Abstract
Objective
The study was conducted to investigate the dephosphorylation of Pseudomonas aeruginosa flagellin (PA FLA) by sweet potato purple acid phosphatase (PAP) and the effect of the enzyme on the flagellin-mediated inflammatory response in the A549 lung epithelial cell line.
Methods
The activity of sweet potato PAP on PA FLA was assayed at different pH (4, 5.5, 7, and 7.5) and temperature (25°C, 37°C, and 55°C) conditions. The release of interleukin-8 (IL-8) and the activation of nuclear factor kappa- light-chain-enhancer of activated B cells (NF-κB) in A549 cells exposed to PA FLA treated with or without sweet potato PAP was measured using IL-8 and NF-κB ELISA kits, respectively. The activation of toll-like receptor 5 (TLR5) in TLR5-overexpressing HEK-293 cells exposed to PA FLA treated with or without sweet potato PAP was determined by the secreted alkaline phosphatase-based assay.
Results
The dephosphorylation of PA FLA by sweet potato PAP was favorable at pH 4 and 5.5 and highest at 55°C. PA-FLA treated with the enzyme decreased IL-8 release from A549 cells to about 3.5-fold compared to intact PA FLA at 1,000 ng/mL of substrate. Moreover, PA-FLA dephosphorylated by the enzyme repressed the activation of NF-κB in the cells compared to intact PA FLA. The activation of TLR5 by PA-FLA was highest in TLR-overexpressing HEK293 cells at a substrate concentration of 5,000 ng/mL, whereas PA FLA treated with the enzyme strongly repressed the activation of TLR5.
Conclusion
Sweet potato PAP has the potential to be a new alternative agent against the increased antibiotic resistance of P. aeruginosa and may be a new conceptual feed additive to control unwanted inflammatory responses caused by bacterial infections in animal husbandry.
Keywords: A549 Cell, Flagellin, Inflammatory Response, Pseudomonas aeruginosa, Sweet Potato Purple Acid Phosphatase
INTRODUCTION
Pseudomonas aeruginosa is a gram-negative bacterium that is widely known to account for up to 10% of all human and animal infections and is one of the main causes of numerous diseases such as hemorrhagic pneumonia, mastitis, urinary tract infections, otitis, and endometritis in domestic animals, causing tremendous damage to animal husbandry [1–3]. Not only does P. aeruginosa causes respiratory diseases in animals such as chickens and cattle, but it also causes mastitis in Holstein cows, and according to Banerjee et al [4], mastitis is a known cause of the loss of milk production, which makes it a major problem in the industry [4–7]. Infection with P. aeruginosa is stimulated by the microbial molecules called pathogen-associated molecular patterns (PAMPs) [8], and the bacterial flagellin is a well-known PAMP, shown to upregulate pro-inflammatory mediators [9,10]. Also, as it is widely known that the activity and properties of certain proteins vary greatly depending on the phosphorylation of corresponding protein [11], dephosphorylating bacterial flagellin might lead to the regulation of inflammation in flagellin-mediated infections. Structurally, P. aeruginosa possesses an unusual surface filament-like flagellum made up of post-translationally phosphorylated flagellin protein [12].
Sweet potato purple acid phosphatase (PAP) is a binuclear metal-containing phosphatase, meaning that it has enzymatically active binuclear metal sites (Fe-Mn center) [13]. The structure of sweet potato PAP is very similar to that of enzyme called phosphoprotein phosphatases (PPPs), binuclear metallohydrolases that catalyze the dephosphorylation of threonine and serine remains [14]. While previous studies indicated that the catalytic cycle and mechanism of PPPs depended upon its crystal structure [15,16], Zhang et al [17] reported the structural similarities of the active sites between PPPs and PAPs, suggesting that PAPs might share the catalytic dephosphorylation mechanism with PPPs. Even though sweet potato PAP had shown phosphatase activity toward eight substrates such as ATP, ADP, AMP, NADP, glucose-6-phosphate, sucrose-6-phophate, fructose-6-phosphate, and p-NPP, studies on the dephosphorylation of sweet potato PAP toward relatively large phosphoproteins are limited [18]. The objective of this study was to investigate the dephosphorylation of bacterial flagellin protein by sweet potato PAP, and the effect of sweet potato PAP on the flagellin-mediated inflammatory response in the A549 lung epithelial cell line.
