Abstract
Objective
This study investigated the effects of in ovo feeding of γ-aminobutyric acid (GABA) and embryonic thermal manipulation (ETM) on growth performance, organ indices, plasma biochemical parameters, hepatic antioxidant levels, and expression of lipid metabolism-related genes in broilers.
Methods
Two hundred and fifty eggs were assigned to one of four treatments: control eggs incubated under standard conditions (CON); eggs that received an in ovo injection of 10% GABA on day 17.5 of incubation (G10); thermally manipulated eggs between days 10 and 18 of incubation at 39.6°C for 6 h daily (TM); and eggs that received both treatments during incubation (G10+TM). After 28 days of rearing, five birds per treatment were selected for blood and organ sampling.
Results
No differences were found in hatchability or growth parameters among different treatment groups. Hepatic gene expression of catalase (CAT) and glutathione peroxidase 1 (GPx1) was upregulated (p = 0.046 and p = 0.006, respectively) in the G10+TM group, while that of nuclear factor erythroid 2–related factor 2 (NRF2) was upregulated (p = 0.039) in the G10 group. In addition, the relative gene expression of NADPH oxidase 1 (NOX1) was significantly lower (p = 0.007) in all treatment groups than that in the CON group. Hepatic fatty acid synthase (FAS) levels and average daily feed intake (ADFI) of last week showed a positive correlation (r = 0.50, p = 0.038). In contrast, the relative gene expression of the extracellular fatty acid-binding protein (EXFAB) and peroxisome proliferator-activated receptor-γ (PPAR-γ) were positively correlated (r = 0.48, p = 0.042 and r = 0.50, p = 0.031) with the overall ADFI of birds.
Conclusion
Taken together, the results of this study suggest that the combination of in ovo feeding of GABA and ETM can enhance hepatic antioxidant function in broilers.
Keywords: Antioxidant Status, Broiler, γ-Aminobutyric acid (GABA), In ovo Feeding, Thermal Manipulation
INTRODUCTION
The poultry production industry faces several challenges that negatively affect birds: stocking density, disease infection, and heat stress (HS). Recent literature indicates that these stressors are often associated with the overproduction of reactive oxygen species (ROS), leading to oxidative stress [1,2]. Birds have developed various antioxidant defense systems to adapt to and survive in the environment. Indeed, in avian species, cell signaling and stress adaptation are maintained by the redox status [3]. The redox balance can be regulated by various systems. The first step is to lower the action of enzymes such as NADPH oxidase (NOX), which is responsible for ROS production, thus limiting the production of free radicals by decreasing oxygen accessibility. The combined action of three antioxidant enzymes ensures another line of defense: catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) [4]. SOD catalyzes the dismutation of O2− into O2 and H2O2. Furthermore, GPx and CAT catalyze the breakdown of H2O2 into H2O and oxygen [5]. These defense systems are triggered by the activation of nuclear factor (erythroid-derived 2)-like 2 (NRF2), which is responsible for the expression of antioxidant and detoxification-related genes [6].
Several strategies have been proposed to alleviate oxidative stress in broiler chickens. One approach was dietary supplementation with γ-aminobutyric acid (GABA) [7]. GABA is a neurotransmitter that regulates vital functions such as blood pressure and respiration [8]. Supplementation with GABA is particularly effective against heat-induced oxidative stress [9]. To mitigate HS, previous studies have used GABA as a feed additive for broilers [10] or provided it to the drinking water of laying hens [11]. Furthermore, our previous study showed that in ovo GABA feeding can improve the antioxidant status in chicks exposed to acute HS [12].
Another approach against heat-induced oxidative stress is epigenetic temperature adaptation. This method can be applied during pre- or early- post-hatch ontogenesis to modulate gene expression and provide long-lasting adaptation to the environment [13]. The most recent of these techniques is embryonic thermal manipulation (ETM). Furthermore, studies have demonstrated that a particular pattern of incubation temperature can affect the metabolic rate of hatchlings. Indeed, the results showed that ETM improves defense mechanisms by enhancing the antioxidant and immune responses of broilers [14].
In the present study, we hypothesized that in ovo feeding of GABA combined with ETM could modulate post-hatch gene expression in broilers. Thus, we evaluated the growth performance, organ indices, plasma biochemical parameters, and hepatic expression of genes related to antioxidant defenses and lipid metabolism in broilers.
MATERIALS AND METHODS
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Gyeongsang National University (GNU-200916-C0058).
Incubation, in ovo feeding procedure and feeding trial
Two hundred and fifty hatching eggs were obtained from 37-week-old Indian River breeder hens housed at a local breeder farm (Hapcheon, Korea). Following standard incubation conditions, the eggs were incubated in an incubator (Rcom Co., Ltd., Gimhae, Korea). Briefly, from embryonic day (ED) 1 to 18, eggs were subjected to 37.8°C and 56% relative humidity (RH), and from ED 18 until hatching, the incubation temperature was maintained at 36.8°C with 70% RH. On ED 10, after candling, non-fertilized eggs were removed from the incubator. The eggs were distributed in four groups of equal numbers (n = 48) with similar weights (61.0± 2 g) using the Solver module of Microsoft Excel (Microsoft Excel 2016; Microsoft Corp., Washington, USA). Each group consisted of eight replicates with six eggs. In our previous study, we found no significant differences (hatching and biological parameters) between the sham control (distilled water injected) and the non-injected control; therefore, we did not include a sham treatment in this trial. The groups were the followings: i) control eggs without in ovo injection and incubated at standard temperature (CON); ii) eggs injected at 17.5 days of incubation with 0.6 mL of 10% (0.1 g/mL) GABA (#A2129; Sigma-Aldrich Inc., St. Louis, CA, USA) dissolved in distilled water (G10); iii) thermally manipulated eggs exposed to 39.6°C for 6 h (from 10:00 h to 16:00) daily from ED 10 to 18 (TM); and iv) eggs that received both previous treatments during incubation (G10+TM).
The solution and methodology used for the in ovo feeding procedure were the same as those previously described [15,16]. Briefly, a hole was drilled from the blunt end of the eggs and subsequently injected with a 1 mL syringe with a 23 G and 1-inch needle (KOVAX-SYRINGE; Korea Vaccine Co., Ltd. Seoul, Korea). Therefore, the injection targeted the amnion with a depth of approximately 2.5 cm.
