Abstract
Tumor necrosis factor (TNF) and the type I TNF receptor (TNFRI), p55, are critical for resistance against primary infections with the intracellular bacterial pathogen Listeria monocytogenes. Importantly, however, susceptibility to primary listeriosis in cytokine-deficient mice does not preclude the development or expression of effective adaptive immunity against virulent L. monocytogenes. We used TNFRI−/− mice to study adaptive antilisterial immunity in the absence of interactions between TNF and TNFRI. Our experiments indicate that TNFRI−/− mice survive and clear high-dose challenges with an attenuated strain of L. monocytogenes that is incapable of cell-to-cell spread. Furthermore, TNFRI−/− mice immunized with attenuated L. monocytogenes go on to develop potent adaptive immunity to subsequent high-dose challenges with virulent L. monocytogenes. Interestingly, CD8+ T-cell depletion in vivo inhibits immunity to L. monocytogenes in the spleen but not in the liver of TNFRI−/− mice. The adaptive immune response in these animals is characterized by activation of listeriolysin O-specific CD8+ T cells, which are capable of transferring antilisterial immunity to naive wild-type C57BL/6 host mice. These experiments demonstrate the development and expression of potent CD8+ T-cell-mediated antilisterial immunity in the absence of TNFRI.
A number of cytokine and cytokine receptor deficiencies have been described which render mice more or less susceptible than wild-type animals to primary infection with the intracellular bacterial pathogen Listeria monocytogenes (13, 40). These studies have helped establish the importance of cytokines in the innate immune response to infection with L. monocytogenes. Tumor necrosis factor (TNF) and the type I TNF receptor (TNFRI), p55, comprise a cytokine-cytokine receptor pair that is clearly important in the normal immune response to L. monocytogenes. TNF is produced shortly following infection with L. monocytogenes, and neutralization of TNF with specific antibodies exacerbates listeriosis in mice (16, 17, 27). Administration of recombinant human TNF can also reduce the severity of primary infection with L. monocytogenes in mice (20, 33). The importance of this cytokine-receptor pair during the primary immune response to L. monocytogenes was confirmed when TNF−/− (28) and TNFRI−/− (9, 30, 34) mice were found to be highly susceptible to primary listeriosis. More recently, a functional death domain of TNFRI−/− has been shown to be required for antilisterial resistance (31).
The observation that TNF is critical during the early stages of the immune response to L. monocytogenes suggests that cells involved in the innate immune response produce the requisite TNF. This interpretation is further supported by studies in which nude (athymic) mice (15) or SCID mice (2), which lack mature T cells, were rendered more susceptible to L. monocytogenes infection by neutralization of TNF. A series of studies from Unanue's group using SCID mice helped uncover the basis of innate immunity to L. monocytogenes and defined an axis of cytokine-driven interactions between NK cells and macrophages which leads to the activation of listericidal activity in macrophages and is responsible for the early control of L. monocytogenes replication in normal mice (40, 41). TNF is a key mediator of macrophage activation in this process.
Importantly, susceptibility to primary listeriosis does not necessarily correlate with susceptibility to a secondary challenge (14). In fact, the most pronounced immunodeficiency described to date, as measured by susceptibility to primary challenge with virulent L. monocytogenes, occurs in mice with a targeted disruption of the gamma interferon (IFN-γ) gene. The 50% lethal dose (LD50) of virulent L. monocytogenes in these animals is approximately 10 CFU (12). However, adaptive immunity can be elicited by immunization with attenuated L. monocytogenes, which confers resistance in IFN-γ−/− mice to high-dose challenges with virulent L. monocytogenes (12).
In addition to its role in the innate response, neutralization studies suggested that TNF is important during a secondary response to L. monocytogenes in wild-type mice (35). This suggested that TNF, produced by cells of the adaptive immune system, may be involved in adaptive immunity to L. monocytogenes. Since CD8+ T cells readily produce TNF in response to antigen-specific stimulation and since CD8+ T cells are important mediators of adaptive immunity to L. monocytogenes, we hypothesized that TNF-TNFRI interactions might be required in adaptive immunity to L. monocytogenes. This hypothesis was further suggested by experiments showing that CD8+ T cells from perforin knockout mice provide antilisterial immunity in hosts with depleted IFN-γ but fail to do so in hosts with depleted TNF (43).
In the present studies, we used attenuated L. monocytogenes to immunize TNFRI−/− mice and study adaptive immunity to L. monocytogenes in the absence of interactions between TNF and TNFRI. We provide evidence that neither the development nor the expression of adaptive immunity to L. monocytogenes requires TNFRI. We further demonstrate that, at least in the spleen, adaptive immunity to L. monocytogenes in TNFRI−/− mice requires CD8+ T cells, indicating that CD8+ T-cell-mediated immunity to L. monocytogenes in the spleen can function independently of TNFRI.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6, H-2b major histocompatibility complex [MHC]) mice were obtained from the National Cancer Institute (Frederick, Md.). TNFRI-deficient (TNFRI−/−) mice (30) were the kind gift of Amgen, Inc., Toronto, Canada. B6 and TNFRI−/− mice were housed at the University of Iowa animal care unit. Mice were matched for age and sex and used at 8 to 16 weeks of age.
Bacteria.
Virulent L. monocytogenes strains 10403s (4) and XFL204, attenuated L. monocytogenes strains DP-L1942 (ActA−) (5) and DP-L1936 (PlcA−B−) (38), and Salmonella enterica serovar Typhimurium strain SL1344 (18) are all resistant to streptomycin. Recombinant L. monocytogenes XFL204 was kindly provided by H. Shen, University of Pennsylvania. XFL204 is derived from 10403s and was engineered using previously described strategies (37) to secrete a fusion protein consisting of dihydrofolate reductase and amino acids 396 to 404 of the nucleoprotein (NP) of lymphocytic choriomeningitis virus (LCMV) (X. Fan, unpublished data). NP396–404 is a well-characterized H-2Db-restricted CD8+ T-cell epitope from LCMV (42). Frozen stocks of bacteria were diluted in TSB and grown in a bacterial shaker at 37°C to an optical density at 600 nm of approximately 0.1 (approximately 108 CFU/ml), diluted in pyrogen-free saline (Abbott Laboratories, North Chicago, Ill.), and injected intravenously (i.v.) or intraperitoneally (i.p.) as indicated in 0.2-ml volumes per animal. Aliquots were plated onto tryptic soy agar containing 50 μg of streptomycin per ml (TSA-Strep) to verify the number of CFU injected.
