Abstract
The protein toxin of Pasteurella multocida PMT is a potent mitogen and activator of phospholipase Cβ. In this study different toxin fragments were investigated. A C-terminal fragment encompassing amino acids 581 through 1285 (PMT581C) was constructed, which was inactive toward intact embryonic bovine lung (EBL) cells after addition to culture medium but caused reorganization of the actin cytoskeleton and rounding up of cells when introduced into the cells by electroporation. As the holotoxin, the toxin fragment PMT581C induced an increase in total inositol phosphate levels after introduction into the cell by electroporation. A C-terminal fragment shorter than PMT581C as well as N-terminal fragments were inactive. Exchange of cysteine-1165 for serine in the holotoxin resulted in a complete loss of the ability to increase inositol phosphate levels. Correspondingly, the mutated toxin fragment PMT581C.C1165S was inactive after cell introduction by electroporation, suggesting an essential role of Cys-1165 in the biological activity of the toxin.
The bacterium Pasteurella multocida is the causative agent of atrophic rhinitis in animals and causes wound infections in humans. PMT is sufficient to induce all major symptoms of atrophic rhinitis in animals (3, 10). PMT appears to act on the α-subunit of the Gq,11 family of heterotrimeric G proteins (25), which stimulates phosphatidylinositol hydrolysis, resulting in an increase in inositol phosphate and diacylglycerol levels (21) and a mobilization of intracellular calcium pools. Moreover, the toxin is a potent mitogen for several cell types (4, 13, 16) and stimulates anchorage-independent DNA synthesis and growth in soft agar in Rat1 fibroblasts (9). The mitogenic effect of PMT on HEK293 cells seems to be caused by stimulation of the mitogen-activated protein kinase pathway via Gq,11-dependent transactivation of the epidermal growth factor receptor (20). In Swiss 3T3 cells, this mitogenic effect of PMT is transient and is followed by a blockade of the cell cycle progression (23).
In cultured cells, PMT induces pronounced cytoskeletal changes. Treatment of Swiss 3T3 fibroblasts results in stress fiber formation and focal adhesion assembly (4, 11), which has been proposed to be caused by an activation of the small GTP-binding protein Rho (11). Similarly, stress fiber formation and an increased permeability of endothelial monolayers of human umbilical vein endothelial cells induced by PMT are dependent on the activation of the Rho-signaling pathway (5). A different cytopathic effect of PMT is observed in Vero and embryonic bovine lung (EBL) cells, which is characterized by rounding up of cells (15, 17).
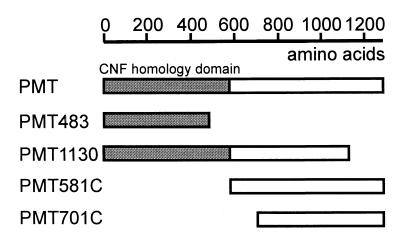
PMT consists of 1,285 amino acid residues and the primary structure of the N terminus of the toxin has 24 and 27% identity at the amino acid level to the N terminus of the cytotoxic necrotizing factors CNF1 and CNF2, respectively, of Escherichia coli. CNF1 and CNF2 are deamidases acting on Rho proteins (7, 18). The catalytic domain of CNF1 is localized to the C-terminal part of the protein (12, 19), whereas the N-terminal part harbors the receptor-binding domain (6, 12). In keeping with this domain structure of CNF1, an amino acid in the C-terminal part of PMT was proposed to be important for biological activity of the toxin (22). In a recent study, however, the biological activity of PMT was localized to the N-terminal part of the holotoxin (24). To clarify this discrepancy, we studied the effects of various fragments of PMT on EBL cells in more detail. Here we report that a C-terminal fragment of PMT elicits the same effects in EBL cells as the holotoxin, suggesting that this part of the toxin is responsible for its biological effects.
MATERIALS AND METHODS
Abbreviations used in this paper.
CNF1 and CNF2, E. coli cytotoxic necrotizing factors 1 and 2; EBL cells, embryonic bovine lung cells; GST, glutathione S-transferase; PMT, P. multocida toxin; MEM, modified Eagle's medium; FCS, fetal calf serum; TRITC, tetramethylrhodamine-5-isothiocyanate.
Materials.
[3H]inositol was obtained from DuPont NEN (Dreieich, Germany). PCR primers were from MWG Biotech (Ebersberg, Germany). All other reagents were of analytical grade and were purchased from commercial sources.
PCR amplification.
