Abstract
The secreted 30-kDa antigen (Ag) of Mycobacterium tuberculosis directly stimulates Th1-type protective cytokine responses in healthy tuberculin reactors but not in patients with active tuberculosis (TB). To examine the cytokine profiles attributable to Th1 suppression associated with active TB, interleukin-12 (IL-12), IL-18, and IL-10 production in response to a 30- or 32-kDa Ag in 16 patients with active pulmonary TB and 24 healthy controls was investigated by enzyme-linked immunosorbent assay. In TB patients, production of IL-12 p40, as well as gamma interferon (IFN-γ), by 30- or 32-kDa Ag-stimulated peripheral blood mononuclear cells (PBMC) was significantly decreased compared with that in healthy tuberculin reactors. There were no significant differences in IL-18 production between patients and controls early during stimulation (16 h). However, PBMC from patients showed significantly enhanced IL-18 proteins after 96 h of stimulation. Similarly, higher IL-10 production was observed in the TB patients than in healthy tuberculin reactors. After 2 months of anti-TB therapy, the mean IFN-γ and IL-12 p40 production and the mean blastogenic responses were significantly increased in PBMC in the 10 TB patients who were followed up. Our findings provide evidence that depressed IL-12 in response to the 30- or 32-kDa Ag is involved in the immunopathogenesis of human active pulmonary TB.
Patients with active tuberculosis (TB) often have ineffective protective immunity to mycobacterial antigens (Ag) and show depressed production of the Th1 cytokine gamma interferon (IFN-γ) compared with healthy tuberculin reactors (HTR) (25, 40). However, little is known about which factors are implicated in the Th1 suppression after in vitro stimulation with the mycobacterial Ag in active TB.
At present, two distinct cytokines, interleukin-12 (IL-12) and IL-18, are thought to be the critical factors skewing the immune response toward a Th1 cytokine profile (26, 33, 34). IL-12 is a 70-kDa heterodimeric cytokine composed of covalently linked p35 and p40 chains (33, 34) and was originally described as having activity in the maturation of cytolytic T lymphochtes (37) and the enhancement of natural killer (NK) cell function (14). It has a crucial role in IFN-γ induction by T lymphocytes (1, 24, 35), and mice deficient in IL-12 are highly susceptible to infection from Mycobacterium tuberculosis (3).
IL-18 is a recently described cytokine known as IFN-γ-inducing factor (22). It markedly stimulates IFN-γ production in Th1 cells (22) and granulocyte-macrophage colony-stimulating factor (15) and inhibits IL-10 production (15, 18, 22). IL-18 shares with IL-12 the role of activating NK cells and polarizing T cells toward Th1 cell function (27, 33, 35). In addition, IL-12 has a synergistic effect with IL-18 on the production of IFN-γ by anti-CD3-activated T cells (15, 18). Moreover, a recent study has shown a combined effect of IL-12 and IL-18 in promoting Mycobacterium leprae-specific Th1 responses (7). On the other hand, IL-18 may act as a strong coinducer of Th1 or Th2 cytokines and has a different role in the regulation of gene expression in NK and T cells (10).
Presently there is great interest in the secreted protein Ag of M. tuberculosis in relation to the immune response to infection, since these proteins are particularly important candidates for development of protective immunity as well as clinical symptoms and complications of the disease (36). The Ag85 complex is the major secreted protein constituent of mycobacterial culture fluids (2, 19), and a recent study has shown that vaccine containing 30-kDa Ag (Ag85B) was very effective in stimulating protective immunity in animals (12). The 30- and 32-kDa Ag were selected for our study on the basis of observations of blastogenic responses and IFN-γ production in tuberculin purified protein derivative (PPD)-reactive donors, whereas active-TB patients showed unresponsiveness to this Ag stimulation (32, 40). The present study was undertaken to further characterize the Th1 regulatory cytokine profiles (Th1 stimulatory cytokines IL-12 and IL-18 and Th1 inhibitory cytokine IL-10) in TB patients in response to a 30- or 32-kDa Ag, as compared with healthy controls. We have found that IL-12, but not IL-18, production was significantly decreased by peripheral blood mononuclear cells (PBMC) from patients with active pulmonary TB in response to the 30- or 32-kDa Ag. We also found that IL-12 and IFN-γ production was greatly increased after 2 months of anti-TB treatment.
MATERIALS AND METHODS
Subjects.
Human subjects were recruited from Catholic University Hospital, Taejon, Korea. A total of 16 patients consented to take part in this study. Their diagnoses were bacteriologically or biopsy-confirmed active TB. All of the patients had parenchymal TB but had no miliary or pleural TB. All of the patients had a positive sputum smear for acid-fast bacilli, and all had a positive sputum culture for M. tuberculosis. Chest radiographs were reviewed by two investigators, and all of the patients had parenchymal abnormalities. They had no previous history of diabetes mellitus or steroid therapy and all were human immunodeficiency virus negative. All of the PBMC from TB patients were obtained before treatment for pulmonary TB in this study.
PBMC were obtained on a voluntary basis from 12 HTR and 12 tuberculin nonreactors (NR), as determined by the Mantoux test, who had no previous history of clinical TB. Skin reactions in healthy volunteers of more than 10 mm after an intradermal test with 5 U of PPD-RT23 (Statens Seruminstitut, Coppenhagen, Denmark) were considered to be positive, and reactions of less than 5 mm were considered to be negative. All of the individuals showing positive tuberculin reactions in this study exhibited reactions of more than 15 mm, while individuals showing negative tuberculin reactions had no reactivity on the skin test. Each of these healthy controls had received Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccination as a child.
Thirty- and 32-kDa Ag of M. tuberculosis H37Rv.
The 30- and 32-kDa Ag of M. tuberculosis were purified as described previously (16). In brief, M. tuberculosis H37Rv was grown for 6 weeks at 37°C as surface pellicles on Sauton's medium. The 30- and 32-kDa Ag were purified to homogeneity by a combination of chromatography on hydroxylapatite, DEAE-Sepharose, and DEAE-Sephacel and gel filtration from M. tuberculosis culture filtrate. The isolated 30- or 32-kDa protein was identified as a single band by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stored in sterile aliquots at −80°C. The endotoxin content was measured by Limulus amebocyte lysate assay and was below 1.5 pg/ml in all Ag preparations.
