Abstract
COVID‐19 vaccine is critical in preventing SARS‐CoV‐2 infection and transmission. However, obesity's effect on immune responses to COVID‐19 vaccines is still unknown. We performed a meta‐analysis of the literature and compared antibody responses with COVID‐19 vaccines among persons with and without obesity. We used Pubmed, Embase, Web of Science, and Cochrane Library to identify all related studies up to April 2022. The Stata.14 software was used to analyze the selected data. Eleven studies were included in the present meta‐analysis. Five of them provided absolute values of antibody titers in the obese group and non‐obese group. Overall, we found that the obese population was significantly associated with lower antibody titers (standardized mean difference [SMD] = −0.228, 95% CI [−0.437, −0.019], P < 0.001) after COVID‐19 vaccination. Significant heterogeneity was present in most pooled analyses but was reduced after subgroup analyses. No publication bias was observed in the present analysis. The Trim and Fill method did not change the results in the primary analysis. The present meta‐analysis suggested that obesity was significantly associated with decreased antibody responses to SARS‐CoV‐2 vaccines. Future studies should be performed to unravel the mechanism of response to the COVID‐19 vaccine in obese individuals.
Keywords: COVID‐19 vaccine, meta‐analysis, obesity
1. INTRODUCTION
Coronavirus disease (COVID‐19) is an infectious disease caused by acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). It is prevalent worldwide, with over 48 million confirmed cases and over 6.1 million deaths reported globally. 1 In the present COVID‐19 pandemic, individuals with obesity have an increased risk of testing positive for SARS‐CoV‐2, with the severity of COVID‐19, and with COVID‐19 mortality. 2 , 3 , 4 , 5 , 6 The exact mechanisms underlying the strong association between obesity and COVID‐19 were not clarified to date. At present, it has been documented that due to increased expression of proteins facilitating viral entry into cells and hyper‐glycosylation of those proteins, patients with obesity have an increased risk of becoming infected with SARS‐CoV‐2. In addition, due to an impaired pulmonary immune response, hyper‐inflammatory systemic responses, increased risk of thrombosis, and increased viral load, patients with obesity also develop severe complications upon SARS‐CoV‐2 infection with significant morbidities and mortalities. 7 Currently, different vaccines against SARS‐CoV‐2 have been implemented worldwide to reduce COVID‐19 cases. However, obesity is associated with reduced memory immune responses. 8 It is unknown whether the protection would be affected by this reduced immune response.
Several vaccines have been designed against the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus, with different mechanisms of action. The main classes of coronavirus vaccines in research and development include (1) messenger ribonucleic acid (mRNA) vaccines. In December 2020, the FDA authorized the emergency use of two SARS‐CoV‐2 mRNA vaccines that utilize a 2‐dose schedule: BNT162b2/Pfizer and mRNA‐1273/Moderna. 9 , 10 (2) Viral vector vaccines: The Oxford‐AstraZeneca COVID‐19 vaccine, also known as AZD1222 or ChAdOx1 nCoV‐19 (ChAdOx1), was one of the earliest authorized. 11 , 12 (3) Inactivated vaccines and subunit vaccines: The inactivated virus cannot replicate but can still produce immunogenicity. Subunit vaccines usually contain protein or peptide antigens derived from pathogens. 13
The efficacy of a vaccine is determined by the difference in the incidence of specific diseases among vaccinated and unvaccinated subjects for the disease. The immune responses to vaccines are evaluated by serological and/or immunological markers. The immune response could be conveniently partitioned into innate and adaptive immunity, in which the adaptive immunity is divided into 2 classes, including cell‐mediated and humoral responses. 14 Humoral responses, only a part of immune responses, are easier to detect than other responses because of wide availability and standardization. The COVID‐19 vaccine induces detectable humoral antibodies against different antigens of severe acute SARS‐CoV‐2. One of the major immunogenic antigens in the post‐vaccine immune response is the transmembrane Spike (S), a receptor‐binding domain (RBD) that protrudes from the surface of the spherical virions and mediates virus entry into host cells. 15 However, the antibody titers after vaccination remain significantly unpredictable, and many factors may influence it. Age, 16 gender, 17 and the number of doses 18 have been shown to influence antibody titers following COVID‐19 vaccination.
