Abstract
In the present study, the effect of modified atmosphere packaging (MAP) on microbial (total aerobic count, yeasts and molds, Enterobacteriaceae, psychrotrophic bacteria, and lactic acid bacteria), physicochemical (pH, moisture content, and color), and sensory properties of raw ready‐to‐eat leek was investigated after disinfection using Percidin–Nanosil disinfectants. There were six different treatments, of which four samples were packaged as MAP (T1 to T4) and two samples were packaged as non‐MAP (T5 and T6). The highest and the lowest reduction in the number of microorganisms after disinfection was observed in psychrotrophic bacteria and total aerobic count, respectively. The count of microbial variables in T2 sample (87% N2, 3% CO2, and 10% O2) was lower than in other treatments. No significant difference was observed in the physcochemical properties (moisture and pH) of treated samples except for the color of T6 sample (cling film) compared to other treatments (p < .05). It is noteworthy that the panelists did not recognize differences among T1, T2, and T5 samples. However, T2 treatment was effective in maintaining microbial, physicochemical, and sensory properties of leek compared to the other treatments. Also, T6 sample showed the lowest quality in all parameters. Disinfection combined with MAP was able to maintain the physicochemical, microbial, and sensory properties of raw ready‐to‐eat leek during storage.
Keywords: food safety, postharvest, quality evaluation, ready to eat, vegetable
The disinfection alongside modified atmosphere packaging (hurdle effect) was able to maintain physicochemical, microbial and sensory properties of raw ready‐to‐eat leek (in T2 sample) during storage.

1. INTRODUCTION
Fruits and vegetables constitute an important part of the human diet (Yousuf et al., 2018) and are good sources of biologically active compounds, which may prevent some chronic diseases (Granado‐Lorencio et al., 2008). Leek (Allium porrum L.) is one of the most commercial leafy vegetables in the world. It belongs to the Allium genus (Alliaceae family) along with onion and garlic (Ozgur et al., 2011). The shelf life of fresh ready‐to‐eat vegetables is greatly limited by the increase in metabolic activity, color destruction, and microbial growth (Char et al., 2012). A wide range of microorganisms including bacteria, yeasts, and molds affect the safety and quality of fresh vegetables and fresh‐cut fruits. Gram‐negative bacteria especially Pseudomonas and Enterobacteriaceae species constitute 80 to 90% of bacterial flora. Lactic acid bacteria (LAB) can be the natural intrinsic flora of fruits and vegetables which can be correlated with fresh product spoilage and unpleasant odors. The number of yeasts and molds (YM) is usually smaller than bacteria; however, as they grow in large numbers, they can contribute to soft rot spoilage and decomposed fermented products (Oliveira et al., 2015).
Washing with water can lower the potential of contamination, although it can transfer pathogenic microorganisms (Gil et al., 2009). Disinfecting agents are known to ensure quality and safety in the food industry (Tirpanalan et al., 2011). Their efficiency varies depending on the type, dosage, and duration of the disinfecting process (Yarahmadi et al., 2012). Peracetic acid (acetic acid–hydrogen peroxide) solution with the chemical formula of C2H4O3 is introduced into the market with several brand names such as Proxitane, Prasan, and Percidin. This achromatic liquid, which belongs to the broad‐spectrum oxidizing compounds family, can eradicate bacteria, viruses, fungi, and spores and is considered a cold sterilizer (Najafi & Arbabi, 2013). Another disinfectant is commercialized with the commercial name of Nanosil (hydrogen peroxide–silver ion) (Barikgugjlu et al., 2016). Nanosil is composed of hydrogen peroxide similar to Percidin. As an advantage, it does not have any dangerous side effects on both humans and the environment (Khazaei et al., 2008).
To package ready‐to‐eat food products, modified atmosphere packaging (MAP) can be employed. The concept of this method is to package a perishable product in an atmosphere containing a different mixture of atmosphere gases (Mousavi‐Baigi & Sedaghat, 2014). Oxygen (O2), carbon dioxide (CO2), and nitrogen (N2) are the most commonly used gases in MAP (Oliveira et al., 2015). Nitrogen has three functions in MAP: displacement of O2 to delay oxidation, postponing of the growth of aerobic spoilage organisms, and acting as a filler to maintain package conformity (Farber et al., 2003). The effectiveness of CO2 as an antimicrobial agent depends significantly on the temperature, type of microorganism, growth phase, water activity, and chemical composition of the product (Putnik et al., 2017). The use of MAP for fresh‐cut products requires careful selection of the film and package type for each commodity (Oliveira et al., 2015). Important factors in the selection of suitable packaging film include clarity, mechanical strength, machinability, seal ability, printability, and gas barrier properties (Farber et al., 2003). Nowadays, in the development of newly packaged ready‐to‐eat products with extended shelf‐life, it is recommended to use multiple methods simultaneously because of the “hurdle effect.” This study aimed to evaluate the shelf life, physicochemical and sensory characteristics, and microbiological safety of fresh leek disinfected by commercial Percidin (peracetic acid) and Nanosil, packaged as MAP.