Immediately after the injection, eggs were sealed using surgical tape (3M Micropore, Saint Paul, MN, USA) and incubated. The control eggs were removed from the incubator for the same period but returned without injection. After hatching, one-day-old broilers (n = 140) with similar weights were selected to equalize the starting weight between the cages and treatments. The birds were raised in battery brooders in a thermally controlled environment at 34°C±1°C and 50% RH for one-day-old broilers, and then the temperature was gradually decreased to 22°C±1°C on day 28. Commercial feed (Nonghuyp Feed Inc., Seoul, Korea) (Table 1) and water were provided ad libitum under continuous lighting. Each treatment consisted of five cages with seven chicks each.
Table 1.
Feed composition of the experimental diets from 0 to 28 days of age1)
| Items | Starter (0 to 7 d) | Grower (8 to 21 d) | Finisher (22 to 28 d) |
|---|---|---|---|
| Ingredients (%) | |||
| Corn grain | 38.97 | 45.87 | 39.68 |
| Wheat grain | 15.00 | 15.00 | 25.00 |
| Soybean meal (42.6% CP) | 32.00 | 25.60 | 20.60 |
| Corn gluten | 3.00 | 2.64 | 3.00 |
| Meat and bone meal | 2.00 | 2.00 | 2.50 |
| Animal fat | 4.00 | 3.88 | 4.54 |
| Salt | 0.25 | 0.25 | 0.25 |
| Tricalcium phosphate | 1.30 | 1.04 | 0.86 |
| Limestone | 1.26 | 1.22 | 1.26 |
| Sodium bicarbonate | 0.00 | 0.02 | 0.00 |
| L-threonine | 0.12 | 0.16 | 0.16 |
| Lysine | 1.23 | 1.43 | 1.32 |
| DL-methionine | 0.33 | 0.34 | 0.29 |
| Choline chloride (50.0%) | 0.03 | 0.03 | 0.03 |
| Premix2) | 0.20 | 0.20 | 0.20 |
| Phytase | 0.05 | 0.05 | 0.05 |
| Anti-coccidia | 0.01 | 0.01 | 0.01 |
| Calculated nutrients (%) | |||
| Crude protein | 23.00 | 20.50 | 19.50 |
| Crude fat | 6.31 | 6.36 | 6.90 |
| Crude fiber | 3.01 | 2.80 | 2.68 |
| Crude ash | 5.99 | 5.34 | 5.02 |
| Calcium | 1.01 | 0.90 | 0.86 |
| Available phosphorus | 0.60 | 0.53 | 0.49 |
| Digestible lysine | 1.43 | 1.24 | 1.09 |
| Digestible methionine+cystine | 1.07 | 0.95 | 0.86 |
| Copper (ppm) | 82.21 | 81.04 | 80.78 |
| Zinc (ppm) | 100.27 | 96.63 | 97.33 |
| Metabolizable energy (kcal/kg) | 3,050 | 3,150 | 3,200 |
Nonghuyp Feed Inc. (Seoul, Korea).
Trace minerals and vitamins provided per kilogram of premix: vitamin A, 12,000,000 IU; vitamin D3, 3,000,000 IU; vitamin E, 40,000 IU; vitamin K3, 2,000 IU; vitamin B1, 2,000 mg; vitamin B2, 5,000 mg; vitamin B6, 3,000 mg; vitamin B12, 20 mg; niacin, 40,000 mg; pantothenic acid, 10,000 mg; folic acid, 1,000 mg, iron, 88,000 mg; copper, 72,600 mg; zinc, 60,000 mg; manganese, 66,000 mg; iodine, 990 mg; selenium, 220 mg; cobalt, 330 mg.
Blood, tissue sampling, and plasma biochemical parameters analysis
On day 28, one bird from each cage was randomly selected for blood and tissue sampling. The birds were euthanized with carbon dioxide before sampling. Blood was collected by heart puncture and transferred into heparinized vacuum containers (#367874; BD Co., Ltd., Franklin Lakes, NJ, USA). The blood samples were then centrifuged at 2,000×g for 10 min at 4°C, and plasma was collected and stored at −20°C for later analysis. Tissues were sampled and weighed (liver, spleen, bursa, and heart), and the liver samples were snap-frozen in liquid nitrogen and stored at −80°C for further analysis. Plasma metabolite concentrations were measured using a VetTest Chemistry Analyzer (IDEXX Co., Ltd., Westbrook, CT, USA) with dry-slide technology, following the manufacturer’s instructions.
Real-time polymerase chain reaction for mRNA quantification
Following the manufacturer’s protocol, total RNA was extracted from the liver using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). The concentrations and purities of the samples were determined by measuring the optical density of each sample using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). Subsequently, reverse transcription was conducted using the PrimeScript first-strand cDNA synthesis kit (Takara, Tokyo, Japan) following the manufacturer’s instructions. The cDNA was then used for performing real-time polymerase chain reaction (PCR) using the StepOnePlus real-time PCR system (Life Technologies, Carlsbad, CA, USA) according to the following protocol: 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Each reaction well was composed of a 20 μL volume containing Power SYBR Green PCR master mix (Life Technologies, USA) and 10 pmol concentration of forward and reverse primers specific for each gene and cDNA. Table 2 lists the primer sequences used in this study. Gene quantification was normalized to the Ct values of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin. Relative expression was determined using the 2−ΔΔct algorithm. The geometric means of both references were used to calculate the expression levels of the target genes.
Table 2.
