Abstract
Background:
Physical inactivity is common in young adult cancer survivors (YACS), but evidence regarding effects of physical activity (PA) interventions among YACS is limited. The IMPACT trial evaluated a theory-based mobile PA intervention on total PA min/wk (primary) and secondary outcomes (moderate-to-vigorous PA (MVPA), light PA, steps, sedentary behaviors) at 6 months in YACS.
Methods:
YACS (N=280) were randomized to an intervention group or self-help group. All participants received digital tools (activity tracker, smart scale, access to arm-specific Facebook group) and an individual videochat session. Intervention participants also received a 6-month program with behavioral lessons, adaptive goal-setting, tailored feedback, tailored text messages, and Facebook prompts. PA was assessed via accelerometry and questionnaires at baseline and 6 months. Generalized estimating equation analyses tested between-group differences in changes over time.
Results:
Of 280 YACS, 251 (90%) completed 6-month accelerometry measures. Accelerometer-measured total PA min/wk changed from 1974.26 at baseline to 2024.34 at 6 months in the intervention (mean change, 55.14 (95% CI, −40.91, 151.19)) and from 1814.93 to 1877.68 in the self-help group (40.94 (95% CI: −62.14, 144.02); between-group P=.84). Increases in MVPA were +24.67 min/wk (95% CI: 14.77, 34.57) in the intervention versus +11.41 min/wk in the self-help (95% CI: 1.44, 21.38; between-group P=.07).
Conclusion:
Although the intervention did not result in significant differences in total PA, the increase in MVPA relative to the self-help group might be associated with important health benefits. Future research should examine moderators to identify for whom, and under what conditions, the intervention might be effective.
Keywords: young adults, cancer survivors, physical activity, digital health, social media, intervention
Lay summary:
Physical inactivity is common in young adult cancer survivors. However, few interventions have focused on helping young adult cancer survivors to get more physical activity. The Improving Physical Activity after Cancer Treatment (IMPACT) Trial compared an mHealth physical activity intervention with a self-help group on total amount of physical activity at 6 months in a nationwide sample of young adult cancer survivors. Intervention participants did not improve their total amount of physical activity, but they did increase their moderate-to-vigorous intensity physical activity by twice as much as the self-help participants. This increase in activity may be associated with health benefits.
Precis:
Young adult cancer survivors participating in an mHealth intervention did not significantly increase accelerometer-measured total physical activity after 6 months and did not differ from self-help participants who only received digital tools (activity tracker, smart scale, access to a Facebook group). However, the intervention doubled the increase in accelerometer-measured moderate-to-vigorous physical activity relative to the self-help group, which may be associated with important health benefits.
INTRODUCTION
Current guidelines recommend that cancer survivors avoid inactivity and engage in both aerobic and resistance-based physical activity (PA) to improve cancer-related health outcomes, including health-related quality of life, physical functioning, fatigue, anxiety, and depressive symptoms.1 Young adult cancer survivors (YACS), diagnosed between ages 18-39, are a subpopulation of survivors that face unique challenges and are at risk for adverse behavioral, physical, and psychosocial outcomes after treatment.2,3 The need for survivorship research focused on YACS was recognized by the US Congress in 2018,4 and the National Institutes of Health has prioritized development of targeted interventions to mitigate adverse outcomes and increase their access to care.5
Most YACS report unmet information and service needs related to PA;6,7 yet, few interventions have been designed specifically for this population.8,9 In particular, digital interventions have the potential to promote healthy lifestyle changes in YACS,10,11 by overcoming reported barriers of time,12 finances,7,13 and distance to treatments,14. while facilitating preferences for individualization,7,14 flexibility,12,14 and provision of peer support.7,14 To date, digital PA interventions for cancer survivors have been limited by small sample sizes,9–11,15–17 short duration,9,15,17,18 self-reported outcomes,17,18 and mixed evidence on their effects on total activity.16
We previously showed among 86 YACS that a Facebook-based intervention was feasible and produced greater increases in total and light PA relative to a self-help group at 12 weeks.19 Building on this potential, we enhanced the intervention with self-regulation strategies, behavior change techniques, and use of commercially-available digital tools.20 It is unknown whether using Facebook and providing digital tools alone, or enhanced with adaptive goal-setting, tailored feedback, and text messages, would increase PA among YACS over 6 months. The Improving Physical Activity after Cancer Treatment (IMPACT) randomized controlled trial was designed to address this gap and limitations of previous intervention research among YACS, including small samples, short study periods, lack of social media, and limited intervention effects on PA.19,21–25 The primary objective of this study was to test the effects of a 6-month theory-based, mobile PA intervention, compared to a self-help group that received digital tools, on total PA among YACS. We hypothesized that the intervention group would have greater increases in the primary outcome of total PA min/wk at 6 months relative to the self-help group. Additionally, we hypothesized that the intervention group would demonstrate greater improvements in secondary outcomes of light and moderate-to-vigorous intensity PA (MVPA) min/wk, steps per day, and sedentary behaviors relative to the self-help group.
METHODS
Study Design
The IMPACT study protocol and methods have been published elsewhere.20 Briefly, this two-arm, single-blinded, parallel groups, randomized controlled trial compared a theory-based, mobile intervention to a self-help group with primary outcome of total PA min/wk objectively-assessed at 6 months. Study procedures were reviewed and approved by the Lineberger Comprehensive Cancer Center Protocol Review Committee and Institutional Review Board of the University of North Carolina at Chapel Hill (Study #16-3409).
Study Participants
Participants were recruited from around the United States between August 2018 to October 2019 through social media, direct mail to individuals identified through a local tumor registry, and clinical- and community-based approaches, using methods reported previously.26 Interested individuals were screened online and by telephone. Eligible individuals were ages 18-39, diagnosed with cancer between ages 18-39 in the previous 10 years, had completed active treatment, and were engaging in <150 min/wk of moderate-to-vigorous intensity PA (MVPA). Additional criteria considered safety (e.g., ability to walk unassisted) and program participation (e.g., English speaking, Internet and mobile access, text messaging plan).20
Randomization
Following informed consent and baseline assessments, participants were randomly assigned with equal probability to the self-help or intervention group using a computerized random numbers list created in REDCap (by a project manager with no participant contact).27
Study Interventions
Intervention details have been described elsewhere.20 Briefly, both self-help (Digital Tools) and IMPACT intervention (Digital Tools Plus) groups received an activity tracker with companion mobile app (Fitbit Alta or Inspire, San Francisco, CA), cellular-enabled scale (BodyTrace, New York, NY), a kickoff individual videochat session to discuss PA recommendations for cancer survivors, and access to an arm-specific secret Facebook group (i.e., 1 for intervention, 1 for self-help group). Within the arm-specific Facebook groups, interventionists announced the addition of new cohorts; participant interaction was not specifically encouraged in the self-help Facebook group, whereas interventionists posted up to 5 discussion prompts per week in the Facebook group for the intervention arm.
