Abstract
Objective:
The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system was developed to stratify the risk of 1-year major amputation. Recently, the WIfI scores were used to define the estimated revascularization benefit quartiles ranging from high benefit (Q1) to questionable benefit (Q4). The aim of our study was to evaluate the revascularization benefit quartiles in a cohort of diabetic patients presenting with chronic limb-threatening ischemia (CLTI).
Methods:
All diabetic patients presenting to our multidisciplinary diabetic foot and wound clinic (June 2012 to May 2020) who underwent lower extremity revascularization for CLTI were included. The affected limbs were graded using the WIfI system and assigned to an estimated benefit of revascularization quartile as previously published. One-year major amputation, complete foot healing, secondary patency, and amputation-free survival were calculated among the quartiles using Kaplan-Meier curve analyses and compared using Cox proportional hazards models.
Results:
Overall, 136 diabetic patients underwent revascularization of 187 limbs (mean age, 64.9 ± 11.2 years; 63.2% male; 58.8% black). The limbs were revascularized using an endovascular approach for 66.8% and open surgery for 33.2%. Of the 187 limbs, 27.3% had a high estimated benefit of revascularization (Q1), 31.6% had a moderate estimate benefit of revascularization (Q2), 20.3% had a low estimated benefit of revascularization (Q3), and 20.9% had a questionable benefit of revascularization (Q4). The estimated 1-year major amputation rates were 7.2% ± 4.1% for Q1, 3.8% ± 2.6% for Q2, 7.0% ± 4.8% for Q3, and 25.7% ± 7.5% for Q4 (P = .006). The estimated 1-year foot healing rates were 87.3% ± 5.7% for Q1, 84.8% ± 5.6% for Q2, 83.8% ± 7.4% for Q3, and 68.2% ± 9.1% for Q4 (P = .06). The overall secondary patency (P = .23) and amputation-free survival (P = .33) did not significantly differ among the groups. Using Cox proportional hazard modeling, the Q4 group had a significantly greater risk of major amputation compared with Q1 (hazard ratio, 4.26; 95% confidence interval, 1.15-15.70). Of the 14 limbs requiring major amputation, 9 (56.3%) had a patent revascularization at the time of amputation, including one of three limbs in Q1, two of two limbs in Q2, no limb in Q3, and six of nine limbs in Q4.
Conclusions:
The questionable estimated revascularization benefit quartile using the WIfI classification system is significantly associated with 1-year major amputation in diabetic patients presenting with CLTI. Limbs with a questionable benefit of revascularization (Q4) will frequently require major amputation despite a patent revascularization, suggesting that the wound size and infection burden are the driving factors behind the elevated risk of major amputation in this group. Our findings support the previously described use of the WIfI classification system to predict revascularization benefit among diabetic patients with CLTI.
Keywords: Revascularization benefit, WIfI, Chronic limb-threatening ischemia, Diabetic foot ulcer
The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system was developed to stratify the risk of 1-year major amputation for patients presenting with chronic limb-threatening ischemia (CLTI).1 The same report defined the WIfI-based estimated likelihood of benefit and/or requirement for revascularization stages, although this classification system has not been widely applied to date. However, the WIfI system was developed using the Delphi method, which relies on expert consensus rather than evidence-based data, and the risk classification for major amputation at 1 year is based on the assumption that revascularization was not performed.
Recently, Mayor et al2 used the WIfI classification system to identify CLTI patients who were most likely to benefit from revascularization. Using data from 10 centers collected from 2005 to 2015, they calculated the difference between the predicted and observed major amputation risk for patients who had undergone revascularization. The WIfI scores for those patients were then used to define the estimated revascularization benefit quartiles, ranging from high benefit (Q1) to questionable benefit (Q4).
If the revascularization benefit quartiles can be replicated in additional populations, they could improve the risk stratification of patients presenting with CLTI. This would help surgeons provide an evidence-based estimate of limb prognosis with and without revascularization. The aim of our study was to evaluate the revascularization benefit quartiles in a cohort of diabetic patients who had presented with CLTI.
METHODS
Study cohort.