Preparation and stimulation of PBMC.
Venous blood was obtained from volunteers in sterile blood collection tubes, and PBMC were isolated by density sedimentation over Histopaque-1077 (Sigma, St. Louis, Mo.). They were then suspended at a density of 106 viable cells/ml in a complete medium (RPMI 1640 [GIBCO BRL, Gaithersburg, Md.]) with 10% fetal bovine serum [GIBCO BRL], sodium pyruvate, nonessential amino acids, penicillin G [100 IU/ml], streptomycin (100 μg/ml), and 5 × 10−5 M 2-mercaptoethanol). Cells were then stimulated with 30- or 32-kDa Ag (1.0 μg/ml), phytohemagglutinin (PHA) (10 μg/ml; Sigma), or lipopolysaccharide (LPS) (0.1 μg/ml; Sigma) and incubated at 37°C in an atmosphere of 5% CO2 in humidified air until used for either isolation of RNA or supernatants.
Lymphocyte proliferation assay.
An amount of 2.5 × 104 PBMC was placed in each well of a round-bottom microtiter tissue culture plate (Falcon Products, Becton Dickinson, Oxnard, Calif.). The blastogenic response was measured at various concentrations of the 30- or 32-kDa Ag for 5 days at 37°C in 5% CO2. On the basis of dose-response studies, 1.0 μg of the 30- or 32-kDa Ag per ml in the final cultures was chosen as the optimum concentration (data not shown). PHA was used at a concentration of 10 μg/ml as a positive control for cell reactivity. Cells were incubated for 5 days at 37°C in an atmosphere of 5% CO2 in humidified air; for the last 18 h, 2 μCi of [3H]thymidine (Amersham, Buckinghamshire, United Kingdom) was present. The cells were harvested on fiberglass paper using a cell harvester (Cambridge Technology, Watertown, Mass.), and the incorporated radioactivity was measured in a liquid scintillation counter (Beckman, Somerset, N.J.). Mean counts per minute ± standard error for triplicate cultures were obtained for each donor. The stimulation index (SI) was calculated by using this value and the counts per minute obtained for unstimulated cultures. The results are expressed as SI.
Enzyme-linked immunosorbent assay (ELISA) for IFN-γ, IL-12, IL-18, and IL-10.
Supernatants were collected at 16 h (for IL-12 and IL-18), 48 h (for IL-10), and 96 h (for IFN-γ) from cultures of PBMC stimulated with 30- or 32-kDa Ag and frozen at −80°C. The frozen supernatants were thawed at room temperature, and the levels of cytokines in culture supernatants were determined with commercial kits for IFN-γ, IL-12 p70, IL-12 p40, and IL-10 assay (PharMingen, San Diego, Calif.) and for IL-18 assay (R&D Systems, Minneapolis, Minn.) in accordance with the manufacturer's instructions. Concentrations of cytokines in the samples were calculated with the standard curve generated from recombinant cytokines, and results are expressed in picograms per milliliter. The difference between values for the duplicate wells was consistently less than 100% of the mean.
RT-PCR and Southern hybridization.
Cells were cultured as described above. After 6 h (for IL-12 p40, IL-12 p35, and IL-18) and 96 h (for IFN-γ), culture cells were collected and washed and total RNA from cells was isolated from PBMC using an RNAgent kit (Promega, Madison, Wis.). First-strand cDNA synthesis and PCR were performed as described elsewhere (5). Briefly, cDNA was synthesized starting from 1 μg total RNA and using 25 U of murine leukemia virus reverse transcriptase (RT) and oligo d(T)16 (Perkin-Elmer, Norwalk, Conn.). All PCRs were performed using 1 μl of cDNA and 2 U of AmpliTaq DNA polymerase (Perkin-Elmer) in 50-μl reaction mixtures on a thermocycler (Biometra Inc., Tampa, Fla.), in which each cycle consists of 40 s at 95°C, 40 s at 60°C, and 40 s at 72°C with a final extension step of 5 min at 72°C. Before starting PCR, an external control, the housekeeping β-actin gene, was used in order to normalize the starting amount of cDNA for each sample.
Primer sequences were as follows: (i) IFN-γ (5′, TGGCTTTTCAGCTCTGCATCG; 3′, TCGACCTCGAAACAGCATCTG), (ii) IL-12 p40 (5′, CCAAGAACTTGCAGCAGCTGAAG; 3′, TGGGTCTATTCCGTTGTGTC); (iii) IL-12 p35 (5′, CCTCAGTTTGGCCAGAAACC; 3′, GGTCTTTCTGGAGGCCAGGC); (iv) IL-18 (5′, GCCTGGACAGTCAGCAAGGAATTG); 3′, CACATTATGAATTTTTTATTTGTT), and (iv) β-actin (5′, TCATGCCATCCTGCGTCTGGACCT; 3′, CGGACTCATCGTACTCCTGCTTG). The IFN-γ, IL-12 p40, IL-12 p35, IL-18, and β-actin amplified products give PCR fragment sizes of 465, 355, 296, 1,102, and 582 bp, respectively. Semiquantitative PCR was performed after determining for each gene product the number of cycles at which the plateau phase became apparent. The plateau phase for β-actin was seen after 35 cycles, and that for other primers was seen after 38 cycles (data not shown). Therefore, we selected the number of cycles from the linear phase of the amplification, for instance, 35 cycles for all cytokines and 32 cycles for β-actin. As a positive control for PCR amplification, reverse-transcribed RNAs isolated from either PHA- or phorbol myristate acetate-ionomycin-stimulated PBMC were included in each PCR assay.
