Abstract
Objective
Postoperative pancreatic fistula (POPF) following distal pancreatectomy (DP) is a serious complication. In the present study, we aimed to identify the risk factors associated with clinically relevant postoperative pancreatic fistula (CR-POPF) and establish a nomogram model for predicting CR-POPF after DP.
Methods
In total, 115 patients who underwent DP at the General Hospital of Northern Theater Command between January 2005 and December 2020 were retrospectively studied. Univariate and multivariable logistic regression analyses were used to identify the independent risk factors associated with CR-POPF. Then, a nomogram was formulated based on the results of multivariable logistic regression analysis. The predictive performance was evaluated with receiver operating characteristic (ROC) curves. Decision curve and clinical impact curve analyses were used to validate the clinical application value of the model.
Results
The incidence of CR-POPF was 33.0% (38/115) in the present study. Multivariate logistic regression analysis identified the following variables as independent risk factors for POPF: body mass index (BMI) (OR 4.658, P = 0.004), preoperative albumin level (OR 7.934, P = 0.001), pancreatic thickness (OR 1.256, P = 0.003) and pancreatic texture (OR 3.143, P = 0.021). We created a nomogram by incorporating the above mentioned risk factors. The nomogram model showed better predictive value, with a concordance index of 0.842, sensitivity of 0.710, and specificity of 0.870 when compared to each risk factor. Decision curve and clinical impact curve analyses also indicated that the nomogram conferred a high clinical net benefit.
Conclusion
Our nomogram could accurately and objectively predict the risk of postoperative CR-POPF in individuals who underwent DP, which could help clinicians with early identification of patients who might develop CR-POPF and early development of a suitable fistula mitigation strategy and postoperative management.
Keywords: Distal pancreatectomy, Pancreatic fistula, Risk factors, Nomogram
Introduction
Distal pancreatectomy (DP) is the standard procedure for the removal of benign or malignant tumours from the pancreatic body or tail. With the development of preoperative management and improvements in surgical techniques, the mortality associated with DP has decreased in the last decade, yet the major morbidity rate remains high, especially in patients who undergo open approaches [1–8]. The most common and severe complication is postoperative clinically relevant pancreatic fistula (CR-POPF), which further causes intraperitoneal abscesses and subsequent lethal haemorrhage [9]. Great efforts have been made to reduce the incidence of CR-POPF in the last decade [4, 10–15]. However, the incidence of CR-POPF after DP is still high, ranging from 9.7 to 39% [4, 10–18]. Therefore, how to effectively reduce the incidence of CR-POPF and conduct timely treatment thereafter is an urgent clinical issue to be solved.
Risk prediction models are increasingly advocated as tools to assist risk stratification and guide prevention and treatment decisions relating to common health conditions [19, 20]. Risk prediction models were constructed to predict the risk of POPF following pancreaticoduodenectomy (PD). For example, the fistula risk score (FRS) and alternative fistula risk score (a-FRS) have been widely reported to predict CR-POPF with high accuracy [21–25]. The use of FRS to determine the risk of CR-POPF can facilitate management-related decision-making, especially drainage strategy [26–29]. The nomogram was also developed to predict CR-POPF following PD [30–33]. It should be noted that the incidence of CR-POPF is higher in patients who undergo DP than in those who undergo PD, but risk prediction models are rarely constructed for CR-POPF in patients who undergo DP. Although numerous risk factors have been previously associated with CR-POPF following DP, a single risk factor does not accurately predict CR-POPF. The development of a risk prediction for CR-POPF following DP is of utmost importance and could help surgeons anticipate, identify, and manage this severe complication from the outset.
In the present study, we aimed to analyse the risk factors contributing to CR-POPF following DP and then develop and validate a nomogram for predicting CR-POPF.
Methods
Patients
Between January 2005 and December 2020, data on consecutive patients who underwent DP were retrospectively collected from the electronic medical record system at the General Hospital of Northern Theater Command. Eligibility criteria were as follows: (1) DP procedure performed; (2) complete preoperative examinations and postoperative 90-day follow-up data; (3) and no history of pancreatectomy. This study was approved by the institutional Ethics Committee of the General Hospital of Northern Theater Command (No.: Y (2021) 056). Written informed consent was obtained from all the patients or patients’ relatives before the surgery.
Surgical procedures and postoperative management
The surgical methods of an open approach and laparoscopic approach were included in the present study. The choice of surgical procedures was decided by consultation among surgeons of our department, and the underlying disease condition was evaluated by preoperative radiological imaging. All resections were performed by senior consultant surgeons with more experience (≥ 20 pancreatectomies per year). The operative techniques were conducted as reported in previous studies [34]. During the operation, a nasogastric tube (NGT) was placed. Splenectomy was performed when malignant neoplasms were diagnosed by preoperative evaluation or spleen-preserving surgery could not be performed because of invasion of blood vessels. Two tubes were generally placed at the end of an operation for drainage of fluid, one tube near the pancreatic stump remnant and another drainage tube in the surgical field.
All patients received routine anti-infection, inhibition of pancreatic exocrine secretion, inhibition of gastric acid secretion, and nutritional support after surgery. Routine blood and biochemical examinations were performed on postoperative day (POD) 1 and then every 3 days until discharge. The amylase level in drainage fluid was routinely measured on POD 3, 5 and 7. Abdominal computed tomography (CT) was usually performed on POD 5 and any time patients had complex abdominal complications. The drainage tube was removed according to the Chinese consensus [35].