Several systematic reviews of studies on COVID‐19 vaccine efficacy and effectiveness have been published. 19 , 20 , 21 , 22 Nevertheless, none focused on the efficacy of COVID‐19 vaccination in people with obesity. Therefore, the present study sought to investigate the associations between obesity and serum antibodies after COVID‐19 vaccination.
2. METHOD
This study was designed following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 23 The protocol of this systematic review was registered in PRISMA (CRD42022373514).
3. SEARCH STRATEGY
A systematic search in four online scientific databases (PubMed, EMBASE, Web of Science, and Cochrane Library) was performed in April 2022. The following search key was used in all databases without filters or restrictions: “COVID‐19 Vaccines or SARS‐CoV‐2 Vaccine” and “obesity or overweight or body mass index.” We have provided a detailed search strategy in Table S1.
4. SELECTION AND ELIGIBILITY CRITERIA
Four independent reviewers (XDO, JLL, BQL, and QYL) screened the titles and abstracts of identified studies for eligibility. The included studies met the following eligibility criteria: (1) Participants were previously COVID‐19 vaccinated; (2) studies included SARS‐CoV‐2 antibody levels; (3) the obesity prevalence or body mass index (BMI) data of the patients were reported. Studies were excluded for the following reasons: (1) Participants included pregnant women, cancer patients, and patients with immunodeficiency; (3) the outcomes did not include antibody titer data; (3) results were not stratified by BMI category.
5. DATA EXTRACTION
For eligible studies, both review authors independently extracted the data. All disagreements were resolved by the third investigator (Junping Wen). The following data were extracted from each included study: first author, publication year, country, vaccine type, number of patients in each reported BMI range, days after vaccination, history of the previous infection with COVID‐19, and outcomes. In this meta‐analysis, quality assessment of included studies was conducted using the criteria of the Newcastle‐Ottawa Scale (NOS). 24 The NOS scores ranged from 0 to 9. A study with NOS scores ≥6 was indicated to be of high quality.
6. STATISTICAL ANALYSIS
A meta‐analysis was performed by Stata14.0 software (Stata Corp, College Station, TX, USA). The primary outcomes of this analysis were the titers of post‐vaccination antibodies presented as mean with standard deviation. The I 2 statistic was used to evaluate heterogeneity across the studies. When I 2 < 50%, the fixed effect model was used to combine data sets. Otherwise, the random effect model was applied. We planned to carry out the following subgroup analyses for the primary outcomes. Moreover, we conducted a subgroup meta‐analysis when heterogeneity was ultra (I 2 > 50%). The following subgroup analyses were planned for the primary outcomes: vaccine type, elapsed time since vaccination, and history of previous SARS‐CoV‐2 infection. To assess the robustness of the combined results and evaluate the effect of individual studies on this meta‐analysis, we conducted a sensitivity analysis. Subsequently, the publication bias was assessed by the Funnel Plots analysis, and Egger's regression asymmetry test was further complemented. The Trim and Fill method was used to adjust the significant publication bias.
7. RESULTS
We identified 1131 potentially relevant records through literature searches; 145 were duplicated articles and were excluded. After title and abstract screening, we retrieved 193 full‐text reports for further review. Finally, 11 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 articles met our inclusion criteria. All included studies were of high quality (NOS > 6). The selection of the literature is summarized in Figure 1. To reduce error and explore the differences between the groups, we decided not to pool the data.
FIGURE 1.
