Abstract
Neutralizing antibody (nAb) responses are attenuated in solid organ transplant recipients (SOTRs) despite severe acute respiratory syndrome-coronavirus-2 vaccination. Preexposure prophylaxis (PrEP) with the antibody combination tixagevimab and cilgavimab (T+C) might augment immunoprotection, yet in vitro activity and durability against Omicron sublineages BA.4/5 in fully vaccinated SOTRs have not been delineated. Vaccinated SOTRs, who received 300 + 300 mg T+C (ie, full dose), within a prospective observational cohort submitted pre and postinjection samples between January 31, 2022, and July 6, 2022. The peak live virus nAb was measured against Omicron sublineages (BA.1, BA.2, BA.2.12.1, and BA.4), and surrogate neutralization (percent inhibition of angiotensin-converting enzyme 2 receptor binding to full length spike, validated vs live virus) was measured out to 3 months against sublineages, including BA.4/5. With live virus testing, the proportion of SOTRs with any nAb increased against BA.2 (47%-100%; P < .01), BA.2.12.1 (27%-80%; P < .01), and BA.4 (27%-93%; P < .01), but not against BA.1 (40%-33%; P = .6). The proportion of SOTRs with surrogate neutralizing inhibition against BA.5, however, fell to 15% by 3 months. Two participants developed mild severe acute respiratory syndrome-coronavirus-2 infection during follow-up. The majority of fully vaccinated SOTRs receiving T+C PrEP achieved BA.4/5 neutralization, yet nAb activity commonly waned by 3 months postinjection. It is critical to assess the optimal dose and interval of T+C PrEP to maximize protection in a changing variant climate.
Keywords: COVID-19, monoclonal antibody, tixagevimab, cilgavimab, organ transplant, Omicron variant
1. Introduction
Many solid organ transplant recipients (SOTRs) exhibit a poor antibody response and plasma neutralizing capacity against severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) variants of concern despite repeated administration of vaccine doses.1, 2, 3 This, in part, underlies the higher rates of infection after vaccination (“breakthrough”) in SOTRs.4 , 5 Consequently, the injectable monoclonal antibody combination tixagevimab and cilgavimab (T+C) was authorized by the US Food and Drug Administration (FDA) as preexposure prophylaxis (PrEP) for SARS-CoV-2 and as a complementary strategy to reduce coronavirus disease 2019 (COVID-19) in immunocompromised persons.6 Supporting data, however, were based on trials of unvaccinated, immunocompetent persons and preceded the rise of the Omicron lineage variants, which exhibit significant immune evasion.7 , 8
The neutralizing activity and effectiveness of T+C in the prevention of COVID-19 in SOTRs remain uncertain, partly owing to studies varying in the dose of the drug and context of Omicron sublineage. For example, in vitro data indicate a >100-fold decrease in the neutralization of BA.1,9, 10, 11 supported by poor protection against severe disease during the BA.1 wave in one series of kidney transplant recipients following low-dose T+C PrEP (150 mg of tixagevimab and 150 mg of cilgavimab [150 + 150 mg]).12 In contrast, the in vitro neutralization of BA.2 by T+C appears largely preserved, and its effectiveness was supported by studies that overlapped the BA.2 sublineage wave.13 , 14 Informed by pharmacologic modeling and early data, the FDA recommended the doubling of T+C dose to 300 + 300 mg in February 2022.15 Equipoise remains, however, regarding the potential of 300 + 300 mg T+C to augment neutralization in fully vaccinated SOTRs against the now-dominant BA.4/5 sublineages, which share a common spike protein and have additional mutations compared to BA.1/2.16 Furthermore, although binding and neutralizing antibodies (nAb) against earlier Omicron sublineages may be detected for several months following 150 + 150 mg T+C,17 the durability of the neutralizing activity against BA.4/5 in SOTRs, particularly nonkidney recipients, is unknown. This has major implications regarding the optimal T+C dosing interval; as of June 2022, the FDA has recommended repeat dosing every 6 months.10
To address these knowledge gaps, we sought to evaluate the following: (1) the change in nAb following 300 + 300 mg T+C injection against Omicron sublineages, including BA.4/5, among fully vaccinated SOTRs and (2) the durability of the neutralizing activity over time.