Intervention design and components were guided by social cognitive theory,28 a self-regulation framework employed in an effective weight gain prevention intervention for young adults,29 and behavior change techniques used in previous PA interventions.30,31 Facebook discussion prompts were intended to promote peer interaction and target behavior change techniques; posts included educational content, motivational messages, questions about PA, stories about role models overcoming PA barriers, and monthly PA challenges. The intervention also provided a mobile responsive website with weekly lessons (i.e., psychoeducation and behavioral skills training), weekly active minutes (i.e., Fitbit equivalent of MVPA minutes) and daily step goals that adapted to individuals’ activity in the prior week, weekly tailored automated feedback, and 5 text messages per week. Intervention participants were instructed to gradually increase their total PA over time and could accept or change their recommend goals.
Data Collection
Assessments occurred at baseline, 3, and 6 months. At each assessment, participants received an email with a unique REDCap survey link with online questionnaires. At baseline and 6 months, participants were mailed an accelerometer, wrist bands, instruction sheet, and pre-paid return envelope. Participants received honoraria of $25, $10, and $50 for completing baseline, 3-, and 6-month assessments, respectively.
Measures
PA was measured using the ActiGraph GT3X+, which participants wore on their wrist (non-dominant) for 7 days. Data were inspected for good wear (≥4 days with at least one weekend day).32 At baseline, participants with inadequate wear were mailed another device to complete measurement. Minute-level data were used to process, clean, and estimate outcomes. Waking wear was identified using a combination of standard wear/non-wear, sleep/wake detection algorithms, wear logs, and visual inspection of data.32,33 Each minute of waking wear was classified using cut-offs applied to the vector magnitude: sedentary (<1800), light (1800–7499), and moderate or vigorous (≥7500).34,35 Next, ≥10 minute bouts of total PA (light, moderate, or vigorous intensity), light, and MVPA were identified using a standard bout counting algorithm.33,36 All days with ≥10 hrs of waking wear were used to compute participant-level outcomes. Finally, bout min/wk were calculated as (5*weekday average) + (2*weekend day average). Steps and sedentary min/d were summarized over all waking minutes. MVPA bout min/wk was used to create an indicator of meeting PA guidelines (≥150 MVPA min/wk).
Self-reported PA and sedentary behaviors were collected as secondary outcomes at baseline and 6 months. Although subjective, self-report measures were pre-specified in our protocol to permit comparisons to earlier studies of digital interventions for cancer survivors, the majority of which used self-reported PA data as primary outcomes.17 A modified version of the Godin Leisure Time Exercise Questionnaire assessed frequency of light, moderate, and vigorous exercise, and average duration during a typical week (min/wk).37,38 The Sedentary Behavior Questionnaire assessed the number of hours spent engaging in 8 different sedentary activities on a typical weekday and weekend day (min/d).39
Baseline measures included sociodemographics, height, health and cancer history. Process measures included days of Fitbit activity monitoring (defined as ≥200 steps/d), Facebook group interactions, and website use. Medical events were collected at 3 and 6 months by self-report and participant-initiated contacts.
Sample Size
The sample size of 280 was estimated to provide at least 80% power to detect a mean between-group difference in total PA of 161 min/wk (α=0.05, two-tailed test) while accommodating 25% attrition at 6 months.20 This estimated effect size was based on increases in total PA in our previous study (d=0.39),19 in which a clinically meaningful between-group change in light PA of 135 min/wk was observed, given that 60-minute increases in light PA have been associated with a 16% decrease in mortality risk.40
Statistical Analyses
Descriptive statistics were used to summarize participant characteristics. Fisher’s exact and Wilcoxon rank sum tests were used to evaluate associations between dropout and baseline categorical and continuous participant characteristics, respectively. Pearson correlations were used to describe the correlation between the accelerometer-measured and self-reported PA. Linear model repeated measures analyses, estimated using generalized estimating equations (GEE), were used to assess between-group differences in activity changes over time, as well as changes in primary and secondary outcomes from baseline to 6 months within groups. All models included covariates, time effect, treatment effect, and the interaction between time and treatment. Given documented associations with PA behaviors41 and as pre-specified in the study protocol, adjusted models included education, time since diagnosis, and age as covariates, and wear time (for accelerometer-measured outcomes).20 Unless otherwise specified, all reported linear model repeated measures results are from adjusted models. Sensitivity analyses were conducted to examine effects of removing potential outliers and only including completers (i.e., participants with complete PA data at baseline and 6 months). Log binomial models were used to estimate intervention effects on the probability of meeting PA guidelines (≥150 MVPA min/wk) at 6 months. In post-hoc analyses, the moderating impact of baseline PA was estimated by fitting separate GEE models for low (0 min/wk), middle (1-30 min/wk), and high (≥31 min/wk) tertile groups of baseline accelerometer-measured MVPA min/wk. Analyses were performed using SAS (Version 9.4, Cary, NC) on the intent-to-treat sample, including data from all randomized participants who received their group assignment (N=280).
RESULTS
We randomly assigned 280 YACS to the self-help or intervention groups (Figure 1 displays participant flow). Table 1 shows baseline participant characteristics; 18% were men, and 23% identified as racial/ethnic minority individuals. On average, participants were 33.4 (SD=4.8) years old and 3.66 (SD=2.41) years from diagnosis. Most were working full-time (63%), and the most common diagnoses were breast, gynecologic, and lymphomas. Retention was 90% at 6 months, with no differences by baseline participant characteristics (Ps>.05) or group (P=.24). There were no study-related adverse events and no between-group differences in number of events.
Figure 1.
