Abstract
Objective
We conducted a pilot study assessing the feasibility of a personalized out-of-pocket cost communication, remote financial navigation, and counseling (CostCOM) intervention in cancer patients.
Methods
Twenty-three adult, newly diagnosed cancer patients at a single community oncology practice were asked to complete a survey and participate in a CostCOM intervention, including patient-specific out-of-pocket cost communication, remote financial navigation, and counseling. Feasibility was defined as patient participation in CostCOM, and its impact on financial worry measured using the Comprehensive Score for Financial Toxicity (COST) (higher score = less worry) was assessed. Eight patients’ and two providers’ experience with CostCOM was evaluated using qualitative interviews.
Results
Mean patient age was 61 (78.3% female; 100% white). Of 23 CostCOM patients, 86.9% completed CostCOM, 60% of them completed a financial assistance application, and 25% of those who applied were enrolled in a co-pay assistance program. Patients’ financial worry significantly improved following CostCOM (COST score of 10.0 ± 9.6 at enrollment vs. 16.9 ± 8.1 at follow-up; p < 0.001). Mean general satisfaction (out of 5) with CostCOM was 4.1 ± 0.7. In qualitative interviews following OOPC communication, 75% felt a positive impact on their mental health, and all patients reported no change in their treatment plan; 83.3% found financial navigation beneficial. In providers’ interviews, buy-in from relevant stakeholders, integration of the CostCOM with existing workflow, and larger studies to assess the effectiveness of CostCOM were identified as factors needed for CostCOM implementation in practice.
Conclusion
CostCOM interventions are feasible and acceptable and decrease financial worry in patients with cancer.
Keywords: Financial hardship, Cancer, Out-of-pocket communication, Financial navigation
Introduction
Cancer is associated with financial hardship due to high medical out-of-pocket costs (OOPC), lost productivity, and changes in employment, income, and insurance [1-4]. Financial hardship is characterized by care non-adherence, material hardship (e.g., medical debt), and financial worry [5] and is linked to poor quality of life (QOL) and survival as well as low care satisfaction in cancer patients [6-9].
With increased cost-sharing, many patients experience higher rates of unexpected medical bills [10-12] and are therefore interested in obtaining OOPC before receiving medical services [13]. To facilitate access to costs, steer patients toward lower-cost care, and decrease overall healthcare spending [14, 15], the Centers for Medicare and Medicaid Services (CMS) mandated price transparency. However, the current mandate is limited only to disclosure of charges, negotiated and cash prices for 300 shoppable services in a patient-friendly format as opposed to OOPCs for different tests and treatments a patient may need. Furthermore, financial navigation and counseling which have also been shown to mitigate costs of care [16-19] may be needed in tandem with price transparency interventions.
We aimed to test the feasibility of OOPC communication and financial navigation (CostCOM) among patients with cancer, partnering with a commercially available price estimator and financial navigation platform, TailorMed Medical Inc. [20]. We further explored the impact of CostCOM intervention on patients’ short-term financial worry.
Methods
This study was conducted according to the guidelines in the Declaration of Helsinki. Procedures for obtaining informed consent and protecting participants were approved and monitored by the Institutional Review Board (IRB#. STUDY00002218). The study was Health Insurance Portability and Accountability Act (HIPAA) compliant. Written informed consent was obtained from all participants. A data use agreement was obtained to allow for sharing of patient information among institutions involved.
Study population
Patients were studied eligible if they were English speaking, 18 years or older, within 90 days of a new diagnosis of any solid cancer of any stage, being treated at a single community oncology practice in Tennessee, had at least one visit at the medical oncology clinic, and were either receiving or planned to receive anticancer therapy (surgery, radiation therapy, chemo or biologic therapy) within 30 days after enrollment. Patients with indolent cancer undergoing observation alone including active surveillance, those with an ECOG performance status > 2, or receiving palliative care or hospice alone were excluded.
Price estimator and financial navigation platform
Before study enrollment, we worked with TailorMed to launch the online HIPPA-compliant estimator and navigation platform at the community oncology practice. The launch of the platform took 5 weeks and included creating a practice-specific interface, user login, and passwords for the practice research team, and training of study coordinators on how to use the platform. At the time of the study, TailorMed did not have access to our practice-negotiated prices with payers. Therefore, OOPCs were estimated using patients’ insurance benefits and Medicare allowable rates.
Patient recruitment
Patients were recruited between May 26, 2021, and September 14, 2021. Eligible patients were identified by the practice study coordinator from outpatient oncology clinics and were introduced to the study and consented during their clinic visit. For patients who declined to participate, limited demographic information was collected from their clinic visits.
At baseline, all patients were asked to complete a 20-min online or paper survey of their sociodemographic, insurance, and clinical information and baseline financial hardship. Additionally, patient insurance information, ICD-10 diagnoses, and ordered medications were entered in the TailorMed platform by the study coordinator. Patients were scheduled for a session with a remote TailorMed financial counselor. All patients received usual care which included routine oncology visits, use of available ancillary staff, and internal or external resources for co-pay assistance or free medication per normal clinic procedures.