2. Methods
2.1. Cohort
SOTRs were enrolled in a national, prospective observational study of SARS-CoV-2 vaccine response (Johns Hopkins IRB00248540) as previously described.18 , 19 All participants were contacted in January 2022 and again in March 2022 to identify participants with a prior or planned receipt of T+C. The study team neither administered T+C nor encouraged its receipt; doses were independently administered in the community according to the decision of the local provider. Participants were enrolled and consent was obtained electronically; consent into the parent cohort permitted additional surveys and sample collection for events such as changes in medical status, including incident COVID-19 infection, as well as the receipt of immunoprophylaxis.
All fully vaccinated participants (≥3 SARS-CoV-2 vaccine doses) who received a total of 300 + 300 mg T+C between February 2, 2022, and April 7, 2022, either as a single 300 + 300 mg dose, or as 2 150 + 150 mg doses, spaced ≤30 days apart, were eligible. Restricting the time between 150 + 150 mg doses ensured post-T+C sampling to be representative of the full neutralizing capacity and to reduce the risk period for COVID-19 without full prophylaxis. Participants were excluded from the analytical cohort if they had not provided an evaluable pre-T+C (“baseline”) sample and both a 2-week (“peak”) and 3-month longitudinal sample post-T+C injection. Participants receiving other monoclonal antibody products active against Omicron 90 days prior to T+C were excluded. Participants receiving the SARS-CoV-2 vaccine at any time following the receipt of T+C were excluded from the primary analysis but were evaluated in a supplementary posthoc analysis. Participants who developed COVID-19 during the follow-up remained in the cohort but were removed from the calculations of neutralizing capacity following the incident infection.
2.2. Sample collection and processing
Participants provided whole-blood samples via an at-home phlebotomy service ≤2 weeks before and 2 weeks, 4 weeks, and 3 months following T+C receipt. Blood was collected in acid citrate dextrose tubes and shipped overnight to Johns Hopkins University. Plasma was separated via centrifugation and stored at –80 °C.
2.3. Binding antibody measurements
Plasma samples were tested using the Meso Scale Diagnostics (MSD) assay to measure the binding antibody against full-length spike, the receptor–binding domain (RBD), and nucleocapsid proteins with the V-PLEX COVID-19 Coronavirus Panel 3 Kit at 1:5000 dilution per manufacturer’s protocol. Conversion to World Health Organization binding antibody units (BAU)/mL was done by multiplying the value obtained with the manufacturer’s recommended conversion factor. The upper limit of quantification for the MSD anti-RBD assay was 4500 BAU/mL. The imputed value from the manufacturer’s software was used if a signal was above the upper limit of quantification.
2.4. Neutralization assays
A subset of paired cohort samples (n = 15) was evaluated for the gold-standard live virus nAb before and 2 weeks after T+C injection against SARS-CoV-2 variants of concern, including Omicron sublineages BA.1, BA.2, BA.2.12.1, and BA.4. nAb titers were determined as previously described,8 using 2-fold dilutions of plasma (starting at 1:20); the highest dilution that eliminated the cytopathic effect in 50% of wells was reported, after which the area under the curve of the neutralization function (nAb AUC; positive if >10) was calculated. All nAb samples in the parent observational cohort (ie, including unpaired samples) were ultimately utilized for the validation of surrogate neutralization testing (ancestral [n = 44]; BA.1 [n = 58]; BA.2 [n = 44]; BA.2.12.1 [n = 27]; BA.4 [n = 27]; see section 2.5.).
2.5. Surrogate neutralization (%ACE2 inhibition)
The MSD chemiluminescent assay was used to measure the inhibition of angiotensin–converting enzyme 2 (%ACE2 inhibition) as previously described.3 Samples were assayed on MSD V-PLEX SARS-CoV-2 panels 25 and 27 at a dilution of 1:100 and tested against the ancestral strain (vaccine) and Omicron sublineages BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Prior work in SOTRs had determined ≥20% ACE2 inhibition as consistent with “neutralizing inhibition,” given the strong association with the detectable live virus nAb.3 We further assessed the discrimination of several thresholds of %ACE2 inhibition in this study to detect the presence of the live virus nAb against Omicron sublineages (see section 2.7.).
