Abstract
Purpose:
To compare the accuracy and safety of 0.56 GBq resin yttrium-90 (90Y) (scout90Y) microspheres with those of technetium-99m macroaggregated albumin (MAA) in predicting the therapeutic 90Y (Rx90Y) dose for patients with hepatocellular carcinoma (HCC).
Materials and Methods:
This prospective single-arm clinical trial (Clinicaltrials.gov: NCT04172714) recruited patients with HCC. Patients underwent same-day mapping with MAA and scout90Y. Rx90Y activity was administered 3 days after mapping. Using paired t test and Pearson correlation, the tumor-to-normal ratio (TNR), lung shunt fraction (LSF), predicted mean tumor dose (TD), and nontumoral liver dose (NTLD) by MAA and scout90Y were compared with those by Rx90Y. Bland-Altman plots compared the level of agreement between the TNR and LSF of scout90Y and MAA with that of Rx90Y. The safety of scout90Y was evaluated by examining the discrepancy in extrahepatic activity between MAA and scout90Y.
Results:
Thirty patients were treated using 19 segmental and 14 nonsegmental (ie, 2 contiguous segments or nonsegmental) therapies. MAA had weak LSF, moderate TNR, and moderate TD linear correlation with Rx90Y. Scout90Y had a moderate LSF, strong TNR, strong TD, and very strong NTLD in correlation with those of Rx90Y. Furthermore, the TNR and LSF of scout90Y had a stronger agreement with those of Rx90Y than with those of MAA. In the nonsegmental subgroup, MAA had no significant correlation with the TD and NTLD of Rx90Y, whereas scout90Y had a very strong correlation with both of these factors. In the segmental subgroup, both MAA and scout90Y had a strong linear correlation with the TD and NTLD of Rx90Y.
Conclusions:
Compared with MAA, scout90Y is a more accurate surrogate for Rx90Y biodistribution for nonsegmental therapies.
Most patients with hepatocellular carcinoma (HCC) are unable to undergo liver transplantation or curative resection (1), necessitating the use of liver-directed therapies, including yttrium-90 (90Y) radioembolization.
A critical factor in optimizing radioembolization therapy is the delivery of a sufficient radioactive dose to the tumor. A recent study by Gabr et al (2) examining explanted livers indicated that in patients treated with glass 90Y microspheres (Therasphere; Boston Scientific, Marlborough, Massachusetts), complete pathological necrosis is more likely to occur when a dose of >400 Gy is delivered to the tumor. Garin et al (3) also demonstrated that personalized dosimetry with single-photon emission computed tomography (SPECT) or computed tomography (CT) with a goal of ≥205 Gy to a targeted HCC tumor with glass 90Y resulted in prolonged survival. Additionally, a post hoc dosimetric analysis of the SorAfenib versus Radio-loembolization in Advanced Hepatocellular Carcinoma (SARAH) trial demonstrated significantly prolonged overall survival among patients with tumors treated with ≥100 Gy compared with that among patients treated with other doses (4). The aforementioned studies and other emerging data have led to increased emphasis on prospective treatment planning with adequate target dose in recent years.
Despite the routine use of technetium-99m (99mTc) macroaggregated albumin (MAA) for planning purposes, evidence suggests that this radiotracer is a suboptimal surrogate for 90Y microspheres (5). Several studies (6-9) have demonstrated that 99mTc MAA is an inconsistent or inaccurate predictor of 90Y microsphere biodistribution, with a significant discrepancy in a substantial percentage of patients owing to size differences, particle number differences, and clumping during delivery. Because tumor dose (TD) directly affects patient outcomes, predictive dosimetric inaccuracies with MAA may result in worsened tumor response, survival, and toxicity, particularly in lobar setting.
Low-dose “scout” 90Y microspheres administered during angiographic mapping may serve as a more accurate marker for 90Y biodistribution and as a bioidentical surrogate. In this prospective single-arm clinical trial, the accuracy and safety of scout90Y administration were compared with those of 99mTc MAA administration during 90Y preprocedural mapping.
MATERIALS AND METHODS
Study Design and Patient Selection
This study was a prospective single-arm clinical trial (clinicaltrials.gov identifier: NCT04172714). The study was approved by the Emory Institutional Review Board. Written consent was obtained for each participant.
All patients recruited were those with treatment-naïve, surgically unresectable HCC and deemed to benefit from 90Y radioembolization as either a potential curative or palliative therapy by a multidisciplinary institutional tumor board. The inclusion criteria involved adult patients with the following criteria: (a) life expectancy of ≥6 months, (b) presence of HCC confirmed by Liver Reporting and Data System (LIRADS) on CT or magnetic resonance (MR) imaging (10), (c) ≤3 lesions confined to a single lobe, (d) a targeted lesion measuring ≥2 cm and ≤8 cm, (e) absence of extrahepatic metastatic disease, (f) and an Eastern Cooperative Oncology Group (ECOG) performance status of <2. The required laboratory values included a bilirubin level of ≤2 mg/dL for nonsegmental (ie, 2 contiguous segments or lobar) therapies and ≤3 mg/dL for segmental therapies, an albumin level of ≥3 g/dL, a prothrombin time/international normalized ratio of <2, aspartate aminotransferase and alanine transaminase levels of ≤3 times the upper limit of the normal range, and a platelet count of >50,000/μL. The exclusion criteria included patients with an elevated lung shunt fraction (LSF) by MAA, which would result in dose modification, thereby resulting in an inadequate TD.
