Abstract
The presence of CD8+ T cells with a memory phenotype in nonimmunized mice has been noted for decades, but it was not until about 2 decades ago that they began to be studied in greater depth. Currently called virtual memory CD8+ T cells, they consist of a heterogeneous group of cells with memory characteristics, without any previous contact with their specific antigens. These cells were identified in mice, but a few years ago, a cell type with characteristics equivalent to the murine ones was described in healthy humans. In this review, we address the different aspects of its biology mainly developed in murine models and what is currently known about its cellular equivalent in humans.
Keywords: TVM cells, TIM cells, Ag-specific T cells, infection, cancer, human TVM cells
General Aspects of Memory-Like CD8+ T Cells
Traditionally, both in mouse and human, it has been considered that CD8+ T cells housed in secondary lymphoid organs (SLO) transit through a single differentiation pathway that begins as “naive” T cells (TN). Then, after the recognition of their cognate antigen (Ag), they are activated and differentiate into effector T cells (TEFF), whose number contracts after the antigen/pathogen is eliminated, leaving a “pool” of conventional Ag-experienced memory T cells (TMEM) (Sallusto and others 1999; Boyman and others 2009; Mueller and others 2013). The TMEM cell population consists mainly of effector memory T cells (TEM), which are mainly found in peripheral tissues, especially in mucosa, and recirculate by blood and lymph. These cells respond quickly to newly encountered Ags, and central memory T cells (TCM) that are found mainly in lymph nodes (LNs) and are responsible for self-renewal and supply of TEFF cells (Boyman et al. 2009, Gerlach et al. 2015, Mueller et al. 2013, Sallusto et al. 1999).
More recently, another population of memory cells has been defined, which does not recirculate and resides mainly in the tissues where the primary infection has occurred and is called resident memory T cells (TRM) (Mueller and others 2013; Gerlach and others 2015).
Two decades later, a new population of CD8+ T cells was identified in unmanipulated mice and exhibited phenotypic characteristics similar to conventional memory T cells by rapidly responding to stimuli, either innate or through their TCRs (Thiele and others 2020). What is surprising about these cells is that they achieve this “memory-like” phenotype and respond as quickly as TMEM without previously having contacted their specific Ags (Sallusto and others 1999). All these features defined these cells as “memory-like” CD8+ T cells and currently are called virtual memory cells (TVM cells). Similar to TMEM cells, TVM cells express high levels of CD44 and CD122 (β chain of IL-2/IL-15 receptor), as well as high levels of Eomesodermin (Eomes), a T-box family transcription factor known for regulating CD8+ T effector and memory cell fate and function (Pearce and others 2003; Intlekofer and others 2005).
The high CD44 and CD62L expression in TVM cells, combined with the absence of markers specifically distinguishing them from TMEM cells, led to TVM being mistakenly included in the TCM cell group for many years (Lee and others 2013a). Fortunately today, TVM can be differentiated since memory-like cells show low expression of CD49d, a component of the VLA-4 and LPAM homing receptors, which is only positively regulated in TMEM cells after strong TCR antigen recognition (Haluszczak and others 2009; Lee and others 2013a; Quinn and others 2020). This point is quite critical, especially considering it has been reported that the vast majority (≥85%) of cells believed to be TCM are actually CD49dlo and therefore TVM cells (Quinn and others 2020).
Even though the existence of memory-like T cells in SLO of nonimmunized animals has long been described, the source and composition of these cells were undefined for many years. Originally, it was suggested that TVM cells are specific for Ags derived from the microbiota. However, commensal microorganisms are not involved in the generation of TVM cells since this population exists in SLO from both germ-free (GF) (Huang and others 2005; Haluszczak and others 2009) and feral mice (Moudra and others 2021) in similar frequency.
Today, an abundance of new evidence has emerged that clarifies some of these “unknowns.” Kedl and others proposed the term “Virtual Memory” (TVM), based on the similarity to a computing term that means “alternative use of disc space,” to describe this novel repertoire of Ag-inexperienced memory T cells present in unprimed mice (Haluszczak and others 2009; White and others 2016, 2017).
When analyzing the frequency of memory-like T cells in nonimmunized mice, this number could reach up to 15%–20% of total CD8+ T cells in SLO (Lee and others 2011, 2013a; White and others 2017). The evaluation of memory-like CD8+ T cells in SLO is quite complex since it represents a heterogeneous group of cells from different origins. This diverse cellular pool comprised the following: (1) CD8+ innate T cells (TIM) that develop in the thymus and depend on IL-4 for their maturation. Contrary to conventional TN cells, TIM cells acquire a memory phenotype within the thymus without previous cognate Ag encounter. (2) Lymphopenia-induced memory cells (TLIM) or homeostatic proliferation memory T cells (THP) arise from homeostatic mechanisms in situations of extreme lymphopenia, which are mediated by either irradiation or genetic T cell deficiency.
TLIM can also result from physiological lymphopenia occurring in the neonatal period in mice (Min and others 2003; Schuler and others 2004). The generation of TLIM cells can occur without foreign antigen activation and is thought to be driven by reduced competition for limiting resources, including IL-7 and low-affinity TCR ligands (Tan and others 2001). (3) TVM cells develop in the periphery; however, these cells arise from specific precursors in the thymus and appear soon after birth in normal mice (Lee and others 2011, 2013a; White and others 2017; Quinn and others 2020). TVM cells highly depend on IL-15 for their generation/maintenance mainly through IL-15 trans presentation by CD8α+ dendritic cells (Sosinowski and others 2013).
There have been a considerable number of publications over the years reporting Ag-independent memory cells with an array of defining names such as innate T cells, memory-like T cells, bystander T cells, virtual memory T cells, and Ag-inexperienced CD8+ T cells being the most commonly used. However, currently, there is consensus to call all 3 cell subsets (TIM, THP/LIM, and TVM) “Virtual Memory” when they reside in SLO since they cannot be distinguished at a phenotypic level (White and others 2017). In this review, we have compiled the current knowledge about TIM and TVM cell populations and address different aspects of their biology. Distinction between THP/TLIM and TVM have been mainly addressed by the reviews from *Pribikova and others (2018) and Hussain and Quinn (2019). In this work, we have also included a complete section about their more recently described human counterparts.
TIM Cells, Phenotype, Origin, and Differentiation
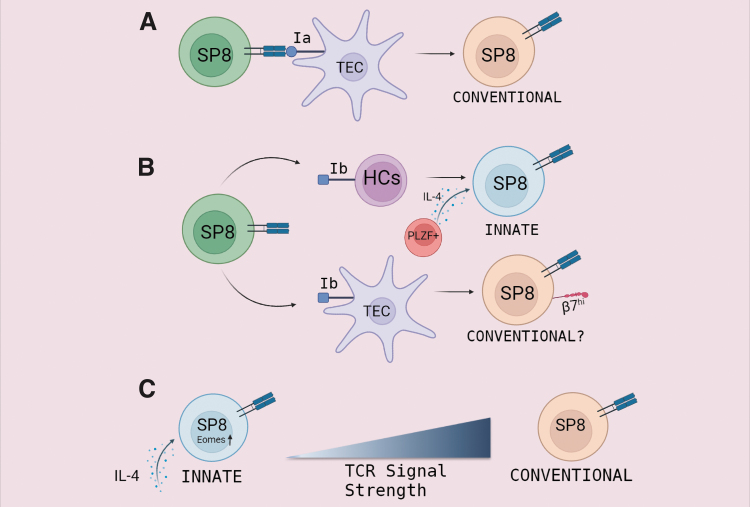
As is the case of conventional single positive CD8 thymocytes (SP8), TIM cells also require particular developmental conditions when it comes to MHC class I (MHC I) and cytokine interactions. Several laboratories have offered valuable information about these points. For instance, it has been demonstrated that MHC Ib-restricted CD8+ T cells are more prone to develop into an “innate-like” phenotype than MHC Ia-restricted T cells (Urdahl and others 2002; Cho and others 2011). Even more, it has been reported that innate CD8+ T cells can be positively selected by MHC class Ib molecules expressed on hematopoietic cells (HCs), and not necessarily by thymic epithelial cells (TEC), as is the case for development of conventional single positive CD8 (SP8) thymocytes (Cho and others 2011; Huang and others 2013; Urdahl and others 2002).
Other investigators have arrived at similar conclusions using Kb−/−Db−/− mice expressing MHC class Ib, but lacking MHC class Ia. Urdahl and others demonstrated that selection of SP8 thymocytes (specific for L. monocytogenes), that acquired a CD44hi activated phenotype was the result of intrathymic interactions of MHC-class Ib but not MHC-class Ia restricted CD8+ T cells with MHC class I-expressing HCs (Urdahl and others 2002). Moreover, the adaptor molecule SAP (SH2D1A) is required for innate CD8+ T cell selection on HCs in ITK KO mice (Horai and others 2007). Cho and others (2011) expanded this concept by using transgenic (Tg) mice expressing a TCR specific for the listerial peptide LemA (D7 Tg) presented by MHC-linked H2-M3 (M3), a MHC class Ib molecule.
The authors show that M3-restricted CD8+ T cells can be successfully selected by either TECs or HCs. Interestingly, the same M3-restricted CD8+ T precursors selected from 2 distinct cell types led to clones with different phenotype and functional characteristics. While M3-restricted CD8+ T cells selected on TECs have a less activated phenotype with high expression of β7 integrin that efficiently migrates to the gut, the same precursors selected by HCs preferentially present features of innate cells (Cho and others 2011). Because both types of T cells generated expressed distinct patterns of integrin receptors, the authors speculate that they occupy different immunological niches and could ultimately play unique roles during an immune response (Cho and others 2011).
Due to the fact that innate CD8+ T cells carry an effector/memory phenotype (CD44hiCD122hi), one concern that has arisen is the possibility that TIM cells represent mature T cells from SLO, which have migrated to the thymus as previously described by our and other laboratories (Chau and others 2002; Hale and Fink 2009; Hodge and others 2012). To answer that question, Rafei and others (2011) have used the RAG2p-GFP mouse model (B6 background). This system allows one to discern between local (GFP+) or recirculating T cells (GFP−). Rafei and others (2011) report that up to 10% of total SP8 thymocytes (GFP+) develop into the innate phenotype (GFP+ CD44hi) in steady-state conditions and are not recirculating mature T cells from SLO.
Interestingly and contrary to other types of innate T cells that develop in the thymus, TIM cells present a nonrestricted TCR repertoire (Rafei and others 2011). In this work, by using OT-I RAG2p-GFP mice, the investigators demonstrate that expression of a given TCR could give rise to both conventional and innate SP8 thymocytes. This demonstrates that the generation of both lineages is not dictated merely by the nature of the TCR and it is possible that conventional and innate SP8 thymocytes may have overlapping TCR repertoires (Rafei and others 2011).
Signaling through MHC class I is important in the selection of conventional and TIM cells, but a role has also been reported for TCR signaling-associated molecules (kinases). In this context, data presented by Atherly and others (2006) indicate that conventional CD8+ T cell maturation is highly dependent on signaling through ITK and RLK, members of the Tec family tyrosine kinases. ITK and RLK, are expressed on thymocytes and regulate T cell receptor signaling thresholds during positive and negative selection.
The investigators showed that ITK KO and RLK/ITK double KO mice are almost devoid of conventional SP8 thymocytes and contain a large number of SP8 thymocytes that express consensus lineage markers indicative of TIM cells (CD44hi, CD122+, and EOMEShi). These cells also produce large amounts of IFNγ ex vivo and depend on IL-15 for maturation (Atherly and others 2006). Similar results were published by Broussard and others (2006), who additionally demonstrated that when ERK is reconstituted in ITK KO mice, they exhibited normalized thymic features and SP8 cells appeared phenotypically more similar to conventional SP8 cells from wild-type (WT) mice.
Most of the current knowledge about TIM biology was gained through studies in mutant mice where this population is dramatically expanded. For instance, after the discovery that mice deficient in Tec family proteins have biased thymic development toward TIM cells, new studies began to appear showing that deficiency in other T cell signaling molecules or transcription factors can also enrich the thymic innate CD8 T cell lineage. In the review by Lee and others (2011), the authors present a complete summary of specific molecules downstream of the TCR pathway involved in innate CD8 T cell development.