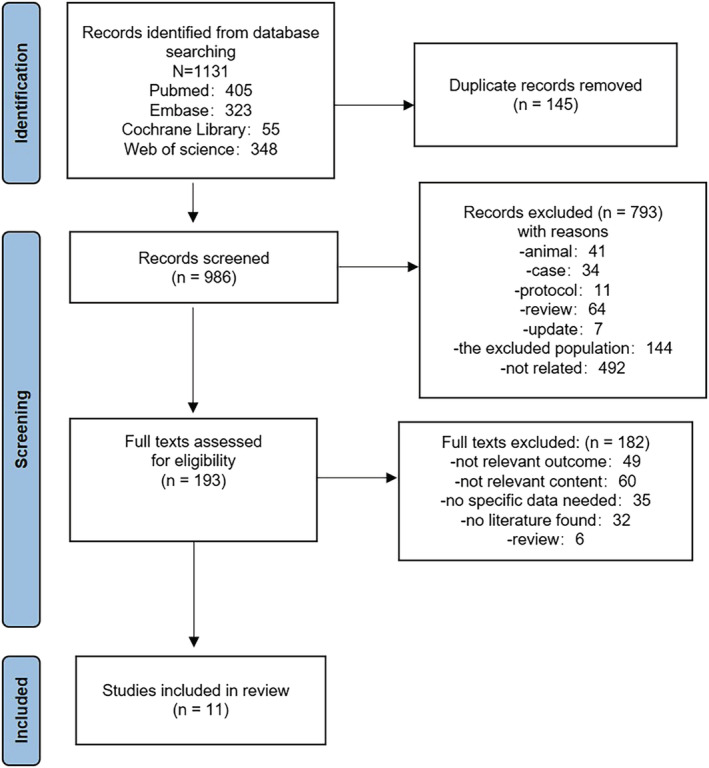
Flowchart of literature screening
7.1. Characteristics of the included studies
Characteristics of participants included in the final analysis are shown in Table 1. A total of 7956 participants were included in the meta‐analysis. The age range was 18–73 years. Of these individuals, 1869 were obese. Out of 12 articles, four 25 , 26 , 27 , 34 articles reported the mRNA vaccines (BNT162b2): two 28 , 35 that of adenovirus‐vector vaccines (ChAdOx1 nCoV‐19) and two 29 , 31 that of inactivated virus vaccines (Sinovac, Sinopharm). The remaining four 31 , 32 , 33 articles do not exclusively contain one type of vaccine. The range of publication years of the included studies was 2021–2022.
TABLE 1.
Basic characteristics of the included studies
| Num | Study, year | N (obesity) | Age (year) | Country | Vaccine | Assessment after administration | History of COVID‐19 infection, n | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 27 | Alexis, 2021 | 1060 (492) | 41.42 ± 12.95 | Italy | BNT162b2 | The first dose, the second dose | Yes, n = 240 |
|
| 2 26 | Raul, 2021 | 248 (22) | 23–69 | Italy | BNT162b2 | The second dose | No |
|
| 3 28 | Awadhesh, 2021 | 552 (67) | 44.8 ± 13.1 | India | ChAdOx1 nCoV‐19 | The first dose, the second dose | Yes, n = 60 |
|
| 4 25 | Shohei Yamamoto, 2022 | 2435 (66) | / | Japan | BNT162b2 | The second dose | Yes, n = 13 |
|
| 5 35 | Jer‐Hwa Chang, 2022 | 270 (59) | 23–68 | China | ChAdOx1 nCoV‐19 | The first dose, the second dose | No |
|
| 6 34 | Andrea Lombardi, 2021 | 1218 (187) | / | Italy | BNT162b2 | Second dose | Undetermined |
|
| 7 33 | Engy Mohamed El‐Ghitany, 2022 | 143 (117) | 43 ± 11.1 | Egypt | AZD1222/BBIBP‐CorV/others | Second dose | Yes, n = 143 |
|
| 8 32 | Rami Alqassieh, 2021 | 288 (249) | / | Jordan | Pfizer‐BioNTech/Sinopharm | Second dose | Yes, n = 8 |
|
| 9 31 | Xiaoguang Li, 2021 | 127 (44) | 22–73 | China | Inactivated vaccines/vector | Second dose | No |
|
| 10 30 | Hui Zhang, 2022 | 1156 (356) | 18–60 | China | Inactivated vaccine | Second dose | No |
|
| 11 29 | İlker İnanc Balkan, 2022 | 169 (86) | 22–66 | China | CoronaVac (Sinovac) | Second dose | Yes, n = 37 |
|
7.2. Relationship between obesity and antibody titers of COVID‐19 vaccination
Among 11 selected studies, five 25 , 26 , 27 , 28 , 35 studies provided absolute values of antibody titer in the obese group and non‐obese group, and two 26 , 27 studies offered the baseline antibody titers. The results of the meta‐analysis of the effectiveness indicator were as follows, and the random‐effects model was used for analysis: When compared with the non‐obese group, the antibody titers of the obese group were lower (SMD = −0.228, 95% CI [−0.437, −0.019], P < 0.001) (Figure 2). However, due to the high heterogeneity (I 2 = 87.9%), sensitivity analysis found that three subgroups of one 27 article had the large offsets but still within the credible interval (Figure S1). We carefully reviewed the full text of this article. It was found that the population was infected with COVID‐19 previously. In addition, the following subgroups were performed in several aspects: vaccine type, elapsed time since vaccination, and history of previous SARS‐CoV‐2 infection.