We included all patients with diabetes who had presented to our multidisciplinary diabetic foot and wound clinic from June 2012 through May 2020 with CLTI and underwent lower extremity revascularization. Patients with claudication and those who did not undergone revascularization were excluded. We also did not include patients with WIfI stage 5 limbs (unsalvageable) or patients with CLTI but without diabetes, because the aim of our study was to evaluate the revascularization benefit quartiles in a cohort of diabetic patients who presented with CLTI. The patients were referred via both inpatient and outpatient consultations and were enrolled in a prospective database that collected longitudinal demographic, comorbidity, wound, revascularization, and outcomes data. A small subset of the limbs included in the present study (through November 2017) had also been included in the study by Mayor et al.2 All the patients provided written informed consent before enrollment in the database. The institutional review board approved the present study.
Treatment paradigm.
The details of our multidisciplinary limb preservation service have been described previously.3 In brief, the patients were initially evaluated by a vascular surgeon, surgical podiatrist, endocrinologist, and wound care nurse. The comorbidities were managed, and wound care was initiated.
All the patients underwent noninvasive vascular laboratory testing to assess their lower extremity perfusion at their initial presentation to our clinic. Patients with a toe pressure of ≤60 mm Hg typically underwent diagnostic angiography with the intent to perform a peripheral vascular intervention. Our practice of pursuing revascularization even for moderate ischemia was determined by our previous experience of poor foot collateral circulation in diabetic patients that led to inferior outcomes in those who did not undergo revascularization. For those patients with a toe pressure >60 mm Hg, revascularization was reserved for those wounds that fail to heal despite aggressive wound care measures. The revascularization approach was left to the surgeon’s discretion, and postoperative vascular laboratory test results were monitored closely to ensure appropriate improvement in perfusion. After revascularization, patients underwent debridement or minor amputation of their foot wounds (if present) back to healthy tissue and/or bone. The patients were then followed up regularly on an outpatient basis in the multidisciplinary clinic. Home nursing was used liberally to assist with wound care. Intravenous antibiotics were used for all those who underwent proximal bone resection (ie, involving the metatarsal or more proximally) according to input from our infectious diseases colleagues.
Definitions.
At their vascular intervention, all the patients’ limbs were graded using the WIfI classification system by provider consensus.1 The limbs were not reclassified after debridement or revascularization. We used this classification to assign the individual limbs to risk difference quartiles according to their estimated benefit from revascularization.2 Quartile (Q) 1 represents WIfI scores with the greatest estimated benefit of revascularization, Q2 represents WIfI scores with a moderate estimated benefit of revascularization, Q3 represents WIfI scores with a low benefit of revascularization, and Q4 represents WIfI scores with a questionable benefit of revascularization (Supplementary Table I, online only). The estimated 1-year lower extremity amputation rates are 4.4% for Q1, 14.8% for Q2, 28.1% for Q3, and 51.2% for Q4 as defined by the data reported by Mayor et al.2 However, Mayor et al2 only classified the revascularization benefit for 49 of the 64 potential WIfI combinations. In our cohort, only two limbs had a WIfI combination that was not included in the report by Mayor et al.2 One limb was WIfI 113 and one was WIfI 123. These patients were placed into the revascularization benefit according to the original WIfI report1 (WIfI 113, moderate benefit; WIfI 123, high benefit).
The primary outcome of our study was 1-year major amputation, which was chosen to match the primary outcome of the original WIfI classification description1 and the report by Mayor et al.2 The secondary outcomes included 1-year complete foot healing, secondary patency, and amputation-free survival. Complete foot healing was defined as complete epithelialization of all foot wounds with sustained functional and anatomic continuity for ≥6 weeks.4,5 Secondary patency was determined from the duplex ultrasound findings (performed at 6 weeks postoperatively and every 3 months thereafter for the first year) using the Rutherford recommended reporting standards.6
Statistical analysis.
All descriptive variables were summarized using counts and percentages or the mean ± standard error, as appropriate. The 1-year outcomes were estimated using life table analyses and Kaplan-Meier curves and compared between the estimated benefit of revascularization quartiles using log-rank tests. We then used Cox proportional hazards models clustered by patient to calculate the hazard ratios (HRs) with 95% confidence intervals (CIs) for the association of the revascularization benefit quartiles (Q1-Q4) with the primary and secondary outcomes.