P. multocida serovar D, strain P824 (kindly donated by D. Schimmel, Jena, Germany), was used as a source of chromosomal DNA. PMT was amplified with a PCR System 2400 from Perkin Elmer (Überlingen, Germany), using the primer pairs PMTsen and PMTanti (5′-AGATCTATGAAAACAAAACATTTTTTTAACTC-3′ and 5′-GGATCCTAGTGCTCTTGTTAAGCGAGG-3′). The reaction was carried out with 4 U of Taq polymerase (Roche, Mannheim, Germany), 600 nM each primer, and 300 ng of chromosomal DNA for 30 cycles (denaturation, 94°C for 10 s; annealing, 48°C for 30 s; elongation, 68°C for 6 min) in a total volume of 100 μl. The amplified DNA fragment was cloned into pCR2.1 (Invitrogen BV, Groningen, The Netherlands). After mobilization with BamHI, the fragment was cloned into the expression vector pGEX-2T.
Cloning of truncated PMT fragments.
PMT in pGEX-2T was used for subcloning the truncated fragments PMT483 and PMT1130, consisting of amino acid residues 1 through 483 and 1 through 1130, respectively. For the generation of PMT1130.pGEX-2T, the PMT.pGEX-2T construct was digested with EcoRI and the truncated vector construct was purified and religated. PMT483.pGEX-2T was obtained by digesting PMT.pGEX-2T with HindIII-BamHI. The truncated vector construct fragment was isolated, treated with the Klenow fragment of DNA polymerase I to generate blunt ends, and religated.
The C-terminal fragments PMT581C and PMT701C were obtained by PCR amplification using PMT.pGEX-2T as a template. Amplicons were cloned into pGEX-2T. The primers used were PMT581Csen (5′-AGATCTAGTCCTTTCCGTATTGGATTA-3′) and PMTanti for PMT581C.pGEX-2T and PMT701Csen (5′-GCGGGATCCGAAATGGCTGGAAAAACCAG-3′) and PMTanti for PMT701C.pGEX2T.
Site-directed mutagenesis of PMT and PMT581C.
The QuikChange kit (Stratagene, Amsterdam, The Netherlands) was used for mutating one nucleotide in PMT.pGEX-2T and PMT581C.pGEX-2T, resulting in PMT.Cys1165Ser and PMT581C.Cys1165Ser, respectively. Procedures were carried out as specified by the manufacturer, using the primer pair PMT.C1165Ssen and PMT.C1165Santi (5′-GAAGCTGGCTCTTCTGATTCAGTAAGC-3′ and 5′-GCTTACTGAATCAGAAGAGCCAGCTTC-3′).
Sequencing.
Sequencing of PMT.pGEX-2T and all its truncated derivatives was done with the ABI PRISM Dye Terminator cycle-sequencing ready-reaction kit (Perkin Elmer, Überlingen, Germany) to check for correct cloning and mutations due to PCR amplification. Sequencing was performed with overlapping DNA fragments.
Cell culture.
Embryonic bovine lung cells (17) were grown as monolayers in MEM containing streptomycin (100 μg/ml), penicillin (100 U/ml), and 15% FCS in a humidified atmosphere of 5% CO2 and 95% air. Cells were subcultured every 2 to 3 days.
Expression of recombinant proteins.
Recombinant PMT and toxin fragments were expressed in E. coli strain TG1 and purified as GST fusion proteins as specified by the manufacturer. GST fusion proteins from the E. coli vector pGEX2T were isolated by affinity chromatography with glutathione-Sepharose (Amersham Pharmacia Biotech, Freiburg, Germany) followed by proteolytic cleavage using 3.25 U of thrombin/mg of recombinant GST fusion protein. Thrombin was removed by incubation with benzamidine-Sepharose (Amersham Pharmacia Biotech).
Analysis of total inositol phosphates.
EBL cells were grown in 24-well plates for 2 to 3 days. Then cultures were labeled with 2 μCi of [2-3H]inositol per ml in serum-free medium (MEM-Waymouth medium [1:1]) overnight. Subsequently, PMT or PMT fragments at the indicated concentrations and LiCl (20 mM) were added at the indicated time points, and the cells were incubated for the indicated times. Thereafter, the medium was replaced by 750 μl of ice-cold 10 mM formic acid (pH 3). After 30 min, the extract was neutralized with 3 ml of 5 mM NH3 (pH 8 to 9). Total inositol phosphates were analyzed by anion-exchange chromatography. Samples were loaded onto columns containing 1 ml of AG1-X8 resin (200 to 400 mesh; Bio-Rad, Munich, Germany) preequilibrated with 5 mM NH3 (pH 8 to 9). After the columns were washed with 4 ml of 40 mM ammonium formate, total inositol phosphates were eluted with 4 ml of 2 M ammonium formate. The eluate was counted in 4 ml of Ultima Gold cocktail (Packard Bioscience e.v., Groningen, The Netherlands).
Electroporation of EBL cells.