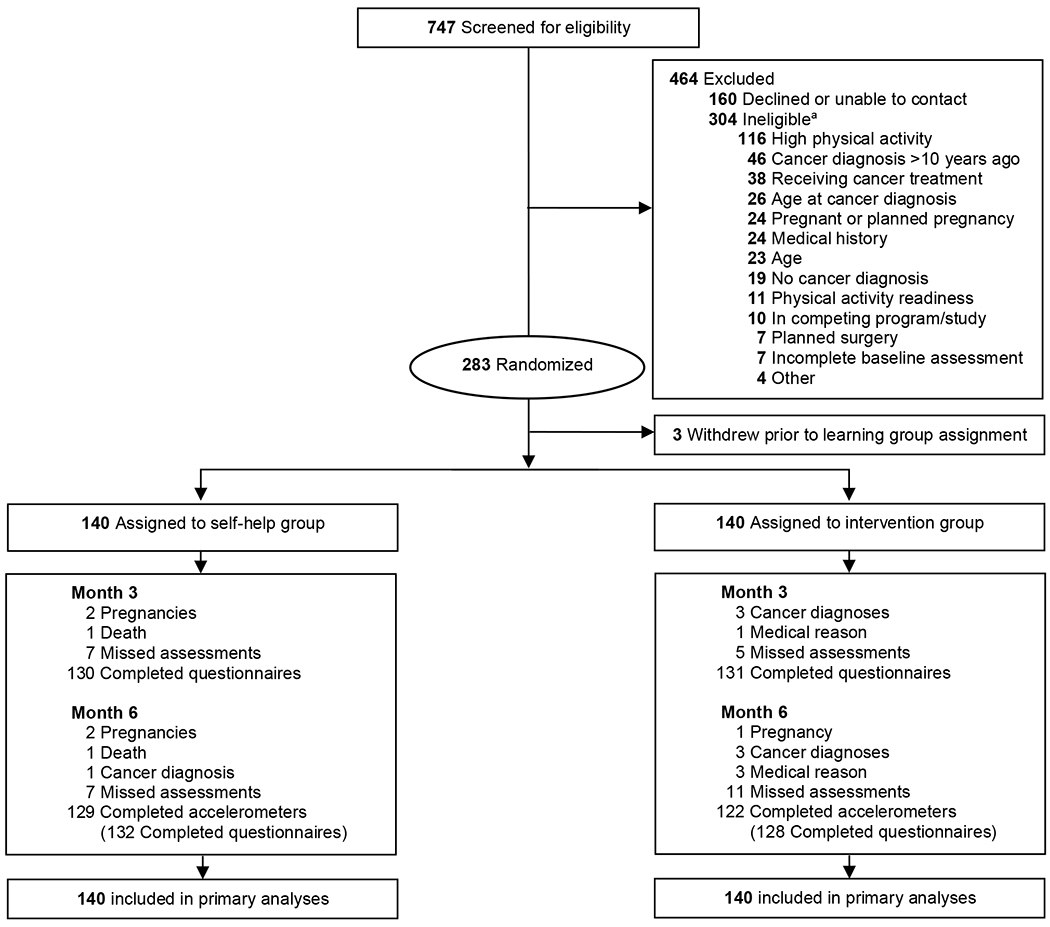
Consolidated Standards of Reporting Trials diagram of participant flow. aThere may be ≥1 reason for ineligibility.
Table 1.
Baseline Characteristics of 280 IMPACT Trial Participantsa
| Variable | Total (n=280) | Self-help (n=140) | Intervention (n=140) |
|---|---|---|---|
| Age, years, Mean (SD) | 33.40 (4.80) | 32.83 (5.13) | 33.98 (4.38) |
| Gender, n (%) | |||
| Male | 51 (18.2) | 24 (17.1) | 27 (19.3) |
| Female | 229 (81.8) | 116 (82.9) | 113 (80.7) |
| Race, n (%) | |||
| American Indian or Alaska Native | 1 (0.4) | 0 (0) | 1 (0.7) |
| Asian | 5 (1.8) | 1 (0.8) | 4 (2.9) |
| Black or African American | 32 (11.4) | 14 (10) | 18 (12.9) |
| White (non-Hispanic) | 215 (77.0) | 112 (80.0) | 103 (74.1) |
| White (Hispanic) | 6 (2.1) | 1 (0.8) | 5 (3.6) |
| Multiple races | 10 (3.6) | 7 (5.0) | 3 (2.2) |
| Otherb | 10 (3.6) | 5 (3.6) | 5 (3.6) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 23 (8.2) | 11 (7.9) | 12 (8.6) |
| Non-Hispanic | 255 (91.1) | 129 (92.1) | 126 (90.0) |
| Marital Status, n (%) | |||
| Single | 93 (33.2) | 48 (34.3) | 45 (32.1) |
| Living with partneror married | 173 (61.8) | 85 (60.7) | 88 (62.9) |
| Separated, divorced, or widowed | 14 (5.0) | 7 (5.0) | 7 (5.0) |
| Education level, n (%) | |||
| ≤ High school graduate or Technical School | 21 (7.5) | 10 (7.1) | 11 (7.9) |
| Some college | 59 (21.1) | 31 (22.1) | 28 (20.0) |
| ≥College degree | 200 (71.4) | 99 (70.7) | 101 (72.1) |
| Household Income, n (%) | |||
| <$30,000 | 45 (16.1) | 25 (17.9) | 20 (14.3) |
| $30,000 to <$60,000 | 70 (25.0) | 40 (28.6) | 30 (21.4) |
| ≥$60,000 | 147 (52.5) | 70 (52.5) | 77 (55.0) |
| Prefer not to answer | 18 (6.4) | 5 (3.6) | 13 (9.3) |
| Insurance Status, n (%) | |||
| Yes | 260 (92.9) | 127 (90.7) | 133 (95.0) |
| No | 20 (7.1) | 13 (9.3) | 7 (5.0) |
| Smoking status, n (%) | |||
| Never smoker | 223 (79.6) | 115 (82.1) | 108 (77.1) |
| Former smoker | 49 (17.5) | 20 (14.3) | 29 (20.7) |
| Current smoker | 8 (2.9) | 5 (3.6) | 3 (2.1) |
| Time Since Diagnosis, years, Mean (SD) | 3.66 (2.41) | 3.41 (2.28) | 3.91 (2.52) |
| Cancer Stage | |||
| Stage I | 60 (21.4) | 27 (19.6) | 33 (24.4) |
| Stage II | 77 (27.5) | 39 (28.3) | 38 (27.9) |
| Stage III | 48 (17.1) | 24 (17.4) | 24 (17.6) |
| Stage IV | 27 (9.6) | 15 (10.9) | 12 (8.8) |
| Other | 12 (4.3) | 5 (3.6) | 7 (5.1) |
| I don’t know | 28 (10.2) | 17 (12.3) | 11 (8.1) |
| Not staged | 22 (7.9) | 11 (8.0) | 11 (8.1) |
| Cancer Type, n (%) | |||
| Breast | 63 (22.5) | 31 (22.1) | 32 (22.9) |
| Colorectal | 10 (3.6) | 4 (2.9) | 6 (4.3) |
| Gynecologic (cervical, ovarian, endometrial) | 40 (13.9) | 17 (11.4) | 23 (16.4) |
| Leukemia / blood cancer | 15 (5.4) | 6 (4.3) | 9 (6.4) |
| Lymphoma (Hodgkin, Non-Hodgkin) | 49 (17.5) | 27 (19.3) | 22 (15.7) |
| Melanoma | 27 (9.6) | 12 (8.6) | 15 (10.7) |
| Testicular | 16 (5.7) | 9 (6.4) | 7 (5.0) |
| Thyroid | 30 (10.7) | 17 (12.1) | 13 (9.3) |
| Otherc | 30 (10.7) | 18 (12.9) | 12 (8.6) |
| Cancer Treatment | |||
| Chemotherapy | 172 (61.4) | 85 (60.7) | 87 (62.1) |
| Surgery | 242 (86.4) | 120 (85.7) | 122 (87.1) |
| Radiation | 128 (45.7) | 69 (49.3) | 59 (42.1) |
| Bone marrow transplant | 23 (8.2) | 11 (7.9) | 12 (8.6) |
| Hormone therapy | 14 (5.0) | 8 (5.7) | 6 (4.3) |
| Immunotherapy | 12 (4.3) | 9 (6.4) | 3 (2.1) |
| Other (radioactive iodine, vaccine, medication) | 13 (4.6) | 7 (5.0) | 6 (4.3) |
| Body Mass Index (kg/m2), Mean (SD) | 30.14 (8.33) | 30.52 (8.72) | 29.76 (7.94) |
Abbreviatons: SD, standard deviation.