Cell lines and cell culture.
EL4 is a C57BL/6-derived thymoma cell line (H-2b MHC; ATCC TIB-39); EL4-LLO refers to EL4 cells stably transfected with a plasmid construct expressing listeriolysin O (LLO) and G418 resistance (11). Cell lines were maintained in RPMI 1640 (Gibco BRL, Grand Island, N.Y.) supplemented with 10% fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 50 μg of gentamicin per ml, 10 mM HEPES, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol (RP10). Transfected cells were maintained in RP10 supplemented with 400 μg of G418 per ml.
Hybridomas and monoclonal Abs.
Our studies utilized the following monoclonal antibodies (Abs), which were purified from hybridoma supernatants: rat anti-mouse CD8 (2.43 [36]) and rat anti-mouse CD4 (GK1.5 [7]). Control polyclonal rat immunoglobulin G (IgG) was purchased from Sigma (St. Louis, Mo.). Monoclonal Abs were purified from culture supernatants using protein G affinity chromatography as recommended by the manufacturer (Pharmacia). Protein concentrations were determined using the bicinchoninic acid assay (Pierce). Flow cytometric analysis was performed using fluorescein isothiocyanate-conjugated anti-CD8 (53.6-7) (Sigma) and phycoerythrin PE-conjugated anti-CD4 (H129.19) (Sigma).
T-cell subset depletion in vivo was carried out by injecting mice i.p. with a total of 1 mg of 2.43, GK1.5, or control rat IgG per animal in divided doses for two or three consecutive days prior to L. monocytogenes challenge (12). CD8+ and CD4+ T-cell subset depletions were quantitated by flow cytometry by dividing the percentage of cells in the relevant subset of a depleted spleen by the percentage of cells in the same subset in a control Ig-treated spleen.
Generation and maintenance of CD8+ T-cell lines.
H-2b MHC CD8+ T-cell lines specific for LLO were derived from B6 mice and H-2b TNFRI−/− mice and were restimulated with EL4-LLO cells. A total of 2 × 107 to 4 × 107 splenocytes from mice injected 7 to 10 days previously with the indicated dose of virulent L. monocytogenes 10403s or attenuated L. monocytogenes DP-L1942 were incubated with 3 × 106 irradiated (150 Gy) EL4-LLO stimulator cells in RP10 at 37°C under 7% CO2. In some experiments (as indicated), infected mice were treated i.p. with ampicillin at 2 mg/mouse/day on days 1 to 3 postinfection. For T-cell lines specific for NP396–404, EL4 cells supplemented with 100 nM synthetic NP396–404 were used as stimulators. Subsequent weekly restimulations were carried out by combining 3 × 106 to 5 × 106 responder cells with 3 × 106 irradiated (150 Gy) stimulator cells and approximately 4 × 107 irradiated (30 Gy) syngeneic splenocytes in RP10 supplemented with 5% supernatant from concanavalin A-stimulated rat spleen cells and 50 mM α-methylmannoside.
51Cr release assays.
51Cr release assays were performed by labeling 1.1 × 106 target cells (EL4 or EL4-LLO) for 1 h at 37°C under 7% CO2 in 0.2 ml of RP10 with 100 μCi of sodium [51Cr] chromate (NEN, Boston, Mass.) and rinsing them three times with 10 ml of phosphate-buffered saline. Then 104 labeled target cells per well were combined with effector cells at the indicated ratios in RP10 in round-bottom 96-well plates. Following a 4-h incubation, supernatant was harvested and assayed for 51Cr release in a gamma counter (Wallac, Turku, Finland). Spontaneous and total release were determined by incubating target cells alone in RP10 or 0.5% Triton X-100, respectively. The percent specific release of 51Cr was calculated by the formula 100 × (experimental cpm − spontaneous cpm)/(total cpm − spontaneous cpm). Spontaneous release was less than 15% of total in all assays.
Adoptive transfer experiments.
The capacity of CD8+ T cells to mediate antilisterial immunity in vivo was quantitated using adoptive-transfer assays. CD8+ T cells restimulated in vitro 7 to 9 days previously were harvested, washed in antibiotic-free buffer, and resuspended in pyrogen-free saline. The cells were delivered i.v. in 0.2- to 0.5-ml volumes into naive host mice. Within 2 h, host mice, including uninjected controls, were challenged i.v. with the indicated dose of bacteria. The numbers of CFU per spleen and per liver were determined 3 days postchallenge by homogenizing the spleens and livers in 0.2% IGEPAL (Sigma), plating 10-fold serial dilutions onto TSA-Strep and calculating mean colony counts after overnight incubation at 37°C. Data are presented as mean log10 CFU ± standard deviation per spleen or per gram of liver. Student's t test was used in statistical analysis; P values are shown for each group compared to the control group in the same experiment which did not receive protective T cells.
Survival assays.
The susceptibility of different strains of mice to infection with virulent L. monocytogenes was quantitated by estimating the lethal dose of 10403s in 50% of the animals (LD50) by the method of Reed and Muench (32).
RESULTS
Estimation of the LD50 of virulent L. monocytogenes 10403s in TNFRI−/− mice.