Confluent EBL cells, which had or had not been prelabeled with 2 μCi of [2-3H]inositol per ml, were trypsinized as usual. A 400-μl volume of cell suspension in MEM plus 15% FCS containing approximately 5 × 105 cells and the indicated proteins or substances was placed in a 0.4-cm-gap-width electroporation cuvette (Bio-Rad). Electroporation was performed using a Gene Pulser transfection apparatus (Bio-Rad), which was set at a capacitance of 950 μF and a voltage of 200 V at room temperature. After electroporation, 100-μl aliquots of the cell suspension were transferred to 24-well plastic dishes containing 500 μl of prewarmed MEM plus 15% FCS and incubated for the indicated times at 37°C under 5% CO2. Then photographs were obtained or the amount of total inositol phosphates was determined, respectively.
Actin cytoskeleton staining.
Cells were electroporated in the presence of the indicated agents as described above. They were then seeded on coverslips and incubated for 6 h at 37°C under 5% CO2. After removal of the medium, the cells were fixed for 30 min with a solution containing 3.7% formaldehyde and 1% Triton X-100 and incubated with TRITC-conjugated phalloidin (4 μg/ml) for 45 min (each step was preceded by three washes in phosphate-buffered saline). After the inverted coverslips were mounted in gelatine on glass slides, the cells were examined and photographed.
RESULTS
Recently, contradictory data have been reported on the localization of the domain carrying the biological activity in PMT. While the N terminus of the toxin was described to elicit effects dependent on Gq in Xenopus oocytes (24), the replacement of a cysteine residue at the C terminus (Cys1165) by serine was reported to result in a complete loss of toxic activity in vivo (22).
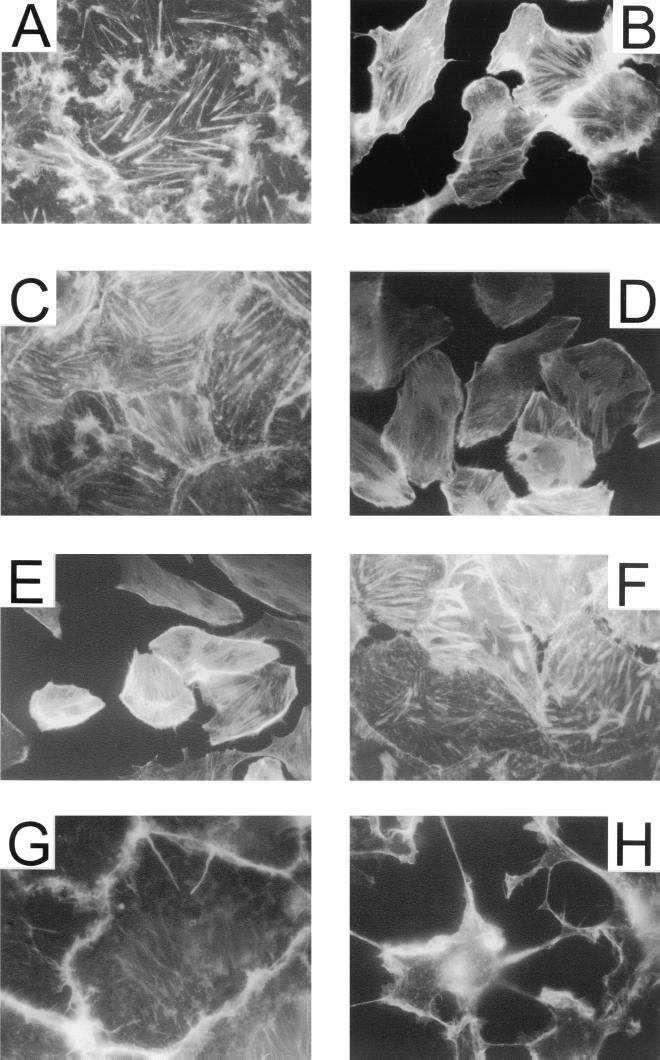
To clarify this issue, we studied the effects of the holotoxin and different toxin fragments in EBL cells. To this end, the PMT gene was cloned in the expression vector pGEX2T and various toxin fragments were constructed (Fig. 1). After expression in E. coli and purification by affinity chromatography, GST fusion proteins were cleaved by thrombin to obtain the recombinant proteins. A sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the toxin and the fragments is shown in Fig. 2. Next we tested the recombinant PMT for its ability to induce morphological effects in EBL cells. As described previously (17), the holotoxin provoked dramatic changes in cell morphology and actin cytoskeleton organization in this cell type. In particular, shrinking of the cell body and formation of lamellipodia-like protrusions were observed. Moreover, PMT led to a reorganization of the actin cytoskeleton. While control cells exhibited thick actin stress fibers (Fig. 3A), PMT-treated cells showed a dense network of often parallel-orientated, delicate fibers (Fig. 3B). During the process of rounding, these fibers disappeared and completely rounded cells showed very little actin structures (data not shown). These effects were clearly different from those elicited by the rho-inactivating fusion toxin Clostridium botulinum C3 transferase (1) (Fig. 3H) and the E. coli cytotoxic necrotizing factor 1 (CNF1) (Fig. 3G), which activates rho proteins. On CNF1 treatment, actin was translocated to the cell-cell contacts, associated with an enlargement of the cell body. On the other hand, inactivation of Rho by the C3 fusion toxin resulted in a total loss of actin structures and a shrinking of the cells.