Clinicopathological variables
Based on previous studies and the potential association between variables and CR-POPF, clinicopathological variables were selected. All clinicopathological characteristics were extracted from electronic medical records. BMI was calculated as weight (in kg)/height2 (in m2). Pancreatic thickness was measured as previously reported [36]. In brief, pancreatic thickness was measured at the resection line in preoperative computed tomography (CT) by 1 researcher who was blinded to the POPF result. The resection line was evaluated with postoperative CT at 5 days after the operation. The pancreatic texture was determined by the surgeon’s tactile response and confirmed from the histopathological reports based on the fibrosis grade of the pancreatic tissues. When the patients underwent laparoscopic DP, the texture of the pancreas was determined by the tactile feedback of the instrument and was reassured after being pulled out from the abdominal cavity. CR-POPF was defined in accordance with the updated 2016 ISGPF consensus guidelines [9]. Briefly, an external fistula with a drain output of any measurable volume of fluid after postoperative Day 3 with an amylase level more than three times the upper limit was associated with a clinically relevant development/condition related directly to POPF. The clinicopathological variables in this study are reported in Table 1.
Table 1.
Clinicopathological characteristics of 115 patients undergoing DP
| Variable | All patients (n = 115) |
|---|---|
| Gender, n (%) | |
| Male | 34 (29.6) |
| Female | 81 (70.4) |
| Age (y), median (IQR) | 53.0 (45.5–62.0) |
| BMI (kg/m2), n (%) | |
| < 25 | 63 (54.8) |
| ≥ 25 | 52 (45.2) |
| Hypertension, n (%) | |
| No | 96 (83.5) |
| Yes | 19 (16.5) |
| Diabetes, n (%) | |
| No | 95 (82.6) |
| Yes | 20 (17.4) |
| Smoking, n (%) | |
| No | 93 (80.9) |
| Yes | 22 (19.1) |
| Alcohol abuse, n (%) | |
| No | 99 (86.1) |
| Yes | 16 (13.9) |
| Hemoglobin (g/L), median (IQR) | 131 (122–140) |
| Prealbumin (g/L), median (IQR) | 210 (184–251) |
| Albumin (g/L), n (%) | |
| ≥ 35 | 90 (78.3) |
| < 35 | 25 (21.7) |
| CA199 (KU/L), median (IQR) | 16.2 (5.80–76.8) |
| Operation time (min), median (IQR) | 287 (231–352) |
| Blood loss (mL), median (IQR) | 300 (200–550) |
| Surgical approach, n (%) | |
| Laparoscopic | 16 (13.9) |
| Open | 99 (86.1) |
| Ligation of main pancreatic duct, n (%) | |
| No | 54 (47.0) |
| Yes | 61 (53.0) |
| Pancreatic stump treatment, n (%) | |
| Endo GIA stapler | 30 (26.1) |
| Suture | 85 (73.9) |
| Pathology, n (%) | |
| PDAC | 36 (31.3) |
| Cystic | 45 (39.13) |
| Pancreatitis | 10 (8.7) |
| Neuroendocrine | 13 (11.3) |
| SPTP | 9 (7.83) |
| Others | 2 (1.74) |
| Pancreas thickness (mm), median (IQR) | 17.2 (15.3–20.2) |
| Pancreas texture, n (%) | |
| Hard | 69 (60.0) |
| Soft | 46 (40.0) |
| Splenectomy, n (%) | |
| No | 56 (48.7) |
| Yes | 59 (51.3) |
| CR-POPF, n (%) | |
| No | 77 (67.0) |
| Yes | 38 (33.0) |
BMI body mass index; IQR interquartile range; CA199 cancerantigen199; PDAC pancreatic ductal adenocarcinoma; SPTP solid pseudopapillary tumor of the pancreas
Statistical analysis
Continuous variables were expressed as the means and standard deviations or medians and interquartile ranges (IQR) and compared by the Mann–Whitney U test, as appropriate. Categorical variables are presented as the counts and percentages in each category and were compared by the chi-squared test or Fisher’s exact test. All variables associated with CR-POPF at a significant level were candidates for stepwise multivariate analysis. A nomogram was formulated based on the results of multivariate logistic regression analysis and by using the vrpm package of R version 4.1.3 (http://mirror.bjtu.edu.cn/cran/bin/windows/base/). The predictive performance of the nomogram was measured by the concordance index (C index) and calibration with 1000 bootstrap samples to decrease the overfit bias. The clinical application value of this model was validated using decision curve and clinical impact curve analyses. All statistical analyses were performed using R software studio (version 4.1.3), and a P value of less than 0.05 was considered to be statistically significant [37].
Results
Clinicopathologic characteristics of the study cohort
In total, 115 patients were included in the present study, of which 34 were men and 81 women, with a median age of 53.0 years (45.5–62.0). Of these 115 patients, 25 (21.7%) had preoperative hypoalbuminemia, and 46 (40.0%) had a soft pancreas. The median pancreas thickness was 17.2 mm. Among these patients, 99 underwent open surgery, and 16 underwent laparoscopic surgery. The median operative time was 287 min, and the median blood loss was 300 ml. There were 59 patients who underwent combined splenectomy, whereas the spleen was preserved in 56 patients. During the postoperative follow-up and management, 77 (67.0%) patients did not present CR-POPF, while 38 (33.0%) developed CR-POPF. The clinicopathologic characteristics are presented in Table 1.