Oligonucleotide primer sequences for RT-qPCR
| Gene | Sequence | Accession number |
|---|---|---|
| ACC | F: CACTTCGAGGCGAAAAAC R: GGAGCAAATCCATGACCA |
XM_015295697.2 |
| CAT | F: ACCAAGTACTGCAAGGCGAA R: TGAGGGTTCCTCTTCTGGCT |
NM_001031215.1 |
| EXFABP | F: GGAGGACCTTGCACATGA R: GTGTAGTTCCGCTCCCTA |
NM_205422.1 |
| FAS | F: CAATGGACTTCATGCCTC R: GCTGGGTACTGGAAGACA |
NM_205155.3 |
| GPx1 | F: AACCAATTCGGGCACCAG R: CCGTTCACCTCGCACTTCTC |
NM_001277853.2 |
| NOX1 | F: GCGAAGACGTGTTCCTGTAT R: GAACCTGTACCAGATGGACTTC |
NM_001101830.1 |
| NOX4 | F: CCTCTGTGCTTGTACTGTGTAG R: GACATTGGAGGGATGGCTTAT |
NM_001101829.1 |
| NRF2 | F: CAGAAGCTTTCCCGTTCATAGA R: GACATTGGAGGGATGGCTTAT |
NM_205117 |
| PPAR-γ | F: TCAGGTTTGGGCGAATGC R: CGCTCGCAGATCAGCAGA |
XM_040646063.1 |
| SOD | F: AGGGGGTCATCCACTTCC R: CCCATTTGTGTTGTCTCCAA |
NM_205064.1 |
| β-actin | F: ACCGGACTGTTACCAACA R: GACTGCTGCTGACACCTT |
NM_205518.1 |
| GAPDH | F: TTGGCATTGTGGAGGGTCTTA R: GTGGACGCTGGGATGATGTT |
NM_204305.1 |
RT-qPCR, real-time quantitative polymerase chain reaction; ACC, acetyl-CoA carboxylase; CAT, catalase; EXFABP, extracellular fatty acid-binding protein; FAS, fatty acid synthase; GPx1, glutathione peroxidase 1; NOX1, nicotinamide adenine dinucleotide phosphate oxidase 1; NOX4, nicotinamide adenine dinucleotide phosphate oxidase 4; NRF2, nuclear factor erythroid 2-related factor 2; PPAR-γ, peroxisome proliferator-activated receptor gamma; SOD, superoxide dismutase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
Before the analysis, all assumptions related to the application of parametric tests were assessed. Data were analyzed via one-way analysis of variance (ANOVA) using the “general linear model procedure” in the SAS software (version 9.4; SAS Institute Inc., 2009). A Tukey’s post hoc test was performed following a significant p-value to assess the differences among means. Concerning hepatic gene expression, orthogonal planned contrasts were made to evaluate specific comparisons using the “Contrast statement” of the SAS software [17]. Differences were considered statistically significant at p≤0.05, and p≤0.1 was considered a trend. Moreover, gene expression patterns were detected by hierarchical clustering [18] using the package “ComplexHeatmap” in R software version 4.0.3 (R Core Team, 2020). Finally, Pearson’s correlation analysis was performed for the evaluated parameters to develop a correlation matrix using the “CORR procedure” in the SAS software.
RESULTS
Hatching parameters, growth performance, and organ indexes
No significant differences were observed in the hatching parameters (Table 3) among different treatment groups. In ovo feeding of GABA and ETM did not significantly affect hatchability or the average hatchling weight.
Table 3.
Effects of in ovo feeding of γ-aminobutyric acid and embryonic thermal manipulation on hatchability parameters
| Parameters | Treatments1) | SEM | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| CON | G10 | TM | G10+TM | |||
| Number of fertile eggs | 48 | 48 | 48 | 48 | NA | NA |
| Number of hatchlings | 46 | 43 | 45 | 41 | NA | NA |
| Hatchability (%) | 95.8 | 89.6 | 93.7 | 85.4 | 1.9 | 0.255 |
| Hatchling weight (g) | 40.9 | 40.4 | 39.7 | 40.0 | 0.23 | 0.296 |
Hatchability was calculated based on n = 8 repetitions per treatment.
SEM, standard error of the mean; NA, not applicable.
The treatments are described as follows: CON, control eggs without in ovo injection and incubated at standard temperature; G10, eggs injected at 17.5 days of incubation with 0.6 mL of 10% γ-aminobutyric acid dissolved in distilled water; TM, thermally manipulated eggs exposed to 39.6°C for 6 h daily from embryonic day 10 to 18; G10+TM, eggs that received both previous treatments during incubation.
Body weight, average daily weight gain (ADWG), and aver age daily feed intake (ADFI) were similar in all treatment groups regardless of the period. However, the G10+TM group had an overall higher (p = 0.018) feed conversion ratio (FCR) than the CON group from days 8 to 21 of the post-hatch period (Table 4).
Table 4.
Effects of in ovo feeding of γ-aminobutyric acid and embryonic thermal manipulation on growth, feed intake, and feed conversion ratio in broiler chickens
| Parameters | Treatment1) | SEM | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| CON | G10 | TM | G10+TM | |||
| BW (g) | ||||||
| Day old | 40.3 | 40.2 | 39.9 | 40.0 | 0.07 | 0.149 |
| 7 d | 155.1 | 154.3 | 152.2 | 167 | 0.10 | 0.161 |
| 21 d | 909.1 | 901.7 | 879.8 | 881.7 | 8.10 | 0.528 |
| 28 d | 1,450.2 | 1,493.8 | 1,431.3 | 1,419 | 18.3 | 0.526 |
| ADWG (g) | ||||||
| 1 to 7 d | 16.4 | 16.2 | 16.1 | 18.2 | 0.37 | 0.151 |
| 8 to 21 d | 53.8 | 53.4 | 52 | 51.1 | 0.52 | 0.211 |
| 22 to 28 d | 77.3 | 84.6 | 78.8 | 76.8 | 2.31 | 0.649 |
| 1 to 28 d | 49.1 | 51.4 | 48.9 | 48.6 | 1.40 | 0.628 |
| ADFI (g) | ||||||
| 1 to 7 d | 15.4 | 14.8 | 14.6 | 16.9 | 0.35 | 0.084 |
| 8 to 21 d | 67.6 | 69.1 | 66.1 | 67.3 | 0.58 | 0.337 |
| 22 to 28 d | 116 | 125.8 | 119.9 | 112.1 | 1.96 | 0.067 |
| 1 to 28 d | 66.3 | 69.9 | 66.8 | 65.4 | 1.20 | 0.095 |
| FCR (g/g) | ||||||
| 1 to 7 d | 0.94 | 0.91 | 0.91 | 0.92 | 0.01 | 0.585 |
| 8 to 21 d | 1.25b | 1.28ab | 1.26ab | 1.30a | 0.02 | 0.018 |
| 22 to 28 d | 1.51 | 1.49 | 1.52 | 1.51 | 0.03 | 0.984 |
| 1 to 28 d | 1.23 | 1.23 | 1.23 | 1.25 | 0.02 | 0.873 |
Values were obtained from n = 5 repetitions per treatment.
SEM, standard error of the mean; BW, body weight; ADWG, average daily weight gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
The treatments are described as follows: CON, control eggs without in ovo injection and incubated at standard temperature; G10, eggs injected at 17.5 days of incubation with 0.6 mL of 10% γ-aminobutyric acid dissolved in distilled water; TM, thermally manipulated eggs exposed to 39.6°C for 6 h daily from embryonic day 10 to 18; G10+TM, eggs that received both previous treatments during incubation. Chicks obtained were grown for 28 days.