Baseline Demographics
From December 2019 to January 2021, 30 patients with 33 HCCs were recruited and treated (Table 1). The mean tumor volume was 44.9 cm3. The mean age was 66.4 years, with 90.3% of patients being men. The most common etiology of HCC was hepatitis C (54.8%), followed by alcoholic liver cirrhosis (22.6%). The mean baseline model for end-stage liver disease and albuminbilirubin scores were 10 and −2.62, respectively. The ECOG performance status was 0, and the Child-Pugh score was A in 77.4% of patients (Table 1). Overall, 19 tumors were treated with radiation segmentectomy, and 14 tumors were treated in a nonsegmental fashion. The mean tumor volume in the segmental subgroup was 33.7 cm3 (median, 21.8 cm3; SD ± 30.3 cm3) and that in the nonsegmental subgroup was 58.4 cm3 (median, 16.8 cm3; SD ± 92.7 cm3).
Table 1.
Baseline Characteristics of the Entire Cohort
| Variable | Frequency | Percentage |
|---|---|---|
| Age (y) | ||
| Mean | 66.4 | |
| Median | 68.2 | |
| Male sex | 28 | 90.3 |
| Etiology of HCC | ||
| Hepatitis B virus | 4 | 12.9 |
| Hepatitis C virus | 17 | 58.8 |
| EtOH | 7 | 22.6 |
| NAFLD | 4 | 12.9 |
| MELD | ||
| Mean | 10 | |
| Median | 10 | |
| Child-Pugh class | ||
| A | 24 | 77.4 |
| B | 6 | 19.4 |
| ALBI | ||
| Mean score | −2.65 | |
| Grade | ||
| 1 | 17 | 54.8 |
| 2 | 13 | 41.9 |
| ECOG PS | ||
| 0 | 24 | 77.4 |
| 1 | 6 | 19.4 |
| 2 | 0 | 0.0 |
| 3 | 0 | 0.0 |
| 4 | 0 | 0.0 |
| Radioembolization type by tumor | ||
| Segmental | 19 | 58 |
| Nonsegmental | 14 | 42 |
| Tumor distribution | ||
| Solitary | 27 | 90 |
| Multifocal | 3 | 10 |
| Scout90Y activity (GBq) | ||
| Mean | 0.56 | |
| Median | 0.56 | |
| 90Y therapeutic activity (GBq) | ||
| Mean | 1.17 | |
| Median | 0.78 | |
| BCLC Stage | ||
| A | 16 | 53.3 |
| B | 9 | 30.0 |
| C | 5 | 16.7 |
ALBI = albumin-bilirubin; BCLC = Barcelona Clinic Liver Cancer; ECOG PS = Eastern Cooperative Oncology Group performance status; EtOH = ethanol; HCC = hepatocellular carcinoma; MELD = model for end-stage liver disease; NAFLD = non-alcoholic fatty liver disease.
Two patients required coil embolization of the right gastric artery before MAA administration. The mean scout90Y activity administered was 0.56 GBq, and the mean therapeutic 90Y (Rx90Y) activity was 1.17 GBq (Table 1). The mean lung dose for the entire cohort was 6.8 Gy (median, 5.8 Gy; SD ± 4.7 Gy). The mean lung dose for the segmental subgroup was 7.2 Gy and that of nonsegmental subgroup was 6.3 Gy.
Angiographic Mapping Procedures
All angiographic procedures were performed by a fellowship-trained interventional radiologist (N.K.) with at least 5 years of experience. All patients underwent prospective angiographic mapping as described in previous literature (11), using coaxial arterial access with a 5-F base catheter and a 2.4–2.8-F microcatheter. This included the use of cone-beam CT with real-time 3-dimensional reconstruction performed in all cases to evaluate tumoral supply to the targeted tumor(s) and ensure complete perfusion of the tumor. From the microcatheter location(s) perfusing the entirety of the tumor, 148 MBq of 99mTc MAA was administered for nonsegmental therapy and 74 MBq of 99mTc MAA was administered for segmental therapy. If 2 segmental administrations were needed, 74 MBq was administered in each supplying vessel. The catheters were then removed. The access site was secured and left in place with a running heparinized saline. The patient was then transferred to the nuclear medicine department to undergo 99mTc MAA planar and SPECT/CT scans. After scanning, the patient was brought back to the interventional radiology department for the second mapping procedure. A typical patient flowchart used in the study is depicted in Figure 1.
Figure 1.
Study flowchart for each recruited patient. 90Y = yttrium-90; CBCT = cone-beam computed tomography; CT = computed tomography; f/u = follow-up; HCC = hepatocellular carcinoma; MAA = macroaggregated albumin; MR = magnetic resonance; PET = positron emission tomography; SIRT = selective internal radiation therapy; SPECT = single-photon emission computed tomography; Tc99 = technetium-99m; w/ = with.
Using the aforementioned techniques, the second mapping procedure was performed using resin 90Y microspheres (SIR-Spheres; Sirtex, Woburn, Massachusetts). From the same microcatheter location and using the same type of microcatheter that was used in MAA mapping, 0.56-GBq 3-day precalibrated 90Y microspheres were administered as the scout dose (scout90Y). This equated to 3.24 million 90Y microspheres administered at mapping for a single dose. If there was dual blood supply to the tumor, 0.37 GBq of 90Y was administered to each artery, totaling to 4.32 million microspheres for patients who required a split dose. The 0.56 GBq of scout activity was determined on the basis of a previously conducted phantom study (12) demonstrating that 0.48 GBq of 90Y activity was needed for accurate imaging on both SPECT and positron emission tomography (PET).
Catheters and sheaths were then removed. Hemostasis was achieved with a transradial band or a femoral artery closure device. The patient was then transferred to the nuclear medicine department for 90Y PET with CT (Fig 1), after which they were discharged home.