In this context, Nayar and others (2012) evaluated the role of IFN regulatory factor 4 (IRF4), a transcription factor that is upregulated following TCR stimulation in WT T cells, and observed that, in contrast to WT thymocytes, activation of SP8 IRF4 KO cells leads to a high and rapid expression of Eomes and the acquisition of a memory phenotype. These data may indicate that, contrary to innate SP8 cells, in conventional SP8 thymocytes, TCR activation induces a high ITK signaling, which in turn promotes IRF4 upregulation and suppression of Eomes expression (Nayar and others 2012).
The reason why different mutations bias SP8 development through the TIM lineage was quite mysterious until it was shown that these particular mutations lead to an overproduction of IL-4 by thymic NKT or CD4+ T cells. IL-4 produced by NKT or CD4+ T cells acts in a cell-extrinsic way promoting Eomes expression on SP8 thymocytes, a hallmark of innate CD8 T cells (Lee and others 2011; Min and others 2011). In this context, Carty and others investigated the signaling pathways required for IL-4-dependent Eomes induction in TIM cells.
They demonstrated that IL-4 is sufficient to drive Eomes expression through STAT6- and Akt-dependent pathways. Very interestingly, the authors suggest that IL-4 signaling pathways may direct cell fate when TCR signals are limiting as IL-4 has little effect on Eomes induction when T cells receive a strong TCR signal; however, IL-4 effectively promotes Eomes during attenuated TCR stimulation (Carty and others 2014).
A study performed by Lee and others demonstrated that in several mouse strains (especially BALB/c mice), a subpopulation of thymic iNKT cells, named NKT2 cells, was abundant and able to produce IL-4 in the absence of stimulation. The authors demonstrate that these physiological amounts of IL-4 produced by NKT2 cells are sufficient to induce a “memory-like” phenotype in SP8 thymocytes during steady-state conditions (Lee and others 2013b); moreover, the transcription factor promyelocytic leukemia zinc finger (PLZF) was ultimately responsible for this effect (D'Cruz and others 2010; Weinreich and others 2010). IL-4 production is not exclusive to NKT cells since it has been reported that other PLZF+ T cells can also drive innate CD8 T cell development (Weinreich and others 2010; Min and others 2011). Particularly, levels of PLZF in the thymus seem to be crucial to generate TIM cells.
Park and others reported that the amounts of PLZF are able to control not only the generation but also the subset composition of thymic iNKT cells. The authors demonstrate that compared to WT mice, PLZFGFPcre+/wt BALB/c mice (mice that produced lower quantity of PLZF) present a dramatic decrease in the frequency of PLZFhi NKT2 cells, leading to lower numbers of innate SP8 thymocytes (Park and others 2019). Data addressing the different components that participate in TIM thymic development are schematically summarized in Fig. 1.
FIG. 1.
Mechanisms involved in thymic differentiation of TIM cells. T cell development in the thymus produces many lineages of mature T cells. (A) When the TCR of a single positive CD8 (SP8) cell recognizes an MHC-Ia molecule expressed on TEC, it results in the development of a conventional CD8+ T cell. (B) In the context of MHC-Ib expression, SP8 thymocytes can be successfully selected either by TECs or HCs. While SP8 cells selected on TECs have “a more naive” phenotype, the same precursors selected by HCs and exposed to interleukin-4 (IL-4) derived from PLZF-expressing thymocytes convert into TIM cells. (C) Reduced TCR signaling in SP8 cells in the presence of IL-4 induces Eomes expression and TIM phenotype acquisition. IL-4 has little effect on Eomes induction when T cells receive a strong TCR signal giving rise mainly to conventional naive SP8 cells. TEC, thymic epithelial cell; HC, hematopoietic cell.
Exploring the role of Eomes in TIM cell formation, Istaces and others (2019) performed an exhaustive analysis at the transcriptomic level, which highlighted a distinct epigenetic program during conventional and unconventional memory CD8+ T cell formation. The investigators demonstrated that even though TIM cells acquire classical features of memory cells, they are only partially programmed toward memory fate at the epigenetic level. They also provide evidence that Eomes contributes to this epigenetic programming in TIM cells (Istaces and others 2019).
To further analyze these data, the investigator developed a transgenic mouse model that overexpressed Eomes in developing thymocytes. Their results demonstrated that SP8 cells in this mouse model presented most of the phenotypic, functional, and transcriptional features of TIM cells. They also identified the transcription factor RUNX3 and the epigenetic regulator BRG1 as partners that interact with EOMES to promote innate memory cell phenotype acquisition (Istaces and others 2019).
Following these comprehensive reports in genetically modified animals, the most curious question was whether innate CD8 T cells existed in WT animals, and if so, if similar mechanisms operated in normal mice. In this regard, Weinreich and others (2010) investigated the phenotype of SP8 thymocytes in BALB/c and C57BL/6 (B6) mice. They found that BALB/c mice present a larger percentage of PLZF+ thymocytes than B6 mice and this finding was in accordance with increased numbers of the memory-phenotype SP8 cells in BALB/c over B6 mice (Weinreich and others 2010). Further studies from this laboratory demonstrated that the large percentage of CD44hi, CD122hi SP8 cells (TIM) in BALB/c mice was IL-4 dependent since IL-4 KO BALB/c mice had lower numbers of thymic TIM cells than WT BALB/c mice (Weinreich and others 2010).
The same laboratory further investigated this point. They speculated that because iNKT lineage diversification is different between mouse strains, the frequency of TIM cells should also be dissimilar. They compared 6 commonly used different inbred strains of mice and showed variability in the frequency and number of total thymic iNKT cells. To this point, B6 presented more NKT1 cells and fewer NKT2, whereas BALB/c presented larger number of NKT2 cells (Lee and others 2013b). From these results, the authors concluded that higher numbers of TIM cells correlated with a higher NKT2/NKT1 index in the different strains of mice (Lee and others 2013b).
Innate CD8 T cell development can be reproduced in vitro. Rafei and others (2013) have developed a novel in vitro model that is suitable for analysis of positive selection and T cell differentiation based on co-culture of CD69neg (preselected) DP thymocytes from OT-I–transgenic mice with peptide-pulsed OP9 stromal cells. By using synthetic peptides that are described to induce positive selection in OT-I cells (Santori and others 2002), they report that culture of DP OT-I cells in the presence of recombinant IL-4 (rIL-4) induced high levels of CD69, PD-L1, Eomes, and CD44, some of the consensus markers of innate polyclonal TCRαβ CD8+ T cells (Rafei and others 2013).
In alignment with this observation, our laboratory has reported that co-culture of either DP CD69neg or CD69pos OT-I cells with a bulk population of thymocytes obtained from Trypanosoma cruzi-infected mice was sufficient to generate SP8 cells with innate characteristics in 48 h. We demonstrated that this effect is IL-4 and IL-15 dependent. Moreover, in the context of T. cruzi infection, SP4 CD44hi cells and thymic myeloid cells are responsible for the local production of IL-4 and IL-15 at the thymus, respectively (Baez and others 2019). Data confirming that TIM cells are dependent not only on IL-4 but also IL-15 for their development were shown by Atherly and others. Their investigative analysis demonstrated that the high accumulation of SP8 CD44hi cells observed in the thymus of ITK KO mice was significantly reduced in the absence of IL-15 in ITK−/− IL-15−/− mice (Atherly and others 2006).
It is not well known how TIM cells are exported from the thymus; however, in the review by White and others (2017) a possible explanation is offered. They speculate that IL-15 can upregulate the expression of the KLF2 transcription factor in TIM cells, which in turn induces the expression of sphingosine 1-phosphate receptor 1 (S1P1), a crucial molecule that allows mature thymocytes to egress the thymus and migrate to SLO. To expand this topic, our preliminary data tracking thymocytes after being intrathymically (i.t.) stained with the dye eFluor670 (eF) indicate that during T. cruzi infection, a reduced number of mature SP4 and SP8 thymocytes are exported to SLO compared to control-uninfected mice.
When we analyzed CD8+ T eF+ cells in LNs and spleen 5 days after i.t. injection, we found that about 15–25% of the cells adopt a memory-like phenotype (CD44hi), as previously reported for WT mice (Haluszczak and others 2009). Interestingly, only 5 days after exportation, a higher proportion of CD8+ T eF+ cells became TMEM or TEFF (CD44hiCD49dhi) than TVM (CD44hiCD49dlo) in T. cruzi-infected mice compared to control mice (unpublished data). We have not yet determined if these CD44hiCD49dloCD8+eF+ cells are TIM cells or TVM cells that convert upon arriving to SLO since both populations are phenotypically indistinguishable once they arrive to the periphery.
Other than these few reports, there is a substantial lack of information on the mechanism that TIM cells utilize to leave the thymus and reach SLO. This important issue certainly deserves a more in-depth analysis, especially in the context of pathological triggers.
The information that accumulated over the years in relation to the origin and development of TIM cells led us to think that the fate of a developing T cell is not quite predictable and even random. The process is highly dependent on the type of presenting cell they contact during positive selection and the surrounding cytokine microenvironment. Still, what type of signals SP8 receive from TECs or HCs, which makes them divert to different lineages, requires deeper investigation.
TVM Cells, Phenotype, Origin, and Differentiation
The TVM population is composed of cells from different origins. This was demonstrated by Kurzweil and others (2014) in a study where they used mice deficient in Nedd4 family-interacting protein 1 (Ndfip1 KO mice) and showed that even though overproduction of IL-4 in the periphery leads to an expanded TVM population, not all memory-like cells are IL-4 dependent as ablation of IL-4 only partially affects the number of TVM cells. This topic was also evaluated by Akue and others (2012), By using 2 well-characterized peptide/MHC complexes (B8R/Kb and HSVgB/Kb), the authors evaluated the frequency of TVM cells with these specificities in unmanipulated mice and demonstrated that their numbers are reduced (but not eliminated) in IL-4 KO mice.
These results support the concept that part of the TVM cell population in SLO could be TIM, which are actually IL-4 dependent and migrate from the thymus to SLO, and once there, they become undistinguished from other TVM cell types (Akue and others 2012). However, this is not completely true since the composition of IL-4-dependent memory-like cells in SLO is not exclusively of TIM cells (Park and others 2016). In a work reported by Park and others (2016), an IL-4/anti-IL-4 antibody complex (IL-4C) was administered for over 1 week, resulting in an induction of innate CD8 T cell-like phenotype in peripheral CD8+ T cells.
The investigators then asked if IL-4-dependent TVM cells could arise in SLO; then, they adoptively transferred CD44loCXCR3− (naive) or CD44hiCXCR3+ (TVM) CD8+ T cells into B6 mice, followed by IL-4C treatment for 1 week. After this period of time, Park and others (2016) showed that IL-4C treatment induced proliferation of both types of transferred cells and simultaneously caused differentiation of naive CD8 T cells into CD44hiCXCR3+ cells during their proliferation. These results indicate that IL-4 is able to induce both memory-like CD8+ T cells from naive CD8+ T cells and expand pre-existing TVM cells in the periphery (Park and others 2016).
Along the same line of investigation, Tripathi and others (2016) asked if the composition and origin of TVM cells between mice from different genetic backgrounds are similar. This point of inquiry was based on the report that BALB/c mice carry a larger number of TIM cells in the thymus and more IL-4-dependent TVM in periphery than C57BL/6 mice (Lee and others 2011). This is mainly due to the fact that BALB/c mice have a larger number of IL-4 producer NKT2-type cells in the thymus than C57BL/6 mice (Lee and others 2013b). In their report, by using IL-4 KO or IL-15 KO mice from both mouse strains, Tripathi and others (2016) demonstrated that the TVM compartment in BALB/c mice relies more on IL-4 than IL-15, whereas the TVM compartment in C57BL/6 mice is more highly dependent on IL-15 and minimally on IL-4.
Moreover, IL-15 is known to be responsible for the long-term survival of memory CD8 T cells. In addition, the prominent CD122 expression of TVM cells provides them with a competitive advantage to respond to these cytokines. In accordance with these data, TVM cells are highly enriched in the liver, representing up to 60%–70% of total memory T cells present in the organ (Nakagawa and others 2004). This is physiologically important as the liver is a rich source of IL-15 and provides TVM cells access to this cytokine (Correia and others 2009).
Although IL-15 is very important in the development and maintenance of TVM cells, reduction in TVM cells in the absence of IL-4 and/or IL-15 is not complete. As such, it is proposed that IL-4 and IL-15 are not the only regulators of TVM cell homeostasis. Indeed, Martinet and others (2015) demonstrated that the phenotype, function, and age-dependent expansion of TVM cells are greatly disturbed in the absence of type I IFN signaling. In this context, the authors showed that type I IFNs are able to directly activate Eomes gene expression, a key transcription factor expressed by TVM cells as mentioned earlier. This study points out that type I IFNs represent an important cytokine during TVM and TIM maturation (Martinet and others 2015).