The PCR product (10 μl) was electrophoresed through a 1.5% agarose gel, denatured for 30 min (1.5 M NaCl, 0.5 M NaOH), neutralized for 30 min (1.5 M NaCl, 1.0 M Tris-HCl), and transferred onto nylon (Hybond NF; Amersham, Arlington Heights, Ill.) with 10× standard saline citrate. DNA was cross-linked to membranes with UV light (Stratolinker; Stratagene, La Jolla, Calif.), dried, prehybridized (68°C for 2 hr), hybridized overnight at 68°C with digoxigenin-labeled oligonucleotides, and processed as recommended by the manufacturer (3′-oligonucleotide-labeling system and enhanced chemiluminescence; Boehringer Mannheim, Indianapolis, Ind.).
The probe sequences were as follows: for the IFN-γ probe, 5′ GGCAGTAACAGCCAAGAGAACCCAAAACGATGCAGAGCTG 3′; for the IL-12 p40 probe, 5′ TGGCTGAGGTCTTGTCCGTGAAGACTCTAT 3′; for the IL-12 p35 probe, 5′ TCTGAAGAGATTGATCATGAAG 3′; for the IL-18 probe, 5′ GAGATAATGCACCCCGGACC 3′; and for the β-actin probe, 5′ GCATCCACGAAACTACCTTC 3′.
Statistical methods.
Results are presented as means and standard deviations (SDs). Statistical significance was calculated using either analysis of variance, Student's t test, or linear regression analysis.
RESULTS
Lymphoproliferative responses and IFN-γ production in healthy controls and patients.
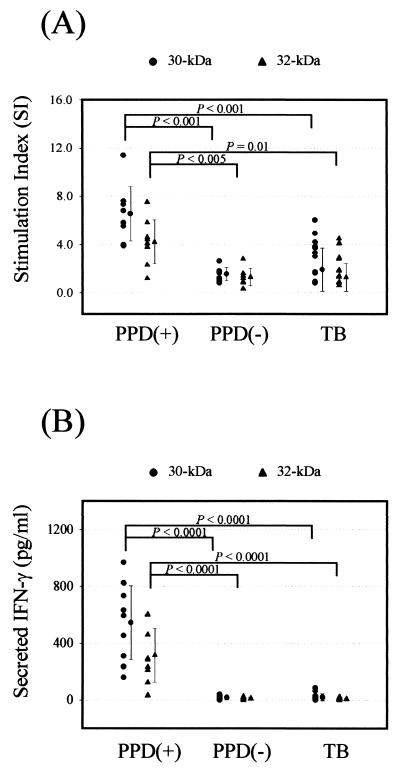
The lymphoproliferative responses and IFN-γ production response to the 30- or 32-kDa Ag from HTR and NR were compared to those from TB patients (Fig. 1). In 30-kDa Ag-stimulated PBMC from TB patients, the blastogenic responses (SI, >4.0) were significantly lower than those in cells from HTR (mean of 6.5 ± 2.2 versus 1.9 ± 1.6; P < 0.001). Similarly, the lymphoproliferative responses to the 32-kDa Ag was lower in TB patients than in HTR (mean of 4.2 ± 1.8 versus 1.3 ± 1.2; P = 0.01). Furthermore, the majority of TB patients did not recognize (SI, <4.0) either of the antigens (13 of 16 [81.3%]). In HTR, the background counts per minute incorporated was 600 ± 300, and the range of positive responses obtained were from 1,200 to 10,620.
FIG. 1.
Proliferative responses and IFN-γ production of PBMC in response to the 30- or 32-kDa Ag of M. tuberculosis in TB patients and healthy controls. (A) PBMC were stimulated for 5 days with the 30- or 32-kDa Ag of M. tuberculosis at a concentration of 1.0 μg/ml. Proliferative responses were assessed as [3H]thymidine incorporation by PBMC from healthy controls and TB patients during the last 18 h of culture after 5 days. Unstimulated PBMC served as a control. The results are expressed as the SI for triplicate cultures. (B) IFN-γ production by PBMC after in vitro stimulation with mycobacterial Ag in TB patients and healthy controls. Supernatants were prepared after 96 h of incubation, and IFN-γ production was measured by ELISA. Values are means and SDs for triplicate supernatant samples.
All of the NR showed either no stimulation (SI, <2.0) or only a marginal increase (SI, 2.0 to 4.0) in the lymphocyte response to the 30- or 32-kDa Ag. These responses were significantly lower than those from HTR in response to both Ag (30-kDa Ag, P < 0.001; 32-kDa Ag, P < 0.005) but did not show any statistically significant difference compared with those from TB patients.
The individual data on the production of IFN-γ by PBMC with Ag stimulation are shown in Fig. 1B. The mean IFN-γ concentrations in supernatants of 30- or 32-kDa Ag-stimulated PBMC from TB patients were significantly lower than the corresponding values for HTR (mean of 19.3 ± 24.9 pg/ml versus 543.9 ± 260.3 pg/ml [P < 0.0001] for 30-kDa Ag; mean of 7.5 ± 5.7 pg/ml versus 314.7 ± 188.8 pg/ml [P < 0.0001] for 32-kDa Ag, respectively). However, the mean IFN-γ production levels in response to the 30- or 32-kDa Ag were similar in TB patients and NR (mean of 19.3 ± 24.9 pg/ml versus 17.0 ± 13.6 pg/ml [P > 0.1] for 30-kDa Ag; mean of 7.5 ± 5.7 pg/ml versus 11.0 ± 8.6 pg/ml [P > 0.1] for 32-kDa Ag, respectively).
The 30-kDa Ag showed a significantly higher activity to induce lymphoproliferation (P < 0.05) and IFN-γ production (P < 0.05) in HTR compared with the 32-kDa Ag.
Production of IL-12, IL-18, and IL-10 in PBMC in healthy controls and patients after in vitro stimulation with the mycobacterial Ag. (i) IL-12 p40 and p70.