FIG. 1.
Schematic representation of the PMT fragments used in this study. The regions homologous to E. coli CNF1 and CNF2 are marked in gray.
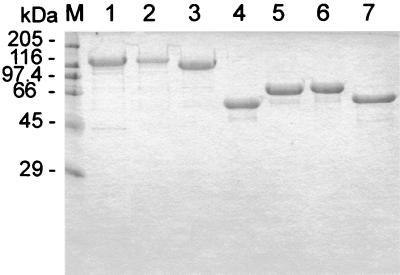
FIG. 2.
Purified recombinant PMT, toxin fragments, and mutants. The recombinant toxin and fragments were constructed as GST fusion proteins, expressed in E. coli, purified by affinity chromatography, and cleaved by treatment with thrombin. PMT (lane 1), PMT.Cys1165Ser (lane 2), PMT1130 (lane 3), PMT483 (lane 4), PMT581C (lane 5), PMT581C.Cys1165Ser (lane 6), and PMT701C (lane 7) are shown. Protein was loaded at a rate of 3 μg for lanes 1 and 3 to 7 and 1 μg on lane 2.
FIG. 3.
Actin staining of EBL cells. (A and B) Effect of PMT on EBL cells after addition to the medium. Cells were incubated with PMT (1 μg/ml) (A) or control buffer (B) for 6 h at 37°C under 5% CO2 in serum-free medium. (C to H) Effect of PMT and toxin fragments on EBL cells after electroporation. EBL cells were electroporated in the presence of control buffer (C), 1 μg of PMT per ml (D), 1 μg of PMT581C per ml (E), or 100 μg of PMT1130 per ml (F) as described in Materials and Methods. The cells were then seeded on coverslips and incubated for 6 h at 37°C under 5% CO2. After fixation of the cells, cellular actin was stained with TRITC-phalloidin. Other cells were incubated in the presence of 1 μg of CNF1 per ml (G) or the fusion toxin of the N-terminal part of the C. botulinum C2I toxin component fused to the C3 transferase (100 ng/ml) together with the activated form of C2II (200 ng/ml) (H). After 4 h, the cells were fixed and stained by TRITC-phalloidin.
To test the PMT fragments for activity on cell morphology, we added them to the culture medium of EBL cells. However, none of the proteins was cytopathic towards EBL cells (data not shown).
To circumvent the uptake process necessary for intracellular toxin action, we introduced the toxin and the fragments into EBL cells by electroporation. When suspended EBL cells were electroporated in the presence of control buffer, they attached to the culture dish within 90 min, resulting in a dense monolayer like that formed by untreated cells 90 min after seeding (Fig. 3C and 4A). The differences in morphology in Fig. 3A and C are explained by different cultivation times after seeding (24 h for the cells in Fig. 3A, and 90 min for those in Fig. 3C). To test electroporation as a method of introducing cytotoxic proteins into cells, we studied the effects of the enzymatically active fragment of Clostridium difficile toxin B (B546), which is incapable of cell binding and entry. EBL cells electroporated in the presence of the toxin B fragment failed to attach to the matrix and exhibited a rounded morphology typical of intoxication with toxin B (data not shown). Therefore, we concluded that electroporation allows the introduction of protein toxins into EBL cells.
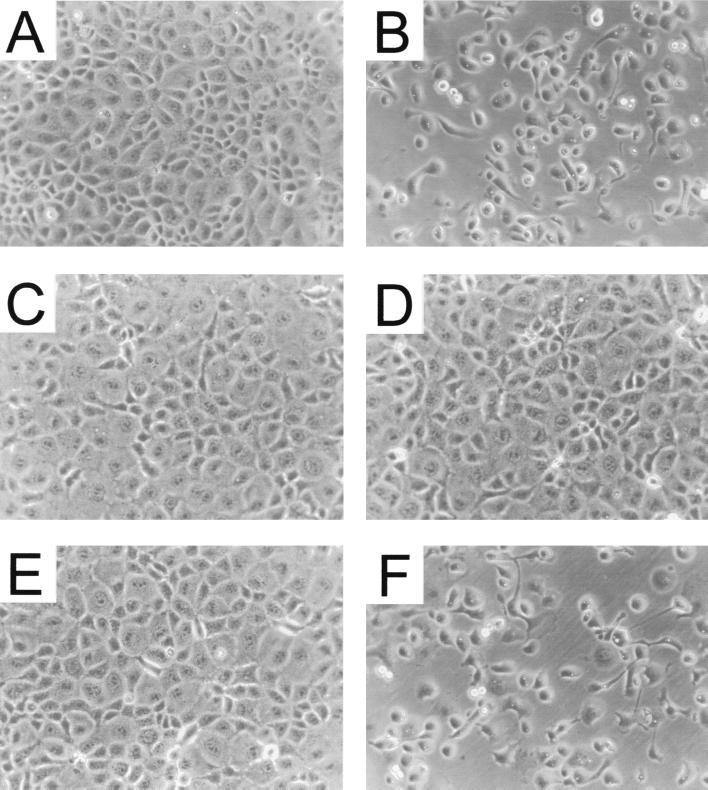
FIG. 4.