One question that has arisen over the years is whether TN and TVM cells share the same TCR profile or do they develop from different T cell clones. In this matter, Haluszczak and others (2009) investigated the TCR repertoire of TVM cells residing in SLO. By using different class I MHC tetramers loaded with various peptide antigens (OVA-Kb/SIINFEKL, vaccinia virus B8R-Kb/TSYKFESV, and HSV1gB-Kb/SSIEFARL), they showed that CD8+ T cells present in SLO of unimmunized animals display a mix of naive and memory phenotype for each clone, but at different ratios. They determined that the percentage of cells with a memory phenotype (CD44hi cells) varied from 10% of OVA-specific CD8+ T cells to 30%–40% in HSV1-specific CD8+ T cells (Haluszczak and others 2009).
These results agree with what was stated for TIM cells in the previous section, where it is demonstrated that an SP8 cell with the same TCR can give rise to a naive or an innate T cell according to the maturation context in which it is surrounded (positive selection by TEC vs. HC, levels of IL-4, TCR signaling strength, etc.). Haluszczak and others (2009) also compared the frequency of CD44hi versus CD44lo cells in gnotobiotic (germ free) and specific pathogen-free (SPF) animals. Notably, they showed that the MHC-I tetramer-bound populations from GF animals contained CD44hi cells with a similar frequency to those found in SPF mice (Haluszczak and others 2009). These data demonstrate that microbial antigens do not contribute to the appearance of memory-phenotype CD8+ T cells in SLO.
Similar peptide/MHC class I tetramers experiments were later performed by the same laboratory to evaluate TVM cell frequencies to 5 different epitopes recognized in the B6 response to murine cytomegalovirus (MCMV). By using 3 highly immunodominant epitopes and 2 that are hardly detectable in the acute MCMV response, the authors concluded that there was a lack of correlation between the frequency of TVM cells and the immunodominance characteristics of the tetramer specificities (Akue and others 2012).
To further understand the origin of these non-IL-4-dependent TVM cells, the authors performed tetramer enrichment assays on thymic and peripheral lymphoid tissues from mice 1 to 4 weeks after birth and demonstrated that tetramer+ TVM cells appeared in the periphery in advance of the thymus, thereby supporting the idea that memory-like cells are generated in SLO after being exported from the thymus (Akue and others 2012). They also found that the frequency of pre-existing TVM cells is stable in both steady-state conditions and after a greatly expanded Ag-driven memory CD8+ T cell immune response. In addition, TVM cells can be maintained long term by undergoing basal proliferation (Akue and others 2012).
Even though it is well accepted that TVM cells develop in SLO, the concept that the thymus is not involved has been changing in recent years. White and others (2016) provide evidence that T cells emerging from the thymus with high affinity for self-antigens (these cells express high levels of CD5) are more likely to become TVM cells when reaching SLO than their CD5lo counterpart. Their results revealed that the bulk population of TVM cells shows a statistically significant increase in the expression of CD5 than in the bulk population of naive cells in unprimed B6 mice, suggesting that the affinity of a T cell to its selecting ligands during thymic positive selection could dictate the fate of a SP8 thymocyte toward the TVM lineage (White and others 2016).
To demonstrate preferential conversion to the TVM phenotype, White and others (2016) adoptively transferred CD44loCD5hiCD8+ T cells into lymphoreplete WT mice, and after 3 weeks, they show that the donor cells acquired a TVM phenotype (CD44hi CD49dlo), and this conversion seems to occur irrespective of the TCR since transferred CD44loCD5hi TCR transgenic gBT cells were significantly more likely to became TVM cells than transferred CD44loCD5lo gBT cells. These data reinforce the concept that the origin of TVM initiates at the thymus since a cell with one particulate TCR could undergo thymic egression with a CD5hi or CD5lo phenotype depending on the signal strength received during positive selection. This could ultimately define its fate in SLO.
Aligned with these data, Drobek and others (2018) explored this concept at the TCR signaling level. By using T cells from CD8.4 knock-in mouse (T cells that carry a CD8 molecule formed by the extracellular portion of CD8α fused to the intracellular part of CD4) (Erman and others 2006), the authors generated T cells that strongly bind Lck, an initiating TCR signal transduction kinase. They demonstrated that when CD8.4 is associated with a low self-reactivity (CD5lo) TCR, like F5 (specific for the influenza Ag NP68), T cells did not preferentially develop into the TVM lineage.
Instead, when they associate CD8.4 co-receptor to CD8+ T cells from OT-I cells, whose TCR has a strong self-reactive avidity (CD5hi), most CD8.4 cells became TVM (up to 80%), indicating that only the most highly self-reactive T cells have the potential to develop into TVM cells (Drobek and others 2018). Based on their data, Drobek and others (2018) speculated that naive and TVM cell compartments could contain T cell clones with different TCR repertoires. To probe their hypothesis, they utilized a Vβ5 transgenic mouse model with fixed TCRβ from the OT-I TCR (Fink and others 1992) that generates a polyclonal population of T cells with one type of TCRβ chain, but variable TCRα chains (Drobek and others 2018).
When they analyzed the frequency of TVM versus TN cells, they observed that, while the frequency of TCRVα3.2+ T cells is slightly enriched for the TVM cell subset, the TCRVα8.3+ T cells have lower frequency of TVM cells than the overall population. Accordingly, OVA-specific TCRVα2+ had higher levels of CD5 than TCRVα8.3+ cells (Drobek and others 2018). More recent work by Miller and others (2020) has extended those previous findings using a clonal approach. The authors sequenced the complete TCR repertoires of TN cells and TVM cells from SLO of B6 mice expressing a fixed transgenic TCRβ chain and variable TCRα chains. Their data revealed that the TCR repertoire of TVM cells is largely dissimilar to that of TN cells, and interestingly, was highly recurrent between individual mice (Miller and others 2020).
By using retrogenic mice expressing either TCR obtained from several recurrent TVM or TN CD8+ T cell clones, they report that the memory phenotype in the periphery was only adopted by CD8+ T cells that expressed TVM, but not TN TCRs. Interestingly, not every CD8+ T cell that carries a high-frequency TVM TCR clone adopts a memory-like phenotype, suggesting that TVM cell development is also restricted to limited niches in SLO (Miller and others 2020). Collectively, the information from these works show that the generation of a TVM cell has both fixed and stochastic aspects that depend upon first, the positive selection received in the thymus, and second, the possibility of accessing specific niches in SLO. The origin, phenotype, location, and cytokine requirement of different subsets of TVM cells discussed in this section are schematically summarized in Fig. 2.
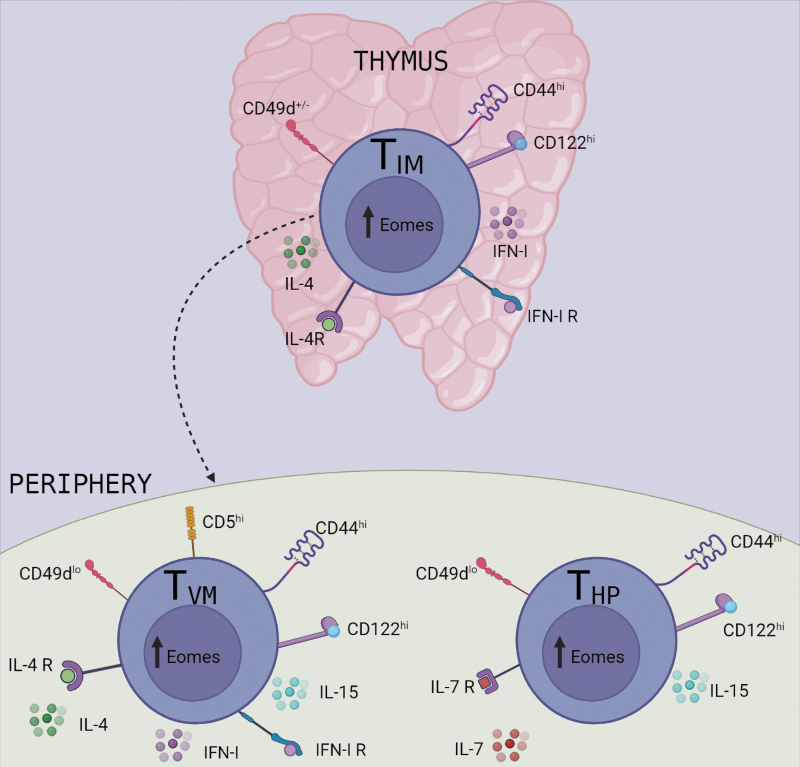
FIG. 2.
Heterogeneous origin and types of cell subsets that compose the TVM population. TIM cell development from SP8 thymocytes when they are exposed to IL-4 and MHC-Ib in the thymus. TVM cells are generated in the periphery from conventional naive cells that emerge from the thymus with a high self-recognition avidity (CD5hi) and their generation is requisitely dependent on IL-15 signaling. While TVM cells and TIM cells were originally distinguished from one another by thymic differential expression of CD44 and CD49d and their dependence on IL-15 versus IL-4, once they emerge into the periphery, the 2 populations are phenotypically indistinguishable. HP memory CD8+ T cells (THP) are produced by homeostatic mechanisms rather than conventional priming and express markers typical of TVM cells (high expression of CD44 and CD122 and low expression of CD49d). THP can be considered part of the TVM pool that resides in SLO and is dependent on IL-7 for survival.
By a different approach, Smith and others (2018) provided new evidence on the origin and fate of TVM cells. They use a CD4 promoter-driven tamoxifen-inducible cre (CD4cre-ERT2) that is able to drive expression of the red fluorescent protein TdTomato (RFP) in CD8+ T cells undergoing thymic selection at the DP stage of T cell development. By this strategy, they can permanently “timestamp” different waves of CD8+ T cells made in the thymus every time mice are exposed to tamoxifen (Tx). Interestingly, the authors could evaluate peripheral waves of CD8+ T cells at fetal, neonatal, and adulthood stages (Smith and others 2018). They showed that in 8-week-old mice, those that received Tx at 1 day of life had 5 times more TVM cells that preferentially localized in the liver than those who received Tx at 28 days of life.
An important question they asked was whether CD8+ T cells from 1-day-old Tx-exposed mice could more easily become TVM cells due to the well-known neonatal lymphopenia that occurred when reaching SLO. To answer this question, the authors transplanted a newborn timestamp thymus into an adult lymphoreplete timestamp mouse and simultaneously evaluated waves of newborn (RFP) and adult (YFP) thymocytes at the same time and in the same peripheral environment. They reported that 4 weeks after Tx exposure, RFP+ cells that originated from the newborn transplanted thymus and matured in adult hosts had a similar proportion of TVM cells to that seen in the intact neonatal mice (Smith and others 2018).
This result correlates with their RNA-seq data that demonstrate CD8+ T cells from 1-day-old Tx mice express a significantly higher proportion of genes typically found in effector and memory cells, while in 28-day-old Tx mice, CD8+ T cells express more genes characteristic of naive and late memory cells (Smith and others 2018).
Another very interesting question that Smith and others (2018) posed is how these cells behave during an infectious state. They reported that cells produced early in life (mostly TVM cells) proliferate and differentiate more quickly than those produced later in life (most TN cells) at 5 days post-LM infection. Moreover, cells from 1-day-old Tx mice present a greater proportion of terminally differentiated short-lived effector cells and make more IFNγ in the early phases of the infection than cells from 28-day-old Tx mice (Smith and others 2018).
One concern that arises from TVM cells is whether they could trigger autoimmunity since they recognized self-Ags with high avidity. In response to this point, Drobek and others (2018) demonstrate that TVM cells are tolerant to self-Ags they encountered in the thymus during positive selection. They show that OT-I cells co-cultured with dendritic cells loaded with the endogenous Ags Catnb and Mapk8 [proposed as positive selecting Ags for OT-I T cells (Santori and others 2002)] do not show significant response in in vitro proliferative assays or in vivo during infection with LM-Catnb (Drobek and others 2018).
To confirm these data in a murine model of autoimmunity, Drobek and others (2018) sorted either TN or TVM cells from OVA-specific clones V14-C1 and V14-C2, so both types of cells express the same TCRs. They then adoptively transferred these cells into RIP.OVA mice (mice that exhibit detectable ovalbumin in pancreatic islets) followed by infection with LM-OVA. Outcomes, in the case of both clones, demonstrate that naive T cells were more efficient in inducing autoimmune diabetes than TVM cells. To explain this effect, the authors speculate that TVM cells could develop suppressive mechanisms that contribute to self-tolerance, such is the case of lower expression of both CD49d integrin and CD25 upon activation (Drobek and others 2018).