Independent risk factors associated with CR-POPF
All 115 patients were divided into the CR-POPF group (n = 38) and the non-CR-POPF group (n = 77) based on the diagnosis of CR-POPF. The results of univariate analysis showed that patients with higher BMI (P = 0.004), hypertension history (P = 0.024), lower serum prealbumin level (P = 0.032), lower serum albumin level (P = 0.001), a thicker pancreas (P < 0.001) and a soft pancreas (P = 0.001) were more likely to develop CR-POPF (Table 2). All of the abovementioned significant parameters were then put into multivariate logistic regression analysis. The results showed that higher BMI (OR 4.658, 95% CI 1.716–14.10, P = 0.004), lower serum albumin level (OR 7.934, 95% CI 2.548–28.292, P = 0.001), thicker pancreas (OR 1.256, 95% CI 1.086–1.470, P = 0.003) and soft pancreas (OR 3.143, 95% CI 1.203–8.497, P = 0.021) were independent risk factors associated with CR-POPF following DP (Table 3).
Table 2.
Univariate analysis of risk factors for postoperative pancreatic fistula after DP
| Variable | No CR-POPF (N = 77) | CR-POPF (N = 38) | P value |
|---|---|---|---|
| Gender, n (%) | 0.908 | ||
| Male | 22 (28.6) | 12 (31.6) | |
| Female | 55 (71.4) | 26 (68.4) | |
| Age (y), n (%) | 0.514 | ||
| < 65 | 66 (85.7) | 30 (78.9) | |
| ≥ 65 | 11 (14.3) | 8 (21.1) | |
| BMI (kg/m2), n (%) | 0.004 | ||
| < 25 | 50 (64.9) | 13 (34.2) | |
| ≥ 25 | 27 (35.1) | 25 (65.8) | |
| Hypertension, n (%) | 0.024 | ||
| No | 69 (89.6) | 27 (71.1) | |
| Yes | 8 (10.4) | 11 (28.9) | |
| Diabetes, n (%) | 0.130 | ||
| No | 67 (87.0) | 28 (73.7) | |
| Yes | 10 (13.0) | 10 (26.3) | |
| Smoking, n (%) | 0.908 | ||
| No | 63 (81.8) | 30 (78.9) | |
| Yes | 14 (18.2) | 8 (21.1) | |
| Alcohol abuse, n (%) | 0.487 | ||
| No | 68 (88.3) | 31 (81.6) | |
| Yes | 9 (11.7) | 7 (18.4) | |
| Hemoglobin (g/L), median (IQR) | 130 (122–140) | 131 (124–138) | 0.917 |
| Prealbumin (mg/L), median (IQR) | 210 (191–253) | 192 (175–240) | 0.032 |
| Albumin (g/L), n (%) | 0.001 | ||
| ≥ 35 | 68 (88.3) | 22 (57.9) | |
| < 35 | 9 (11.7) | 16 (42.1) | |
| CA199 (kU/L), median (IQR) | 14.6 (5.1–36.0) | 28.9 (13.6–296) | 0.061 |
| Operation time (min), median (IQR) | 285 (240–345) | 314 (214–364) | 0.861 |
| Blood loss (mL), median (IQR) | 300 (200–500) | 320 (200–600) | 0.691 |
| Surgical approach, n (%) | 0.903 | ||
| Laparoscopic | 10 (13.0) | 6 (15.8) | |
| Open | 67 (87.0) | 32 (84.2) | |
| Ligation of main pancreatic duct, n (%) | 0.794 | ||
| Yes | 35 (45.5) | 19 (50.0) | |
| No | 42 (54.5) | 19 (50.0) | |
| Pancreatic stump treatment, n (%) | 0.791 | ||
| Endo GIA stapler | 19 (24.7) | 11 (28.9) | |
| Suture | 58 (75.3) | 27 (71.1) | |
| Pathology, n (%) | 0.715 | ||
| PDAC | 22 (28.6) | 14 (36.8) | |
| Cystic | 30 (39.0) | 15 (39.5) | |
| Pancreatitis | 7 (9.1) | 3 (7.9) | |
| Neuroendocrine | 11 (14.3) | 2 (5.3) | |
| SPTP | 6 (7.8) | 3 (7.9) | |
| Others | 1 (1.2) | 1 (2.6) | |
| Pancreas thickness (mm), median (IQR) | 16.4 (14.9–18.6) | 19.5 (16.8–22.5) | < 0.001 |
| Pancreas texture, n (%) | 0.001 | ||
| Hard | 55 (71.4) | 14 (36.8) | |
| Soft | 22 (28.6) | 24 (63.2) | |
| Splenectomy, n (%) | 0.693 | ||
| No | 36 (46.8) | 20 (52.6) | |
| Yes | 41 (53.2) | 18 (47.4) |
BMI body mass index; IQR interquartile range; CA199 cancerantigen199; PDAC pancreatic ductal adenocarcinoma; SPTP solid pseudopapillary tumor of the pancreas
Table 3.