2.6. SARS-CoV-2 infection ascertainment
Participants were surveyed at days 7 and 90 following T+C injection for incident COVID-19 infection using the positive antigen or PCR testing. Additionally, serial anti–nucleocapsid (anti–N) testing was performed (see section 2.3.) to detect subclinical infections. A breakthrough infection was defined as anti–N seroconversion or a positive COVID-19 test occurring ≥14 days after the receipt of full-dose T+C injection. Infections not meeting criteria, such as those occurring between 150 + 150 mg doses, were recorded but not classified as breakthrough.
2.7. Statistical analysis
Wilcoxon matched-pairs signed-rank test was used to compare the live virus nAb before and 2 weeks after T+C injection. Surrogate neutralization vs live virus nAb correlation, by variant, was assessed using scatter plots and Spearman rank testing. Area under the receiver-operator curve (AUROC) testing was performed to evaluate the sensitivity and specificity of %ACE2 inhibition cutoffs, which maximized the classification of low- (nAb AUC > 10) and high-level (nAb AUC > 100) nAbs against Omicron sublineages. Given BA.4/5 nAbs share identical spike protein sequences, BA.4 nAb was used as the gold-standard comparator against BA.5 spike surrogate neutralization; the correlation between BA.4 and BA.5 surrogate neutralization was assessed. The McNemar test was used to compare the frequency of achieving surrogate neutralizing inhibition above threshold over time; persons developing incident COVID-19 infection were removed from subsequent timepoint calculations. Analyses were conducting using Stata/SE, version 17.0 (StataCorp).
3. Results
3.1. Demographics and vaccination history
The median (interquartile range [IQR]) age of the 36 participants was 61 years (58-66 years), and 20 (65%) of the participants were female and 3 (8%) were non-White (Table ). Twenty (56%) participants were kidney recipients and 10 (28%) were thoracic recipients (5 [14%] heart and 5 [14%] lung). The median time from transplant to T+C injection was 4.1 years (1.7-9.2 years), and 19 (53%) participants reported taking triple immunosuppression (corticosteroids, calcineurin/mammalian target of rapamycin inhibitor, and antimetabolite).
Table.
Clinical, transplant, and vaccination characteristics of solid organ transplant recipients who received tixagevimab and cilgavimab.
| N | 36 |
|---|---|
| T+C dose, n (%) | |
| 2 150 + 150 doses | 13 (36%) |
| 1 300 + 300 dose | 23 (64%) |
| Age (y), median (IQR) | 60.7 (58.1, 66.1) |
| Transplanted organ(s), n (%) | |
| Kidney | 20 (56%) |
| Liver | 3 (8%) |
| Lung | 5 (14%) |
| Heart | 4 (11%) |
| Multiplea | 4 (11%) |
| Sex, n (%) | |
| Male | 16 (44%) |
| Female | 20 (56%) |
| Race, n (%) | |
| White | 33 (92%) |
| Other | 3 (8%) |
| Years since transplant, median (IQR) | 4.1 (1.7, 9.2) |
| Maintenance immunosuppression, n (%) | |
| Triple immunosuppressionb | 19 (53%) |
| Mycophenolate | 30 (83%) |
| Corticosteroids | 23 (64%) |
| Calcineurin inhibitor | 30 (83%) |
| mTOR inhibitors | 6 (17%) |
| Belatacept | 1 (3%) |
| Number of vaccine doses prior to T+C, n (%) | |
| 3 | 8 (22%) |
| 4 | 27 (75%) |
| 5 | 1 (3%) |
| Days from last vaccination to T+C, median (IQR) | 38.5 (26, 114.5) |
| History of prior COVID-19c, n (%) | 2 (6%) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; mTOR, mammalian target of rapamycin inhibitor; T+C, tixagevimab and cilgavimab.
Includes heart-kidney (1), liver-kidney (1), and kidney-pancreas (2).
Defined as corticosteroids plus calcineurin or mTOR inhibitor plus antimetabolite.
Defined as self-reported prior to a positive COVID-19 test or baseline (pre-T+C)-positive antinucleocapsid antibody.