A recent publication by Hou and others (2021) demonstrates a very revealing phenomenon in the field of immunology, which is that TVM cells could give rise to tissue-resident memory cells (TRM) during the course of influenza infection. The author presents evidence that TVM cells that recognize viral antigen can rapidly migrate to the lungs during the first 24 h postinfection period to provide early infection control, but are retained in the organ and give rise to TRM independent of SLO (Hou and others 2021).
The knowledge that is emerging in the field of TIM and TVM cells in recent years is providing enlightening evidence about the role of these Ag-independent (or not) T cells in the immune system. These latest findings lead one to think about the flexibility of the immune system in adapting different lineages of T cells according to the system's need, especially in infectious and pathological contexts.
Effector Mechanisms of TIM/TVM Cells
One of the greatest enigmas about TVM cells is which cytotoxic mechanisms operate in these cells. This topic has been approached with some laboratories, through ingenious methodologies and strategies, shedding light on this mystery. An interesting question is how TVM cells distinguish their targets and whether the recognition and signaling through the TCR are involved. We will mention in more detail in the next section how early IFNγ production by TIM/TVM cells participates in the control of pathogen dissemination and burden load; however, other classical CD8+ T cell mechanisms, such as perforin/granzyme release, are less investigated. Moreover, typical NK “killing” receptors, such as NKG2D, have been proposed to participate in TVM cell effector mechanisms. In the following section, we comment on reports that address the role of these TVM effector molecules and their actions.
Chu and others (2013), by using Nur77-GFP reporter mice (whose GFP level is proportional to the TCR stimulus strength and independent of inflammatory signals), demonstrated that after 48 h post-LM-OVA infection, TVM cells (termed bystander-activated CD8+ T cells by this group) slightly upregulated GFP expression compared to naive CD8+ T cells from the same mice. However, GFP expression was much lower than in mice treated with αCD3 as a positive control and potent inducer of TCR signaling (Chu and others 2013). Their data support the idea that a weak TCR signal could activate TVM cells. However, as demonstrated by other laboratories (see Role of TVM cells in infectious processes section), TVM cells could respond to Ag stimulation during primary and secondary immune responses giving rise to TEFF and TCM cells.
Another important question addressed by Chu and others (2013) is whether NKG2D is a bona fide effector receptor in TVM cells. NKG2D is an activating receptor commonly expressed in NK cells and in activated memory CD8+ T cells after TCR stimulation (Slifka and others 2000). RNA-seq data have shown that TVM cells express IL-12R, IL-18R, IFNγ, granzyme B (GrzB), and NKG2D, molecules known to enhance innate-like effector functions (Hussain and others 2019; White and others 2016). Exposure to IL-12 and IL-18 enables TIM/TVM cells to produce IFNγ early in Th1 inflammatory/infectious processes (Berg and others 2002, 2003; Haluszczak and others 2009), while GrzB and NKG2D expression can mediate Ag-independent cytotoxicity (Chu and others 2013).
Based on data demonstrating that TVM cells are primed to rapidly respond to IL-12, IL-15, and IL-18 through constitutive receptor expression, Chu and others (2013) asked if exposure to these stimuli was able to induce both GrzB and NKG2D upregulation. Results demonstrated that IL-12, IL-15, and IL-18 can upregulate GrzB expression as early as 6 h after in vitro stimulation, but the cytokines are not primary regulators of NKG2D expression (Chu and others 2013). The situation might be different in an in vivo context as White and others (2016) reported that when gBT TVM cells are adoptively transferred into IL-15 KO hosts and then challenged with LM-OVA, the transferred cells showed substantially reduced levels of GrzB, NKG2D, and IFNγ compared to the same cells transferred to WT mice.
In TMEM cells, NKG2D is associated with a senescent phenotype (Prajapati and others 2018). However, despite being a marker of senescence and TCR-mediated dysfunction, NKG2D activity in TVM-like cells (CD8+ CD25−) facilitates enhanced innate responsiveness (Tietze and others 2012). In this context, Chu and others (2013) presented evidence that TVM-like cells can attack target cells in an NKG2D-dependent manner. In addition, a role for NKG2D in both infectious and tumor mouse models has been reported as follows: after primary influenza infection, a rapid arrival of non-Ag-specific OT-I cells is found in the lung, which are able to upregulate NKG2D, but not CD25, expression.
Accordingly, the in vivo blockage of NKG2D induces a lack of control in early viral replication in these mice (Sckisel and others 2014). In a tumor model, Tiezte and others (2012) co-treated Renca-bearing mice with anti-CD40/IL-2 and an NKG2D blocking antibody and demonstrated that blockade of NKG2D in mice receiving immunotherapy led to a significant decrease in the control of tumor growth. NKG2D could also be induced with other innate stimuli. To demonstrate this, Tiezte and others (2012) sorted NKG2D−CD25−CD8+CD44high T cells from congenic Ly5.1 mice and adoptively transferred them into WT C57BL/6 mice.
Two days after transfer, mice were treated with anti-CD40/IL-2, and NKG2D expression was analyzed 11 days later. The investigators found that CD8+ T cells from immunotherapy-treated mice were able to expand and upregulate NKG2D expression (Tietze and others 2012). It is worth clarifying that, between the different subsets constituting the pool of TVM cells in SLO as mentioned above, some laboratories have reported that IL-4-dependent TVM cells exhibit reduced or absent NKG2D expression, although they do produce IFNγ after IL-12 + IL-18 stimulation (Ventre and others 2012; Jameson and others 2015).
Lee and others (2013a) have focused their attention on the functional characteristics of TVM cells with reported differences from both naive and Ag-specific cells. For example, Lee and others (2013a) showed that in vitro, TVM cells manifest certain TMEM cell functions such as increased T-box transcription factor expression and advanced G1 cell cycle status and present naive-like properties, such as low IFNγ production after Ag stimulation. In all cases, Eomes expression seemed to be essential for the development of functional memory-like characteristics of the innate memory population. Eomes expression has been shown to bind to the il2rb promoter leading to increases in CD122 expression and then driving memory CD8+ T cell sensitivity to IL-15 (Intlekofer and others 2005). Also, Eomes increases the ability of memory CD8+ T cells to rapidly produce IFNγ (Intlekofer and others 2005).
NKG2D is not an exclusive functional mediator of TVM cells. For instance Lanzer and others (2018) have demonstrated that TVM cells obtained from the lung of aged mice infected with influenza virus developed a strong GrzB response and mediated viral clearance similar to that observed in young mice. In another study, Wang and others (2021) presented evidence that co-culturing TVM cells with A20 lymphoma cells, pre-treated with the chemotherapeutic drug cytarabine (Ara-C) or doxorubicin (DOX), resulted in the ability of TVM cells to substantially induce GrzB expression.
Moreover, adding a GrzB inhibitor (Z-AAD-CMK) to the co-cultures significantly reduced TVM-medicated tumor cell death when compared to vehicle control-treated cells (Wang and others 2021). Collectively, data presented in this section demonstrate that TVM cells are capable of exhibiting several mechanisms of cytotoxicity in different infectious or tumor settings. The fact that some types of CD8+ T cells present as TVM cells that develop into rapid effectors of the innate immune response, whereas other conventional CD8+ T cells, with the same TCRs, can develop later during the adaptive immune response, is a fascinating and creative tool of the immune system.
Role of TIM/TVM Cells in Infectious Processes
TIM and TVM cells as an early source of IFNγ
During the early phase of certain infectious processes, innate cells are the main contributors of IFNγ, a critical cytokine for the control of multiple pathogens (Lukin and others 2000; Lertmemongkolchai and others 2001; Berg and others 2002, 2003). To date, TIM/TVM-like lymphocytes have been found to play a very important first-line defense role in viral (Sckisel and others 2014; Lee and others 2015), bacterial (Lertmemongkolchai and others 2001; Berg and others 2003; Chu and others 2013), and parasitic (Baez and others 2019) infections. For many years, it was believed that NK and NKT cells were the primarily sources of IFNγ in response to pathogen-derived inflammatory triggers occurring in the absence of antigenic immune responses.
However, more recently, Kambayashi and others (2003) demonstrated that other cell types were involved in early IFNγ production through identification of a population of spleen and lymph node IFNγ-secreting CD8+ T cells in LPS-injected mice (Kambayashi and others 2003). Moreover, Kambayashi and others (2003) reported that production of IFNγ by this CD8+ T cell population was MHC class I independent and restricted to CD44hi (memory phenotype) cells. IFNγ production by memory-like CD8+ T cells was indirectly induced through macrophage/dendritic cell-derived IFNα/β, IL-15, IL-12, and IL-18 in an Ag-independent way (Yajima and others 2001; Kambayashi and others 2003; Haluszczak and others 2009; Bou Ghanem and others 2011; Martinet and others 2015).
Pathogen-associated molecular patterns and danger signals can promote the production of inflammatory cytokines by different innate immune cells, such as dendritic cells and macrophages that are capable of producing large amounts of IL-12 and IL-18 in the early phase of an infection. The receptors for both IL-12 and IL-18 are constitutively expressed on TIM/TVM cells (White and others 2016, 2017), thus providing a mechanism whereby these innate cells can rapidly respond by producing large amounts of IFNγ early in infectious or Th1 inflammatory processes. Interestingly, a recent work demonstrates a higher frequency of IFNγ-producing TVM cells in B6 than BALB/c mice after in vitro stimulation with IL-12/IL-18 due to a larger expression of IL-18R in TVM cells from B6 mice (Moudra and others 2021).
The role of innate TIM/TVM-like cells during certain bacterial infections, such as Burkholderia pseudomallei (BP) and LM, where resistance is strictly dependent upon IFNγ production, has been appreciated for some time (Lertmemongkolchai and others 2001; Berg and others 2002). Approximately 20 years ago, 2 different laboratories demonstrated that in vitro exposure of splenocytes to BP or LM induced IFNγ production from pre-existing CD8+ TCRαβ+ CD44hi T cells and this effect was triggered by bacterially induced IL-12 and IL-18 (Lertmemongkolchai and others 2001).
In the case of viral infections, Lee and others (2015) have shown that the large number of IL-4-induced innate CD8+ T cells (currently called TIM cells) present in CIITAtg mice produce high levels of both IFNγ and TNFα. These cells fully control the viremia upon infection with clone 13 of lymphocytic choriomeningitis virus (LCMV) and are dependent upon IL-4 since CIITAtgIL-4KO mice are not capable of clearing the virus (Lee and others 2015).
Our laboratory reported similar results using a parasitic infection model. We demonstrated that during the acute phase of T. cruzi infection, thymic cells enriched with TIM cells have substantial capacity to produce IFNγ after IL-12 + IL-18 stimulation and induce protection when adoptively transferred to T. cruzi-infected mice (Baez and others 2019).
IL-12+IL-18-induced IFNγ production in memory-like CD8+ T cells seems to be regulated differently than in NK cells. Martinet and others (2015) demonstrated that after in vitro rIL-12 + rIL-18 stimulation, or following LM infection in vivo, memory-like CD8+ T cells, and not NK cells, from IRF9 knockout (KO) mice produced significantly lower amounts of IFNγ than their WT counterparts.
Berg and others (2003) further explored this topic and found that besides their rapid capacity to produce IFNγ, innate CD8+ T cells can also protect mice from L. monocytogenes by mechanisms that are TCR and IFNγ independent. Moreover, Hu and others (2007) demonstrated that adoptively transferred CD8+ CD44hi cells from ITK KO mice exhibited enhanced response to L. monocytogene infection by reducing bacterial burden in IFNγ KO mice. Overall, these data support the concept that memory-like CD8+ T cells act in a rapid and efficient manner early during infection phase to provide an additional source of IFNγ, which alongside innate cytotoxic mechanisms collaborate to resolve or resist infections until an adaptive immune response can initiate. Even more interesting is the notion that contrary to other innate cell types, TIM/TVM cells may also produce IFNγ through TCR signaling mechanisms. This is examined in the next section.