CostCOM session
CostCOM consisted of an hour of interactive one-on-one sessions with a single remote financial counselor at TailorMed within two weeks after enrollment. Sessions were offered as either phone or video (i.e., Zoom), but all patients opted for phone sessions. Each session included the following.
OOPC communication, a review of patient-specific insurance benefits and education of the estimated OOPC for anticipated ordered medications during the fiscal year. Patients were notified that the discussed OOPC may differ from the final bill and is an estimate.
Financial navigation, real-time professional guidance to identify financial assistance programs that alleviate costs of care and discuss information to improve insurance coverage. TailorMed financial navigation platform consists of a compiled list of available financial assistance programs offered by different organizations and is continuously getting updated. With details of the patient’s insurance, diagnosis, and treatment, TailorMed is able to automate the process of searching for the best financial assistance programs. With additional details of the patient’s household income and household size obtained by the financial counselor at the time of the CostCOM session, the search will be refined to the programs that the patient is most eligible to apply for.
Financial counseling to address patients’ financial concerns and enroll them in financial assistance programs. Fidelity was assessed by a second study coordinator who participated in all CostCOM sessions to monitor the duration and content using a checklist.
All participants had access to the TailorMed remote financial counselor contact information to initiate a phone call in case of any financial concern. Furthermore, all participants received at least one follow-up phone call by the TailorMed financial counselor within 1 to 3 months after the initial session to get an update on the status of their financial assistance application, get enrolled in new financial assistance programs if available, and discuss any additional financial issues.
All TailorMed financial counselors received training on financial navigation and best practices to use TailorMed products to optimize their use as part of their employment with TailorMed. The two financial counselors who participated in the current study received additional training regarding the intervention components to ensure consistency in intervention delivery.
Patient follow-up
All patients were surveyed one month after the CostCOM session to assess changes in financial worry and their satisfaction and experience with the CostCOM session. Additionally, eight randomly selected patients stratified by estimated OOPC for the fiscal year (n = 4 OOPCs of equal to or more than $2500 and n = 4 OOPC of less than $2500) participated in 60-min one-on-one qualitative interviews through phone within one to three months after receipt of the intervention to assess their experience with the CostCOM session and barriers and facilitators to participation. Participants in the qualitative interviews were mailed $40 gift cards.
Provider follow-up
After completion of all patient follow-ups, two practice providers (study coordinator and practice oncologist) were surveyed to assess practice adoption of intervention using the Organizational Readiness for Implementation Change (ORIC) scale (score 1–5; higher score = higher readiness) [21]. They participated in 30-min one-on-one qualitative interviews to assess their thoughts on CostCOM integration with clinical workflow, barriers and facilitators to delivery, perceived intervention impact, and suggestions to ease future maintenance and dissemination. Participants in the qualitative interviews were mailed $40 gift cards.
Outcomes
Feasibility outcomes were the percentage of enrolled patients participating in the CostCOM session, enrollment in financial supportive services, and approved monetary amount of assistance.
Financial worry was measured by the 11-item Comprehensive Score for Financial Toxicity (COST) survey (score 0–44; higher score = less worry) [22, 23]. Cost-related care non-adherence was defined as a self-reported positive response to any (a) delaying, (b) foregoing, (c) stopping, or (d) changing prescribed medications due to cost, (e) refusing recommended imaging or tests, or (f) skipping office visits due to cost in the last 3 months, adapted from Medical Expenditure Panel Survey (MEPS) [24] and was measured both for cancer care as well as care related to other health conditions including preventive services. Material financial hardship was defined as a self-reported positive response to any minor conditions such as (a) withdrawal from savings or retirement accounts or (b) selling stocks or other investments, or major conditions such as home sale, refinance or move to affordable rental, or car sale, (c) incurrence of debt, and (d) bankruptcy because of cancer care, or treatment in the last 3 months, adapted from MEPS [24]. Patient satisfaction with CostCOM was assessed with an adapted Patient Satisfaction Questionnaire [25] (mean score 1–5; higher score = greater satisfaction).
Patients were queried on self-reported age, gender, race, ethnicity, marital status, education, employment status, income, and financial self-efficacy (i.e., how respondents manage certain financial problems; score = 6–24; higher score = higher self-efficacy) [26], which was previously administered in patients with cancer [4], insurance health literacy (score = 20–80; higher score = higher literacy) [27], insurance, cancer type, stage, recommended treatment, numbers of relapses, emergency department (ED) visit or inpatient hospitalization in the last 3 months, prior history of cancer diagnosis, and presence of comorbidities.
Data analyses
Descriptive statistics were used to report baseline characteristics and feasibility outcomes. For financial worry, financial self-efficacy, and health insurance literacy, we used paired t-tests to assess changes in outcomes at enrollment and post-CostCOM. All statistical tests were performed using the STATA software package (Stata/MP 17.0 for Mac; StataCorp, TX). A p value < 0.05 was considered significant.