Numbers may not sum to total due to missing data, percentages may not sum to 100% due to rounding.
Other self-reported race included: Asian Indian, Hispanic/Latino/Cape Verdean
Other cancers included: gastrointestinal stromal tumors, bone, brain, liver, multiple myeloma, oral, pancreatic, sarcomas
All participants completed a kickoff session. Fitbit activity monitoring was higher among intervention participants, who tracked on a median of 174.5 (of 182) total days [Mdn (IQR): 96% (83%, 99%)] compared to 163.5 days [90% (50%, 98%)] among self-help participants (between-group P=.0006). Intervention participants engaged within the Facebook group (i.e., viewed, reacted to (e.g., like, love), or posted ≥1 comment) during a median of 11 (of 26) total weeks [Mdn (IQR): 42% (23%, 62%)], whereas self-help participants engaged during 6 weeks [23% (15%, 38%)] (between-group P<.0001). Among intervention participants, median total weeks with ≥1 login onto the intervention website was 23 (of 26) weeks [Mdn (IQR): 88.5% (63.5%, 100%)]. On average, participants had 7.0 days of accelerometer wear, with waking wear averaging 15.4 h/d (intervention) and 15.5 h/d (self-help) at baseline, and 15.5 h/d (intervention, self-help) at 6 months, with no differences by group or time point.
Mean accelerometer-measured total PA min/wk increased by 55.1 min/wk (95% CI: −40.9, 151.2) in the intervention group compared to an increase of 40.9 min/wk (95% CI: −62.1, 144.0) in the self-help group (Table 2), with no between-group difference (14.2 min/wk; P=.84). MVPA min/wk increased by 24.7 min/wk (95% CI, 14.77, 34.57) in the intervention group and 11.4 min/wk (95% CI: 1.44, 21.38) in the self-help group; the between-group difference was not statistically significant (13.3 min/wk; 95% CI: −0.9, 27.4; P=.07). The intervention group increased steps/d by 670.7 (95% CI: 373.1, 968.3) compared to 299.9 (95% CI: −40.1, 640.0) in the self-help group (between-group P=.11), which represents ~2500 additional steps/wk in the intervention group. There were no statistically significant between-group changes in accelerometer-measured light PA or sedentary behavior (Ps=.96 to .99).
Table 2.
Changes in Physical Activity and Sedentary Behavior Within and Between Intervention and Self-help Groups in the IMPACT Trial
| Outcome variable and group | Mean (SD)a | Within-Group Changeb | Between-Group Difference in Changeb | |||
|---|---|---|---|---|---|---|
| Baseline | 6 Months | Value (95% CI) | P | Value (95% CI) | P | |
| Accelerometer measured outcomes | ||||||
| Total PA (min/wk) | 14.20 (−126.69, 155.09) | .84 | ||||
| Intervention | 1974.26 (673.86) | 2024.34 (686.74) | 55.14 (−40.91, 151.19) | .26 | ||
| Self-help | 1814.93 (704.51) | 1877.68 (758.19) | 40.94 (−62.14, 144.02) | .44 | ||
| Moderate-to-Vigorous PA (min/wk) | 13.26 (−0.86, 27.39) | .07 | ||||
| Intervention | 24.75 (31.21) | 49.43 (59.89) | 24.67 (14.77, 34.57) | <.01 | ||
| Self-help | 27.42 (34.43) | 39.46 (57.14) | 11.41 (1.44, 21.38) | .03 | ||
| Light PA (min/wk) | −.0067 (−139.58, 139.57) | .99 | ||||
| Intervention | 1949.51 (664.55) | 1974.91 (676.69) | 30.14 (−64.31, 124.59) | .54 | ||
| Self-help | 1787.51 (695.55) | 1838.21 (750.37) | 30.15 (−72.66, 132.95) | .57 | ||
| Steps per day | 370.77 (−80.66, 822.19) | .11 | ||||
| Intervention | 8917.18 (2206.00) | 9629.07 (2141.19) | 670.69 (373.09, 968.29) | <.01 | ||
| Self-help | 8394.47 (2116.27) | 8779.33 (2343.83) | 299.92 (−40.10, 639.95) | .08 | ||
| Sedentary Behavior (min/d) | −0.44 (−17.80, 16.92) | .96 | ||||
| Intervention | 546.20 (89.83) | 538.04 (104.13) | −7.57 (−19.00, 3.85) | .19 | ||
| Self-help | 557.43 (89.53) | 550.99 (99.27) | −7.13 (−20.23, 5.95) | .29 | ||
| Self-report measured outcomes | ||||||
| Total PA (min/wk) | 39.88 (−4.63, 84.40) | .08 | ||||
| Intervention | 118.92 (111.29) | 242.45 (181.62) | 123.28 (94.45, 152.12) | <.01 | ||
| Self-help | 130.10 (187.39) | 214.63 (180.65) | 83.40 (49.30, 117.50) | <.01 | ||
| Moderate-to-Vigorous PA (min/wk) | 5.36 (−20.86, 31.58) | .69 | ||||
| Intervention | 56.64 (66.88) | 134.28 (113.18) | 77.02 (57.05, 96.98) | <.01 | ||
| Self-help | 51.65 (75.44) | 123.77 (111.20) | 71.66 (54.64, 88.67) | <.01 | ||
| Light PA (min/wk) | 33.76 (−2.06, 69.58) | .06 | ||||
| Intervention | 62.29 (73.49) | 108.17 (126.46) | 46.01 (24.39, 67.64) | <.01 | ||
| Self-help | 78.45 (153.94) | 90.86 (107.66) | 12.25 (−16.52, 41.03) | .40 | ||
| Sedentary Behavior (min/d) | −20.16 (−76.13, 35.80) | .48 | ||||
| Intervention | 600.81 (257.09) | 590.02 (222.17) | −20.24 (−60.69, 20.22) | .33 | ||
| Self-help | 572.55 (236.53) | 576.40 (218.77) | −0.07 (−38.70, 38.55) | .99 | ||
| Weekday Sedentary Behavior (min/d) | −35.14 (−96.37, 26.10) | .26 | ||||
| Intervention | 638.11 (277.21) | 628.36 (234.09) | −20.66 (−65.18, 23.85) | .36 | ||
| Self-help | 601.99 (255.51) | 620.37 (244.27) | 14.48 (−27.52, 56.47) | .50 | ||
| Weekend Sedentary Behavior (min/d) | 17.75 (−42.81, 78.32) | .57 | ||||
| Intervention | 507.54 (253.80) | 494.18 (252.20) | −18.20 (−59.34, 22.95) | .39 | ||
| Self-help | 498.95 (249.95) | 466.45 (214.53) | −35.95 (−80.40, 8.50) | .11 | ||
Abbreviations: SD, standard deviation; CI, confidence interval.