Previous studies have demonstrated that TNFRI−/− mice succumb to primary infection with low doses of virulent LM (250 to 500 CFU) that are sublethal for wild-type mice (9, 30, 34). It was of interest to estimate the lower limit of susceptibility of these animals to virulent L. monocytogenes and to explore the possibility that very low challenges (<250 CFU) with virulent L. monocytogenes might cause chronic but nonlethal infections in TNFRI−/− animals. To estimate the LD50 of virulent L. monocytogenes 10403s in TNFRI−/− mice, naive TNFRI−/− animals were injected i.v. with graded doses of 10403s and monitored for survival. CFU analyses were performed in triplicate on the bacterial suspensions which were used to inject the mice. These indicated that 80 to 115% of the expected dose was delivered. Whereas all wild-type B6 mice that received 103 CFU of virulent L. monocytogenes survived at least 27 days, all TNFRI−/− mice that received the same dose of virulent L. monocytogenes died within 8 days of challenge. Within 11 days, 43% (3 of 7) of the TNFRI−/− mice that received 102 CFU of virulent L. monocytogenes had died. All TNFRI−/− mice that were challenged with 101 CFU of virulent L. monocytogenes survived at least 27 days. These results reveal that the LD50 of virulent L. monocytogenes 10403s in naive TNFRI−/− is approximately 102.
The LD50 of virulent strain 10403s administered i.v. to B6 mice was approximately 104.7 (data not shown). Thus, compared to wild-type mice, TNFRI−/− mice are highly susceptible to primary infection with virulent L. monocytogenes. The LD50 of 10403s is approximately 500-fold lower in TNFRI−/− mice than in wild-type animals.
Chronic L. monocytogenes infection in immunocompromised mice has been observed previously (1, 23, 40). To test whether TNFRI−/− mice are susceptible to chronic infections with virulent L. monocytogenes, all surviving mice were sacrificed at 27 or 33 days postchallenge and examined for the presence of L. monocytogenes. CFU analysis performed on spleen and liver homogenates with limits of detection of 50 CFU/spleen and 100 CFU/g of liver failed to detect persistent L. monocytogenes infection in any mice.
These results verify the extreme susceptibility of TNFRI−/− to primary challenge with virulent L. monocytogenes compared to that of control B6 mice. Additionally, the data indicate that TNFRI−/− mice can clear infections with small numbers of virulent L. monocytogenes, since there was no evidence of chronic infection in TNFRI−/− mice that survived near-lethal challenges.
TNFRI−/− mice survive high-dose challenges with an attenuated strain.
Secondary immunity to L. monocytogenes is mediated most efficiently by CD8+ T cells in wild-type and IFN-γ−/− mice (12, 13). The susceptibility of TNFRI−/− mice to primary L. monocytogenes infection suggests that immunization of these mice using virulent L. monocytogenes may be problematic since necessarily low (and therefore highly variable with respect to the LD50) challenge doses of virulent L. monocytogenes may not adequately or consistently prime T-cell responses. To determine the feasibility of immunizing TNFRI−/− mice with attenuated L. monocytogenes, two attenuated strains, DP-L1942 (5) and DP-L1936 (38), were used at high doses to challenge TNFRI−/− mice. DP-L1942 carries an engineered in-frame deletion in the actA gene, which encodes a protein involved in actin polymerization and cell-to-cell spread (8, 22). DP-L1942 is effective for immunization of IFN-γ−/− mice and activates the CD8+ T-cell compartment in IFN-γ−/− and wild-type mice (10, 12). DP-L1936 carries an engineered in-frame deletion in the genes for phospholipases A and B (plcA and plcB), which are involved in the escape of the bacterial cell from the primary (plcB) and secondary (plcA and plcB) phagosomes (38, 39). Both DP-L1942 and DP-L1936 are attenuated in wild-type mice, with LD50s of approximately 107 and 106.5, respectively (5, 38). TNFRI−/− and B6 mice were challenged with high doses of both attenuated strains and monitored for survival (Table 1). Whereas all of the animals survived high-dose challenge with DP-L1942, all TNFRI−/− mice challenged with 106 CFU of DP-L1936 succumbed to infection. Mortality was also observed with DP-L1936 at doses as low as 104 CFU per animal.
TABLE 1.
TNFRI−/− mice survive high-dose challenges with attenuated L. monocytogenes DP-L1942 (actA) but are more susceptible than B6 mice to attenuated DP-L1936 (plcAB)a
| Strain (genotype) | L. monocytogenes dose (CFU) | Mouse strain | No. of mice that survived/total no. |
|---|---|---|---|
| DP-L1942 (actA) | 106 | C57BL/6 | 3/3 |
| 106 | TNFRI−/− | 3/3 | |
| DP-L1936 (plcAB) | 107 | C57BL/6 | 2/3 |
| 107 | TNFRI−/− | 0/3 | |
| 106 | TNFRI−/− | 0/5 | |
| 105 | TNFRI−/− | 3/4 | |
| 104 | TNFRI−/− | 3/4 |
B6 and TNFRI−/− mice were infected i.v. with the indicated doses of attenuated L. monocytogenes strain DP-L1942 or DP-L1936 and monitored for survival for 20 to 30 days. All nonsurviving mice died within 9 days of challenge. Mice that survived for 20 days after the challenge with DP-L1936 were analyzed for L. monocytogenes CFU in the spleen and liver, and no colonies were detected (limit of detection, 50 CFU/spleen and approximately 100 CFU/g of liver). The data from DP-L1942-immunized mice are representative of at least six independent experiments. The remaining data are pooled from two independent experiments.
In a separate experiment, one of three TNFRI−/− mice had detectable L. monocytogenes (∼103 CFU per spleen and per g of liver) at 7 days after infection with 106 CFU DP-L1942 while none of three wild-type B6 mice had detectable organisms (limit of detection, 100 CFU/organ). The levels of L. monocytogenes were below these limits in TNFRI−/− mice at 10 days after infection with 106 CFU of DP-L1942. Similar results, indicating a slight delay in clearance of DP-L1942 in immunocompromised mice, were found with IFN-γ−/− mice (V. P. Badovinac, A. Tvinnereim, and J. T. Harty, submitted for publication). However, in all cases examined to date, clearance of the actA mutant was complete by 10 days postinfection (p.i.). This is consistent with the course of sublethal L. monocytogenes infections in wild-type mice, which are cleared by approximately 10 days p.i. In contrast, chronic infections are observed in SCID mice, which lack adaptive immune systems (3), and slp-76−/− mice, which lack T cells (26). Thus, the results with DP-L1942 are consistent with those of previous studies in wild-type and IFN-γ−/− mice and indicate that (i) clearance of attenuated LM DP-L1942 from TNFRI−/− mice is rapid and complete and (ii) immunization of TNFRI−/− mice results in an adaptive immune response which may provide protection against secondary challenges with virulent LM.