In-depth interviews were audio-recorded and professionally transcribed verbatim. NVivo, version 12, was used to manage and analyze qualitative data. A codebook was developed based on the evaluation questions and emergent themes identified through open coding of the first few transcripts and team discussions [28]. After codebook development, each interview was coded by a primary coder, with a secondary coder checking all codes for accuracy, and discrepancies were resolved through discussion. Node reports (e.g., text associated with a specific code) were developed using NVivo to facilitate the identification of sub-themes and similarities and differences by group (i.e., estimated OOPC). Patient data were summarized, with illustrative quotes, into matrices by patients’ estimated OOPC levels to identify patterns [29].
Results
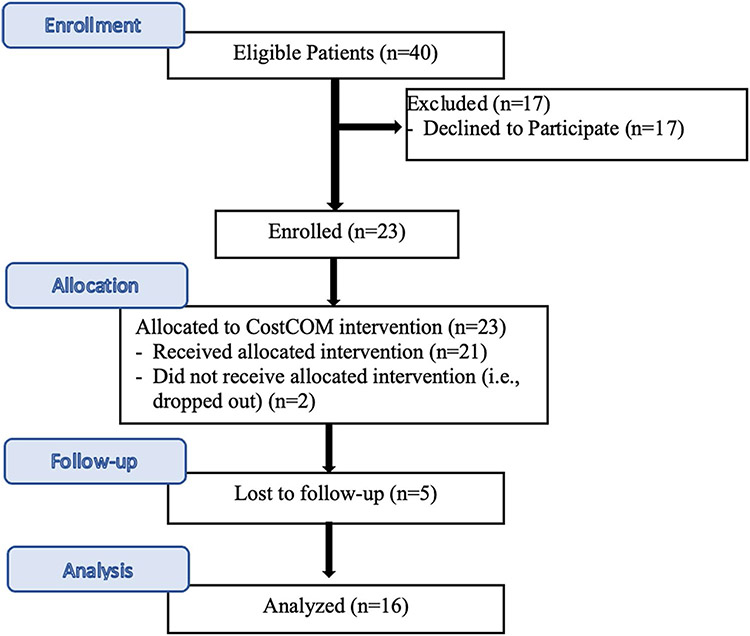
Of 40 eligible patients who were approached to participate, 23 (57.5%) consented and were enrolled (Fig. 1). There was no significant difference in age, gender, race, ethnicity, marital status, and insurance of the participants when compared to those who did not consent (n = 17). Two patients dropped out of the study, one after completing the baseline survey and the second after completing the intervention session. An additional 5 patients were lost to follow-up and did not complete the post-intervention survey. Patients who were not married (vs. those married) were more likely to be lost to follow-up after intervention (45.4% vs. 0%). There was no significant difference in other baseline characteristics as well as baseline financial worry, financial self-efficacy, and health insurance literacy of patients who completed the follow-up survey and lost to follow-up patients.
Fig. 1.
Study flowchart
Baseline demographics and clinical characteristics
Table 1 outlines the baseline characteristics of the 23 enrolled patients who completed baseline surveys. The mean age at enrollment was 61 ± 10.1 years (range 27–73), and 78.3% (n = 18) were female. All enrolled patients were White, and 4.4% (n = 1) were Hispanic. Majority (87%; n = 30) were not employed, and 70% (n = 14) had an annual household income of less than $30,000. A total of 52.2% (n = 12) had Medicare as primary insurance, followed by 34.8% (n = 8) who had commercial insurance.
Table 1.
Baseline characteristics of participants
| Total (n = 23) | |
|---|---|
| Demographics | |
| Age, years (mean ± SD) | 61.0 ± 10.1 |
| Gender | Missing = 0 |
| Male | 5 (21.7%) |
| Female | 18 (78.3%) |
| Race | Missing = 0 |
| White | 23 (100%) |
| Ethnicity | Missing = 0 |
| Hispanic, Latino, or Spanish | 1 (4.4%) |
| Not Hispanic, Latino, or Spanish | 22 (95.6%) |
| Marital status | Missing = 0 |
| Married or living with a partner | 11 (47.8%) |
| Single, separated, divorced, widowed | 12 (52.2%) |
| Education | Missing = 0 |
| High school graduate or less | 13 (56.5%) |
| More than high school graduate | 10 (43.5%) |
| Employment status | Missing = 0 |
| Full-time or part-time | 3 (13.0%) |
| Unemployed, retired, disabled | 20 (87.0%) |
| Income | Missing = 3 |
| < $30 k | 14 (70.0%) |
| > = $30 k | 6 (30.0%) |
| Insurance | Missing = 0 |
| Medicare | 12 (52.2%) |
| Medicaid | 1 (4.3%) |
| Commercial | 8 (34.8%) |
| Self-pay | 2 (8.7%) |
| Clinical characteristics | |
| Cancer type | Missing = 0 |
| Breast | 7 (30.4%) |
| Lung | 4 (17.4%) |
| GI | 6 (26.1%) |
| GU | 2 (8.7%) |
| Other | 4 (17.4%) |
| Cancer stage | Missing = 2 |
| Non-metastatic | 18 (85.7%) |
| Metastatic | 3 (14.3%) |
| Recommended cancer treatment | |
| Surgery | 12 (52.2%) |
| Chemotherapy | 23 (100%) |
| Radiation therapy | 14 (60.9%) |
| ED or inpatient hospitalization in the last 3 months | 11 (57.9%) |
| Prior history of cancer diagnosis | 4 (21.7%) |
| Presence of comorbidities | 12 (52.2%) |
| Health insurance literacy*, mean ± SD | 51.0 ± 15.5 |
| Financial self-efficacy**, mean ±SD | 11.2 ± 4.8 |
Breast cancer was the most common cancer type among enrolled patients (30.4%; n = 7), and majority of patients had non-metastatic cancer (n = 85.7%; n = 18). A total of 21.7% (n = 4) had a prior history of another cancer diagnosis, and 52.2% (n = 12) had other comorbidities.