Morphological effects of recombinant PMT, toxin fragments, and mutants on EBL cells after electroporation. Electroporation was performed as described in Materials and Methods. Cells were electroporated in the presence of control buffer or recombinant proteins. They were then seeded in 3-cm-diameter dishes and incubated for 4 h at 37°C under 5% CO2. Photographs were taken. (A) Control buffer; (B) PMT (200 ng/ml); (C) PMT.Cys1165Ser (2 μg/m); (D) PMT1130 (2 μg/ml); (E) PMT483 (2 μg/ml); (F) PMT581C (200 ng/ml).
When electroporated in the presence of PMT, EBL cells reattached to the matrix, as did control cells. Within 3 h, however, these cells adopted the same morphology as that observed after application of PMT to intact cells; e.g., the cells rounded up and showed a redistribution of the actin cytoskeleton (Fig. 3D and 4B). On introduction of the N-terminal fragments PMT483 (Fig. 4E) and PMT1130 (Fig. 3F and 4D) into the cells, no morphological changes were observed. However, the C-terminal fragment PMT581C, encompassing amino acids 581 through 1285, elicited the same morphological effects as did the holotoxin when introduced by electroporation (Fig. 4F). Moreover, this fragment led to a redistribution of actin structures identical to that of the holotoxin (Fig. 3E), while a C-terminal fragment shorter than PMT581C (PMT701C) had no effect on cell morphology and the actin cytoskeleton (data not shown).
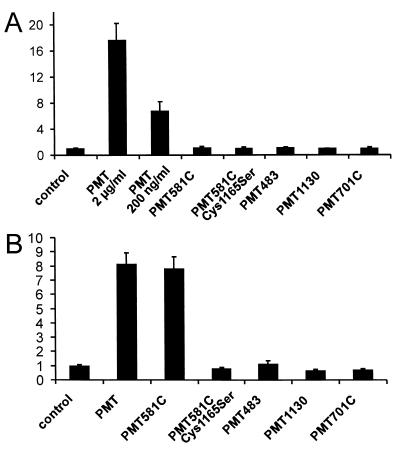
To further characterize the toxin fragments, we investigated their effects on inositol phosphate accumulation in EBL cells using [3H]inositol-labeled monolayers. As found for other cell types (13, 14, 21), addition of the holotoxin to the culture medium led to an increase in the amount of total inositol phosphates, indicating an increased activity of phospholipase C (Fig. 5A). Addition of the toxin fragments to the culture medium had no effect, even when they were added at a more than 500-fold higher concentration than required for an effect of the holotoxin (Fig. 5A). Next, we tested the effects of the different fragments on inositol phosphate accumulation after electroporation. Again, the N-terminal fragments had no effect. However, the C-terminal fragment PMT581C provoked an increase in inositol phosphate levels to the same extent as that observed with the holotoxin. However, PMT701C was inactive (Fig. 5B).
FIG. 5.
Effects of PMT, toxin fragments, and mutants on inositol phosphate accumulation in EBL cells. (A) PMT, fragments, or mutants were applied to cultured EBL cells in the presence of 20 mM LiCl. After 90 min, the total amount of inositol phosphates in the cells was measured. Data are given as fold induction of buffer control plus 20 mM LiCl (means and standard error; n = 3). Proteins were applied at 2 μg/ml or 200 ng/ml (PMT), 20 μg/ml (PMT701C), or 100 μg/ml (PMT581C, PMT581C.Cys1165Ser, PMT483, and PMT1130). (B) PMT, fragments, or mutants were introduced into EBL cells by electroporation in the presence of 20 mM LiCl. After 90 min, the total amount of inositol phosphates in the cells was measured as described in Materials and Methods. Data are given as fold induction of buffer control plus 20 mM LiCl (means and standard error; n = 3). Proteins were applied at a concentration of 5 μg/ml (PMT and PMT581C), 20 μg/ml (PMT701C), or 100 μg/ml (PMT581C.Cys1165Ser, PMT483, and PMT1130).