All statistical tests were two sided, with an α of 0.05. Statistical analyses were performed using Stata, version 14 (StataCorp LP, College Station, Tex).
RESULTS
Overall, 136 diabetic patients underwent revascularization of 187 limbs (Table 1). Their mean age was 64.9 ± 11.2 years, 63.2% were men, and 58.8% were black. Most of the patients had non–insulin-dependent diabetes (91.9%). Hypertension (91.2%), dyslipidemia (72.1%), and coronary artery disease (45.6%) were also common. The median follow-up time was 18.0 months (interquartile range, 4.2-28.2 months).
Table I.
Baseline patient characteristics
| Variable | Overall (n = 136) |
|---|---|
| Age, years | 64.9 ± 1.0 |
| Male sex | 86 (63.2) |
| Race | |
| White | 49 (36.0) |
| Black | 80 (58.8) |
| Other/unknown | 7 (5.2) |
| Insurance status | |
| Medicare/Medicaid | 103 (75.7) |
| Private | 31 (22.8) |
| Other | 2 (1.5) |
| Diabetes mellitus | |
| Insulin dependent | 11 (8.1) |
| Non-insulin dependent | 125 (91.9) |
| Baseline hemoglobin A1c, % | 8.25 ± 2.2 |
| Comorbidity | |
| Hypertension | 124 (91.2) |
| Dyslipidemia | 98 (72.1) |
| Coronary artery disease | 92 (45.6) |
| Congestive heart failure | 28 (20.6) |
| Chronic obstructive pulmonary disease | 7 (5.2) |
| Cerebrovascular disease | 26 (19.1) |
| Chronic kidney disease | 31 (22.8) |
| Dialysis | 23 (16.9) |
| Retinopathy | 45 (33.1) |
| Neuropathy with loss of protective sensation | 133 (97.8) |
| Smoking status | |
| Current | 28 (20.6) |
| Former | 51 (37.5) |
| Never | 57 (41.9) |
Data presented as mean ± standard error or number (%).
The indication for lower extremity revascularization was a diabetic foot ulcer in 50.8%, gangrene in 42.9%, and rest pain in 6.4%. The limbs were revascularized using an endovascular approach in 66.8% and open surgery in 33.2% (Table II). More than one half of the limbs treated had been classified as WIfI stage 4 (59.9%), followed by stage 3 (24.1%), stage 2 (9.6%), and stage 1 (6.4%). A breakdown of limbs by individual WIfI grade is provided in Supplementary Table II (online only). Of the 187 limbs, 27.3% had a high estimated benefit of revascularization (Q1), 31.6% had a moderate estimate benefit of revascularization (Q2), 20.3% had a low estimated benefit from revascularization (Q3), and 20.9% had a questionable benefit of revascularization (Q4).
Table II.
Description of treated diabetic limbs stratified by quartile of estimated likelihood of benefit of revascularization
| Variable | Overall | Q1 (highest benefit) |
Q2 (moderate benefit) |
Q3 (low benefit) |
Q4 (questionable benefit) |
P value |
|---|---|---|---|---|---|---|
| Limbs treated, No. | 187 (100) | 51 (27.3) | 59 (31.6) | 38 (20.3) | 39 (20.9) | NA |
| WIfI stage | <.001 | |||||
| 1 | 12 (6.4) | 0 (0) | 0 (0) | 11 (29.0) | 1 (2.6) | |
| 2 | 18 (9.6) | 0 (0) | 7 (11.9) | 9 (23.7) | 2 (5.1) | |
| 3 | 45 (24.1) | 18 (35.3) | 10 (17.0) | 16 (42.1) | 1 (2.6) | |
| 4 | 112 (59.9) | 33 (64.7) | 42 (71.2) | 2 (5.3) | 35 (89.7) | |
| Revascularization approach | .09 | |||||
| Endovascular | 125 (66.8) | 37 (72.6) | 32 (54.2) | 29 (76.3) | 27 (69.2) | |
| Open surgery | 62 (33.2) | 14 (27.5) | 27 (45.8) | 9 (23.7) | 12 (30.8) |
NA, Not applicable; Q, quartile; WIfI, Wound, Ischemia, and foot Infection.