All participants completed 3 doses of primary SARS-CoV-2 vaccination pre-T+C injection, including 28 (75%) who received 4 or more doses (1 [3%] received 5 doses). Thirty-four out of the 36 participants (94%) received the 3-dose mRNA primary series (BNT162b2 or mRNA-1273), and 27 out of 28 (96%) and 1 out of 1 (100%) participants received mRNA vaccine as the fourth and fifth dose, respectively (other doses were with Ad26.COV2.S) (Table). The median (IQR) time from the last vaccine to the first T+C dose was 38.5 days (26-114.5 days); however, 0 participants received a vaccine <30 days of peak (2-week post full dose) testing. Two participants had prior COVID-19 infection (self-reported positive test [1] or anti–N testing [2]); the confirmed diagnosis occurred in December 2022 during the BA.1 wave.
Regarding T+C dosing, 23 (64%) of the participants received a single 300 + 300 mg dose and 13 (36%) of them received 2 150 + 150 mg doses, a median of 16 days (17-22 days) apart; demography and transplant factors did not differ by the T+C regimen except that those who received 2 150 + 150 mg doses had used more triple immunosuppression and received a more recent dose of SARS-CoV-2 vaccine (Supplementary Table S1).
3.2. Change in binding antibodies
The median (IQR) anti-RBD titer increased from 669 (31, 2892) to 8091 (5067, 10322) BAU/mL at 2 weeks post-T+C injection (P < .001) (Supplementary Fig. S1); all 8 (22%) participants with a negative antibody test prior to T+C injection had a positive antibody test following T+C injection. The median anti-RBD titer waned to 4155 (3174, 4957) BAU/mL by 3 months post-T+C injection. The peak titer was similar for those receiving a single 300 + 300 mg dose and those receiving 2 150 + 150 mg doses (median [IQR] 7554 [4715, 9075] vs 8912 [8185, 10657]; P = .08).
3.3. Live virus nAb
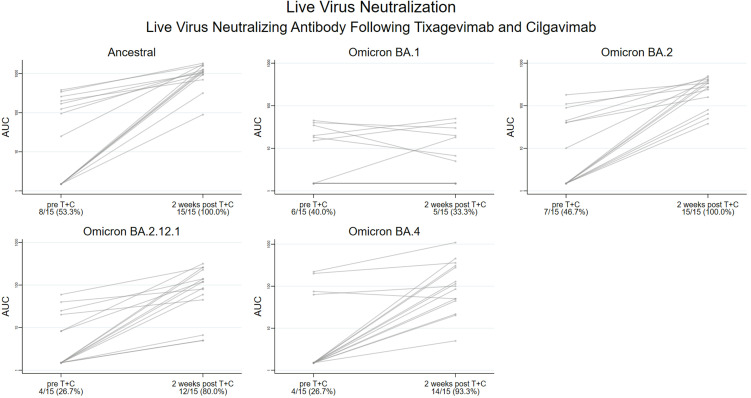
Among the 15 participants from the analytical cohort with paired live virus testing, nAb AUC significantly increased against the ancestral variant, from median (IQR) 25 (1.5, 201) to 1103 (945, 1583) (P < .001), yet it did not increase against BA.1, from 1.5 (1.5, 20) to 1.5 (1.5, 20) (P = .8). In contrast, nAb AUC significantly increased from 1.5 (1.5, 45) to 279 (81, 399) vs BA.2 (P < .001), from 1.5 (1.5, 20) to 120 (45, 230) vs BA.2.12.1 (P < .001), and from 1.5 (1.5, 63) to 101 (45, 309) vs BA.4 (P < .001) (Fig. 1 ). Median nAb AUCs against Omicron BA.1, BA.2, BA.2.12.1, and BA.4 sublineages at 2 weeks post-T+C injection were 735-, 4-, 9-, and 11-fold lower, respectively, than the nAb AUC against the ancestral variant. Notably, transplant and demographic characteristics did not statistically differ between participants who did and did not have the live virus nAb test performed (Supplementary Table S2).
Figure 1.