Baseline financial hardship
At enrollment, the mean COST score for financial worry was 10.0 ± 9.6, with 77.0% (n = 20) reporting some degree of financial worry. Only 4.3% (n = 1) reported cost-related non-adherence to cancer care, which was the refusal of recommended imaging. However, 17.4% (n = 4) reported cost-related non-adherence to non-cancer-related care (i.e., medication, office visit, tests) and preventive services (8.7%). A total of 34.8% (n = 8) reported material hardship, and 50% of them were major (Table 2).
Table 2.
Baseline financial hardship of participants
| Financial hardship | Total (n = 23) |
|---|---|
| Financial worry COST score*, mean ± SD | 10.0 ± 9.6 |
| Financial worry**, n (%) | 20 (77%) |
| Cost-related cancer care non-adherence, n (%) | 1 (4.3%) |
| Cost-related non-cancer care non-adherence, n (%) | 4 (17.4%) |
| Material conditions, n (%) | 8 (34.8%) |
For financial worry, COST scores range between 0 and 44, with a higher score representing higher worry
It represents any expression of financial worry to the question: “My Illness has been a financial hardship to my family and me”
Feasibility outcomes
At enrollment, only 8.7% (n = 2) of patients reported discussion of treatment OOP cost with their healthcare provider or oncology clinic staff, 26.1% (n = 6) met a clinic financial counselor or social worker, and 17.4% (n = 4) were enrolled in financial assistance programs (free medication, assistance with transportation, or living expenses).
Of 23 patients, 86.9% (n = 20) completed the CostCOM session. Two patients dropped out of the study prior to the CostCOM session, and one patient was a no show to the session despite three rescheduling attempts. Patients had an annual median $1688 estimated out-of-pocket cost (IQR, $470–$3958) for their anticipated IV or oral anticancer therapy regimen. As part of the CostCOM intervention, a financial assistance application was completed by the counselor for 60% (n = 12) of patients participating in the CostCOM session, and 25% (n = 3/12) were enrolled in financial assistance programs (including assistance with medication co-pays, living and transportation expenses), resulting in a median of $14,500 in cost savings (a total of $49,900 for the 3 patients).
Impact of CostCOM on financial worry
Compared to financial worry at enrollment, COST scores significantly increased after the CostCOM session (i.e., financial worry decreased) (COST score of 10.0 ± 9.6 at enrollment vs. 16.9 ± 8.1 at follow-up; p < 0.001) (Table 3). While financial self-efficacy also improved from enrollment to follow-up (11.2 ± 4.8 vs. 13.2 ± 3.8), the difference was not significant (p = 0.08). Furthermore, we did not observe any significant difference in insurance health literacy between enrollment and follow-up. Care non-adherence and material hardship were not measured at follow-up.
Table 3.
Impact of CostCOM on financial worry, financial self-efficacy, and health insurance literacy among 16 participants with a follow-up
| Pre- CostCOM (baseline) |
Post-CostCOM | P value | |
|---|---|---|---|
| Financial worry cost score | 10 ± 9.7 | 16.9 ± 8.1 | < 0.001 |
| Financial self-efficacy | 11.2 ± 4.5 | 13.2 ± 3.8 | 0.08 |
| Insurance health literacy | 52.3 ± 13.9 | 52.9 ± 11.9 | 0.87 |
Patients’ experience with CostCOM
Table 4 shows patients’ experience with CostCOM. Among 16 patients with follow-up surveys, patients’ mean general satisfaction with CostCOM financial navigation was 4.1 ± 0.7 (out of 5). A total of 93.7% (n = 15) patients reported recommending the financial counseling they received to others. However, only 25% (n = 4) and 18.7% (n = 3) had a good understanding of their estimated OOP cost or financial assistance programs they might be eligible for.
Table 4.