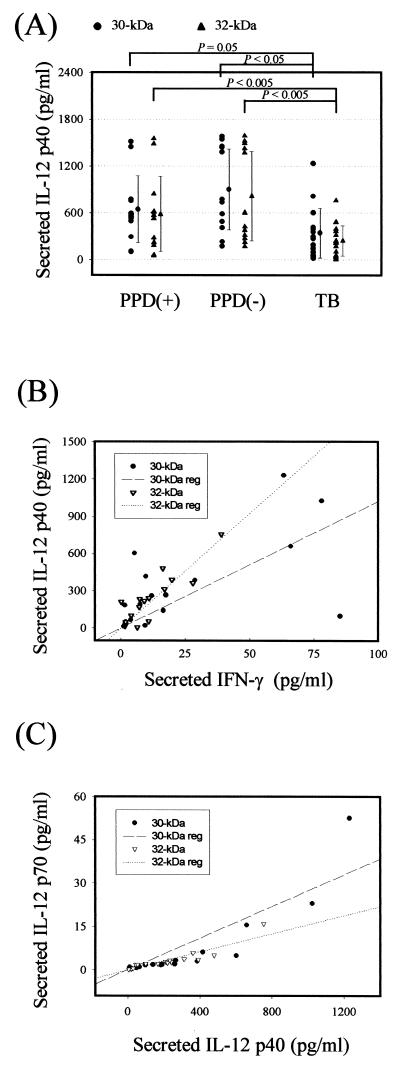
To evaluate IL-12 production in PBMC in response to the 30- or 32-kDa Ag, we performed ELISA with anti-IL-12 p70 and anti-IL-12 p40. Preliminary experiments using PBMC from three HTR and three NR showed that IL-12 p40 was not produced in freshly isolated cells but was detectable 3 h after and peaked from 12 to 18 h after stimulation with 30- or 32-kDa Ag (data not shown). We therefore used the 16-h time point for studying the IL-12 p40 production after stimulation with the mycobacterial Ag.
PBMC from healthy controls and TB patients produced comparable concentrations of IL-12 p40, as determined by ELISA (Fig. 2A). As shown in Fig. 2A, although the highest mean IL-12 p40 production was observed in the NR group (means of 896.5 ± 519.2 pg/ml for the 30-kDa Ag and 812.9 ± 571.8 pg/ml for the 32-kDa Ag), there was no statistically significant difference between the HTR and NR groups. However, the mean IL-12 p40 concentrations in supernatants of 30- or 32-kDa Ag-stimulated PBMC from TB patients were significantly lower than the corresponding values in HTR (mean of 339.5 ± 317.1 pg/ml versus 641.6 ± 427.3 pg/ml [P = 0.05] for the 30-kDa Ag; mean of 240.5 ± 193.7 pg/ml versus 581.9 ± 480.3 pg/ml [P < 0.05] for the 32-kDa Ag, respectively). In addition, those from TB patients showed a more significantly depressed IL-12 p40 production than those from NR subjects (P < 0.005 for both Ag). However, there were no significant differences between the two Ag in NR (P > 0.1).
FIG. 2.
IL-12 production by PBMC after in vitro stimulation with the 30- or 32-kDa Ag of M. tuberculosis. (A) IL-12 p40 production by PBMC after in vitro stimulation with mycobacterial Ag in TB patients and healthy controls. Supernatants were prepared after 16 h and IL-12 p40 production was measured by ELISA. Values are means and SDs for of triplicate supernatant samples. (B) Linear regression analysis of the data for TB patients presented in panel A and Fig. 1B. IL-12 p40 and IFN-γ production induced by the 30- or 32-kDa Ag are correlated in a significant manner (n = 16, r = 0.61, and P = 0.01 for the 30-kDa Ag; n = 16, r = 0.88, and P < 0.001 for the 32-kDa Ag). (C) Linear regression analysis of IL-12 p40 and p70 production in TB patients. IL-12 p40 and p70 release was assayed by ELISA after stimulation with the Ag for 16 h. Significant correlation was found for IL-12 p40 and p70 production induced by the 30- or 32-kDa Ag (n = 16, r = 0.89, and P < 0.0001 for the 30-kDa Ag; n = 16, r = 0.91, and P < 0.0001 for the 32-kDa Ag).
We also observed that the individual production of IL-12 p40 in all of the patients was significantly correlated with 30- or 32-kDa Ag-induced IFN-γ secretion at 96 h after stimulation (Fig. 2B).
In addition, we determined the release of IL-12 p70, which is a more reliable measurement of biologically active IL-12 production. Even though excess IL-12 p70 protein (from 20.0 to 55.0 pg/ml) was detected in some donors whose IL-12 p40 protein levels were higher than 1,000 pg/ml, 30- or 32-kDa Ag-induced levels of IL-12 p70 were very low. However, IL-12 p70 release was correlated in a significant manner with the release of IL-12 p40 (n = 16, r = 0.89, and P < 0.0001 for the 30-kDa Ag; n = 16, r = 0.91, and P < 0.0001 for the 32-kDa Ag) (Fig. 2C). Unstimulated PBMC showed no detectable levels of IL-12 p40 (<100 pg/ml) and IL-12 p70 (<0.2 pg/ml) (data not shown). Stimulation of PBMC with LPS resulted in the secretion of IL-12 p40 (1,000 to 2,500 pg/ml). LPS induced similar IL-12 titers in the healthy controls and TB patients (data not shown).
(ii) IL-18.