Multivariate logistic regression analysis for postoperative pancreatic fistula after DP
| Variable | β | S.E. | Wald | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| BMI, kg/m2, (≥ 25 vs < 25) | 1.539 | 0.531 | 2.898 | 0.004 | 4.658 | 1.716–14.10 |
| Albumin, g/L, (< 35 vs ≥ 35) | 2.071 | 0.607 | 3.413 | 0.001 | 7.934 | 2.548–28.292 |
| Pancreas thickness, mm | 0.228 | 0.076 | 2.984 | 0.003 | 1.256 | 1.086–1.470 |
| Pancreas texture, (soft vs hard) | 1.145 | 0.495 | 2.314 | 0.021 | 3.143 | 1.203–8.497 |
BMI body mass index; β regression coefficient; S.E. standard error of regression coefficient; Wald Wald chi-square value; CI confidence interval; OR odds ratio
Construction of a predictive nomogram incorporating risk factors for CR-POPF
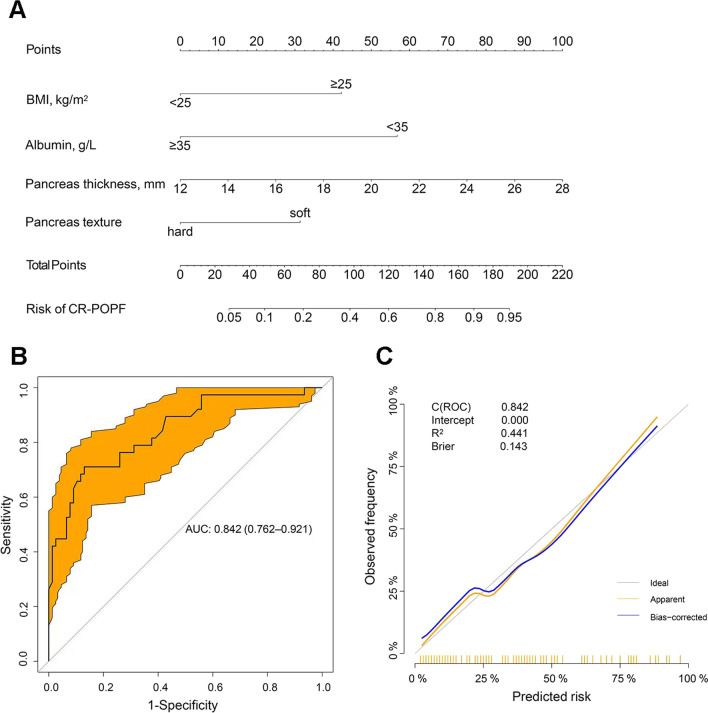
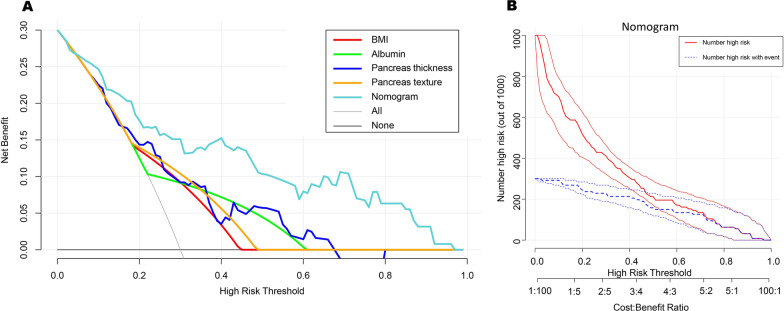
The independent risk factors associated with CR-POPF were used to construct a nomogram (Fig. 1A). The nomogram demonstrated good accuracy in estimating the risk of CR-POPF, with a C-index of 0.842 (95% CI 0.762–0.921) (Fig. 1B). Calibration plots graphically exhibited good consistency between actual observations and nomogram-predicted CR-POPF (Fig. 1C). The predictive value of the nomogram, including AUC, sensitivity, specificity, positive predictive value and negative predictive value, was compared with each risk factor in the present study. The optimal cut-off value of total nomogram scores was determined to be 102. The results showed that the C-index of the nomogram was 0.842 (95% CI 0.762–0.921), which was significantly higher than that of each indictor alone [BMI: 0.654 (95% CI 0.560–0.747), albumin: 0.652 (95% CI 0.565–0.739), pancreas thickness: 0.722 (95% CI 0.620–0.824), pancreas texture: 0.673 (95% CI 0.580–0.766)]. Compared to each indictor, the nomogram predicted CR-POPF with a sensitivity of 0.710 and specificity of 0.870, yielding a PPV of 0.730 and NPV of 0.859, indicating that the nomogram had better discriminatory performance (Table 4). Moreover, decision curve analysis (DCA) and clinical impact curve (CIC) were used to validate the clinical application value of the model. As shown in Fig. 2A, B, the nomogram also showed greater clinical net benefits, which further demonstrated that the nomogram had better predictive and accuracy values.
Fig. 1.
A Nomogram for preoperative prediction of CR-POPF following DP. Points indicate BMI, preoperative serum albumin level, pancreatic thickness and pancreatic texture. The score for each value was assigned by drawing a line upwards to the “Points” line, and the sum of the four scores was plotted on the “Total points” line (probability of CR-POPF). B Receiver operating characteristic (ROC) curves were used to evaluate the nomogram model performance. C The calibration curve of the nomogram model
Table 4.
Discriminatory performance of BMI, albumin, indication of surgery, pancreas thickness, pancreas texture, and the formulated nomogram for detecting patients with CR-POPF after PD
| Variables | AUC (95% CI) | Cut off | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| BMI | 0.654 (0.560–0.747) | – | 0.658 | 0.649 | 0.481 | 0.794 |
| Albumin | 0.652 (0.565–0.739) | – | 0.421 | 0.883 | 0.640 | 0.756 |
| Pancreas thickness | 0.722 (0.620–0.824) | 19.3 | 0.579 | 0.779 | 0.564 | 0.789 |
| Pancreas texture | 0.673 (0.580–0.766) | – | 0.632 | 0.714 | 0.522 | 0.797 |
| Nomogram | 0.842 (0.762–0.921) | 102 | 0.710 | 0.870 | 0.730 | 0.859 |
BMI body mass index; AUC area under the receiver-operating-characteristic curve; CI confidence interval; PPV positive predictive value; NPV negative predictive value
Fig. 2.