MATERIALS AND METHODS
Reagents and cell culture
Purified flagellin from P. aeruginosa (PA FLA) encoded by the fliC gene [19], and sweet potato PAP were purchased from InvivoGen (Sandiego, CA, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively. Both were reconstituted in endotoxin-free water (Sigma-Aldrich, USA). PiColor Lock phosphate detection reagent was purchased from Novusbio (Centennial, CO, USA) while the Cymax Human IL-8 ELISA kit was obtained from Ab Frontier (Seoul, Korea). The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) p65 (Total) InstantOne ELISA kit was procured from Invitrogen, Thermo-Fisher Scientific (Carlsbad, CA, USA).
The human alveolar carcinoma epithelial cell line (A549 cells) was procured from American Type Culture Collection (ATCC CCL-185) and was maintained at F-12K Medium Ham’s F-12K (Kaighn’s) Medium (Gibco Life technologies; Gaithersburg, MD, USA) with 10% fetal bovine serum (Gibco Life technologies, USA) and 1% penicillin-streptomycin (Gibco Life technologies, USA) at 37°C in a 5% CO2 humidified incubator.
HEK-Blue hTLR5 cells (Invitrogen, Thermo-Fisher Scien tific, USA) were cultured at 37°C in Dulbecco modified eagle medium (DMEM; Gibco Life technologies, USA) with 30 μg/mL of blasticidin (Invitrogen, Thermo-Fisher Scientific, USA) and 100 μg/mL of Zeocin (Invitrogen, Thermo-Fisher Scientific, USA).
Dephosphorylation of flagellin by sweet potato PAP at various pHs and temperatures
The phosphatase assay was performed at 37°C for 4 hours in a 1-mL reaction mixture containing 4 μL of the enzyme (46.4 μg), 1 μg of PA FLA substrate, and 50 mM Na-acetate (pH 4.0 and 5.5) or Bis-Tris (pH 7.0) or Tris-HCl (pH 7.5). The assay was also performed using the same protocol at conditions of pH 5.5 (Na-acetate) for 4 hours at 25°C, 37°C, and 55°C. The released inorganic phosphates (Pi) were read at optical density (OD) 635 nm, using PiColorLock detection kit (Novusbio; Centennial, CO, USA) following the manufacturer’s instructions. Enzyme activity was defined as the amount of enzyme required to liberate 1 nmol of inorganic phosphate per min under the assay conditions divided by mg of the protein.
Effect of sweet potato PAP on flagellin-induced pro-inflammatory mediators in A549 cells
A549 cells were initially seeded onto 96-well plates at a density of 1.5×104 cells per well and cultured to 80% confluency at 37°C in a 5% CO2 incubator. Various concentrations of FLA (0, 0.1, 1, 10, 100, 1,000 ng/mL) were treated with 1 μL of sweet potato PAP (11.6 μg) for 6 h. Then, enzyme-treated FLA and intact FLA at each concentration were applied to the cells for 12 h. The levels of interleukin-8 (IL-8) and NF-κB were assayed at OD 450 nm using Cymax Human IL-8 ELISA kit and NF-κB p65 (Total) InstantOne ELISA Kit, respectively, according to the manufacturer’s instructions.
Effect of sweet potato PAP on flagellin-induced TLR5 activation in TLR5 overexpressing HEK-293 cells
Human TLR5-overexpressing HEK-293 cells (HEK-Blue hTLR5 cells) were cultured in 96-well plates at an initial concentration of 1.5×104 cells per well until 80% confluency. Different concentrations of PA FLA (0, 0.1, 1, 10, 100, 1,000, 5,000 ng/mL) were treated with 1 μL of sweet potato PAP (11.6 μg) for 6 hours. Then, enzyme treated FLA and intact FLA at each concentration were added to the cells. Activated toll-like receptor 5 (TLR5) was monitored at OD 620 nm using the secreted alkaline phosphatase-based assay according to the manufacturer’s protocols.