The TCR is involved in TIM/TVM cell immune response
As mentioned above, TIM/TVM cells are more prone to respond in a bystander manner to cytokine stimuli during the course of an infection rather than by TCR activation and signaling. However, these cells carry a wide TCR repertoire that is completely functional. Data that support this point were provided by White and others (2016), who demonstrated that OTI TVM cells (CD44hi CD49dlo OTI cells) are able to protect against LM-OVA by inducing a substantial reduction in splenic bacterial CFUs. Interestingly, when they evaluated protection by a different TCR transgenic mouse model that does not recognize bacteria in an Ag-specific way (gBT cells), White and others (2016) observed a surprisingly high protection after LM-OVA infection, similar to the one mediated by OTI cells.
Thus, the investigators concluded that TVM cells are capable of mediating potent immunological protection against bacterial challenge in the presence or absence of their cognate antigen (White and others 2016). Interestingly, this effect is highly dependent on IL-15 in an Ag-independent context; since using IL-15−/− mice as recipients, only the antigen-specific TVM OTI-transferred cells demonstrate a protective effect in this system, while nonspecific gBT TVM cells show a substantial reduction in granzyme B, NKG2D, and IFNγ expression, which compromises their functional capacity (White and others 2016). These latest data suggest that memory-like cells could rapidly respond early in infections, in an Ag-specific or nonspecific manner, to support the innate immune response until Ag-experienced adaptive immunity develops.
If recognition by the TCR in TVM cells is possible, then it would be reasonable to ask whether TVM cells can become effector cells during an infectious process and generate memory-phenotype progeny. In this matter, several laboratories have demonstrated that both TIM and TVM cell subsets are poised to produce IFNγ when they encounter cognate antigen. As a result, both cell types trigger an antigen-specific protective immune response against infection that is far better than CD8+ TN cells (Lee and others 2013a; White and others 2016).
Moreover, when comparing in vitro IFNγ production by OVA-specific TN, TVM, and TMEM cells after a 5-h Ag stimulation, TVM cells produced higher levels of IFNγ compared to TN cells, but significantly lower than TMEM cells (Lee and others 2013a). Lee and others (2013a) also tested the same populations after LM-OVA in vivo infection and observed that in the early infection phase, TVM cells expand more rapidly than TN cells, but this difference was lost at later times. Interestingly, when evaluating a recall response to LM-OVA, no advantage in the number of memory OVA-specific TVM cells was observed compared to the memory TN cell counterpart (Lee and others 2013a).
When studying cytokine production, Quinn and others (2018) demonstrated that following TCR stimulation, TVM cells could give rise to TEFF cells; however, TVM-derived TEFF cells produced predominantly more IFNγ alone compared to TN-derived TEFF that were more multifunctional through production of a broad spectrum of cytokines. Moreover, TEFF cells that arise from TVM cells adopt a short-lived effector cell phenotype, while TN-derived TEFF cells are more likely to develop into stable TMEM populations (Lee and others 2013a; Smith and others 2018). When evaluating Ag-specific secondary immune responses by TMEM and TVM cells, Lee and others (2013a) showed that both subsets expand equally; however, TVM cells produced significantly larger numbers of TCM cells than TMEM cells.
Collectively, these data demonstrate that TVM cells are not only capable of responding in a TCR-specific manner to generate effector cells with rapid IFNγ production capacity early in infection, but are also able to respond to secondary challenge by differentiating mainly into TCM cells.
Role of TIM/TVM in Cancer
Numerous studies on cancer immunotherapy show that the antitumor effects depend on the generation of antigen-specific T cells. However, there is evidence that antitumor properties can also be mediated by alternatively activated T cells that are not tumor specific. Tietze and others (2012) treated mice with anti-CD40 Ab + IL-2 and observed significant antitumor effects in 3 different murine tumor models. Their data correlated with a massive expansion of splenic CD8+CD44hiCD122hiCD25− at 11 days after this regimen administration, where, as referred by the authors, no expression of CD25 on T cells mainly indicates activation independent of TCR engagement as demonstrated by the same authors in in vitro assays (Tietze and others 2012).
They reported that the CD8+ memory-like T cells that responded to the treatment also exert high cytolytic activity toward tumor targets partially recognized through NKG2D (Tietze and others 2012). The authors also evaluated the phenotype of CD8+ T cells present in melanoma biopsies from patients receiving local treatment with the TLR7 agonist imiquimod, a nonantigenic immunotherapy. Interestingly, immunohistologic analyses of the biopsies demonstrated a marked infiltration of CD8+CD25− T cells within the tumors compared with tumors treated with the vehicle alone (Tietze and others 2012).
Similarly, Hu and others (2014) also reported, years ago, a substantial benefit in the antitumor activity of NKG2D+CD8+ T cells in 4T1 tumor-bearing mice after a nonantigenic treatment with doxorubicin (Dox) + IL-12. Their analysis shows a large number of NKG2D+CD8+ T cells colonizing the tumors of mice that received the treatment compared to control mice (Tietze and others 2012; Hu and others 2014). In addition, they demonstrate that blocking NKG2D completely reversed Dox plus IL-12-mediated inhibition of tumor growth (Tietze and others 2012; Hu and others 2014).
By their site, Xu and others (2013) show that a single dose of ALT-803, a complex of an interleukin (IL)-15 superagonist mutant and a dimeric IL-15 receptor, is able to eliminate 5T33P and MOPC-315P myeloma cells present in the bone marrow of tumor-bearing mice. Also, ALT-803 treatment promoted in vivo expansion of CD8+CD44high that upregulates NKG2D, but not CD25 expression, and secretes large amounts of IFNγ (Xu and others 2013). Furthermore, ALT-803-activated CD8+ memory-like T cells exhibited in vitro nonspecific cytotoxic activity against myeloma and other tumor cell lines (Xu and others 2013).
Even though the number of these “memory-like” cells described in these reports increased in response to alternative activation with inflammatory cytokines in the absence of immunization with specific Ags, whether these cells belong to the virtual CD8+ T cell lineage is not determined in these studies, as they were not characterized with the markers that now define this population.
The role of TIM/TVM cells is particularly important in cancer immune response since once tumors miss their MHC class I expression, they cannot be recognized by CD8+ T cells in a TCR-specific manner. This point is quite vital because the loss of MHC-I occurs frequently in many different types of human cancers (Garrido and others 2010; Challa-Malladi and others 2011). Wang and others (2021), by using several tumor models, demonstrated that chemotherapeutic treatment significantly increases TVM TILs in tumors (CD44+CD122+NKG2D+Eomes+CD49d−).
Interestingly, in their work, TVM cells were activated in the presence of tumors cells treated in vitro with the chemotherapeutic drug Ara-C or Dox and produced large amounts of granzyme B, which can mediate the apoptosis of target cells (Wang and others 2021). The authors also validated their results in a humanized murine model and demonstrated that chemotherapy-treated human tumors also activated human TVM cells, independent of tumor-derived MHC-I (Wang and others 2021).
In our group, we have demonstrated that IL-12 and IL-18 systemic expression in tumor-bearing OT-I mice are able to induce high infiltration of CD8+ T cells with a TVM phenotype into non-OVA B16 or pancreatic ductal adenocarcinoma tumor cells (KPC). Moreover, cells obtained from LNs of IL-12+IL-18-treated OT-I mice showed activation features after in vitro exposure and contact with KPC cells (unpublished data).
In the work previously mentioned by Miller and others (2020), the investigators examined the presence of TVM recurrent clones in tumors and draining LNs from TRAMP-bearing mice. The author co-transfected polyclonal TVM and TN cells into the prostatic adenocarcinoma-bearing mice, and 4 months later analyzed the transferred cells. They found that TVM cells represent a substantial fraction of the tumor-infiltrating CD8+ T cells. They isolated CD8+ T cells from the prostate tumors and using a TCR sequencing approach, they identified numerous TVM cell clones that were enriched in TRAMP prostate tumors (Miller and others 2020).
Comparing the frequency of those intratumor TVM clones with the ones present in SLO of tumor-free mice, they found that the prevalence of those clones is quite different between SLO and tumors. They conclude that TRAMP prostate tumors favor the recurrent enrichment of “tumor-associated” TVM cells that are uncommon in the periphery (Miller and others 2020).
The control of tumor growth through Ag-independent pathways is a topic of growing interest, considering that several tumors lose the expression of MHC type I as an evasion mechanism, which makes the tumor less susceptible to Ag-specific lysis, but more susceptible to innate control mechanisms such as NK cells and now to TVM cells.
TVM Cells During Aging
In young mice, TVM cell functional capacity is optimum with the ability to rapidly proliferate and produce cytokines after TCR or innate stimulation compared to TN, as previously mentioned. However, over time, the proportion of TVM cells accumulates and becomes dysfunctional. Recent work examined peripheral TVM cells in both young and aged germ-free B6 and BALB/c mice that found that the frequency of TVM cells increases with age and is independent of genetic background. The investigators also examined different hygienic conditions that included cohousing laboratory and feral mice with results demonstrating only minimal effects on peripheral TVM cells. This suggests that a common homeostatic mechanism exists during aging that is independent of mouse strain or commensal microbiota (Moudra and others 2021).
Several investigators have addressed the cause of these changes throughout the TVM cell lifespan. Renkema and others (2014) evaluated the long-term maintenance of TVM cells in unimmunized old mice. They found that TVM cells from old OT-I or WT mice (≥14-month old) displayed several different characteristics not observed in younger mice (2–4-month old) (Renkema and others 2014). TVM cell increased in frequency from 20% in young mice to up to 70% in old mice (Rudd and others 2011). Moreover, by using CD44, CD62L, and CD49d markers, Chiu and others (2013) determined that 90% of the TCM CD8+ T cells were indeed TVM cells in aged mice.
This age-related accumulation in TVM cells correlates with an increased proliferation capacity, exhibiting significantly higher propensity to divide 4 or more times in response to IL-7 and IL-15. In contrast, TVM cells in old mice are less capable of proliferating in response to cognate peptide (TCR). Moreover, the cells undergo increased apoptosis specifically in response to peptide stimulation (Renkema and others 2014). However, preferential enrichment of TVM cells with high avidity in older animals that exhibited strong antimicrobial function could compensate for functional defects during aging (Rudd and others 2011). Consistent with the age-associated increase in TVM cells, it was reported that the de novo response to influenza virus in aged mice was dominated by TVM cells, in contrast to the response in young mice (Lanzer and others 2018).
In a comparative study to evaluate functional defects characteristic of TN versus TVM cells with age, Quinn and others (2018) sorted TN and TVM cells from unimmunized WT mice, polyclonally stimulated in vitro with coated aCD3ɛ, and evaluated cell numbers up to 8 days post-stimulus. They found that, while young TN and TVM cells could extensively proliferate, only aged TN cells exhibited a slight age-related defect; however, aged TVM cells displayed a severe reduced proliferative capacity mainly caused by decreases in cell cycle division (Quinn and others 2018).
When they evaluated young and aged TN and TVM cell proliferation following in vitro IL-15 stimulation, Quinn and others (2018) found that only young and aged TVM cells proliferated robustly. This indicated that in TVM cells, TCR and cytokine-specific proliferative responses are regulated independently (Quinn and others 2018). To determine if environment is responsible for the defect observed in aged TVM cells, the author performed adoptive transfer experiments by administering young TN and TVM cells into aged C57BL/6 recipient mice. Results indicated that, while TN versus TVM cell proportions were stable for over 2 months after transfer, both subsets exhibited a severe reduction in proliferative capacity.
In addition, adoptive transfer of aged TVM cells to young WT recipient mice did not recover the TVM cell defect (Quinn and others 2018). Evaluation of exhaustion-associated markers demonstrated that age-related changes in TVM cells were not consistent with exhaustion, but rather senescence and associated with upregulation of NKRs, Bcl-2 expression, and increased phosphorylation of MAPK signaling pathway proteins (Quinn and others 2018). Based on these data, the author surmises that the “inflammaging” state commonly observed in elderly people allows TVM cells to survive relatively well in the aged environment as they respond better to cytokines than to TCR engagement.
The collective data on the preferential accumulation of aged TVM cells and their functional role in this stage suggest that, while aging, organisms become more dependent on innate immune responses that efficiently and rapidly act through cytokines rather than by an impaired Ag-specific response.
TIM/TVM Cell in Humans
Discovery and phenotype
In humans, demonstrating the existence of CD8 T+ cells having a memory phenotype without encountering any antigen is quite challenging, and to date, only 4 studies have addressed this question using human cord blood samples. In 2006, a first study identified CD8+ T cells expressing either KIR receptors or the inhibitory NKG2A receptor with an EMRA phenotype (CD45RA+ CCR7−) in cord blood, intact from viral infection or placental pathology (Warren and others 2006). Later, a second study identified CD8+ T cells in fetal human thymus and spleen expressing CD45RA, CD161, and CD122 markers at their surface and the transcription factor Eomes intracellularly (Min and others 2011). In these first 2 studies, the identified TIM/TVM cells were functional, with the capacity to secrete IFNγ in response to a nonspecific stimulation by phorbol myristate acetate and ionomycin.