A Decision curve analysis of the nomogram model. B A clinical impact curve of the nomogram model
Discussion
CR-POPF following DP has been considered a potential precursor of more serious events. In the present study, the incidence of CR-POPF was 33.0%, which is similar to the results of previous studies [4, 10–18]. Our study also suggested that BMI, preoperative serum albumin level, pancreatic thickness and pancreatic texture are significantly associated with CR-POPF after DP.
Previous studies [38–40] have attempted to use drain fluid amylase levels on the first postoperative day to predict CR-POPF following DP, but further clinical validation is needed. There is a wide range in cut-off values between these studies, which would limit its wide use. One study [41] reported a postoperative score that incorporated four factors (i.e., operation time, BMI, amylase level on drains on postoperative Day 3 and pancreatic thickness) to predict the risk of developing CR-POPF. However, other risk factors that have been recognized as important for CR-POPF development were not included in the model. Nomograms, an easy-to-use prediction tool, have been widely used to predict clinical events. In the present study, we constructed a nomogram by incorporating four comprehensive and easily available variables. Importantly, this nomogram showed satisfactory discriminative ability and accuracy.
All four risk factors used to construct this nomogram have been reported in previous studies. A higher BMI is generally accepted as an important risk factor for the development of POPF following DP [18, 42]. It is well known that a higher BMI increases intraoperative technical difficulty and influences the physiology of the pancreas because of pancreatic fatty infiltration [43, 44]. Increased fat in the pancreas would intuitively increase the softness of the gland. Indeed, the soft pancreatic texture is known to be an important risk factor for fistula development following pancreatomy [17, 18]. Although there are no standardized criteria to define the texture of the pancreas, pancreatic texture has already been used to evaluate the risk of POPF after pancreatic resection, especially PD. Intraoperative ultrasound elastography may be useful for determining the pancreatic texture [45], but it is not routinely used in the operation, and its diagnostic performance needs to be further validated. In the present study, as a conventional approach, the pancreatic texture was determined by two experienced surgeons during the operation. Moreover, there is compelling evidence proving that the POPF rate increases as thickness increases [18, 36, 46]. Patients with a thicker pancreas would increase the technical difficulties of suturing or stapling, which may be why recent technological innovations do not significantly decrease the rate of CR-POPF after DP. Preoperative serum albumin levels have also been demonstrated to be a predictive factor for CR-POPF after DP [18]. Preoperative hypoalbuminemia is often correlated with increased morbidity after surgery, as reported in many studies [47, 48]. The explanations of this result include poor tissue healing, decreased collagen synthesis in surgical wounds, delayed return of bowel function and suppression of the systemic inflammatory response. Unsurprisingly, the proposed nomogram, which incorporated the abovementioned variables, performed well, as supported by the C index values of 0.842, and showed better discriminatory performance to determine CR-POPF, with a sensitivity of 0.710, specificity of 0.870, PPV of 0.730 and NPV of 0.859 when compared to each variable. Furthermore, the nomogram also presented a high clinical net benefit in predicting CR-POPF.
All variables used in this nomogram are universal and easily available. Based on the predictive accuracy of CR-POPF, we believe this nomogram model would help surgeons make reasonable decisions to prevent the occurrence of severe adverse events. First, patients with albumin < 35 g/L should have nutrition supplementation prior to surgery. Second, it is difficult to close the stump of the remnant pancreas completely when patients have a soft pancreas or a thick pancreas. A pancreatic duct stent was placed prior to surgery, and a combination of linear stapling plus continuous suturing of the stump would be optimal to decrease the incidence of CR-POPF. Third, this nomogram model could accurately stratify patients with different risks of CR-POPF, which enables surgeons to choose a reasonable drainage strategy. For patients with a low risk of CR-POPF, early drain removal should be performed safely, whereas patients with total scores greater than 102 should receive more attention and take more effective measures.
This study has some limitations that should be mentioned. First, this was a single-centre retrospective study with a limited sample size. Moreover, this model was not externally validated. Further validation needs to be performed in other institutions with large sample sizes. Second, other factors, which might be correlated with postoperative CR-POPF, such as drain fluid amylase level, were not included in this study. Finally, although the nomogram exhibited good predictive accuracy, the false-positive rate was 0.270, and the false-negative rate was 0.141 for predicting CR-POPF presence, which remains high if major clinical decisions are needed.
Conclusion
We identified BMI, preoperative serum albumin level, pancreatic thickness and pancreatic texture as the preoperative factors for CR-POPF following DP. By combining these preoperative factors, a nomogram was constructed. The nomogram provides an optimal preoperative estimation of CR-POPF presence in patients who undergo DP.
Acknowledgements
The authors thank Ph. Zhengqing Lei (Department of General Surgery, the affiliated Zhongda Hospital, Southeast University, Nanjing, China) for providing help with statistical details. The authors also thank Prof. Xingshun Qi (Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China) for critically revising the manuscript.