Baseline sample n=140; 6-month sample: Accelerometer n=122 (intervention), n=129 (self-help); Self-Report n=128 (intervention), n=132 (self-help).
Adjusted for education, time since diagnosis, age, and wear time (accelerometer only).
Self-reported total PA and MVPA increased in both groups (between-group Ps=.08-69). Self-reported and accelerometer-measured total PA min/wk were not correlated (baseline: ρ=−0.016, P=.79; 6 months: ρ=0.056, P=.38), while self-reported and accelerometer-measured MVPA were (baseline: ρ=0.135, P=.02; 6 months: ρ=0.293, P<.0001). Intervention participants increased self-reported light PA (46.0 min/wk) versus no change in self-help participants (between-group P=.06). There were no within- or between-group changes in self-reported sedentary behaviors (Ps=.11 to .99).
The intervention group had slightly higher odds of meeting PA guidelines compared to the self-help group, with no between-group differences. Using accelerometry, at 6 months, 6.6% of intervention participants and 4.6% of self-help participants achieved ≥150 MVPA min/wk, up from 0% at baseline (RR (95% CI): 1.50 (0.5, 4.2); P=.43). Self-reported MVPA indicated that 40.6% of intervention and 32.6% of self-help participants met guidelines (RR (95% CI): 1.23 (0.89, 1.70); P=.20) at 6 months.
Removing statistical outliers (≥3 SDs away from the mean outcome change over time) did not impact conclusions for total PA or self-reported MVPA outcomes. After removing outliers from accelerometer-measured MVPA analyses (n=2 intervention, n=4 self-help), change in the self-help group was no longer evident (4.3 min/wk; 95%CI: −3.0, 11.6)), and the between-group difference was statistically significant (16.3 min/wk; 95% CI: 5.3, 27.3; P=.0037). Models that examined outcomes for study completers had similar results to the intent-to-treat analyses for all total PA and self-reported MVPA outcomes. For accelerometer-measured MVPA, change over time was significantly different between groups (14.8 min/wk; 95% CI: 0.7, 29.1; P=.04) among completers.
Figure 2 depicts changes in MVPA min/wk over time by group and baseline MVPA tertiles (Supplementary Material). Participants who started with no MVPA responded similarly to the two conditions, increasing by about 30 min/wk (between-group P=.80). Among participants in the middle tertile, the intervention group increased by 27.9 min/wk (95% CI: 6.6, 49.1) versus 6.5 min/wk (95% CI: −2.6, 15.7) in the self-help group (between-group difference, 21.3 min/wk; 95% CI: −1.6, 44.3; P=.07). For those in the highest tertile, on average intervention participants improved by 13.8 min/wk (95% CI: −4.4, 32.1), while self-help participants decreased by −6 min/wk (95% CI: −27.1, 14.4).
Figure 2.
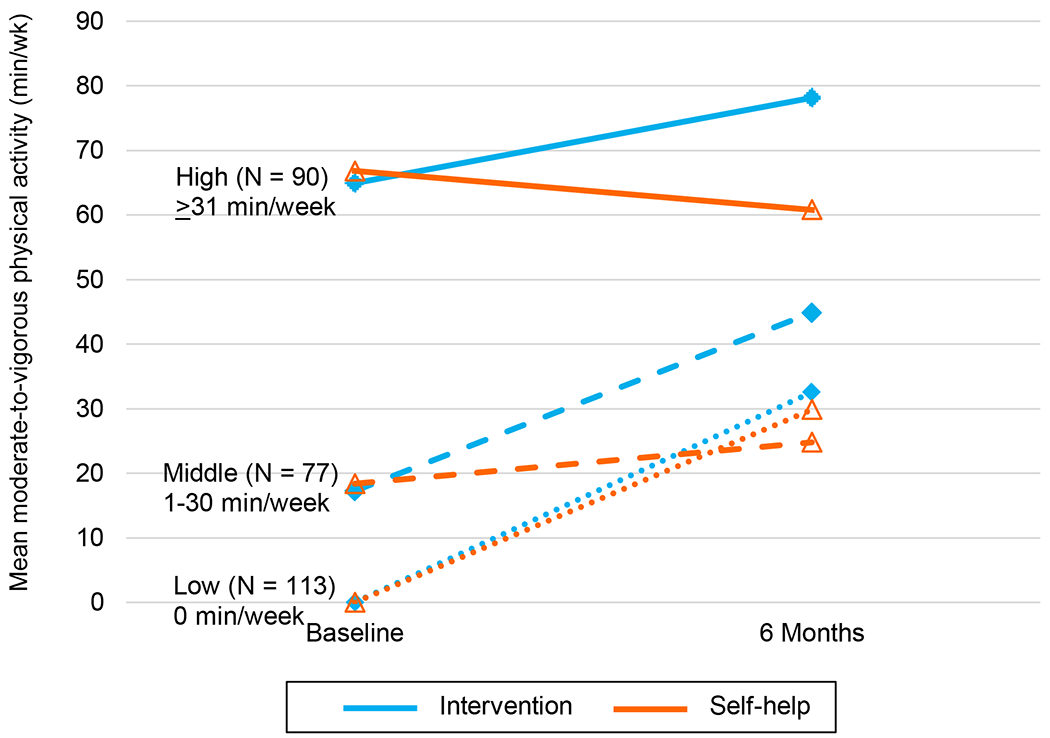
Changes in Accelerometer-Measured MVPA by Group Stratified by Baseline MVPA. Abbreviation: MVPA, moderate-to-vigorous physical activity. N in each MVPA tertile at baseline: low = 113 (40.4%); middle = 77 (27.5%); high = 90 (32.1%).
