Abstract
BACKGROUND:
Young adults diagnosed with colorectal cancer (CRC) comprise a growing, yet understudied, patient population. We estimated 5-year relative survival of early-onset CRC and examined disparities in survival by race-ethnicity in a population-based sample.
METHODS:
We used the National Cancer Institute’s Surveillance, Epidemiology, and End Results program of cancer registries to identify patients diagnosed with early-onset CRC (20–49 years of age) between January 1, 1992, and December 31, 2013. For each racial-ethnic group, we estimated 5-year relative survival, overall and by sex, tumor site, and stage at diagnosis. To illustrate temporal trends, we compared 5-year relative survival in 1992–2002 vs 2003–2013. We also used Cox proportional hazards regression models to examine the association of race-ethnicity and all-cause mortality, adjusting for age at diagnosis, sex, county type (urban vs rural), county-level median household income, tumor site, and stage at diagnosis.
RESULTS:
We identified 33,777 patients diagnosed with early-onset CRC (58.5% White, 14.0% Black, 13.0% Asian, 14.5% Hispanic). Five-year relative survival ranged from 57.6% (Black patients) to 69.1% (White patients). Relative survival improved from 1992–2002 to 2003–2013 for White patients only; there was no improvement for Black, Asian, or Hispanic patients. This pattern was similar by sex, tumor site, and stage at diagnosis. In adjusted analysis, Black (adjusted hazard ratio [aHR], 1.42; 95% confidence interval [CI], 1.36–1.49), Asian (aHR, 1.06; 95% CI, 1.01–1.12), and Hispanic (aHR, 1.16; 95% CI, 1.10–1.21) race-ethnicity were associated with all-cause mortality.
CONCLUSION:
Our study adds to the well-documented disparities in CRC in older adults by demonstrating persistent racial-ethnic disparities in relative survival and all-cause mortality in patients with early-onset CRC.
Keywords: Colorectal Cancer, Survival, Disparities, Race-Ethnicity, Population-Based
Young adults diagnosed with colorectal cancer (CRC) comprise a growing, yet understudied, patient population.1,2 Incidence rates of early-onset CRC (<50 years of age) have increased in the United States since the early 1990s; mortality rates have improved little during the same time period.3
Early-onset CRC disproportionately affects racial and ethnic minorities. We previously reported in this journal differences in incidence rates of early-onset CRC by race and ethnicity, noting consistently higher rates in Black compared with White persons and large, relative increases in young (20–29 years of age) Hispanic persons.4 The extent to which these differences in incidence rates translate into differences in cancer survival remains less clear. Differences in cancer survival may reveal the influence of both upstream determinants and downstream factors across the cancer care continuum, ranging from social and environmental conditions to cancer treatment to biological response.5 A growing literature suggests Black patients diagnosed with early-onset CRC have experienced smaller gains in survival over time compared with White patients,6-8 but few studies have examined disparities in cancer survival in other patient populations with early-onset CRC, particularly Asian and Hispanic patients. To address this gap and extend the findings of our prior work, we estimated 5-year relative survival and examined disparities in survival by race and ethnicity in a diverse, population-based sample of patients diagnosed with early-onset CRC.
Materials and Methods
We identified patients newly diagnosed with incident, invasive CRC at 20–49 years of age using population-based data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program of cancer registries. We combined concepts of race and ethnicity as race-ethnicity to include patients who were non-Hispanic White (“White”), non-Hispanic Black (“Black”), non-Hispanic Asian or Pacific Islander (“Asian”), or Hispanic (of any race, “Hispanic”). For each racial-ethnic group, we estimated 5-year relative survival, overall and by sex, tumor site, and stage at diagnosis. To illustrate temporal trends, we plotted estimates of 5-year relative survival in 2 time periods: January 1, 1992, to December 31, 2002, and January 1, 2003, to December 31, 2013. We used Cox proportional hazards regression models to examine the association of race-ethnicity and all-cause mortality, adjusting for age at diagnosis, sex, county type (urban vs rural), county-level median household income (≤$49,999 vs $50,000–$74,999 vs ≥$75,000), tumor site, and stage at diagnosis. See the Supplementary Methods for details.
Results
We identified 33,777 patients diagnosed with early-onset CRC, of whom 19,759 (58.5%) were White, 4728 (14.0%) were Black, 4388 (13.0%) were Asian, and 4902 (14.5%) were Hispanic. Table 1 summarizes characteristics of the study population by race-ethnicity.
Table 1.