Abbreviations
- POPF
Postoperative pancreatic fistula
- DP
Distal pancreatectomy
- CR-POPF
Clinically relevant postoperative pancreatic fistula
- ROC
Receiver operating characteristic
- BMI
Body mass index
- FRS
Fistula risk score
- NGT
Nasogastric tube
- CT
Computed tomography
- IQR
Interquartile ranges
- DCA
Decision curve analysis
- CIC
Clinical impact curve
Author contributions
CH, YZ, YT and CW designed and performed the research and wrote the paper; LL and MZ collected the data; CH and YZ performed the statistical analysis; and CW and YT designed the study and revised the paper. All authors read and approved the final manuscript.
Funding
This study was supported by the Natural Science Foundation of Liaoning Province (Grant No. 20180551256) and the Postdoctoral Science Foundation of China (Grant No. 2018T111168).
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
The study was conducted in accord with the ethical standards of the Helsinki Declaration of 1975. This study was approved by the institutional Ethics Committee of the General Hospital of Northern Theater Command (No.: Y (2021) 056). Written informed consent was obtained from all the patients or patients’ relatives before the surgery.
Consent for publication
Not applicable.
Competing interests
The authors confirm that there are no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenchen He and Yibing Zhang have contributed equally to this work
Contributor Information
Chunhui Wang, Email: wangchh_2013@163.com.
Yufu Tang, Email: tangyufu0227@163.com.
References
- 1.Magge D, Gooding W, Choudry H, Steve J, Steel J, Zureikat A, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg. 2013;148(6):525–531. doi: 10.1001/jamasurg.2013.1673. [DOI] [PubMed] [Google Scholar]
- 2.Lee SY, Allen PJ, Sadot E, D’Angelica MI, DeMatteo RP, Fong Y, et al. Distal pancreatectomy: a single institution’s experience in open, laparoscopic, and robotic approaches. J Am Coll Surg. 2015;220(1):18–27. doi: 10.1016/j.jamcollsurg.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 3.van Hilst J, de Rooij T, Klompmaker S, Rawashdeh M, Aleotti F, Al-Sarireh B, et al. Minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA): a Pan-European Propensity Score Matched Study. Ann Surg. 2019;269(1):10–17. doi: 10.1097/SLA.0000000000002561. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. 2019;269(1):2–9. doi: 10.1097/SLA.0000000000002979. [DOI] [PubMed] [Google Scholar]
- 5.Bagaria SP, Swallow C, Suraweera H, Raut CP, Fairweather M, Cananzi F, et al. Morbidity and outcomes after distal pancreatectomy for primary retroperitoneal sarcoma: an analysis by the Trans-Atlantic Australasian Retroperitoneal Sarcoma Working Group. Ann Surg Oncol. 2021;28(11):6882–6889. doi: 10.1245/s10434-021-09739-9. [DOI] [PubMed] [Google Scholar]
- 6.Klompmaker S, de Rooij T, Koerkamp BG, Shankar AH, Siebert U, Besselink MG, et al. International validation of reduced major morbidity after minimally invasive distal pancreatectomy compared with open pancreatectomy. Ann Surg. 2021;274(6):e966–e973. doi: 10.1097/SLA.0000000000003659. [DOI] [PubMed] [Google Scholar]
- 7.Korrel M, Vissers FL, van Hilst J, de Rooij T, Dijkgraaf MG, Festen S, et al. Minimally invasive versus open distal pancreatectomy: an individual patient data meta-analysis of two randomized controlled trials. HPB (Oxford) 2021;23(3):323–330. doi: 10.1016/j.hpb.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Lof S, van der Heijde N, Abuawwad M, Al-Sarireh B, Boggi U, Butturini G, et al. Robotic versus laparoscopic distal pancreatectomy: multicentre analysis. Br J Surg. 2021;108(2):188–195. doi: 10.1093/bjs/znaa039. [DOI] [PubMed] [Google Scholar]
- 9.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Kawai M, Hirono S, Okada K, Sho M, Nakajima Y, Eguchi H, et al. Randomized controlled trial of pancreaticojejunostomy versus stapler closure of the pancreatic stump during distal pancreatectomy to reduce pancreatic fistula. Ann Surg. 2016;264(1):180–187. doi: 10.1097/SLA.0000000000001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uemura K, Satoi S, Motoi F, Kwon M, Unno M, Murakami Y. Randomized clinical trial of duct-to-mucosa pancreaticogastrostomy versus handsewn closure after distal pancreatectomy. Br J Surg. 2017;104(5):536–543. doi: 10.1002/bjs.10458. [DOI] [PubMed] [Google Scholar]
- 12.Aoki T, Mansour DA, Koizumi T, Matsuda K, Kusano T, Wada Y, et al. Preventing clinically relevant pancreatic fistula with combination of linear stapling plus continuous suture of the stump in laparoscopic distal pancreatectomy. BMC Surg. 2020;20(1):223. doi: 10.1186/s12893-020-00876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang JY, Shin YC, Han Y, Park JS, Han HS, Hwang HK, et al. Effect of polyglycolic acid mesh for prevention of pancreatic fistula following distal pancreatectomy: a randomized clinical trial. JAMA Surg. 2017;152(2):150–155. doi: 10.1001/jamasurg.2016.3644. [DOI] [PubMed] [Google Scholar]