Means in a row that possess different superscripts differ significantly (p<0.05).
The organ index results are presented in Table 5. The ab solute spleen, bursa, and heart weights did not differ across different treatment groups. In contrast, absolute liver weight was statistically different (p = 0.046) among the treatment groups, with the highest value recorded in the G10 group and the lowest in the G10+TM group. Significant differences were observed in the relative weight of the bursa among the treatment groups. Chicks in the G10+TM group had a higher (p = 0.008) relative bursa weight than those in the CON group did. Finally, the relative liver, spleen, and heart weights were similar among all the treatment groups.
Table 5.
Effects of in ovo feeding of γ-aminobutyric acid and embryonic thermal manipulation on absolute and relative organs weight in broiler chickens
| Parameters | Treatments1) | SEM | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| CON | G10 | TM | G10+TM | |||
| Absolute weight (g) | ||||||
| Liver | 36.9ab | 41.2a | 35.6ab | 33.3b | 1.1 | 0.046 |
| Spleen | 1.2 | 1.1 | 1.4 | 1.01 | 0.2 | 0.460 |
| Bursa | 1.7 | 2.1 | 1.8 | 2.3 | 0.1 | 0.093 |
| Heart | 7.3 | 8.2 | 6.4 | 6.8 | 0.3 | 0.080 |
| Relative weight (%) | ||||||
| Liver | 2.9 | 3.3 | 3.1 | 3.1 | 0.08 | 0.264 |
| Spleen | 0.09 | 0.09 | 0.12 | 0.09 | 0.02 | 0.442 |
| Bursa | 0.13B | 0.17AB | 0.15B | 0.22A | 0.01 | 0.008 |
| Heart | 0.57 | 0.66 | 0.54 | 0.65 | 0.02 | 0.138 |
Values were obtained from n = 5 repetitions per treatment.
The treatments are described as follows: CON, control eggs without in ovo injection and incubated at standard temperature; G10, eggs injected at 17.5 days of incubation with 0.6 mL of 10% γ-aminobutyric acid dissolved in distilled water; TM, thermally manipulated eggs exposed to 39.6°C for 6 h daily from embryonic day 10 to 18; G10+TM, eggs that received both previous treatments during incubation. Chicks obtained were grown for 28 days.
Means in a row that possess different superscripts differ significantly (p<0.05).
Means in a row that possess different superscripts differ significantly (p<0.01).
Plasma biochemical parameters
Table 6 presents the effects of in ovo feeding with GABA and thermal manipulation on the plasma biochemical parameters of broilers. Plasma glucose, triglyceride, and cholesterol levels were not affected by any treatment. However, plasma total protein levels were higher (p = 0.019) in chicks in the G10+TM group than those in the CON group.
Table 6.
Effects of in ovo feeding of γ-aminobutyric acid and embryonic thermal manipulation on plasma biochemical parameters in broiler chickens
| Parameters | Treatments1) | SEM | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| CON | G10 | TM | G10+TM | |||
| Glucose (mg/dL) | 251.8 | 274.4 | 274.8 | 266.6 | 6.7 | 0.624 |
| Triglycerides (mg/dL) | 41.4 | 27.6 | 26.6 | 37.6 | 3.2 | 0.267 |
| Total protein (g/dL) | 2.48b | 2.62ab | 2.58ab | 3.1a | 0.1 | 0.019 |
| Cholesterol (mg/dL) | 114.8 | 96.2 | 88.2 | 111 | 6.8 | 0.506 |
Values were obtained from n = 5 repetitions per treatment.
SEM, standard error of the mean.
The treatments are described as follows: CON, control eggs without in ovo injection and incubated at standard temperature; G10, eggs injected at 17.5 days of incubation with 0.6mL of 10% γ-aminobutyric acid dissolved in distilled water; TM, thermally manipulated eggs exposed to 39.6°C for 6 h daily from embryonic day 10 to 18; G10+TM, eggs that received both previous treatments during incubation. Chicks obtained were grown for 28 days.
Means in a row that possess different superscripts differ significantly (p<0.05).
Hepatic mRNA relative expression
Tables 7 and 8 show the results of the ANOVA procedure and contrast analysis performed on the hepatic mRNA relative expression of the genes studied. Significant differences among the treatment groups were revealed for the expression of antioxidant-related genes. The glutathione peroxidase 1 (GPx1) gene was significantly upregulated (p = 0.039) in chicks in the G10+TM group compared to those in the CON group. In contrast, significantly lower (p = 0.039) expression of the NADPH oxidase 1 (NOX1) gene was observed in the G10 and G10+TM treatment groups than in the CON treatment group. Furthermore, the contrast analysis results showed that the chicks from the G10+TM group exhibited higher (p = 0.046) CAT gene expression than the CON group chicks. Although not statistically significant, NOX4 gene expression showed a decreasing tendency (p = 0.077) in the G10 group. In addition, orthogonal contrast analysis showed that the NRF2 gene was significantly upregulated (p = 0.039) in birds fed GABA in ovo (G10). Although a numerically lower relative expression of the lipid metabolism gene in the G10+TM group was continuously observed, neither the ANOVA procedure nor the contrast analysis revealed significant differences among all groups.
Table 7.
Effects of in ovo feeding of γ-aminobutyric acid and embryonic thermal manipulation on relative hepatic gene expression in broiler chickens
| Genes | Treatments1) | SEM | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| CON | G10 | TM | G10+TM | |||
| SOD | 1.00 | 1.58 | 1.28 | 2.36 | 0.33 | 0.522 |
| CAT | 1.00 | 1.59 | 3.22 | 4.41 | 0.61 | 0.171 |
| GPx1 | 1.00a | 1.31ab | 1.59ab | 2.04b | 0.19 | 0.039 |
| NOX1 | 1.00B | 0.28A | 0.52AB | 0.43A | 0.09 | 0.007 |
| NOX4 | 1.00 | 0.41 | 0.55 | 0.57 | 0.11 | 0.274 |
| ACC | 1.00 | 2.26 | 2.17 | 0.41 | 0.52 | 0.573 |
| FAS | 1.00 | 1.89 | 1.02 | 0.84 | 0.17 | 0.124 |
| NRF2 | 1.00 | 2.26 | 1.01 | 1.23 | 0.22 | 0.123 |
| EXFABP | 1.00 | 0.91 | 0.51 | 0.21 | 0.11 | 0.283 |
| PPAR-γ | 1.00 | 0.99 | 0.99 | 0.43 | 0.14 | 0.497 |
Values were obtained from n = 5 repetitions per treatment.
