue [15, 16]. In mouse models, elevated eosinophils in adipose tissue are ostensibly favourable to metabolic health, leading to enhanced thermogenesis and glucose sensitivity and decreased weight gain, while a lack of eosinophils at homeostasis has been correlated with negative impacts on metabolic health, such as reduced production of thermogenic fat [3, 17]. However, the data are unclear regarding the role of eosinophils in adipose tissue and homeostasis in humans [18–20]. Differences in the timing and method of eosinophil alteration may explain some of these conflicting results. To date, there is no evidence that eosinophil depletion in humans results in gastrointestinal issues, change in adipose tissue, change in body weight or other homeostatic dysregulation [3, 21].
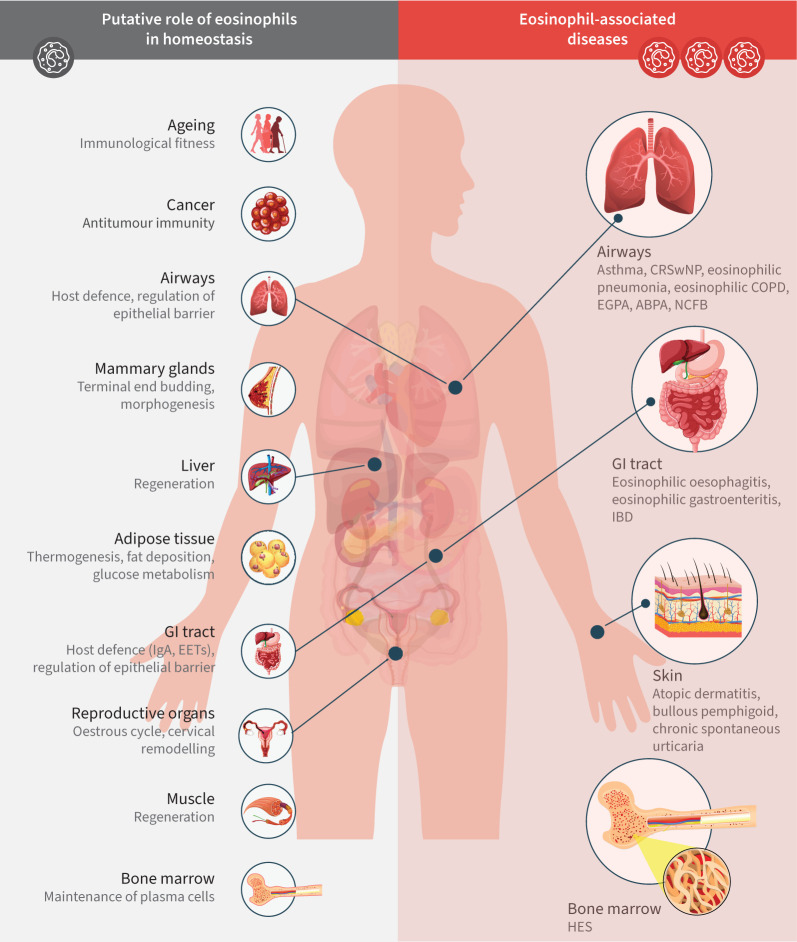
FIGURE 2.
Putative role for eosinophils in homeostasis and eosinophil-associated diseases. Homeostatic roles for eosinophils illustrated in this figure are supported by data from animal models and human in vitro studies. ABPA: allergic bronchopulmonary aspergillosis; CRSwNP: chronic rhinosinusitis with nasal polyps; EET: extracellular trap; EGPA: eosinophilic granulomatosis with polyangiitis; GI: gastrointestinal; HES: hypereosinophilic syndrome; IBD: inflammatory bowel disease; NCFB: non-cystic fibrosis bronchiectasis. Adapted from Jackson and Munitz [11].
A particular concern for the clinical community regarding use of a therapy that suppresses eosinophil numbers is the observation of eosinophil infiltration in various tumour types. Whether this finding is favourable, unfavourable or neutral to the host currently remains unclear and is likely to vary across cancer types.
Standing in contrast to the protective and homeostatic functions ascribed to eosinophils is their involvement in disease pathogenesis. Eosinophils have long been associated with a functional duality: exerting protective effects against parasitic infection yet contributing to pathology in allergic disease [22, 23]. As effector cells, eosinophils respond to immune cascade activation triggered by antigens presented by certain allergens, helminths or other microbial pathogens. The resulting eosinophil degranulation releases cytotoxic substances that are believed to be beneficial in combatting pathogens but damaging to surrounding healthy tissue. The damaging effect is tissue specific. For example, epithelial denudation and mucus plugging is functionally most important in the lower airway [24] and tissue oedema in the upper airway. The presence of peripheral or tissue-specific eosinophilia and/or eosinophilic inflammation not related to infective sources is found in conditions such as asthma, COPD, chronic rhinosinusitis with nasal polyps (CRSwNP), eosinophilic granulomatosis with polyangiitis (EGPA) and hypereosinophilic syndrome (HES) (figure 2) [22, 23, 25, 26]. As eosinophils are believed to have a direct, causal relationship to the tissue damage and disease manifestations observed in these conditions, eosinophil depletion is a logical means of therapeutic intervention. Indeed, clinical trials have yielded positive results across a variety of conditions whose pathogenesis is believed to be related to eosinophilic immune dysfunction (table 1) [27–47] and several eosinophil-targeted therapies are approved for clinical use [48, 49].
TABLE 1.
Extent of eosinophil depletion with medical therapies
| Drug | Target | Population | Eosinophil reduction | |
| Blood | Other | |||
| Benralizumab | IL-5R | Asthma [27, 28] | ↓↓↓↓ | ↓↓↓ (airway), |
| ↓↓↓↓ (sputum) | ||||
| COPD [29] | ↓↓↓↓ | ↓↓↓↓ (sputum) | ||
| CRSwNP [30] | ↓↓↓↓ | |||
| EGPA [31] | ↓↓↓↓ | |||
| HES [32, 33] | ↓↓↓↓ | ↓↓↓↓ (GI) | ||
| Mepolizumab | IL-5 | Asthma [28, 34, 35] | ↓↓↓ | ↓ (sputum) |
| COPD [36] | ↓↓↓ | |||
| CRSwNP [37] | ↓↓↓ | |||
| EGPA [38] | ↓↓↓ | |||
| HES [39] | ↓↓↓ | |||
| Reslizumab | IL-5 | Asthma [40–42] | ↓↓↓ | ↓↓ (sputum) |
| Dexpramipexole | Unknown mechanism | CRSwNP [43] | ↓–↓↓↓↓# | ↓↓↓↓ (nasal tissue) |
| HES [44] | 0–↓↓↓↓¶ | |||
| Imatinib | Tyrosine kinase | CEL/HES [45] | 0–↓↓↓↓¶ | |
| Systemic glucocorticoids | Glucocorticoid receptors | Asthma [46, 47] | ↓–↓↓↓↓+ | |
↓: <50% reduction; ↓↓: 50–79% reduction; ↓↓↓: 80–94% reduction; ↓↓↓↓: ≥95% reduction/near-complete depletion. IL-5R: interleukin-5 receptor; CRSwNP: chronic rhinosinusitis with nasal polyps; EGPA: eosinophilic granulomatosis with polyangiitis; HES: hypereosinophilic syndrome; GI: gastrointestinal; CEL: chronic eosinophilic leukaemia. #: approximately two-thirds of patients demonstrated >95% eosinophil reduction and the other one-third were partial responders (30–50% eosinophil reduction); ¶: responses varied from profound reductions (e.g. eosinophil counts ≤10 cells·µL−1) to no effect; +: reductions in eosinophils are dose dependent and related to baseline levels [47].
Earlier research suggested that eosinophils acquire distinct phenotypes, and both resident and inflammatory eosinophils were identified in the lungs of mouse models [50]. Biologics directed at IL-5 were believed to target and deplete inflammatory eosinophils while sparing resident eosinophils. Evidence from a recent study of allergen-challenged mice contradicts previous research, demonstrating that anti-IL-5 therapy reduces all lung populations of eosinophils regardless of phenotype [51]. In view of that, these new data also indicate that eosinophilic phenotypes may exist on a continuum rather than as distinct cellular subtypes.