LSF and Dosimetry Analysis
All imaging evaluation was performed by nuclear medicine physicians and physicists (D.B. and I.S.) with more than 10 years of experience. The LSF of 99mTc MAA was assessed using planar and SPECT/CT. Attenuation-corrected images of the liver and lungs obtained using SPECT/CT were used for treatment planning, including LSF calculation, over the classically performed planar scintigraphy, given its higher accuracy. Briefly, semiautomated segmentation of the liver and lung parenchyma with manual manipulation, if needed, was performed with MIM, version 6.9 (MIM Software, Beachwood, Ohio) (13). MAA tumor-to-normal liver ratio (TNR) was also calculated using SPECT/CT. The perfused area of the liver and the targeted tumor(s) was contoured. Using the respective volume and activity count in each of the compartment, the TNR was calculated.
The LSF and TNR for the scout90Y and Rx90Y were assessed with PET/CT using the aforementioned method. PET/CT was selected over 90Y SPECT/CT because of its ability to provide high-resolution images, as validated by several studies (13,14).
Prospective Dosimetry Treatment Planning
The therapeutic 90Y activity to be administered was calculated based on MAA TNR and LSF data from SPECT/CT as the current standard of care. A partition model was used to calculate 90Y dosages in all patients who underwent nonsegmental therapies with the goal of delivering a >200 Gy dose to tumors and <70 Gy dose to the perfused nontumoral liver (15). A single-compartment dose of 200 Gy to the segment was used for segmentectomies. A dose of the prescribed 90Y activity minus the scout (.56 GBq) activity was then administered within 3 days from the mapping procedures for Rx90Y. All dosimetry planning was performed using MIM SurePlan (MIM Software) (13).
Therapeutic Selective Internal Radiotherapy Procedure
Patients underwent Rx90Y 3 days after the mappings as an outpatient. Using the aforementioned technique and from the same microcatheter location where MAA and Scout90Y were administered and using the same type of microcatheter, the prescribed activity was administered using 3-day precalibrated resin 90Y for segmentectomies or 2 day precalibrated resin 90Y for nonsegmental therapies. The patient was then transferred to the nuclear medicine department for 90Y PET/CT to confirm adequate tumor coverage and assess for extrahepatic activity (Fig 1).
Accuracy of 99mTc MAA versus Scout90Y in Predicting Dosimetry after Therapeutic 90Y Radioembolization
Using voxel-based dosimetry, the prescribed activity and biodistribution of MAA and scout90Y, which included the predicted mean TD and nontumoral liver dose (NTLD), were calculated with SurePlan. The TNR and LSF from MAA and scout90Y were used in the partition model using the cumulative prescribed 90Y activity (scout + therapeutic) to calculate the respective predicted TD and NTLD.
Safety of Scout90Y versus 99mTc-MAA
The safety of the scout dose resin microsphere was evaluated by determining nontarget embolization seen on scout90Y PET that was not detected and mitigated during mapping cone-beam CT and evaluating for a discrepancy in extrahepatic activity between MAA SPECT/CT and scout dose 90Y PET/CT. All adverse events were reported using Common Terminology for Clinical Adverse Events (CTCAE), version 5 (16). Furthermore, the potential embolic effect of scout90Y was evaluated, as outlined in Appendix A (available online on the article’s Supplemental Material page at www.jvir.org).
Statistical Analysis
The mean TNR and LSF and dose predicted by MAA (MAA TD and MAA NTLD) and scout90Y (scout TD and scout NTLD) were compared with those of Rx90Y using the paired Student t test. The linear correlation coefficients between these variables were compared using the Pearson correlation coefficient. The degree of linear correlation was defined as follows: 0–0.3, very weak; 0.3–0.5, weak; 0.5–0.7, moderate; 0.7–0.9, strong; and 0.9–1.0, very strong. Subanalyses for all dosimetric factors were then performed for patients who underwent segmentectomy versus those treated with nonsegmental treatments. Additionally, Bland-Altman plots were created to evaluate the degree of agreement between the TNR and LSF of Rx90Y and those of MAA and scout90Y. Bland-Altman analysis was performed using R software, version 4 (R Foundation for Statistical Computing, Vienna, Austria) (Table E1, available online at www.jvir.org). All other statistical analyses were performed using SPSS Software, version 28 (IBM, Armonk, New York).
RESULTS
Tumor-to-Normal Liver Ratio
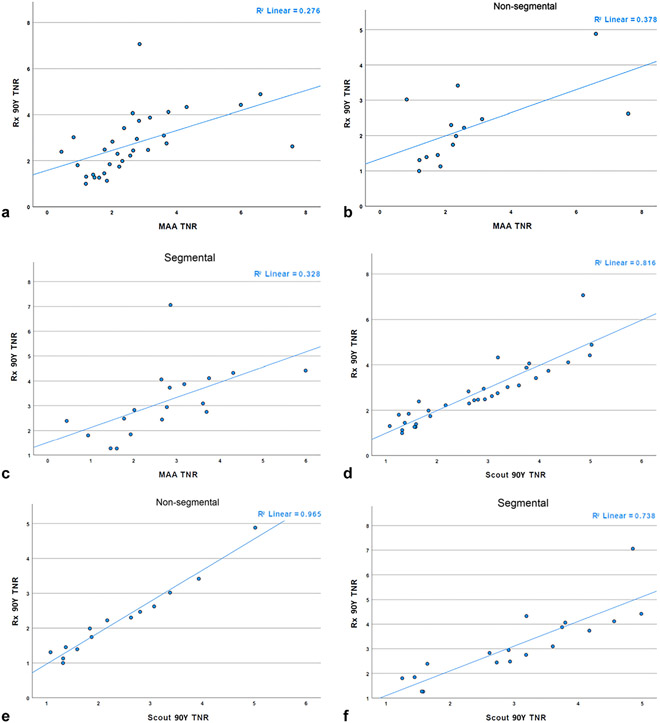
For the entire cohort, the mean TNR for MAA, scout90Y, and Rx90Y was 2.67, 2.75, and 2.74, respectively (for all, P > .05). There was a moderate linear correlation between the TNRs of MAA and Rx90Y (r = 0.53; P = .002) and a very strong linear correlation between the TNRs of scout90Y and Rx90Y (r = 0.904; P = .001) (Table 2 and Fig 2a, d)
Table 2.