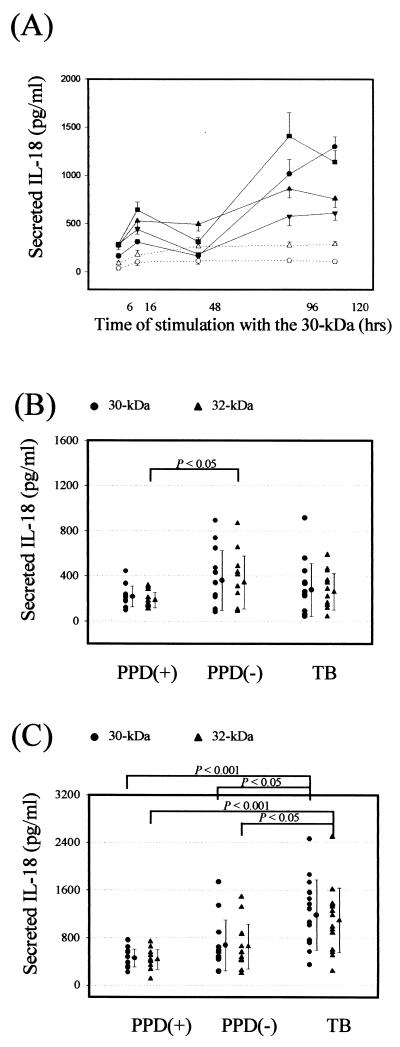
Next we wished to evaluate IL-18 production in PBMC in response to the 30- or 32-kDa Ag. Significant time-dependent IL-18 production was observed in PBMC after in vitro stimulation with the 30-kDa Ag. IL-18 was detectable 6 h after stimulation with 30-kDa Ag, peaked at 16 h, and had slightly decreased or similar levels to 48 h, and then maximal IL-18 protein was measured at 96 or 120 h. IL-18 production in the absence of Ag was less than 250 pg/ml at 96 h of stimulation (Fig. 3A). We used the 16- and 96-h time points for studying the IL-18 production after stimulation with the mycobacterial Ag.
FIG. 3.
IL-18 production by PBMC after in vitro stimulation with the 30- or 32-kDa Ag of M. tuberculosis. (A) Kinetics of IL-18 production by PBMC in HTR and TB patients after in vitro stimulation with the 30-kDa Ag or medium alone. PBMC were stimulated with the 30-kDa Ag (solid lines) or medium alone (dotted lines), and supernatants were harvested after 6, 16, 48, 96, and 120 h, respectively. Secreted IL-18 in supernatants was measured by ELISA as described in Materials and Methods. Results are expressed as means (and SDs) for three experiments with supernatants from four donors. Similar data were obtained using cells from three other donors. IL-18 production in the absence of Ag (dotted lines) was less than 250 pg/ml. Square and circle, TB patients; triangles, HTR. (B and C) IL-18 production by PBMC after in vitro stimulation with mycobacterial Ag in TB patients and healthy controls. Supernatants were prepared after 16 h (B) and 96 h (C), and IL-18 production was measured by ELISA. Values are means and SDs for triplicate supernatant samples.
At 16 h of stimulation with Ag, there were no significant differences in IL-18 production among the three groups (Fig. 3B). We found that incubation of unstimulated PBMC (without Ag) for 16 h also produced comparative values of IL-18 (195.8 ± 66.7 pg/ml). Therefore, IL-18 production early during stimulation (16 h) with mycobacterial Ag was not shown to be up-regulated compared with that with other stimuli, such as adherence alone.
However, the mean concentration of IL-18 in PBMC from TB patients was significantly increased after a 96-h stimulation with the 30- or 32-kDa Ag compared with those from HTR (mean of 1,183.7 ± 589.7 pg/ml versus 457.1 ± 152.1 pg/ml [P < 0.001] for the 30-kDa Ag; mean of 1,094.0 ± 544.5 pg/ml versus 432.1 ± 166.8 pg/ml [P < 0.001] for the 32-kDa Ag, respectively) (Fig. 3C). The IL-18 production from unstimulated PBMC cultured for 96 h was not very different from that after 16 h of stimulation. In addition, there was no significant difference in IL-18 production between the HTR and NR groups (P > 0.05 for both antigens).
Stimulation of PBMC with LPS resulted in the secretion of IL-18 (1,000 to 2,000 pg/ml). LPS induced similar IL-18 titers in the healthy controls and TB patients (data not shown).
(iii) IL-10.
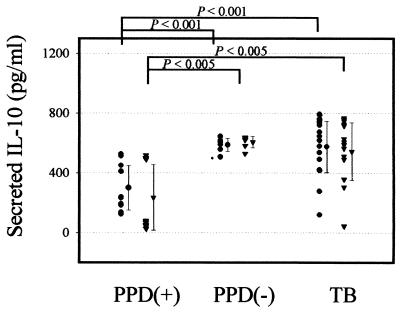
In contrast to data showing IFN-γ and IL-12 to be impaired in the TB patients, IL-10 was produced in significantly increased amounts by all patients after stimulation with 30- or 32-kDa Ag compared with HTR (mean of 575.6 ± 173.6 pg/ml versus 298.7 ± 149.8 pg/ml [P < 0.001] for the 30-kDa Ag; mean of 543.3 ± 192.8 pg/ml versus 235.3 ± 219.2 pg/ml [P < 0.005] for the 32-kDa Ag, respectively) (Fig. 4). Moreover, the IL-10 produced by the NR was significantly increased in supernatants in response to the 30- or 32-kDa Ag compared with that produced by HTR (P < 0.001 for the 30-kDa Ag; P < 0.005 for the 32-kDa Ag). Stimulation of PBMC with LPS preferentially induced the secretion of IL-10 (500 to 1,000 pg/ml). LPS induced similar IL-10 titers in the healthy controls and TB patients (data not shown).
FIG. 4.
IL-10 production by PBMC after in vitro stimulation with the 30- or 32-kDa Ag of M. tuberculosis in TB patients and healthy controls. Supernatants were prepared after 48 h and IL-10 production was measured by ELISA. Values are means and SDs for triplicate supernatant samples.
Cytokine mRNA expression in healthy controls and patients after in vitro stimulation with the 30- or 32-kDa Ag.