Honing in on the pathogenic aspects of eosinophils neglects their putative protective role and raises an important question: would the potential benefits of eosinophil depletion be offset by functional loss? In this review, we approach this question by delving into the current state of knowledge regarding the effects of eosinophil depletion, deriving insights from mouse models of eosinophil lineage ablation, naturally occurring conditions associated with eosinophil absence and clinical data from medical therapy-induced eosinophil reduction/depletion in humans. Although eosinophils have been implicated in multiple aspects of health and homeostasis, for the purposes of determining the benefits and risks of eosinophil depletion, we focus mainly on infection and malignancy, given the clinical concerns in these areas and the lack of any direct human data supporting a key role for eosinophils in other aspects of physiology.
Methods
Relevant studies were identified through query of the MEDLINE database for English language articles published from 2000 to 2021 using the terms “eosinophil depletion”, “eosinophil lineage ablation” or “eosinophil” and “PHIL”, “ΔdblGATA”, “GATA-1 deletion”, “benralizumab”, “mepolizumab”, “reslizumab”, “dexpramipexole”, “imatinib”, “glucocorticoid” or “systemic corticosteroid”. Publications describing the safety of eosinophil-depleting therapies were identified through searches for “benralizumab”, “mepolizumab” or “reslizumab” in combination with “long-term”, “safety”, “infection”, “helminth”, “malignancy”, “cancer”, “coronavirus disease 2019 (COVID-19)” or “pregnancy”. Studies were selected for discussion in this review based on topical relevance. Publications cited by articles identified through the search strategy were included, as appropriate.
Predicting the consequences of eosinophil depletion
Helminth and opportunistic infections
The foundation of concern regarding eosinophil depletion is rooted in the putative function of these cells in combatting infection by parasites and other pathogens. Support for the importance of eosinophils comes from the observation that blood and tissue eosinophil counts increase in response to helminth infection [5]. Emerging data from in vitro studies and animal models also suggest that eosinophils have antiviral properties, but the applicability and relevance of these findings to the immune response in humans has not been established [52]. Nonetheless, if a primary function of eosinophils is host defence against parasitic and other infections, reducing or depleting eosinophils would be expected to increase infection risk.
Beyond the mechanistic rationale, there are clinical reasons to regard therapeutic eosinophil depletion with scepticism when viewed through the lens of infectious disease risk. Opportunistic infections, particularly Pneumocystis jirovecii pneumonia, herpes zoster and tuberculosis, are known hazards associated with glucocorticoids [53], a drug class affecting eosinophil generation, survival and function [54]. Glucocorticoids are also associated with the development of hyperinfection syndrome, a potentially life-threatening condition in which helminth autoinfection is accelerated, leading to excessive worm burden [55]. However, an important caveat when discussing immune function and infection risk associated with glucocorticoids is that eosinophil suppression is only one aspect of their broad immunosuppressive effects [53]. Glucocorticoids alter the differentiation, activation and/or function of multiple immune cell types, including eosinophils, macrophages, neutrophils, lymphocytes and dendritic cells [53]. Hence, observations regarding infection risk with glucocorticoids cannot be attributed solely to their effects on eosinophils.
Malignancies
Another question regarding eosinophil depletion is its potential effect on malignancy risk. As noted earlier, data are mixed regarding whether eosinophil infiltration is potentially a favourable, unfavourable or neutral finding [56, 57]. Mechanistically, the forces driving tumour infiltration by eosinophils and the role eosinophils play in tumour biology have not been established [58]. Consequently, any proposed effect of eosinophil depletion on malignancy risk is currently speculative.
Comparing and contrasting mouse and human eosinophils
Mouse and human eosinophils share many common features, including a similar developmental pathway from haematopoietic stem cell to IL-5Rα+ eosinophil progenitor and, ultimately, mature eosinophil [1–3]. In mice, granulocyte macrophage progenitors (GCPs) become eosinophil progenitors, whereas in humans, GCPs do not differentiate into eosinophils. Although generally similar in structure and appearance, mature human and mouse eosinophils have some notable differences (figure 1). Whether these differences are important in relation to the behaviour of the eosinophil in health or disease, or in the context of eosinophil depletion through actions on other immune mediators, remains to be determined. However, it is reasonable to assume that at least some of the species-related differences described herein underpin why the theoretical consequences of eosinophil depletion predicted by some experimental mouse models have not been observed in humans.
Mouse models of eosinophil depletion
Heretofore unknown insights into the role of eosinophils in health and disease were made possible by the creation of two mouse models, ΔdblGATA and PHIL, in the early 2000s [59, 60]. The consequences (or lack thereof) of eosinophil depletion were observed both in the general health of the ΔdblGATA and PHIL mice and from subsequent study of these animals under various experimental conditions (table 2) [59–77].
TABLE 2.
Highlights of findings from mouse models of eosinophil depletion
| Mouse model | Study | Intervention | Key findings |
| ΔdblGATA | Yu et al. [59], 2002 | NA | Eosinophils absent; no overt defects (viable and fertile; normal lifespan) |
| PHIL | Lee et al. [60], 2004 | NA | Eosinophils absent; no overt defects (viable and fertile; normal lifespan); reduced pulmonary mucus accumulation and airway hyperresponsiveness in response to allergen challenge |
| ΔdblGATA and PHIL | Swartz et al. [61], 2006 | Helminth infection (Schistosoma mansoni) | No effect on traditional measures of infection; increased IL-5 expression versus wild-type controls |
| PHIL | O’Connell et al. [62], 2011 | Helminth infection (Strongyloides stercoralis) | Similar primary and secondary immune responses versus wild-type controls; larval killing was impaired in PHIL mice treated with neutrophil-depleting antibodies |
| PHIL | Cadman et al. [63], 2014 | Helminth infection (Brugia malayi) | Compared with wild-type controls, infected PHIL mice had longer survival of microfilariae during primary infection, augmented parasite-induced IgE response, increased goblet cell mucus production and reduced airway physiology changes |
| ΔdblGATA | Knott et al. [64], 2007 | Helminth infection (Nippostrongylus brasiliensis) | Compared with wild-type controls, infected ΔdblGATA mice had greater worm burden and egg deposition, less resistance to secondary lung infection, and similar expulsion of worms from primary/secondary infection |
| ΔdblGATA | Huang et al. [65], 2015 | Helminth infection (Trichinella spiralis) | Compared with wild-type controls, infected ΔdblGATA mice had similar adult worm clearance in primary/secondary infection and reduced resistance to skeletal muscle larvae accumulation |
| ΔdblGATA | Frohberger et al. [66], 2019 | Helminth infection (Litomosoides sigmodontis) | Compared with wild-type controls, infected ΔdblGATA mice had greater microfilaraemia and higher adult worm counts |
| ΔdblGATA and PHIL | Fabre et al. [67], 2009 | Helminth infection (T. spiralis) | Increased death of larvae in skeletal muscle was observed in ΔdblGATA and PHIL mice compared with wild-type controls |
| ΔdblGATA and PHIL | Gebreselassie et al. [68], 2012 | Helminth infection (T. spiralis) | Transfer of eosinophils from infected IL-5 transgenic mice to infected ΔdblGATA and PHIL mice improved larvae growth and survival |
| PHIL | Drake et al. [69], 2016 | Viral infection (parainfluenza 1) | Reduced parainfluenza virus RNA after ovalbumin sensitisation and resulting eosinophil recruitment were observed in virus-infected wild-type controls but not PHIL mice |
| ΔdblGATA | Ma et al. [70], 2018 | Viral infection (influenza A/HK/1/68) | ΔdblGATA and wild-type controls both succumbed to influenza infection despite repeated exposure to the fungus Alternaria alternata to stimulate eosinophil recruitment to nasal passages |
| ΔdblGATA | Yordanova et al. [71], 2021 | Protozoan parasite infection (Giardia muris) | Measures of parasite control were similar in infected ΔdblGATA mice and wild-type controls |
| PHIL | Arnold et al. [72], 2018 | Bacterial infection (Helicobacter pylori, Citrobacter rodentium) | Compared with wild-type controls, infected PHIL mice had lower (H. pylori) or higher (C. rodentium) bacterial colonisation in the gastrointestinal tract, increased inflammation and Th1 response, and more severe C. rodentium-induced colitis (consistent with greater immune activation) |
| ΔdblGATA | Gestal et al. [73], 2018 | Bacterial infection (Bordetella bronchiseptica) | No difference was observed in colonisation dynamics between ΔdblGATA mice and wild-type controls; differences were observed when mice were infected with a mutant B. bronchiseptica strain |
| ΔdblGATA | O’Dea et al. [74], 2014 | Fungal infection (Aspergillus fumigatus) | In this neutropenic infection model, ΔdblGATA mice experienced reduced fungal burden and mortality compared with wild-type controls |
| ΔdblGATA | Amarsaikhan et al. [75], 2017 | Fungal infection (A. fumigatus) | In this neutropenic infection model, caspofungin-induced pulmonary aspergillosis was less severe and fungal burden was lower in ΔdblGATA mice versus wild-type controls |
| ΔdblGATA | Dietschmann et al. [76], 2020 | Fungal exposure (A. fumigatus) | Compared with wild-type controls, infected ΔdblGATA mice had lower IL-4, IL-17 and IL-23 expression in the lung and reduced number of Th2 cells in the lung parenchyma, and similar expression of goblet cell markers, number of mucin-positive cells and lung weight increase |
| ΔdblGATA | Wang et al. [77], 2021 | Fungal exposure (A. fumigatus) | Compared with wild-type controls, infected ΔdblGATA mice had lower IL-4, IL-5 and IL-13 expression in bronchoalveolar lavage fluid and lung tissue, reduced expression of endoplasmic reticulum stress markers, milder increases in apoptosis and less lung autophagy |
NA: not applicable; IL: interleukin; Th1/2: T-helper 1/2.