FIGURE 2.
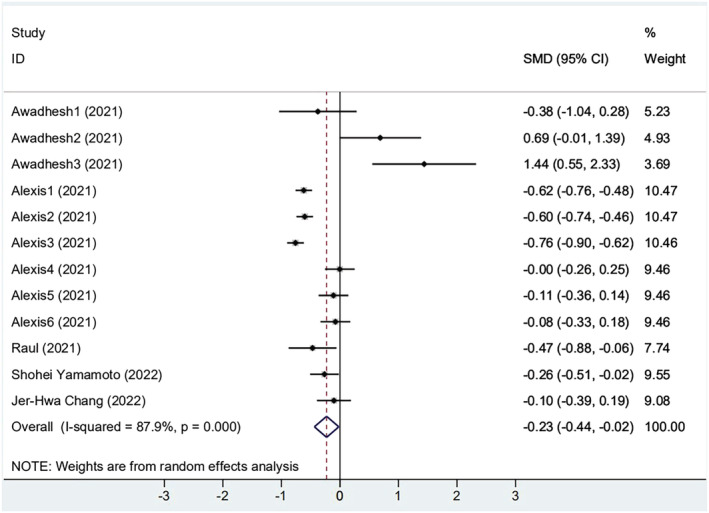
The forest plot of antibody titers
Among the five included articles, three 25 , 26 , 27 reported BNT162b2 mRNA vaccination, and two 28 , 35 were vaccinated with ChAdOx1 nCoV‐19 vaccine. As the results show, both vaccinations were significantly associated with obesity (Figure 3A). However, in the participants receiving the CoronaVac vaccine, antibody titers were higher in the obese group than in the non‐obese group (SMD = 0.340, 95% CI [−0.333, 1.013], P = 0.002). We found that most subjects received the first dose in the group of ChAdOx1 nCoV‐19 vaccination. Next, two subgroups were designed by a different number of vaccinations: Group 1 received only one dose of vaccine; group 2 received 2 doses of vaccine. We found that two groups were significantly correlated with obesity. Antibody titers in the obese group were lower than those in the non‐obese group (Dose 1: SMD = −0.133, 95% CI [−0.636, 0.371], P < 0.001; Dose 2: SMD = −0.272, 95% CI [−0.548, 0.005], P < 0.001) (Figure 3B). It both showed lower, but the second dose was more pronounced than the first. The articles were divided into two groups according to the presence or absence of COVID‐19 infection. As shown in Figure 3C, the two groups' heterogeneity decreased. The results showed that the subgroups explained part of the heterogeneity, but notable between‐study heterogeneity still existed, so the random effect model processed the data.
FIGURE 3.