Characteristics of 33,777 Young Adults (20–49 Years of Age) Diagnosed With Colorectal Cancer by Race-Ethnicity (SEER 13, 1992–2013)
| Non-Hispanic White (n = 19,759 [58.5%]) |
Non-Hispanic Black (n = 4728 [14.0%]) |
Non-Hispanic Asian/Pacific Islander (n = 4388 [13.0%]) |
Hispanic (n = 4902 [14.5%]) |
All (N = 33,777 [100%]) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Age | ||||||||||
| 20–29 y | 864 | 4.4 | 194 | 4.1 | 217 | 4.9 | 350 | 7.1 | 1625 | 4.8 |
| 30–39 y | 3969 | 20.1 | 967 | 20.5 | 1003 | 22.9 | 1266 | 25.8 | 7205 | 21.3 |
| 40–49 y | 14,926 | 75.5 | 3567 | 75.4 | 3168 | 72.2 | 3286 | 67.0 | 24,947 | 73.9 |
| Sex | ||||||||||
| Male | 10,671 | 54.0 | 2328 | 49.2 | 2271 | 51.8 | 2561 | 52.2 | 17,831 | 52.8 |
| Female | 9088 | 46.0 | 2400 | 50.8 | 2117 | 48.2 | 2341 | 47.8 | 15,946 | 47.2 |
| Stage | ||||||||||
| Local | 6998 | 35.4 | 1493 | 31.6 | 1474 | 33.6 | 1593 | 32.5 | 11,558 | 34.2 |
| Regional | 7504 | 38.0 | 1656 | 35.0 | 1760 | 40.1 | 1829 | 37.3 | 12,749 | 37.7 |
| Distant | 4725 | 23.9 | 1372 | 29.0 | 1029 | 23.5 | 1293 | 26.4 | 8419 | 24.9 |
| Missing | 532 | 2.7 | 207 | 4.4 | 125 | 2.8 | 187 | 3.8 | 1051 | 3.1 |
| Tumor site | ||||||||||
| Proximal colon | 5820 | 29.5 | 1650 | 34.9 | 916 | 20.9 | 1325 | 27.0 | 9711 | 28.8 |
| Distal colon | 5629 | 28.5 | 1317 | 27.9 | 1422 | 32.4 | 1504 | 30.7 | 9872 | 29.2 |
| Rectum | 7795 | 39.4 | 1577 | 33.4 | 1961 | 44.7 | 1906 | 38.9 | 13,239 | 39.2 |
| Missing | 515 | 2.6 | 184 | 3.9 | 89 | 2.0 | 167 | 3.4 | 955 | 2.8 |
SEER, Surveillance, Epidemiology, and End Results.
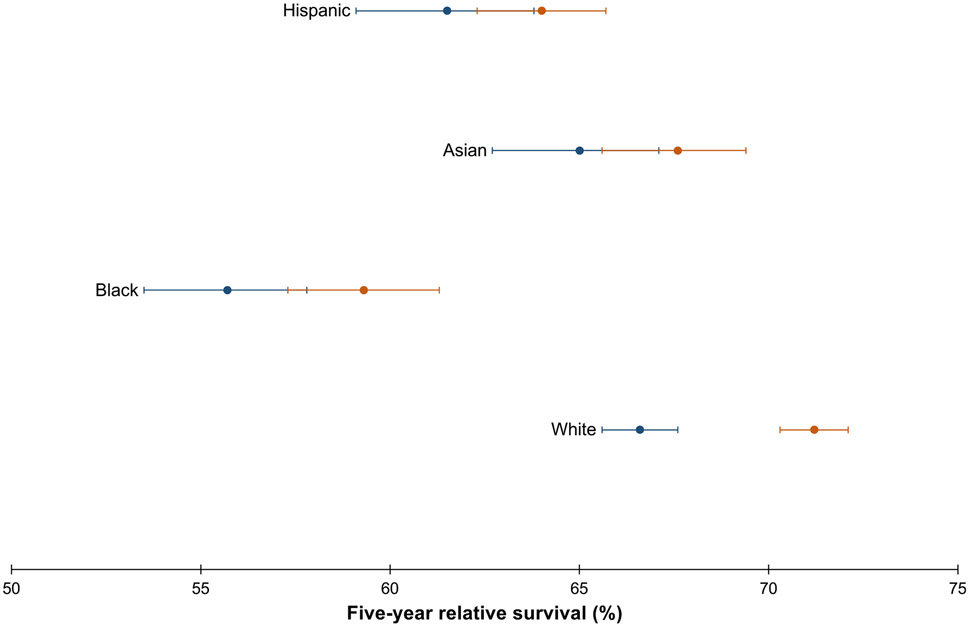
As shown in Table 2, 5-year relative survival ranged from 57.6% (Black patients) to 69.1% (White patients). Relative survival increased from 1992–2002 to 2003–2013 for White patients only, and there was no improvement in survival for the other racial-ethnic groups over time (Figure 1, Supplementary Table 1). Survival for Black patients diagnosed in 2003–2013 (59.3%; 95% confidence interval [CI], 57.3%–61.3%) remained lower compared with survival for White (66.6%; 95% CI, 65.6%–67.6%) and Asian (65.0%; 95% CI, 62.7%–67.1%) patients diagnosed in 1992–2002, as well as compared with Hispanic patients diagnosed in 2003–2013 (64.0%; 95% CI, 62.3%–65.7%).
Table 2.