Statistical analysis
Statistical analyses were conducted using one-way analysis of variance (PROC GLM; SAS 9.4, SAS Inst. Inc., Cary, NC, USA) to search for significant differences between the treatments with Duncan’s multiple range test. The probability level used for statistical significance was p<0.05. The results were presented as the means and standard errors from three experiments.
RESULTS
Determination of PA FLA-dephosphorylating activity of sweet potato PAP
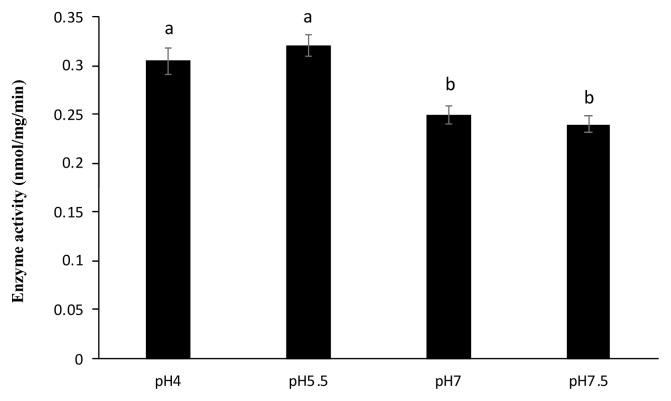
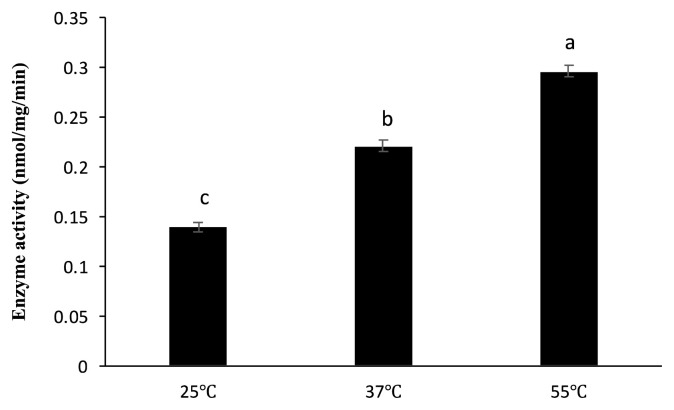
As shown in Figure 1, the dephosphorylation of PA FLA by sweet potato PAP was more effective in acidic conditions (pH 4 and 5.5) compared to neutral conditions (pH 7 and 7.5). Moreover, the phosphatase activity was highest at 55°C (Figure 2).
Figure 1.
Dephosphorylation of PA FLA by sweet potato PAP in different pH conditions. The phosphatase assay was performed at 37°C for 4 hours in a 1-mL reaction mixture containing 4 μL of the enzyme (46.4 μg), 1 μg of PA FLA substrate, and 50 mM Na-acetate (pH 4.0 and 5.5) or Bis-Tris (pH 7.0) or Tris-HCl (pH 7.5). Enzyme activity was defined as the amount of enzyme required to liberate 1 nmol of inorganic phosphate per min divided by mg of the protein. The data were expressed as the mean and standard errors from three experiments. PA FLA, Pseudomonas aeruginosa flagellin; PAP, purple acid phosphatase. a,b Means lacking common letters differ significantly (p<0.05).
Figure 2.
Dephosphorylation of PA FLA by sweet potato PAP in different temperatures. The phosphatase assay was performed in a 1-mL reaction mixture containing 4 μL of the enzyme (46.4 μg) and 1 μg of PA FLA substrate at pH 5.5 (Na-acetate) for 4 hours at 25°C, 37°C, and 55°C. Enzyme activity was defined as the amount of enzyme required to liberate 1 nmol of inorganic phosphate per min divided by mg of the protein. The data were expressed as the mean and standard errors from three experiments. PA FLA, Pseudomonas aeruginosa flagellin; PAP, purple acid phosphatase. a–c Means lacking common letters differ significantly (p<0.05).