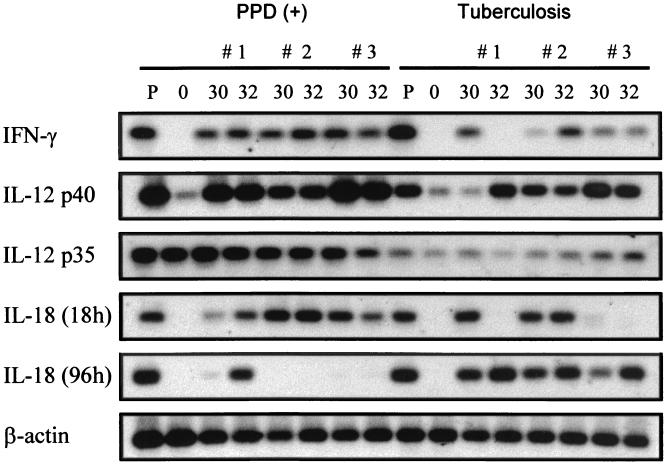
The levels of mRNA in PBMC from HTR and TB patients were determined by RT-PCR and Southern hybridization (Fig. 5). After the 30- or 32-kDa Ag treatment, IFN-γ mRNA levels in PBMC in HTR subjects were prominently induced at 6 h and greatly induced at 96 h (data not shown). IL-12 p40 and IL-18 mRNAs were also clearly detected at 6 h, but IL-12 p40 mRNA was significantly depressed at 96 h, whereas IL-18 mRNA was detectable at 96 h (data not shown). We therefore used the 96-h time point for studying IFN-γ mRNA expression and the 6-h time point for studying for IL-12 p40 and IL-18 mRNA expression after stimulation with the mycobacterial Ag.
FIG. 5.
Southern blot analysis of cytokine cDNA after in vitro stimulation with the 30- or 32-kDa Ag of M. tuberculosis. PBMC were isolated from healthy controls and TB patients and cultured for 0, 6 (for IL-12 p40 and IL-18), and 96 (for IFN-γ) h in the presence of the 30- or 32-kDa Ag (1 μg/ml). Following reverse transcription, a PCR with primers specific for each cytokine was performed. The PCR products were electrophoresed on a 2% agarose gel and hybridized with a digoxigenin-labeled probe. Lanes P, mRNA from PBMC stimulated with PHA or LPS; lanes O, mRNA from freshly isolated PBMC.
Figure 5 shows a Southern blot from a representative experiment using cells obtained from three separate donors in each group. TB patients usually elicited little or no detectable IFN-γ mRNA at 96 h with the 30- or 32-kDa Ag. However, PBMC from the HTR examined showed IFN-γ mRNA expression at 96 h in response to the 30- or 32-kDa Ag. In addition, Ag-stimulated PBMC from HTR exhibited much higher IL-12 p40 mRNA expressions at 6 h than those from TB patients. More strikingly, IL-12 p35 mRNA expression was also reduced in TB patients compared to healthy controls. However, the IL-12 p35 mRNA expression showed a tendency to be constitutive before and after stimulation with Ag.
Most HTR and TB patients did not exhibit detectable IL-18 mRNA prior to stimulation with the mycobacterial Ag. Even though there are some discrepancies between individual subjects, the frequencies of IL-18 mRNA expression in TB patients were similar to those in HTR in response to the 30- or 32-kDa Ag. In addition, protein expression paralleled mRNA expression in separate donors. None of the differences in mRNA induction were due to variations in the RNA isolation procedure or to the process of reverse transcription, since equivalent amounts of β-actin mRNA (a housekeeping gene) were expressed by PBMC from the same subject.
Lymphoproliferation and cytokine production after anti-TB therapy.
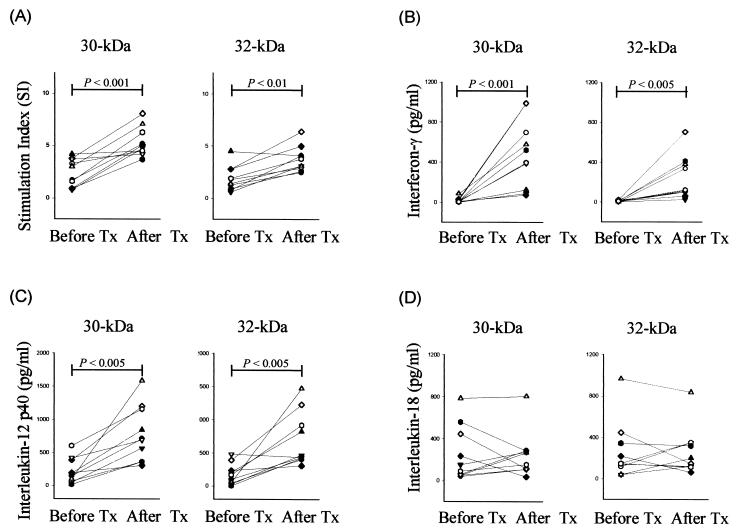
Follow-up data are reported for 10 of the original 16 patients with TB. After 2 months of treatment, the lymphoproliferative responses either to the 30- or 32-kDa Ag were increased in all patients. Blastogenic responses of the 30-kDa Ag-stimulated PBMC from TB patients were more significantly increased (P < 0.001) after 2 months compared to those of the 32-kDa Ag-stimulated PBMC (P < 0.01) (Fig. 6A).
FIG. 6.
Lymphoproliferative responses and cytokine production after anti-TB therapy. PBMC from TB patients (n = 10) were stimulated with the 30- or 32-kDa Ag (1 μg/ml) before treatment (Tx) and at 2 months after treatment. Lymphoproliferative responses (A) and IFN-γ (B), IL-12 p40 (C), and IL-18 production (D) in PBMC were determined.
Figure 6 shows the individual data on the production of cytokines after anti-TB therapy. The patients showed significant increases in mean production of IFN-γ in response to the mycobacterial Ag (from 17.0 ± 24.0 pg/ml to 484.1 ± 322.2 pg/ml [P < 0.001] for the 30-kDa Ag; from 7.6 ± 6.1 pg/ml to 235.6 ± 205.1 pg/ml [P < 0.005] for the 32-kDa Ag) after 2 months of treatment (Fig. 6B). Furthermore, the mean production of IL-12 p40 was significantly increased in response to the 30-kDa Ag (from 214.7 ± 181.3 pg/ml to 775.9 ± 400.4 pg/ml [P < 0.005]) or the 32-kDa Ag (from 171.7 ± 151.7 pg/ml to 690.4 ± 380.6 pg/ml [P < 0.005]) (Fig. 6C). Even though there were differences in the magnitude of cytokine increase, the supernatants from all patients showed up-regulation of IL-12 p40 protein after 2 months of therapy.