2. MATERIALS AND METHODS
2.1. Leek preparation and disinfection
Fresh leeks were prepared from a local farm located in Shiraz, Iran (during the months of October and November 2019), and processed the same day under aseptic conditions. Following the removal of physically damaged, dehydrated, or discolored (yellowed) leaves, leek stalks were cut. At the beginning of the experiment, 10 g of raw leeks were subjected to microbial evaluation. The leek stalks were immersed in the distilled water for 5 min, afterward treated with 0.66 ml/L Percidin (acetic acid–hydrogen peroxide) for 3 min (according to manufacturer's instructions) and 3.75 ml/L Nanosil (hydrogen peroxide ‐silver ion) for 30 minutes (according to manufacturer's instructions). The disinfected samples were drained in a laminar flow biosafety cabinet until no visible water droplets were present and then a 10 g sample was taken for microbial evaluation.
2.2. Modified atmosphere packaging
Washed and disinfected fresh leeks were placed in 60 g portions in polypropylene (PP) trays (15 cm width × 20 cm length × 3 cm depth). Two commercial moisture absorbent pads (60 ml/L) were placed in each tray in order to absorb any condensed moisture and to keep samples fresh. Modified atmosphere packaging (DZ‐400, Wenzhou Zhonghuan Packaging Machine Co., Ltd, China) of these trays was then performed using a two‐layer laminated film (20 cm width × 30 cm length; 90 μm thickness) made of polyester (PS) as the outer layer and polyethylene (PE) as the inner layer. The permeability of the film to oxygen was 113 ml.μm/m2.day.Kpa.
A total of six different treatments (Table 1) were applied in two replicates. T1 to T4 treatments included MAP by injecting a mixture of gases (oxygen, carbon dioxide, and nitrogen). Two other treatments (T5 and T6) were packaged using ambient air. T5 group was sealed in the PS/PE plastic bag as the “MAP control” sample and T6 sample was packaged using cling film as the package control sample. The packages were stored at 5.0 ± 0.5°C for 9 days. All microbial experiments, including total aerobic count, yeasts and molds, Enterobacteriaceae, psychrotrophic bacteria, and lactic acid bacteria, as well as sensory evaluation, were carried out on days 2, 6, and 9 of the experiment. Physicochemical characteristics including pH, moisture content, and color were measured on days 1, 5, and 9 of the experiment.
TABLE 1.
Packaging treatments used in this study.
| Sample | N2% | CO2% | O2% | Packaging material |
|---|---|---|---|---|
| T1 (MAP) | 67.00 ± 0.10 | 3.00 ± 0.10 | 30.00 ± 0.10 | PS/PE |
| T2 (MAP) | 87.00 ± 0.10 | 3.00 ± 0.10 | 10.00 ± 0.10 | PS/PE |
| T3 (MAP) | 94.00 ± 0.10 | 3.00 ± 0.10 | 3.00 ± 0.10 | PS/PE |
| T4 (MAP) | 30.00 ± 0.10 | 0.00 + 0.01 | 70.00 ± 0.10 | PS/PE |
| T5 (Control –Ambient air) | 78.00 ± 0.01 | 0.04 ± 0.01 | 21.00 ± 0.01 | PS/PE |
| T6 (Cling film –Ambient air) | 78.00 ± 0.01 | 0.04 ± 0.01 | 21.00 ± 0.01 | Cling film |
2.3. Microbial evaluation
Microbiological determinations were done using 10 g sample of leek per replicate on days 2, 6, and 9. The samples were mixed with 90 ml of sterile normal saline in a sterile bag and homogenized in a laboratory stomacher (Easy Mix, AES Chemunex) for 90 s. Regarding psychrotrophic bacteria, cultured plate count agar (PCA) Petri dishes were incubated for 7 days at 7.0 ± 0.5°C, and for the total aerobic count test, they were incubated for 3 days at 30.0 ± 0.5°C. Enterobacteriaceae were determined using violet red bile glucose agar (VRBG) incubated at 37 ± 0.5°C for 1 day. Lactic acid bacteria (LAB) were enumerated on De Man–Rogosa–Sharpe Agar (MRS), followed by incubation at 37 ± 0.5°C for 48 h. Yeast and mold count (incubated for 5 days at 25 ± 0.5°C) were determined on yeast extract glucose chloramphenicol agar (YGC).
2.4. Physicochemical evaluation
2.4.1. pH
Leek samples (10 g) were homogenized in distilled water (100 ml/L) using a blender (LB20ES, Waring) and the pH of the solution was measured using a pH meter (Metrohm 827, Switzerland). Three measurements were taken on every sample. The results were expressed as units of pH (Feldsine et al., 2002).