Live virus neutralizing antibody (nAb) against severe acute respiratory syndrome-coronavirus-2 variants pre and post tixagevimab and cilgavimab (T+C) injection among fully vaccinated solid organ transplant recipients (SOTRs). Live virus assays against the ancestral variant and Omicron sublineages BA.1, BA.2, BA.2.12.1, and BA.4 were performed in a subset of SOTRs (n = 15) before and 2 weeks following T+C injection. The Y axis denotes the area under the curve of the neutralization function (nAb AUC) on the log10 scale, with AUC > 10 denoting a positive antibody test (above the dashed orange line). The proportion with detectable nAbs is displayed on the X axis pre- and post-T+C injection. nAb AUC significantly increased for ancestral, BA.1, BA.2, BA.2.12.1, and BA.4 variants (P < .001 by the Wilcoxon paired signed-rank test), but not for BA.1 (P = .8).
3.4. Assessment of surrogate neutralization vs live virus neutralization
Correlation of neutralizing assessments was performed among all individuals in the parent cohort with available samples, revealing a high positive correlation between %ACE2 inhibition and the live virus nAb AUC across variants (Supplementary Fig. S2; 0.91 [BA.1], 0.92 [BA.2], 0.92 [BA.2.12.1], and 0.78 [BA.4]), particularly above the a priori 20% ACE2 inhibition threshold. ROC curves showed an excellent discrimination of nAbs across thresholds of %ACE2 inhibition for Omicron sublineages (Supplementary Fig. S3). Twenty-five percent ACE2 inhibition was selected to define neutralizing inhibition, given better discrimination for nAbs against BA.2 and BA.4/5 sublineages; eg, sensitivity/specificity/AUROC (95% CI) for BA.4 nAb > 10 was 72%/95%/0.959 (0.906-1.00) and for BA.4 nAb > 100 was 94%/74%/0.882 (0.794-0.969). Given we imputed BA.5 sensitivity and specificity from BA.4 live virus data, we compared %ACE2 inhibition values of BA.4 and BA.5 and confirmed an excellent correlation (Supplementary Fig. S4; r = 0.98).
3.5. Durability of neutralizing activity against SARS-CoV-2 variants
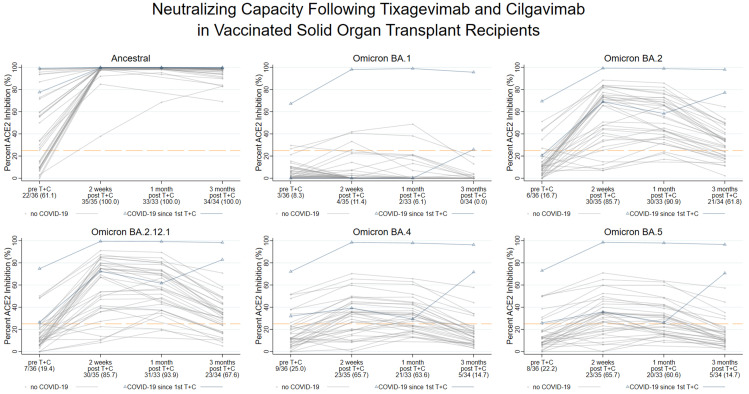
Using the 25% ACE2 inhibition threshold, we calculated the proportion of participants demonstrating neutralizing inhibition against SARS-CoV-2 variants from the baseline to 2 weeks and 3 months post-T+C injection (Fig. 2 ). After the exclusion of persons who developed incident COVID-19 infection (n = 2; see section 3.6.), neutralization of the ancestral variant increased from 61% (22/36) pre-T+C injection to 100% (35/35) at 2 weeks and 100% (34/34) at 3 months (Exact McNemar, P < .001). Neutralization did not increase against BA.1, as observed in 8% (3/36) pre-T+C vs 11% (4/35) at 2 weeks and 0% (0/34) at 3 months (Exact McNemar, P = .6). Neutralization of BA.2, however, increased from 17% (6/36) pre-T+C to 86% (30/35) at 2 weeks and 62% (21/34) at 3 months (Exact McNemar, P < .001). BA.2.12.1 neutralization similarly increased from 19% (7/36) pre-T+C to 85% (30/35) at 2 weeks and 68% (23/34) at 3 months (Exact McNemar, P < .001). Neutralization of BA.4 increased from 25% (9/36) pre-T+C to 66% (23/35) at 2 weeks, but it was observed in only 15% (5/34) at 3 months (Exact McNemar, P < .001). These findings were extremely similar to those for BA.5, with 22% (8/36) pre-T+C vs 66% (23/35) at 2 weeks showing neutralization, falling to only 15% (5/34) at 3 months (Exact McNemar, P < .001).