However, there were no statistically significant differences between the IL-18 production before and after treatment in response to the 30- or 32-kDa Ag (P > 0.1). Decreases in IL-18 protein were observed in some patients after treatment, but in none of the patients did this correlate well with the IFN-γ or IL-12 p40 production (Fig. 6D). Similarly, a decreasing pattern in IL-10 production was observed in some TB patients after treatment (data not shown) but did not reach statistical significance (P > 0.1 for the 30- and 32-kDa Ag). All 10 patients showed responsiveness to the new antimycobacterial agents in the course of 6 months of treatment, converted to negative on direct smear and/or culture, and clinically and radiographically improved.
DISCUSSION
In a previous study, we found a difference in the intracellular IFN-γ and lymphoproliferative responses between TB patients and HTR using 30-kDa Ag along with PPD. When PBMC cultures were stimulated with 30-kDa Ag, the mean intracellular IFN-γ production in T cells from TB patients was significantly lower than that of HTR, but there were no significant differences between the two groups after stimulation with PPD (11).
The basis for this observation was investigated in the present study by examining the selected Th1-driving cytokine (IL-12 and IL-18) and Th2-driving cytokine (IL-10) responses to the purified 30- or 32-kDa Ag in PBMC from patients with active pulmonary TB and from HTR and NR controls.
The mean IL-12 p40 concentrations in supernatants and IL-12 p40 mRNA expression from TB patients were significantly lower than the corresponding levels in HTR and NR in response to both Ag (Fig. 2A and 5). We also observed that the individual production of IL-12 p40 in most patients was specifically correlated with 30- or 32-kDa Ag-induced IFN-γ secretion at 96 h after stimulation (Fig. 2B). Our data suggest that the early stage of active pulmonary TB may be associated with depressed IL-12 expression, which plays a pivotal role in Th1 protective immunity. Protective immunity against M. tuberculosis requires activated mononuclear phagocytes and T cells, and IL-12 may provide a crucial link between these two cell populations by regulating IFN-γ production and the cytotoxic effector function of mycobacterial antigen-specific T cells (5, 33, 34, 38).
Our findings support earlier indications that IL-12 production might be reduced in the periphery, if not at the site of disease (38). In another study, Zhang et al. (39) observed reduced expression of the IL-12 receptor β1 and β2 subunits on peripheral blood T cells from patients with pulmonary TB, which would agree with the reduced production of IL-12 detected in this study. In addition, our data partially correlate with a previous study on TB in children by Swaminathan et al. (31), in which the increased reduction in IFN-γ production in children with TB of increasing clinical severity did not correlate with greater IL-12 production.
Our RT-PCR results showing reduced IL-12 p35 mRNA expression in TB patients compared to healthy controls strengthens the importance of IL-12 p35 expression in protective immunity in TB, as suggested by earlier experiments with human monocytes infected with M. tuberculosis H37Ra (5). In those studies, the authors suggested that IFN-γ may provide an afferent feedback signal for enhancing IL-12 p70 expression through selective up-regulation of IL-12 p35 mRNA levels and that the conditions that augment early or constitutive IL-12 p35 expression may augment the development of protective immunity against M. tuberculosis (5).
After therapy, IL-12 production, as well as lymphoproliferative activity and IFN-γ production, was significantly increased in PBMC from TB patients in response to the 30- or 32-kDa Ag. Fulton et al. (6) suggested that IL-12 released by infected macrophages in turn can further up-regulate M. tuberculosis-specific CD4+-T-cell effector function. They also report that IFN-γ may provide an afferent feedback signal that enhances IL-12 p70 expression (5). These results and our data led us to hypothesize that patients with active TB show increased IL-12 production after therapy due to an afferent feedback signal, IFN-γ, and that the resultant IL-12 can up-regulate more IFN-γ.
Using a sensitive ELISA specific for the IL-12 p70 heterodimer, we could detect very low levels of IL-12 production by PBMC from TB patients stimulated with 30- or 32-kDa Ag. However, IL-12 p70 release was correlated in a significant manner with the release of IL-12 p40 (Fig. 2C) in our assay system. Others have reported that p40 mRNA and free p40 protein are produced by cells producing bioactive IL-12 (8, 28).
The production of IL-18 by HTR, NR, and TB patients after in vitro stimulation with 30- or 32-kDa Ag was determined. Early during stimulation (16 h), no Ag-specific IL-18 production was observed in PBMC from TB patients or healthy controls. However, IL-18 production by TB patients at 96 h after stimulation was more significantly increased in response to the 30- or 32-kDa Ag than that by HTR or NR. The normal or increased production of IL-18 supports results obtained using IL-18 knockout mice. In this case, although splenic IFN-γ concentrations were reduced, the mice did not develop acute disseminated disease (30).
Although IL-18 was originally discovered as a factor which induced IFN-γ (20, 22), IL-18 and IL-12 do not mutually stimulate each other's expression. The synthesis and degradation of the mRNAs for these cytokines are regulated independently of each other. In addition, there may be different subsets of macrophages producing IL-12 and IL-18 (21).
In addition, many different cell sources play a role in producing IL-18, including monocytes, macrophages, and dendritic cells, especially in bacterial infections (4, 17), and even CD4 and CD8 T cells (13), while IL-12 is produced by activated macrophages (35). It is likely that our late IL-18 induction in TB patients is associated with a T-cell-specific stimulation. Others have also reported that T-cell-specific stimulation led to an induction of IL-18 mRNA expression after more than 24 h (13). However, questions have remained about the role of increased IL-18 production in TB patients 96 h after stimulation. Recently, Hoshino et al. suggested that IL-18 may act as a strong coinducer of Th1 or Th2 cytokines (10). Others (23) have indicated that IL-18 does not drive Th1 development but synergizes with IL-12 for IFN-γ production. In fact, the most consistent conclusion that can be made about IFN-γ production is that IL-12 is needed for IL-18-induced IFN-γ production (29).