Tumor-to-Normal Liver Ratio and Lung Shunt Fraction Data: Entire Cohort and Nonsegmental and Segmental Groups
| Variable | Mean TNR | Paired t test with Rx90Y (P value) |
Correlation with Rx90Y (r) |
P value | Mean LSF (%) |
Paired t test with Rx90Y (P value) |
Correlation with Rx90Y (r) |
P value |
|---|---|---|---|---|---|---|---|---|
| Entire cohort: MAA | 2.67 | .796 | 0.53 | .020 | 5.68 | .418 | 0.39 | .031 |
| Entire cohort: Scout90Y | 2.75 | .893 | 0.90 | <.001 | 5.79 | .058 | 0.76 | <.001 |
| Entire cohort: Rx90Y | 2.74 | 5.21 | ||||||
| Nonsegmental: MAA | 2.65 | .312 | 0.62 | .019 | 5.67 | .653 | 0.53 | .062 |
| Nonsegmental: Scout90Y | 2.38 | .015 | 0.98 | <.001 | 6.31 | .023 | 0.89 | <.001 |
| Nonsegmental: Rx90Y | 2.21 | 5.25 | ||||||
| Segmental: MAA | 2.69 | .136 | 0.57 | .013 | 5.68 | .511 | 0.19 | .471 |
| Segmental: Scout90Y | 3.04 | .525 | 0.86 | <.001 | 5.39 | .598 | 0.43 | .083 |
| Segmental: Rx90Y | 3.15 | 5.18 |
90Y = yttrium-90; BCLC = Barcelona Clinic Liver Cancer; LSF = lung shunt fraction; MAA = macroaggregated albumin; Rx90Y = therapeutic yttrium-90; TNR = tumor-to-normal liver ratio.
Figure 2.
Correlation of tumor-to-normal liver ratio (TNR) by yttrium-90 (90Y) mapping and 90Y treatment type: (a) macroaggregated albumin (MAA) versus therapeutic 90Y (Rx90Y) entire cohort, (b) MAA versus Rx90Y nonsegmental treatment, (c) MAA versus Rx90Y segmental treatment, (d) Scout90Y versus Rx90Y entire cohort, (e) Scout90Y versus Rx90Y nonsegmental treatment, and (f) Scout90Y versus Rx90Y segmental treatment.
For the nonsegmental subgroup, the mean TNR was not significantly different between MAA and Rx90Y (2.65 vs 2.21; P = .312); whereas, there was a statistically significant difference between the TNRs of scout90Y and Rx90Y (2.38 vs 2.21; P = .015). However, in the same subgroup, the TNR of MAA was moderately correlated with the TNR of Rx90Y (r = 0.615; P = .019), and the TNR of scout90Y was very strongly correlated with the TNR of Rx90Y (r = 0.982; P < .001) (Table 2 and Fig 2).
For the segmental subgroup, the TNR was 2.69 for MAA, 3.04 for scout90Y, and 3.15 for Rx90Y, which were not statistically different (for all, P > .05). Again, there was a moderate correlation of MAA TNR (r = 0.57; P = .013) and a high correlation of Scout90Y TNR with Rx90Y TNR (r = 0.86; P < .001) (Table 2 and Fig 2).
Additionally, the descriptive Bland-Altman analysis demonstrated that the TNR of scout90Y for the entire cohort had a clearly superior agreement with the TNR of Rx90Y (mean difference, −0.01; standard difference, 0.57) compared with that of MAA TNR with Rx90Y TNR (mean difference, 0.07; standard difference, 1.45) (Fig E1, available online at www.jvir.org).
Lung Shunt Fraction
For the entire cohort, the LSF for MAA, scout90Y, and Rx90Y was 5.7%, 5.8%, and 5.2%, respectively (for all, P > .05). There was a weak linear correlation between the LSF of MAA and the LSF of Rx90Y (r = 0.39; P = .032) and a strong linear correlation between the LSF of scout90Y and the LSF of Rx90Y (r = 0.76; P < .001) (Table 2; Fig E2, available online at www.jvir.org).
In the nonsegmental subgroup, the mean LSF was not statistically different between MAA (5.7%) and Rx90Y (5.2%; P = .65) but was different between scout90Y (5.8%) and Rx90Y (5.2%; P = .02). However, there was no correlation between the LSF of MAA and the LSF of Rx90Y (r = 0.53; P = .062); whereas, there was a strong correlation between the LSF of scout90Y and the LSF of Rx90Y (r = 0.885; P < .001) (Table 2; Fig E2, available online at www.jvir.org).
For the segmentectomies, the mean LSFs were not statistically different (MAA, 5.6%; scout90Y, 5.4%; and Rx90Y, 5.2%; for all, P > .05). There was no significant correlation between the LSF of MAA and the LSF of Rx90Y (P = .47). However, there was a week correlation between the LSF of scout90Y and the LSF of Rx90Y (r = 0.43; P = .042) (Table 2; Fig E2, available online at www.jvir.org).
The descriptive Bland-Altman analysis demonstrated that the LSF of scout90Y for the entire cohort had a marginally superior agreement with the LSF of Rx90Y (mean difference, −0.01; standard difference, 0.02) compared with that of MAA LSF with Rx90Y LSF (mean difference, 0; standard difference, 0.03) (Fig E1, available online at www.jvir.org).