Data presented as number (%).
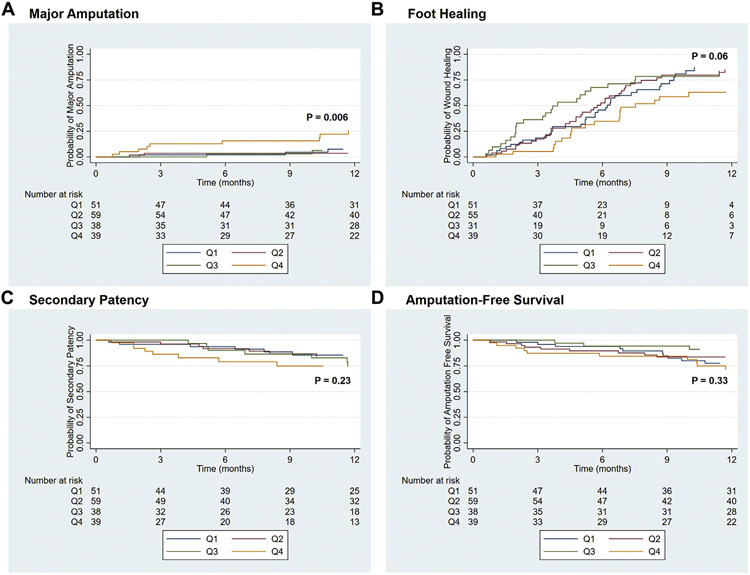
The estimated 1-year major amputation rates were 7.2% ± 4.1% for Q1, 3.8% ± 2.6% for Q2, 7.0% ± 4.8% for Q3, and 25.7% ± 7.5% for Q4 (P = .006; Fig, A). The estimated 1-year foot healing rates were 87.3% ± 5.7% for Q1, 84.8% ± 5.6% for Q2, 83.8% ± 7.4% for Q3, and 68.2% ± 9.1% for Q4 (P = .06; Fig, B). The 1-year secondary patency (P = .23; Fig, C) and amputation-free survival (P = .33; Fig, D) did not significantly differ among the groups. Also, no significant difference was found in the 1-year survival among the groups (P = .47; Supplementary Fig, online only).
Fig.
Kaplan-Meier curves showing major amputation (A), complete foot healing (B), secondary patency (C), and amputation-free survival (D) for diabetic patients who underwent lower extremity revascularization stratified by quartiles (Q) of estimated benefit of revascularization. All standard errors were <10%.
Cox proportional hazard modeling showed that the Q4 group had a significantly greater risk of major amputation compared with the Q1 group (HR, 4.26; 95% CI, 1.15-15.70). No significant differences were found in the risk of major amputation for Q2 or Q3 compared with Q1. The Q4 limbs had a slightly lower HR for complete foot healing (HR, 0.61; 95% CI, 0.35-1.05); however, the difference was not statistically significant (P = .08). Patency and amputation-free survival were not significantly different among the groups (Table III).
Table III.
Cox proportional hazards models for 1-year complete foot healing and limb salvage outcomes stratified by quartile of estimated benefit of revascularization
| HR (95% CI) | ||||
|---|---|---|---|---|
| Outcome | Q1 (highest benefit) |
Q2 (moderate benefit) |
Q3 (low benefit) |
Q4 (questionable benefit) |
| Major amputation | Reference | 0.65 (0.11-3.91) | 1.03 (0.17-6.15) | 4.26 (1.15-15.7) |
| Complete foot healing | Reference | 1.06 (0.67-1.67) | 1.34 (0.80-2.25) | 0.61 (0.35-1.05) |
| Loss of patency | Reference | 1.04 (0.35-3.11) | 1.59 (0.54-4.74) | 2.45 (0.87-6.90) |
| Loss of amputation-free survival | Reference | 0.89 (0.36-2.19) | 0.46 (0.13-1.68) | 1.44 (0.60-3.47) |
CI, Confidence interval; HR, hazard ratio; Q, quartile.