Antigen-specific adaptive immunity to virulent L. monocytogenes in TNFRI−/− mice.
Survival of TNFRI−/− mice following high-dose challenge with attenuated L. monocytogenes might only reflect the activity of the innate immune response and does not demonstrate the development of secondary immunity. To test whether a high-dose challenge with attenuated L. monocytogenes DP-L1942 leads to the development of secondary resistance in TNFRI−/− mice, naive and DP-L1942-immunized TNFRI−/− mice were challenged with graded doses of virulent strain 10403s and monitored for survival (Table 2). Naive and immunized B6 animals were included as controls. Consistent with the experiments described above, all naive TNFRI−/− mice challenged with 2 × 103 CFU of virulent L. monocytogenes died within 8 to 9 days of challenge. In contrast, all TNFRI−/− mice that had been previously immunized with 106 CFU of attenuated strain DP-L1942 survived challenges with 2 × 105 CFU of virulent strain 10403s. Four of five of the immunized TNFRI−/− mice (80%) survived challenges with 2 × 106 CFU of virulent L. monocytogenes, and one of three TNFRI−/− mice (33%) survived challenge with 10-fold more L. monocytogenes CFU. These data indicate that resistance to virulent strain 10403s in TNFRI−/− mice undergoing secondary challenge is at least 10,000-fold greater than resistance to this strain in naive TNFRI−/− mice.
TABLE 2.
Previously immunized TNFRI−/− mice exhibit high levels of resistance to secondary challenge with virulent L. monocytogenesa
| Mouse strain | Challenge dose (CFU) | No. of mice that survived/total no. |
|---|---|---|
| B6 | ||
| Naive | 2 × 106 | 2/6 |
| Immune | 2 × 106 | 6/6 |
| TNFRI−/− | ||
| Naive | 2 × 103 | 0/2 |
| Immune | 2 × 105 | 5/5 |
| Immune | 2 × 106 | 4/5 |
| Immune | 2 × 107 | 1/3 |
B6 or TNFRI−/− mice which had been immunized i.p. with 106 CFU of attenuated L. monocytogenes DP-L1942 28 days previously were challenged i.v. with the indicated dose of virulent strain 10403s, and survival was monitored for at least 43 days. These data are pooled from two independent experiments, the second of which did not include a repeat analysis for naive TNFRI−/− mice or immune TNFRI−/− mice challenged with 2 × 107 CFU of virulent L. monocytogenes.
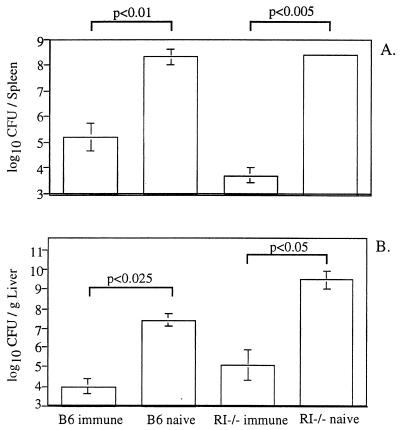
To examine the severity of infection in naive and immune TNFRI−/− mice challenged with virulent L. monocytogenes, CFUs in the spleen (Fig. 1A) and the liver (Fig. 1B) were measured 3 days after a high-dose challenge with virulent strain 10403s. TNFRI−/− mice that had been previously immunized with attenuated L. monocytogenes showed dramatic reductions in CFUs in both the spleen and the liver compared to naive animals. Immune TNFRI−/− mice and immune B6 mice were equally capable of controlling secondary infection with virulent L. monocytogenes by day 3 p.i. Both B6 and TNFRI−/− mice that had not been previously exposed to attenuated L. monocytogenes suffered severe listeriosis in the spleen and liver, with high levels of infection. Interestingly, while the primary infection in the livers of naive TNFRI−/− mice (109.5 CFU) was more severe than the primary infection in the livers of naive B6 mice (107.4 CFU), there was no apparent difference in the severity of primary infection in the spleen. However, with lower doses of virulent L. monocytogenes administered i.p., a more severe infection has been observed in both the spleens and the livers of naive TNFRI−/− mice than in those of naive B6 mice 3 days following primary infection (9). Combined with the survival studies, these results demonstrate that immunization of TNFRI−/− mice with attenuated L. monocytogenes leads to the development of adaptive immunity to high-dose challenges with virulent L. monocytogenes.
FIG. 1.
Immunized TNFRI−/− mice exhibit high levels of resistance to secondary challenge with virulent L. monocytogenes. B6 or TNFRI−/− (RI−/−) mice, which were naive or had been immunized i.p. with 106 CFU attenuated L. monocytogenes DP-L1942 28 days previously, were challenged i.v. with 1.7 × 105 CFU of virulent strain 10403s. CFUs in the spleen (A) and liver (B) were measured 3 days later. Data are presented as mean log10 CFU and standard deviation for two to four mice per group. Student's t test was used to calculate P values. These data are representative of two independent experiments with similar results.
To verify the antigen specificity of secondary resistance to L. monocytogenes in TNFRI−/− mice, groups of five to seven TNFRI−/− mice were immunized with 106 CFU of attenuated strain DP-L1942 and 7 weeks later the immune mice and naive controls were challenged with 104 CFU of virulent L. monocytogenes 10403s or 102 CFU of the unrelated bacterium S. enterica serovar Typhimurium SL1344 (18). All the animals were then monitored for survival. Consistent with the data in Table 2, five of five immunized TNFRI−/− mice survived challenge with virulent strain 10403s while six of six naive TNFRI−/− mice challenged with virulent strain 10403s died. Immune mice were not resistant to challenge with virulent S. enterica serovar Typhimurium SL1344, since all seven of these mice also succumbed. Similarly, five of six naive TNFRI−/− mice challenged with virulent S. enterica serovar Typhimurium SL1344 died.