The first of these models, ΔdblGATA, was generated by deletion of a high-affinity, double GATA binding site located in the promoter region of GATA-1 (erythroid transcription factor), which is presumed to be involved in autoregulation of GATA-1 expression [59]. GATA-1 is a transcription factor expressed in several haematopoietic cell lineages, including erythroid cells, megakaryocytes, mast cells and eosinophils [78, 79]. In vitro studies have shown that the level of GATA-1 expression is linked to lineage specification: low GATA-1 protein concentrations fail to induce mature phenotypes in avian myelomonocytic cell lines, whereas intermediate concentrations result in formation of eosinophils and higher concentrations yield thromboblasts and probably erythroblasts [78]. Complete absence of GATA-1 during development, as observed in a separate transgenic mouse model, results in embryonic lethality [79]. Homozygous (female)/hemizygous (male) ΔdblGATA mice lack eosinophils, yet erythroid cells, megakaryocytes and mast cells are unaffected or only modestly perturbed [59]. ΔdblGATA mice are viable and fertile with no overt defects aside from the absence of eosinophils.
The PHIL mouse was created by introducing a transgenic construct in which the eosinophil peroxidase promoter was used to drive diphtheria toxin A expression, resulting in the death of eosinophil lineage-committed cells [60]. PHIL mice lack eosinophils but not other haematopoietically derived cells. Like ΔdblGATA mice, PHIL mice are viable and fertile with no overt health issues and a normal lifespan. Allergen challenge in these mice demonstrated that eosinophils contribute to pulmonary mucus accumulation and are necessary for airway hyperresponsiveness, both of which are characteristic features of asthma.
Eosinophil depletion in humans
The consequences of eosinophil depletion in humans can be studied through two routes: 1) by assessing the effects of naturally occurring inherited or acquired cases of eosinophil absence, or 2) by reducing/depleting eosinophil counts via medical therapies. Naturally occurring eosinophil deficiency is uncommon in humans [80]. Rare cases have been reported, as reviewed by Gleich et al. [80], which generally occur in the context of other immune system deficiencies (e.g. basophil absence) and/or gammaglobulinaemia. Hence, the conclusions that can be drawn regarding eosinophil depletion in these patients are limited by the coexistence of other immune system irregularities. A small number of patients have allergic disease and absence of eosinophils without evidence of other immune system abnormalities [80]. The clinical course of these patients in most cases was unremarkable aside from the presence of allergic disease.
Multiple currently available medical therapies reduce eosinophil counts in blood and tissue. These include the anti-IL-5 monoclonal antibodies (mAbs) mepolizumab and reslizumab, the anti-IL-5R mAb benralizumab, the tyrosine kinase inhibitor imatinib, the oral therapy dexpramipexole and corticosteroids. The degree of eosinophil reduction varies by agent, consistent with differences in their mechanisms of action (table 1). Eosinophil reduction with dexpramipexole and imatinib depends on the individual, with some patients demonstrating high levels of eosinophil suppression and others only modest or moderate effects [43–45]. Therapies not specifically designed to target eosinophils are also prone to affecting multiple immune cell types such as reduction of basophil counts and, in some cases, neutrophil counts by dexpramipexole [81] or the alteration of differentiation, activation, apoptosis and/or function of macrophages, neutrophils, lymphocytes and dendritic cells by glucocorticoids [53].
Overall, the most rapid and profound eosinophil-specific depletion occurs with the anti-IL-5Rα mAb benralizumab [27, 28]. Benralizumab has been studied in patients with asthma [82–86], COPD [29, 87], CRSwNP [30], EGPA [31] and HES [32], accumulating thousands of patient-years of exposure data. In clinical trials and real-world studies, treatment with benralizumab resulted in complete or near-complete depletion of blood eosinophils [27–32, 88, 89]. When compared directly in patients with severe eosinophilic asthma and a baseline blood eosinophil count >300 cells·µL−1, the onset of effect for benralizumab, as measured by time to achieve a 50% reduction in blood eosinophil count, was rapid (1.7±0.7 h) and similar to that of prednisone (2.5±0.3 h) but significantly faster than mepolizumab (25.8±14.3 h; p<0.001) [28]. 30 days after receiving treatment, geometric mean blood eosinophil counts were 8±2.8 cells·µL−1 in the benralizumab treatment group and 92±1.7 cells·µL−1 in the mepolizumab treatment group (p=0.002 for the comparison between treatment groups). Benralizumab also significantly depleted tissue eosinophils [27, 32, 33]. Among adults with eosinophilic asthma, benralizumab reduced airway eosinophil counts by a median of 95.8% [27]. In a study of patients with HES and gastrointestinal tissue eosinophilia, eosinophils were completely depleted in all segments of the gastrointestinal tract following treatment with benralizumab [33]. Given these observations, patients who have received benralizumab are a valuable source of data regarding the effects of eosinophil depletion in humans.
Effect of eosinophil depletion on susceptibility to helminth infections
Evidence from mouse models
Susceptibility to and immune responses of PHIL and ΔdblGATA mice have been tested using a variety of helminths, including those that naturally infect rodents (e.g. Trichinella spiralis) and those that do not (e.g. Strongyloides stercoralis) (table 2). In general, mice lacking eosinophils displayed appropriate primary and secondary responses to helminth infections. Absence of eosinophils in ΔdblGATA and PHIL mice had no influence on measures of infection by the helminth Schistosoma mansoni [61]. Worm burden and egg deposition in S. mansoni-infected eosinophil lineage-ablated mice were similar to those in wild-type controls. Granuloma formation in the liver only differed in cell type composition, with a notable absence of eosinophils in ΔdblGATA and PHIL mice. Similarly, primary and secondary immune responses were comparable in PHIL mice and wild-type controls infected with S. stercoralis [62]. Larval killing was impeded, however, in PHIL mice treated with a neutrophil-depleting antibody [62].
These findings suggest that response to helminth infection has intrinsic redundancy between the functions of eosinophils and neutrophils [90]. Helminths are one of numerous pathogens that are likely drivers of immune system diversification, which is an essential component of surviving infection. In tissue, helminths may be open to attack by the full range of innate immune responders, including neutrophils, eosinophils, basophils, macrophages and platelets, and the role of each immune cell type depends on the tissue type and the helminth species involved. This is likely to create a degree of immune redundancy. In general, eosinophils appear to contribute to and augment the immune response rather than functioning as a central component and thus are not absolutely required for helminth larval killing.
In response to infection with Brugia malayi, PHIL mice experienced longer survival of microfilariae during primary infection, augmented parasite-induced IgE response and increased goblet cell mucus production compared with wild-type controls, yet airway physiology changes were reduced in PHIL mice [63]. ΔdblGATA mice infected with Nippostrongylus brasiliensis had greater worm burden and egg deposition and demonstrated less resistance to secondary lung infection compared with wild-type controls; however, expulsion of worms from primary and secondary infection was unaffected [64]. Similarly, adult worm clearance was unaffected in ΔdblGATA mice with primary or secondary T. spiralis infection, but resistance to accumulation of larvae in skeletal muscle was reduced compared with wild-type controls [65]. Greater microfilaraemia and higher adult worm counts were also reported in ΔdblGATA mice infected with Litomosoides sigmodontis compared with wild-type controls [66].