DISCUSSION
To our knowledge, this is the largest trial to report on an mHealth PA intervention with accelerometer-measured outcomes among YACS. The study enabled asynchronous nationwide study participation by an underrepresented subpopulation of survivors, using techniques with potential to broaden public health impact. Contrary to our hypotheses, the intervention did not increase accelerometer-measured total PA over 6 months compared to the self-help group which received digital tools only. Of note, both groups increased accelerometer-measured and self-reported MVPA over 6 months, underscoring the potential for digital tools and social media alone to offer benefit to some YACS. Among intervention participants, the mean estimated increases in accelerometer-measured and self-reported MVPA were approximately 25 and 77 min/wk respectively, which is double the amount of MVPA at baseline and considered a clinically meaningful change.42,43 Among those who were inactive at baseline, both groups improved MVPA, while among those with low MVPA at baseline, the intervention increased MVPA. These findings demonstrate the potential for mHealth interventions to increase MVPA among YACS and underscore the need to better tailor interventions to adapt to their dynamic lifestyle contexts and for additional research on how to promote achievement of PA guidelines within a scalable mHealth program.
Previous trials among YACS19,21–25 and trials that evaluated digital interventions or wearable devices among cancer survivors15,16,18 have been smaller, shorter, and used self-reported measures. The lack of an intervention effect on total PA is consistent with previous studies among cancer survivors; a recent review indicated that only two of six randomized controlled trials of mHealth interventions effectively increased total PA.16,43,44 The increases in accelerometer-measured MVPA min/wk in both groups may have limited our ability to observe significant group differences at 6 months. However, it is notable that group differences were significant in sensitivity analyses and among study completers. Intervention participants received additional components designed to capitalize on activity tracker functions, including weekly adaptive goals, tailored feedback, and text messages. These, along with higher activity monitoring and Facebook engagement, may have contributed to increases in MVPA and steps among intervention participants. The MVPA changes were similar to 6-month improvements in a recent mHealth PA intervention among breast cancer survivors of 25 min/wk.45 These could represent meaningful improvements, as guidelines for cancer survivors indicate that a ≥90 min/wk dose of MVPA improves cancer-related fatigue, health-related quality of life, physical function, anxiety, and depression.1 Mean self-reported MVPA in both groups met this threshold at 6 months (124-134 min/wk). These increases are higher than reported in a meta-analysis which found that digital interventions produced self-reported MVPA increases of ~40 min/wk among survivors.17
The MVPA increase observed in the self-help group is an important finding, suggesting that using digital tools alone or in combination with peer support without additional intervention might be of benefit for some YACS. The provision of digital tools to the self-help group, rather than the use of a no-treatment control, was consistent with previous recommendations for advancing eHealth research and facilitated evaluation of the added effects of theory-based intervention enhancements beyond those resulting from use of the digital tools alone.20,46 Future research should characterize engagement with intervention components and digital tools, and evaluate additional moderators of effects to identify for whom, and under what conditions, these interventions are effective. Previous mHealth interventions on MVPA in cancer survivors have typically lasted 12 weeks, and a recent review of such interventions noted that this duration may be sufficient and feasible to affect PA change.16 While we did not assess PA at 3 months, it is possible that there were group differences or greater PA increases at that timepoint. Additional research should examine Fitbit tracker data to evaluate week-to-week PA changes.
This trial extends previous work that demonstrated feasibility of PA interventions that have used Facebook among YACS.19,47 The findings on self-reported increases in light PA min/wk in the intervention group are consistent with our earlier work.20 Though the intervention encouraged light PA and reduction of sedentary behavior in tailored feedback, this feedback was provided only periodically, and participants did not receive specific goals for these outcomes. Given evidence that high levels of sedentary behavior are associated with poor health outcomes in cancer survivors,48 future research should focus on reducing sedentary behaviors concurrent with increasing PA, as few studies have done so.16,49,50
Study findings should be considered in the context of limitations. The study sample comprised predominantly females who were highly-educated, identified as white, and were likely motivated to make behavior changes, thus limiting generalizability of findings. Our recruitment methods and/or the English-only requirement may have limited the reach or relevance of the program to groups recruited in proportions that do not reflect national demographics (e.g., males, Hispanics). The multicomponent package limits ability to determine the effects of individual intervention components. Additionally, the trial did not assess PA outcomes at 3 months, which may have been a missed opportunity to detect short-term effects. Despite these limitations, this trial had several strengths, including the randomized design and use of accelerometry to screen potential participants and assess PA outcomes. High baseline PA was the most common reason for study ineligibility, which may reflect a strong interest in supportive programs regardless of PA levels. We efficiently recruited an at-risk sample with 40% engaging in no MVPA at baseline, reached an understudied population of survivors, and tested remotely-delivered interventions which enabled nationwide participation. The active comparison group received commercially-available tools, allowing us to examine the added effect of theory-based intervention components designed to enhance the effect of these tools. Furthermore, study retention was high at 6 months, and study duration was longer than most previous trials.
In conclusion, this study found that YACS participating in a remotely-delivered mHealth intervention increased self-reported total PA, but not accelerometer-measured total PA, and they did not differ from self-help participants receiving digital tools, who also reported increased total PA. However, the intervention doubled the increase in MVPA compared to the self-help group. In post-hoc analyses, both groups improved MVPA among those who were inactive at baseline, while the intervention was beneficial for those with low levels of MVPA at baseline. Future work will examine maintenance of intervention effects at 12 months. Further research should examine potential benefits derived from engagement with social media and other intervention components. Finally, future evaluations of hypothesized mediators and potential moderators of mHealth intervention effects to identify for whom, and under what conditions, effectiveness varied are warranted.