Five-Year Relative Survival of Colorectal Cancer (20–49 Years of Age) by Race-Ethnicity, Overall and by Sex, Tumor Site, and Stage at Diagnosis (SEER 13, 1992–2013)
| Non-Hispanic White (n = 19,759) |
Non-Hispanic Black (n = 4728) |
Non-Hispanic Asian/Pacific Islander (n = 4388) |
Hispanic (n = 4902) | |
|---|---|---|---|---|
| Overall | 69.1 (68.4–69.7) | 57.6 (56.2–59.1) | 66.5 (65.0–67.9) | 63.1 (61.7–64.5) |
| Sex | ||||
| Male | 67.8 (66.9–68.7) | 56.5 (54.4–58.6) | 65.7 (63.6–67.6) | 61.7 (59.7–63.7) |
| Female | 70.6 (69.6–71.5) | 58.8 (56.7–60.8) | 67.3 (65.2–69.3) | 64.7 (62.6–66.6) |
| Tumor site | ||||
| Proximal colon | 66.1 (64.8–67.3) | 55.3 (52.8–57.7) | 65.2 (61.9–68.2) | 65.4 (62.7–68.0) |
| Distal colon | 69.8 (68.5–71.0) | 59.0 (56.2–61.7) | 65.1 (62.5–67.6) | 63.6 (61.0–66.1) |
| Rectum | 72.2 (71.2–73.2) | 61.3 (58.8–63.8) | 69.3 (67.1–71.3) | 63.5 (61.2–65.7) |
| Stage at diagnosis | ||||
| Local | 94.2 (93.6–94.8) | 89.2 (87.3–90.9) | 94.4 (93.0–95.6) | 90.9 (89.2–92.3) |
| Regional | 76.8 (75.8–77.8) | 66.1 (63.7–68.4) | 72.5 (70.3–74.6) | 72.4 (70.2–74.5) |
| Distant | 19.7 (18.5–20.8) | 13.1 (11.4–15.0) | 16.1 (13.9–18.5) | 17.0 (14.9–19.2) |
Values in parentheses are 95% confidence interval.
SEER, Surveillance, Epidemiology, and End Results.
Figure 1.
Five-year relative survival of CRC (20–49 years of age) with corresponding 95% CIs, by race-ethnicity, shown over the period 1992–2002 (blue dot) vs 2003–2013 (orange dot). Source: SEER 13.
Racial-ethnic disparities in relative survival by sex
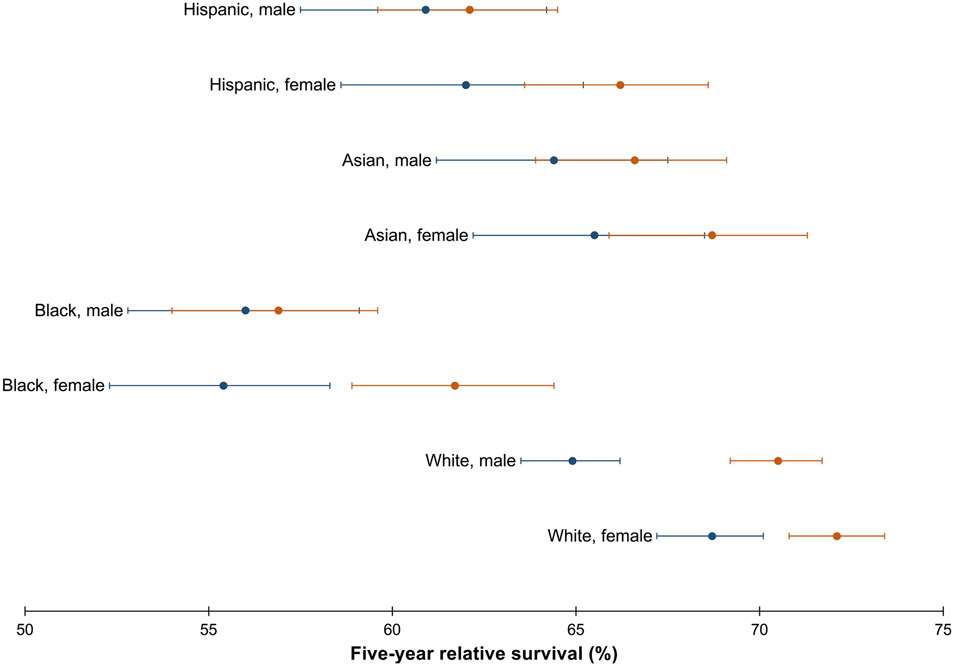
Relative survival was generally higher for women compared with men across all racial-ethnic groups (Table 2). Specifically, survival was lowest for Black men (56.5%; 95% CI, 54.4%–58.6%) and highest for White women (70.6%; 95% CI, 69.6%–71.5%). This pattern persisted over time (Figure 2). Survival increased from 1992–2002 to 2003–2013 for White men and women and Black women but not for other groups (Supplementary Table 1).
Figure 2.
Five-year relative survival of CRC (20–49 years of age) with corresponding 95% CIs, by race-ethnicity and sex, shown over the period 1992–2002 (blue dot) vs 2003–2013 (orange dot). Source: SEER 13.