Interestingly, experimental data from ΔdblGATA and PHIL mice suggest that eosinophils may support parasite growth and survival [67, 68]. Fabre et al. [67] reported that T. spiralis larvae that had infiltrated skeletal muscle cells died in large numbers between 15 and 22 days post-infection in both ΔdblGATA and PHIL mice compared with wild-type controls, suggesting that eosinophils are necessary for helminth survival and sustaining chronic infection. In a follow-up study, Gebreselassie et al. [68] observed that transfer of eosinophils from T. spiralis-infected IL-5 transgenic mice (which overexpress IL-5) to T. spiralis-infected ΔdblGATA and PHIL mice improved larvae growth and survival. These data stand in sharp contrast with the traditional view that eosinophils combat rather than foster helminth infection and may suggest that certain helminths have evolved to utilise the eosinophilic environment they promote as a mechanism of survival.
The interpretation of these results must take into account the caveats of the model system. First, the helminths used to evaluate immune response in eosinophil-depleted mice were not all pathogens that normally infect rodents. For example, mice are not a natural host for S. stercoralis and although this parasite can infect mice, it does not complete a normal infection cycle [91]. Notably, the experiments in which eosinophils appeared to support helminth growth and survival did involve a natural rodent pathogen (T. spiralis). Second, strain background effects account for some differences in findings between ΔdblGATA and PHIL mice [92], and suggest an influence of genetic variation on infection response. Third, to the frustration of the scientific community, observations in mice are not always replicated in humans.
Data from clinical trials
To date, biologic therapies that specifically reduce eosinophil counts have not been associated with an increase in helminth infections (table 3) [48, 82, 85, 93–99]. In placebo-controlled and long-term studies of benralizumab, mepolizumab and reslizumab, no cases of helminth infections were reported. Given the extent of effect, benralizumab, which generates complete or near-complete depletion of circulating eosinophils [27–32], affords the best opportunity to examine the safety of eosinophil depletion in humans. Across patient populations, studies have shown that treatment with benralizumab did not increase helminth or opportunistic infection risk, even in geographic areas where parasitic infections are more common, such as South America, Africa and Southeast Asia [48]. Indeed, to date, not a single case of helminth infection during treatment with benralizumab has been reported in the literature. However, the fact that hyperinfection syndrome is a well-recognised complication of systemic corticosteroid use in individuals infected with S. stercoralis [100] signifies that broad inhibition of T2 inflammation has the ability to significantly impact antihelminth immunity, even if more targeted antieosinophil therapy does not. Interestingly, the only phase 3 trial of an anti-T2 biologic to highlight a numerically increased rate of parasitic infection was with the anti-IL-4R mAb dupilumab in the setting of paediatric moderate-to-severe asthma. In this trial, seven cases of parasitic infections were recorded in the dupilumab arm (2.6%) compared with zero cases in the placebo arm [101]. Mechanistically, this aligns with the results of experimental infection of IL-4/IL-13-deficient mice with N. brasiliensis, showing impaired worm expulsion and the finding that IL-4/IL-13 produced by innate cells is required and sufficient for effective T2 immune responses against infection in this setting [102].
TABLE 3.
Infection and malignancy findings from long-term biologic therapy clinical trials
| Drug and study | Population (exposure duration) | Helminth infections | Opportunistic infections | Malignancy |
| Benralizumab | ||||
| BORA [82] | Asthma (2 years) | None | Incidence similar in years 1 and 2 (no percentage cited) | Incidence <1% |
| SIROCCO/CALIMA/BORA [48, 93] | Asthma (2 years) | None | No overall incidence cited (0.6% herpes zoster) | Incidence <1% |
| ZONDA/BORA [94] | Asthma (1.6 years) | None | No overall incidence cited | None |
| MELTEMI [85] | Asthma (up to 5 years) | None | Infection incidence did not increase with longer drug exposure | Incidence <1% |
| Mepolizumab | ||||
| COSMOS [95] | Asthma (1.5 years) | None | No overall incidence cited | None |
| COLUMBA [96] | Asthma (3.5 years) | None | Incidence 7% (2% herpes zoster; one serious event) | Incidence 2%, consistent with age- and sex-adjusted SEER data |
| COSMEX [97] | Asthma (up to 4.8 years) | None | Incidence 4% (0.9% herpes zoster) | Incidence 2% |
| MHE100901 [98] | HES (4.8 years) | None | Incidence 1% | Greater incidence versus SEER may reflect risk in HES |
| Reslizumab | ||||
| Long-term extension [99] | Asthma (up to 2 years) | None | None | Incidence 1%, consistent with population expectations |
SEER: Surveillance, Epidemiology, and End Results; HES: hypereosinophilic syndrome.
Influence of eosinophil depletion on susceptibility to non-helminth infections
Studies in mouse models
Multiple studies have assessed responses of ΔdblGATA and PHIL mice to viruses, protozoa and bacteria (table 2). Results for viral and protozoan pathogens have generally shown no differences between eosinophil-depleted mice and wild-type controls [69–71]. Regarding the effects of eosinophil depletion on bacterial colonisation, differences have been reported in gastrointestinal microbiota composition of eosinophil-deficient mice compared with wild-type controls [12–14]. The significance of this finding is unknown, as the differences are not associated with deleterious effects on health [14]. In an experimental infection model using Helicobacter pylori or Citrobacter rodentium, PHIL mice demonstrated greater inflammatory and T-helper 1 responses compared with wild-type controls, yet whether eosinophil depletion increased or decreased gastrointestinal colonisation differed between the bacterial pathogens [72]. No difference in colonisation was observed when eosinophil-deficient mice and wild-type controls were challenged with Bordetella bronchiseptica [73]. Overall, the sparse and inconsistent data provide insufficient support for a key role of eosinophils in host defence against viral, protozoan and bacterial pathogens.
Response to fungal infections in eosinophil-deficient mice has been most widely studied in the context of bronchopulmonary aspergillosis. In a neutropenic mouse model of invasive aspergillosis, eosinophil-deficient mice experienced reduced fungal burden and mortality compared with wild-type mice [74]. A subsequent study showed that caspofungin-induced pulmonary aspergillosis was less severe and had a lower fungal burden in eosinophil-deficient neutropenic mice versus wild-type controls, suggesting that eosinophils are detrimental to fungal clearance in this context [75]. In non-neutropenic mice, eosinophil deficiency was associated with attenuated T2 inflammatory response to intranasal inoculation with Aspergillus fumigatus [76, 77]. These data suggest that eosinophils are involved in response to fungal infections but that their absence may in fact be beneficial.
Data from clinical trials
Eosinophil-depleting, anti-IL-5/5R therapies have not been associated with an increased risk for non-helminth infections, including protozoan, bacterial or fungal pathogens (table 3). Among patients with asthma, a population already at increased pneumonia risk through use of inhaled corticosteroids, the incidence of pneumonia in long-term studies was low [48, 82, 93–97, 99]. Moreover, data from an integrated analysis of the MELTEMI long-term extension and its predecessor studies showed that greater benralizumab exposure did not increase serious infection risk [85].
However, there have been conflicting data suggesting that eosinophils may have a role in host antiviral response. The MATERIAL study, which evaluated experimental rhinovirus-induced immune responses in patients with mild asthma (n=37) receiving mepolizumab or placebo, reported an increase in virus load on day 7 post-infection for mepolizumab-treated patients [103]. Although the inference is that there is an anti-IL-5-driven impairment of the mechanisms limiting viral replication, the investigators did not measure virus load at any earlier time post-inoculation. Critically, rhinovirus replication is known to peak at day 2–3 following inoculation in patients with asthma, making an isolated day 7 measure very difficult to interpret [104]. Notably, although it is reasonable to assume that most individuals experience one or more upper respiratory tract viral infections each year (the dominant cause of asthma exacerbations), the integrated MELTEMI analysis revealed that of patients who received benralizumab, ≥75% had zero asthma exacerbations annually and 59% had zero exacerbations throughout the entire integrated analysis period (up to 5 years). These observations are in keeping with the finding that the pattern recognition receptor Toll-like receptor 7, which is known to be important in sensing rhinovirus, is inhibited by eosinophilic inflammation, resulting in impairment of antiviral interferon responses [105].
Interestingly, there are data to suggest that eosinophils from patients with asthma, particularly those with severe disease, are less able to bind and inactivate respiratory viruses [106]; hence, eosinophils from these patients may play a lesser role in host defence in this context. However, because eosinophils in patients with asthma are already primed, they may have enhanced cytotoxic effects that contribute to virus-induced asthma exacerbations [106].