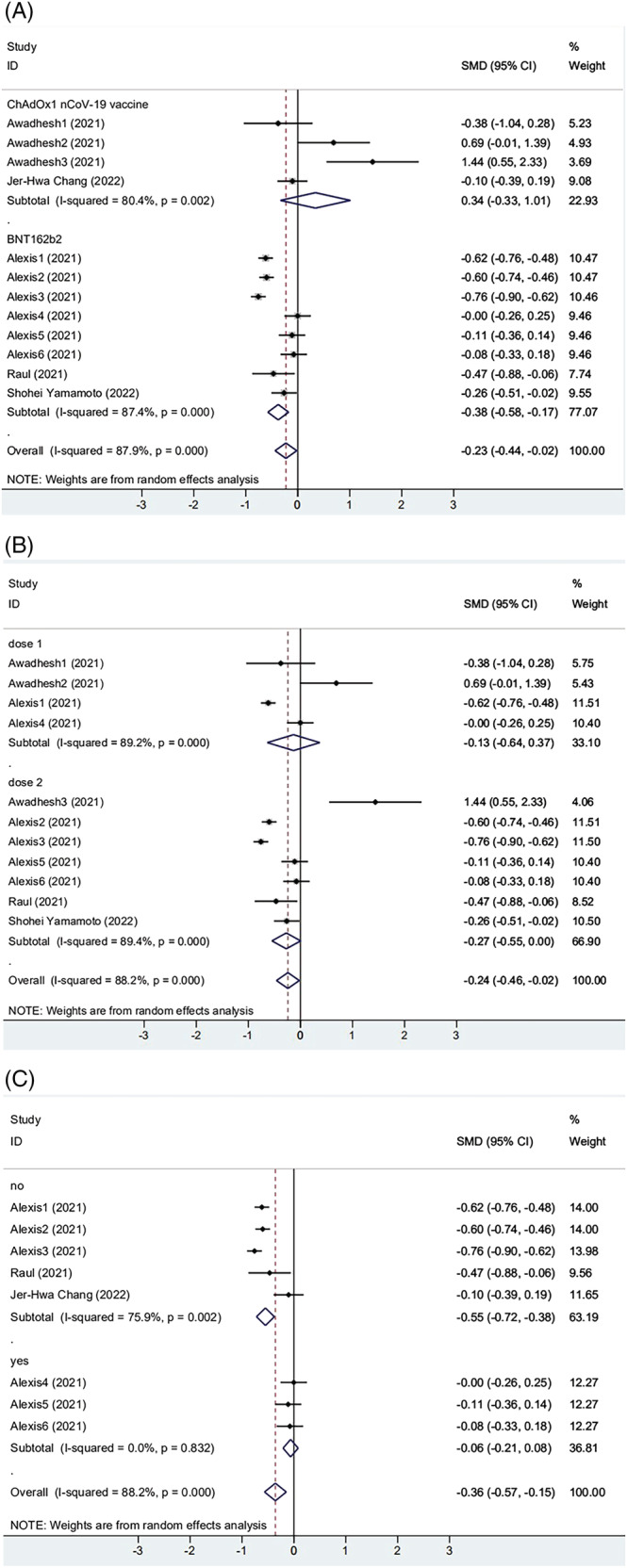
(A) The forest plot of antibody titers induced by different vaccines. (B) The forest plot of antibody titers after different doses of vaccination. (C) The forest plot of antibody titers from different populations
Two studies reported the baseline antibody titers. One of these contained six small groups. As shown in Figure 4, participants with obesity increase had a smaller increase of antibody titer compared with the non‐obese group (SMD = −0.465, 95% CI [−0.612, −0.318], P = 0.001), yet heterogeneity was still present (I 2 = 73.5%). Sensitivity analysis of the meta‐analysis showed that they deviated symmetrically from the center axis (Figure S2). The result indicated that patients infected with COVID‐19 already had a certain antibody level. Not only that, antibody levels in individuals with obesity were higher. The above results show that immune response to the vaccine seems to be affected by the elapsed time since vaccination and history of previous SARS‐CoV‐2 infection.
FIGURE 4.