2.4.2. Moisture content
Moisture content was determined gravimetrically according to the 984.25 AOAC method (Feldsine et al., 2002) in duplicate, and results were expressed as a percent. Around 3 g of a homogenized sample was dried at 103 ± 2°C (Oven OT 140, Iran) to constant weight.
| (1) |
Where W*1 is the sample weight before heating and W*2 is the sample weight after heating.
Color measurement
The color of leek samples was determined using a Konica Minolta Colorimeter (CR 400, Minolta). The results were expressed in terms of L*, a*, and b* Hunter Lab values, where L* represents the lightness, a* represents chromaticity on a green (−) to red (+) axis, and b* represents chromaticity on a blue (−) to yellow (+) axis. Also, the total color difference (∆E) between color parameters of the samples on day 1 and day 9, for each sample, was calculated using the following equation:
| (2) |
Where L * 1, a * 1, and b * 1 are the color parameters of the samples on day 1 and L * 9, a * 9, and b * 9 are the color parameters of the samples on day 9.
2.5. Sensory evaluation
Nine untrained panel members aged 22–40 years were recruited among the students, faculty, and staff of the School of Nutrition and Food Sciences, Shiraz, Iran. The samples were evaluated under white illumination at room temperature. Leek characteristics including texture, aroma, color, and overall acceptability were scored on a 1–5 scale, where 5 = very good, 4 = good, 3 = normal, 2 = poor quality, and 1 = not acceptable (Waghmare & Annapure, 2013).
2.6. Statistical analysis
Statistical analyses were performed using SPSS 26 (IBM SPSS Software) and Microsoft Excel. All microbial counts were log‐transformed for statistical analyses. The microbial evaluation was performed via Freidman nonparametric test, and for comparing the days for each treatment, Kruskal–Wallis test (p < .01) was run. The Mann–Whitney U nonparametric test was employed to analyze the significance of the difference (disinfecting effect) for all features. Physicochemical properties (pH, moisture, and color) of samples were tested by repeated measures and Duncan's comparison test (p < .05). In addition, the analysis of sensory test results was performed using the Kruskal–Wallis test.
3. RESULTS
3.1. Microbiology enumeration
3.1.1. Evaluation of the efficacy of disinfectants
Raw leeks were treated with 0.66 ml/L Percidin (acetic acid–hydrogen peroxide) for 3 minutes and 3.75 ml/L Nanosil (hydrogen peroxide–silver ion) for 30 minutes before packaging. Microbial load in intended treatment (B) reduced compared to raw leek (A). Total aerobic count, yeasts and molds, Enterobacteriaceae, psychrotrophic bacteria, and LAB were decreased by 0.37, 0.48, 0.69, 1.05, and 0.77 log cfu g−1, respectively. The most and least amount of microbial load reduction was reported for the psychrotrophic bacteria and Enterobacteriaceae (Table 2).
TABLE 2.
Efficacy of disinfectants on the microbial load of raw leek.
| Factor | A | B | p‐value |
|---|---|---|---|
| Mean ± SD (log CFU g‐1) | Mean ± SD (log CFU g‐1) | ||
| Total aerobic count | 6.59 ± 0.308 | 5.814 ± 0.368 | <0.01 |
| Enterobacteriaceae | 4.739 ± 0.510 | 4.362 ± 0.380 | 0.008 |
| Lactic acid bacteria | 4.089 ± 0.389 | 3.606 ± 0.544 | 0.002 |
| Yeasts and molds | 4.640 ± 0.254 | 3.950 ± 0.579 | <0.01 |
| Psychrotrophic bacteria | 5.978 ± 0.326 | 4.921 ± 0.434 | <0.01 |
Note: A: Before washing + disinfection; B: After washing + disinfection.
3.1.2. Effects of MAP on microorganisms
During the storage period, increases in the microbial count were observed in all samples. At the end of the storage, the number of yeasts and molds for different treatments was different and there was a significant difference between them (p < .01). The highest and lowest contents of yeasts and molds were related to T6 (5.7 log cfu g−1) and T2 (5.1 log cfu g−1) samples, respectively (Figure 1b). The number of LAB in the leek's samples was increased in all treatments after the storage time ranged between 4.09 and 4.79 log cfu g−1. The lowest and highest count of LAB were observed in T2 (4.09 log cfu g−1) and T4 samples, respectively (4.79 log cfu g−1) (Figure 2).
FIGURE 1.

The effect of modified atmospheric packing on the total number of aerobic bacteria (a), yeasts, and molds (b) during 9 days of storage at 5°C. T1: (67% N2, 3% CO2, 30% O2) ( ), T2: (87% N2, 3% CO2, 10% O2) (
), T2: (87% N2, 3% CO2, 10% O2) ( ), T3: (94% N2, 3% CO2, 3% O2) (
), T3: (94% N2, 3% CO2, 3% O2) ( ), T4: Super atmospheric oxygen (SAO) (30% N2, 0% CO2, 70% O2) (
), T4: Super atmospheric oxygen (SAO) (30% N2, 0% CO2, 70% O2) ( ), T5: Control sample (
), T5: Control sample ( ), and T6: Cling film (
), and T6: Cling film ( ).