Among opportunistic infections, herpes zoster was the only pathogen whose occurrence was specifically noted in clinical trials of anti-IL-5/5R therapies (table 3). Herpes zoster infections were infrequent (≤2%) and the risk of opportunistic infection did not appear to increase with time on treatment [48, 82, 85, 93–99]. In benralizumab clinical trials, no increase in opportunistic infection risk was observed [48]. Analysis of phase 3 data showed similar rates of viral infections in patients who received benralizumab versus placebo [48, 87]. Although disseminated herpes zoster infection following benralizumab treatment has been described in a case report [107], an increased infection rate has not been observed in clinical trials [48, 85, 94] or post-marketing surveillance [108]. The patient described in the case report was also receiving prednisolone (unspecified dose) and cyclophosphamide [107], which will have contributed to infection susceptibility.
Biologic therapies and COVID-19
Early in the COVID-19 pandemic, blood eosinopenia findings were reported among hospitalised patients. The significance of this has been debated; the question of whether to maintain eosinophil-depleting therapies amid the COVID-19 pandemic was an initial topic of discussion within the clinical community using these therapies [109]. The greater likelihood of COVID-19 signs and symptoms at hospital admission and the longer hospitalisation and course of disease in patients with low versus normal eosinophil counts have marked eosinopenia as a putative prognostic indicator [109]. However, a nationwide cohort study conducted in Israel found that use of biologic therapies was not associated with increased risk of moderate-to-severe COVID-19 or of the composite end-point of moderate-to-severe COVID-19 or all-cause 90-day mortality in a population of patients with asthma who had tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (n=8242) [110]. Neither biologic therapies nor systemic corticosteroids were associated with increased risk for SARS-CoV-2 infection, but recent chronic systemic glucocorticoid use was associated with both increased risk for moderate-to-severe COVID-19 and increased risk for the composite end-point of moderate-to-severe COVID-19 or all-cause 90-day mortality. A single-centre analysis of 24 adults in the UK who developed confirmed SARS-CoV-2 infection (9% of the overall study population) while on treatment with benralizumab for severe eosinophilic asthma reported no serious outcomes of infection (i.e. requirement for intensive care unit admission, use of mechanical ventilation or death) [111]. In total, 75% of patients had mild infections that did not require hospitalisation; however, this is likely to have been underestimated because other patients in the study population may have experienced asymptomatic infections or not pursued confirmatory PCR testing if they experienced only very mild symptoms.
Interestingly, benralizumab was successfully used in the treatment of drug rash with eosinophilia and systemic symptoms (DRESS) in three patients with COVID-19 [112, 113]. All three patients were unresponsive to glucocorticoid treatment but experienced rapid decreases in eosinophil counts and clinical improvement in DRESS after initiating benralizumab. These findings have led to exploration of benralizumab for the treatment of DRESS outside the context of COVID-19 [114].
Eosinophil depletion and risk of malignancy
Studies in mouse models
The role of eosinophils in tumour development has been an area of extensive research and interest over recent years, driven by the observation that eosinophil infiltration is detected in many tumour types [57]. Some of the same factors that attract eosinophils to sites of inflammation or infection could serve to recruit eosinophils to the tumour micro-environment (TME). The role of eosinophils within the TME is controversial and potentially complicated by functional heterogeneity. Studies of cytokines and other factors secreted by eosinophils have diametrically shown them to have antitumour effects or to stimulate tumour progression [56, 57]. Whether many of these factors are generated by eosinophils within the TME remains to be determined.
There is no evidence that eosinophil lineage-ablated mice are at naturally increased risk for development of carcinomas or other malignant tumours, with neither ΔdblGATA nor PHIL transgenic mouse lines prone to spontaneous malignancies [80]. There is, however, evidence that eosinophils play a role in tumour surveillance in mice within the context of experimentally induced tumours. Simson et al. [115] reported that ΔdblGATA mice on a BALB/c genetic background that were treated with methylcholanthrene displayed increased fibrosarcoma development compared with syngeneic wild-type controls. Similar findings were observed for mice that were eosinophil deficient due to knockout of both the IL-5 and CCL11 (eotaxin-1) genes (il5/ccl11–/– mice). Interestingly, Stathopoulos et al. [116] discovered that intrapleural injection of Lewis lung cancer (LLC) or mouse colon adenocarcinoma (MC38) cells into IL-5-deficient (il5–/–) C57BL/6 mice reduced the occurrence of malignant pleural effusions (MPEs) versus the same treatment in syngeneic wild-type controls. Provision of exogenous IL-5 to il5–/– mice after introduction of LLC or MC38 cells restored MPE formation capability. Subsequent experiments revealed that IL-5 deficiency in mice protected against development of pulmonary metastasis in experimentally induced tumour models [117]. In these models, eosinophil infiltration in the lungs was greatly reduced in IL-5 knockout mice versus wild-type controls. As has been observed in other experimental systems, the role of eosinophils, eosinophilia and eosinophil recruitment in the context of cancer and the TME is complex and warrants further investigation [56, 57].
Data from clinical trials
In contrast to an infection signal, which should become evident within the timescale of a phase 3 clinical trial, an increased incidence of malignancy may only become apparent over a longer time. To date, anti-IL-5/5R therapies have shown no effect on malignancy risk [48, 82, 85, 93–99]; frequencies of new malignancies in long-term studies of benralizumab, mepolizumab and reslizumab are generally consistent with expectations based on characteristics of the study population. Additional reassurance is provided by two very large (around 3000 patients), placebo-controlled COPD trials of benralizumab, which included a population at intrinsically higher cancer risk (mean age 65 years; one-third active smokers) [87]. In these studies, the incidences of malignancies in patients treated with benralizumab were numerically less versus placebo. Overall, the incidence of malignancies during long-term treatment with benralizumab has been low (<1%), showed no pattern with respect to affected organ or tissue type and was consistent with expectations for the patient population (table 3) [48, 82, 85, 93, 94].
Although an elevated incidence of malignancy compared with Surveillance, Epidemiology, and End Results (SEER; https://seer.cancer.gov) data was observed in a mepolizumab study of patients with HES, the authors noted that baseline malignancy risk may be higher in patients with HES than in the general population (from which SEER data are drawn) [98]. Ongoing long-term surveillance registries of anti-IL-5/5R and other biologic therapies remain important, especially for rarer and slower-growing malignancies.
Eosinophil depletion and pregnancy
Experience with exposure to biologic therapies that reduce eosinophil counts during pregnancy is limited. Pre-clinical studies showed that benralizumab administered at supratherapeutic doses throughout gestation did not result in adverse sequelae or have teratogenic effects in non-human primates [108, 118]. Eosinophil suppression was observed in the neonates, with gradual recovery to normal counts by 6 months post-partum in all but one subject. Measures of other leukocytes, haemoglobin concentration, platelet counts and antibody responses were similar to those in controls.
A recent case report describes a patient who received benralizumab for the treatment of HES throughout pregnancy [118]. The patient's baby was healthy, with normal growth and development and without evidence of atopic disease. Peripheral eosinophils remained undetectable in the child until age 7 months; at a 1-year assessment, eosinophils appeared phenotypically normal. Appropriate immunological response was elicited after administration of pneumococcal conjugate vaccine at 4, 6, 9 and 12 months. Evidence to date suggests that therapeutic eosinophil reduction does not have adverse effects in utero; however, limitations of the currently available data preclude a true determination of the impact of eosinophil depletion during pregnancy at this time.
Conclusions
Despite the traditionally held view of eosinophils as protective against helminths and possibly other microbial pathogens, data from animals and humans, individually and in aggregate, do not suggest deleterious effects of life without eosinophils. Mice lacking eosinophils appear viable and healthy without a predisposition to infection or malignancy. Although some alterations in the course of, or in immune response to, helminth infection were observed in certain experimental infection models tested in eosinophil lineage-ablated mice, the overall ability to defend against helminth infection appears to be intact. Moreover, emerging data suggest that eosinophils may harbour rather than hamper helminth infections, presumably a consequence of evolutionary pressure to survive. In humans, near-complete depletion of eosinophils has not been shown to have adverse effects on health, to promote susceptibility to helminth or opportunistic infections, or to increase the risk for malignancy. These findings suggest that, at the very least, there appears to be sufficient inherent redundancy in key host defence mechanisms to prevent the loss of eosinophils from disrupting normal immune function. As such, based on current data, the benefits of a therapeutic strategy that specifically targets eosinophils do not appear to be offset by any unintended immune or key homeostatic dysfunction, and these therapies should remain at the forefront of treating eosinophil-mediated disorders.
Shareable PDF
Acknowledgements
Writing and editing support, including preparation of the draft manuscript under the direction and guidance of the authors, incorporating author feedback and manuscript submission, was provided by Crystal Murcia (CiTRUS Health Group, Philadelphia, PA, USA) and funded by AstraZeneca (Cambridge, UK).