Supplementary Material
Acknowledgements:
We would like to thank the members of the UNC Weight Research Program for their valuable support, including Karen Hatley, Kristen Polzien, Lindsey Camp, Erin Coffman, Lex Hurley, Susanna Choi, Miriam Chisholm, Kayla Warechowsi, and Juhi Chinthapatla. We wish to acknowledge Dr. Donald Rosenstein, Dr. Eliza Park, Dr. Andrew Smitherman, Lauren Lux, and community-based organizations, that graciously assisted with study recruitment. We are most grateful to the young adult cancer survivors who participated in the study.
Funding:
This work was supported by the National Cancer Institute (R01CA204965 to CGV), the UNC Connected Health for Applications and Interventions Core (funded by P30DK056350 to UNC Nutrition Obesity Research Center or P30CA016086 to the UNC Lineberger Comprehensive Cancer Center), the University Cancer Research Fund, and the National Center for Advancing Translational Sciences (UL1TR002489). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
ClinicalTrials.gov Identifier: NCT03569605
Conflict of Interest Disclosures: None.
References
- 1.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Policy Forum, Board on Health Care Services, A Livestrong and Institute of Medicine Workshop, Institute of Medicine. Identifying and Addressing the Needs of Adolescents and Young Adults with Cancer: Workshop Summary. Washington (DC): National Academies Press (US); 2014. doi: 10.17226/18547 [DOI] [PubMed] [Google Scholar]
- 3.Ketterl TG, McCabe MS, Rosenstein DL, et al. Impact of cancer on physical and mental activities of daily living in young adult (YA) survivors. J Clin Oncol. 2019;37. [Google Scholar]
- 4.Text - S.292 - 115th Congress (2017-2018): Childhood Cancer Survivorship, Treatment, Access, and Research Act of 2018 | Congress.gov | Library of Congress. https://www.congress.gov/bill/115th-congress/senate-bill/292/text?q=%7B%22search%22%3A%5B%22S292%22%5D%7D&r=1. Accessed November 19, 2021. [Google Scholar]
- 5.RFA-CA-20-027: Research to Reduce Morbidity and Improve Care for Pediatric, and Adolescent and Young Adult (AYA) Cancer Survivors (R01 Clinical Trial Optional). https://grants.nih.gov/grants/guide/rfa-files/rfa-ca-20-027.html. Accessed November 19, 2021.
- 6.Zebrack BJ, Block R, Hayes-Lattin B, et al. Psychosocial service use and unmet need among recently diagnosed adolescent and young adult cancer patients. Cancer. 2013;119(1):201–214. doi: 10.1002/cncr.27713 [DOI] [PubMed] [Google Scholar]
- 7.Pugh G, Hough RE, Gravestock HL, Jackson SE, Fisher A. The health behavior information needs and preferences of teenage and young adult cancer survivors. J Adolesc Young Adult Oncol. 2017;6(2):318–326. doi: 10.1089/jayao.2016.0089 [DOI] [PubMed] [Google Scholar]
- 8.Bradford NK, Chan RJ. Health promotion and psychological interventions for adolescent and young adult cancer survivors: A systematic literature review. Cancer Treat Rev. 2017;55:57–70. doi: 10.1016/j.ctrv.2017.02.011 [DOI] [PubMed] [Google Scholar]
- 9.Kopp LM, Gastelum Z, Guerrero CH, Howe CL, Hingorani P, Hingle M. Lifestyle behavior interventions delivered using technology in childhood, adolescent, and young adult cancer survivors: A systematic review. Pediatr Blood Cancer. 2017;64(1):13–17. doi: 10.1002/pbc.26166 [DOI] [PubMed] [Google Scholar]
- 10.Devine KA, Viola AS, Coups EJ, Wu YP. Digital health interventions for adolescent and young adult cancer survivors. JCO Clin Cancer Inform. 2018;2:1–15. doi: 10.1200/CCI.17.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCann L, McMillan KA, Pugh G. Digital interventions to support adolescents and young adults with cancer: systematic review. JMIR Cancer. 2019;5(2):e12071. doi: 10.2196/12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabin C, Simpson N, Morrow K, Pinto B. Behavioral and Psychosocial Program Needs of Young Adult Cancer Survivors. Qualitative health research. 2011;21(6):796. [DOI] [PubMed] [Google Scholar]
- 13.Wu YP, Yi J, McClellan J, et al. Barriers and facilitators of healthy diet and exercise among adolescent and young adult cancer survivors: implications for behavioral interventions. J Adolesc Young Adult Oncol. 2015;4(4):184–191. doi: 10.1089/jayao.2015.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabin C, Simpson N, Morrow K, Pinto B. Intervention format and delivery preferences among young adult cancer survivors. Int J Behav Med. 2013;20(2):304–310. doi: 10.1007/s12529-012-9227-4 [DOI] [PubMed] [Google Scholar]
- 15.Coughlin SS, Caplan LS, Stone R. Use of consumer wearable devices to promote physical activity among breast, prostate, and colorectal cancer survivors: a review of health intervention studies. J Cancer Surviv. 2020;14(3):386–392. doi: 10.1007/s11764-020-00855-1 [DOI] [PubMed] [Google Scholar]
- 16.Khoo S, Mohbin N, Ansari P, Al-Kitani M, Müller AM. mHealth Interventions to Address Physical Activity and Sedentary Behavior in Cancer Survivors: A Systematic Review. Int J Environ Res Public Health. 2021;18(11). doi: 10.3390/ijerph18115798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts AL, Fisher A, Smith L, Heinrich M, Potts HWW. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2017;11(6):704–719. doi: 10.1007/s11764-017-0632-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberlin C, O’Dwyer T, Mockler D, Moran J, O’Donnell DM, Broderick J. The use of eHealth to promote physical activity in cancer survivors: a systematic review. Support Care Cancer. 2018;26(10):3323–3336. doi: 10.1007/s00520-018-4305-z [DOI] [PubMed] [Google Scholar]
- 19.Valle CG, Tate DF, Mayer DK, Allicock M, Cai J. A randomized trial of a Facebook-based physical activity intervention for young adult cancer survivors. J Cancer Surviv. 2013;7(3):355–368. doi: 10.1007/s11764-013-0279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valle CG, Pinto BM, LaRose JG, et al. Promoting physical activity in young adult cancer survivors using mHealth and adaptive tailored feedback strategies: Design of the Improving Physical Activity after Cancer Treatment (IMPACT) randomized controlled trial. Contemp Clin Trials. 2021;103:106293. doi: 10.1016/j.cct.2021.106293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabin C, Dunsiger S, Ness KK, Marcus BH. Internet-Based Physical Activity Intervention Targeting Young Adult Cancer Survivors. J Adolesc Young Adult Oncol. 2011;1(4):188–194. doi: 10.1089/jayao.2011.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabin C, Pinto B, Fava J. Randomized trial of a physical activity and meditation intervention for young adult cancer survivors. J Adolesc Young Adult Oncol. 2016;5(1):41–47. doi: 10.1089/jayao.2015.0033 [DOI] [PubMed] [Google Scholar]