Patients’ experience with CostCOM
| Survey results of 16 participants | N = 16 |
| Satisfaction with CostCOM, mean ± SD | |
| General satisfaction | 4.1 ± 0.7 |
| Satisfaction with counselors’ interpersonal manner | 4.5 ± 0.7 |
| Satisfaction with counselors’ communication | 4.7 ± 0.4 |
| Satisfaction with time spent with patients | 4.4 ± 0.7 |
| Recommending CostCOM financial counseling | 15 (93.7%) |
| Appropriate duration of CostCOM financial counseling | 16 (100%) |
| Good understanding of the estimated OOP cost after the CostCOM session | 4 (25%) |
| Understanding the financial assistance programs they might be eligible for | 3 (18.7%) |
| Preference for components of CostCOM to be included in the session | |
| Overview of insurance benefits | 7 (43.7%) |
| Discussion of OOP costs | 12 (75%) |
| Review of available financial assistance programs | 11 (68.8%) |
| Help with enrollment in assistance programs | 12 (75%) |
| Qualitative interview of 8 participants | N = 8 |
| Remembering OOPC communication | 3 (37.5%) |
| Impact of OOPC on the decision on a treatment plan | Missing = 2 |
| No changes in the treatment plan | 6 (100%) |
| Impact on mental health | |
| Positive impact (“felt fortunate” or “relieved”) | 6 (75%) |
| Negative impact (“felt anxious” or “scared”) | 2 (25%) |
| Remembering receipt of financial navigation | 7 (87.5%) |
| Impact of financial navigation | Missing = 2 |
| Found beneficial | 5 (83.3%) |
| Did not find beneficial | 1 (16.7%) |
Discussion of OOP costs (75% (n = 12)) and help with enrollment in assistance programs (75% (n = 12)) were the most common components of CostCOM recommended to be included in the sessions. A total of 62.5% (n = 10) reported a preference to discuss treatment OOP costs with their healthcare provider (n = 5) or oncology staff personnel (n = 5) on the day of their visit. The majority (81.2%; n = 13) also preferred to receive their OOP cost estimates written on paper and handed to them during their office visit or mailed to them as opposed to verbal communication (in person or over the phone) (50%; n = 8) or electronic communication via portal, text message, or email (43.4%; n = 7).
Qualitative interviews with patients
Of 8 patients participating in qualitative interviews, the majority (62.5%; n = 5) could not remember OOPC communication during their CostCOM session (Table 4). When queried about the impact of OOPC communication on treatment plan or mental health, those not remembering OOPC communication during CostCOM were asked to respond as “if they have had OOPC communication.” Six patients (100% of those with a response to the question) reported that OOPC discussion (even if they could not remember the CostCOM session) did not have any impact on their decision for their treatment plan; 75% (4 patients with < $2500 OOPC and 2 patients with ≥ $2500) reported that OOPC discussion had or would have had (if not remembering the communication) a positive impact on their mental health, and they “felt fortunate” or “relieved.” In contrast, 2 patients with ≥ $2500 OOPC (both of them could not remember OOPC communication) felt that OOPC discussion would have made them more “anxious” or “scared.”
The majority of patients (87.5%; n = 7) remembered receipt of financial navigation and preferred financial navigation through the phone as opposed to in person. Furthermore, 83.3% (n = 5; all of whom remembered receipt of navigation) reported that they thought the financial navigation was beneficial for the “information received,” “peace of mind,” and “financial assistance enrolled.”
Providers’ experience with CostCOM
The two participating providers’ age was 49.5 (SD, 17.7), and both were males with a mean of 22 years in practice (SD, 8). Practice usual care was described as disclosure of medication OOPC only to some patients based on their treatment, and meeting with an onsite financial navigator only if a patient requests or the provider makes a referral. However, no financial navigation platform was available.
Table 5 shows the practice’s usual care needs and barriers for improvement, areas where CostCOM intervention can be improved, barriers, facilitators, and resources needed for implementation of CostCOM in practice. Overall, the providers reported a mean of 3.5 (SD, 0.6) (out of 5) for organizational readiness to implement change, such as implementing CostCOM.
Table 5.
Providers’ experience with CostCOM
| The following areas were identified by two providers as practice needs, and CostCOM barriers and facilitators for implementation in the practice |
|---|
Needs for improvement practice usual care
|
Barriers to improvement in the practice of usual care
|
Areas where CostCOM intervention can be improved
|
Facilitators for CostCOM implementation in the practice
|
Barriers to CostCOM implementation in the practice
|
Resources needed for CostCOM implementation
|
Discussion
In this pilot study, we found that remote delivery of personalized OOPC communication, financial navigation, and counseling intervention to patients with cancer in a community oncology practice is feasible, with 86.9% of patients completing the CostCOM session, 60% of them completing a financial application program, and 25% of those with an application being enrolled in a co-pay assistance program during the 4-month study period.
