Abstract
Background:
Chemoradiotherapy (CRT) for head and neck cancer (HNC) induces side-effects, including trismus, which impairs quality of life by causing difficulty to eat, speak, and maintain good oral hygiene, and by altering social life. Given the wide variation of reported trismus prevalence and as a first mandatory step for the preventive physiotherapy OPEN program (NCT03979924) this study evaluated trismus occurrence and its link with radiation doses.
Methods:
Study population was non-larynx HNC patients with epidermoid carcinoma treated with CRT, with or without surgery. A physiotherapist measured maximal interincisal distance before, during and after CRT, at 10 weeks and 6 months. The proportion of patients with trismus (with a 95% confidence interval) was estimated. Irradiation doses were analyzed between patients with and without trismus using non-parametric Kruskal-Wallis test.
Results:
We included 45 patients (77.8% male), median age 61 years (range 41-77). The proportion of trismus at baseline was 24.4%, 26.8% at 10 weeks and 37.1% at 6 months. During radiotherapy, it was 27.9% at week 3 and increased to 41.9% at week 6. Trismus occurrence at 10 weeks was higher when the radiation dose to the ipsilateral lateral pterygoid muscle was above the median value, that is, 36.8 grays.
Conclusion:
Trismus occurrence differed according to radiation dose and cancer location. These findings highlight the necessity of early preventive physiotherapy programs to reduce trismus occurrence. The second step, of the interventional multicenter OPEN program, is currently evaluating the impact of preventive physiotherapy and patient education on trismus in a sample of 175 patients.
Keywords: head and neck cancer, trismus, physiotherapy, radiotherapy, supportive care
Introduction
Head and neck cancers (HNC) include cancers of the oral cavity, pharynx, larynx, paranasal sinuses, nasal cavity, and the salivary glands. In 2017, 15 000 new cases of HNC were diagnosed in France, of which 75% were in men.1,2 In the past years, the incidence of these cancers has increased, especially in women, due to an increase in smoking and alcohol consumption as well as the higher rate of human papillomavirus (HPV) infections favoring the development of HNCs.3 -5
Patients are treated mainly by chemo-radiotherapy (CRT) with or without surgery. Various severe secondary effects are reported during and after the treatment with burdensome effects on patients’ quality of life: dry mouth, swallowing difficulties, restricted tongue, lips and neck mobility, lymphedema, pain, tissue and scar tightness, fibrosis and restricted jaw mobility, in particular the mouth opening.6 -9 Trismus is defined as limited mouth opening that is, maximum interincisal opening (MIO) ≤35 mm.10,11 It is known to induce a major decrease in quality of life, including poor oral hygiene, limited access for dental care and medical surveillance, difficulties to communicate and eat often requiring gastrostomy to avoid weight loss.12,13 Moreover, the risk of social isolation and associated symptoms such as depression and anxiety, often underestimated, are also reported.12 Despite its heavy impact, few studies report quantitative and precise data on trismus prevalence and consequences. Observational studies remain heterogeneous and of variable quality. Thus, in treated patients (surgery and/or CRT) for HNC the prevalence rate of trismus varies between 6% and 79%.13 -17
The beneficial effects of physiotherapy programs on trismus prevalence have not been fully confirmed and there are no validated protocols or guidelines regarding exercise therapy.18 A randomized study on 374 patients dating back to 2011 found no positive effect on trismus prevalence.19 More recently, a randomized study evaluated a comprehensive rehabilitation program (eg, 45 minutes per week with a physiotherapist and 7 exercises 5 times/day at home) that did not seem to provide additional beneficial effects.20 These 2 studies concluded that rehabilitation programs, although burdensome for patients, should nonetheless be implemented in order to further explore their potential effect on reducing trismus occurrence. Some studies have evaluated whether passive tools could improve compliance and efficacy of rehabilitation.21 -23 In 2015, a systematic review showed a wide variability in the delay between radiotherapy and the beginning of rehabilitation programs.24 Indeed, the efficacy of exercises, with an influence on mouth opening, may be reduced in case of well-installed trismus, which is often related to fibrosis, scar and tissue tightness. Few studies report beneficial results in terms of trismus prevalence following early physiotherapy.22,25
Because trismus prevalence, its rehabilitation and the efficacy of preventive physiotherapy programs are poorly documented, particularly in France, we conducted a monocentric prospective study at the Institut du Cancer de Montpellier (ICM) in the South of France, to estimate the trismus occurrence in patients treated with CRT for HNC. In this paper, as a mandatory step before the interventional phase, we report as a primary objective trismus occurrence 10 weeks after the end of CRT in a consecutive series of non-larynx HNC patients. As secondary objectives, we intended to (i) evaluate change in mouth opening before, during and after treatment, (ii) assess the associated loco-regional toxicities and, (iii) evaluate potential correlations with radiation doses at different locations The next step is an on-going multicenter study which includes 175 patients and examines the impact of a preventive physiotherapy program combined with patient education.
Methods
Study Design and Objectives
This study was performed in the frame of a large interventional multicenter prospective program called OPEN “Mouth Opening, Prevention, Education, Nutrition” registered as NCT03979924 (https://clinicaltrials.gov). The aim was to offer early and preventive management of trismus to HNC patients and evaluate the efficacy of the intervention on trismus throughout the duration of treatment.