).
FIGURE 2.

The effect of modified atmosphere packaging on Enterobacteriaceae (a) psychrotrophic bacteria (b) during 9 days of storage at 5°C. T1: (67% N2, 3% CO2, 30% O2) (  ), T2: (87% N2, 3% CO2, 10% O2) (
), T2: (87% N2, 3% CO2, 10% O2) ( ), T3: (94% N2, 3% CO2, 3% O2) (
), T3: (94% N2, 3% CO2, 3% O2) ( ), T4: Super atmospheric oxygen (SAO) (30% N2, 0% CO2, 70% O2) (
), T4: Super atmospheric oxygen (SAO) (30% N2, 0% CO2, 70% O2) ( ), T5: Control sample (
), T5: Control sample (  ), and T6: Cling film (
), and T6: Cling film (  ).
).
After 9 days of storage at 5°C, Enterobacteriaceae count in different samples was 5.6–6 log cfu g−1 with the highest count in T6. The microbial count of Enterobacteriaceae was controlled in T2 (87% N2, 3% CO2, and 10% O2) and T4 samples (30% N2, 0% CO2, and 70% O2) which contained superatmospheric oxygen (SAO). Initially, the count of psychrotrophic bacteria ranged from 5.5–5.6 log cfu g−1, and after the end of storage time, it reached 5.9–7.4 log cfu g−1. T6 sample showed the highest amount of psychrotrophic bacteria. T2 sample controlled the psychrotrophic bacteria better than other treatments; it showed a nonsignificant difference on different days (Figure 3b). After the storage period, the total aerobic count in T1 to T6 samples was 6.3, 6.2, 7.1, 6.5, 6.6, and 7.6 log cfu g−1, respectively. The total aerobic count of all treatments increased and there was a significant difference in microbial load in different treatments after the end of shelf life (p < .01) (Figure 1a).
FIGURE 3.

The effect of modified atmosphere packaging on lactic acid bacteria during 9 days of storage at 5°C. T1: (67% N2, 3% CO2, 30% O2) (  ), T2: (87% N2, 3% CO2, 10% O2) (
), T2: (87% N2, 3% CO2, 10% O2) ( ), T3: (94% N2, 3% CO2, 3% O2) (
), T3: (94% N2, 3% CO2, 3% O2) ( ), T4: Super atmospheric oxygen (SAO) (30% N2, 0% CO2, 70% O2) (
), T4: Super atmospheric oxygen (SAO) (30% N2, 0% CO2, 70% O2) ( ), T5: Control sample (
), T5: Control sample (  ), and T6: Cling film (
), and T6: Cling film (  ).
).
3.2. pH and moisture content
The pH of all samples except T2 sample with a slight increase declined during storage at 5°C. The pH of the samples was between 6.7 and 6.9. There was no significant difference among days and treatments at the end of the storage period. (Figure 4a).
FIGURE 4.

(a) The pH of the samples was measured using pH meters. Day 1: (  ), day 5: (
), day 5: (  ), and day 9: (
), and day 9: (  ). (b) Moisture (%) according to AOAC 984.25 method in the samples kept at 5 °C. T1: (67% N2, 3% CO2, 30% O2), T2: (87% N2, 3% CO2, 10% O2), T3: (94% N2, 3% CO2, 3% O2), T4: (30% N2, 0% CO2, 70% O2), and T5: control sample, T6: cling film (p < .05).
). (b) Moisture (%) according to AOAC 984.25 method in the samples kept at 5 °C. T1: (67% N2, 3% CO2, 30% O2), T2: (87% N2, 3% CO2, 10% O2), T3: (94% N2, 3% CO2, 3% O2), T4: (30% N2, 0% CO2, 70% O2), and T5: control sample, T6: cling film (p < .05).
The moisture content of samples had a significant difference on different days, but the interaction between the treatment and the storage time showed no significant difference (p < .05). The moisture content of samples was between 92.84 and 94.19% at the end of the storage period. (Figure 4b).
3.3. Color changes
Statistical analysis showed significant differences (p < .05) for L* value, among the treatments, interaction of treatments, and storage time. The lightness (L*) in the T6 sample was raised to the highest level, which showed the positive effect of (MAP) packaging in maintaining this color parameter (Figure 5). The a* value also showed a decreasing trend during the storage. A significant reduction in the b* value was also observed during storage. The total color difference (∆E) of the samples was measured with T5 (control) and T6 (cling film) samples as a reference after 9 days of storage.
FIGURE 5.

The L, a, and b values of leek samples on days 1, 5, and 9 of storage at 5°C. Day 1: (  ), Day 5: (
), Day 5: (  ), and Day 9: (
), and Day 9: (  ).