These results indicate that immunization of TNFRI−/− mice with attenuated L. monocytogenes does not result in resistance, as measured by survival, to an unrelated intracellular bacterial pathogen. Since the LD50 of SL1344 in wild-type mice is very low (∼25 organisms) (29), we did not perform a direct comparison of the virulence of this organism in naive wild-type versus naive TNFRI−/− mice.
These data are also consistent with other experiments (results not shown) in which TNFRI−/− mice remain resistant to high-dose challenges with virulent L. monocytogenes for up to 16 weeks after immunization with DP-L1942. Thus, clearance of DP-L1942 by TNFRI−/− mice not only is rapid and complete but also results in long-lasting immunity to challenges with otherwise lethal doses of virulent L. monocytogenes.
CD8+ T cells in TNFRI−/− mice respond in an antigen-specific fashion following immunization with L. monocytogenes.
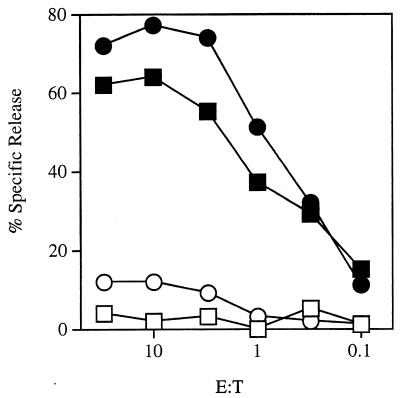
Since the CD8+ T-cell response plays an important role in adaptive antilisterial immunity in wild-type mice (25), it was of interest to determine whether a CD8+ T-cell response develops in TNFRI−/− mice following infection with L. monocytogenes. Wild-type or perforin-deficient H-2b mice infected with L. monocytogenes mount H-2Kb-restricted CD8+ T-cell responses to LLO, a protein antigen secreted by L. monocytogenes (11). Splenocytes from TNFRI−/− mice, previously immunized with 106 CFU of attenuated strain DP-L1942, were restimulated in vitro with irradiated syngeneic stimulator EL4-LLO cells. Following two restimulations in vitro, these effector cells (which were 98% CD4− CD8+ by flow cytometric analysis [data not shown]) were tested for antigen-specific cytolytic activity in a standard 51Cr release assay (Fig. 2). TNFRI−/−-derived CD8+ T cells specific for LLO efficiently lysed target cells in an antigen-specific fashion. These results indicate that L. monocytogenes infection in TNFRI−/− mice activates LLO-specific CD8+ T cells.
FIG. 2.
CD8+ T cells from L. monocytogenes-immunized TNFRI−/− mice exhibit antigen-specific cytolysis of target cells expressing LLO. LLO-specific CD8+ T cells derived from TNFRI−/− mice (squares) or B6 mice (circles) were incubated at the indicated effector-to-target ratio (E:T) with 51Cr-labeled EL4 (open symbols) or 51Cr-labeled EL4-LLO (solid symbols) cells for 3.5 h. Specific lysis was determined by measuring 51Cr in the supernatant by standard techniques. These data are representative of two independent experiments with similar results.
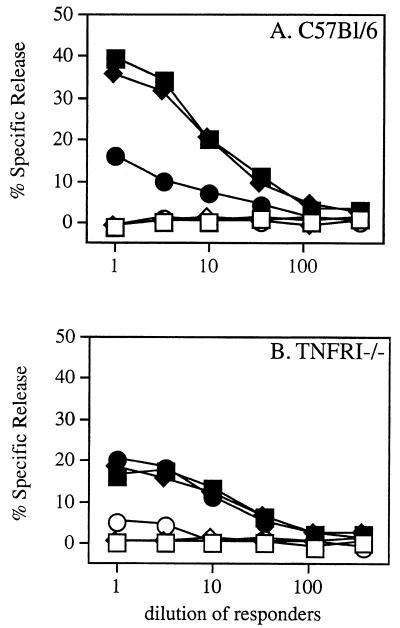
One limitation of our studies of listeriosis in TNFRI−/− mice (which bear MHC molecules of the H-2b haplotype) is that no endogenous MHC class Ia-restricted CD8+ T-cell epitopes have been defined in the H-2b system. It was of interest to measure the CD8+ T-cell response in TNFRI−/− mice against a defined H-2b-restricted epitope. Toward that end, TNFRI−/− and B6 mice were immunized with high doses of virulent L. monocytogenes XFL204. Strain XFL204 secretes a fusion protein containing a known H-2Db-restricted CD8+ T-cell epitope derived from the nucleoprotein of LCMV (NP396–404) (H. Shen et al., unpublished data). Challenge doses of XFL204 in B6 and TNFRI−/− mice were normalized to approximately 10 LD50 (data not shown). To allow survival of the animals following high-dose challenge with virulent strain XFL204, the animals were injected i.p. with ampicillin (2 mg/animal/day) on days 1 to 3 p.i. All B6 and TNFRI−/− animals subjected to this regimen survived for at least 1 week post challenge. At 7 days postchallenge, splenocytes from each animal were cultured in vitro with the NP396–404 peptide. Following 6 days of restimulation in vitro, responders were analyzed for antigen-specific cytolytic activity in a standard 51Cr release assay (Fig. 3). The results reveal NP396–404-specific CD8+ T-cell expansion and cytolytic activity in all animals, regardless of genotype, subjected to primary infection with a high dose of virulent L. monocytogenes XFL204 and given antibiotic therapy. The 51Cr release assay used in these experiments is not sufficiently quantitative to conclude that differences in the level of response are significant.
FIG. 3.