Although the baseline financial worry (i.e., COST score) in our patient population was higher than what has been reported for in other cancer studies [23, 30], our result showed that patients’ financial worry improved following the CostCOM intervention, confirming that price transparency when delivered in an appropriate setting and with financial navigation can mitigate patients’ anxiety about cancer treatment. In our study, while financial self-efficacy also improved following CostCOM, it did not reach statistical significance. This suggests that our intervention may improve financial worry through improving self-efficacy, a modifiable patient variable that is shown to correlate with financial worry [31], but our study did not have sufficient statistical power.
Our qualitative interviews attempted to assess the separate effects of OOPC communication and financial navigation. Interview results showed that despite consistent, standardized communication methods regarding OOPC and financial navigation and tailoring of OOPC information to the individual patient, many participants did not remember communication of costs occurring. Of those interviewed (within one to three months after intervention), 62.5% did not remember receipt of OOPC communication; 16.7% did not remember receiving financial navigation. Furthermore, of those surveyed (within one month after intervention), only 25% and 18.7% of patients had a good understanding of their estimated OOPC and financial assistance programs they might be eligible for, respectively. Our results are consistent with prior studies showing sometimes patients do not correctly recall much of the recommendations and information given by healthcare providers or may require prompting to recall [32, 33]. This also likely reflects how overwhelming a cancer diagnosis for the patients can be and highlights the need for enhancing the CostCOM session through teach back (i.e., asking the patient to repeat information in his or her own words) and an information summary sheet of the session and follow-up sessions as the participating providers have suggested.
Curiously, most patients reported feeling “relieved” by getting information on OOPC, even among those who did not remember receipt of OOPC. The majority (83.3%) of patients found financial navigation beneficial, even among those all of whom remembered receipt of navigation services. Anxiety relief identified during the qualitative interviews is consistent with prior studies; for example, among 60 patients with head and neck cancer, 43.1% felt that talking about cancer-related costs would reduce their anxiety, and 36.2% felt it would increase their anxiety [30]. It is reassuring that our interview participants did not report changes in their treatment plan as a result of OOPC communication. Similarly, others have demonstrated that while cost discussion results in lower OOP expenditure, cost discussion did not impact the receipt of patient-reported appropriate care [34]. Future studies with larger populations are needed to better quantify any independent benefits of OOPC communication and financial navigation.
Our interviews with the providers identified the existing gaps in the practice of usual care including a lack of sufficient staff with adequate training for OOPC communication and financial navigation which results in patients meeting with the onsite financial navigation only by request. This highlights the need for standardized pathways for financial screening and referral in practices where resources are limited. Furthermore, buy-in from relevant stakeholders, integration of the proposed intervention with existing workflow, and larger studies to assess the effectiveness of CostCOM were identified as some of the factors that are needed for CostCOM implementation in practice.
While the assessment was beyond the scope of this study, financial navigation also mitigates financial losses for the health system ($2.1 million savings per year in one study) [35], facilitating financial sustainability in return for investment in financial navigation [36, 37]. In the study of 244 cancer patients at Cowell Family Cancer Center, financial navigators secured $259,593 in health system savings [37]. The average cost of commercially available price estimators and financial navigation platforms such as the one used in the current study is driven by the providers’ active patient volume, payers mix, and medication mix. However, the average cost to practice is generally compared favorably with the patient and system savings arguing for sustainability. The practices may opt to use their onsite or off-site financial counselor, who will only require training on financial navigation and best practices to use the price estimator and financial navigation platform. If a practice does not have a financial counselor, remote financial counselors are available at an additional cost.
Our study had limitations: the small sample size, a non-randomized design, and short follow-up period limited assessment of CostCOM intervention effectiveness in mitigating all domains of financial hardship. The price transparency platform did not have access to hospital-negotiated prices with payers; the OOPC estimates may have underestimated the actual OOPC for patients with commercial insurance. Integration of the platform with the practice as well as the new CMS price transparency rule allowing for access to negotiated prices would improve this process. Study participants were recruited from a single clinic and were required to communicate in English which may limit the generalizability of our findings. Although patients’ caregiver could be present at the time of intervention, we did not keep track of what percentage of participants had a caregiver present. Lastly, it is possible that the study coordinator and oncologists’ experience with the intervention is biased as they facilitated the study recruitment; however, we believe their input for future implementation of such interventions was informative.
Conclusion
Our study showed that remote delivery of intervention providing OOPC estimates tailored to patient-specific insurance benefits coupled with financial navigation is feasible among newly diagnosed cancer patients receiving care in community oncology practice. Early results suggest that this intervention decreased financial worry. Larger randomized controlled trials are needed to assess the effectiveness of the proposed intervention in mitigating financial hardship and improving patient outcomes.