SEM, standard error of the mean; SOD, superoxide dismutase; CAT, catalase; GPx1, glutathione peroxidase 1; NOX1, nicotinamide adenine dinucleotide phosphate oxidase 1; NOX4, nicotinamide adenine dinucleotide phosphate oxidase 4; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; NRF2, nuclear factor erythroid 2-related factor 2; EXFABP, extracellular fatty acid-binding protein; PPAR-γ, peroxisome proliferator-activated receptor-gamma.
The treatments are described as follows: CON, control eggs without in ovo injection and incubated at standard temperature; G10, eggs injected at 17.5 days of incubation with 0.6 mL of 10% γ-aminobutyric acid dissolved in distilled water; TM, thermally manipulated eggs exposed to 39.6°C for 6 h daily from embryonic day 10 to 18; G10+TM, eggs that received both previous treatments during incubation. Chicks obtained were grown for 28 days.
Means in a row that possess different superscripts differ significantly (p<0.05).
Means in a row that possess different superscripts differ significantly (p<0.01).
Table 8.
Results of planned contrasts of hepatic gene expression analysis (p-value)
| Genes | Planned contrast1) | ||
|---|---|---|---|
|
| |||
| CON vs G10 | CON vs TM | CON vs G10+TM | |
| SOD | NS | NS | NS |
| CAT | NS | NS | 0.046 |
| GPx1 | NS | 0.092 | 0.006 |
| NOX1 | 0.001 | 0.019 | 0.007 |
| NOX4 | 0.077 | NS | NS |
| ACC | NS | NS | NS |
| FAS | 0.065 | NS | NS |
| NRF2 | 0.039 | NS | NS |
| EXFABP | NS | NS | 0.087 |
| PPAR-γ | NS | NS | NS |
The treatments are described as follows: CON, control eggs without in ovo injection and incubated at standard temperature; G10, eggs injected at 17.5 days of incubation with 0.6 mL of 10% γ-aminobutyric acid dissolved in distilled water; TM, thermally manipulated eggs exposed to 39.6°C for 6 h daily from embryonic day 10 to 18; G10+TM, eggs that received both previous treatments during incubation. Chicks obtained were grown for 28 days.
SOD, superoxide dismutase; NS, not significant; CAT, catalase; GPx1, glutathione peroxidase 1; NOX1, nicotinamide adenine dinucleotide phosphate oxidase 1; NOX4, nicotinamide adenine dinucleotide phosphate oxidase 4; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; NRF2, nuclear factor erythroid 2-related factor 2; EXFABP, extracellular fatty acid-binding protein; PPAR-γ, peroxisome proliferator-activated receptor-gamma.
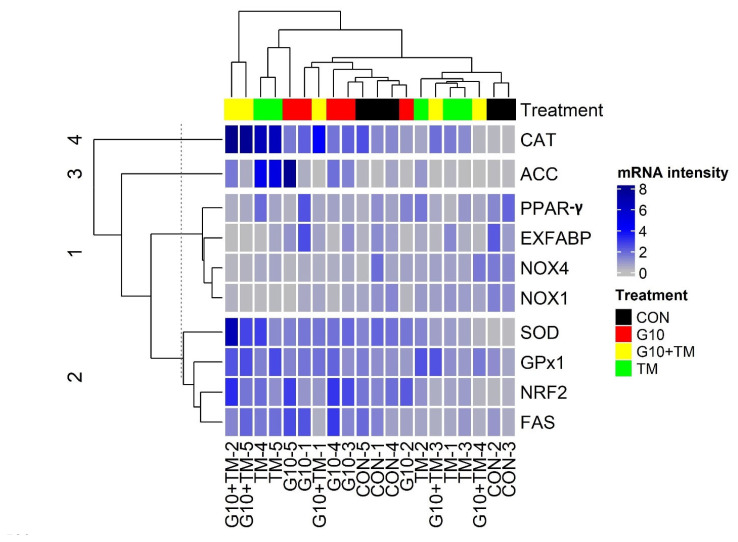
Gene expression data were clustered into four groups based on Euclidean distances (Figure 1). Two large clusters were identified: the first was composed of NOX1, NOX4, extracellular fatty acid-binding protein (EXFABP), and peroxisome proliferator-activated receptor-γ (PPAR-γ), with NOX1, NOX4, and EXFABP being downregulated (bright gray bars), whereas PPAR-γ expression was decreased or unchanged compared to the control. The second-largest cluster included SOD, GPx1, NRF2, and fatty acid synthase (FAS) genes, which were moderately upregulated (blue bars). Finally, CAT and acetyl-CoA carboxylase (ACC) were the only genes in separate clusters. CAT was robustly upregulated (dark blue bars) in the treatment groups relative to the control group.
Figure 1.
Hierarchical cluster tree of hepatic gene expression. The genes panel included antioxidant genes (CAT, SOD, GPx1, NOX1, NOX4, and NRF2) and lipid metabolism genes (ACC, FAS, EXFABP, and PPAR-γ). Each row represents a gene and each column represents an experimental unit belonging to a specific treatment. The treatments are described as follows: CON, control eggs without in ovo injection and incubated at standard temperature; G10, eggs injected at 17.5 days of incubation with 0.6 mL of 10% γ-aminobutyric acid dissolved in distilled water; TM, thermally manipulated eggs exposed to 39.6°C for 6 h daily from embryonic day 10 to 18; G10+TM, eggs that received both previous treatments during incubation. Gene expression analysis was performed using real-time quantitative polymerase chain reaction (RT-qPCR) with GAPDH and β-actin as reference genes. The tree was constructed using the package “ComplexHeatmap” of the R software version 4.0.3 (R Core Team, 2020). CAT, catalase; ACC, acetyl-CoA carboxylase; PPAR-γ, peroxisome proliferator-activated receptor-gamma; EXFABP, extracellular fatty acid-binding protein; NOX4, nicotinamide adenine dinucleotide phosphate oxidase 4; NOX1, nicotinamide adenine dinucleotide phosphate oxidase 1; SOD, superoxide dismutase; GPx1, glutathione peroxidase 1; NRF2, nuclear factor erythroid 2-related factor 2; FAS, fatty acid synthase.