Racial-ethnic disparities in relative survival by tumor site
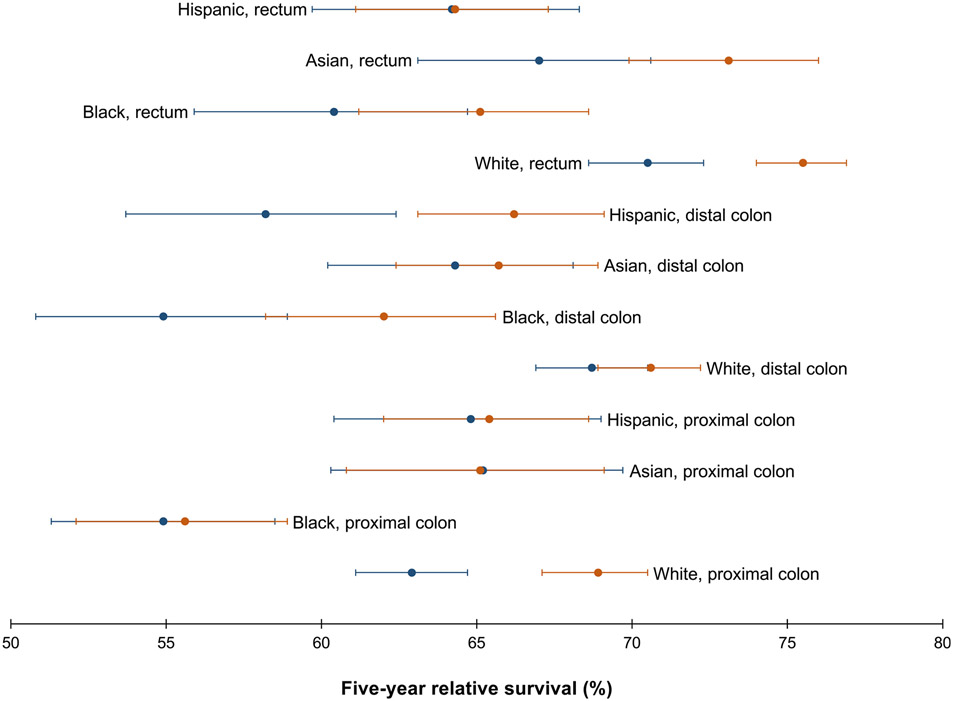
Relative survival differed by race-ethnicity and tumor site (Table 2), and was lowest for Black patients with proximal colon tumors (55.3%; 95% CI, 52.8%–57.7%) and highest for White patients with rectal tumors (72.2%; 95% CI, 71.2%–73.2%). Asian patients with rectal tumors (69.3%; 95% CI, 67.1%–71.3%) had better survival than Hispanic (63.5%; 95% CI, 61.2%–65.7%) and Black (61.3%; 95% CI, 58.8%–63.8%) patients with rectal tumors but similar to White patients (72.2%; 95% CI, 71.2%–73.2%). Survival differed by tumor site for White (proximal colon < distal colon < rectum) and Black (proximal colon < rectum) patients but was similar across tumor sites (ranging from 63.9% to 69.3%) for Asian and Hispanic patients. Improvements in survival from 1992–2002 to 2003–2013 (Figure 3) were limited to White patients with proximal colon (from 62.8% [95% CI, 61.0%–64.6%] to 69.1% [95% CI, 67.4%–70.8%]) and rectal (from 69.2% [95% CI, 67.6%–70.8%] to 74.6% [95% CI, 73.2%–75.9%]) tumors and Hispanic patients with distal colon tumors (from 58.2% [95% CI, 53.7%–62.5%] to 66.4% [95% CI, 63.2%–69.3%]) (Supplementary Table 1).
Figure 3.
Five-year relative survival of CRC (20–49 years of age) with corresponding 95% CIs, by race-ethnicity and tumor site, shown over the period 1992–2002 (blue dot) vs 2003–2013 (orange dot). Source: SEER 13.