Patients
Study population consisted of cancer patients recruited according to the following inclusion criteria: age ≥18 years old, histologically proved epidermoid carcinoma of the oral cavity, oropharynx and cavum and scheduled treatment with ≥54 Gy radiotherapy with concomitant chemotherapy with or without prior surgery. Patients were not included if they presented a lack of the median or lateral incisors, any diseases or trauma affecting jaw mobility with permanent trismus or metastases, a legal inability to participate and medical or psychological conditions interfering with their consent. All patients were well informed and provided written consent prior to the study.
Based on previous data from the literature, the trismus prevalence was expected to be around 30% in HNC patients. In this observational study, we included 45 patients to ensure having at least 40 evaluable patients (10% considered non-evaluable). The values obtained were used as reference values for the sample size calculation in the second interventional step.
The study protocol was approved by the ethical committee (Comité de Protection des Personnes Sud Méditerrannée III, June 1st, 2016) and by an institutional review board and was conducted in accordance with Helsinki Declaration and the Good Clinical Practice requirements.
Treatments
Surgery and CRT
A proportion of 33% of patients underwent surgery. Irradiation was delivered with an IMRT method, and more precisely by volumetric-modulated arc therapy (VMAT) with a RapidArc planning and delivery techniques (Varian Medical Systems, Palo Alto, CA, USA). Radiotherapy was administered for 7 weeks totaling a minimum dose of 54 Gy. Patients received concomitant chemotherapy composed of 3 cycles of cisplatin (100 mg/m²/day at week 1, 4, and 7 of radiotherapy) or 8 injections of cetuximab (loading dose of 400 mg/m² 1 week before starting radiation and then 250 mg/m² weekly during radiation). For each patient, the muscles involved in jaw movements (medial and lateral pterygoid, masseter, and temporalis) were delineated on the contrast-enhanced computed tomography (CECT) scan and used to plan the radiotherapy by the radiotherapy physicist. The delivered dose to each of these muscles and to the temporo-mandibular joint was then calculated according to homo- or contralateral feature, defined as ipsilateral nearest structures to the tumor location.
Nutrition
According to national guidelines in case of radiotherapy ≥50 Gy in the oral cavity with concomitant chemotherapy and to avoid treatment interruption, a preventive gastrostomy was programed prior to CRT.26,27
Physiotherapy evaluation and follow-up
A detailed physiotherapy assessment was performed 4 times during treatment and the follow-up periods: within 30 days before CRT, at the end of CRT, at 10 weeks and 6 months after CRT (Supplemental Figure S1). The recorded variables included weight and artificial nutritional status, degree of physical activity, state of the skin, the scars, the oral cavity and mobility of lips, tongue, and jaw. The physiotherapist evaluated the mouth opening at every detailed physiotherapy assessment and once a week during the 7 weeks of CRT. In case of trismus, the physiotherapist provided a Physiotherapy Guidebook and, in accordance with patient education and patient’s choice, recommendations were to be followed at home.
Endpoints and Assessments
The primary endpoint was the mouth opening at 10 weeks after CRT defined as the distance (mm) between the upper left median incisor (#21) and the lower left median incisor (#31), measured by the physiotherapist using Therabite Range of Motion Scale® (ATOS Medical, Sweden, Malmö). In the absence of one of these teeth, measurements were performed using other median or lateral incisors, provided that the up to down axis remained vertical. Secondary endpoints included the evolution of mouth opening during CRT. The occurrence of loco-regional symptoms (edema, erythema, scar, and tissue adherence), were assessed during physiotherapy evaluation at baseline (within 30 days before radiotherapy), at the end of CRT and at 10 weeks and 6 months after the end of CRT (Supplemental Figure S1). Toxicities were reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) criteria v4.03. Medical history and demographic data were collected at the initial screening. A physical examination was conducted in a standardized follow-up program during radiotherapy with a weekly evaluation by a radiation oncologist and scoring for acute toxicity. Thus, we were able to assess the correlation between the radiation doses and trismus occurrence.
Statistical Methods
Statistical analyses were performed according to a plan. Quantitative variables were expressed as means with 95% CI, or medians with ranges. The trismus occurrence at 10 weeks after treatment was estimated and is presented as a proportion with its 95% confidence interval (95% CI, estimated using the exact Binomial method). Mouth opening during treatment was evaluated using a linear mixed model. In the subgroup of patients free of trismus at inclusion, the time to trismus occurrence was analyzed using the Kaplan-Meier method. Irradiation doses were compared between patients with and without trismus using non-parametric Kruskal-Wallis test. The predictive value of dosimetry on trismus occurrence was assessed using univariate logistic regression model providing P values, odd ratios (OR) and 95% CI for each radiation site. Statistical significance was set at P < .05. All analyses were performed with Stata software v13 (StataCorp LP, College Station, TX).
Results
Patient characteristics
Between October 20th 2016 and December 31st 2017, 45 patients were included in the study. Their clinical features, social status and habits are presented in Table 1. Median age was 61 years (range: 41-77) and a majority of participants (77.8%) were male. The primary tumor was localized in the oropharynx (73.3%) or the oral cavity (20.0%). Patients had mainly a WHO performance status of 0 (54.6%) and 1 (31.8%). One patient was excluded before the CRT treatment due to complete dental avulsion immediately after inclusion. In total, we were able to evaluate 41 patients for mouth opening at 10 weeks after completion of CRT because 2 patients missed the visit (logistic reasons) and 1 patient could not wear his dental appliance.