Acknowledgements
Assistance with data acquisition was provided by Justin Reynolds, BS CCRP at Ballad Health Cancer Center. Assistance with IRB approval and data use agreement at Ballad Health Cancer Center was provided by Asheesh Shipstone, MD. Research reported in this publication was supported in part by the Intervention Development, Dissemination and Implementation Developing Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This work was supported by ECOG-ACRIN Cancer Care Delivery Research Pilot Grant. TailorMed Medical Inc. provided in-kind support including access to their price transparency and financial navigation platforms as well as an hour of remote financial counseling for the enrolled participants for free. TailorMed and ECOG-ACRIN had no role in the design of the study; in the collection of surveys, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Footnotes
Conflict of interest Drs. Sadigh, Coleman, and Switchenko received related research support from ECOG-ACRIN CCDR Pilot. Dr. Sadigh receives research support from the Harvey L. Neiman Health Policy Institute and an honorarium from the Journal of the American College of Radiology in her role as Associate Editor. Dr. Carlos reports a conflict of interest as GERRAF Board of Review Chair and as ECOG-ACRIN CCDR Committee Chair. Dr. Carlos reports salary support from the Journal of the American College of Radiology as Editor in Chief and funding from the National Cancer Institute during the conduct of this study.
References
- 1.Brown ML, Riley GF, Schussler N, Etzioni R (2002) Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care 40:104–117. 10.1097/00005650-200208001-00014 [DOI] [PubMed] [Google Scholar]
- 2.Short PF, Moran JR, Punekar R (2011) Medical expenditures of adult cancer survivors aged <65 years in the United States Cancer 117: 2791–2800 10.1002/cncr.25835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Tangka FK, Trogdon JG, Sabatino SA, Richardson LC (2009) The personal financial burden of cancer for the working-aged population. Am J Manag Care 15:801–806 [PubMed] [Google Scholar]
- 4.Sadigh G, Switchenko J, Weaver KE, Elchoufi D, Meisel J, Bilen MA, Lawson D, Cella D, El-Rayes B, Carlos R (2022) Correlates of financial toxicity in adult cancer patients and their informal caregivers. Support Care Cancer 30:217–225. 10.1007/s00520-021-06424-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR (2017) Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 10.1093/jnci/djw205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D (2015) Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract 11:145–150. 10.1200/JOP.2014.001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D (2016) Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol 34:1732–1740. 10.1200/JCO.2015.63.2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey SD, Bansal A, Fedorenko CR, Blough DK, Overstreet KA, Shankaran V, Newcomb P (2016) Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol 34:980–986. 10.1200/JCO.2015.64.6620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chino F, Peppercorn J, Taylor DH Jr, Lu Y, Samsa G, Abernethy AP, Zafar SY (2014) Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist 19:414–420. 10.1634/theoncologist.2013-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankaran V, Jolly S, Blough D, Ramsey SD (2012) Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: a population-based exploratory analysis. J Clin Oncol 30:1608–1614. 10.1200/JCO.2011.37.9511 [DOI] [PubMed] [Google Scholar]
- 11.Claxton G, Rae M, Panchal N, Whitmore H, Damico A, Kenward K (2014) Health benefits in 2014: stability in premiums and coverage for employer-sponsored plans Health Aff (Millwood) 33:1851–1860 10.1377/hlthaff.2014.0792 [DOI] [PubMed] [Google Scholar]
- 12.Lopes L, Kearney A, Hamel L, Bordie M. Data note: public worries about and experience with surprise medical bills. Available at: https://www.kff.org/health-costs/poll-finding/data-note-public-worries-about-and-experience-with-surprise-medical-bills/. Accessed on September 27, 2021 [Google Scholar]
- 13.Sadigh G, Carlos RC, Krupinski EA, Meltzer CC, Duszak R Jr (2017) Health care price transparency and communication: implications for radiologists and patients in an era of expanding shared decision making. AJR Am J Roentgenol 209:959–964. 10.2214/AJR.17.18360 [DOI] [PubMed] [Google Scholar]
- 14.Wu SJ, Sylwestrzak G, Shah C, DeVries A (2014) Price transparency for MRIs increased use of less costly providers and triggered provider competition. Health Aff (Millwood) 33:1391–1398. 10.1377/hlthaff.2014.0168 [DOI] [PubMed] [Google Scholar]
- 15.Sinaiko AD (2019) What is the value of market-wide health care price transparency? JAMA 322:1449–1450. 10.1001/jama.2019.11578 [DOI] [PubMed] [Google Scholar]
- 16.Sadigh G, Gallagher K, Obenchain J, Benson A 3rd, Mitchell E, Sengupta S, Carlos RC (2019) Pilot feasibility study of an oncology financial navigation program in brain cancer patients. J Am Coll Radiol 16:1420–1424. 10.1016/j.jacr.2019.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankaran V, Leahy T, Steelquist J, Watabayashi K, Linden H, Ramsey S, Schwartz N, Kreizenbeck K, Nelson J, Balch A, Singleton E, Gallagher K, Overstreet K (2018) Pilot feasibility study of an oncology financial navigation program. J Oncol Pract 14:e122–e129. 10.1200/JOP.2017.024927 [DOI] [PubMed] [Google Scholar]