).
Based on the data, among the MAP samples (T1 to T4), T2 and T3 samples had a narrower color difference than the others, after 5 and 9 days, respectively. T6 samples showed the most color change after 9 days of storage. Nonetheless, the T5 sample showed the lowest ∆E. A comparison of the color difference between T5 and other MAP samples (∆ET5) also showed that T3 sample had a minor color difference from the control sample after 9 days of storage. This means the modified atmosphere had a positive effect on this parameter compared to cling film packaging (Table 3).
TABLE 3.
Total color difference (∆E) between the fresh samples (day 1) and the samples after 5 (∆ED5) and 9 (∆ED9) days of storage; and total color difference of the samples after 9 days of storage with T5 (∆ET5) and T6 (∆ET6) samples as reference.
| Sample | ∆ED5 | ∆ED9 | ∆ET5 | ∆ET6 |
|---|---|---|---|---|
| T1 | 6.266092 | 7.402701 | 9.902399 | 12.54764 |
| T2 | 4.326678 | 6.604704 | 8.304573 | 11.61587 |
| T3 | 6.61689 | 5.141433 | 5.998241 | 10.38957 |
| T4 | 10.13688 | 6.320539 | 7.55229 | 12.64573 |
| T5 | 2.933993 | 4.796814 | 0 | 5.677076 |
| T6 | 5.135274 | 10.07281 | 5.677076 | 0 |
Note: T1: (67% N2, 3% CO2, 30% O2), T2: (87% N2, 3% CO2, 10% O2), T3: (94% N2, 3% CO2, 3% O2), T4: (30% N2, 0% CO2, 70% O2), T5: control sample, T6: cling film.
3.4. Sensory evaluation
Figure 6 represents the odor, texture, color, and overall acceptability scores for all the treated and nontreated leek. The most important parameter in the sensory evaluation of leek was the smell and tissue of leek. All the treatments showed quality reduction during the storage period. Measurement of sensory parameters was an indicator of significant difference among treatments (p < .05). The highest overall acceptability score was related to T1, T2, and T5, and the lowest was related to T6 sample.
FIGURE 6.
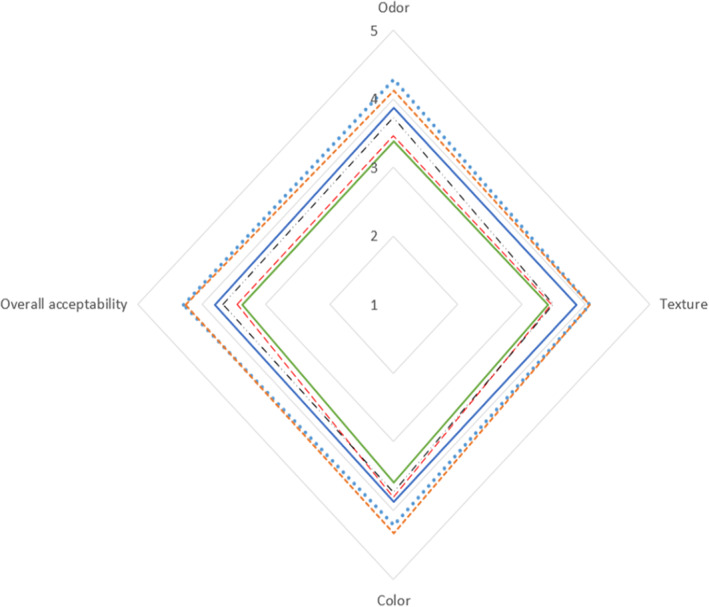
The effect of modified atmosphere packaging on sensory attributes of leek during storage at 5 °C for 9 days. T1: (67% N2, 3% CO2, 30% O2), T2: (87% N2, 3% CO2, 10% O2), T3: (94% N2, 3% CO2, 3% O2), T4: (30% N2, 0% CO2, 70% O2), T5: control sample, and T6: cling film (p < .05). T1: (  ), T2: (
), T2: (  ), T3: (
), T3: (  ), T4: (
), T4: ( ), T5: (
), T5: (  ), T6: (
), T6: (  ).
).
4. DISCUSSION
This study was conducted to improve the quality of leek using a combination of disinfectant (Percidin–Nanosil) and MAP during storage. Microorganisms are one of the most important factors contributing to the reduction in shelf life and the quality of vegetables. Therefore, it is necessary to suppress the growth of microorganisms (Murcia et al., 2009). A wide range of disinfectants are currently used to reduce the microbial load of fresh produce, and each of them has its own advantages and disadvantages. The efficiency of disinfectants depends on various factors, such as dosage, contact time, and type of product. In the present study, Percidin and Nanosil disinfectants led to a significant reduction of the total aerobic count, and psychrotrophic bacteria, yeasts, and molds. Leeks were first disinfected by Percidin due to its unpleasant odor (Barikgugjlu et al., 2016).