Effect of sweet potato PAP on PA FLA-induced IL-8 secretion in A549 cells
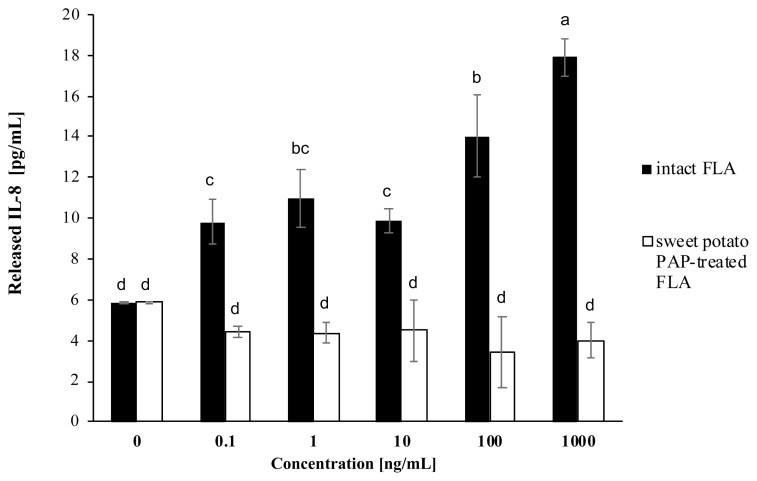
As shown in Figure 3, PA-FLA stimulated the release of IL-8 in A549 cells. However, PA-FLA treated with sweet potato PAP inhibited the secretion of IL-8 in the cells, decreasing the IL-8 release to about 3.5-fold compared to that of intact PA-FLA, even at 1,000 ng/mL of substrate.
Figure 3.
Effect of sweet potato PAP on PA FLA-induced IL-8 secretion in A549 cells. A549 cells were initially seeded onto 96-well plates at a density of 1.5×104 cells per well and cultured to 80% confluency at 37°C in a 5% CO2 incubator. Various concentrations of FLA (0, 0.1, 1, 10, 100, 1,000 ng/mL) were treated with 1 μL of sweet potato PAP (11.6 μg) for 6 h. Then, enzyme-treated FLA and intact FLA at each concentration were applied to the cells for 12 h. The level of IL-8 was assayed at OD 450 nm using Cymax Human IL-8 ELISA kit. The data were expressed as the mean and standard errors from three experiments. PAP, purple acid phosphatase; PA FLA, Pseudomonas aeruginosa flagellin; IL-8, interleukin-8; OD, optical density; ELISA, enzyme-linked immunosorbent assay. a–d Means lacking common letters differ significantly (p<0.05).
Effect of sweet potato PAP on PA FLA-induced NF-κB activation in A549 cells
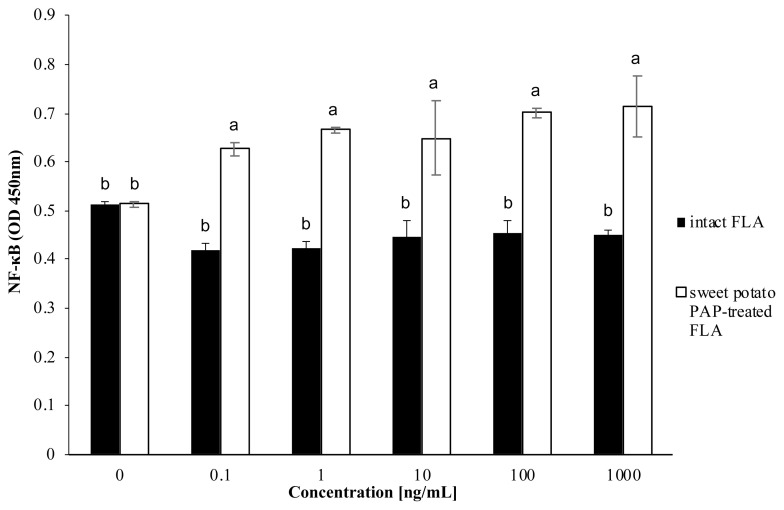
Figure 4 showed that PA FLA dephosphorylated by sweet potato PAP repressed the activation of NF-κB in A549 cells compared to intact PA FLA, but there was no significant difference between the substrate levels.