Footnotes
Conflict of interest: D.J. Jackson reports advisory board and speaker fees from AstraZeneca, Sanofi, Novartis, Chiesi Pharmaceuticals, Boehringer Ingelheim and GSK. I.D. Pavord reports honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Aerocrine AB, Almirall, Novartis, Teva, Chiesi, Sanofi/Regeneron, Menarini and GSK; payments for organising educational events from AstraZeneca, GSK, Sanofi/Regeneron and Teva; honoraria for attending advisory panels with Genentech, Sanofi/Regeneron, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Circassia, Chiesi and Knopp; payments to support FDA approval meetings from GSK; and sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca, Teva and Chiesi.
Support statement: This study was supported by AstraZeneca. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Lee JJ, Jacobsen EA, Ochkur SI, et al. . Human versus mouse eosinophils: “That which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol 2012; 130: 572–584. doi: 10.1016/j.jaci.2012.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klion AD, Ackerman SJ, Bochner BS. Contributions of eosinophils to human health and disease. Annu Rev Pathol 2020; 15: 179–209. doi: 10.1146/annurev-pathmechdis-012419-032756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobsen EA, Jackson DJ, Heffler E, et al. . Eosinophil knockout humans: uncovering the role of eosinophils through eosinophil-directed biological therapies. Annu Rev Immunol 2021; 39: 719–757. doi: 10.1146/annurev-immunol-093019-125918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park YM, Bochner BS. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol Res 2010; 2: 87–101. doi: 10.4168/aair.2010.2.2.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Appleton JA. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol 2016; 32: 798–807. doi: 10.1016/j.pt.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariyaratne A, Finney CAM. Eosinophils and macrophages within the Th2-induced granuloma: balancing killing and healing in a tight space. Infect Immun 2019; 87: e00127-19. doi: 10.1128/IAI.00127-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigo-Munoz JM, Sastre B, Canas JA, et al. . Eosinophil response against classical and emerging respiratory viruses: COVID-19. J Investig Allergol Clin Immunol 2021; 31: 94–107. doi: 10.18176/jiaci.0624 [DOI] [PubMed] [Google Scholar]
- 8.Ondari E, Calvino-Sanles E, First NJ, et al. . Eosinophils and bacteria, the beginning of a story. Int J Mol Sci 2021; 22: 8004. doi: 10.3390/ijms22158004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueiredo RT, Neves JS. Eosinophils in fungal diseases: an overview. J Leukoc Biol 2018; 104: 49–60. doi: 10.1002/JLB.4MR1117-473R [DOI] [PubMed] [Google Scholar]
- 10.Marichal T, Mesnil C, Bureau F. Homeostatic eosinophils: characteristics and functions. Front Med 2017; 4: 101. doi: 10.3389/fmed.2017.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson DJ, Munitz A. Safety of eosinophil depletion. In: Jackson DJ, Wechsler ME, eds. Eosinophilic Lung Diseases (ERS Monograph). Sheffield, European Respiratory Society, 2022; pp. 238–252. [Google Scholar]
- 12.Chu VT, Beller A, Rausch S, et al. . Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 2014; 40: 582–593. doi: 10.1016/j.immuni.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 13.Jung Y, Wen T, Mingler MK, et al. . IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol 2015; 8: 930–942. doi: 10.1038/mi.2014.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh G, Brass A, Knight CG, et al. . Gut eosinophils and their impact on the mucus-resident microbiota. Immunology 2019; 158: 194–205. doi: 10.1111/imm.13110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu D, Molofsky AB, Liang HE, et al. . Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011; 332: 243–247. doi: 10.1126/science.1201475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Y, Nguyen KD, Odegaard JI, et al. . Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014; 157: 1292–1308. doi: 10.1016/j.cell.2014.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung KJ, Nati M, Chavakis T, et al. . Innate immune cells in the adipose tissue. Rev Endocr Metab Disord 2018; 19: 283–292. doi: 10.1007/s11154-018-9451-6 [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen EA, De Filippis E. Can eosinophils in adipose tissue add fuel to the fire? Immunol Cell Biol 2021; 99: 13–16. doi: 10.1111/imcb.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moussa K, Gurung P, Adams-Huet B, et al. . Increased eosinophils in adipose tissue of metabolic syndrome. J Diabetes Complications 2019; 33: 535–538. doi: 10.1016/j.jdiacomp.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 20.Brigger D, Riether C, van Brummelen R, et al. . Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat Metab 2020; 2: 688–702. doi: 10.1038/s42255-020-0228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cusack RP, Huang C, LaVigne N, et al. . Effect of anti-IL-5 biologics on weight and body mass index. Allergy 2021; 76: 2913–2916. doi: 10.1111/all.14956 [DOI] [PubMed] [Google Scholar]
- 22.Sastre B, Rodrigo-Munoz JM, Garcia-Sanchez DA, et al. . Eosinophils: old players in a new game. J Investig Allergol Clin Immunol 2018; 28: 289–304. doi: 10.18176/jiaci.0295 [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Jung Y. The emerging role of eosinophils as multifunctional leukocytes in health and disease. Immune Netw 2020; 20: e24. doi: 10.4110/in.2020.20.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunican EM, Elicker BM, Gierada DS, et al. . Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest 2018; 128: 997–1009. doi: 10.1172/JCI95693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bafadhel M, Pavord ID, Russell REK. Eosinophils in COPD: just another biomarker? Lancet Respir Med 2017; 5: 747–759. doi: 10.1016/S2213-2600(17)30217-5 [DOI] [PubMed] [Google Scholar]
- 26.Bachert C, Marple B, Schlosser RJ, et al. . Adult chronic rhinosinusitis. Nat Rev Dis Primers 2020; 6: 86. doi: 10.1038/s41572-020-00218-1 [DOI] [PubMed] [Google Scholar]
- 27.Laviolette M, Gossage DL, Gauvreau G, et al. . Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 2013; 132: 1086–1096. doi: 10.1016/j.jaci.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran AM, Ramakrishnan S, Borg CA, et al. . Blood eosinophil depletion with mepolizumab, benralizumab, and prednisolone in eosinophilic asthma. Am J Respir Crit Care Med 2020; 202: 1314–1316. doi: 10.1164/rccm.202003-0729LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brightling CE, Bleecker ER, Panettieri RA Jr, et al. . Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med 2014; 2: 891–901. doi: 10.1016/S2213-2600(14)70187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachert C, Han JK, Desrosiers MY, et al. . Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: a randomized, placebo-controlled trial. J Allergy Clin Immunol 2022; 149: 1309–1131. doi: 10.1016/j.jaci.2021.08.030 [DOI] [PubMed] [Google Scholar]
- 31.Nanzer AM, Dhariwal J, Kavanagh J, et al. . Steroid-sparing effects of benralizumab in patients with eosinophilic granulomatosis with polyangiitis. ERJ Open Res 2020; 6: 00451-2020. doi: 10.1183/23120541.00451-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuang FL, Legrand F, Makiya M, et al. . Benralizumab for PDGFRA-negative hypereosinophilic syndrome. N Engl J Med 2019; 380: 1336–1346. doi: 10.1056/NEJMoa1812185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuang FL, De Melo MS, Makiya M, et al. . Benralizumab completely depletes gastrointestinal tissue eosinophils and improves symptoms in eosinophilic gastrointestinal disease. J Allergy Clin Immunol Pract 2022; 10: 1598–1605. doi: 10.1016/j.jaip.2022.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavord ID, Korn S, Howarth P, et al. . Mepolizumab for severe eosinophilic asthma (dream): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 35.Ortega HG, Liu MC, Pavord ID, et al. . Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 36.Pavord ID, Chanez P, Criner GJ, et al. . Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. doi: 10.1056/NEJMoa1708208 [DOI] [PubMed] [Google Scholar]
- 37.Han JK, Bachert C, Fokkens W, et al. . Mepolizumab for chronic rhinosinusitis with nasal polyps (synapse): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021; 9: 1141–1153. doi: 10.1016/S2213-2600(21)00097-7 [DOI] [PubMed] [Google Scholar]