Mean TD Prediction using MAA and Scout90Y
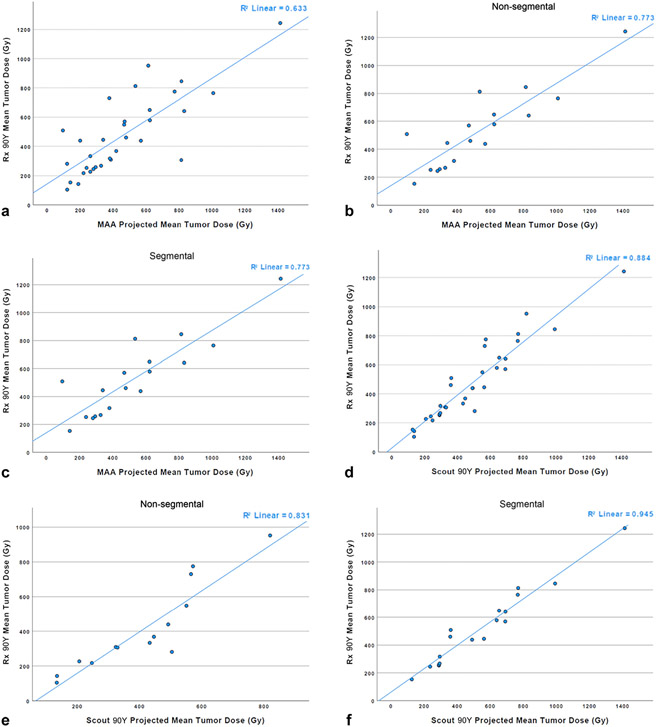
For the entire cohort, the mean TDs predicted using MAA and scout90Y were 474 and 470 Gy, respectively, and were not statistically different from the TD of Rx90Y, which was 491 Gy (for all, P > .05). There was a moderate linear correlation between the TD of MAA and the TD of Rx90Y (r = 0.74; P < .001) and a very strong linear correlation between the TNR of scout90Y and the TNR of Rx90Y (r = 0.900; P < .001).
For the nonsegmental subgroup, there was no significant difference between the mean TDs (396 Gy for MAA vs 404 Gy for scout90Y vs 412 Gy for Rx90Y; for all, P > .05). The TD of scout90Y was very strongly correlated with the TD of Rx90Y (r = 0.93; P < .001), whereas MAA had no correlation with Rx90Y (r = 0.341; P = .232) (Table 3 and Fig 3).
Table 3.
Tumor Dose and Nontumor Liver Dose
| Variable | Mean TD (Gy) |
Paired t test with Rx90Y (P value) |
Correlation with Rx90Y (r) |
P value |
Mean NTLD (Gy) |
Paired t test with Rx90Y (P value) |
Correlation with Rx90Y (r) |
P value |
|---|---|---|---|---|---|---|---|---|
| Entire cohort: MAA | 474 | .68 | 0.74 | <.001 | 117 | .003 | 0.93 | <.001 |
| Entire cohort: Scout90Y | 470 | .44 | 0.9 | <.001 | 91 | .163 | 0.97 | <.001 |
| Entire cohort: Rx90Y | 491 | 87 | ||||||
| Nonsegmental: MAA | 396 | .83 | 0.341 | .232 | 67 | .14 | 0.44 | .114 |
| Nonsegmental: Scout90Y | 404 | .75 | 0.93 | <.001 | 62 | .19 | 0.95 | <.001 |
| Nonsegmental: Rx90Y | 412 | 61 | ||||||
| Segmental: MAA | 534 | .71 | 0.87 | <.001 | 164 | .005 | 0.92 | <.001 |
| Segmental: Scout90Y | 521 | .49 | 0.91 | <.001 | 117 | .23 | 0.97 | <.001 |
| Segmental: Rx90Y | 552 | 110 |
90Y = yttrium-90; MAA = macroaggregated albumin; NTLD = nontumor liver dose; Rx90Y = therapeutic yttrium-90; TD = tumor dose. Statistically significant values are set in bold.
Figure 3.
The correlation of mean tumor dose by yttrium-90 (90Y) mapping and 90Y treatment type: (a) macroaggregated albumin (MAA) versus therapeutic 90Y (Rx90Y) entire cohort, (b) MAA versus Rx90Y nonsegmental treatment, (c) MAA versus Rx90Y segmental treatment, (d) Scout90Y versus Rx90Y entire cohort, (e) Scout90Y versus Rx90Y nonsegmental treatment, and (f) Scout90Y versus Rx90Y segmental treatment.
For the segmental subgroup, the mean predicted TDs by MAA (534 Gy) and scout90Y (521 Gy) were statistically similar to that of Rx90Y (552 Gy; for all, P > .05). Additionally, MAATD had a strong correlation with Rx90Y TD (r = 0.87; P < .001), whereas scout90Y TD was very strongly correlated with Rx90Y TD (r = 0.91; P < .001) (Table 3 and Fig 3).
Mean NTLD Prediction by MAA and Scout90Y
For the entire cohort, NTLDs predicted by MAA were significantly different from those predicted by Rx90Y (117 vs 87 Gy; P = .003), whereas the predicted NTLDs by scout90Yand Rx90Y were similar (91 vs 87 Gy; P = .163). The scout90Y NTLD was very strongly correlated with the Rx90Y NTLD (r = 0.972; P < .001). In addition, there was a very strong linear correlation between the MAA NTLD and the Rx90Y NTLD (r = 0.933; P < .001).