Figure 4.
Effect of sweet potato PAP on PA FLA-induced NF-κB activation in A549 cells. A549 cells were initially seeded onto 96-well plates at a density of 1.5×104 cells per well and cultured to 80% confluency at 37°C in a 5% CO2 incubator. Various concentrations of FLA (0, 0.1, 1, 10, 100, 1,000 ng/mL) were treated with 1 μL of sweet potato PAP (11.6 μg) for 6 h. Then, enzyme-treated FLA and intact FLA at each concentration were applied to the cells for 12 h. The level of NF-κB was assayed at OD 450 nm using NF-κB p65 (Total) InstantOne ELISA Kit. The data were expressed as the mean and standard errors from three experiments. PAP, purple acid phosphatase; PA FLA, Pseudomonas aeruginosa flagellin; NF-κB, nuclear factor kappa- light-chain-enhancer of activated B; OD, optical density; ELISA, enzyme-linked immunosorbent assay. a,b Means lacking common letters differ significantly (p<0.05).
Effect of sweet potato PAP on PA FLA-induced TLR5 activation in TLR5 overexpressing HEK-293 cells
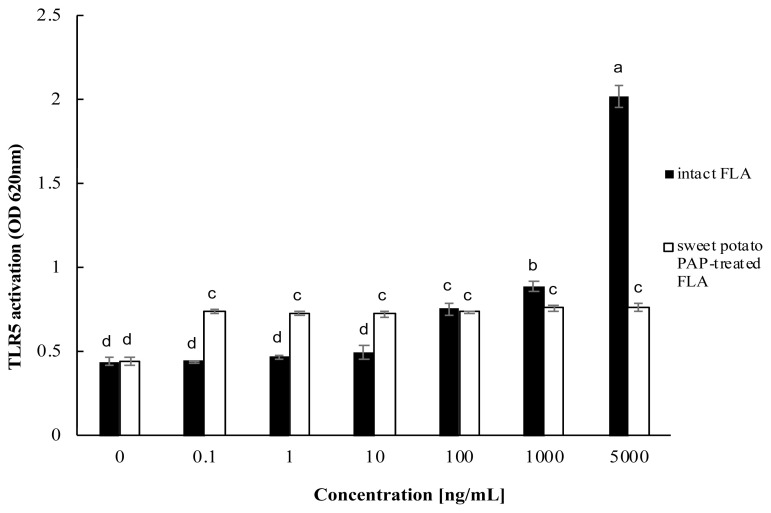
As shown in Figure 5, the activation of TLR5 by PA FLA was effective in TLR5-overexpressing HEK293 cells at substrate concentrations over 100 ng/mL and was the highest at a substrate concentration of 5,000 ng/mL, where PA FLA treated with sweet potato PAP strongly repressed the activation of TLR5.
Figure 5.
Effect of sweet potato PAP on PA FLA-induced TLR5 activation in HEK-Blue hTLR5 cells. HEK-Blue hTLR5 cells were cultured in 96-well plates at an initial concentration of 1.5×104 cells per well until 80% confluency. Different concentrations of PA FLA (0, 0.1, 1, 10, 100, 1,000, 5,000 ng/mL) were treated with 1 μL of sweet potato PAP (11.6 μg) for 6 hours. Then, enzyme treated FLA and intact FLA at each concentration were added to the cells. Activated TLR5 was monitored at OD 620 nm using the SEAP-based assay. The data were expressed as the mean and standard errors from three experiments. PAP, purple acid phosphatase; PA FLA, Pseudomonas aeruginosa flagellin; TLR5, toll-like receptor 5; HEK-Blue hTLR5 cells, human TLR5-overexpressing HEK-293 cells; OD, optical density; SEAP, secreted alkaline phosphatase. a–d Means lacking common letters differ significantly (p<0.05).
DISCUSSION
P. aeruginosa infections cause animal diseases and pose a great threat to animal husbandry. The bacteria is responsible for various respiratory diseases, mastitis, otitis, and many others [1,3]. However, the frequently used antibiotics have allowed these bacteria to acquire antibiotic resistance [20,21]. Thus, the importance of finding new alternatives targeting antibiotic resistant bacteria like P. aeruginosa has grown. Although flagellin had been reported to be a PAMP in P. aeruginosa, there are insufficient studies regarding the inactivation of PA FLA.