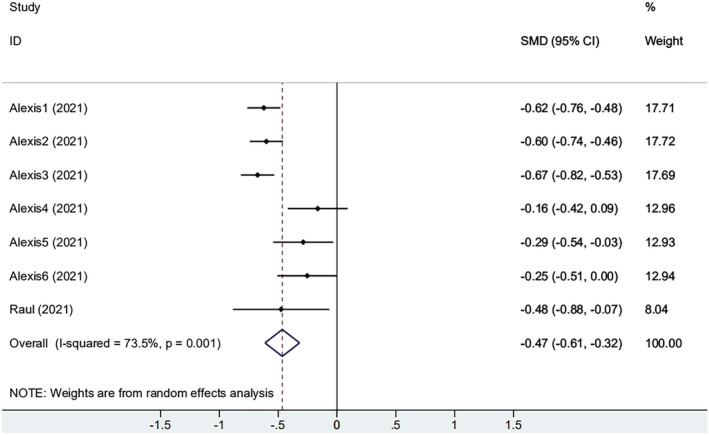
The forest plot of change from baseline of antibody titers
7.3. Relationship between obesity and seroprevalence of antibodies after COVID‐19 vaccination
Further, we analyzed how the immune response of the obese group differed from that of the control groups. Among the 11 papers, five reported differences in antibody positivity rates between obese and control groups. The antibody positivity rate of the obese was lower than that of the overall control group, with an odds ratio (OR) of 0.965 (95% CI, 0.662–1.406) (Figure 5). When BMI = 25 was used as a cut‐off value, the results showed that the immunogenicity of overweight individuals was significantly lower than that of controls (pooled OR from seven studies, 0.874; 95% CI, 0.602–1.267, P = 0.001) (Figure 5). The former is not statistically significant, and the latter is statistically significant, which could be attributed to the small sample size of obese groups.
FIGURE 5.
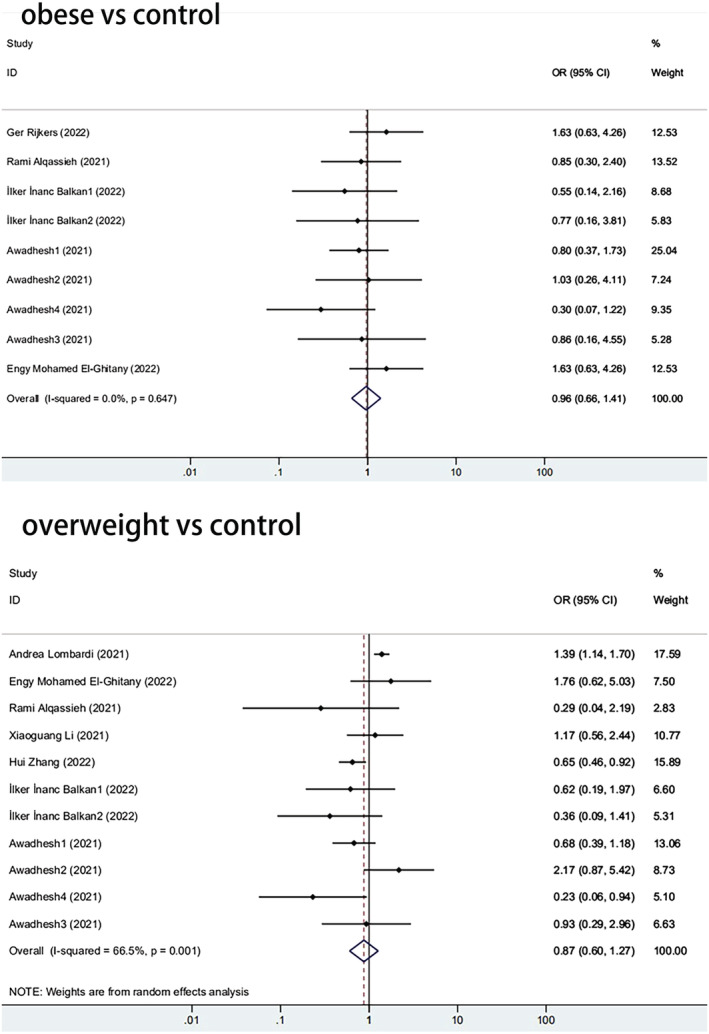
The forest plot of antibody positivity rates
7.4. Risk of bias of included studies
In the meta‐analysis of antibody positivity rates, the funnel plot was not strongly suggestive of publication bias (Figure 6). Findings of Egger's test (P = 0.290, P = 0.138) suggested that publication bias was not significant. Furthermore, we applied the trim‐and‐fill method to this meta‐analysis by using Metatrim. It has been presented in Figures S3 and S4, and the results showed that no trimming was performed, further suggesting no publication bias. Also, we adopted the trim‐and‐fill method to the meta‐analysis of antibody titers, and six assumed studies with favorable effects were added, and the pooled result was still robust (SMD = −0.581, 95% CI [−0.803, 0.358], P < 0.001) (Figure 7).