Of the 137 limbs with ≥1 year of follow-up, 16 (11.7%) ultimately required major amputation at 1 year (Table IV; Supplementary Table III, online only). Of those 16 limbs, 9 (56.3%) had been classified as Q4 (questionable benefit), 3 (18.8%) as Q1 (highest benefit), 2 (12.5%) as Q2 (moderate benefit), and 2 as Q3 (low benefit). At the time of amputation, 9 (56.3%) limbs had a patent revascularization, including 1 of 3 in the Q1 group (33.3%), 2 of 2 in the Q2 group (100%), none in the Q3 group (0%), and 6 of 9 in the Q4 group (66.7%). Stratification of the individual WIfI grades (Supplementary Table III, online only) showed that major amputation was more common among limbs with either more extensive wounds (wound grade 3) or more ischemia (ischemia grade 3). Severe foot infection (foot infection grade 3) was uncommon among the limbs requiring major amputation.
Table IV.
Patency of diabetic limbs requiring major amputation at 1 year stratified by quartile of estimated benefit of revascularizationa
| Quartile | 1-year Major amputation |
Revascularization patent at major amputation |
|---|---|---|
| Overall | 16/137 (11.7) | 9/16 (56.3) |
| Q1 (highest benefit) | 3/34 (8.8) | 1/3 (33.3) |
| Q2 (moderate benefit) | 2/42 (4.8) | 2/2 (100) |
| Q3 (low benefit) | 2/39 (6.7) | 0/2 (0) |
| Q4 (questionable benefit) | 9/31 (29.0) | 6/9 (66.7) |
Q, Quartile.
Data presented as number per total (%).
Total number of patients was 137 because those without 1 year of follow-up were excluded from the subanalysis.
The association of various prognostic classification schemes with major amputation is provided in Supplementary Table IV (online only). The major amputation rates for the WIfI clinical stages (classified according to amputation risk) ranged from 8.3% for stage 1 to 12.5% for stage 4. The major amputation rates for the WIfI clinical stages (classified according to the theoretical revascularization benefit) ranged from 18.2% for stage 1 to 12.7% for stage 4. The major amputation rates for the revascularization benefit quartiles as defined by Mayor et al2 ranged from 8.8% for Q1 to 29.0% for Q4. Although the 95% CIs for all observed/expected ratios were large because of the small event numbers, the largest differential in major amputation risk was observed with the revascularization benefit quartiles.
DISCUSSION
The WIfI classification system has recently been used to identify which patients with CLTI would be most likely to benefit from lower extremity revascularization.2 Using the individual scores for each of the WIfI components (ie, wound, ischemia, and foot infection), the quartiles of potential revascularization benefit were described. In the present study, we sought to evaluate these quartiles in a contemporary cohort of CLTI patients with diabetes. We found a significant association between the revascularization quartile and the need for a major amputation. Specifically, patients with WIfI scores corresponding to Q4, indicating a questionable benefit of revascularization, had a significantly greater risk of major amputation compared with those with Q1 to Q3. The foot healing rates were also slightly lower in the Q4 group, although the difference was not statistically significant (P = .06). Overall, our data support the use of the previously described WIfI benefit of revascularization quartiles for estimating the 1-year major amputation risk for diabetic patients presenting with CLTI.2
The estimated risk of major amputation in the original study by Mayor et al2 ranged from 4.4% for Q1 (highest benefit of revascularization) to 51.2% for Q4 (questionable benefit of revascularization) and increased linearly across the groups. We found a significantly greater risk of major amputation for the Q4 patients (29.0%) compared with the other groups. However, our 1-year major amputation rate was one half of that expected for Q4 limbs,2 and the risk of major amputation was relatively similar, and low, for the Q1 through Q3 limbs. Our data suggest that a clear disadvantage exists for a Q4 WIfI score but that the outcomes for the other quartiles of revascularization benefit tend to overlap. The discrepancy for the outcomes of the Q1 to Q3 groups in our study compared with the data reported by Mayor et al2 might have been because our unique patient cohort was limited to patients with diabetes, the high burden of WIfI grade 2 and 3 wounds, insufficient power to detect a difference in outcomes among the Q1 to Q3 groups, and/or our care delivery model. Although we did not directly assess the association of our care model with the revascularization outcomes in the present study, all our patients with diabetes and CLTI are treated by our multidisciplinary team, which has been previously shown to have robust limb salvage outcomes regardless of the patient comorbidity burden or socioeconomic disadvantage.7,8 Multidisciplinary teams have been associated with better limb salvage outcomes in a wide variety of patient populations after revascularization,9-12 which might have contributed to the lower rates of major amputation in our study.