Table 1.
Clinical Features and Social Habits of Included Patients at Baseline.
| Feature | Patients (n = 45) |
|---|---|
| Age (years), median [range] | 61 [41-77] |
| Sex, n (%) | |
| Male | 35 (77.8) |
| Female | 10 (22.2) |
| Tumor localization, n (%) | |
| Oropharynx | 33 (73.3) |
| Oral cavity | 9 (20.0) |
| Cavum | 3 (6.7) |
| Previous surgery, n (%) | 15 (33.3) |
| WHO performance status, n (%) | |
| 0 | 24 (54.6) |
| 1 | 14 (31.8) |
| 2-3 | 6 (13.6) |
| Missing | 1 |
| Marital status, n (%) | |
| Single | 12 (26.7) |
| Married or equivalent | 31 (68.9) |
| Living with a family member | 2 (4.4) |
| Occupation, n (%) | |
| Working | 12 (26.7) |
| Not working: unemployed, without activity, disabled | 10 (22.2) |
| Not working: retired | 23 (51.1) |
| Academic degree, n (%) | |
| Secondary/high school | 29 (61.9) |
| Bachelor’s degree | 2 (4.6) |
| First certificate and higher | 13 (29.6) |
| Missing | 1 |
| Smoking status, n (%) | |
| Non-smoker | 7 (15.6) |
| Former smoker | 30 (66.7) |
| Smoker | 8 (17.8) |
| Alcohol consumption | |
| None | 15 (33.3) |
| Former drinker | 11 (24.4) |
| Current drinker | 19 (42.2) |
| Physical activity* | |
| None | 16 (35.6) |
| Occasionala | 11 (24.4) |
| Frequentb | 18 (40.0) |
1 to 3 times a week.
4 times/week and more.
Activity equivalent to a 30-min walk.
Treatments
A total of 44 patients received concomitant CRT and 33% of them had previously undergone surgery.
Target volumes were treated with 3 different dose levels (simultaneous integrated boost) according to the risk of relapse. High, medium and low-risk volumes received 60 to 70 Gy (2.00 Gy/fraction), 63 Gy (1.80 Gy/fraction) and 56 Gy (1.60 Gy/fraction), respectively, in 30 to 35 fractions delivered 5 days/week over a 6 to 7 week period. The median radiotherapy dose was 70 Gy (range 60-70) delivered in a median number of 35 fractions (range 30-35). As for chemotherapy, most patients received cisplatin (74.4%, n = 32) or cetuximab (18.6%, n = 8).
In accordance with national guidelines, a gastrostomy was systematically proposed to all patients. Thus, 43 gastrostomies were placed before the beginning of CRT. Of the 2 patients who did not receive a gastrostomy, one refused but received it anyway during treatment as it became necessary, whereas for the other it was contraindicated because of previous abdominal surgery, and a nasogastric probe was placed during treatment. For 12 patients (28%) the gastrostomy was used from the beginning of CRT and for 31 patients (72%) the gastrostomy was prophylactic. Among these 31 patients, 5 patients (16%) never used the enteral nutrition.
Trismus Occurrence, Mouth Opening and Evolution During Treatment
At baseline, trismus was recorded in 11 patients (24%) (Table 2). This value increased to 45.2% at the end of CRT treatment and then decreased to 26.8% (95% CI [14.2-42.9]) at 10 weeks after the end of CRT reaching 37.1% at 6 months. At the inclusion, the mean mouth opening was 40.2 mm (95% CI [37.5-43.0]), it slightly decreased between weeks 2 and 3 (40.9-37.8 mm, respectively) and then continuously decreased throughout the treatment arriving at less than 35 mm at week 7 (Figure 1A). According to the adjusted model, mouth opening decreased significantly over the weeks during CRT treatment (β = −.96; P < .001). The proportion of trismus increased over the treatment period and reached more than 40% at weeks 6 and 7 (41.9% and 41.4%, respectively) (Figure 1B). Trismus-free survival was assessed in patients without trismus at baseline (n = 34). By the week 1 of treatment, trismus-free survival rate was 97.1%, 95% CI [80.9-99.6] and by week 7, this value decreased to 59.7%, 95% CI [40.4-74.5]. Among the 11 patients with trismus at inclusion for different raisons (previous surgery for 5 and tumor size or location for others), 8 had trismus at 10 weeks after CRT (4 of them had previous surgery), 2 recovered from trismus and one was missing at 10 weeks. Moreover, 3 patients developed trismus during treatment (Supplemental Table S4).
Table 2.
Mouth Opening and Trismus Occurrence at Inclusion, at End of CRT, at 10 Weeks and 6 Months After CRT.
| Inclusion | End of CRT | 10 wk after CRT | 6 mo after CRT | |
|---|---|---|---|---|
| Mouth opening mean (mm) [95% CI] | 40.2 [37.5-43.0] | 33.9 [30.6-37.2] | 39.0 [36.3-41.8] | 37.8 [34.9-40.6] |
| Trismus, n (%) | 11 (24.4) | 19 (45.2) | 11 (26.8) | 13 (37.1) |
| [95% CI for the %] | [12.9-39.5] | [29.8-61.3] | [14.2-42.9] | [21.5-55.1] |
| Missing data | None | 3 | 4* | 10 |
Data were missing at 10 weeks for 4 patients: 3 patients were not evaluable (see Patients section) and 1 patient died after treatment.