In the nonsegmental subgroup, NTLDs were not significantly different among MAA, scout90Y, and Rx90Y (67, 62.2, and 61.1 Gy, respectively; P = .19). However, the MAA NTLD had no correlation with the Rx90Y NTLD (r = 0.442; P = .114), whereas the scout90Y NTLD had a very strong correlation with the Rx90Y NTLD (r = 0.95; P < .001) (Fig E3, available online at www.jvir.org).
In the segmentectomy subgroup, the mean MAA NTLD was significantly higher than the mean Rx90Y NTLD (164 vs 111 Gy; P < .001). There was no statistically significant difference in the mean scout90Y NTLD and Rx90Y NTLD (117 vs 110 Gy; P = .228) (Table 3; Fig E3, available online at www.jvir.org). Both the MAA NTLD (r = 0.92) and scout90Y NTLD (r = 0.97) had a very strong linear correlation with the Rx90Y NTLD (for all, P < .001) (Table 3; Fig E3, available online at www.jvir.org).
Safety
No extrahepatic nontarget embolization was seen on scout90Y PET. Specifically, there was no discrepancy between extrahepatic activity seen on MAA SPECT/CT and scout90Y PET/CT. Additionally, there was no nontarget embolization seen on Rx90Y PET/CT. The clinical and laboratory adverse events at 3 and 6 months after 90Y are reported in Table 4. The embolic effect of scout90Y is outlined in Appendix A (Fig E4, available online at www.jvir.org).
Table 4.
Frequencies and Percentages of Any Adverse Event Reported after Yttrium-90 Radioembolization at 3 and 6 Months
| Toxicity | Frequency of any CTCAE |
|||
|---|---|---|---|---|
| 3 mo | % | 6 mo | % | |
| Clinical toxicities | ||||
| Fatigue | 2 | 7 | 1 | 3 |
| Abdominal pain | 2 | 7 | 4 | 13 |
| Ascites | 1 | 3 | 2 | 7 |
| Encephalopathy | 1 | 3 | 1 | 3 |
| Anorexia | 0 | 0 | 0 | 0 |
| Fever | 0 | 0 | 0 | 0 |
| Nausea | 3 | 10 | 0 | 0 |
| Vomiting | 0 | 0 | 1 | 3 |
| Death | 1 | 3 | 1 | 3 |
| Any CTCAE | 6 | 20 | 4 | 13 |
| Severe >3 CTCAE | 0 | 0 | 1 | 3 |
| Laboratory toxicities | ||||
| Total bilirubin | 5 | 17 | 3 | 10 |
| ALT | 1 | 3 | 2 | 7 |
| AST | 4 | 13 | 2 | 7 |
| Alkaline phosphatase | 5 | 17 | 1 | 3 |
| Albumin | 4 | 13 | 3 | 10 |
| Creatinine | 3 | 10 | 2 | 7 |
| Hyponatremia | 7 | 23 | 2 | 7 |
| WBC | 3 | 10 | 1 | 3 |
| Anemia | 3 | 10 | 2 | 7 |
| Platelets | 7 | 23 | 5 | 17 |
| Any CTCAE | 26 | 87 | 18 | 60 |
| Severe >3 CTCAE | 6 | 20 | 4 | 13 |
Note–The one patient death over the study period was secondary to transplantation complications.
ALT = alanine transaminase; AST = aspartate aminotransferase; CTCAE = Common Terminology for Clinical Adverse Events; WBC = white blood cell.
DISCUSSION
Personalized 90Y dosimetry is critical for improving outcomes in patients with primary and metastatic liver cancers. This has led to the use of 99mTc MAA as a surrogate marker to predict doses to the tumor, liver, and lung prospectively. However, several studies (5,6,9) have demonstrated limitations with 99mTc MAA as a surrogate marker for Rx90Y. For example, a study by Wondergem et al (6) comparing 99mTc MAA SPECT and 90Y bremsstrahlung SPECT/CT found a difference in activity of >10%, 20%, and 30% in 68%, 43%, and 32% of contoured segments, respectively. When examining activity per milliliter. In another comparison of more than 1,000 lesions of various pathologies by Ilhan et al (9), 99mTc MAA SPECT/CT was found to have a weak correlation with 90Y bremsstrahlung SPECT/CT TNR. A study by Villalobos et al (5) on 190 patients with HCC treated with glass microspheres showed that the MAA TNR had a moderate correlation with that of 90Y on SPECT/CT, with a weak correlation in the segmentectomy subset and moderate correlation in lobar treatment. The result of the current study is concordant with previously published data by Villalobos et al (5), that overall, the MAA TNR has a moderate linear correlation with the Rx90Y TNR.
There are multiple proposed mechanisms by which 99mTc MAA falls short as a 90Y surrogate. MAA has significant discrepancies in size, particle number, and morphology compared with 90Y particles. Most (90%) MAA particle sizes range from 10 to 100 μm, with a mean particle diameter smaller than that of 90Y microspheres (6). Smaller MAA particles may more easily pass through hepatic capillaries, resulting in overestimation of shunting and falsely depict nontumoral uptake (17). In addition, the significantly lower number of MAA particles delivered may not accurately simulate 90Y flow dynamics (6). Finally, quality control issues related to the preparation of 99mTc and albumin particles, including clumping, can also contribute to inaccuracies (18). Additionally, a potential cause of discrepancy between MAA and 90Y in a large number of studies is the comparison of 99mTc MAA SPECT/CT to 90Y Bremsstrahlung SPECT/CT, which has a lower spatial resolution than that of 90Y PET/CT (5,6,9). In this study, to eliminate the inherent inaccuracies of 90Y SPECT/CT, the LSF and TNR of MAA SPECT/CT were compared with those of 90Y PET/CT.