More than one half of the patients who ultimately required amputation in our study had a patent lower extremity revascularization at the time of their amputation. Among the patients in the Q4 group, more than two thirds required major amputation despite the presence of a patent revascularization. This finding suggests that patients with more extensive wounds and/or more infection might have a lower benefit of revascularization. Although patients with worse ischemia also had increased amputation rates, this was typically related to bypass thrombosis. According to the Global Vascular Guidelines for Chronic Limb-Threatening Ischemia, “revascularization should not be performed in the absence of significant ischemia (WIfI ischemia grade 0) unless an isolated region of poor perfusion in conjunction with major tissue loss (eg, WIfI wound grade 2 or 3) can be effectively targeted.”13 In our study, most limbs requiring amputation had had either extensive wounds (WIfI wound grade 3) or a failed revascularization in the setting of extensive preoperative ischemia (ischemia grade 3). Using the WIfI scoring system, Mayor et al2 reported that wound severity was most strongly associated with major amputation risk after revascularization. Consistent with this finding, ulcer size has been associated with worse amputation and wound healing outcomes in previous diabetic foot ulcer studies.7,14,15 Together, these data suggest that patients with extensive foot wounds should be counseled that their risk of major amputation is high even if revascularization is achieved.
The slightly lower rate of wound healing that we found for Q4 limbs in our study is novel. Although this finding was not quite significant (P = .06), the Q4 limbs had a 39% lower likelihood of achieving complete foot healing at 1 year compared with the Q1 limbs. The clinical implications are clear. If the WIfI revascularization benefit quartiles are associated with both major amputation and foot healing, the limbs in the questionable benefit category should be carefully scrutinized before the patient is offered a revascularization procedure. Lower extremity revascularization is costly to the healthcare system,16 especially for advanced staged wounds.17,18 If clinical benefit is unlikely to be achieved, perhaps primary amputation would be the most appropriate for patients presenting with advanced Q4 stage wounds, especially if the patient is functionally impaired or chronically ill.19
The concept of using WIfI scores to estimate the revascularization benefit for diabetic patients presenting with CLTI is clinically relevant. Diabetic patients with peripheral artery disease have a significantly greater risk of mortality compared to patients with peripheral artery disease but without diabetes,20 and the morbidity occurring after lower extremity revascularization in this population is high.11,21,22 Although good long-term outcomes with both open and endovascular revascularization ap-proaches are possible,11,21,23 appropriate patient selection is critical. We have been using the WIfI classification system in our multidisciplinary diabetic foot and wound clinic since 2015 to provide patients with an estimated likelihood of wound healing.3 However, we have not previously observed a correlation between the WIfI stage and the risk of major amputation in our patient population,3,7 which made conversations about the appropriateness of revascularization for patients with extensive wounds quite difficult. We anticipate that we will be able to incorporate the WIfI revascularization benefit classification scheme into our patient conversations, thereby allowing us to have more quantitative conversations about the risks and benefits of aggressive revascularization procedures for patients at particularly high risk of surgery. Thus, when a patient presents with a Q4 limb, we will be able to have a greater discussion, not only about the probability of limb salvage, but also about patient-centered functional outcomes in the long term. The revascularization benefit quartiles evaluated in the present study provided a larger differential of the major amputation estimates compared with the traditional WIfI classification system for major amputation, suggesting that both tools have relevance in the clinical setting.