Figure 1.
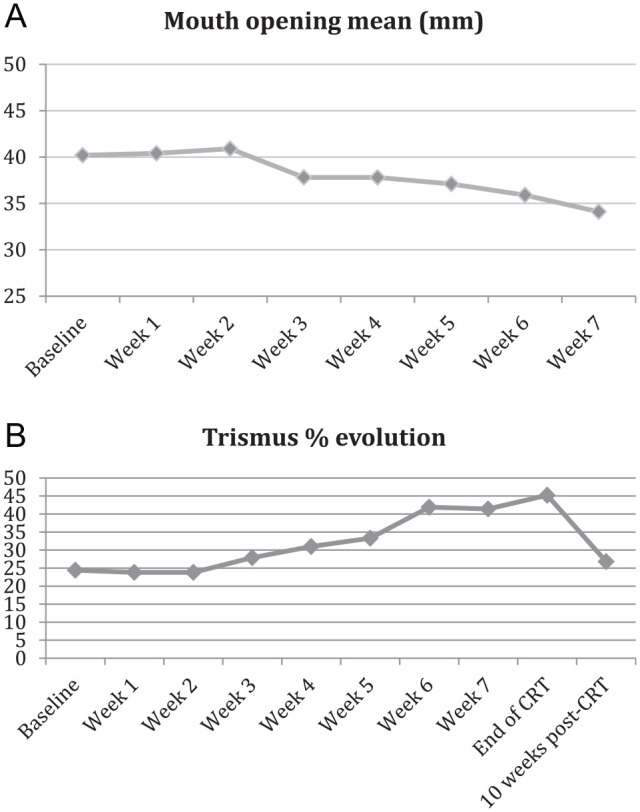
Evolution of mouth opening (A) and proportion of trismus (B) during CRT treatment from baseline.
Link Between Radiation Doses and Trismus
The median radiation doses at each radiation site are presented in Table 3. Compared to patients without trismus, patients with trismus at 10 weeks after treatment had received significantly higher radiation doses for all ipsilateral locations compared to contralateral locations, except contralateral temporalis, which also had increased doses. At 6 months, doses were significantly higher in patients with trismus than in patients without, for all locations except the contralateral medial pterygoid location.
Table 3.
Irradiation Doses at Different Radiation Sites and Link Between Doses and Trismus Occurrence at End of CRT, at 10 Weeks and 6 Months After CRT.
| Localization | Median dose (Gy) [range] | End of treatment n = 42 |
At 10 wk after treatment
n = 41 |
At 6 mo after treatment
n = 35 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trismusn = 19 | No. trismusn = 23 | P-value | Trismusn = 11 | No. trismusn = 30 | P-value | Trismusn = 13 | No. trismusn = 22 | P-value | ||
| Ipsilateral temporo-mandibular joint | 18.1 [2.2-69.8] | 22.2 [2.8-69.8] | 14.5 [2.2-67.1] | .206 | 22.9 [14.7-69.8] | 14.4 [2.2-67.1] | .049 | 22.2 [4.4-69.8] | 12.1 [2.2-59.4] | .014 |
| Controlateral temporo-mandibular joint | 11.5 [2.6-57.4] | 14.0 [2.7-36.6] | 10.0 [2.6-57.4] | .397 | 14.0 [7.8-35.8] | 10.3 [2.6-57.4] | .126 | 14.4 [4.4-57.4] | 7.2 [2.6-36.6] | .013 |
| Ipsilateral medial pterygoid | 63.3 [23.8-71.4] | 65.6 [36-71.4] | 61.7 [23.8-69.6] | .165 | 65.9 [59.4-70.8] | 61.7 [23.8-71.4] | .031 | 66.2 [47.1-70.8] | 57.7 [23.8-71.4] | .012 |
| Controlateral medial pterygoid | 45.5 [25.4-67.9] | 48.3 [35.5-63.8] | 45.1 [25.4-67.9] | .714 | 48.5 [35.5-61.3] | 44.7 [25.4-67.9] | .462 | 48.5 [31.9-67.9] | 44.7 [25.4-63.8] | .101 |
| Ipsilateral lateral pterygoid | 36.8 [4.3-71.3] | 42.9 [4.3-71.3] | 29.2 [4.3-70] | .043 | 45.4 [34.4-68.9] | 26.4 [4.3-71.3] | .001 | 45.3 [18.7-70] | 21.3 [4.3-71.3] | .001 |
| Controlateral lateral pterygoid | 16.0 [3.4-59.0] | 21.1 [3.5-51.3] | 14.3 [3.4-59] | .337 | 21.5 [10.8-51.3] | 14.1 [3.4-59] | .080 | 25.9 [6.3-59] | 11.4 [3.4-48.3] | .011 |
For almost all locations (except the masseter and the contralateral medial pterygoid locations), a radiation dose above the median value was associated with a higher risk of trismus at 10 weeks after treatment (Figure 2B and Supplemental Table S1). Similarly, at 6 months, high radiation doses at all locations (except contralateral masseter and contralateral temporalis) were significant predictive factors for trismus (Figure 2C and Supplemental Table S2).