In a study conducted by Vandekinderen et al. (2009), disinfection of freshly chopped leek using 80 ml/L of disinfectant for 60 s reduced the initial microbial contamination of leeks (between 5.58 and 7.29 log cfu g−1) by 1 log cfu g−1. Barikgugjlu et al. (2016) investigated the effect of Nanosil (120, 150, and 180 ppm) and peracetic acid (40, 60, and 80 ppm) on the level of Salmonella enteritidis and E. coli O157: H7 on raw mixed vegetables (coriander, parsley, basil, coriandrum, spearmint, leek, and radish). The contact times (5 and 10 min) showed no significant difference in the reduction in test organisms. At the highest concentration, peracetic acid reduced the number of Salmonella enteritidis and E. coli O157: H7 by 1.65 and 1 log cfu g−1, respectively. The reduction values by Nanosil were 2.24 and 2.04 log cfu g−1, respectively. Overall, Nanosil showed higher activity against test organisms. In another study, the total microbial count of freshly cut strawberries reduced by 2.6 log cfu g−1 after disinfection with 100 ml/L of peracetic acid for 120 s (Van de Velde et al., 2016). Dai et al. found a reduction of 2.16 and 2.20 log cfu g−1 in the total aerobic count after disinfection of freshly chopped scallion with peracetic acid at the concentrations of 150 ml/L (2 min) and 100 ml/L (5 min), respectively (Dai et al., 2012).
MAP approach is applied to reduce respiration of fresh produce without leading to anaerobic respiration; as a result, the oxygen content is generally recommended to be between 1 and 5%. The low oxygen content in the packages may lead to the production of ethanol, acetaldehyde, and organic acids which is accompanied by discoloration, texture change, and unpleasant taste (Granado‐Lorencio et al., 2008).
The count of LAB was at the highest level in T3 sample (with very low oxygen content) compared to the other treatments because they were microaerophilic bacteria. The total aerobic count was at the highest level in packaging groups with low CO2 content (in treatments of T3 to T5, and T6). It seems that the antimicrobial properties of CO2 can be an effective factor in reducing the total aerobic count. Among all treatments, T2 group (87% N2, 3% CO2, and 10% O2) showed lower microbial content after 9 days of storage. As expected, the growth of yeasts and molds (aerobic microorganisms) was at the highest level in T4 sample (with the highest oxygen level). However, different treatments showed no significant differences in pH and moisture content.
In terms of color, peracetic acid as an oxidizing agent had no negative effect on leek (T1 to T5 groups). Therefore, T6 group exhibited a significant difference from the other groups, suggesting a positive effect of packaging film and MAP. Tsouvaltzis et al. (2008) reported that the amount of L* value (lightness parameter) in minimally processed leek in control packaging (ambient air) decreased further compared to the MAP packaging (1% O2 + 14% CO2) during 14 days of storage at 6.5°C.
Sensory evaluation done by the untrained panelists showed better overall acceptability of all samples of leek till day 6, but on day 9, it was difficult to distinguish any difference among T1, T2, and T5 samples. In the study of Gómez and Artés (2005), the sensory evaluation of celery sticks in two MAP packaging conditions (6 kPa O2 + 7 kPa CO2 and 9 kPa O2 + 5 kPa CO2) was better maintained than the control (ambient air) sample at 5°C. There are several studies on the efficacy of MAP approach alone or in combination with chemicals in shelf‐life extension. Exama et al. (1993) reported that O2 concentrations of less than 1% and CO2 concentrations of more than 15% are critical concentrations for agricultural products. However, the critical concentrations for each product depend on several variables such as temperature, physiological conditions, maturity, and preparation processes.
In a study, the effect of MAP approach on the freshly cut leek was investigated. The results showed that storing leek at 5°C under 5% O2, 5% CO2, and 90% N2 had moderate effectiveness (Mousavi‐Baigi & Sedaghat, 2014). Arvanitoyannis et al. (2011) investigated microbiological and sensory properties of rocket salad (Arugula) alone and in combination with “Lollo Verde” lettuce under MAP conditions (5% O2 and 10% CO2 as MAP‐A treatments and 2% O2 and 5% CO2 as MAP‐B treatments) for 10 days. The color was better preserved in both MAP treatments compared with the passive sample.
Rosa et al. (2007) studied the effect of the improved atmosphere (88% N2, 2% CO2, and 10% O2) and inactive atmosphere packaging on the physicochemical, microbial, and sensory properties of the freshly cut parsley during storage at 5 °C. They showed that parsley had better quality stability under inactive atmosphere packaging compared with MAP conditions during a 6‐day time period. The inefficiency of MAP in increasing parsley shelf life can be at least partly attributed to different respiration rates of products which can even be affected by season.