The limitations of our study deserve discussion. Although our study was based on a single institution’s experience, we made our best efforts to replicate the study by Mayor et al2 as closely as possible. We were able to confirm the decreased benefit of revascularization for Q4 and believe that this group of patients should be carefully evaluated before committing them to aggressive limb salvage efforts that might not change their amputation rate. However, validation of these findings in a larger cohort is necessary. We also included a very specific patient population (all with diabetes) treated using a well-established care paradigm. Although this approach likely reduced the possibility of residual confounding, whether our findings will translate to other patient populations and other treatment settings is unclear. In addition, the revascularization procedures were heterogeneous and determined by surgeon discretion. We did not have data to compare how the timing of revascularization might affect the major amputation and patency outcomes or whether postprocedural hemodynamic data played a role. Although we did measure the toe pressure before and after revascularization, the number of missing values because of toe amputations resulted in a low-powered comparison. Thus, we did not include these data in our analysis. However, we have previously shown that the long-term outcomes did not significantly differ for open surgery vs endovascular interventions in this cohort.11 We were not able to compare the outcomes for patients who had undergone revascularization vs wound care alone because the number of patients in the latter group was extremely low. A comparison of patients with similar WIfI classifications with vs without revascularization would be helpful to better understand the true benefits of revascularization. Also, we had a low number of major amputation events overall, which precluded us from performing adjusted Cox models in assessing the association of the revascularization benefit quartile with the need for major amputation. Finally, in the present study, similar to the study by Mayor et al,2 we did not assess patient-centered outcomes, which should also be considered. The strengths of our study included the extensive wound details we collected, the longitudinal follow-up of the patients included in our cohort, and the consistent method with which they were treated by our multidisciplinary team.
CONCLUSIONS
The estimated revascularization benefit quartiles using the WIfI classification system were significantly associated with 1-year major amputation in diabetic patients presenting with CLTI. Limbs with a questionable benefit of revascularization (ie, Q4) frequently required major amputation despite having a patent revascularization, suggesting that wound size and infection burden are the driving factors behind the elevated risk of major amputation in this group. Our findings support the previously described use of the WIfI classification system to predict the revascularization benefit, especially among diabetic patients with CLTI.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: A retrospective analysis of a prospectively maintained institutional database
Key Findings: Studying data from 187 lower extremity revascularization procedures, we found that the estimated revascularization benefit quartiles using the Wound, Ischemia, and foot Infection (WIfI) classification system are significantly associated with 1-year major amputation in diabetic patients presenting with chronic limb-threatening ischemia. Limbs with a questionable benefit of revascularization will frequently require major amputation despite patent revascularization.
Take Home Message: Our findings support the previously described use of the WIfI classification system to predict the revascularization benefit for diabetic patients with chronic limb-threatening ischemia.
Acknowledgments
C.W.H. has received funding from the National Institute of Diabetes and Digestive and Kidney Diseases via a K32 grant (grant K23DK12451).
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on Wound, Ischemia, and foot Infection (WIfI). J Vasc Surg 2014;59. 220–34.e1-2. [DOI] [PubMed] [Google Scholar]
- 2.Mayor J, Chung J, Zhang Q, Montero-Baker M, Schanzer A, Conte MS, et al. Using the Society for Vascular Surgery Wound, Ischemia, and foot Infection classification to identify patients most likely to benefit from revascularization. J Vasc Surg 2019;70:776–85.e1. [DOI] [PubMed] [Google Scholar]