- 14.Landoni L, De Pastena M, Fontana M, Malleo G, Esposito A, Casetti L, et al. A randomized controlled trial of stapled versus ultrasonic transection in distal pancreatectomy. Surg Endosc. 2021 doi: 10.1007/s00464-021-08724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Buren G, Bloomston M, Schmidt CR, Behrman SW, Zyromski NJ, Ball CG, et al. A prospective randomized multicenter trial of distal pancreatectomy with and without routine intraperitoneal drainage. Ann Surg. 2017;266(3):421–431. doi: 10.1097/SLA.0000000000002375. [DOI] [PubMed] [Google Scholar]
- 16.Fukami Y, Saito T, Osawa T, Hanazawa T, Kurahashi T, Kurahashi S, et al. Which is the best predictor of clinically relevant pancreatic fistula after pancreatectomy: drain fluid concentration or total amount of amylase? Ann Gastroenterol Surg. 2021;5(6):844–852. doi: 10.1002/ags3.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang GQ, Yadav DK, Jiang W, Hua YF, Lu C. Risk factors for clinically relevant postoperative pancreatic fistula (CR-POPF) after distal pancreatectomy: a single center retrospective study. Can J Gastroenterol Hepatol. 2021;2021:8874504. doi: 10.1155/2021/8874504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ecker BL, McMillan MT, Allegrini V, Bassi C, Beane JD, Beckman RM, et al. Risk factors and mitigation strategies for pancreatic fistula after distal pancreatectomy: analysis of 2026 resections from the International, Multi-institutional Distal Pancreatectomy Study Group. Ann Surg. 2019;269(1):143–149. doi: 10.1097/SLA.0000000000002491. [DOI] [PubMed] [Google Scholar]
- 19.Damen JA, Hooft L, Schuit E, Debray TP, Collins GS, Tzoulaki I, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi: 10.1136/bmj.i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh SM, Stefani KM, Kim HC. Development and application of chronic disease risk prediction models. Yonsei Med J. 2014;55(4):853–860. doi: 10.3349/ymj.2014.55.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM., Jr A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216(1):1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Mungroop TH, van Rijssen LB, van Klaveren D, Smits FJ, van Woerden V, Linnemann RJ, et al. Alternative Fistula Risk Score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg. 2019;269(5):937–943. doi: 10.1097/SLA.0000000000002620. [DOI] [PubMed] [Google Scholar]
- 23.Angrisani M, Sandini M, Cereda M, Paiella S, Capretti G, Nappo G, et al. Preoperative adiposity at bioimpedance vector analysis improves the ability of Fistula Risk Score (FRS) in predicting pancreatic fistula after pancreatoduodenectomy. Pancreatology. 2020;20(3):545–550. doi: 10.1016/j.pan.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed A, Nicolais L, Fitzgerald TL. Revisiting the Pancreatic Fistula Risk Score: clinical nomogram accurately assesses risk. Am Surg. 2021 doi: 10.1177/00031348211047471. [DOI] [PubMed] [Google Scholar]
- 25.Trudeau MT, Casciani F, Ecker BL, Maggino L, Seykora TF, Puri P, et al. The Fistula Risk Score Catalog: toward precision medicine for pancreatic fistula after pancreatoduodenectomy. Ann Surg. 2022;275(2):e463–e472. doi: 10.1097/SLA.0000000000004068. [DOI] [PubMed] [Google Scholar]
- 26.McMillan MT, Fisher WE, Van Buren G, McElhany A, Bloomston M, Hughes SJ, et al. The value of drains as a fistula mitigation strategy for pancreatoduodenectomy: something for everyone? Results of a randomized prospective multi-institutional study. J Gastrointest Surg. 2015;19(1):21–30. doi: 10.1007/s11605-014-2640-z. [DOI] [PubMed] [Google Scholar]
- 27.McMillan MT, Malleo G, Bassi C, Butturini G, Salvia R, Roses RE, et al. Drain management after pancreatoduodenectomy: reappraisal of a prospective randomized trial using risk stratification. J Am Coll Surg. 2015;221(4):798–809. doi: 10.1016/j.jamcollsurg.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 28.McMillan MT, Malleo G, Bassi C, Allegrini V, Casetti L, Drebin JA, et al. Multicenter, prospective trial of selective drain management for pancreatoduodenectomy using risk stratification. Ann Surg. 2017;265(6):1209–1218. doi: 10.1097/SLA.0000000000001832. [DOI] [PubMed] [Google Scholar]
- 29.Mungroop TH, Klompmaker S, Wellner UF, Steyerberg EW, Coratti A, D'Hondt M, et al. Updated Alternative Fistula Risk Score (ua-FRS) to include minimally invasive pancreatoduodenectomy: pan-European validation. Ann Surg. 2021;273(2):334–340. doi: 10.1097/SLA.0000000000003234. [DOI] [PubMed] [Google Scholar]