Figure 2.
Longitudinal neutralizing inhibition against severe acute respiratory syndrome-coronavirus-2 variants following tixagevimab and cilgavimab (T+C) injection among fully vaccinated solid organ transplant recipients (SOTRs). Surrogate neutralization (%ACE2 inhibition) against the spike protein of the ancestral and Omicron BA.1, BA.2, BA.2.12.1, BA.4, and BA.5 sublineages was performed on all participant samples pre-T+C injection, and at 2 weeks, 1 month, and 3 months post-T+C injection. Each trajectory represents an individual SOTR, and those experiencing an infection following the first dose of T+C are highlighted with blue triangles. The Y axis represents %ACE2 inhibition (0%-100%). The proportion of participants demonstrating neutralizing inhibition at each timepoint (above the dashed orange line) is presented on the X axis; individuals developing incident COVID-19 infection prior to a given timepoint are excluded from the denominator. Neutralizing inhibition increased against the ancestral, BA.2, BA.2.12.1, BA.4, and BA.5 variants (P < .001 by the McNemar Exact test), but not against the BA.1 variant (P = .6), by 2 weeks. Neutralizing inhibition waned by 3 months post-T+C injection, as seen in <20% of participants against the BA.4/5 variant. %ACE2 inhibition, the inhibition of angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019.
Regarding posthoc subgroup analysis, a high inhibition of BA.4/5 at 3 months post-T+C injection was observed in individuals who developed incident SARS-CoV-2 infection (blue triangles, Fig. 2 [see section 3.6.]). Among the participants who received 2 150 + 150 mg doses and provided intercurrent blood samples (n = 7), a second 150 + 150 mg dose transiently improved neutralization across variants except for BA.1 (Supplementary Fig. S5). Within the supplemental group that received SARS-CoV-2 booster vaccination at any point following T+C injection (n = 5), 2 demonstrated approximately stable or increased crossvariant neutralization between 1 and 3 months post-T+C injection, including 1 participant who demonstrated high-level neutralizing inhibition against BA.1 (Supplementary Fig. S6).
3.6. SARS-CoV-2 infections
All (36/36) participants filled out 7-day and 3-month follow-up surveys. Two participants were diagnosed with SARS-CoV-2 infection during the follow-up, including 2 breakthrough infections. Specifically, 1 participant showed anti–N seroconversion at day 14 following the first 150 + 150 mg dose; no COVID-19 symptoms or positive tests were reported. The participant with a symptomatic breakthrough infection reported a positive COVID-19 test at day 65 following 300 + 300 mg dosing and anti–N seroconversion at day 90; the participant reported mild symptoms and received bebtelovimab for treatment. No other participant reported a positive COVID-19 test or demonstrated anti–N seroconversion.
4. Discussion
This real-world study of fully vaccinated SOTRs indicates that the 300 + 300 mg dose of T+C, currently under emergency use authorization in the USA, increases plasma neutralizing capacity against most Omicron sublineages, including BA.4/5. For example, using sensitive live virus assays at the peak drug concentration, 93% of the participants showed BA.4 nAbs, which increased 67-folds compared to the pre-T+C levels. In contrast, only one-third of the participants showed BA.1 nAbs despite high levels of binding antibodies. Importantly, using a validated surrogate neutralization assay, we demonstrated a relatively short duration of neutralizing inhibition against BA.4/5, with only 15% SOTRs showing inhibition by 3 months post-T+C injection.
Important aspects of this study include the confirmation of substantial variation in the neutralizing activity against Omicron sublineages after T+C injection using plasma samples from fully vaccinated, majority-boosted SOTRs; this serves as a real-world complement to the in vitro data presented in FDA emergency use authorization materials and prior lab-based studies. These data also support a mechanism behind the varying results of the studies on T+C effectiveness, namely, better evidence of the effect during the periods of BA.2 predominant circulation, particularly when using higher dosing of the drug, as recently shown by Al Jurdi et al.13 As extrapolation, 300 + 300 mg T+C may, therefore, augment short-term protection against SARS-CoV-2 infection in the current BA.4/5 era. It is also notable that surrogate neutralization testing (%ACE2 inhibition) showed a good discrimination and high correlation with the presence of live virus nAbs across Omicron sublineages. These data contextualize the use of such surrogate assays, which, like pseudoviruses, are of higher throughput and do not require specialized biosafety procedures.