Figure 2.
Mean radiation dose by irradiated ipsilateral site, in patients with or without trismus, at different time points. Radiation dose are presented in grays (Gy) in patients without trismus (light gray) or with trismus (dark gray), at the end of treatment (A), at 10 weeks after CRT treatment (B) or at 6 months after treatment (C).
Abbreviations: TMJ, temporo-mandibular joint; MP, medial pterygoid; LT, lateral pterygoid; Mass, masseter; Temp, temporalis.
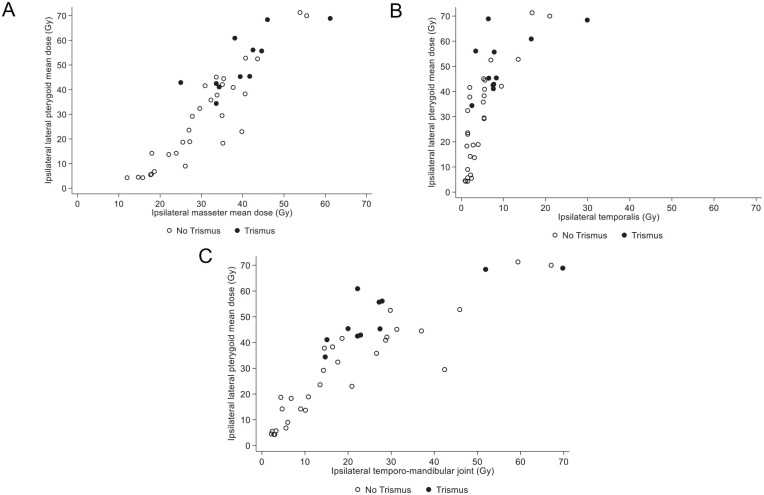
The distribution of patients with and without trismus at 10 weeks after treatment according to mean radiation doses at different ipsilateral locations (masseter, temporalis and temporo-mandibular joint) versus lateral pterygoid is presented in Figure 3. A proportion of 90% of patients (10/11) with trismus at 10 weeks had received a radiation dose >30 Gy at the ipsilateral lateral pterygoid and >30 Gy at the ipsilateral masseters (Figure 3A), 81.8% (9/11) had received a dose <10 Gy at the ipsilateral temporalis (Figure 3B), and 81.8% (9/11) between 10 and 30 Gy at the ipsilateral temporo-mandibular joint (Figure 3C).
Figure 3.
Distribution of patients with or without trismus at 10 weeks after treatment according to the radiation dose administered at different ipsilateral locations. Radiation doses are presented in grays (Gy) in patients without trismus (white circles) or with trismus (black circles). (A) Lateral pterygoid and masseter, (B) lateral pterygoid and temporalis, and (C) lateral pterygoid and temporo-mandibular joint.
Toxicity
No grade 4 toxicity was reported. Grade 3 toxicities were reported at the end of CRT and at 10 weeks after treatment (Supplemental Table S3). In all, 18.2% (n = 8) displayed mucositis at the end of CRT but only 2.3% (n = 1) at 10 weeks after treatment (evaluated according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v4.03).
Discussion
To the best of our knowledge, this study is the first to investigate mouth opening and trismus occurrence at precise moments in time, before, during and after concomitant CRT for non-larynx HNC. More than one-fourth of patients (27%) had trismus at 10 weeks after CRT and more than one-third of patients (37%) had trismus at 6 months after treatment. The study was able to evaluate mouth-opening evolution during CRT treatment indicating a peak of trismus at week 6 of CRT starting at week 3. In addition, our study found that trismus at the end of treatment was linked with higher doses of radiation at the ipsilateral lateral pterygoid muscle. These data are important in the context of the wide variation of trismus prevalence reported in the literature and can be considered representative given that ICM treats more than 200 HNC patients per year (eg, 2021). Moreover, our data may represent a solid basis for interventional studies such as the on-going OPEN program.
We focused in this study on the exploration of trismus in specific locations of head and neck malignancies such as oral cavity, oropharynx and cavum. Numerous previous studies have indicated that tumor primary site is one of the most predictive clinical parameters for occurrence of trismus.15,28 Tumors of the larynx and hypopharynx are to be distinguished from those of the oropharynx and nasopharynx and this is easily explained by the immediate proximity of the muscles for mastication.