- 38.Wechsler ME, Akuthota P, Jayne D, et al. . Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017; 376: 1921–1932. doi: 10.1056/NEJMoa1702079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roufosse F, Kahn JE, Rothenberg ME, et al. . Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol 2020; 146: 1397–1405. doi: 10.1016/j.jaci.2020.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castro M, Mathur S, Hargreave F, et al. . Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2011; 184: 1125–1132. doi: 10.1164/rccm.201103-0396OC [DOI] [PubMed] [Google Scholar]
- 41.Castro M, Zangrilli J, Wechsler ME, et al. . Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355–366. doi: 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 42.Bernstein JA, Virchow JC, Murphy K, et al. . Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid-dependent asthma: results from two phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 2020; 8: 461–474. doi: 10.1016/S2213-2600(19)30372-8 [DOI] [PubMed] [Google Scholar]
- 43.Laidlaw TM, Prussin C, Panettieri RA, et al. . Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope 2019; 129: E61–E66. doi: 10.1002/lary.27564 [DOI] [PubMed] [Google Scholar]
- 44.Panch SR, Bozik ME, Brown T, et al. . Dexpramipexole as an oral steroid-sparing agent in hypereosinophilic syndromes. Blood 2018; 132: 501–509. doi: 10.1182/blood-2018-02-835330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metzgeroth G, Walz C, Erben P, et al. . Safety and efficacy of imatinib in chronic eosinophilic leukaemia and hypereosinophilic syndrome: a phase-II study. Br J Haematol 2008; 143: 707–715. doi: 10.1111/j.1365-2141.2008.07294.x [DOI] [PubMed] [Google Scholar]
- 46.Austin D, Pouliquen I, Keene O, et al. . Blood eosinophil dose response to oral corticosteroids in a population of patients with severe asthma. Eur Respir J 2016; 48: Suppl. 60, PA1110. doi: 10.1183/13993003.congress-2016.PA1110 [DOI] [Google Scholar]
- 47.Ortega H, Llanos JP, Lafeuille MH, et al. . Effects of systemic corticosteroids on blood eosinophil counts in asthma: real-world data. J Asthma 2019; 56: 808–815. doi: 10.1080/02770903.2018.1502301 [DOI] [PubMed] [Google Scholar]
- 48.Jackson DJ, Korn S, Mathur SK, et al. . Safety of eosinophil-depleting therapy for severe, eosinophilic asthma: focus on benralizumab. Drug Safety 2020; 43: 409–425. doi: 10.1007/s40264-020-00926-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hearn AP, Kent BD, Jackson DJ. Biologic treatment options for severe asthma. Curr Opin Immunol 2020; 66: 151–160. doi: 10.1016/j.coi.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 50.Mesnil C, Raulier S, Paulissen G, et al. . Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 2016; 126: 3279–3295. doi: 10.1172/JCI85664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dolitzky A, Grisaru-Tal S, Avlas S, et al. . Mouse resident lung eosinophils are dependent on IL-5. Allergy 2022; 77: 2822–3825. doi: 10.1111/all.15362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol 2020; 146: 1–7. doi: 10.1016/j.jaci.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am 2016; 42: 157–176. doi: 10.1016/j.rdc.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schleimer RP, Bochner BS. The effects of glucocorticoids on human eosinophils. J Allergy Clin Immunol 1994; 94: 1202–1213. doi: 10.1016/0091-6749(94)90333-6 [DOI] [PubMed] [Google Scholar]
- 55.Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis 2012; 25: 458–463. doi: 10.1097/QCO.0b013e3283551dbd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakkal S, Miller S, Apostolopoulos V, et al. . Eosinophils in cancer: favourable or unfavourable? Curr Med Chem 2016; 23: 650–666. doi: 10.2174/0929867323666160119094313 [DOI] [PubMed] [Google Scholar]
- 57.Grisaru-Tal S, Itan M, Klion AD, et al. . A new dawn for eosinophils in the tumour microenvironment. Nat Rev Cancer 2020; 20: 594–607. doi: 10.1038/s41568-020-0283-9 [DOI] [PubMed] [Google Scholar]
- 58.Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res 2014; 2: 1–8. doi: 10.1158/2326-6066.CIR-13-0196 [DOI] [PubMed] [Google Scholar]
- 59.Yu C, Cantor AB, Yang H, et al. . Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med 2002; 195: 1387–1395. doi: 10.1084/jem.20020656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JJ, Dimina D, Macias MP, et al. . Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004; 305: 1773–1776. doi: 10.1126/science.1099472 [DOI] [PubMed] [Google Scholar]
- 61.Swartz JM, Dyer KD, Cheever AW, et al. . Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood 2006; 108: 2420–2427. doi: 10.1182/blood-2006-04-015933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Connell AE, Hess JA, Santiago GA, et al. . Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun 2011; 79: 2770–2778. doi: 10.1128/IAI.00931-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cadman ET, Thysse KA, Bearder S, et al. . Eosinophils are important for protection, immunoregulation and pathology during infection with nematode microfilariae. PLoS Pathog 2014; 10: e1003988. doi: 10.1371/journal.ppat.1003988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knott ML, Matthaei KI, Giacomin PR, et al. . Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol 2007; 37: 1367–1378. doi: 10.1016/j.ijpara.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 65.Huang L, Gebreselassie NG, Gagliardo LF, et al. . Eosinophils mediate protective immunity against secondary nematode infection. J Immunol 2015; 194: 283–290. doi: 10.4049/jimmunol.1402219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frohberger SJ, Ajendra J, Surendar J, et al. . Susceptibility to L. sigmodontis infection is highest in animals lacking IL-4R/IL-5 compared to single knockouts of IL-4R, IL-5 or eosinophils. Parasit Vectors 2019; 12: 248. doi: 10.1186/s13071-019-3502-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fabre V, Beiting DP, Bliss SK, et al. . Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol 2009; 182: 1577–1583. doi: 10.4049/jimmunol.182.3.1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gebreselassie NG, Moorhead AR, Fabre V, et al. . Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol 2012; 188: 417–425. doi: 10.4049/jimmunol.1101980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drake MG, Bivins-Smith ER, Proskocil BJ, et al. . Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am J Respir Cell Mol Biol 2016; 55: 387–394. doi: 10.1165/rcmb.2015-0405OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma M, Redes JL, Percopo CM, et al. . Alternaria alternata challenge at the nasal mucosa results in eosinophilic inflammation and increased susceptibility to influenza virus infection. Clin Exp Allergy 2018; 48: 691–702. doi: 10.1111/cea.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yordanova IA, Lamatsch M, Kuhl AA, et al. . Eosinophils are dispensable for the regulation of IgA and TH17 responses in Giardia muris infection. Parasite Immunol 2021; 43: e12791. doi: 10.1111/pim.12791 [DOI] [PubMed] [Google Scholar]
- 72.Arnold IC, Artola-Boran M, Tallon de Lara P, et al. . Eosinophils suppress TH1 responses and restrict bacterially induced gastrointestinal inflammation. J Exp Med 2018; 215: 2055–2072. doi: 10.1084/jem.20172049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gestal MC, Blas-Machado U, Johnson HM, et al. . Disrupting Bordetella immunosuppression reveals a role for eosinophils in coordinating the adaptive immune response in the respiratory tract. Microorganisms 2020; 8: 1808. doi: 10.3390/microorganisms8111808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Dea EM, Amarsaikhan N, Li H, et al. . Eosinophils are recruited in response to chitin exposure and enhance TH2-mediated immune pathology in Aspergillus fumigatus infection. Infect Immun 2014; 82: 3199–3205. doi: 10.1128/IAI.01990-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amarsaikhan N, Sands EM, Shah A, et al. . Caspofungin increases fungal chitin and eosinophil and γδ T cell-dependent pathology in invasive aspergillosis. J Immunol 2017; 199: 624–632. doi: 10.4049/jimmunol.1700078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dietschmann A, Schruefer S, Krappmann S, et al. . TH2 cells promote eosinophil-independent pathology in a murine model of allergic bronchopulmonary aspergillosis. Eur J Immunol 2020; 50: 1044–1056. doi: 10.1002/eji.201948411 [DOI] [PubMed] [Google Scholar]
- 77.Wang S, Jiang Z, Li L, et al. . Ameliorative effects of eosinophil deficiency on immune response, endoplasmic reticulum stress, apoptosis, and autophagy in fungus-induced allergic lung inflammation. Respir Res 2021; 22: 173. doi: 10.1186/s12931-021-01770-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev 1995; 9: 1250–1262. doi: 10.1101/gad.9.10.1250 [DOI] [PubMed] [Google Scholar]