Antigen-specific expansion of CD8+ T cells in TNFRI−/− mice following high-dose challenge with virulent L. monocytogenes. Wild-type B6 (A) or TNFRI−/− (B) mice were injected with approximately 10 LD50 of virulent L. monocytogenes XFL204 (105 in B6 mice and 103 in TNFRI−/− mice) followed by a 3-day course of antibiotic therapy. At 7 days postchallenge, splenocytes were harvested from each animal and were cultured in vitro with irradiated EL4 cells and NP396–404 at 100 nM. After 6 days in vitro, responders were incubated at the indicated dilutions with 51Cr-labeled EL4 cells in the absence (open symbols) or presence (solid symbols) of NP396-404 at 100 nM for 4 hours. Each line represents an independent animal.
LLO-specific CD8+ T cells derived from TNFRI−/− mice transfer potent antilisterial immunity to naive wild-type B6 host mice.
LLO-specific CD8+ T cells from wild-type B6 mice mediate potent antilisterial immunity in adoptive-transfer assays (11, 43). To assess the ability of LLO-specific CD8+ T cells derived from TNFRI−/− mice to mediate antilisterial immunity, naive B6 host mice were injected i.v. with LLO-specific CD8+ T cells from TNFRI−/− mice and then given a high-dose challenge with virulent L. monocytogenes 10403s. CFU analyses of spleen and liver homogenates were performed 3 days postchallenge to assess the level of infection in T-cell-injected mice and in noninjected control animals (Table 3). The results show that LLO-specific CD8+ T cells from TNFRI−/− mice mediated dramatic reductions in L. monocytogenes CFU in both the spleens and the livers of recipient animals. Multiple experiments have demonstrated previously that similar reductions in CFUs correlate with the survival of T-cell-injected animals whereas unprotected mice die 4 to 6 days after a challenge with similar doses of virulent L. monocytogenes 10403s (43, 44). Thus, LLO-specific CD8+ T cells from TNFRI−/− mice mediate antilisterial immunity in naive B6 host mice.
TABLE 3.
LLO-specific CD8+ T cells from TNFRI−/− mice mediate antilisterial immunity in wild-type B6 host micea
| No. of TNFRI−/− CD8+ T cells | Bacterial counts (log10 CFU) per:
|
|
|---|---|---|
| Spleen | g of liver | |
| 0 | 8.3 | 8.4 |
| 0 | 8.3 | 8.4 |
| 0 | 8.7 | 8.3 |
| 12 × 106 | <4.4 | <5.2 |
| 12 × 106 | 4.7 | <5.2 |
| 12 × 106 | <4.4 | <5.2 |
Three or four B6 mice were injected i.v. with the indicated number of LLO-specific CD8+ T cells from TNFRI−/− mice followed by 1.8 × 105 CFU of virulent L. monocytogenes 10403s. CFU analyses on spleen and liver homogenates from all mice were performed 3 days postchallenge. In the majority of cases, no colonies were detected in mice that had received TNFRI−/− CD8+ T cells (the limit of detection was 4.4 log10 CFU/spleen and 5.2 log10 CFU/g of liver). One of four control animals (not shown) died before the CFU analysis.
Adaptive immunity to L. monocytogenes in TNFRI−/− mice involves CD8+ T cells.
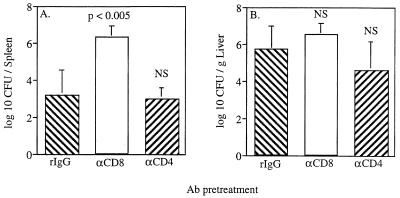
While both CD4+ and CD8+ T cells respond in an antigen-specific fashion to infection with virulent L. monocytogenes, adoptive-transfer experiments (4, 6, 24) and studies in mice deficient in CD4+ or CD8+ T cells (21, 25) indicate that CD8+ T cells are the most effective mediators of specific antilisterial immunity in wild-type mice. To determine whether CD8+ T cells play a role in the expression of secondary immunity to L. monocytogenes in the absence of TNFRI, TNFRI−/− mice were immunized with attenuated L. monocytogenes DP-L1942 and allowed to rest for at least 28 days; then the CD4+ or CD8+ T cells of the mice were depleted with injections of depleting monoclonal Ab, and the mice challenged with virulent L. monocytogenes. At 3 days postchallenge, CFU analyses were performed to determine bacterial loads in the spleen and liver. In addition, a subset of animals from each group was monitored for survival for 14 days (see below). Results of CFU analyses indicate that depletion of CD8+ cells exacerbated infection in the spleens (Fig. 4A) but not significantly in the livers (Fig. 4B) of TNFRI−/− mice undergoing a secondary response to L. monocytogenes. Depletion of CD4+ cells, in contrast, did not result in increased bacterial loads in either the spleen (Fig. 4A) or the liver (Fig. 4B).
FIG. 4.
Depletion of CD8+ T cells, but not CD4+ T cells, diminishes secondary immunity to L. monocytogenes in the spleen in TNFRI−/− mice. Five to six TNFRI−/− mice per group were immunized i.p. on day 0 with 106 CFU of attenuated L. monocytogenes DP-L1942. On day 30 to 32, immune mice received control polyclonal rat IgG (rIgG), rat anti-mouse CD8 (αCD8), or rat anti-mouse CD4 (αCD4) as indicated at 0.3 mg/mouse/day i.p. On day 33, all mice were challenged i.v. with 106 CFU of virulent strain 10403s. At 3 days p.i., the animals were sacrificed and analyzed for CFU in the spleen (A) and liver (B). The efficiency of in vivo depletion averaged 86 and 91% for CD8+ and CD4+ cells, respectively, as determined by flow cytometric analysis of splenocytes. These data are pooled from two independent experiments and are given as mean and SD. NS, not significant.
DISCUSSION
Adaptive immunity to L. monocytogenes in the absence of TNFRI.