The trismus occurrence reported here can be compared to previous studies in the literature. Indeed, several meta-analyses and studies have assessed trismus prevalence after CRT in HNC patients but results are heterogeneous with wide variations.16 Such variations can be explained by multiple factors. First, trismus was not always defined and measured in the same way. Most studies, including ours, used the Dijkstra definition of trismus as mouth-opening of 35 mm or less measured with a ruler,29 a sliding caliper or the Therabite Range of motion scale®.10,11,20 However, some other studies assessed trismus using the CTCAE toxicity scale based on functional outcomes and not “only” an interincisal distance.30 This last procedure is less precise and not reproducible. Second, study populations were heterogeneous in terms of tumor location with, for instance, the inclusion of patients with nasopharynx and larynx tumors16,31 or treated with different strategies (CRT alone, surgery plus CRT or surgery alone).13,16,32 Finally, most of the previous studies assessed trismus at different time points, most often at baseline, at 6 months and 1 year.13,14,28,32 Some studies were not precise regarding the time of trismus evaluation. For instance, a study reported trismus “during a visit at the department”15 and another evaluated trismus between 3 and 48 months after treatment.33 In contrast with these studies, our investigation measured the mouth opening and trismus occurrence at precise moments before treatment (baseline), every week during treatment, 10 weeks and 6 months after end of CRT. In our study, the baseline occurrence of trismus was 24.4%, while previous studies indicate rates between 9% and 17.3%.16 At 6 months, our occurrence of 37% is in accordance with 3 other studies (meta-analysis) reporting rates of 38%, 42%, and 44.1%,16 but higher than the value of 23.6% reported in the wide cross-sectional study of van der Geer et al on 730 patients with very heterogeneous profiles and where the time point of trismus assessment was not specified.28
To our knowledge, there are no other studies in the literature assessing mouth opening and trismus occurrence at precise moments before, during and after CRT treatment. Repeated measures are however essential to evaluate change over time and the effect of intervention.34,35 During CRT, we observed a peak of trismus occurrence at week 6 starting 3 weeks after beginning of treatment. Indeed, trismus occurrence during treatment increased in our study from 23.8% at week 1 to more than 40% at week 6. These data highlight the importance of early and active physiotherapy programs, which should start early at the beginning of treatment and not once the trismus is recorded. Prediction of trismus occurrence is thus central to preventing its occurrence and consequences on the patients’ quality of life. It should be noted that clinical tumoral and treatment-related predictive factors have previously been identified in the literature.15 The main factors are: (i) age—older age was shown to be predictive of trismus occurrence15,30; (ii) tumor location—2 studies identified tumor locations with a high risk of developing trismus such as oropharynx,15 maxilla15,32 mandible cheek15,32 and major salivary glands15; (iii) tumor size and/or stage—patients with T3 or T4 tumors showed a higher risk of trismus than patients with T1 or T2 tumors32,36; (iv) patients with limited mouth opening before treatment were shown to be at higher risk of developing trismus.32 Trismus prevalence also increased among patients receiving both surgery and radiotherapy treatment,32 surgery alone especially after flap reconstruction15,36 and radiotherapy.15,36 Van der Geer et al proposed an individual risk-score to estimate the risk to present trismus for a given patient, based on the above indicated predictive factors (age, dentition, tumor location, treatment with reconstruction, radiotherapy, or chemotherapy). Our study suggests that radiation sites and doses received also seem to be predictive factors of trismus occurrence. Regarding the radiation sites, irradiated ipsilateral masseter,30,37-39 the paired masseter38 and ipsilateral lateral pterygoid30,38,39 have been shown to be linked with a higher risk of trismus occurrence. Studies have also determined cut-off levels of radiation dose.30 Kraaijenga et al showed that doses higher than 58 Gy at the lateral pterygoid muscle and higher than 22 Gy at the masseter were predictive factors of trismus.39 Another study showed a link between receiving more than 40 Gy to all ipsilateral sites and trismus occurrence at 6 months.40 In a very large retrospective series on 421 patients, Rao et al found a trismus rate of around 11%.30 It should be noted that this incidence rate did not correspond to a trismus defined according to the measurement of the mouth opening but grade >1 using the CTCAE version 4.0, in which grade 1 toxicity was defined as decreased range of motion without impaired eating. This measurement technique induces a confounding factor in the data analysis. In our study, the ipsilateral lateral pterygoid muscle was associated with early trismus occurrence, as early as the end of radiotherapy treatment.
Obviously, our study has some limitations, inherent to its observational design and its relatively small number of patients. However, the sample size is comparable to other previous studies. It should be pointed out that our study was mandatory to precisely document the trismus occurrence as the first step of the multicenter interventional OPEN program. We were careful in the setting up of the study, especially in terms of homogeneity of recruited patients, even if at baseline, some had undergone surgery and some had not, some had trismus and some had not and that 2 different CT were used. We were also rigorous as for the quality and precision of both trismus measurement and time-points.
Conclusion
Our study indicates a high trismus occurrence among HNC patients during and after concomitant CRT starting at week 3 with a peak at 6 weeks of CRT. This highlights the necessity of early preventive physiotherapy programs to reduce trismus occurrence. We hope to find less trismus occurrence in the second step of this ongoing interventional multicenter study in which patients are invited to follow an early preventive program based on active educational physiotherapy starting at the beginning of CRT treatment.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354221147283 for Trismus Occurrence and Link With Radiotherapy Doses in Head and Neck Cancer Patients Treated With Chemoradiotherapy by Kerstin Faravel, Marta Jarlier, Pierre Senesse, Marie-Eve Huteau, Chloé Janiszewski, Anne Stoebner and Pierre Boisselier in Integrative Cancer Therapies
Acknowledgments
The authors thank all the patients who participated in the study for their trust and physicians involved in care delivery. We also extend our thanks to Caroline Constant for her significant contribution as clinical research assistant, and to Annelys Niel, Nathalie Chet and Léa Zambernardi, oncology nurse coordinators, for patient screening during inclusion phase. We also acknowledge Florin Grigorescu, Hélène de Forges and Joanna Norton for their contribution in writing the paper and Anne Lainé and physiotherapist students, Valène Metge, Marius Crayssac and Emilie Alauzet, who helped us collecting data.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This ongoing study is financially supported by the French Health Ministry, Direction générale de l’offre de soins (grant no PHRIP-15-0526) who did not contribute to design, data collection, interpretation and decision to publish.