- 30.Shen J, Guo F, Sun Y, Zhao J, Hu J, Ke Z, et al. Predictive nomogram for postoperative pancreatic fistula following pancreaticoduodenectomy: a retrospective study. BMC Cancer. 2021;21(1):550. doi: 10.1186/s12885-021-08201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang XT, Huang CS, Liu C, Chen W, Cai JP, Cheng H, et al. Development and validation of a new nomogram for predicting clinically relevant postoperative pancreatic fistula after pancreatoduodenectomy. World J Surg. 2021;45(1):261–269. doi: 10.1007/s00268-020-05773-y. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Pu N, Chen Q, Mei Y, Wang D, Jin D, et al. Comprehensive diagnostic nomogram for predicting clinically relevant postoperative pancreatic fistula after pancreatoduodenectomy. Front Oncol. 2021;11:717087. doi: 10.3389/fonc.2021.717087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honselmann KC, Antoine C, Frohneberg L, Deichmann S, Bolm L, Braun R, et al. A simple nomogram for early postoperative risk prediction of clinically relevant pancreatic fistula after pancreatoduodenectomy. Langenbecks Arch Surg. 2021;406(7):2343–2355. doi: 10.1007/s00423-021-02184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez-Cruz L. Distal pancreatic resection: technical differences between open and laparoscopic approaches. HPB (Oxford) 2006;8(1):49–56. doi: 10.1080/13651820500468059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Study Group of Pancreatic Surgery in Chinese Society of Surgery of Chinese Medical A, Pancreatic Disease Committee of Chinese Research Hospital A, Editorial Board of Chinese Journal of S A consensus statement on the diagnosis, treatment, and prevention of common complications after pancreatic surgery (2017) Zhonghua Wai Ke Za Zhi. 2017;55(5):328–334. doi: 10.3760/cma.j.issn.0529-5815.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Jang JY, Son D, Lee S, Han Y, Shin YC, et al. Optimal stapler cartridge selection according to the thickness of the pancreas in distal pancreatectomy. Medicine (Baltimore) 2016;95(35):e4441. doi: 10.1097/MD.0000000000004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang ZY, Zhu Z, Zhang Y, Ni L, Lu B. A nomogram for predicting feasibility of laparoscopic anterior resection with trans-rectal specimen extraction (NOSES) in patients with upper rectal cancer. BMC Surg. 2021;21(1):296. doi: 10.1186/s12893-021-01290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maggino L, Malleo G, Bassi C, Allegrini V, Beane JD, Beckman RM, et al. Identification of an optimal cut-off for drain fluid amylase on postoperative day 1 for predicting clinically relevant fistula after distal pancreatectomy: a multi-institutional analysis and external validation. Ann Surg. 2019;269(2):337–343. doi: 10.1097/SLA.0000000000002532. [DOI] [PubMed] [Google Scholar]
- 39.Pecorelli N, Guarneri G, Palucci M, Gozzini L, Vallorani A, Crippa S, et al. Early biochemical predictors of clinically relevant pancreatic fistula after distal pancreatectomy: a role for serum amylase and C-reactive protein. Surg Endosc. 2022 doi: 10.1007/s00464-021-08883-3. [DOI] [PubMed] [Google Scholar]
- 40.Linnemann RJA, Patijn GA, van Rijssen LB, Besselink MG, Mungroop TH, de Hingh IH, et al. The role of abdominal drainage in pancreatic resection—a multicenter validation study for early drain removal. Pancreatology. 2019;19(6):888–896. doi: 10.1016/j.pan.2019.07.041. [DOI] [PubMed] [Google Scholar]
- 41.Rollin N, Cassese G, Pineton DE, Chambrun G, Serrand C, Navarro F, Blanc P, et al. An easy-to-use score to predict clinically relevant post-operative pancreatic fistula after distal pancreatectomy. Minerva Surg. 2021 doi: 10.23736/S2724-5691.21.09001-8. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, Drake J, Deneve JL, Behrman SW, Dickson PV, Shibata D, et al. Rising BMI Is associated with increased rate of clinically relevant pancreatic fistula after distal pancreatectomy for pancreatic adenocarcinoma. Am Surg. 2019;85(12):1376–1380. doi: 10.1177/000313481908501232. [DOI] [PubMed] [Google Scholar]
- 43.Kühn JP, Berthold F, Mayerle J, Völzke H, Reeder SB, Rathmann W, et al. Pancreatic steatosis demonstrated at MR imaging in the general population: clinical relevance. Radiology. 2015;276(1):129–136. doi: 10.1148/radiol.15140446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khoury T, Asombang AW, Berzin TM, Cohen J, Pleskow DK, Mizrahi M. The clinical implications of fatty pancreas: a concise review. Dig Dis Sci. 2017;62(10):2658–2667. doi: 10.1007/s10620-017-4700-1. [DOI] [PubMed] [Google Scholar]
- 45.Kawabata Y, Okada T, Iijima H, Yoshida M, Iwama H, Xu J, et al. Intraoperative ultrasound elastography is useful for determining the pancreatic texture and predicting pancreatic fistula after pancreaticoduodenectomy. Pancreas. 2020;49(6):799–805. doi: 10.1097/MPA.0000000000001576. [DOI] [PubMed] [Google Scholar]
- 46.Kang MK, Kim H, Byun Y, Han Y, Choi YJ, Kang JS, et al. Optimal stapler cartridge selection to reduce post-operative pancreatic fistula according to the pancreatic characteristics in stapler closure distal pancreatectomy. HPB (Oxford) 2021;23(4):633–640. doi: 10.1016/j.hpb.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara Y, Shiba H, Shirai Y, Iwase R, Haruki K, Furukawa K, et al. Perioperative serum albumin correlates with postoperative pancreatic fistula after pancreaticoduodenectomy. Anticancer Res. 2015;35(1):499–503. [PubMed] [Google Scholar]
- 48.Kelly KJ, Greenblatt DY, Wan Y, Rettammel RJ, Winslow E, Cho CS, et al. Risk stratification for distal pancreatectomy utilizing ACS-NSQIP: preoperative factors predict morbidity and mortality. J Gastrointest Surg. 2011;15(2):250–259. doi: 10.1007/s11605-010-1390-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.