Racial-ethnic disparities in relative survival by stage at diagnosis
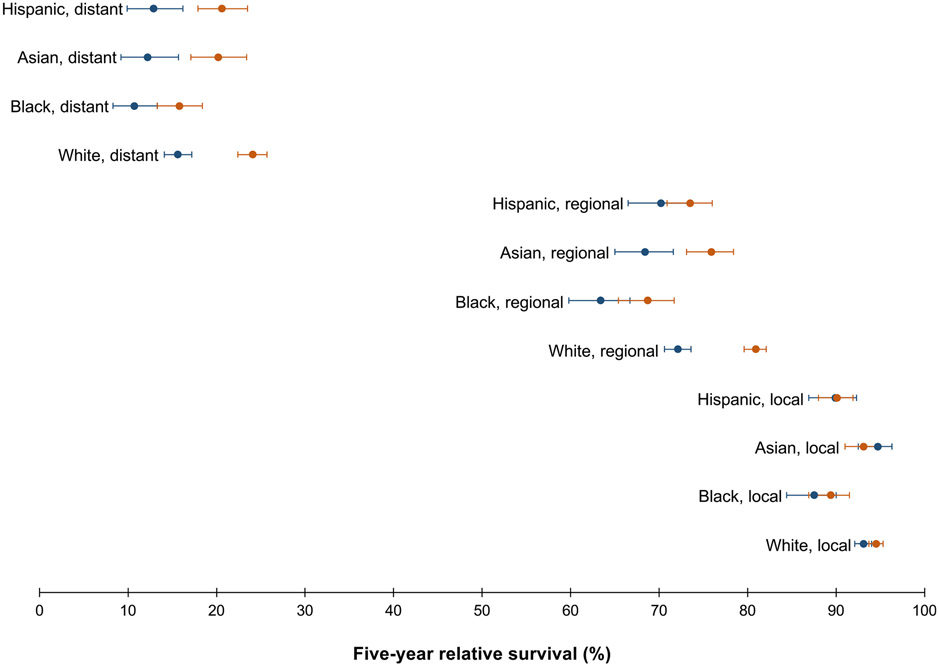
Across all racial-ethnic groups, 5-year relative survival was lowest for distant-stage disease, ranging from 13.1% (95% CI, 11.4%–15.0%) for Black patients to 19.7% (95% CI, 18.5%–20.8%) for White patients (Table 2). As shown in Figure 4, relative survival of distant-stage disease increased from 1992–2002 to 2003–2013 for all racial-ethnic groups except Black patients (from 10.5% [95% CI, 8.2%–13.2%] to 15.1% (95% CI, 12.7%–17.8%]). There was no improvement in survival over time for local-stage disease, although survival was generally high (Supplementary Table 1).
Figure 4.
Five-year relative survival of CRC (20–49 years of age) with corresponding 95% CIs, by race-ethnicity and stage at diagnosis, shown over the period 1992–2002 (blue dot) vs 2003–2013 (orange dot). Source: SEER 13.
Adjusted models of all-cause mortality
Supplementary Table 2 shows crude and adjusted hazard ratios (aHRs) demonstrating the association of race-ethnicity and all-cause mortality, adjusted for age at diagnosis, sex, county type, county-level household median income, tumor site, and stage at diagnosis. In adjusted analysis, Black (aHR, 1.40; 95% CI, 1.33–1.47), Asian (aHR, 1.07; 95% CI, 1.02–1.13), and Hispanic (aHR, 1.14; 95% CI, 1.08–1.19) race-ethnicity were associated with higher all-cause mortality.
Discussion
Our study demonstrates striking racial-ethnic disparities in relative survival and all-cause mortality in patients with early-onset CRC. Specifically, we observed lower 5-year relative survival of Hispanic and Asian patients diagnosed with early-onset CRC compared with White patients, and this difference persisted across sex, tumor site, and stage at diagnosis. Black patients also had worse survival than White patients. Disparities remained in models adjusted for other clinical and sociodemographic factors.
Relative survival was consistently worse for Hispanic and Asian compared with White patients, and Black patients recently diagnosed with early-onset CRC in our study had worse survival than White patients diagnosed a decade earlier. Notably, Hispanic and Asian patients had worse survival than White patients, despite the comparatively lower incidence rates of early-onset CRC in these 2 groups.4 Although racial-ethnic disparities in survival may be, in part, due to late stage diagnoses, differences remained in adjusted models accounting for clinical and sociodemographic factors. It is likely that these disparities reflect unequal access to, receipt of, and timeliness of cancer treatment; limited access to information,9 treatment,10,11 and high-quality care facilities12 impacts cancer survival in medically underserved and minority populations. For example, studies show Black, Asian, and Hispanic patients are less likely to receive adjuvant chemotherapy for colon cancer13,14 or radiation therapy for advanced rectal cancer15 compared with White patients. Health insurance coverage and pre-existing chronic conditions, such as obesity and diabetes,16-18 may also contribute to differences in survival, particularly the stark disparities among patients with distant disease.19
A limited number of population-based studies have examined CRC survival (across all ages) among Hispanic and Asian patients. We observed lower relative survival of Asian and Hispanic patients diagnosed with early-onset CRC compared with White patients. By contrast, Liang et al20 observed higher relative survival of Asian compared with White patients, although this study was conducted only among patients diagnosed at ≥65 years of age. Others report Asian and Hispanic patients have equivalent or better survival compared with White patients at comparable levels of income, education, and insurance, regardless of age at diagnosis.21 Hispanic and Asian populations are heterogeneous,22 and there may be differences in survival within these groups that have not yet been examined in the setting of early-onset CRC.