Several studies on the functions of mammalian PAP have already been conducted and suggested that PAP was involved in physiological events like osteoclastic bone resorption and erythrophagocytosis through the dephosphorylation of certain proteins [22]. Nevertheless, a precise study on the dephosphorylating activity of sweet potato PAP with relatively high molecular weight substances like proteins has not yet been reported. In the present study, sweet potato PAP successfully exhibited phosphatase activity against PA FLA (Figures 1, 2), which was a novel finding. Moreover, sweet potato PAP was highly active at acidic pH range (4 to 5.5) (Figure 1), which was compatible with the result of chickpea PAP (CaPAP7), verifying the typical property of acid phosphatase [23]. Previously, the phosphatase activity of sweet potato PAP for the substrate, p-nitrophenyl phosphate (pNPP) exhibited the highest activity at 50°C [18], which is almost similar to our result of 55°C for PA PLA (Figure 2).
Indeed, bacterial flagellins elicit pro-inflammatory cyto kines such as IL-6 and IL-8 in various cell types [24–27]. As shown in Figure 3, PA FLA provoked the secretion of the universal inflammatory marker IL-8 in A549 cells. However, treatment of the cells with every concentration of substrate dephosphorylated by sweet potato PAP reduced the secretion of IL-8, suggesting that the enzyme exerted anti-inflammatory activity. To some extent, the dephosphorylation of flagellin appears to be related to dysfunction. For example, dephosphorylation of the profission Drp1 (dynamin-related protein 1) protein by the cytosolic phosphatase calcineurin led to the fragmentation of depolarized mitochondria during the cell cycle, differentiation, and death [28].
Moreover, PA FLA dephosphorylated by the enzyme re pressed the activation of NF-κB (Figure 4), which is widely known to mediate IL-8 gene expression [29]. Bacterial flagellin provokes inflammation via stimulating the NF-κB pathway [30,31].
TLRs regulate the innate immune response and contribute to enhancing antibacterial defenses in host cells [32]. Among the TLRs, TLR5 is well-known to recognize FLA on the bacterial cell surface, eliciting inflammatory signaling by activating the NF-κB pathway [31]. When a bacterial infection takes place, host cells over-produce TLR5 to defend against such attacks [32].
As shown in Figure 5, intact PA FLA clearly induced the activation of TLR5 in TLR5- overexpressing HEK-293 cells at higher doses of more than 1,000 ng/mL, which was in good agreement with the previous result observed in 293T cells exposed to purified Salmonella typhimurium flagellin [33], but PA FLA dephosphorylated by sweet potato PAP repressed it. Bacterial flagellins structurally possess two highly conserved N-terminal and C-terminal domains and one central hypervariable domain [32]. In the case of Salmonella enterica-derived flagellin, one variant with a deletion in the N-terminus (FliCΔ1–180) failed to stimulate TLR5 [32]. Presumably, phosphotyrosine exists within the N-terminus of PA FLA and the dephosphorylation of the residue inhibited the interaction of PA FLA with TLR5 [12].
Just as exogenous bovine intestinal alkaline phosphatase has been extensively applied to clinical human trials [34], the conception of the administration of sweet potato PAP by oral delivery or by intravenous injection can be positively suggested to maintain good health for farm and companion animals against the gastrointestinal inflammatory disorder and antibiotics-related infections [34,35]. In conclusion, sweet potato PAP has the potential to be a new alternative agent against the increased antibiotic resistance of P. aeruginosa and may be a new conceptual feed additive to control unwanted inflammatory responses caused by bacterial infections in animal husbandry.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), (2019R1F1A1043769).