FIGURE 6.
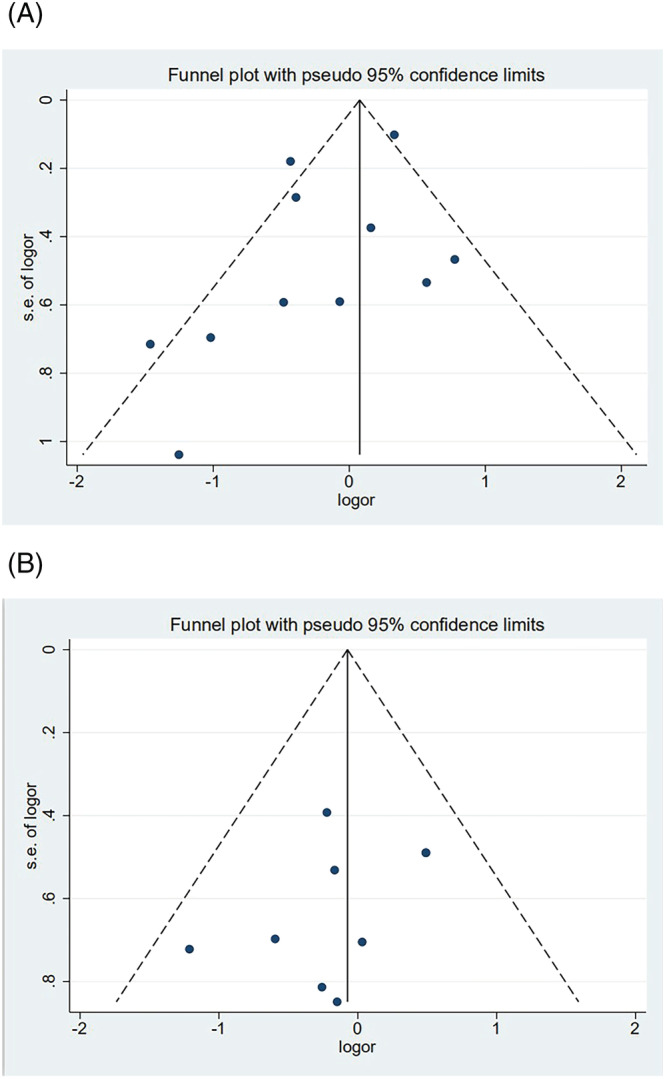
(A) The forest plot of antibody positivity rates compared with the overweight population. (B) The forest plot of antibody positivity rates compared with the obese population
FIGURE 7.
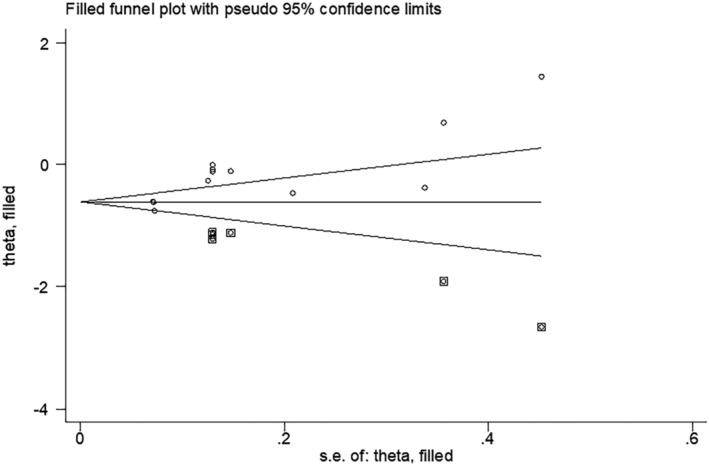
Findings of Egger's test after the Trim and Fill method
8. DISCUSSION
To further understand the impaired responses to COVID‐19 vaccination, more and more studies were performed to evaluate the effectiveness of the new coronavirus vaccine in different populations, including immunocompromised patients, 36 children and adolescents, 37 older adults, 38 and pregnant women. 39 This systematic review and meta‐analysis identified 11 studies evaluating the association between patients with obesity and the receipt of COVID‐19 vaccination.