Some have suggested genetic and biological differences also drive disparities in early-onset CRC,23,24 based on the observation that epigenetic aging of the normal colon and immune response profiles of colon tumors25,26 differ between Black and White patients. Although differences in biological response may contribute to the disparities in early-onset CRC survival that we and others20,27 have observed, these factors are likely downstream consequences of upstream determinants—a broad set of social and environmental conditions that influence health.28 Upstream determinants are increasingly recognized as important influences across the cancer care continuum.29 For example, emerging research in epigenetics suggests that neighborhood characteristics (eg, poverty, safety, social cohesion)30 influence DNA methylation and may drive differences in cancer survival.31,32 Similarly, unequal access to and receipt of treatment may be related to interpersonal and structural racism.10,33 Mistrust of the healthcare system,34,35 language barriers,36 and cultural factors may also play a role.34,37,38
Relative survival was lowest among Black men and remained stagnant over time, and we observed a similar pattern for Hispanic men, albeit to a lesser degree. Relative survival was generally higher for women compared with men across all racial-ethnic groups. Reasons for these differences are not well understood but may be driven by both sex-related biologic factors (eg, endogenous sex hormones, immune response)39 and gender-related factors (eg, cultural belief systems, health-seeking behavior).34,38 Literature on sex and gender differences in early-onset CRC is limited: few studies have examined gender-related factors, and no studies have examined the intersection of gender and race.40 Instead, most studies report sex-related differences in survival, and survival is generally higher or similar in women vs men. For example, in a study conducted in the United Kingdom, men and women with early-onset CRC had similar survival,41 but a German study reported lower 5-year relative survival in men vs women with early-onset CRC.42 U.S. studies have also shown differences in survival between men and women.7
Our study adds to the well-documented disparities in survival of Black and White patients diagnosed with CRC.20,43-45 We previously reported smaller improvements in relative survival of proximal colon cancer but larger improvements in rectal cancer survival over time for younger Black compared with White patients.6 In the current study, we observed a similar pattern for proximal colon cancer but persistent Black-White disparities for rectal cancer, consistent with results from Acuna-Villaorduna et al.27 Elsewhere, Holowatyj et al7 observed lower cancer-specific survival in Black vs White patients with early-onset CRC but no difference in survival between Hispanic and White patients. Similar findings were noted by others.8,46 The differences in findings between these studies may be related to differences in time periods or populations examined.
An important strength of our study is the large and diverse (41% Black, Asian, or Hispanic) population-based sample. The diversity of our study population allowed us to examine relative survival of Asian and Hispanic patients diagnosed with early-onset CRC, groups that are underrepresented in research on this disease. There were some limitations. We used information on race and ethnicity collected by cancer registries as an imperfect proxy for social constructs; similarly, we used county-level measures of income and urban vs rural as proxies for socioeconomic status. The field will benefit from improved collection and use of individual-level information that more accurately capture social determinants of health. We did not include American Indian or Alaska Native patients due to small sample size, and this group generally has lower survival compared with White patients.47 Cancer registry data may not be uniformly accurate for some population groups, including racial-ethnic minorities; however, studies assessing the validity of SEER data show no racial-ethnic differences in case ascertainment (98%)48,49 and high agreement between SEER and self-reported race.50 We also could not examine the contribution of access to and receipt of treatment to disparities in relative survival because population-based cancer registries do not systematically collect this information. Further, information on tumor markers (eg, microsatellite instability) is often incomplete, and missingness differs by race-ethnicity.
In summary, our findings contribute to the growing literature on racial-ethnic disparities in early-onset CRC and extend knowledge by demonstrating lower relative survival and higher all-cause mortality of Asian and Hispanic compared with White patients. These findings serve as a forewarning for worsening disparities as 2 critical events continue to unfold in the United States. First, our study included patients diagnosed with early-onset CRC before the COVID-19 pandemic, and the social and economic impact of the pandemic will likely exacerbate existing cancer health disparities.51 Second, the U.S. Preventive Services Task Force recommended in May 2021 that CRC screening begin at 45 (vs 50) years of age.52 Although differences in the uptake of screening are unlikely to explain the disparities in our study conducted among patients diagnosed prior to new guidelines, implementation of these guidelines may disproportionately benefit the worried well.53 Future research and interventions should address disparities in this growing patient population by targeting both upstream determinants and downstream factors that contribute to inequities in CRC outcomes.
Supplementary Material
What You Need to Know.
Background
Early-onset colorectal cancer (CRC) is rising and disproportionately affects racial-ethnic minorities. We estimated 5-year relative survival of early-onset CRC and examined disparities by race-ethnicity in a diverse population-based sample.
Findings
Relative survival improved over time for White individuals only, and there was no improvement for Black, Asian, or Hispanic individuals. Black, Asian, and Hispanic race-ethnicity were associated with all-cause mortality.
Implications for patient care
Future research and interventions should address disparities in this growing patient population by targeting both upstream determinants and downstream factors that contribute to inequities in CRC outcomes.
Funding
Caitlin C. Murphy is supported by the National Cancer Institute, United States at the National Institutes of Health, United States under award number R01CA242558. Peter S. Liang is supported by the National Cancer Institute at the National Institutes of Health under award number K08CA230162. Folasade P. May is supported by the UCLA Jonsson Comprehensive Cancer Center, United States and Eli and Edythe Broad Center of Regenerative Medicine, United States and Stem Cell Research Ablon Scholars Program. The sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Abbreviations used in this paper:
- aHR
adjusted hazard ratio
- CI
confidence interval
- CRC
colorectal cancer
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Conflicts of Interest
These authors disclose the following: Caitlin C. Murphy reports consulting for Freenome. Folasade P. May reports consulting for Freenome, Medtronic, and Takeda. Peter S. Liang reports research support from Epigenomics and Freenome and consulting for Guardant Health. The remaining author discloses no conflicts.