- 3.Mathioudakis N, Hicks CW, Canner JK, Sherman RL, Hines KF, Lum YW, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system predicts wound healing but not major amputation in patients with diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg 2017;65:1698–705.e1. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus GS, Cooper DM, Knighton DR, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994;2:165–70. [DOI] [PubMed] [Google Scholar]
- 5.Margolis DJ, Berlin JA, Strom BL. Interobserver agreement, sensitivity, and specificity of a "healed" chronic wound. Wound Repair Regen 1996;4:335–8. [DOI] [PubMed] [Google Scholar]
- 6.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26:517–38. [DOI] [PubMed] [Google Scholar]
- 7.Hicks CW, Canner JK, Mathioudakis N, Sherman R, Malas MB, Black JH III, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification independently predicts wound healing in diabetic foot ulcers. J Vasc Surg 2018;68:1096–103. [DOI] [PubMed] [Google Scholar]
- 8.Hicks CW, Canner JK, Mathioudakis N, Sherman RL, Hines K, Lippincott C, et al. Neighborhood socioeconomic disadvantage is not associated with wound healing in diabetic foot ulcer patients treated in a multidisciplinary setting. J Surg Res 2018;224:102–11. [DOI] [PubMed] [Google Scholar]
- 9.Causey MW, Ahmed A, Wu B, Gasper WJ, Reyzelman A, Vartanian SM, et al. Society for Vascular Surgery limb stage and patient risk correlate with outcomes in an amputation prevention program. J Vasc Surg 2016;63:1563–73.e2. [DOI] [PubMed] [Google Scholar]
- 10.Flores AM, Mell MW, Dalman RL, Chandra V. Benefit of multidisciplinary wound care center on the volume and outcomes of a vascular surgery practice. J Vasc Surg 2019;70:1612–9. [DOI] [PubMed] [Google Scholar]
- 11.Hicks CW, Canner JK, Lum YW, Black JH III, Abularrage CJ. Long-term outcomes of an endovascular-first approach for diabetic patients with predominantly tibial disease treated in a multidisciplinary setting. Ann Vasc Surg 2019;60:315–26.e2. [DOI] [PubMed] [Google Scholar]
- 12.Meloni M, Izzo V, Giurato L, Brocco E, Gandini R, Uccioli L. Limb salvage in diabetic patients with ischemic heel ulcers. Int J Low Extrem Wounds 2019;19:275–81. [DOI] [PubMed] [Google Scholar]
- 13.Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019;69(Suppl):3S–125S.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fife CE, Horn SD, Smout RJ, Barrett RS, Thomson B. A predictive model for diabetic foot ulcer outcome: the wound healing index. Adv Wound Care (New Rochelle) 2016;5:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Armstrong DG, Harkless LB, et al. The effects of ulcer size and site, patient’s age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet Med 2001;18:133–8. [DOI] [PubMed] [Google Scholar]
- 16.Hicks CW, Selvarajah S, Mathioudakis N, Sherman RE, Hines KF, Black JH III, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg 2016;33:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hicks CW, Canner JK, Karagozlu H, Mathioudakis N, Sherman RL, Black JH III, et al. Quantifying the costs and profitability of care for diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg 2019;70:233–40. [DOI] [PubMed] [Google Scholar]
- 18.Hicks CW, Selvarajah S, Mathioudakis N, Perler BA, Freischlag JA, Black JH III, et al. Trends and determinants of costs associated with the inpatient care of diabetic foot ulcers. J Vasc Surg 2014;60:1247–54.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor SM, Kalbaugh CA, Blackhurst DW, Cass AL, Trent EA, Langan EM III, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg 2006;44:747–55; discussion: 755-6. [DOI] [PubMed] [Google Scholar]
- 20.Low Wang CC, Blomster JI, Heizer G, Berger JS, Baumgartner I, Fowkes FGR, et al. Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: the EUCLID trial. J Am Coll Cardiol 2018;72:3274–84. [DOI] [PubMed] [Google Scholar]
- 21.Ballotta E, Toniato A, Piatto G, Mazzalai F, Da Giau G. Lower extremity arterial reconstruction for critical limb ischemia in diabetes. J Vasc Surg 2014;59:708–19. [DOI] [PubMed] [Google Scholar]
- 22.Malmstedt J, Leander K, Wahlberg E, Karlstrom L, Alfredsson L, Swedenborg J. Outcome after leg bypass surgery for critical limb ischemia is poor in patients with diabetes: a population-based cohort study. Diabetes Care 2008;31:887–92. [DOI] [PubMed] [Google Scholar]
- 23.Hicks CW, Najafian A, Farber A, Menard MT, Malas MB, Black JH III, et al. Diabetes does not worsen outcomes following infrageniculate bypass or endovascular intervention for patients with critical limb ischemia. J Vasc Surg 2016;64:1667–74.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.