REFERENCES
- 1.Kidd TJ, Gibson JS, Moss S, et al. Clonal complex Pseudomonas aeruginosa in horses. Vet Microbiol. 2011;149:508–12. doi: 10.1016/j.vetmic.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 2.Poonsuk K, Chuanchuen R. Contribution of the MexXY multidrug efflux pump and other chromosomal mechanisms on aminoglycoside resistance in Pseudomonas aeruginosa isolates from canine and feline infections. J Vet Med Sci. 2012;74:1575–82. doi: 10.1292/jvms.12-0239. [DOI] [PubMed] [Google Scholar]
- 3.Salomonsen CM, Themudo GE, Jelsbak L, et al. Typing of Pseudomonas aeruginosa from hemorrhagic pneumonia in mink (Neovison vison) Vet Microbiol. 2013;163:103–9. doi: 10.1016/j.vetmic.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Batabyal K, Joardar SN, et al. Detection and characterization of pathogenic Pseudomonas aeruginosa from bovine subclinical mastitis in West Bengal, India. Vet World. 2017;10:738–42. doi: 10.14202/vetworld.2017.738-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong Q, Ruan MD, Niu MF, Qin CL, Hou Y, Guo JZ. Immune efficacy of DNA vaccines based on oprL and oprF genes of Pseudomonas aeruginosa in chickens. Poult Sci. 2018;97:4219–27. doi: 10.3382/ps/pey307. [DOI] [PubMed] [Google Scholar]
- 6.Coorens M, Banaschewski BJH, Baer BJ, et al. Killing of Pseudomonas aeruginosa by chicken cathelicidin-2 is immunogenically silent, preventing lung inflammation in vivo. Infect Immun. 2017;85:e00546–17. doi: 10.1128/IAI.00546-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan WH, Ibrahim AMK, Shany SAS, Salam HSH. Virulence and resistance determinants in Pseudomonas aeruginosa isolated from pericarditis in diseased broiler chickens in Egypt. J Adv Vet Anim Res. 2020;7:452–63. doi: 10.5455/javar.2020.g441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balloy V, Verma A, Kuravi S, Si-Tahar M, Chignard M, Ramphal R. The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa. J Infect Dis. 2007;196:289–96. doi: 10.1086/518610. [DOI] [PubMed] [Google Scholar]
- 9.Zeng H, Carlson AQ, Guo Y, et al. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol. 2003;171:3668–74. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Xu K, Ambati B, Yu FSX. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–54. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B: possible facilitation by the formation of a ternary complex with the grb2 adaptor protein. J Biol Chem. 2000;275:4283–9. doi: 10.1074/jbc.275.6.4283. [DOI] [PubMed] [Google Scholar]
- 12.Kelly-Wintenberg K, South SL, Montie TC. Tyrosine phosphate in a- and b-type flagellins of Pseudomonas aeruginosa. J Bacteriol. 1993;175:2458–61. doi: 10.1128/jb.175.8.2458-2461.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schenk G, Gahan LR, Carrington LE, et al. Phosphate forms an unusual tripodal complex with the Fe–Mn center of sweet potato purple acid phosphatase. Proc Natl Acad Sci USA. 2005;102:273–8. doi: 10.1073/pnas.0407239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Ma Y, Yu JG. Theoretical studies on the mechanism of activation of phosphoprotein phosphatases and purple acid phosphatases suggest an evolutionary strategy to survive in acidic environments. J Biol Inorg Chem. 2013;18:1019–26. doi: 10.1007/s00775-013-1053-x. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–84. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Kelker MS, Page R, Peti W. Crystal structures of protein phosphatase-1 bound to nodularin-r and tautomycin: a novel scaffold for structure-based drug design of serine/threonine phosphatase inhibitors. J Mol Biol. 2009;385:11–21. doi: 10.1016/j.jmb.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Yang H, Yang Z, et al. Sulfonated compounds bind with prostatic acid phosphatase (pap248–286) to inhibit the formation of amyloid fibrils. ChemistryOpen. 2018;7:447–56. doi: 10.1002/open.201800041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusudo T, Sakaki T, Inouye K. Purification and characterization of purple acid phosphatase PAP1 from dry powder of sweet potato. Biosci Biotechnol Biochem. 2003;67:1609–11. doi: 10.1271/bbb.67.1609. [DOI] [PubMed] [Google Scholar]