Later, Jacomet and others (2015) identified TIM/TVM cells expressing KIRs and/or NKG2A surface markers with an enriched EMRA phenotype and a marked Eomes expression. This result was recently confirmed by an independent study using the same markers to identify TIM/TVM cells in both cord blood and peripheral adult blood (Kasakovski and others 2021). TIM/TVM cells have a highly cytotoxic potential, as they have a high content of perforin and granzyme B (Jacomet and others 2015). Importantly, a new function enlightened by Jacomet and others (2015) was the secretion of IFNγ by TIM/TVM cells in response to a proinflammatory stimulation by IL-12 and IL-18, demonstrating the NK-like function of these cells in cord blood.
Currently, there is no consensus to specifically identify TIM/TVM cells in human peripheral blood; since early 2000, several studies have identified CD8+ T cells with innate-like features and 2 methods seem to emerge concomitantly. The first method uses only surface cell markers: CD8, KIR, NKG2A, and CD45RA, and can be completed by the expression of CD62L and CD122 and the lack of CD27 and CCR7 markers (White and others 2016; Quinn and others 2020). The second method uses surface cell markers (CD8, KIR, and NKG2A) plus the expression of the transcription factor Eomes (Jacomet and others 2015, 2016; Barbarin and others 2017; Kasakovski and others 2021). This second method has the advantage of taking into account that Eomes expression is preferentially linked to the IFNγ secretion function in response to an innate stimulation by IL-12 and IL-18, as CD8+ Eomes− KIR/NKG2A+ T cells poorly secrete IFNγ (Daniel and others 2021).
In these 2 methods, the antibodies used are mainly a mix of anti-NKG2A, anti-panKIR2D (clone NKVSF1), and anti-KIR3DL1/DL2 (clones DX9 and 5.133), resulting in the identification of a heterogeneous population. In an attempt to better define TIM/TVM cells on a functional basis, Barbarin and others (2017) found an association between CD49d and the IFNγ secretion, but not specific for the identification of TIM/TVM cells in humans. Another interesting marker could be the ecto-5′-nucleotidase CD73, which has been demonstrated to delineate a subset of polyfunctional memory T cells expressing Eomes, with increased survival and with the ability to develop into cells resembling tissue-resident memory T cells (Fang and others 2021).
Finally, 3 recent studies looked deeper into the phenotype of TIM/TVM cells.
The first study sorted separately CD8+ CD45RA+ panKIR2D+ KIR3DL1/DL2+ cells (TIM/TVM KIR+ cells) and CD8+ CD45RA+ NKG2A+ cells (TIM/TVM NKG2A+ cells) and performed an RNA-seq analysis of these 2 subsets of TIM/TVM cells (Pieren and others 2021). The authors confirmed that these 2 subsets have different characteristics and suggested different functions. In particular, TIM/TVM KIR+ cells were found to share common features with previously described regulatory CD8+ T cells (Nakagawa and others 2018; Holderried and others 2021; Mishra and others 2021), with the expression of the transcription factor Helios and higher expression of CD122 and TIGIT.
This study identified TIGIT and CD226 as potential markers to better characterize TIM/TVM KIR+ cells and TIM/TVM NKG2A+ cells. Indeed, TIM/TVM KIR+ cells are mainly TIGIT+ CD266− and TIM/TVM NKG2A+ cells are TIGIT− CD226+. Furthermore, they also demonstrated a suppressive activity of TIM/TVM KIR+ cells in an in vitro assay, but did not address the functionality of these 2 subsets in an innate-like stimulation assay (Pieren and others 2021).
The second study, combining gene expression profiles and TCR repertoires, identified a CD8+ T cell subset (CD45RA+ CD45RO− CCR7−), expressing panKIR2D receptors with or without NKG2C (KLRC2) (Schattgen and others 2022). These TIM/TVM KIR2D+ NKG2C+/− cells had a higher frequency of Helios-positive cells than KIR2D− NKG2C− CD8+ T cells (Schattgen and others 2022), hence strengthening the hypothesis of a regulatory function for TIM/TVM KIR+ cells. This last study also confirms the early study by Björkström and others (2012), showing that TIM/TVM KIR+ cells displayed a restricted or biased TCR repertoire.
The third study also performed RNA-seq analysis of CD8+ KIR+ T cells and confirmed (1) their TIGIT+ Helios+ NKG2A+ CCR7− CD27− CD28− phenotype with high cytotoxic content (perforin and granzyme B), (2) their less diverse TCR repertoire, and (3) their regulatory function in autoimmune diseases (Li and others 2022). Taken together, the phenotype TIGIT+ CD266− of TIM/TVM KIR+ cells and their biased TCR repertoire raised the question of their exhaustion status, as it was shown that CD226− CD8+ T cells were dysfunctional (Weulersse and others 2020).
Finally, further characterization and properties of KIR+ CD8+ T cells, in particular KIR2D+ CD8+ T cells, were provided by Gimeno and others (2020). After culture in the presence of their HLA-C ligands, KIR2DL2/L3/S2+ CD8+ T cells showed notably higher expression of IFNγ, TGFβ, and perforin than KIR2DL1/S1+ CD8+ T cells. However, KIR2DL1/S1+ CD8+ T cells had a gene expression profile compatible with an active cancer immunosurveillance, whereas KIR2DL2/L3/S2+ CD8+ T cells had a gene expression profile suggesting a function of suppressive antitumor responses (Gimeno and others 2020).
Table 1 outlines the current knowledge on mouse TVM and its human counterpart. Further studies are needed to determine where each TIM/TVM subset fits in the effector-memory and exhaustion differentiation continuum. This knowledge could aid in determining if these cells could be a target for immunotherapy.
Table 1.
Summary of Mouse and Human Virtual Memory CD8 T Cell Subsets Main Characteristics
| Name | Species | Membrane markers | Transcription factor signature | TCR repertoire | Response to TCR stimulation | Innate-like functions | Central/peripheral differentiation | Homeostasis throughout life | Pathological context |
|---|---|---|---|---|---|---|---|---|---|
| TVM | Mouse | CD8, CD44hi, CD122hi, CD49dlo, IL-4R, IFN-I R | Eomeshi | Diverse, but nonsimilar to TN repertoire | Yes, with low proliferation | Highly responsive to IL-15 IFNγ secretion in response to IL-12/IL-18 |
IL-15 and Eomes dependent, mainly in periphery Precursors in the thymus |
Increase with age | Bacterial and viral infections Tumor infiltration in several cancer models |
| TIM/TVM | Human | CD8, KIR+ and/or NKG2A+, CD62L, CD122, CD45RA+, CCR7− CD27− as defined in Jacomet et al., 2015 |
Eomes+, T-bet+ | Unknown | Yes | IFNγ secretion in response to IL-12/IL-18 CD107a degranulation induced by anti-CD16 or MHC class I- target cells Highly responsive to IL-15 |
Unknown, but present in cord blood, fetal thymus, and spleen | Not consensual | High antitumoral potential (CML, ovarian. and breast cancers) Expanded in HIV patients |
| TIM/TVM NKG2A+ | Human | CD8, NKG2A+, CD45RA+, TIGIT−, CD226+ as defined in Pieren et al., 2021 |
Eomesint | Unknown | Yes, proliferation similar to other CD8 T cells | High IFNγ secretion in response to IL-12/IL-18 | Unknown | Decline with age | High antitumoral potential, synergy with NK cells (NKG2A as a checkpoint inhibitor) |
| TIM/TVM KIR+ | Human | CD8, KIR+, CD45RA+, TIGIT+, CD226− as defined in Pieren et al., 2021 |
Eomeshi, Helios | Less diverse than other CD8 T cells | Yes, proliferation lower than other CD8 T cells | Low IFNγ secretion in response to IL-12/IL-18 | Unknown | Increase with age | Putative CD8 Treg suppressive activity in autoimmune and infectious diseases |
Cytokines and TIM/VM cells
Despite few studies on the subject, by analogy with the mice model (Barbarin and others 2017), it is obvious that human TIM/TVM cells must be dependent on IL-15, IL-4, and type I interferon for their homeostasis/development. Indeed, TIM/TVM cells are enriched in CD122, the common β chain receptor for IL-2 and IL-15, and they express the transcription factor Eomes, both known to confer high sensitivity to IL-15. A recent in vitro study observed that after 10 days of culture, only IL-15 in combination with IL-2 increased the KIR+ CD56− T cell frequency compared to IL-2 + IL-12 stimulation (David and others 2021).
For KIR2DL3+ TIM/TVM cell subset, the authors demonstrated that the implied mechanism was the inhibition of their apoptosis (David and others 2021). In contrast, in an expansion in vitro model using HLA-C ligands, panKIR2D+ CD8+ T cells expanded preferentially in the presence of their ligand HLA-C1 and the proinflammatory cytokine IL-12, but surprisingly not in the presence of IL-15 (Gimeno and others 2020). This observation suggests that depending on the context and the subset of KIR cells, different cytokines are needed for TIM/TVM cell homeostasis/expansion.
IL-4 was also shown by Jacomet and others (2016) to participate in the homeostasis/development of TIM/TVM cells. This point will be further discussed below in the context of iNKT/TIM/TVM cell axis described in chronic myeloid leukemia (CML).
Regarding type I IFN, in the mouse model and as mentioned earlier, it was shown that type I IFNs could induce TVM cell differentiation (Martinet and others 2015). In humans, there is no direct evidence of a role of type I IFNs demonstrated, but type I IFNs were shown to promote IL-15Rα expression in human T cells, resulting in enhanced IL-15 signaling and increased cytotoxicity in vitro in a mixed lymphocyte reaction (Hansen and others 2011). Another study showed that adding IFNα to the proinflammatory IL-12 + IL-18 cytokine cocktail resulted in an IFNγ secretion similar to that found in response to the classical IL-12 + IL-18 stimulation in other innate-like T cell subsets (MAIT, iNKT, and Tγδ cells) (Gutierrez-Arcelus and others 2019).
Another indirect evidence comes from CML patients treated with IFNα before the rise of targeted therapy using tyrosine kinase inhibitors (TKI). A few numbers of patients were able to respond to the IFNα treatment and even maintained a treatment-free remission (TFR) for years (Bonifazi and others 2001). It is easy to hypothesize that the TFR obtained in these rare patients could be partially due to IFNα action on the immune system (de Castro and others 2003). Moreover, a recent study linked durable TFR of CML patients to a higher percentage of TIM/VM cells (Cayssials and others 2019). Despite the small number of subjects, two-thirds of them were patients with an IFNα treatment history, thus raising the question of IFNα in TIM/TVM cells arising and long-term presence in the peripheral blood of these patients. Thus, IFNα could have an effect on TIM/TVM cells that remains to be further confirmed.
NK-like functions of innate CD8 T cells in humans: an antitumoral response?
Although it is well known that the diversity in the repertoire of KIR and the KIR/HLA-ligand interactions determine the susceptibility to autoimmunity, infections, or cancer (Takeshita and others 2013), only a few recent studies have identified CD8+ T cells expressing KIR receptors with innate-like or Treg features and linked it with immunosurveillance of cancer or as possible therapeutic targets. For example, in the peripheral circulation of breast cancer patients, a CD8+ CD25+ CD127− T cell population was identified; this subset decreased with the advancement of cancer (Chakraborty and others 2018).
An elegant in vitro model showed that these cells were present mainly in the early stages of tumor development, expressed high level of KIR and low-level of NKG2A, and produced IFNγ (Chakraborty and others 2018), thus resembling the CD8 Treg KIR+ TIM/TVM cells previously described. The authors hypothesized that CD25+ KIR+ CD127− FOXP3− CD8+ T cells could impede tumor growth at an early stage before being deleted by CD4+ Treg at a later stage (Chakraborty and others 2018).
In another study, the interaction of KIR3DL3 with its ligand HHLA2 was identified as a human immunosuppressive pathway (Wei and others 2021). In this work, KIR3DL3+ CD8+ TEMRA cells could lyse NK-sensitive K562 targets and less-sensitive solid tumor cells by both TCR-dependent and TCR-independent mechanisms. Moreover, tumor mice models demonstrated that KIR3DL3−HHLA2 interaction blockage restored the effector function of the KIR3DL3+ cells and promoted antitumor immunity (Wei and others 2021). It remains to determine the respective contribution of KIR3DL3+ NK cells and KIR3DL3+ CD8 T cells to this antitumoral effect.