Additionally, this study is the first to demonstrate the waning of the neutralizing capacity of T+C against BA.4/5 by 3 months post 300 + 300 mg injection in a diverse cohort of SOTRs (including thoracic and liver recipients). This extends prior work showing the waning of nAbs against earlier sublineages following 150 + 150 mg dosing and indicates that even a higher dosing strategy is subject to evasion by later Omicron sublineages. The potential reopening of an increased risk window for BA.4/5 infection by 3 months following T+C injection is an important factor for transplant clinicians in counseling SOTRs. SOTRs should be aware of the ongoing SARS-CoV-2 infection risk despite PrEP receipt and should not defer booster vaccination if eligible, given this might augment crossvariant neutralization beyond that afforded by these monoclonal antibodies. Importantly, only participants who showed baseline BA.4/5 neutralization pre-T+C injection (ie, an appropriate vaccine-associated humoral immune response) demonstrated neutralizing inhibition of BA.4/5 by 3 months post-T+C injection. Taken together, this study suggests that the recent FDA guideline to redose 300 + 300 mg T+C every 6 months may not represent an optimized interval in the current variant climate.
The limitations of this study include a small sample with variable vaccine responses on study entry, reflecting the real-world use of monoclonal antibody PrEP. Owing to the labor-intensive nature of sensitive live virus assays, these were not performed on all samples, which may lead to a conservative estimate of lower level nAb durability. However, the validation of surrogate neutralization at varying thresholds was performed to confirm the operating characteristics of this assay to specifically detect higher levels of nAb that may be necessary to prevent SARS-CoV-2 infection. We were also unable to precisely separate the neutralizing capacity from preceding vaccination from that of T+C. However, no participant was vaccinated within 30 days of peak sampling; thus, trends in neutralization by that timepoint likely represent the effects of T+C. Furthermore, the assessment of T+C effectiveness was somewhat limited by the reliance upon participant self-report. Survey response rates, however, were excellent and we utilized anti–N antibody testing to augment breakthrough ascertainment, in addition to capturing patient-reported at-home testing. The follow-up time, as with other published studies, predominately predated the BA.4/5 eras, and thus, longer follow-ups are necessary to correlate measures of neutralization with real-world effectiveness. Moreover, this study only reports the prevalence and dynamics of nAbs and does not include measures of cellular immune responses that may play a key role in the prevention of severe disease.
Taken together, these data suggest that T+C is a reasonable adjunct to vaccination for high-risk SOTRs and may augment humoral immunogenicity against Omicron BA.4/5 sublineages, even among those with preceding detectable binding antibodies. The protective effects, however, may not be long-lasting against SARS-CoV-2 infection amid an evolving variant climate. It remains important to consider vaccination as the backbone of immunoprotection for high-risk SOTRs and encourage adherence to the FDA and Centers for Disease Control and Prevention recommendations for dosing, particularly in the era of variant-specific boosters. Further research examining the durability of neutralization against emerging Omicron sublineages, such as BA.4.6, BA.2.75, and BQ.1, is necessary, given evolving mutations and the potential for further immune evasion.15 , 20 , 21
Funding
This work was supported by the Ben-Dov family, the Trokhan Patterson family, grants 5T32DK007713 (J. L. A.) and K01DK101677 (A. B. M.) from the National Institute of Diabetes and Digestive and Kidney Diseases, grants K24AI144954 (D. L. Segey), K08AI156021(A. H. Karaba), U01AI138897, 3U01AI138897-04S1, and K23AI157893(W. A. Werbel) from the National Institute of Allergy and Infectious Diseases, and grant U54CA260491 (A. Pekosz and A. H. Karaba) from the National Cancer Institute.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. D. L. Segey received consulting and/or speaking honoraria from Sanofi, Novartis, Veloxis, Mallinckrodt, Jazz Pharmaceuticals, CSL Behring, Thermo Fisher Scientific, Caredx, Transmedics, Kamada, MediGO, Regeneron, AstraZeneca, Takeda/Shire, Novavax, and Bridge to Life. R. K. Avery received grant/research support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, Regeneron, and Takeda/Shire. A. H. Karaba received consulting fees from Roche. W. A. Werbel received speaking fees from AstraZeneca and advisory board fees from Novavax. The remaining authors of this manuscript have no financial disclosures or conflicts of interest to disclose as described by the American Journal of Transplantation.