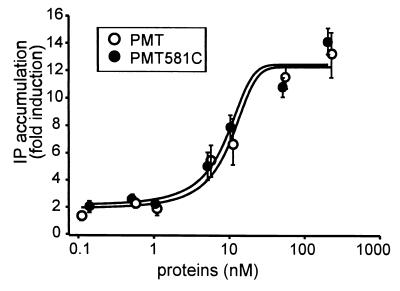
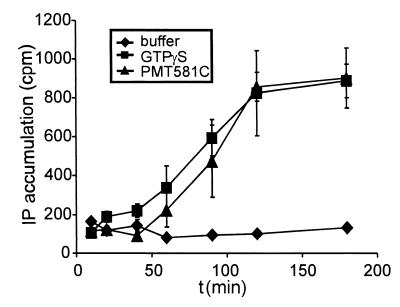
The concentration-effect relationship of the active fragment PMT581C on inositol phosphate accumulation was compared with that of the holotoxin. As shown in Fig. 6, similar concentration-effect curves were obtained for the two proteins. Next, the time dependence of the effect of PMT581C on inositol phosphate accumulation was compared with the stimulation of inositol phosphate production induced by the stable GTP analogue GTPγS. As can be seen in Fig. 7, the two agents caused similar profiles and the same maximal extent of inositol phosphate accumulation over the times given.
FIG. 6.
Concentration-effect dependency of PMT versus PMT581C after electroporation of EBL cells. Electroporation of EBL cells was performed as described in Materials and Methods in the presence of the indicated concentrations of PMT or PMT581C, respectively. Then the cells were seeded in 24-well plates and incubated for 2 h at 37°C. After addition of 20 mM LiCl, the cells were further incubated for 90 min. Subsequently, the total amount of inositol phosphates was determined as described in Materials and Methods. Data are given as means and standard errors (n = 3).
FIG. 7.
Time dependency of inositol phosphate accumulation after introduction of GTPγS or PMT581C into EBL cells. EBL cells were electroporated in the presence of 1 mM GTPγS or 2.5 μg of PMT581C per ml. Then they were incubated at 37°C under 5% CO2 in medium containing 15% FCS and 20 mM LiCl. At the indicated time points, the medium with the nonattached cells was removed and the cells were centrifuged at 800 rpm for 3 min and resuspended in formic acid (10 mM). The suspension was added to the attached cells and incubated for 30 min at 4°C. Subsequently, the total amount of inositol phosphates was determined as described in Materials and Methods. Data are given as means and standard errors (n = 3).
Recently it was shown that the mutant Cys1165Ser of PMT is nontoxic to cells (22). Although the study did not permit clarification the functional role of this cysteine residue, it was suggested that Cys1165 is essential for the biological activity of PMT (22). We confirmed that the Cys1165Ser mutant of PMT had no cytopathic effects on intact cells (data not shown). To clarify the role of the Cys1165, we studied the effects of the cysteine exchange with serine in the holotoxin PMT and in the PMT581C fragment after electroporation. As shown in Fig. 5B, the Cys1165Ser mutant of PMT581C failed to increase the amount of total inositol phosphates and did not influence cell morphology after electroporation (data not shown). The same was found for the holotoxin mutant (Fig. 4C).
DISCUSSION
EBL cells have been used to assess the activity of PMT. These cells show a typical cytopathic morphology on treatment with PMT (17). In this study we investigated the effects of PMT and toxin fragments on EBL cells. Consistent with a previous study (24), we observed that none of the toxin fragments studied induced a cytopathic effect when applied to the cell culture medium of intact cells. However, a C-terminal fragment encompassing amino acids 581 through 1285 (PMT581C) provoked the same morphological changes and redistribution of the actin cytoskeleton as did the holotoxin when introduced into cells by electroporation (Fig. 4). Moreover, this fragment caused an increase in total inositol phosphate levels similar to that observed with the holotoxin (Fig. 5B) and was as efficient as the parent toxin (Fig. 6). PMT581C increased the total inositol phosphate levels to the same extent as GTPγS did (Fig. 7). The stable GTP analogue presumably stimulates inositol phosphate production by direct activation of the Gq protein, which is an activator of phospholipase C (2). In some experiments we observed a lag phase in the onset of inositol accumulation induced by the toxin fragment. However, because measurements at early time points are unreliable (e.g., duration of leakage of the cells after electroporation), we are unable to decide whether an activating step is necessary for the action of PMT581C. Nevertheless, the data indicate that regions of PMT which are responsible for the intracellular biological activity in EBL cells are included in PMT581C. A fragment encompassing amino acids 701 through 1285 (PMT701C) exhibited no activity in our system. Therefore, at least a part of the region covered by amino acids 581 through 700 seems to be important for the biological activity and/or stability of the active fragment. Moreover, even high concentrations of PMT581C had no effect when applied to the cell culture medium. These data indicate that the fragment is not sufficient to mediate binding to the receptor and/or uptake into the target cells. As shown in Fig. 5, the PMT holotoxin was less active in the electroporation experiments than in the experiments using intact cells. This can be explained by a lower activity of the toxin in the presence of serum, which was also observed in whole-cell experiments with serum (data not shown). The electroporation experiments had to be performed in the presence of serum, since the cells did not reattach to the matrix after electroporation in the absence of FCS. Alternatively, an additional activation step might occur during internalization of the toxin, which would result in a higher activity after uptake of the protein.