The requirement for TNF and TNFRI in the primary response to L. monocytogenes has been established previously (9, 28, 30, 34). In the experiments described above, we estimate the LD50 of virulent L. monocytogenes 10403s in naive TNFRI−/− mice to be approximately 102, compared to approximately 104.7 in wild-type mice. The extreme susceptibility of these mice to primary listeriosis complicates studies of secondary resistance to L. monocytogenes since anything but the lowest immunizing dose (which is difficult to estimate precisely at the time of immunization) is lethal. This problem has been overcome previously in IFN-γ−/− mice by immunizing naive animals with an attenuated strain of L. monocytogenes that does not express the virulence factor encoded by the L. monocytogenes gene actA (12). This strain, which invades host cells and escapes from the phagosome into the cytoplasm but fails to spread from cell to cell, elicits protective, CD8+ T-cell-dependent immunity in wild-type and IFN-γ−/− mice (12). Here we demonstrate the versatility of this strategy and use it to study secondary immunity to L. monocytogenes in TNFRI−/− mice. Our results also suggest the feasibility of another approach, which is to immunize susceptible mice with doses of virulent L. monocytogenes that would otherwise be fatal and prevent death by reducing the bacterial load with antibiotics.
TNFRI−/− mice survive high-dose challenges with attenuated actA mutant strains and subsequently develop wild-type levels of resistance to secondary challenge with virulent strains. Thus, neither the development nor the expression of high levels of adaptive immunity to L. monocytogenes requires TNFRI in genetically deficient mice. This is the second instance (IFN-γ being the first) of a cytokine or cytokine receptor being absolutely required for effective innate immunity to L. monocytogenes but nonessential for an effective adaptive immune response.
Role of CD8+ T cells in adaptive immunity to L. monocytogenes in TNFRI−/− mice.
We directly assessed the role of CD8+ T cells in adaptive immunity to L. monocytogenes in TNFRI−/− mice by depleting the CD8+ T cells of immune TNFRI−/− mice prior to secondary challenge with virulent L. monocytogenes. The depletion of CD8+ T cells increased the severity of secondary listeriosis in the spleens of TNFRI−/− mice (Fig. 4). We also demonstrated that CD8+ T cells in the spleens of TNFRI−/− mice respond in an antigen-specific fashion to L. monocytogenes (Fig. 2 and 3). Thus, CD8+ T cells can mediate antilisterial immunity in the spleen by a mechanism that is independent of TNFRI. The dependence of antilisterial immunity on CD8+ T cells in the spleens of TNFRI−/− mice is consistent with previous studies which showed that perforin plays a role in CD8+ T-cell-mediated immunity to L. monocytogenes in the spleen (19, 43, 44).
Depletion of CD8+ T cells diminishes secondary immunity to L. monocytogenes in both the spleens and livers of wild-type mice (25). In contrast, depletion of CD8+ T cells did not result in significant exacerbation of listeriosis in the livers of immune TNFRI−/− mice. These data could result from differential depletion of CD8+ cells in wild-type and TNFRI−/− mouse livers or less dependence on CD8+ T cells for antilisterial immunity in the livers in the absence of TNFRI. Preliminary evidence revealed low or undetectable levels of CD8+ cells in the livers of wild-type and TNFRI−/− mice at 28 days after infection with strain DP-L1942 (D. W. White, A. Schlueter, and J. T. Harty, unpublished data), and thus the CD8+ T cells that participate in immunity in the livers of wild-type mice must be recruited from the peripheral pool. Since the peripheral pool of CD8+ T cells in immune TNFRI−/− mice was reduced considerably by the antibody treatment, exacerbating the infection in the spleen, the liver results are most consistent with a compensatory, CD8+ T-cell-independent mode of resistance in TNFRI−/− mice. As has been observed in wild-type mice (25), depletion of CD4+ T cells had no impact on antilisterial immunity in either the spleens or livers of immune TNFRI−/− mice. It should be pointed out that our data do not formally rule out the possibility that CD4+ T cells are able to express some antilisterial activity when CD8+ T cells are depleted in the TNFRI−/− mice. Together, these data are consistent with an organ-specific compensatory mechanisms of antilisterial immunity in mice lacking TNF-TNFRI interactions. Since this immunity does not depend on CD4+ or CD8+ T cells, it may be mediated by altered macrophage function or perhaps by γδ T cells. Immunohistochemical studies to characterize the immune response in the livers of mice lacking TNF and TNFRI are under way to address these issues.
Previous experiments in our laboratory showed that perforin-deficient CD8+ T cells failed to transfer antilisterial immunity into hosts depleted of TNF with neutralizing Ab (43). One hypothesis to explain this result is that TNF must engage TNFRI on the activated CD8+ T cell in vivo for it to mediate antilisterial immunity in an adoptive-transfer assay. Our data demonstrate that TNFRI expression on activated CD8+ T cells is not required for adoptive immunity to L. monocytogenes. Therefore, although TNF may be required for the in vivo antilisterial activity of CD8+ T cells, its direct action on activated CD8+ T cells via their TNFRI is not required.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI36864 and AI42767 (J.T.H.). D.W.W. is a trainee in the Medical Scientist Training Program.
The expert technical assistance of Lori Gorton and Gail Mayfield is greatly appreciated. We thank Amgen, Inc., Toronto, Canada, for TNFRI−/− breeders and Hao Shen, University of Pennsylvania, for XFL204, which was constructed in the laboratory of Jeff F. Miller in the Department of Microbiology and Immunology at UCLA School of Medicine.