In the previous research, MAP approach (5% O2, 10% CO2, and 85% N2) showed higher efficacy in improving the physicochemical and sensory properties of freshly cut papaya during 25 days of storage at 5 °C when used in combination with chemicals emphasizing the use of “hurdle technology” in food preservation (Feldsine et al., 2002). Barbosa et al. (2016) investigated the effect of three MAP treatments with very high CO2 and very low or zero O2 contents on the physicochemical and sensory properties of the cooked vegetables. They found a nonsignificant difference in pH, moisture, and color in different treatments and they observed no significant difference between the samples in terms of sensory parameters.
5. CONCLUSIONS
In this study, the effect of Percidin–Nanosil disinfectants was evaluated along with MAP during 9 days of storage at 5 °C. The results showed that among different treatments, T2 sample (87% N2, 3% CO2, and 10% O2) had the best microbial, physicochemical, and sensory properties. Also, the lowest quality among the treatments in all variables was related to T6 sample. The shelf life of leek without using disinfectants and MAP was limited to 6 days. Therefore, the disinfection alongside MAP (hurdle effect) was able to maintain physicochemical, microbial, and sensory properties of raw ready‐to‐eat leek during storage.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this study.
ACKNOWLEDGMENTS
This study was conducted by the Department of Health and Quality Control of Shiraz University of Medical Sciences. We are very grateful for the help of Dr. Baeghbali and Dr. Ashrafi‐Dehkordi for their contribution to editing and statistical analysis.
Hosseininezhad, B. , Nader, M. , Ramezanian, A. , Niakousari, M. , & Mazloomi, S. M. (2023). A combination of modified atmosphere packaging and two chemical disinfectants: Effects on microbial, sensory, and physicochemical properties of raw ready‐to‐eat leek. Food Science & Nutrition, 11, 148–156. 10.1002/fsn3.3047
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Arvanitoyannis, I. S. , Bouletis, A. D. , Papa, E. A. , Gkagtzis, D. C. , Hadjichristodoulou, C. , & Papaloucas, C. (2011). Microbial and sensory quality of “Lollo verde” lettuce and rocket salad stored under active atmosphere packaging. Anaerobe, 17, 307–309. [DOI] [PubMed] [Google Scholar]
- Barbosa, C. , Alves, M. R. , Rocha, S. , & Oliveira, M. B. P. (2016). Modified atmosphere packaging of precooked vegetables: Effect on physicochemical properties and sensory quality. Food Chemistry, 194, 391–398. [DOI] [PubMed] [Google Scholar]
- Barikgugjlu, R. , Khaniki, G. J. , Nodehi, R. N. , Alimohammadi, M. , & Mousavi, S. M. (2016). Efficacy of nanosil (hydrogen peroxide‐silver ion) and peracetic acid on reduction of Escherichia coli O157: H7 and salmonella entritidis in raw vegetables. Annual Review of Food Science and Technology, 17, 333–339. [Google Scholar]
- Char, C. , Silveira, A. C. , Inestroza‐Lizardo, C. , Hinojosa, A. , Machuca, A. , & Escalona, V. H. (2012). Effect of noble gas‐enriched atmospheres on the overall quality of ready‐to‐eat arugula salads. Postharvest Biology and Technology, 73, 50–55. [Google Scholar]
- Dai, X. , Luo, H. , Jiang, L. , Ling, L. , Xue, Y. , & Yu, Z. (2012). Efficacy of different sanitizing agents and their combination on microbe population and quality of fresh‐cut Chinese chives. Journal of Food Science, 77, 348–353. [DOI] [PubMed] [Google Scholar]
- Exama, A. , Arul, J. , Lencki, R. W. , Lee, L. Z. , & Toupin, C. (1993). Suitability of plastic films for modified atmosphere packaging of fruits and vegetables. Journal of Food Science, 58, 1365–1370. [Google Scholar]
- Farber, J. N. , Harris, L. J. , Parish, M. E. , Beuchat, L. R. , Suslow, T. V. , Gorney, J. R. , Garrett, E. H. , & Busta, F. F. (2003). Microbiological safety of controlled and modified atmosphere packaging of fresh and fresh‐cut produce. Comprehensive Reviews in Food Science and Food Safety, 2, 142–160. [Google Scholar]
- Feldsine, P. , Abeyta, C. , & Andrews, W. H. (2002). AOAC international methods committee guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. Journal of AOAC International, 85, 1187–1200. [PubMed] [Google Scholar]
- Gil, M. I. , Selma, M. V. , López‐Gálvez, F. , & Allende, A. (2009). Fresh‐cut product sanitation and wash water disinfection: Problems and solutions. International Journal of Food Microbiology, 134, 37–45. [DOI] [PubMed] [Google Scholar]
- Gómez, P. A. , & Artés, F. (2005). Improved keeping quality of minimally fresh processed celery sticks by modified atmosphere packaging. LWT‐ Food Science and Technology, 38, 323–329. [Google Scholar]
- Granado‐Lorencio, F. , Olmedilla‐Alonso, B. , Herrero‐Barbudo, C. , Sánchez‐Moreno, C. , de Ancos, B. , Martínez, J. A. , Pérez‐Sacristán, B. , & Blanco‐Navarro, I. (2008). Modified‐atmosphere packaging (MAP) does not affect the bioavailability of tocopherols and carotenoids from broccoli in humans: A cross‐over study. Food Chemistry, 106, 1070–1076. [Google Scholar]
- Khazaei, M. , Nabizadeh, R. , Naddafi, K. , Vaezi, F. , Yunesian, M. , Roshany, M. , & Farzinnia, B. (2008). Qom wastewater disinfection with hydrogen peroxide‐silver ion Complex3GA. Qom University of Medical Sciences Journal, 1, 31–37. [Google Scholar]
- Mousavi‐Baigi, S. F. , & Sedaghat, N. (2014). Application new packaging techniques to maintain the quality and freshness of fresh salads and vegetables. The first national congress on snack foods.