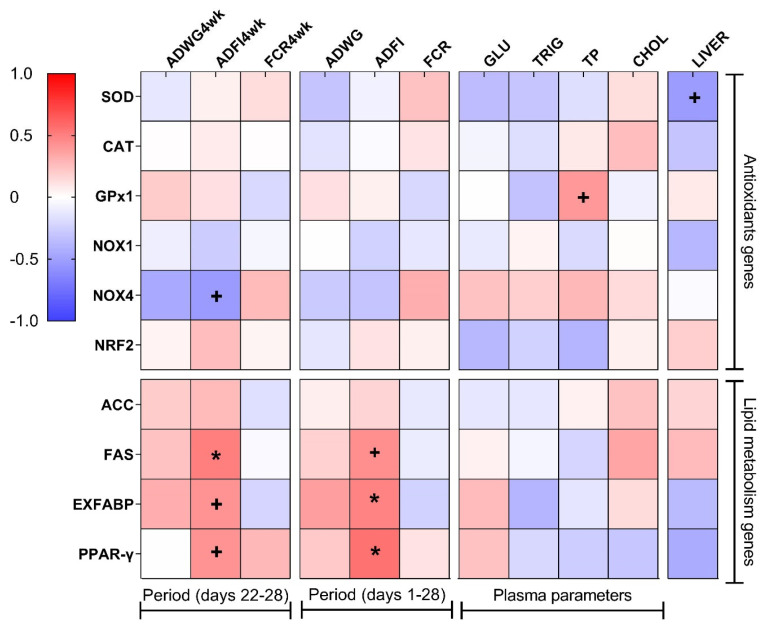
Figure 2 presents the results of Pearson’s correlation anal ysis between relative gene expression, growth parameters, plasma biochemical parameters, and relative liver weight. The overall ADFI was positively correlated (red color) with both relative EXFABP (r = 0.48, p = 0.042) and PPAR-γ (r = 0.55, p = 0.031) gene expression. Interestingly, the same positive correlation was observed between ADFI during the final week of the trial (ADFI4wk) and FAS (r = 0.50, p = 0.038) gene expression.
Figure 2.
Pearson correlation heat map between the relative mRNA levels of the genes studied, the growth parameters, the plasma biochemical parameters, and the relative liver weight in broiler chickens. The red color indicates a positive correlation, the blue color indicates a negative correlation and the white color indicates no correlation. Pearson r values were calculated using the “CORR procedure” of the SAS software version 9.4 (SAS Institute Inc., 2009). ADWG4wk, average daily weight gain of the last week; ADFI4wk, average daily feed intake of the last week; FCR4wk, feed conversion ratio of the last week; ADWG, average daily weight gain; ADFI, average daily feed intake; FCR, feed conversion ratio; GLU, glucose; TRIG, triglycerides; TP, total protein; CHOL, cholesterol; LIVER, relative liver weight; SOD, superoxide dismutase; CAT, catalase; GPx1, glutathione peroxidase 1; NOX1, nicotinamide adenine dinucleotide phosphate oxidase 1; NOX4, nicotinamide adenine dinucleotide phosphate oxidase 4; NRF2, nuclear factor erythroid 2-related factor 2; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; EXFABP, extracellular fatty acid-binding protein; PPAR-γ, peroxisome proliferator-activated receptor-gamma. + Indicates a trend at the 0.1 level. * Correlation is significant at p<0.05 level.
DISCUSSION
Modern broilers have undergone decades of intensive genetic selection for rapid growth and muscle yield, rendering them particularly susceptible to oxidative stress [19]. Oxidative stress occurs when there is an overproduction of free radicals, such that the antioxidant system can no longer act as a neutralizer [20]. There are numerous options for combating oxidative stress in poultry. The first step is to limit ROS production by reducing the activity of NOX enzymes. The NOX protein complex mainly transfers electrons from NADPH to oxygen molecules, resulting in O2− production [21]. The second is to increase the scavenging of free radicals through antioxidant defenses. In chickens, the first line of antioxidant defense consists of SOD, CAT, and GPx [22]. Activation of the NRF2 gene is a critical step in the antioxidant modulation pathway [6]. NRF2 is involved in the synthesis of the three major antioxidant enzymes [1]. This study evaluated the hepatic gene expression of CAT, SOD, GPx1, and NRF2 after ETM and in ovo feeding with GABA. Only birds in the G10 group had significantly higher hepatic gene expression of NRF2 than the CON group. Both GPx1 and CAT were significantly upregulated only in the G10+TM group (Table 8).
Interestingly, SOD, CAT, and GPx1 were upregulated in all treatment groups, with the G10+TM group always showing the highest expression (Table 7). Furthermore, all three treatments downregulated the expression of NOX1. Our previous study highlighted that in ovo feeding of GABA increased the plasma total antioxidant activity in heat-stressed chicks [12]. Others have also reported enhanced GPx activity but reduced oxidation levels in birds fed GABA during stress [23]. The ability of GABA to mitigate oxidative stress is linked to its ability to promote glutamate levels [24], thus promoting the synthesis of antioxidant enzymes, such as GPx [25]. However, the mechanism by which GABA could modulate NRF2 remains unclear and requires further research. Additionally, ETM ameliorated heat-induced oxidative stress by downregulating the hepatic NOX gene family [26]. Another study evaluating the effects of cold-induced oxidative stress in broilers also revealed that ETM could lower the expression of NOX4 in the liver, spleen, and heart [27]. Based on the concept of an antioxidant system integrated into cells, we hypothesized that a combination of antioxidants would be more effective than a single antioxidant [22]. Therefore, these findings indicate the combined effect of in ovo feeding and thermal manipulation on the enhancement of hepatic antioxidant capacity in broilers.
Although no significant results were found across differ ent treatment groups regarding lipid metabolism-related genes, we found correlations among hepatic gene expression of FAS, EXFABP, PPAR-γ, and ADFI (Figure 2). FAS and ACC are two important genes that encode key enzymes in de novo fatty acid synthesis in birds [28]. FAS utilizes malonyl-CoA, catalyzed by ACC, to produce long-chain fatty acids. Similar to our results, FAS expression increased with increased feed intake in chickens [29]. This might explain why the correlation was stronger between ADFI in the last phase of the trial because chicks increased their feed intake with age. PPAR-γ is a transcription factor involved in the regulation of adipogenesis [30]. EXFABP belongs to a family of fatty acid-binding proteins that mediate the metabolism and transportation of lipids. The positive correlation between these genes and the overall ADFI in this study may indicate higher lipid deposition in birds with the highest feed consumption.