- 23.Bélanger LJ, Mummery WK, Clark AM, Courneya KS. Effects of targeted print materials on physical activity and quality of life in young adult cancer survivors during and after treatment: an exploratory randomized controlled trial. J Adolesc Young Adult Oncol. 2014;3(2):83–91. doi: 10.1089/jayao.2013.0021 [DOI] [Google Scholar]
- 24.Keadle SK, Meuter L, Phelan S, Phillips SM. Charity-based incentives motivate young adult cancer survivors to increase physical activity: a pilot randomized clinical trial. J Behav Med. 2021;44(5):682–693. doi: 10.1007/s10865-021-00218-w [DOI] [PubMed] [Google Scholar]
- 25.Johnson AM, Baker KS, Haviland MJ, et al. A Pilot Randomized Controlled Trial of a Fitbit- and Facebook-Based Physical Activity Intervention for Young Adult Cancer Survivors. J Adolesc Young Adult Oncol. October 2021. doi: 10.1089/jayao.2021.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valle CG, Camp LN, Diamond M, et al. Recruitment of young adult cancer survivors into a randomized controlled trial of an mHealth physical activity intervention. Trials. 2022;23(1):254. doi: 10.1186/s13063-022-06148-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandura A. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1 [DOI] [PubMed] [Google Scholar]
- 29.Wing RR, Tate DF, Espeland MA, et al. Innovative Self-Regulation Strategies to Reduce Weight Gain in Young Adults: The Study of Novel Approaches to Weight Gain Prevention (SNAP) Randomized Clinical Trial. JAMA Intern Med. 2016;176(6):755–762. doi: 10.1001/jamainternmed.2016.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009;28(6):690–701. doi: 10.1037/a0016136 [DOI] [PubMed] [Google Scholar]
- 31.Finne E, Glausch M, Exner A-K, Sauzet O, Stölzel F, Seidel N. Behavior change techniques for increasing physical activity in cancer survivors: a systematic review and meta-analysis of randomized controlled trials. Cancer Manag Res. 2018;10:5125–5143. doi: 10.2147/CMAR.S170064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evenson KR, Terry JW. Assessment of differing definitions of accelerometer nonwear time. Res Q Exerc Sport. 2009;80(2):355–362. doi: 10.1080/02701367.2009.10599570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi L, Ward SC, Schnelle JF, Buchowski MS. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med Sci Sports Exerc. 2012;44(10):2009–2016. doi: 10.1249/MSS.0b013e318258cb36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koster A, Shiroma EJ, Caserotti P, et al. Comparison of Sedentary Estimates between activPAL and Hip- and Wrist-Worn ActiGraph. Med Sci Sports Exerc. 2016;48(8):1514–1522. doi: 10.1249/MSS.0000000000000924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamada M, Shiroma EJ, Harris TB, Lee I-M. Comparison of physical activity assessed using hip- and wrist-worn accelerometers. Gait Posture. 2016;44:23–28. doi: 10.1016/j.gaitpost.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 37.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 38.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77(5):359–362. [PubMed] [Google Scholar]
- 39.Rosenberg DE, Norman GJ, Wagner N, Patrick K, Calfas KJ, Sallis JF. Reliability and validity of the Sedentary Behavior Questionnaire (SBQ) for adults. J Phys Act Health. 2010;7(6):697–705. doi: 10.1123/jpah.7.6.697 [DOI] [PubMed] [Google Scholar]
- 40.Loprinzi PD. Light-Intensity Physical Activity and All-Cause Mortality. Am J Health Promot. 2017;31(4):340–342. doi: 10.4278/ajhp.150515-ARB-882 [DOI] [PubMed] [Google Scholar]
- 41.Rabin C. Review of health behaviors and their correlates among young adult cancer survivors. J Behav Med. 2011;34(1):41–52. doi: 10.1007/s10865-010-9285-5 [DOI] [PubMed] [Google Scholar]
- 42.Hair BY, Hayes S, Tse C-K, Bell MB, Olshan AF. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014;120(14):2174–2182. doi: 10.1002/cncr.28630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh B, Spence RR, Sandler CX, Tanner J, Hayes SC. Feasibility and effect of a physical activity counselling session with or without provision of an activity tracker on maintenance of physical activity in women with breast cancer - A randomised controlled trial. J Sci Med Sport. 2020;23(3):283–290. doi: 10.1016/j.jsams.2019.09.019 [DOI] [PubMed] [Google Scholar]
- 44.Hartman SJ, Nelson SH, Myers E, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: The memory & motion study. Cancer. 2018;124(1):192–202. doi: 10.1002/cncr.30987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips SM, Penedo FJ, Collins LM, et al. Optimization of a technology-supported physical activity promotion intervention for breast cancer survivors: Results from Fit2Thrive. Cancer. November 2021. doi: 10.1002/cncr.34012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glasgow RE. eHealth evaluation and dissemination research. Am J Prev Med. 2007;32(5 Suppl):S119–26. doi: 10.1016/j.amepre.2007.01.023 [DOI] [PubMed] [Google Scholar]
- 47.Mendoza JA, Baker KS, Moreno MA, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: A pilot study. Pediatr Blood Cancer. 2017;64(12). doi: 10.1002/pbc.26660 [DOI] [PubMed] [Google Scholar]
- 48.Swain CTV, Nguyen NH, Eagles T, et al. Postdiagnosis sedentary behavior and health outcomes in cancer survivors: A systematic review and meta-analysis. Cancer. 2020;126(4):861–869. doi: 10.1002/cncr.32578 [DOI] [PubMed] [Google Scholar]
- 49.Trinh L, Arbour-Nicitopoulos KP, Sabiston CM, et al. RiseTx: testing the feasibility of a web application for reducing sedentary behavior among prostate cancer survivors receiving androgen deprivation therapy. Int J Behav Nutr Phys Act. 2018;15(1):49. doi: 10.1186/s12966-018-0686-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch BM, Nguyen NH, Moore MM, et al. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer. 2019;125(16):2846–2855. doi: 10.1002/cncr.32143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