- 18.Sherman DE (2017) Transforming practices through the oncology care model: financial toxicity and counseling. J Oncol Pract 13:519–522. 10.1200/JOP.2017.023655 [DOI] [PubMed] [Google Scholar]
- 19.Hung A, Blalock DV, Miller J, McDermott J, Wessler H, Oakes MM, Reed SD, Bosworth HB, Zullig LL (2021) Impact of financial medication assistance on medication adherence: a systematic review. J Manag Care Spec Pharm 27:924–935. 10.18553/jmcp.2021.27.7.924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TailorMed Medical Inc. Available at: https://tailormed.co/. Accessed on September 27, 2021
- 21.Shea CM, Jacobs SR, Esserman DA, Bruce K, Weiner BJ (2014) Organizational readiness for implementing change: a psychometric assessment of a new measure. Implement Sci 9:7. 10.1186/1748-5908-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza JA, Yap BJ, Hlubocky FJ, Wroblewski K, Ratain MJ, Cella D, Daugherty CK (2014) The development of a financial toxicity patient-reported outcome in cancer: the COST measure. Cancer 120:3245–3253. 10.1002/cncr.28814 [DOI] [PubMed] [Google Scholar]
- 23.de Souza JA, Yap BJ, Wroblewski K, Blinder V, Araujo FS, Hlubocky FJ, Nicholas LH, O’Connor JM, Brockstein B, Ratain MJ, Daugherty CK, Cella D (2017) Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST). Cancer 123:476–484. 10.1002/cncr.30369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agency for Healthcare Research and Quality. Medical expenditure panel survey. Available at: https://www.meps.ahrq.gov/mepsweb/. Accessed on September 27, 2021
- 25.Marshall GN, Hays RD. (1994) The Patient Satisfaction Questionnaire Short-Form (PSQ-18) Santa Monica, CA: RAND [Google Scholar]
- 26.Lown JM (2011) Development and validation of a financial self-efficacy scale. J Financ Couns Plan 22(2):54–63 [Google Scholar]
- 27.Paez KA, Mallery CJ, Noel H, Pugliese C, McSorley VE, Lucado JL, Ganachari D (2014) Development of the Health Insurance Literacy Measure (HILM): conceptualizing and measuring consumer ability to choose and use private health insurance. J Health Commun 19(Suppl 2):225–239. 10.1080/10810730.2014.936568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patton MQ (2014) Qualitative research & evaluation methods: integrating theory and practice. Sage publications Inc. Thousands Oak, California. [Google Scholar]
- 29.Miles MB, Huberman AM, Saldana J (2014) Qualitative data analysis: a methods sourcebook. Sage Publications Inc. Thousands Oak, California. [Google Scholar]
- 30.Chino F, Brizel DM, Mowery YM (2021) Patient Reported Outcomes and Financial Toxicity in Head and Neck Cancer (PaRTNer): baseline financial toxicity and attitudes toward costs from a pilot study. J Clin Oncology 39:56–56. 10.1200/JCO.2020.39.28_suppl.56 “ [DOI] [Google Scholar]
- 31.Sadigh G, Lava N, Switchenko J, Duszak R Jr, Krupinski EA, Meltzer C, Hughes D, Carlos RC (2021) Patient-reported financial toxicity in multiple sclerosis: predictors and association with care non-adherence. Mult Scler 27:453–464. 10.1177/1352458520913977 [DOI] [PubMed] [Google Scholar]
- 32.Laws MB, Lee Y, Taubin T, Rogers WH, Wilson IB (2018) Factors associated with patient recall of key information in ambulatory specialty care visits: results of an innovative methodology. PLoS One 13:e0191940. 10.1371/journal.pone.0191940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins V, Solis-Trapala I, Langridge C, Catt S, Talbot DC, Fallowfield LJ (2011) What oncologists believe they said and what patients believe they heard: an analysis of phase I trial discussions. J Clin Oncol 29:61–68. 10.1200/JCO.2010.30.0814 [DOI] [PubMed] [Google Scholar]
- 34.Hong YR, Salloum RG, Yadav S, Smith G, Mainous AG 3rd (2020) Patient-provider discussion about cancer treatment costs and out-of-pocket spending: implications for shared decision making in cancer care. Value Health 23:1592–1598. 10.1016/j.jval.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 35.Yezefski T, Steelquist J, Watabayashi K, Sherman D, Shankaran V (2018) Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care 24:S74–S79 [PubMed] [Google Scholar]
- 36.Rocque GB, Pisu M, Jackson BE, Kvale EA, Demark-Wahnefried W, Martin MY, Meneses K, Li Y, Taylor RA, Acemgil A, Williams CP, Lisovicz N, Fouad M, Kenzik KM, Partridge EE, Patient Care Connect G (2017) Resource use and Medicare costs during lay navigation for geriatric patients with cancer. JAMA Oncol 3:817–825. 10.1001/jamaoncol.2016.6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert C, Legleitner S, Laraia K. Results of a pilot study at one community cancer center. 2019. Available at: https://www.accc-cancer.org/docs/documents/oncology-issues/articles/jf19/jf19-technology-unlocks-untapped-potential-in-a-financial-navigation-program.pdf Accessed on September 27, 2021 [Google Scholar]