Hierarchical clustering revealed that the gene panel could be separated into four clusters, with two larger clusters. The first cluster included a set of downregulated genes (NOX1, NOX4, EXFABP, and PPAR-γ). Since NOXs family genes are responsible for ROS production, we can hypothesize that limited lipid deposition is associated with reduced ROS production. The second largest cluster included the SOD, GPx1, NRF2, and FAS genes, which were predominantly upregulated in our treatment groups. The first line of antioxidant enzymes showed increased gene expression in G10, TM, and G10+TM groups. The synthesis of this array of protective molecules results from the activation of the NRF2 transcription factor to maximize antioxidant protection and maintain internal redox balance [20]. This might explain why these genes were in the same cluster. In addition, the higher feed intake recorded in both G10 and TM during the final phase of our trial accounted for the upregulation of FAS.
This study aimed to not only assess the effects of in ovo feeding of GABA and ETM in broilers, but also to elucidate their potential potentiating effects when treated together. Because all treatments were applied during incubation, the primary outcomes to be measured were the hatching parameters. No significant differences were observed in hatchability or hatchling weight across all the treatment groups. Previous results have shown that in ovo feeding of amino acids [31], especially GABA, does not reduce hatchability in broilers. This result indicates that a high intake of exogenous GABA during incubation is not detrimental to chicken embryos, and thus, in ovo feeding of GABA is safe and can be useful for the poultry industry.
Our results also highlight that the ETM used in this study did not reduce hatchability. On the other hand, the effect of ETM on hatchability is inconsistent in the literature, reporting a significant reduction [32], increase, or no effect [16]. The discrepancies in the results may be attributed to the duration and intensity of thermal manipulation. Indeed, some studies [33] have reported decreased growth and hatchability in embryos exposed to continuous ETM. Therefore, intermittent ETM may be more appropriate to avoid a drastic reduction in hatchability. Finally, the combination of in ovo feeding of GABA and ETM, which does not significantly affect hatchability, indicates that both techniques can be implemented in this configuration without harming the embryo.
Post-hatch growth performance mostly did not show any differences between groups; only FCR during the 8 to 21 days period was increased in the G10+TM group. Studies evaluating in ovo feeding have consistently reported its significant effects on growth within the first two weeks of the rearing period. Growth performance has been reported to improve after in ovo feeding with various amino acids [34]. Although ADWG and FI were higher in the G10 group, no significant differences were found in our trial. Differences in nutrients, concentrations, and dosages may explain these results. Although the literature reports the merits of ETM, its beneficial effects on growth parameters have not been recognized. Likewise, ETM does not affect or reduce the growth performance [35]. Thus, the higher FCR recorded in the G10+TM group may be associated with thermal manipulation rather than in ovo feeding, because the G10 group consistently performed better.
The organ index is an important indicator that reflects the developmental status of organs. The immune organ index is especially significant because it often correlates with the magnitude of immune function [36]. In this study, birds in the G10+TM group had the highest relative bursa weights. Similarly, Tang and Chen [37] found an increased bursa index and improved immune function in broilers supplemented with GABA at 14 and 21 days of rearing. Evidence suggests that GABA plays a role in immunity by modulating cytokine secretion and modifying immune cell secretion [38]. However, none of the trials evaluating the potential effects of GABA on the bursa have conducted an in-depth study to elucidate the mechanism underlying its action. Thus, although GABA effectively enhances the bursa index, the explanations on this topic remain speculative, and further research is needed. In addition, the immunity-promoting abilities of ETM in poultry have already been demonstrated [26]. Thus, these results suggest the concomitant action of in ovo feeding of both GABA and ETM on the immune status of broilers.
In conclusion, the results of this study showed that in ovo GABA feeding and ETM increased the bursa index and improved hepatic antioxidant gene expression in broilers when treated together during incubation. Therefore, further investigation of the potential synergistic effect of the two methods for alleviating heat-induced oxidative stress in broilers will be of great interest.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
The authors are thankful to the Korean Government Scholarship Program (KGSP) and the Brain Pool Program funded by the Ministry of Science and ICT through the National Research Foundation of Korea (2019H1D3A1A01071142).
REFERENCES
- 1.Surai PF. Antioxidant systems in poultry biology: superoxide dismutase. J Anim Res Nutr. 2016;1:8. [Google Scholar]
- 2.Goel A, Ncho CM, Choi YH. Regulation of gene expression in chickens by heat stress. J Anim Sci Biotechnol. 2021;12:11. doi: 10.1186/s40104-020-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surai PF, Kochish II, Kidd MT. Redox homeostasis in poultry: regulatory roles of NF-κB. Antioxidants. 2021;10:186. doi: 10.3390/antiox10020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chueh CC, Lin LJ, Lin WC, et al. Antioxidant capacity of banana peel and its modulation of Nrf2-ARE associated gene expression in broiler chickens. Ital J Anim Sci. 2019;18:1394–403. [Google Scholar]
- 5.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ncho CM, Jeong C, Gupta V, Goel A. The effect of gamma-aminobutyric acid supplementation on growth performances, immune responses, and blood parameters of chickens reared under stressful environment: a meta-analysis. Environ Sci Pollut Res. 2021;28:45019–28. doi: 10.1007/s11356-021-13855-0. [DOI] [PubMed] [Google Scholar]
- 8.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–8. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 9.El-Naggar K, El-Kassas S, Abdo SE, Kirrella AAK, Al Wakeel RA. Role of gamma-aminobutyric acid in regulating feed intake in commercial broilers reared under normal and heat stress conditions. J Therm Biol. 2019;84:164–75. doi: 10.1016/j.jtherbio.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Hu H, Bai X, Shah AA, et al. Interactive effects of glutamine and gamma-aminobutyric acid on growth performance and skeletal muscle amino acid metabolism of 22–42-day-old broilers exposed to hot environment. Int J Biometeorol. 2016;60:907–15. doi: 10.1007/s00484-015-1084-9. [DOI] [PubMed] [Google Scholar]