ORCID iD: Kerstin Faravel  https://orcid.org/0000-0003-1231-1108
https://orcid.org/0000-0003-1231-1108
Supplemental Material: Supplemental material for this article is available online.
References
- 1. INCA. Les cancers en France. n.d. Accessed March 1, 2019. https://www.e-cancer.fr/ressources/cancers_en_france/
- 2. Institut National du Cancer (INCa). [Head and neck cancer: From diagnosis to follow-up]. 2018. [Google Scholar]
- 3. Taberna M, Mena M, Pavón MA, Alemany L, Gillison ML, Mesía R. Human papillomavirus-related oropharyngeal cancer. Ann Oncol. 2017;28:2386-2398. [DOI] [PubMed] [Google Scholar]
- 4. Tumban E. A current update on human papillomavirus-associated head and neck cancers. Viruses. 2019;11: 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewis A, Kang R, Levine A, Maghami E. The new face of head and neck cancer: the HPV epidemic. Oncology. 2015;29:616-626. [PubMed] [Google Scholar]
- 6. van der Molen L, van Rossum MA, Burkhead LM, Smeele LE, Hilgers FJ. Functional outcomes and rehabilitation strategies in patients treated with chemoradiotherapy for advanced head and neck cancer: a systematic review. Eur Arch Otorhinolaryngol. 2009;266:889-900. [DOI] [PubMed] [Google Scholar]
- 7. van der Molen L, van Rossum MA, Rasch CR, Smeele LE, Hilgers FJ. Two-year results of a prospective preventive swallowing rehabilitation trial in patients treated with chemoradiation for advanced head and neck cancer. Eur Arch Otorhinolaryngol. 2014;271:1257-1270. [DOI] [PubMed] [Google Scholar]
- 8. Hutcheson KA, Bhayani MK, Beadle BM, et al. Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: use it or lose it. JAMA Otolaryngol Head Neck Surg. 2013;139:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rathod S, Gupta T, Ghosh-Laskar S, Murthy V, Budrukkar A, Agarwal J. Quality-of-life (QOL) outcomes in patients with head and neck squamous cell carcinoma (HNSCC) treated with intensity-modulated radiation therapy (IMRT) compared to three-dimensional conformal radiotherapy (3D-CRT): evidence from a prospective randomized study. Oral Oncol. 2013;49:634-642. [DOI] [PubMed] [Google Scholar]
- 10. Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35:337-342. [DOI] [PubMed] [Google Scholar]
- 11. van der Geer SJ, van Rijn PV, Kamstra JI, Roodenburg JLN, Dijkstra PU. Criterion for trismus in head and neck cancer patients: a verification study. Support Care Cancer. 2019;27:1129-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson J, Johansson M, Rydén A, Houltz E, Finizia C. Impact of trismus on health-related quality of life and mental health. Head Neck. 2015;37:1672-1679. [DOI] [PubMed] [Google Scholar]
- 13. Pauli N, Johnson J, Finizia C, Andréll P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol. 2013;52:1137-1145. [DOI] [PubMed] [Google Scholar]
- 14. Kamstra JI, van Leeuwen M, Roodenburg JL, Dijkstra PU. Exercise therapy for trismus secondary to head and neck cancer: a systematic review. Head Neck. 2017;39:160-169. [DOI] [PubMed] [Google Scholar]
- 15. van der Geer SJ, van Rijn PV, Kamstra JI, et al. Prevalence and prediction of trismus in patients with head and neck cancer: a cross-sectional study. Head Neck. 2019;41:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watters AL, Cope S, Keller MN, Padilla M, Enciso R. Prevalence of trismus in patients with head and neck cancer: a systematic review with meta-analysis. Head Neck. 2019;41:3408-3421. [DOI] [PubMed] [Google Scholar]
- 17. Abboud WA, Hassin-Baer S, Alon EE, et al. Restricted mouth opening in head and neck cancer: etiology, prevention, and treatment. J Oncol Pract. 2020;16:643-653. [DOI] [PubMed] [Google Scholar]
- 18. Loewen I, Jeffery CC, Rieger J, Constantinescu G. Prehabilitation in head and neck cancer patients: a literature review. J Otolaryngol Head Neck Surg. 2021;50:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahlberg A, Engström T, Nikolaidis P, et al. Early self-care rehabilitation of head and neck cancer patients. Acta Otolaryngol. 2011;131:552-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Høgdal N, Juhl C, Aadahl M, Gluud C. Early preventive exercises versus usual care does not seem to reduce trismus in patients treated with radiotherapy for cancer in the oral cavity or oropharynx: A randomised clinical trial. Acta Oncol. 2015;54:80-87. [DOI] [PubMed] [Google Scholar]