The limitations of 99mTc MAA in accurately predicting 90Y biodistribution have led to investigations of more accurate surrogates (14). Biomarkers with properties more similar to those of 90Y have been proposed, such as resin or albumin microspheres labeled with positron-emitting isotopes, such as fluorine-18 and yttrium-86 (19,20). Rather than identifying other biosimilar markers, this study proposes the use of a bioidentical scout90Y as a more optimal mapping radiotracer, similar to the Holmium-166 microsphere model (17). In addition to potentially superior dosimetry data, the use of 90Y in lieu of other isotopes may be more convenient in centers that are already well adapted to handle and deliver 90Y particles.
This study demonstrated increased accuracy and the predictive value of a scout90Y when compared with 99mTc MAA particles, with a stronger correlation of LSF, TNR, TD, and NTLD to those of Rx90Y. Specifically, for nonsegmental therapies, MAA had no correlation with the mean TD or NTLD when compared with the actual corresponding doses by Rx90Y, whereas scout90Y demonstrated a very strong linear correlation for both. Accurate prospective dosimetry planning is more important in nonsegmental therapies than in segmentectomies to minimize toxic dose to the nontumoral liver. Hence, the results of this study suggest the need for the use of a more accurate surrogate for treatment planning, particularly in the nonsegmental setting.
Selection of the 0.56 GBq of activity of scout90Y was based on the result of a phantom study (12). This dose assured that, even with a hypothetical LSF of 100%, 0.56 GBq of 90Y would cause a 27 Gy dose to the lung, which is less than the recommended limit. In 2 patients, tumors in 2 segments were treated separately. In this subset of patients, 2 separate 0.37 GBq 90Y scout activities were used with no visible difference in the quality of image on 90Y PET/CT.
Other phantom studies have suggested accurate imaging of scout90Y dose with activities as low as 0.10 GBq (21). The authors acknowledge that 0.56 GBq of activity could conceivably cause treatment-related liver toxicity in patients who may not be a further 90Y candidate because of “failure” of mapping. However, this activity was chosen to ensure adequate imaging in this proof-of-concept study. Although this study had no patients with mapping failure, a larger multi-institutional study with at least 12 months follow-up will be necessary to evaluate the true toxicity effects in patients who were scouted but not treated with 90Y.
This study has several additional limitations. The sample size is relatively small; hence, small differences may not be appreciated. The nuclear medicine physicians calculating the MAA and 90Y dosimetry were not blinded, potentially introducing bias. Additionally, given the propensity of 99mTc MAA to overestimate LSF (17), subtle differences between the biomarkers may have been appreciated with a larger cohort. A larger sample size would also be useful to validate the safety profile because no cases of extrahepatic shunting were noted in this study. Furthermore, MAA may be potentially embolic, particularly in a small volume infusion. Although the authors did not observe signs of embolization after MAA injection based on visual assessment of cone-beam CT before scout90Y, this could potentially affect the TNR and LSF, particularly in segmentectomies. Another limitation is the TD calculations, which were calculated as the Rx90Y in addition to Scout90Y dosage, and no residual activities were taken into account. Lastly, the results of this resin scout90Y needs to be replicated for glass 90Y.
In conclusion, this study demonstrated that scout90Y is a safe and feasible surrogate for 90Y treatment planning in patients with HCC. It demonstrated superior predictive accuracy compared with 99mTc MAA for TNR and LSF in both segmental and nonsegmental therapies. Additionally, scout90Y demonstrated superior dosimetry prediction compared with MAA for nonsegmental therapies. Further larger randomized (MAA vs scout90Y) studies with a longer-term follow-up period are needed to elucidate the clinical benefit of using scout90Y instead of MAA.
Supplementary Material
RESEARCH HIGHLIGHTS.
In this single-arm prospective trial in which 30 patients underwent concurrent radioembolization planning using scout yttrium-90 (90Y) and technetium-99m microaggregated albumin, scout90Y treatment had a significantly higher correlation with the therapeutic 90Y tumor-to-normal ratio, lung shunt fraction, mean tumor dose, and nontumor liver dose in patients undergoing nonsegmental 90Y therapy.
There was no discrepancy in nontarget embolization between the scout90Y and technetium-99m microaggregated albumin treatments, suggesting a similar short-term safety profile.
STUDY DETAILS.
Study type:
Prospective, observational, descriptive study
Level of evidence:
4 (SIR-C)
ACKNOWLEDGMENTS
This work was partially sponsored by Sirtex Medical (Woburn, Massachusetts) through an investigator-initiated grant. Additionally, the primary investigator (N.K.) was supported by Radiological Society of North America Research Scholar Grant and Sirtex Medical Ltd. The academic study team performed the analysis and wrote the manuscript independently of the study sponsor.
ABBREVIATIONS
- CT
computed tomography
- HCC
hepatocellular carcinoma
- LSF
lung shunt fraction
- MAA
macroaggregated albumin
- 90Y
yttrium-90
- 99mTc
technetium-99m
- NTLD
nontumoral liver dose
- PET
positron emission tomography
- Rx90Y
therapeutic 90Y
- TNR
tumor-to-normal liver ratio
- TD
tumor dose
- SPECT
single-photon emission computed tomography
Footnotes
N.K. reports grant from Sirtex Medical Ltd, educational grant from Boston Medical, and consulting fees from Sirtex Medical Ltd. None of the other authors have identified a conflict of interest.
From the 2022 SIR Annual Meeting, Abstract No. 25, “Accuracy of Scout Dose Y90 for Prospective Personalized Selective Internal Radiation Therapy Planning.”
Figures E1–E4, Table E1, and Appendix A can be found by accessing the online version of this article on www.jvir.org and selecting the Supplemental Material tab.
Contributor Information
Nima Kokabi, Division of Interventional Radiology and Image-Guided hvMedicine, Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia.
Linzi A. Webster, Division of Interventional Radiology and Image-Guided hvMedicine, Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia.