Regarding NKG2A+ CD8+ T cells, several studies demonstrated their importance in cancer immunosurveillance, but did not investigate the relevance of their innate-like functions in this context. As mentioned above, the antitumoral function of NKG2D+ CD25− CD8+ TIM cells described in mice has been confirmed in human melanoma biopsies. In humans, memory CD25− CD8+ T cells could be induced in vitro either by an IL-2 stimulation without TCR engagement or in melanoma by antigen-nonspecific immunotherapy treatment (Tietze and others 2012).
The strongest evidence linking TIM/TVM cells (KIR/NKG2A+ Eomes+) to an antitumoral function was established by the group of Gombert and Herbelin (Jacomet and others 2016). They demonstrated the implication of TIM/TVM cells in CML. First, they showed that KIR/NKG2A+ Eomes+ TIM/TVM cell subset was defective at disease diagnostic with the loss of antigen-independent cytotoxic activity and IFNγ production in response to innate-like stimulation with IL-12 + IL-18. The percentage and the IFNγ function of KIR/NKG2A+ Eomes+ TIM/TVM cells were partially restored after tyrosine kinase (TKI) therapy in patients achieving disease control (Jacomet and others 2016). Indeed, TIM/TVM cells are targeted by the TKI dasatinib in humans and mice (Barbarin and others 2020).
Moreover, by analogy with the mice model, they hypothesized that KIR/NKG2A+ Eomes+ TIM/TVM cell deficit at CML diagnosis could be linked to iNKT cell deficit already observed, with an impaired IL-4 production by iNKT cells (Rossignol and others 2012). The positive correlation observed between Eomes expression in KIR/NKG2A+ Eomes+ TIM/TVM cells and PLZF expression in iNKT cells, along with the direct effect of IL-4 in vitro on KIR/NKG2A+ Eomes+ TIM/TVM cell proliferation, strengthens this hypothesis (Jacomet and others 2016). Later, they demonstrated in a monocentric retrospective study that long-term TKI treatment discontinuation without relapse could be associated with an elevated presence of KIR/NKG2A+ Eomes+ TIM/TVM cells in the blood, correlated with high NK cell percentage (Cayssials and others 2019). Supranormalized KIR/NKG2A+ Eomes+ TIM/TVM cells were normally functional like in healthy donors, with an intact IFNγ secretion function (Cayssials and others 2019).
Taken together, KIR/NKG2A+ Eomes+ TIM/TVM cells are a critical element for CML immunosurveillance. The role of KIR/NKG2A+ Eomes+ TIM/TVM cells was also demonstrated in chemo-treated human lymphoma cells deprived of MHC-I molecules co-cultured in vitro with purified human CD8+ T cells from healthy donors. An increase in the percentage of KIR/NKG2A+ Eomes+ TIM/TVM cells, associated with an increase of granzyme B content, was observed (Wang and others 2021). KIR/NKG2A+ Eomes+ TIM/TVM cells were also observed in solid cancers: in the draining LNs from breast cancer patients and in primary tumor, carcinosis, and peritoneal ascites from ovarian cancer patients (Barbarin and others 2017).
Even though human TIM/TVM was mainly studied in the context of cancer, a few studies have reported their presence in other pathologies. Indeed, Jin and others (2020) observed an increase of these cells in HIV patients undergoing successful antiretroviral therapy, and demonstrated a suppressive activity dependent on KIR receptors ex vivo. Furthermore, Daniel and others (2021) showed that chronic allo-antigenic stimulation promotes the generation of TIM/TVM cells with a senescent/inflammaging signature (EMRA phenotype, CD27− CD28−, and high perforin content).
Conclusions
The pool of memory CD8+ T cells is composed of a variety of cell subtypes with different phenotypic and functional characteristics. Within this group, we can find the so-called Ag-independent memory cells or virtual memory cells. TVM have long been confused with Ag-specific central memory T cells, which has caused for many years their true role not to be clarified within the immune system.
The discovery of human CD8+ T cells with identical characteristics to murine TVM cells has aroused enormous interest in recent years and has posed a new challenge in trying to understand not only their role in different pathological processes but also to discriminate what roles have been erroneously assigned to specific memory cells instead of virtual memory cells.
Numerous publications are currently emerging, which are clarifying more and more about the origin, functional characteristics and their role in different pathological processes, especially in infections and cancer, which will provide a new edge to understand their key role within the immune system.
Authors' Note
When I arrived at Howard´s laboratory to do my postdoctoral training, I was completely overwhelmed and extremely homesick. However, he never lost faith in me and we have been working together for over 20 years. He was both my mentor and my friend and we have shared scientific and personal life experiences throughout these years.
He introduced me to the field of cytokines, and especially the interferon's role in the immune system. Since then, we have had a productive scientific collaboration during my time in the United States and later in Argentina. His mentorship helped me build both the person and the scientist I am today.
I was so pleased to be invited to contribute to this festschrift to honor Howard's scientific career and hope we continue our friendship for the rest of our lives.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Correction added on October 17, 2022 after first online publication of September 9, 2022: The references in this sentence originally cited Drobek and others (2018) and Hussain and others (2019). These have been updated to Pribikova and others (2018) and Hussain and Quinn (2019). Pribikova and others (2018) is added to the reference list.
References
- Akue AD, Lee JY, Jameson SC. 2012. Derivation and maintenance of virtual memory CD8 T cells. J Immunol 188(6):2516–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherly LO, Lucas JA, Felices M, et al. 2006. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity 25(1):79–91. [DOI] [PubMed] [Google Scholar]
- Baez NS, Cerban F, Savid-Frontera C, et al. 2019. Thymic expression of IL-4 and IL-15 after systemic inflammatory or infectious Th1 disease processes induce the acquisition of “innate” characteristics during CD8+ T cell development. PLoS Pathog 15(1):e1007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarin A, Abdallah M, Lefevre L, et al. 2020. Innate T-alphabeta lymphocytes as new immunological components of anti-tumoral “off-target” effects of the tyrosine kinase inhibitor dasatinib. Sci Rep 10(1):3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarin A, Cayssials E, Jacomet F, et al. 2017. Phenotype of NK-Like CD8(+) T cells with innate features in humans and their relevance in cancer diseases. Front Immunol 8:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RE, Cordes CJ, Forman J. 2002. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol 32(10):2807–2816. [DOI] [PubMed] [Google Scholar]
- Berg RE, Crossley E, Murray S, et al. 2003. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med 198(10):1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkstrom NK, Beziat V, Cichocki F, et al. 2012. CD8 T cells express randomly selected KIRs with distinct specificities compared with NK cells. Blood 120(17):3455–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi F, de Vivo A, Rosti G, et al. 2001. Chronic myeloid leukemia and interferon-alpha: a study of complete cytogenetic responders. Blood 98 (10): 3074–3081. [DOI] [PubMed] [Google Scholar]
- Bou Ghanem EN, Nelson CC, D'Orazio SE. 2011. T cell-intrinsic factors contribute to the differential ability of CD8+ T cells to rapidly secrete IFN-gamma in the absence of antigen. J Immunol 186(3):1703–1712. [DOI] [PubMed] [Google Scholar]
- Boyman O, Letourneau S, Krieg C, et al. 2009. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol 39(8):2088–2094. [DOI] [PubMed] [Google Scholar]
- Broussard C, Fleischacker C, Horai R, et al. 2006. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity 25(1):93–104. [DOI] [PubMed] [Google Scholar]
- Carty SA, Koretzky GA, Jordan MS. 2014. Interleukin-4 regulates eomesodermin in CD8+ T cell development and differentiation. PLoS One 9(9):e106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayssials E, Jacomet F, Piccirilli N, et al. 2019. Sustained treatment-free remission in chronic myeloid leukaemia is associated with an increased frequency of innate CD8(+) T-cells. Br J Haematol 186(1):54–59. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Bhattacharjee P, Panda AK, et al. 2018. Providence of the CD25(+) KIR(+) CD127(-) FOXP3(-) CD8(+) T-cell subset determines the dynamics of tumor immune surveillance. Immunol Cell Biol 96(10):1035–1048. [DOI] [PubMed] [Google Scholar]
- Challa-Malladi M, Lieu YK, Califano O, et al. 2011. Combined genetic inactivation of beta2-Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell 20(6):728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau LA, Rohekar S, Wang JJ, et al. 2002. Thymic re-entry of mature activated T cells and increased negative selection in vascularized allograft recipients. Clin Exp Immunol 127(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu BC, Martin BE, Stolberg VR, et al. 2013. Cutting edge: central memory CD8 T cells in aged mice are virtual memory cells. J Immunol 191(12):5793–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Bediako Y, Xu H, et al. 2011. Positive selecting cell type determines the phenotype of MHC class Ib-restricted CD8+ T cells. Proc Natl Acad Sci U S A 108(32):13241–13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Tyznik AJ, Roepke S, et al. 2013. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep 3(3):701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia MP, Cardoso EM, Pereira CF, et al. 2009. Hepatocytes and IL-15: a favorable microenvironment for T cell survival and CD8+ T cell differentiation. J Immunol 182(10):6149–6159. [DOI] [PubMed] [Google Scholar]
- D'Cruz LM, Yang CY, Goldrath AW. 2010. Transcriptional regulation of NKT cell development and homeostasis. Curr Opin Immunol 22(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel L, Tassery M, Lateur C, et al. 2021. Allotransplantation is associated with exacerbation of CD8 T-Cell senescence: the particular place of the innate CD8 T-Cell component. Front Immunol 12:674016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Willem C, Legrand N, et al. 2021. Deciphering the biology of KIR2DL3(+) T lymphocytes that are associated to relapse in haploidentical HSCT. Sci Rep 11(1):15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro FA, Palma PV, Morais FR, et al. 2003. Immunological effects of interferon-alpha on chronic myelogenous leukemia. Leuk Lymphoma 44(12):2061–2067. [DOI] [PubMed] [Google Scholar]
- Drobek A, Moudra A, Mueller D, et al. 2018. Strong homeostatic TCR signals induce formation of self-tolerant virtual memory CD8 T cells. EMBO J 37(14):e98518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erman B, Alag AS, Dahle O, et al. 2006. Coreceptor signal strength regulates positive selection but does not determine CD4/CD8 lineage choice in a physiologic in vivo model. J Immunol 177(10):6613–6625. [DOI] [PubMed] [Google Scholar]
- Fang F, Cao W, Zhu W, et al. 2021. The cell-surface 5'-nucleotidase CD73 defines a functional T memory cell subset that declines with age. Cell Rep 37(6):109981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink PJ, Swan K, Turk G, et al. 1992. Both intrathymic and peripheral selection modulate the differential expression of V beta 5 among CD4+ and CD8+ T cells. J Exp Med 176(6):1733–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido F, Cabrera T, Aptsiauri N. 2010. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer 127(2):249–256. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Loughhead SM, von Andrian UH. 2015. Figuring fact from fiction: unbiased polling of memory T cells. Cell 161(4):702–704. [DOI] [PubMed] [Google Scholar]
- Gimeno L, Serrano-Lopez EM, Campillo JA, et al. 2020. KIR+ CD8+ T lymphocytes in cancer immunosurveillance and patient survival: gene expression profiling. Cancers (Basel) 12(10):2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Arcelus M, Teslovich N, Mola AR, et al. 2019. Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat Commun 10(1):687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Fink PJ. 2009. Back to the thymus: peripheral T cells come home. Immunol Cell Biol 87(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluszczak C, Akue AD, Hamilton SE, et al. 2009. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med 206(2):435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ML, Woetmann A, Krejsgaard T, et al. 2011. IFN-alpha primes T- and NK-cells for IL-15-mediated signaling and cytotoxicity. Mol Immunol 48(15–16):2087–2093. [DOI] [PubMed] [Google Scholar]
- Hodge DL, Reynolds D, Cerban FM, et al. 2012. MCP-1/CCR2 interactions direct migration of peripheral B and T lymphocytes to the thymus during acute infectious/inflammatory processes. Eur J Immunol 42(10):2644–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderried TA, Kruse M, Serries M, et al. 2021. Helios-expressing CD8(+) T cells are decreased in patients with systemic lupus erythematosus. Lupus 30(6):1022–1024. [DOI] [PubMed] [Google Scholar]