- 21. Zatarain LA, Smith DK, Deng J, et al. A randomized feasibility trial to evaluate use of the jaw dynasplint to prevent trismus in patients with head and neck cancer receiving primary or adjuvant radiation-based therapy. Integr Cancer Ther. 2018;17:960-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamstra JI, Roodenburg JL, Beurskens CH, Reintsema H, Dijkstra PU. TheraBite exercises to treat trismus secondary to head and neck cancer. Support Care Cancer. 2013;21:951-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee R, Yeo ST, Rogers SN, et al. Randomised feasibility study to compare the use of therabite® with wooden spatulas to relieve and prevent trismus in patients with cancer of the head and Neck. Br J Oral Maxillofac Surg. 2018;56:283-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scherpenhuizen A, van Waes AM, Janssen LM, Van Cann EM, Stegeman I. The effect of exercise therapy in head and neck cancer patients in the treatment of radiotherapy-induced trismus: a systematic review. Oral Oncol. 2015;51:745-750. [DOI] [PubMed] [Google Scholar]
- 25. Pauli N, Fagerberg-Mohlin B, Andréll P, Finizia C. Exercise intervention for the treatment of trismus in head and neck cancer. Acta Oncol. 2014;53:502-509. [DOI] [PubMed] [Google Scholar]
- 26. French Speaking Society of Clinical Nutrition and Metabolism (SFNEP). Clinical nutrition guidelines of the French speaking society of clinical nutrition and metabolism (SFNEP): summary of recommendations for adults undergoing non-surgical anticancer treatment. Dig Liver Dis. 2014;46:667-674. [DOI] [PubMed] [Google Scholar]
- 27. Assenat E, Thezenas S, Flori N, et al. Prophylactic percutaneous endoscopic gastrostomy in patients with advanced head and neck tumors treated by combined chemoradiotherapy. J Pain Symptom Manag. 2011;42:548-556. [DOI] [PubMed] [Google Scholar]
- 28. van der Geer SJ, Kamstra JI, Roodenburg JL, et al. Predictors for trismus in patients receiving radiotherapy. Acta Oncol. 2016;55:1318-1323. [DOI] [PubMed] [Google Scholar]
- 29. Pauli N, Svensson U, Karlsson T, Finizia C. Exercise intervention for the treatment of trismus in head and neck cancer—a prospective two-year follow-up study. Acta Oncol. 2016;55:686-692. [DOI] [PubMed] [Google Scholar]
- 30. Rao SD, Saleh ZH, Setton J, et al. Dose-volume factors correlating with trismus following chemoradiation for head and neck cancer. Acta Oncol. 2016;55:99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ozyar E, Cengiz M, Gurkaynak M, Atahan IL. Trismus as a presenting symptom in nasopharyngeal carcinoma. Radiother Oncol. 2005;77:73-76. [DOI] [PubMed] [Google Scholar]
- 32. Wetzels JW, Merkx MA, de Haan AF, Koole R, Speksnijder CM. Maximum mouth opening and trismus in 143 patients treated for oral cancer: a 1-year prospective study. Head Neck. 2014;36:1754-1762. [DOI] [PubMed] [Google Scholar]
- 33. Johnson J, van As-Brooks CJ, Fagerberg-Mohlin B, Finizia C. Trismus in head and neck cancer patients in Sweden: incidence and risk factors. Med Sci Monit. 2010;16:CR278-CR282. [PubMed] [Google Scholar]
- 34. Karsten RT, van der Molen L, Hamming-Vrieze O, et al. Long-term swallowing, trismus, and speech outcomes after combined chemoradiotherapy and preventive rehabilitation for head and neck cancer; 10-year plus update. Head Neck. 2020;42:1907-1918. [DOI] [PubMed] [Google Scholar]
- 35. Karlsson O, Karlsson T, Pauli N, Andréll P, Finizia C. Jaw exercise therapy for the treatment of trismus in head and neck cancer: a prospective three-year follow-up study. Support Care Cancer. 2021;29:3793-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott B, Butterworth C, Lowe D, Rogers SN. Factors associated with restricted mouth opening and its relationship to health-related quality of life in patients attending a Maxillofacial Oncology Clinic. Oral Oncol. 2008;44:430-438. [DOI] [PubMed] [Google Scholar]
- 37. Lindblom U, Gärskog O, Kjellén E, et al. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta Oncol. 2014;53:620-627. [DOI] [PubMed] [Google Scholar]
- 38. Gebre-Medhin M, Haghanegi M, Robért L, Kjellén E, Nilsson P. Dose-volume analysis of radiation-induced trismus in head and neck cancer patients. Acta Oncol. 2016;55:1313-1317. [DOI] [PubMed] [Google Scholar]
- 39. Kraaijenga SA, Hamming-Vrieze O, Verheijen S, et al. Radiation dose to the masseter and medial pterygoid muscle in relation to trismus after chemoradiotherapy for advanced head and neck cancer. Head Neck. 2019;41:1387-1394. [DOI] [PubMed] [Google Scholar]
- 40. Hague C, Beasley W, Garcez K, et al. Prospective evaluation of relationships between radiotherapy dose to masticatory apparatus and trismus. Acta Oncol. 2018;57:1038-1042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354221147283 for Trismus Occurrence and Link With Radiotherapy Doses in Head and Neck Cancer Patients Treated With Chemoradiotherapy by Kerstin Faravel, Marta Jarlier, Pierre Senesse, Marie-Eve Huteau, Chloé Janiszewski, Anne Stoebner and Pierre Boisselier in Integrative Cancer Therapies