Interestingly, a residue in the C-terminal portion of the toxin (Cys1165) has been found by Ward et al. (22) to be essential for toxic activity in vivo. However, these studies were unable to clarify the reason for the functional loss of the mutant. Here it is shown that this residue is most probably involved in the biological activity of the toxin because the mutant Cys1165Ser of the fragment PMT581C failed to elicit any toxic affects after electroporation.
In a recent publication on the effects of PMT (24), the N terminus of the toxin was ascribed a biological activity on Ca2+-dependent chloride currents in Xenopus oocytes and an activity on the morphology of Vero cells after transfection. As reported, N-terminal fragments had no biological effect in our hands. However, PMT might exhibit a domain structure like the exoenzyme S of Pseudomonas aeruginosa. This toxin is a bifunctional protein consisting of separate domains responsible for the GAP activity on Rho and the ADP-ribosyltransferase activity with Ras as a target (8).
From the data presented, we propose a domain structure of PMT. We suggest that the N-terminal part of the toxin contributes to the uptake of the toxin into the target cell because PMT581C is incapable of entering cells. The C-terminal part mediates phospholipase C activation and morphological changes. Cys-1165 within this region is crucial for cellular activity. Interestingly, a similar domain structure was proposed for E. coli CNF1 and CNF2, which have a 24 to 27% homology with PMT at their N termini. The N terminus of CNF1 was shown to mediate the uptake into cells (6, 12), while C-terminal fragments exert deamidase activity in vitro and induce morphological effects after introduction into cells by microinjection (19).
ACKNOWLEDGMENTS
The study was financially supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Barth H, Hofmann F, Olenik C, Just I, Aktories K. The N-terminal part of the enzyme component (C2I) of the binary Clostridium botulinum C2 toxin interacts with the binding component C2II and functions as a carrier system for a Rho ADP-ribosylating C3-like fusion toxin. Infect Immun. 1998;66:1364–1369. doi: 10.1128/iai.66.4.1364-1369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blank J L, Ross A H, Exton J H. Purification and characterization of two G-proteins that activate the beta 1 isozyme of phosphoinositide-specific phospholipase C. Identification as members of the Gq class. J Biol Chem. 1991;266:18206–18216. [PubMed] [Google Scholar]
- 3.Chrisp C E, Foged N T. Induction of pneumonia in rabbits by use of a purified protein toxin from Pasteurella multocida. Am J Vet Res. 1991;52:56–61. [PubMed] [Google Scholar]
- 4.Dudet L L, Chailler P, Dubreuil D, Martineau-Doize B. Pasteurella multocida toxin stimulates mitogenesis and cytoskeleton reorganization in swiss 3T3 fibroblasts. J Cell Physiol. 1996;168:173–182. doi: 10.1002/(SICI)1097-4652(199607)168:1<173::AID-JCP21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Essler M, Hermann K, Amano M, Kaibuchi K, Heesemann J, Weber P C, Aepfelbacher M. Pasteurella multocida toxin increases endothelial permeability via rho kinase and myosin light chain phosphatase. J Immunol. 1998;161:5640–5646. [PubMed] [Google Scholar]
- 6.Fabbri A, Gauthier M, Boquet P. The 5′ region of cnf1 harbours a translational regulatory mechanism for CNF1 synthesis and encodes the cell-binding domain of the toxin. Mol Microbiol. 1999;33:108–118. doi: 10.1046/j.1365-2958.1999.01453.x. [DOI] [PubMed] [Google Scholar]
- 7.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 8.Goehring U-M, Schmidt G, Pederson K J, Aktories K, Barbieri J T. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem. 1999;274:36369–36372. doi: 10.1074/jbc.274.51.36369. [DOI] [PubMed] [Google Scholar]
- 9.Higgins T E, Murphy A C, Staddon J M, Lax A J, Rozengurt E. Pasteurella multocida toxin is a potent inducer of anchorage-independent cell growth. Proc Natl Acad Sci USA. 1992;89:4240–4244. doi: 10.1073/pnas.89.10.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamp E M, Kimman T G. Induction of nasal turbinate atrophy in germ-free pigs, using Pasteurella multocida as well as bacterium-free crude and purified dermonecrotic toxin of P. multocida. Am J Vet Res. 1988;49:1844–1849. [PubMed] [Google Scholar]