CRediT Authorship Contributions
Timothy Andrew Zaki, MD (Conceptualization: Equal; Data curation: Lead; Formal analysis: Equal; Methodology: Equal; Writing – original draft: Lead; Writing – review & editing: Supporting)
Peter S. Liang, MD, MPH (Formal analysis: Supporting; Writing – review & editing: Supporting)
Folasade P. May, MD, PhD, MPhil (Formal analysis: Supporting; Writing – review & editing: Supporting)
Caitlin C. Murphy, PhD, MPH (Conceptualization: Equal; Data curation: Supporting; Formal analysis: Equal; Methodology: Equal; Supervision: Lead; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2022.05.035.
References
- 1.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst 2017;109:djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy CC, Lund JL, Sandler RS. Young-onset colorectal cancer: earlier diagnoses or increasing disease burden? Gastroenterology 2017;152:1809–1812.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER Research Data, 13 Registries, 2020 Sub (1992-2018) - Linked To County Attributes - Time Dependent (1990-2018) Income/Rurality, 1969-2019 Counties. National Cancer Institute, DCCPS, Surveillance Research Program. Available at: http://www.seer.cancer.gov. Accessed May 10, 2021. [Google Scholar]
- 4.Chang SH, Patel N, Du M, et al. Trends in early-onset vs late-onset colorectal cancer incidence by race/ethnicity in the United States Cancer Statistics Database. Clin Gastroenterol Hepatol 2022;20:e1365–e1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzora S, May FP, Ogedegbe G. COVID-19 and social determinants of health in gastroenterology and hepatology. Gastroenterology 2021;161:1373–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy CC, Wallace K, Sandler RS, et al. Racial disparities in incidence of young-onset colorectal cancer and patient survival. Gastroenterology 2019;156:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holowatyj AN, Ruterbusch JJ, Rozek LS, et al. Racial/ethnic disparities in survival among patients with young-onset colorectal cancer. J Clin Oncol 20 2016;34:2148–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arshad HMS, Kabir C, Tetangco E, et al. Racial disparities in clinical presentation and survival times among young-onset colorectal adenocarcinoma. Dig Dis Sci 2017;62:2526–2531. [DOI] [PubMed] [Google Scholar]
- 9.Busch EL, Martin C, DeWalt DA, et al. Functional health literacy, chemotherapy decisions, and outcomes among a colorectal cancer cohort. Cancer Control 2015;22:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bui A, Yang L, Myint A, May FP. Race, ethnicity, and socioeconomic status are associated with prolonged time to treatment after a diagnosis of colorectal cancer: a large population-based study. Gastroenterology 2021;160:1394–1396.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson DR, Martinez ME, Gupta S, et al. Racial disparity in consultation, treatment, and the impact on survival in metastatic colorectal cancer. J Natl Cancer Inst 2013;105:1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breslin TM, Morris AM, Gu N, et al. Hospital factors and racial disparities in mortality after surgery for breast and colon cancer. J Clin Oncol 2009;27:3945–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy CC, Harlan LC, Warren JL, et al. Race and insurance differences in the receipt of adjuvant chemotherapy among patients with stage III colon cancer. J Clin Oncol 2015; 33:2530–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tramontano AC, Chen Y, Watson TR, et al. Racial/ethnic disparities in colorectal cancer treatment utilization and phase-specific costs, 2000-2014. PLoS One 2020;15:e0231599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris AM, Billingsley KG, Baxter NN, et al. Racial disparities in rectal cancer treatment: a population-based analysis. Arch Surg 2004;139:151–155. [DOI] [PubMed] [Google Scholar]
- 16.Ottaiano A, Nappi A, Tafuto S, et al. Diabetes and body mass index are associated with neuropathy and prognosis in colon cancer patients treated with capecitabine and oxaliplatin adjuvant chemotherapy. Oncology 2016;90:36–42. [DOI] [PubMed] [Google Scholar]
- 17.Silber JH, Rosenbaum PR, Ross RN, et al. Racial disparities in colon cancer survival: a matched cohort study. Ann Intern Med 2014;161:845–854. [DOI] [PubMed] [Google Scholar]
- 18.Sinicrope FA, Foster NR, Yothers G, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer 2013; 119:1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser Family Foundation. Health coverage by race and ethnicity, 2010-2019. Available at: https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity/. Accessed December 29, 2021.