Data availability
Data supporting findings of this work are not publicly available due to their containing information that could compromise the privacy of research participants. Access to deidentified data are available on reasonable request from the corresponding author (W. A. Werbel, wwerbel1@jhmi.edu).
Acknowledgments
The authors thank the participants of the Johns Hopkins COVID-19 Transplant Vaccine Study, without whom this research would not have been possible. The authors also thank the members of the study team, including Brian J. Boyarsky, Jonathan Mitchell, Amy Chang, Chunyi Xia, Kim Hall, Mary Sears, Alex, and Jonathan Susilo.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajt.2022.11.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Benotmane I., Bruel T., Planas D., Fafi-Kremer S., Schwartz O., Caillard S. A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the Delta variant in kidney transplant recipients. Kidney Int. 2022;101(5):1073–1076. doi: 10.1016/j.kint.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamar N., Abravanel F., Marion O., et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA–based vaccine in recipients of a solid organ transplant. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaba A.H., Zhu X., Liang T., et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2022;22(4):1253–1260. doi: 10.1111/ajt.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin C.X., Moore L.W., Anjan S., et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105(11):e265–e266. doi: 10.1097/TP.0000000000003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J., Zheng Q., Madhira V., et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182(2):153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronavirus (COVID-19) Update . US Food and Drug Administration; 2021. FDA Authorizes New Long-Acting Monoclonal Antibodies for Pre-exposure Prevention of COVID-19 in Certain Individuals [press release] [Google Scholar]
- 7.Levin M.J., Ustianowski A., De Wit S., et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386(23):2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carreño J.M., Alshammary H., Tcheou J., et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602(7898):682–688. doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]
- 9.Takashita E., Yamayoshi S., Simon V., et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022;387(5):468–470. doi: 10.1056/NEJMc2207519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fact Sheet for Healthcare Providers: Emergency Use Authorization for Evusheld™ (tixagevimab co-packaged with cilgavimab). Food and Drug Administration. Accessed August 1, 2022.
- 11.Bruel T., Hadjadj J., Maes P., et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med. 2022;28(6):1297–1302. doi: 10.1038/s41591-022-01792-5. [DOI] [PubMed] [Google Scholar]
- 12.Benotmane I., Velay A., Gautier-Vargas G., et al. Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients. Am J Transplant. 2022;22(11):2675–2681. doi: 10.1111/ajt.17121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Jurdi A., Morena L., Cote M., Bethea E., Azzi J., Riella L.V. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am J Transplant. Published online. June 21, 2022 doi: 10.1111/ajt.17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaminski H., Gigan M., Vermorel A., et al. COVID-19 morbidity decreases with tixagevimab-cilgavimab preexposure prophylaxis in kidney transplant recipient nonresponders or low-vaccine responders. Kidney Int. 2022;102(4):936–938. doi: 10.1016/j.kint.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fact Sheet for Healthcare Providers: Emergency Use Authorization for Evusheld (tixagevimab co-packaged with cilgavimab) US Food and Drug Administration; 2022. [Google Scholar]
- 16.Wang Q., Guo Y., Iketani S., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benotmane I., Velay A., Vargas G.G., et al. A rapid decline in the anti-receptor-binding domain of the SARS-CoV-2 spike protein IgG titer in kidney transplant recipients after tixagevimab-cilgavimab administration. Kidney Int. 2022;102(5):1188–1190. doi: 10.1016/j.kint.2022.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werbel W.A., Boyarsky B.J., Ou M.T., et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330–1332. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan K., Karim F., Ganga Y., et al. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat Commun. 2022;13(4686) doi: 10.1038/s41467-022-32396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao Y., Jian F., Wang J., et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Preprint (bioRxiv) September 9, 2022 doi: 10.1101/2022.09.15.507787. Posted online. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting findings of this work are not publicly available due to their containing information that could compromise the privacy of research participants. Access to deidentified data are available on reasonable request from the corresponding author (W. A. Werbel, wwerbel1@jhmi.edu).