Dear Editor,
Postprandial glycemic control remains challenging for people with type 1 diabetes (T1D) even with the use of newer technologies such as automated insulin delivery (AID) systems. Newer insulin formulations aim to decrease postprandial glucose excursions through the use of excipients that accelerate insulin absorption. Ultrarapid lispro (URLi) has been found to be superior to traditional analogs at controlling postprandial glucose in adults with diabetes using both multiple daily injections1 and continuous insulin infusion.2
At this time, several reports have been published on the use of ultrarapid acting insulin analogs in the AID systems that are currently available, however, virtually all of these reports found no clear benefit.3–5 Here we report a case of improved glycemic control in an already well-controlled patient while using URLi with Control-IQ. To the best of our knowledge, this is the first report of ultrarapid acting insulin use with Control-IQ.
We compared continuous glucose monitoring (CGM) and pump data in a 19-year-old female with T1D who obtained URLi from her primary care office. She used URLi with her Tandem t:slimX2 pump with Control-IQ technology (13 weeks), then returned to using insulin aspart (4 weeks) without altering the timing of her boluses in relation to meal intake (0–15 min before eating based on CGM trends).
Average total daily insulin was similar with both insulin analogs (48.6 U vs. 46.9 U). The patient followed a low carbohydrate diet throughout the time period analyzed, with slightly more carbohydrates eaten while using URLi (average 43 g vs. 34 g). Time in range (70–180 mg/dL) was higher with the use of URLi (90.9% ± 6.9% vs. 84.9% ± 6.0%) with time in the euglycemic range (70–140 mg/dL) over 10% higher (79.2% vs. 68.8%). Time spent in hypoglycemia was lower when using URLi (2.4% vs. 4.9%) and the patient required fewer average daily boluses (5.9 vs. 6.7).
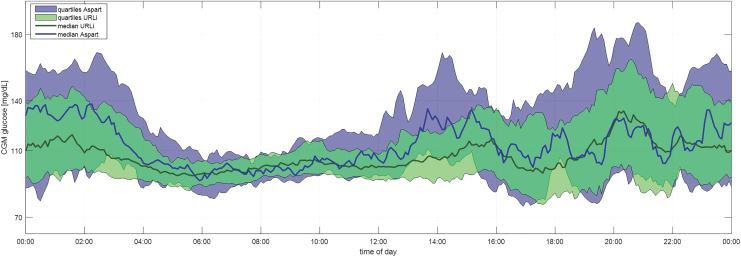
As seen in Figure 1, the daily CGM profile on URLi was notably tighter. Average CGM with URLi was 113 mg/dL compared with 121 mg/dL with aspart. The patient's HbA1c remained <6% during routine clinic visits around this time. No adverse events were reported while using URLi, including no reports of increased occlusions.
FIG. 1.
Average 24-h CGM profiles (URLi vs. Aspart). CGM, continuous glucose monitoring; URLi, ultrarapid lispro.
Postprandial glucose control remains challenging even with use of AID systems, and faster acting insulin formulations continue to be an area of great interest in improving this issue for people with diabetes. Although it appears that ultrarapid acting insulin analogs may not offer clear benefits when used with some AID systems, potential for benefit in other controllers that utilize automated boluses in addition to basal modulations remains to be determined.
Glycemic control was unexpectedly improved in this well-controlled patient consuming a low carbohydrate diet using the Tandem Control-IQ system, suggesting potential for even greater benefits in populations with worse glycemic control and greater postprandial glucose excursions. Larger randomized studies are clearly warranted to determine the impact of using URLi with AID systems, both with the current control algorithms and with modified algorithms that take updated pharmacokinetic and pharmacodynamic profiles into account.
Authors' Contributions
M.J.S. was involved in writing the article—original draft. M.D.B. contributed to formal analysis, writing—review and editing, and visualization. M.D.D. was involved in writing—review and editing. B.P.K. was in charge of supervision and writing—review and editing.
Author Disclosure Statement
M.J.S. reports receiving grant support and supplies, paid to her institution, from Tandem Diabetes Care, Insulet, Medtronic, and Dexcom. M.D.D. reports receiving grant support and supplies, paid to his institution, from Tandem Diabetes Care, Medtronic, and Dexcom. M.D.B. reports research support from Dexcom, Arecor Ltd., Novo Nordisk A/S, and Tandem Diabetes Care; consulting fees from Dexcom, Arecor, and Adocia. B.P.K. reports research support handled by the University of Virginia from Dexcom, Novo Nordisk A/S, and Tandem Diabetes Care. M.D.B. and B.P.K. report patent royalties, managed by the University of Virginia, from Johnson & Johnson, Sanofi, and Dexcom.
Funding Information
No funding was received for this article.
References
- 1. Klaff L, Cao D, Dellva MA, et al. Ultra rapid Lispro improves postprandial glucose control compared with Lispro in patients with type 1 diabetes: Results from the 26-week PRONTO-T1D study. Diabetes Obes Metab 2020;22:1799–1807; doi: 10.1111/dom.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Warren M, Bode B, Cho JI, et al. Improved postprandial glucose control with ultra rapid Lispro versus Lispro with continuous subcutaneous insulin infusion in type 1 diabetes: PRONTO-Pump-2. Diabetes Obes Metab 2021;23:1552–1561; doi: 10.1111/dom.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bode B, Carlson A, Liu R, et al. Ultrarapid Lispro demonstrates similar time in target range to Lispro with a hybrid closed-loop system. Diabetes Technol Ther 2021;23:828–836; doi: 10.1089/dia.2021.0184. [DOI] [PubMed] [Google Scholar]
- 4. Dovc K, Piona C, Yeşiltepe Mutlu G, et al. Faster compared with standard insulin aspart during day-and-night fully closed-loop insulin therapy in type 1 diabetes: A double-blind randomized crossover trial. Diabetes Care 2020;43:29–36; doi: 10.2337/dc19-0895. [DOI] [PubMed] [Google Scholar]
- 5. Boughton CK, Hartnell S, Thabit H, et al. Hybrid closed-loop glucose control with faster insulin aspart compared with standard insulin aspart in adults with type 1 diabetes: A double-blind, multicentre, multinational, randomized, crossover study. Diabetes Obes Metab 2021;23:1389–1396; doi: 10.1111/dom.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]