- 19.Cendra MM, Christodoulides M, Hossain P. Signaling mediated by Toll-like receptor 5 sensing of Pseudomonas aeruginosa flagellin influences IL-1β and IL-18 production by primary fibroblasts derived from the human cornea. Front Cell Infect Microbiol. 2017;7:130. doi: 10.3389/fcimb.2017.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odumosu BT, Adeniyi BA, Chandra R. Analysis of integrons and associated gene cassettes in clinical isolates of multidrug resistant Pseudomonas aeruginosafrom Southwest Nigeria. Ann Clin Microbiol Antimicrob. 2013;12:29. doi: 10.1186/1476-0711-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faldynova M, Videnska P, Havlickova H, et al. Prevalence of antibiotic resistance genes in faecal samples from cattle, pigs and poultry. Vet Med. 2013;58:298–304. doi: 10.17221/6865-VETMED. [DOI] [Google Scholar]
- 22.Oddie GW, Schenk G, Angel NZ, et al. Structure, function, and regulation of tartrate-resistant acid phosphatase. Bone. 2000;27:575–84. doi: 10.1016/s8756-3282(00)00368-9. [DOI] [PubMed] [Google Scholar]
- 23.Bhadouria J, Singh AP, Mehra P, et al. Identification of purple acid phosphatases in chickpea and potential roles of CaPAP7 in seed phytate accumulation. Sci Rep. 2017;7:11012. doi: 10.1038/s41598-017-11490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon JH, Ahn KB, Kim SK, Im J, Yun CH, Han SH. Bacterial flagellin induces IL-6 expression in human basophils. Mol Immunol. 2015;65:168–76. doi: 10.1016/j.molimm.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Schmeck B, Lorenz J, N’Guessan PD, et al. Histone acetylation and flagellin are essential for legionella pneumophila-induced cytokine expression. J Immunol. 2008;181:940–7. doi: 10.4049/jimmunol.181.2.940. [DOI] [PubMed] [Google Scholar]
- 26.Xu M, Xie Y, Jiang C, et al. Treponema pallidum flagellins elicit proinflammatory cytokines from human monocytes via TLR5 signaling pathway. Immunobiology. 2017;222:709–18. doi: 10.1016/j.imbio.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Nakamoto K, Watanabe M, Sada M, et al. Pseudomonas aeruginosa-derived flagellin stimulates IL-6 and IL-8 production in human bronchial epithelial cells: a potential mechanism for progression and exacerbation of COPD. Exp Lung Res. 2019;45:255–66. doi: 10.1080/01902148.2019.1665147. [DOI] [PubMed] [Google Scholar]
- 28.Cereghetti GM, Stangherlin A, Martins de Brito O, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci. 2008;105:15803–8. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayden MS, West AP, Ghosh S. NF-κB and the immune response. Oncogene. 2006;25:6758–80. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 30.Jiang C, Xu M, Kuang X, et al. Treponema pallidum flagellins stimulate MMP-9 and MMP-13 expression via TLR5 and MAPK/NF-κB signaling pathways in human epidermal keratinocytes. Exp Cell Res. 2017;361:46–55. doi: 10.1016/j.yexcr.2017.09.040. [DOI] [PubMed] [Google Scholar]
- 31.Benedikz EK, Bailey D, Cook CNL, et al. Bacterial flagellin promotes viral entry via an NF-kB and toll like receptor 5 dependent pathway. Sci Rep. 2019;9:7903. doi: 10.1038/s41598-019-44263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao Y, Liu F, Yang J, et al. Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cell Mol Immunol. 2015;12:729–42. doi: 10.1038/cmi.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tallant T, Deb A, Kar N, et al. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-κB and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 2004;4:33. doi: 10.1186/1471-2180-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Estaki M, DeCoffe D, Gibson DL. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J Gastroenterol. 2014;20:15650–6. doi: 10.3748/wjg.v20.i42.15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buddington RK, Sangild PT. Companion animals symposium: Development of the mammalian gastrointestinal tract, the resident microbiota, and the role of diet in early life. J Anim Sci. 2011;89:1506–19. doi: 10.2527/jas.2010-3705. [DOI] [PubMed] [Google Scholar]