- 79.Fujiwara Y, Browne CP, Cunniff K, et al. . Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci USA 1996; 93: 12355–12358. doi: 10.1073/pnas.93.22.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gleich GJ, Klion AD, Lee JJ, et al. . The consequences of not having eosinophils. Allergy 2013; 68: 829–835. doi: 10.1111/all.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dworetzky SI, Hebrank GT, Archibald DG, et al. . The targeted eosinophil-lowering effects of dexpramipexole in clinical studies. Blood Cells Mol Dis 2017; 63: 62–65. doi: 10.1016/j.bcmd.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 82.Busse WW, Bleecker ER, FitzGerald JM, et al. . Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med 2019; 7: 46–59. doi: 10.1016/S2213-2600(18)30406-5 [DOI] [PubMed] [Google Scholar]
- 83.Bleecker ER, FitzGerald JM, Chanez P, et al. . Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 84.FitzGerald JM, Bleecker ER, Nair P, et al. . Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 388: 2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 85.Korn S, Bourdin A, Chupp G, et al. . Integrated safety and efficacy among patients receiving benralizumab for up to 5 years. J Allergy Clin Immunol Pract 2021; 9: 4381–4392. doi: 10.1016/j.jaip.2021.07.058 [DOI] [PubMed] [Google Scholar]
- 86.Ferguson GT, FitzGerald JM, Bleecker ER, et al. . Benralizumab for patients with mild to moderate, persistent asthma (BISE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2017; 5: 568–576. doi: 10.1016/S2213-2600(17)30190-X [DOI] [PubMed] [Google Scholar]
- 87.Criner GJ, Celli BR, Brightling CE, et al. . Benralizumab for the prevention of COPD exacerbations. N Engl J Med 2019; 381: 1023–1034. doi: 10.1056/NEJMoa1905248 [DOI] [PubMed] [Google Scholar]
- 88.Kavanagh JE, Hearn AP, Dhariwal J, et al. . Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest 2021; 159: 496–506. doi: 10.1016/j.chest.2020.08.2083 [DOI] [PubMed] [Google Scholar]
- 89.Kavanagh JE, Hearn AP, d'Ancona G, et al. . Benralizumab after sub-optimal response to mepolizumab in severe eosinophilic asthma. Allergy 2021; 76: 1890–1893. doi: 10.1111/all.14693 [DOI] [PubMed] [Google Scholar]
- 90.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 2011; 11: 375–388. doi: 10.1038/nri2992 [DOI] [PubMed] [Google Scholar]
- 91.Breloer M, Abraham D. Strongyloides infection in rodents: immune response and immune regulation. Parasitology 2017; 144: 295–315. doi: 10.1017/S0031182016000111 [DOI] [PubMed] [Google Scholar]
- 92.Walsh ER, Sahu N, Kearley J, et al. . Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med 2008; 205: 1285–1292. doi: 10.1084/jem.20071836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.FitzGerald JM, Bleecker ER, Bourdin A, et al. . Two-year integrated efficacy and safety analysis of benralizumab in severe asthma. J Asthma Allergy 2019; 12: 401–413. doi: 10.2147/JAA.S227170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bourdin A, Shaw D, Menzies-Gow A, et al. . Two-year integrated steroid-sparing analysis and safety of benralizumab for severe asthma. J Asthma 2021; 58: 514–522. doi: 10.1080/02770903.2019.1705333 [DOI] [PubMed] [Google Scholar]
- 95.Lugogo N, Domingo C, Chanez P, et al. . Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther 2016; 38: 2058–2070. doi: 10.1016/j.clinthera.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 96.Khatri S, Moore W, Gibson PG, et al. . Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol 2019; 143: 1742–1751. doi: 10.1016/j.jaci.2018.09.033 [DOI] [PubMed] [Google Scholar]
- 97.Khurana S, Brusselle GG, Bel EH, et al. . Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther 2019; 41: 2041–2056. doi: 10.1016/j.clinthera.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 98.Roufosse FE, Kahn JE, Gleich GJ, et al. . Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol 2013; 131: 461–467. doi: 10.1016/j.jaci.2012.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murphy K, Jacobs J, Bjermer L, et al. . Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol Pract 2017; 5: 1572–1581. doi: 10.1016/j.jaip.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 100.Geri G, Rabbat A, Mayaux J, et al. . Strongyloides stercoralis hyperinfection syndrome: a case series and a review of the literature. Infection 2015; 43: 691–698. doi: 10.1007/s15010-015-0799-1 [DOI] [PubMed] [Google Scholar]
- 101.Bacharier LB, Maspero JF, Katelaris CH, et al. . Dupilumab in children with uncontrolled moderate-to-severe asthma. N Engl J Med 2021; 385: 2230–2240. doi: 10.1056/NEJMoa2106567 [DOI] [PubMed] [Google Scholar]
- 102.Oeser K, Schwartz C, Voehringer D. Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol 2015; 8: 672–682. doi: 10.1038/mi.2014.101 [DOI] [PubMed] [Google Scholar]
- 103.Sabogal Pineros YS, Bal SM, van de Pol MA, et al. . Anti-IL-5 in mild asthma alters rhinovirus-induced macrophage, B-cell, and neutrophil responses (MATERIAL). A placebo-controlled, double-blind study. Am J Respir Crit Care Med 2019; 199: 508–517. doi: 10.1164/rccm.201803-0461OC [DOI] [PubMed] [Google Scholar]
- 104.Jackson DJ, Makrinioti H, Rana BM, et al. . IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med 2014; 190: 1373–1382. doi: 10.1164/rccm.201406-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hatchwell L, Collison A, Girkin J, et al. . Toll-like receptor 7 governs interferon and inflammatory responses to rhinovirus and is suppressed by IL-5-induced lung eosinophilia. Thorax 2015; 70: 854–861. doi: 10.1136/thoraxjnl-2014-205465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sabogal Pineros YS, Bal SM, Dijkhuis A, et al. . Eosinophils capture viruses, a capacity that is defective in asthma. Allergy 2019; 74: 1898–1909. doi: 10.1111/all.13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mishra AK, Sahu KK, James A. Disseminated herpes zoster following treatment with benralizumab. Clin Respir J 2019; 13: 189–191. doi: 10.1111/crj.12998 [DOI] [PubMed] [Google Scholar]
- 108.AstraZeneca Pharmaceuticals LP . Fasenra (benralizumab) injection, for subcutaneous use [prescribing information]. 2017. www.accessdata.fda.gov/drugsatfda_docs/label/2017/F761070s000lbl.pdf Date last accessed: 2 August 2022.
- 109.Xie G, Ding F, Han L, et al. . The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy 2021; 76: 471–482. doi: 10.1111/all.14465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adir Y, Humbert M, Saliba W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: nationwide real-world evidence. J Allergy Clin Immunol 2021; 148: 361–367. doi: 10.1016/j.jaci.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Francis CHR, Hearn AP, Ratnakumar S, et al. . Covid-19 in the absence of eosinophils: the outcome of confirmed SARS-COV-2 infection whilst on treatment with benralizumab. Allergy 2022; 77: 2558–2560. doi: 10.1111/all.15334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmid-Grendelmeier P, Steiger P, Naegeli MC, et al. . Benralizumab for severe DRESS in two COVID-19 patients. J Allergy Clin Immunol Pract 2021; 9: 481–483. doi: 10.1016/j.jaip.2020.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mesli F, Dumont M, Soria A, et al. . Benralizumab: a potential tailored treatment for life-threatening DRESS in the COVID-19 era. J Allergy Clin Immunol Pract 2021; 9: 3529–3531. doi: 10.1016/j.jaip.2021.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lang CCV, Schmid-Grendelmeier P, Maverakis E, et al. . Reply to “Benralizumab: a potential tailored treatment for life-threatening dress in the COVID-19 era”. J Allergy Clin Immunol Pract 2021; 9: 3531–3532. doi: 10.1016/j.jaip.2021.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simson L, Ellyard JI, Dent LA, et al. . Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol 2007; 178: 4222–4229. doi: 10.4049/jimmunol.178.7.4222 [DOI] [PubMed] [Google Scholar]
- 116.Stathopoulos GT, Sherrill TP, Karabela SP, et al. . Host-derived interleukin-5 promotes adenocarcinoma-induced malignant pleural effusion. Am J Respir Crit Care Med 2010; 182: 1273–1281. doi: 10.1164/rccm.201001-0001OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zaynagetdinov R, Sherrill TP, Gleaves LA, et al. . Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res 2015; 75: 1624–1634. doi: 10.1158/0008-5472.CAN-14-2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manetz S, Maric I, Brown T, et al. . Successful pregnancy in the setting of eosinophil depletion by benralizumab. J Allergy Clin Immunol Pract 2021; 9: 1405–1407. doi: 10.1016/j.jaip.2020.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01217-2022.Shareable (225.3KB, pdf)