- Murcia, M. A. , Jiménez‐Monreal, A. M. , García‐Diz, L. , Carmona, M. , Maggi, L. , & Martínez‐Tomé, M. (2009). Antioxidant activity of minimally processed (in modified atmospheres), dehydrated and ready‐to‐eat vegetables. Food and Chemical Toxicology, 47, 2103–2110. [DOI] [PubMed] [Google Scholar]
- Najafi, N. , & Arbabi, A. (2013). Effect of peracetic acid on disinfection and shelf‐life of lettuce after harvest. Third National Conference on Food Security.
- Oliveira, M. , Abadias, M. , Usall, J. , Torres, R. , Teixidó, N. , & Viñas, I. (2015). Application of modified atmosphere packaging as a safety approach to fresh‐cut fruits and vegetables–a review. Trends in Food Science & Technology, 46, 13–26. [Google Scholar]
- Ozgur, M. , Akpinar‐Bayizit, A. , Ozcan, T. , & Yilmaz‐Ersan, L. (2011). Effect of dehydration on several physico‐chemical properties and the antioxidant activity of leek (Allium porrum L.). Notulae Botanicae Horti Agrobotanici Cluj‐Napoca, 39, 144–151. [Google Scholar]
- Putnik, P. , Roohinejad, S. , Greiner, R. , Granato, D. , Bekhit, A. E. D. A. , & Bursać Kovačević, D. (2017). Prediction and modeling of microbial growth in minimally processed fresh‐cut apples packaged in a modified atmosphere: A review. Food Control, 80, 411–419. [Google Scholar]
- Rosa, C. , Sapata, M. , & Guerra, M. M. (2007). Chemical and sensory characteristics and microbiological safety of fresh finely chopped parsley packed in modified atmosphere. Food Control, 18, 1008–1012. [Google Scholar]
- Tirpanalan, O. , Zunabovic, M. , Domig, K. J. , & Kneifel, W. (2011). Mini review: Antimicrobial strategies in the production of fresh‐cut lettuce products. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances, 1, 176–188. [Google Scholar]
- Tsouvaltzis, P. , Brecht, J. K. , Siomos, A. S. , & Gerasopoulos, D. (2008). Responses of minimally processed leek to reduced O2 and elevated CO2 applied before processing and during storage. Postharvest Biology and Technology, 49, 287–293. [Google Scholar]
- Van de Velde, F. , Grace, M. H. , Pirovani, M. É. , & Lila, M. A. (2016). Impact of a new postharvest disinfection method based on peracetic acid fogging on the phenolic profile of strawberries. Postharvest Biology and Technology, 117, 197–205. [Google Scholar]
- Vandekinderen, I. , Devlieghere, F. , De Meulenaer, B. , Ragaert, P. , & Van Camp, J. (2009). Optimization and evaluation of a decontamination step with peroxyacetic acid for fresh‐cut produce. Food Microbiology, 26, 882–888. [DOI] [PubMed] [Google Scholar]
- Waghmare, R. B. , & Annapure, U. S. (2013). Combined effect of chemical treatment and/or modified atmosphere packaging (MAP) on quality of fresh‐cut papaya. Postharvest Biology and Technology, 85, 147–153. [Google Scholar]
- Yarahmadi, M. , Yunesian, M. , Pourmand, M. R. , Shahsavani, A. , Mubedi, I. , Nomanpour, B. , & Naddafi, K. (2012). Evaluating the efficiency of lettuce disinfection according to the official protocol in Iran. Iranian Journal of Public Health, 41, 95–103. [PMC free article] [PubMed] [Google Scholar]
- Yousuf, B. , Qadri, O. S. , & Srivastava, A. K. (2018). Recent developments in shelf‐life extension of fresh‐cut fruits and vegetables by application of different edible coatings: A review. Lwt, 89, 198–209. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