Mohammad Elsayed, Division of Interventional Radiology and Image-Guided hvMedicine, Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia.
Jeffrey M. Switchenko, Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, Georgia.
Bernard Chen, Division of Interventional Radiology and Image-Guided hvMedicine, Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia.
David Brandon, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia.
James Galt, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia.
Ila Sethi, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia.
Mircea Cristescu, Division of Interventional Radiology, Department of Radiology, Medical College of Wisconsin, Milwaukee, Wisconsin.
S. Cheenu Kappadath, Division of Diagnostic Imaging, Department of Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, Texas.
David M. Schuster, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia.
REFERENCES
- 1.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015; 61:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabr A, Riaz A, Johnson GE, et al. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol imaging 2021; 48:580–583. [DOI] [PubMed] [Google Scholar]
- 3.Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021; 6:17–29. [DOI] [PubMed] [Google Scholar]
- 4.Hermann AL, Dieudonné A, Ronot M, et al. Relationship of tumor radiation-absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with 90Y in the SARAH study. Radiology 2020; 296:673–684. [DOI] [PubMed] [Google Scholar]
- 5.Villalobos A, Cheng B, Wagstaff W, et al. Tumor-to-normal ratio relationship between planning technetium-99 macroaggregated albumin and posttherapy yttrium-90 Bremsstrahlung SPECT/CT. J Vasc Interv Radiol 2021;32:752–760. [DOI] [PubMed] [Google Scholar]
- 6.Wondergem M, Smits MLJ, Elschot M, et al. 99 mTc-macroaggregated albumin poorly predicts the intrahepatic distribution of 90Y resin microspheres in hepatic radioembolization. J Nucl Med 2013; 54:1294–1301. [DOI] [PubMed] [Google Scholar]
- 7.Haste P, Tann M, Persohn S, et al. Correlation of technetium-99 m macroaggregated albumin and yttrium-90 glass microsphere biodistribution in hepatocellular carcinoma: a retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. J Vasc Interv Radiol 2017; 28:722–730.e1. [DOI] [PubMed] [Google Scholar]
- 8.Knesaurek K, Machac J, Muzinic M, DaCosta M, Zhang Z, Heiba S. Quantitative comparison of yttrium-90 (90Y)-microspheres and technetium-99 m (99 mTc)-macroaggregated albumin SPECT images for planning 90Y therapy of liver cancer. Technol Cancer Res Treat 2010; 9: 253–262. [DOI] [PubMed] [Google Scholar]
- 9.Ilhan H, Goritschan A, Paprottka P, et al. Predictive value of 99 mTc-MAA SPECT for 90Y-labeled resin microsphere distribution in radioembolization of primary and secondary hepatic tumors. J Nucl Med 2015; 56:1654–1660. [DOI] [PubMed] [Google Scholar]
- 10.Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 2018; 289:816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camacho JC, Moncayo V, Kokabi N, et al. (90)Y Radioembolization: multimodality imaging pattern approach with angiographic correlation for optimized target therapy delivery. Radiographics 2015; 35:1602–1618. [DOI] [PubMed] [Google Scholar]
- 12.Kokabi N, Sethi I, Mir D, et al. Evaluation of utility of low dose yttrium-90 for radioembolization treatment planning using SPECT/CT and PET/CT: a phantom study. Eur J Nucl Med Mol Imaging 2019; 46:S259. [Google Scholar]
- 13.Maughan NM, Garcia-Ramirez J, Arpidone M, et al. Validation of posttreatment PET-based dosimetry software for hepatic radioembolization of yttrium-90 microspheres. Med Phys 2019; 46:2394–2402. [DOI] [PubMed] [Google Scholar]
- 14.Tafti BA, Padia SA. Dosimetry of Y-90 microspheres utilizing Tc-99 m SPECT and Y-90 PET. Semin Nucl Med 2019; 49:211–217. [DOI] [PubMed] [Google Scholar]
- 15.Kim SP, Cohalan C, Kopek N, Enger SA. A guide to 90Y radioembolization and its dosimetry. Phys Med 2019; 68:132–145. [DOI] [PubMed] [Google Scholar]
- 16.NCTE Program. Common Terminology Criteria for Adverse Events (CTCAE). Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed February 6, 2022.
- 17.Elschot M, Nijsen JFW, Lam MGEH, et al. 99mTc-MAA overestimates the absorbed dose to the lungs in radioembolization: a quantitative evaluation in patients treated with 166Ho-microspheres. Eur J Nucl Med Mol Imaging 2014; 41:1965–1975. [DOI] [PubMed] [Google Scholar]
- 18.Spina JC, Hume I, Pelaez A, Peralta O, Quadrelli M, Monaco RG. Expected and unexpected imaging findings after 90Y transarterial radioembolization for liver tumors. Radiographics 2019; 39:578–595. [DOI] [PubMed] [Google Scholar]
- 19.Schiller E, Bergmann R, Pietzsch J, et al. Yttrium-86-labelled human serum albumin microspheres: relation of surface structure with in vivo stability. Nucl Med Biol 2008; 35:227–232. [DOI] [PubMed] [Google Scholar]
- 20.Selwyn RG, Avila-Rodriguez MA, Converse AK, et al. 18F-labeled resin microspheres as surrogates for 90Y resin microspheres used in the treatment of hepatic tumors: a radiolabeling and PET validation study. Phys Med Biol 2007; 52:7397–7408. [DOI] [PubMed] [Google Scholar]
- 21.Kunnen B, Dietze MMA, Braat AJAT, Lam MGEH, Viergever MA, de Jong HWAM. Feasibility of imaging 90Y microspheres at diagnostic activity levels for hepatic radioembolization treatment planning. Med Phys 2020; 47:1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.