- 11.Lacerda H M, Lax A J, Rozengurt E. Pasteurella multocida toxin, a potent intracellularly acting mitogen, induces p125FAK and paxillin tyrosine phosphorylation, actin stress fiber formation, and focal contact assembly in Swiss 3T3 cells. J Biol Chem. 1996;271:439–445. doi: 10.1074/jbc.271.1.439. [DOI] [PubMed] [Google Scholar]
- 12.Lemichez E, Flatau G, Bruzzone M, Boquet P, Gauthier M. Molecular localization of the Escherichia coli cytotoxic necrotizing factor CNF1 cell-binding and catalytic domains. Mol Microbiol. 1997;24:1061–1070. doi: 10.1046/j.1365-2958.1997.4151781.x. [DOI] [PubMed] [Google Scholar]
- 13.Mullan P B, Lax A J. Pasteurella multocida toxin is a mitogen for bone cells in primary culture. Infect Immun. 1996;64:959–965. doi: 10.1128/iai.64.3.959-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy A C, Rozengurt E. Pasteurella multocida toxin selectively facilitates phosphatidylinositol 4,5-bisphosphate hydrolysis by bombesin, vasopressin, and endothelin. Requirement for a functional G protein. J Biol Chem. 1992;267:25296–25303. [PubMed] [Google Scholar]
- 15.Pennings A M, Storm P K. A test in vero cell monolayers for toxin production by strains of Pasteurella multocida isolated from pigs suspected of having atropic rhinitis. Vet Microbiol. 1984;9:503–508. doi: 10.1016/0378-1135(84)90071-3. [DOI] [PubMed] [Google Scholar]
- 16.Rozengurt E, Higgins T, Chanter N, Lax A J, Staddon J M. Pasteurella multocida toxin: potent mitogen for cultured fibroblasts. Proc Natl Acad Sci USA. 1990;87:123–127. doi: 10.1073/pnas.87.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutter J M, Luther P D. Cell culture assay for toxigenic Pasteurella multocida from atrophic rhinitis of pigs. Vet Rec. 1984;114:393–396. doi: 10.1136/vr.114.16.393. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor 1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt G, Selzer J, Lerm M, Aktories K. The Rho-deamidating cytotoxic-necrotizing factor CNF1 from Escherichia coli possesses transglutaminase activity—cysteine-866 and histidine-881 are essential for enzyme activity. J Biol Chem. 1998;273:13669–13674. doi: 10.1074/jbc.273.22.13669. [DOI] [PubMed] [Google Scholar]
- 20.Seo B, Choy E W, Maudsley W E, Miller W E, Wilson B A, Luttrell L M. Pasteurella multocida toxin stimulates mitogen-activated protein kinase via Gq/11-dependent transactivation of the epidermal growth factor receptor. J Biol Chem. 2000;275:2239–2245. doi: 10.1074/jbc.275.3.2239. [DOI] [PubMed] [Google Scholar]
- 21.Staddon J M, Barker C J, Murphy A C, Chanter N, Lax A J, Michell R H, Rozengurt E. Pasteurella multocida toxin, a potent mitogen, increases inositol 1,4,5-triphosphate and mobilizes Ca2+ in Swiss 3T3 cells. J Biol Chem. 1991;266:4840–4847. [PubMed] [Google Scholar]
- 22.Ward P N, Miles A J, Sumner I G, Thomas L H, Lax A J. Activity of the mitogenic Pasteurella multocida toxin requires an essential C-terminal residue. Infect Immun. 1998;66:5636–5642. doi: 10.1128/iai.66.12.5636-5642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson B A, Aminova L R, Ponferrada V G, Ho M. Differential modulation and subsequent blockade of mitogenic signaling and cell cycle progression by Pasteurella multocida toxin. Infect Immun. 2000;68:4531–4538. doi: 10.1128/iai.68.8.4531-4538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson B A, Ponferrada V G, Vallance J E, Ho M F. Localization of the intracellular activity domain of Pasteurella multocida toxin to the N terminus. Infect Immun. 1999;67:80–87. doi: 10.1128/iai.67.1.80-87.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson B A, Zhu X, Ho M, Lu L. Pasteurella multocida toxin activates the inositol triphosphate signaling pathway in Xenopus oocytes via Gqα-coupled phospholipase C-β1. J Biol Chem. 1997;272:1268–1275. doi: 10.1074/jbc.272.2.1268. [DOI] [PubMed] [Google Scholar]