REFERENCES
- 1.Bancroft G J, Bosma M J, Bosma G C, Unanue E R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986;137:4–9. [PubMed] [Google Scholar]
- 2.Bancroft G J, Sheehan K C, Schreiber R D, Unanue E R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989;143:127–130. [PubMed] [Google Scholar]
- 3.Bhardwaj V, Kanagawa O, Swanson P E, Unanue E R. Chronic Listeria infection in SCID mice: requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J Immunol. 1998;160:376–384. [PubMed] [Google Scholar]
- 4.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 5.Brundage R A, Smith G A, Camilli A, Theriot J A, Portnoy D A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci USA. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czuprynski C J, Brown J F. Effects of purified anti-Lyt-2 mAb treatment on murine listeriosis: comparative roles of Lyt-2+ and L3T4+ cells in resistance to primary and secondary infection, delayed-type hypersensitivity and adoptive transfer of resistance. Immunology. 1990;71:107–112. [PMC free article] [PubMed] [Google Scholar]
- 7.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loken M R, Pierres M, Fitch F W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK 1.5: similarity of L3T4 to the human Leu 3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 8.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wachter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endres R, Luz A, Schulze H, Neubauer H, Futterer A, Holland S M, Wagner H, Pfeffer K. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 1997;7:419–432. doi: 10.1016/s1074-7613(00)80363-5. [DOI] [PubMed] [Google Scholar]
- 10.Goossens P L, Milon G. Induction of protective CD8+ T lymphocytes by an attenuated Listeria monocytogenes actA mutant. Int Immunol. 1992;4:1413–1418. doi: 10.1093/intimm/4.12.1413. [DOI] [PubMed] [Google Scholar]
- 11.Harty J T, Bevan M J. CD8 T-cell recognition of macrophages and hepatocytes results in immunity to Listeria monocytogenes. Infect Immun. 1996;64:3632–3640. doi: 10.1128/iai.64.9.3632-3640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harty J T, Bevan M J. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 13.Harty J T, Lenz L L, Bevan M J. Primary and secondary immune responses to Listeria monocytogenes. Curr Opin Immunol. 1996;8:526–530. doi: 10.1016/s0952-7915(96)80041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harty J T, Tvinnereim A R, White D W. Effector mechanisms of CD8+ T cells. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 15.Hauser T, Frei K, Zinkernagel R M, Leist T P. Role of tumor necrosis factor in Listeria resistance of nude mice. Med Microbiol Immunol. 1990;179:95–104. doi: 10.1007/BF00198530. [DOI] [PubMed] [Google Scholar]
- 16.Havell E A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989;143:2894–2899. [PubMed] [Google Scholar]
- 17.Havell E A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987;139:4225–4231. [PubMed] [Google Scholar]
- 18.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 19.Kagi D, Ledermann B, Burki K, Hengartner H, Zinkernagel R M. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- 20.Kato K, Nakane A, Minagawa T, Kasai N, Yamamoto K, Sato N, Tsuruoka N. Human tumor necrosis factor increases the resistance against Listeria infection in mice. Med Microbiol Immunol. 1989;178:337–346. doi: 10.1007/BF00197452. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann S H, Ladel C H. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 22.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 23.Leist T P, Meager A, Exley T, Zinkernagel R M. Evidence for a role of IFN gamma in control of Listeria monocytogenes in T cell deficient mice. Experientia. 1991;47:630–632. doi: 10.1007/BF01949893. [DOI] [PubMed] [Google Scholar]
- 24.Lukacs K, Kurlander R. Lyt-2+ T cell-mediated protection against listeriosis. Protection correlates with phagocyte depletion but not with IFN-gamma production. J Immunol. 1989;142:2879–2886. [PubMed] [Google Scholar]
- 25.Mielke M E, Niedobitek G, Stein H, Hahn H. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J Exp Med. 1989;170:589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myung, P., J. Clements, D. W. White, J. T. Harty, and G. Koretzky. Macrophage function in slp-76−/− mice. Int. Immunol., in press. [DOI] [PubMed]
- 27.Nakane A, Minagawa T, Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988;56:2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF-alpha-deficient mice—a critical requirement for TNF-alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penheiter K L, Mathur N, Giles D, Fahlen T, Jones B D. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer's patches. Mol Microbiol. 1997;24:697–709. doi: 10.1046/j.1365-2958.1997.3741745.x. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 31.Plitz T, Huffstadt U, Endres R, Schaller E, Mak T W, Wagner H, Pfeffer K. The resistance against Listeria monocytogenes and the formation of germinal centers depend on a functional death domain of the 55 kDa tumor necrosis factor receptor. Eur J Immunol. 1999;29:581–591. doi: 10.1002/(SICI)1521-4141(199902)29:02<581::AID-IMMU581>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493. [Google Scholar]
- 33.Roll J T, Young K M, Kurtz R S, Czuprynski C J. Human rTNF alpha augments anti-bacterial resistance in mice: potentiation of its effects by recombinant human rIL-1 alpha. Immunology. 1990;69:316–322. [PMC free article] [PubMed] [Google Scholar]
- 34.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 35.Samsom J N, Langermans J A, Savelkoul H F, van Furth R. Tumour necrosis factor, but not interferon-gamma, is essential for acquired resistance to Listeria monocytogenes during a secondary infection in mice. Immunology. 1995;86:256–62. [PMC free article] [PubMed] [Google Scholar]
- 36.Sarmiento M, Glasebrook A L, Fitch F W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 37.Shen H, Miller J F, Fan X, Kolwyck D, Ahmed R, Harty J T. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 38.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith G A, Portnoy D A. The role of two phospholipases in the pathogenicity of Listeria monocytogenes. Infect Agents Dis. 1993;2:183–185. [PubMed] [Google Scholar]
- 40.Unanue E R. Inter-relationship among macrophages, natural killer cells and neutrophils in the early stages of Listeria resistance. Curr Opin Immunol. 1997;9:35–43. doi: 10.1016/s0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- 41.Unanue E R. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 42.van der Most R G, Murali-Krishna K, Whitton J L, Oseroff C, Alexander J, Southwood S, Sidney J, Chesnut R W, Sette A, Ahmed R. Identification of Db- and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology. 1998;240:158–167. doi: 10.1006/viro.1997.8934. [DOI] [PubMed] [Google Scholar]
- 43.White D W, Harty J T. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J Immunol. 1998;160:898–905. [PubMed] [Google Scholar]
- 44.White D W, MacNeil A, Busch D H, Philip I M, Pamer E G, Harty J T. Perforin-deficient CD8+ T cells: in vivo priming and antigen-specific immunity against Listeria monocytogenes. J Immunol. 1999;162:980–988. [PubMed] [Google Scholar]