- Horai R, Mueller KL, Handon RA, et al. 2007. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity 27(5):775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Shao T, Mao T, et al. 2021. Virtual memory T cells orchestrate extralymphoid responses conducive to resident memory. Sci Immunol 6(62):eabg9433. [DOI] [PubMed] [Google Scholar]
- Hu J, Sahu N, Walsh E, et al. 2007. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur J Immunol 37(10):2892–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhu S, Xia X, et al. 2014. CD8+T cell-specific induction of NKG2D receptor by doxorubicin plus interleukin-12 and its contribution to CD8+T cell accumulation in tumors. Mol Cancer 13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Wei B, Velazquez P, et al. 2005. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4+ T lymphocytes. Clin Immunol 117(3):221–230. [DOI] [PubMed] [Google Scholar]
- Huang W, Hu J, August A. 2013. Cutting edge: innate memory CD8+ T cells are distinct from homeostatic expanded CD8+ T cells and rapidly respond to primary antigenic stimuli. J Immunol 190:2490–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T, Quinn KM. 2019. Similar but different: virtual memory CD8 T cells as a memory-like cell population. Immunol Cell Biol 97(7):675–684. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, et al. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 6(12):1236–1244. [DOI] [PubMed] [Google Scholar]
- Istaces N, Splittgerber M, Lima Silva V, et al. 2019. EOMES interacts with RUNX3 and BRG1 to promote innate memory cell formation through epigenetic reprogramming. Nat Commun 10:3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomet F, Cayssials E, Barbarin A, et al. 2016. The Hypothesis of the Human iNKT/Innate CD8(+) T-cell axis applied to cancer: evidence for a deficiency in chronic myeloid leukemia. Front Immunol 7:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomet F, Cayssials E, Basbous S, et al. 2015. Evidence for eomesodermin-expressing innate-like CD8(+) KIR/NKG2A(+) T cells in human adults and cord blood samples. Eur J Immunol 45:1926–1933. [DOI] [PubMed] [Google Scholar]
- Jameson SC, Lee YJ, Hogquist KA. 2015. Innate memory T cells. Adv Immunol 126:173–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JH, Huang HH, Zhou MJ, et al. 2020. Virtual memory CD8+ T cells restrain the viral reservoir in HIV-1-infected patients with antiretroviral therapy through derepressing KIR-mediated inhibition. Cell Mol Immunol 17(12):1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi T, Assarsson E, Lukacher AE, et al. 2003. Memory CD8+ T cells provide an early source of IFN-gamma. J Immunol 170(5):2399–2408. [DOI] [PubMed] [Google Scholar]
- Kasakovski D, Zeng X, Lai J, et al. 2021. Characterization of KIR + NKG2A + Eomes- NK-like CD8+ T cells and their decline with age in healthy individuals. Cytometry B Clin Cytom 100(4):467–475. [DOI] [PubMed] [Google Scholar]
- Kurzweil V, LaRoche A, Oliver PM. 2014. Increased peripheral IL-4 leads to an expanded virtual memory CD8+ population. J Immunol 192(12):5643–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer KG, Cookenham T, Reiley WW, et al. 2018. Virtual memory cells make a major contribution to the response of aged influenza-naive mice to influenza virus infection. Immun Ageing 15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Park SP, Park CH, et al. 2015. IL-4 Induced Innate CD8+ T Cells Control Persistent Viral Infection. PLoS Pathog 11(10):e1005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Hamilton SE, Akue AD, et al. 2013a. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci U S A 110(33):13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Holzapfel KL, Zhu J, et al. 2013b. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol 14(11):1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jameson SC, Hogquist KA. 2011. Alternative memory in the CD8 T cell lineage. Trends Immunol 32(2):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertmemongkolchai G, Cai G, Hunter CA, et al. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol 166(2):1097–1105. [DOI] [PubMed] [Google Scholar]
- Li J, Zaslavsky M, Su Y, et al. KIR+CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science. 2022. Apr 15;376(6590):eabi9591. doi: 10.1126/science.abi9591. Epub 2022 Apr 15. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukin K, Cosyns M, Mitchell T, et al. 2000. Eradication of Cryptosporidium parvum infection by mice with ovalbumin-specific T cells. Infect Immun 68(5):2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet V, Tonon S, Torres D, et al. 2015. Type I interferons regulate eomesodermin expression and the development of unconventional memory CD8(+) T cells. Nat Commun 6:7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CH, Klawon D E J, Zeng S, et al. 2020. Eomes identifies thymic precursors of self-specific memory-phenotype CD8(+) T cells. Nat Immunol 21(5):567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, McHugh R, Sempowski GD, et al. 2003. Neonates support lymphopenia-induced proliferation. Immunity 18(1):131–140. [DOI] [PubMed] [Google Scholar]
- Min HS, Lee YJ, Jeon YK, et al. 2011. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J Immunol 186(10):5749–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Srinivasan S, Ma C, et al. 2021. CD8(+) Regulatory T Cell - A Mystery to Be Revealed. Front Immunol 12:708874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudra A, Niederlova V, Novotny J, et al. 2021. Phenotypic and clonal stability of antigen-inexperienced memory-like T cells across the genetic background, hygienic status, and aging. J Immunol 206(9):2109–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, et al. 2013. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 31:137–161. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Wang L, Cantor H, et al. 2018. New insights into the biology of CD8 regulatory T cells. Adv Immunol 140:1–20. [DOI] [PubMed] [Google Scholar]
- Nakagawa R, Inui T, Nagafune I, et al. 2004. Essential role of bystander cytotoxic CD122+CD8+ T cells for the antitumor immunity induced in the liver of mice by alpha-galactosylceramide. J Immunol 172(11):6550–6557. [DOI] [PubMed] [Google Scholar]
- Nayar R, Enos M, Prince A, et al. 2012. TCR signaling via Tec kinase ITK and interferon regulatory factor 4 (IRF4) regulates CD8+ T-cell differentiation. Proc Natl Acad Sci U S A 109(41):E2794–E2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Lee A, Lee JI, et al. 2016. Effect of IL-4 on the development and function of memory-like CD8 T cells in the peripheral lymphoid tissues. Immune Netw 16(2):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, DiPalma DT, Kwon J, et al. 2019. Quantitative difference in PLZF protein expression determines iNKT lineage fate and controls innate CD8 T cell generation. Cell Rep 27(9):2548–2557 e2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Mullen AC, Martins GA, et al. 2003. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302(5647):1041–1043. [DOI] [PubMed] [Google Scholar]
- Pieren D K J, Smits N A M, Hoeboer J, et al. 2021. Regulatory KIR(+) RA(+) T cells accumulate with age and are highly activated during viral respiratory disease. Aging Cell 20(6):e13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati K, Perez C, Rojas L B P, et al. 2018. Functions of NKG2D in CD8(+) T cells: an opportunity for immunotherapy. Cell Mol Immunol 15(5):470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribikova M, Moudra A, Stepanek O. 2018. Opinion: Virtual memory CD8 T cells and lymphopenia-induced memory CD8 T cells represent a single subset: Homeostatic memory T cells. Immunol Lett 203:57–61. [DOI] [PubMed] [Google Scholar]
- Quinn KM, Fox A, Harland KL, et al. 2018. Age-related decline in primary CD8(+) T cell responses is associated with the development of senescence in virtual memory CD8(+) T cells. Cell Rep 23(12):3512–3524. [DOI] [PubMed] [Google Scholar]
- Quinn KM, Hussain T, Kraus F, et al. 2020. Metabolic characteristics of CD8(+) T cell subsets in young and aged individuals are not predictive of functionality. Nat Commun 11(1):2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafei M, Hardy MP, Williams P, et al. 2011. Development and function of innate polyclonal TCRalphabeta+ CD8+ thymocytes. J Immunol 187(6):3133–3144. [DOI] [PubMed] [Google Scholar]
- Rafei M, Rouette A, Brochu S, et al. 2013. Differential effects of gammac cytokines on postselection differentiation of CD8 thymocytes. Blood 121(1):107–117. [DOI] [PubMed] [Google Scholar]
- Renkema KR, Li G, Wu A, et al. 2014. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J Immunol 192(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol A, Levescot A, Jacomet F, et al. 2012. Evidence for BCR-ABL-dependent dysfunctions of iNKT cells from chronic myeloid leukemia patients. Eur J Immunol 42(7):1870–1875. [DOI] [PubMed] [Google Scholar]
- Rudd BD, Venturi V, Li G, et al. 2011. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci U S A 108(33):13694–13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, et al. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401(6754):708–712. [DOI] [PubMed] [Google Scholar]
- Santori FR, Kieper WC, Brown SM, et al. 2002. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity 17(2):131–142. [DOI] [PubMed] [Google Scholar]
- Schattgen SA, Guion K, Crawford JC, et al. 2022. Integrating T cell receptor sequences and transcriptional profiles by clonotype neighbor graph analysis (CoNGA). Nat Biotechnol 40(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler T, Hammerling GJ, Arnold B. 2004. Cutting edge: IL-7-dependent homeostatic proliferation of CD8+ T cells in neonatal mice allows the generation of long-lived natural memory T cells. J Immunol 172(1):15–19. [DOI] [PubMed] [Google Scholar]
- Sckisel GD, Tietze JK, Zamora AE, et al. 2014. Influenza infection results in local expansion of memory CD8(+) T cells with antigen non-specific phenotype and function. Clin Exp Immunol 175(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Pagarigan RR, Whitton JL. 2000. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol 164(4):2009–2015. [DOI] [PubMed] [Google Scholar]
- Smith NL, Patel RK, Reynaldi A, et al. 2018. Developmental Origin Governs CD8(+) T cell fate decisions during infection. Cell 174(1):117–130 e114. [DOI] [PubMed] [Google Scholar]
- Sosinowski T, White JT, Cross EW, et al. 2013. CD8alpha+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J Immunol 190(5):1936–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita LY, Gonzalez-Galarza FF, dos Santos EJ, et al. 2013. A database for curating the associations between killer cell immunoglobulin-like receptors and diseases in worldwide populations. Database (Oxford) 2013:bat021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, et al. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A 98(15):8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D, La Gruta NL, Nguyen A, et al. 2020. Hiding in Plain Sight: Virtually Unrecognizable Memory Phenotype CD8(+) T cells. Int J Mol Sci 21(22):8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze JK, Wilkins DE, Sckisel GD, et al. 2012. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood 119(13):3073–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Morris SC, Perkins C, et al. 2016. IL-4 and IL-15 promotion of virtual memory CD8(+) T cells is determined by genetic background. Eur J Immunol 46(10):2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdahl KB, Sun JC, Bevan MJ. 2002. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol 3(8):772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventre E, Brinza L, Schicklin S, et al. 2012. Negative regulation of NKG2D expression by IL-4 in memory CD8 T cells. J Immunol 189(7):3480–3489. [DOI] [PubMed] [Google Scholar]
- Wang X, Waschke BC, Woolaver RA, et al. 2021. MHC class I-independent activation of virtual memory CD8 T cells induced by chemotherapeutic agent-treated cancer cells. Cell Mol Immunol 18(3):723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS, Rana PM, Rieger DT, et al. 2006. CD8 T cells expressing killer Ig-like receptors and NKG2A are present in cord blood and express a more naive phenotype than their counterparts in adult blood. J Leukoc Biol 79(6):1252–1259. [DOI] [PubMed] [Google Scholar]
- Wei Y, Ren X, Galbo PMJr., et al. 2021. KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci Immunol 6 (61):eabf9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich MA, Odumade OA, Jameson SC, et al. 2010. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol 11(8):709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weulersse M, Asrir A, Pichler AC, et al. 2020. Eomes-Dependent Loss of the Co-activating Receptor CD226 Restrains CD8(+) T cell anti-tumor functions and limits the efficacy of cancer immunotherapy. Immunity 53(4):824–839 e810. [DOI] [PubMed] [Google Scholar]
- White JT, Cross EW, Burchill MA, et al. 2016. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat Commun 7:11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JT, Cross EW, Kedl RM. 2017. Antigen-inexperienced memory CD8(+) T cells: where they come from and why we need them. Nat Rev Immunol 17(6):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Jones M, Liu B, et al. 2013. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res 73(10):3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T, Nishimura H, Ishimitsu R, et al. 2001. Memory phenotype CD8(+) T cells in IL-15 transgenic mice are involved in early protection against a primary infection with Listeria monocytogenes. Eur J Immunol 31(3):757–766. [DOI] [PubMed] [Google Scholar]