- 20.Liang PS, Mayer JD, Wakefield J, et al. Trends in sociodemographic disparities in colorectal cancer staging and survival: a SEER-Medicare analysis. Clin Transl Gastroenterol 2020;11: e00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamath SD, Torrejon N, Wei W, et al. Racial disparities negatively impact outcomes in early-onset colorectal cancer independent of socioeconomic status. Cancer Med 2021;10:7542–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastogi N, Xia Y, Inadomi JM, et al. Disparities in colorectal cancer screening in New York City: an analysis of the 2014 NYC Community Health Survey. Cancer Med 2019;8:2572–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holowatyj AN, Perea J, Lieu CH. Gut instinct: a call to study the biology of early-onset colorectal cancer disparities. Nat Rev Cancer 2021;21:339–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller C, Ihionkhan E, Stoffel EM, et al. Disparities in early-onset colorectal cancer. Cells 2021;10:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi AD, Chan AT. Racial differences in epigenetic aging of the colon: Implications for colorectal cancer. J Natl Cancer Inst 2020;113:1618–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paredes J, Zabaleta J, Garai J, et al. Immune-related gene expression and cytokine secretion is reduced among African American colon cancer patients. Front Oncol 2020;10:1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acuna-Villaorduna AR, Lin J, Kim M, et al. Racial/ethnic disparities in early-onset colorectal cancer: implications for a racial-ethnic-specific screening strategy. Cancer Med 2021; 10:2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gehlert S, Sohmer D, Sacks T, et al. Targeting health disparities: a model linking upstream determinants to downstream interventions. Health Aff (Millwood) 2008;27:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alcaraz KI, Wiedt TL, Daniels EC, et al. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin 2020;70:31–46. [DOI] [PubMed] [Google Scholar]
- 30.Danos DM, Ferguson TF, Simonsen NR, et al. Neighborhood disadvantage and racial disparities in colorectal cancer incidence: a population-based study in Louisiana. Ann Epidemiol 2018;28:316–321.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JA, Zhao W, Wang X, et al. Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: the Multi-Ethnic Study of Atherosclerosis. Epigenetics 2017;12:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stirzaker C, Zotenko E, Song JZ, et al. Methylome sequencing in triple-negative breast cancer reveals distinct methylation clusters with prognostic value. Nat Commun 2015;6:5899. [DOI] [PubMed] [Google Scholar]
- 33.Doubeni CA, Selby K, Levin TR. Disparities in preventable mortality from colorectal cancer: are they the result of structural racism? Gastroenterology 2021;160:1022–1025. [DOI] [PubMed] [Google Scholar]
- 34.Hammond WP. Psychosocial correlates of medical mistrust among African American men. Am J Community Psychol 2010; 45:87–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobs EA, Mendenhall E, McAlearney AS, et al. An exploratory study of how trust in health care institutions varies across African American, Hispanic and white populations. Commun Med 2011;8:89–98. [DOI] [PubMed] [Google Scholar]
- 36.Hendren S, Chin N, Fisher S, et al. Patients’ barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. J Natl Med Assoc 2011; 103:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray DM 2nd, Anyane-Yeboa A, Balzora S, et al. COVID-19 and the other pandemic: populations made vulnerable by systemic inequity. Nat Rev Gastroenterol Hepatol 2020; 17:520–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobralske MC. Health care seeking among Mexican American men. J Transcult Nurs 2006;17:129–138. [DOI] [PubMed] [Google Scholar]
- 39.Wichmann MW, Muller C, Hornung HM, et al. Colorectal Cancer Study Group. Gender differences in long-term survival of patients with colorectal cancer. Br J Surg 2001; 88:1092–1098. [DOI] [PubMed] [Google Scholar]
- 40.Crenshaw K. Demarginalizing the intersection of race and sex: a black feminist critique of antidiscrimination doctrine, feminist theory and antiracist politics, 139. Univ Chicago Legal Forum, 1989:8. [Google Scholar]
- 41.White A, Ironmonger L, Steele RJC, et al. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer 2018;18:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majek O, Gondos A, Jansen L, et al. Sex differences in colorectal cancer survival: population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS One 2013;8: e68077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas JS, Brawarsky P, Iyer A, et al. Association of area sociodemographic characteristics and capacity for treatment with disparities in colorectal cancer care and mortality. Cancer 2011; 117:4267–4276. [DOI] [PubMed] [Google Scholar]
- 44.White A, Vernon SW, Franzini L, et al. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer 2010; 116:4622–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salem ME, Puccini A, Trufan SJ, et al. Impact of sociodemographic disparities and insurance status on survival of patients with early-onset colorectal cancer. Oncologist 2021; 26:e1730–e1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang DY, Thrift AP, Zarrin-Khameh N, et al. Rising incidence of colorectal cancer among young Hispanics in Texas. J Clin Gastroenterol 2017;51:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perdue DG, Haverkamp D, Perkins C, et al. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska Native people, 1990-2009. Am J Public Health 2014;104(Suppl 3):S404–S414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer 1995;76:2343–2350. [DOI] [PubMed] [Google Scholar]
- 49.Thoburn KK, German RR, Lewis M, et al. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer 2007; 109:1607–1616. [DOI] [PubMed] [Google Scholar]
- 50.Clegg LX, Reichman ME, Hankey BF, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control 2007;18:177–187. [DOI] [PubMed] [Google Scholar]
- 51.Balzora S, Issaka RB, Anyane-Yeboa A, et al. Impact of COVID-19 on colorectal cancer disparities and the way forward. Gastrointest Endosc 2020;92:946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2022;162:285–299. [DOI] [PubMed] [Google Scholar]
- 53.Liang PS, Allison J, Ladabaum U, et al. Potential intended and unintended consequences of recommending initiation of colorectal cancer screening at age 45 years. Gastroenterology 2018; 155:950–954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.