- 11.Choi YH. Effects of γ-aminobutyric acid on mortality in laying hens during summer time. J Agric Life Environ Sci. 2019;53:131–9. [Google Scholar]
- 12.Ncho CM, Goel A, Jeong CM, Youssouf M, Choi YH. In ovo injection of gaba can help body weight gain at hatch, increase chick weight to egg weight ratio, and improve broiler heat resistance. Animals. 2021;11:1364. doi: 10.3390/ani11051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ncho CM, Gupta V, Goel A. Effect of thermal conditioning on growth performance and thermotolerance in broilers: A systematic review and meta-analysis. J Therm Biol. 2021;98:102916. doi: 10.1016/j.jtherbio.2021.102916. [DOI] [PubMed] [Google Scholar]
- 14.Al-Zghoul MB, Mohammad Saleh KM. Effects of thermal manipulation of eggs on the response of jejunal mucosae to posthatch chronic heat stress in broiler chickens. Poult Sci. 2020;99:2727–35. doi: 10.1016/j.psj.2019.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ncho CM, Goel A, Jeong CM, Gupta V, Choi YH. Effects of in ovo feeding of γ-aminobutyric acid on growth performances, plasma metabolites, and antioxidant status in broilers exposed to cyclic heat stress. Sustainability. 2021;13:11032. doi: 10.3390/su131911032. [DOI] [Google Scholar]
- 16.Goel A, Ncho CM, Jeong CM, Choi YH. Embryonic thermal manipulation and in ovo gamma-aminobutyric acid supplementation regulating the chick weight and stress-related genes at hatch. Front Vet Sci. 2022;8:807450. doi: 10.3389/fvets.2021.807450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Elliott KEC, Durojaye OA, Fatemi SA, WSchilling M, Peebles ED. Effects of in ovo injection of L-ascorbic acid on growth performance, carcass composition, plasma antioxidant capacity, and meat quality in broiler chickens. Poult Sci. 2019;98:3617–25. doi: 10.3382/ps/pez173. [DOI] [PubMed] [Google Scholar]
- 18.Slawinska A, Dunislawska A, Plowiec A, Gonçalves J, Siwek M. TLR-mediated cytokine gene expression in chicken peripheral blood mononuclear cells as a measure to characterize immunobiotics. Genes. 2021;12:195. doi: 10.3390/genes12020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soleimani AF, Zulkifli I, Omar AR, Raha AR. Physiological responses of 3 chicken breeds to acute heat stress. Poult Sci. 2011;90:1435–40. doi: 10.3382/ps.2011-01381. [DOI] [PubMed] [Google Scholar]
- 20.Surai PF, Kochish II, Fisinin VI, Kidd MT. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkman HN, Gaetani GF. Catalase: a tetrameric enzyme with four tightly bound molecules of NADPH. Proc Natl Acad Sci. 1984;81:4343–7. doi: 10.1073/pnas.81.14.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surai P, Kochish I, Fisinin V. Antioxidant systems in poultry biology: nutritional modulation of vitagenes. Eur Poult Sci. 2017;81:214. doi: 10.1399/eps.2017.214. [DOI] [Google Scholar]
- 23.Al Wakeel RA, Shukry M, Abdel Azeez A, Mahmoud S, Saad MF. Alleviation by gamma amino butyric acid supplementation of chronic heat stress-induced degenerative changes in jejunum in commercial broiler chickens. Stress. 2017;20:562–72. doi: 10.1080/10253890.2017.1377177. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Tang J, Sun YQ, Xie J. Protective effect of γ-aminobutyric acid on antioxidation function in intestinal mucosa of Wenchang chicken induced by heat stress. J Anim Plant Sci. 2013;23:1634–41. [Google Scholar]
- 25.Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U. The glutamine-glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem Res. 2015;40:402–9. doi: 10.1007/s11064-014-1473-1. [DOI] [PubMed] [Google Scholar]
- 26.Al-Zghoul MB, Sukker H, Ababneh MM. Effect of thermal manipulation of broilers embryos on the response to heat-induced oxidative stress. Poult Sci. 2019;98:991–1001. doi: 10.3382/ps/pey379. [DOI] [PubMed] [Google Scholar]
- 27.Saleh KM, Tarkhan AH, Al-Zghoul MB. Embryonic thermal manipulation affects the antioxidant response to post-hatch thermal exposure in broiler chickens. Animals. 2020;10:126. doi: 10.3390/ani10010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaefarian F, Abdollahi MR, Cowieson A, Ravindran V. Avian liver: the forgotten organ. Animals. 2019;9:63. doi: 10.3390/ani9020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hicks JA, Trakooljul N, Liu HC. Discovery of chicken microRNAs associated with lipogenesis and cell proliferation. Physiol Genomics. 2010;41:185–93. doi: 10.1152/physiolgenomics.00156.2009. [DOI] [PubMed] [Google Scholar]
- 30.Wang G, Kim WK, Cline MA, Gilbert ER. Factors affecting adipose tissue development in chickens: a review. Poult Sci. 2017;96:3687–99. doi: 10.3382/ps/pex184. [DOI] [PubMed] [Google Scholar]
- 31.Ohta Y, Kidd MT. Optimum site for in ovo amino acid injection in broiler breeder eggs. Poult Sci. 2001;80:1425–9. doi: 10.1093/ps/80.10.1425. [DOI] [PubMed] [Google Scholar]
- 32.Narinç D, Erdoğan S, Tahtabiçen E, Aksoy T. Effects of thermal manipulations during embryogenesis of broiler chickens on developmental stability, hatchability and chick quality. Animal. 2016;10:1328–35. doi: 10.1017/S1751731116000276. [DOI] [PubMed] [Google Scholar]
- 33.Piestun Y, Halevy O, Yahav S. Thermal manipulations of broiler embryos-The effect on thermoregulation and development during embryogenesis. Poult Sci. 2009;88:2677–88. doi: 10.3382/ps.2009-00231. [DOI] [PubMed] [Google Scholar]
- 34.Kadam MM, Bhanja SK, Mandal AB, et al. Effect of in ovo threonine supplementation on early growth, immunological responses and digestive enzyme activities in broiler chickens. Br Poult Sci. 2008;49:736–41. doi: 10.1080/00071660802469333. [DOI] [PubMed] [Google Scholar]
- 35.Piestun Y, Halevy O, Shinder D, Ruzal M, Druyan S, Yahav S. Thermal manipulations during broiler embryogenesis improves post-hatch performance under hot conditions. J Therm Biol. 2011;36:469–74. doi: 10.1016/j.jtherbio.2011.08.003. [DOI] [Google Scholar]
- 36.Riras A, Fabricant J. Indication of immunodepression in chicken infected with various strain of Marck’s dsease virus. Avian Dis. 1988;32:1–8. [PubMed] [Google Scholar]
- 37.Tang J, Chen Z. The protective effect of gamma-aminobutyric acid on the development of immune function in chickens under heat stress. J Anim Physiol Anim Nutr (Berl) 2016;100:768–77. doi: 10.1111/jpn.12385. [DOI] [PubMed] [Google Scholar]
- 38.Jin Z, Mendu SK, Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids. 2013;45:87–94. doi: 10.1007/s00726-011-1193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]