Our data correlate well with those of others on the points that the Th1 response was reduced in the peripheral blood of TB patients (9, 32, 40) and IL-10 production was higher than that by PPD-positive controls (32). However, IL-10 and IL-18 production did not show any statistically significant difference after therapy in response to the Ag.
In conclusion, these data demonstrate that active TB patients showed significantly decreased IL-12 production compared with healthy controls, although their IL-18 levels were similar early (at 16 h) and became higher later (at 96 h) after stimulation with the 30- or 32-kDa Ag. Furthermore, IL-12 and IFN-γ production in response to the Ag was enhanced significantly by anti-TB therapy. The results provide evidence that IL-12 may principally contribute to the human protective immune responses to M. tuberculosis. A further characterization of the role of IL-18 production after in vitro stimulation with the secreted Ag must be performed.
ACKNOWLEDGMENTS
This work was supported by grant 1998-003-F00067 from the Korea Research Foundation and in part by a grant from the Ministry of Health and Welfare of Korea (HMP-98-B-1-003) made in the program year of 1998.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Abou-Zeid C, Ratliff T L, Wiker H G, Harboe M, Bennedsen J, Rook G A W. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988;56:3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulton S A, Cross J V, Toossi Z T, Boom W H. Regulation of interleukin-12 by interleukin-10, transforming growth factor-β, tumor necrosis factor-α, and interferon-γ in human monocytes infected with Mycobacterium tuberculosis H37Ra. J Infect Dis. 1998;178:1105–1114. doi: 10.1086/515698. [DOI] [PubMed] [Google Scholar]
- 6.Fulton S A, Johnsen J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia V E, Uyemura K, Sieling P A, Ochoa M T, Morita C T, Okamura H, Kurimoto M, Rea T H, Modlin R L. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–6121. [PubMed] [Google Scholar]
- 8.Gubler U, Chua A O, Schoenhaut D S, Dwyer C M, McComas W, Motyka R, Nabavi N, Wolitzky A G, Quinn P M, Familletti P C, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havlir D V, Wallis R S, Boom W H, Daniel T M, Chervenak K, Ellner J J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991;59:665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino T, Wiltrout R H, Young H A. IL-18 is a potent coinducer of IL-13 in NK and T cells: a new potential role for IL-18 in modulating the immune response. J Immunol. 1999;162:5070–5077. [PubMed] [Google Scholar]
- 11.Jo E K, Kim H J, Min D, Song Y, Song C H, Paik T H, Suhr J W, Park J K. Dysregulated production of interferon-gamma, interleukin-4 and interleukin-6 in early tuberculosis patients in response to antigen 85B of Mycobacterium tuberculosis. Scand J Immunol. 2000;51:209–217. doi: 10.1046/j.1365-3083.2000.00663.x. [DOI] [PubMed] [Google Scholar]
- 12.Kamath A T, Feng C G, MacDonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein S A, Ottmann O G, Ballas K, Dobmeyer T S, Pape M, Weidmann E, Hoelzer D, Kalina U. Quantification of human interleukin 18 mRNA expression by competitive reverse transcriptase polymerase chain reaction. Cytokine. 1999;11:451–458. doi: 10.1006/cyto.1998.0424. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto L I, Usui M, Ikeda M, Kurimoto M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 16.Lim J H, Park J K, Jo E K, Song C H, Min D, Song Y J, Kim H J. Purification and immunoreactivity of three components from the 30/32-kilodalton antigen 85 complex in Mycobacterium tuberculosis. Infect Immun. 1999;67:6187–6190. doi: 10.1128/iai.67.11.6187-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 18.Micallef M J, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 19.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura K, Okamura H, Wada K, Nagata K, Komatsu T, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun. 1989;38:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 22.Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita K, Torigoe K, Okura T, Fukuda S, Kurimoto M. A novel costimulatory factor for gamma interferon induction found in the liver of mice causes endotoxic shock. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 24.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez F O, Rodriguez J I, Agudelo G, Garcia L F. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infect Immun. 1994;62:5673–5678. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sareneva T, Matikainen S, Kurimoto M, Julkunen I. Influenza A virus-induced IFN-alpha/beta and IL-18 synergistically enhance IFN-gamma gene expression in human T cells. J Immunol. 1998;160:6032–6038. [PubMed] [Google Scholar]
- 27.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260:496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 28.Stern A S, Podlaski F J, Hulmes J D, Pan Y C, Quinn P M, Wolitzky A G, Familletti P C, Stremlo D L, Truitt T, Chizzonite R, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoll S, Jonuleit H, Schmitt E, Muller G, Yamauchi H, Kurimoto M, Knop J, Enk A H. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol. 1998;28:3231–3239. doi: 10.1002/(SICI)1521-4141(199810)28:10<3231::AID-IMMU3231>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun. 1999;67:2585–2589. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swaminathan S, Gong J, Zhang M, Samten B, Hanna L E, Narayanan P R, Barnes P F. Cytokine production in children with tuberculosis infection and disease. Clin Infect Dis. 1999;28:1290–1293. doi: 10.1086/514794. [DOI] [PubMed] [Google Scholar]
- 32.Torres M, Herrera T, Villareal H, Rich E A, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–180. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 34.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 35.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 36.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong H L, Wilson D E, Jenson J C, Familletti P C, Stremlo D L, Gately M K. Characterization of a factor(s) which synergizes with recombinant interleukin 2 in promoting allogeneic human cytolytic T-lymphocyte responses in vitro. Cell Immunol. 1988;111:39–54. doi: 10.1016/0008-8749(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Gately M K, Wang E, Gong J, Wolf S F, Lu S, Modlin R L, Barnes P F. Interleukin 12 at the site of disease in tuberculosis. J Clin Invest. 1994;93:1733–1739. doi: 10.1172/JCI117157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Gong J, Presky D H, Xue W, Barnes P F. Expression of the IL-12 receptor beta 1 and beta 2 subunits in human tuberculosis. J Immunol. 1999;162:2441–2447. [PubMed] [Google Scholar]
- 40.Zhang M, Lin Y, Iyer D V, Gong J, Abrams J S, Barnes P F. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]