In this study, our results suggested that antibody titers were low in the obese group than that in the non‐obese group (SMD = −0.228, 95% CI [−0.437, −0.019], P < 0.001). Moreover, compared with persons with a less than 25 of BMI, people with a BMI greater than 25 had lower antibody positivity rates after being immunized with the COVID‐19 vaccine (OR: 0.874, 95% CI: 0.602–1.267, P = 0.001). Additionally, visual inspection of funnel plots revealed no obvious indication of publication bias, and statistical investigation of publication bias, using the Egger intercept, was nonsignificant. In the present study of antibody titers, applying the trim‐and‐fill method by using the Trim and Fill method indicated that our conclusions were reliable because the results remained unchanged after six assumed studies. However, we observed a higher heterogeneity among 5 included studies about antibody titers. By performing subgroup analyses, we found that the heterogeneity might be caused by vaccine type, elapsed time since vaccination, and history of previous SARS‐CoV‐2 infection. Heterogeneity was lower after subgroup analysis compared with pre‐subgroup analysis.
This study shows that being overweight or obese is associated with lower antibody titers after COVID‐19 mRNA vaccination. Obesity is a metabolic problem and a chronic inflammatory disease. It increases the risk of metabolic disorders such as hyperglycemia, hyperlipidemia, hypercholesterolemia, and diabetes. 40 Accumulating excess fat causes chronic inflammation, damages the immune system, and thus impairs antibody formation. 41 , 42 Accumulating excess fat causes chronic inflammation and damages the immune system, thus impairing antibody formation. The adipose tissue in obese individuals results in systemic inflammation, which increases the serum levels of diversified proinflammatory chemokines, adipokines, and cytokines. The adipose tissue in obese individuals results in systemic inflammation, which increases the serum levels of diversified proinflammatory chemokines, adipokines, and cytokines. 43 The chronic low‐grade state of inflammation disrupts the immune response in obese persons. In obese individuals, chronic inflammation develops due to dysfunctional adipose tissue, negatively affecting T‐cell function, antibody responses, and macrophage migration. 44 Compared with individuals with normal BMI, ACE2 receptor expression is significantly higher in adipose tissue in obese individuals. 45 , 46 , 47 ACE2 is a major receptor of the spike protein of SARS‐CoV‐2, and polymorphisms of the ACE2 gene modulate the susceptibility of SARS‐CoV‐2 infection via an elevation in the expression level of ACE2. 48 Thus, it is hypothesized that immune dysfunction increases the risk of SARS‐CoV‐2 infection and reduces vaccine response in severely obese individuals.
In addition, obesity is associated with reduced immunogenicity after hepatitis B, 49 tetanus, 50 and influenza 51 , 52 , 53 vaccination. A recent study demonstrated that higher BMI is associated with lower Ab titers in response to the COVID‐19 vaccine in Italian healthcare workers. 54 Thus, despite encouraging COVID‐19 vaccination results, obese patients may still be vulnerable to reinfection with SARS‐CoV‐2 long term, affecting herd immunity and SARS‐CoV‐2 elimination. In conclusion, long‐term COVID‐19 vaccine efficacy in these patients should be closely monitored to limit further effects of COVID‐19 on patients and society with obesity.
9. CONCLUSIONS
In summary, the present meta‐analysis suggested that obesity is significantly associated with the decreased antibody response to COVID‐19 vaccines. Patients with obesity generated significantly reduced antibody titers after COVID‐19 vaccines compared to people with normal weight. Future studies should be performed to unravel this relationship to prevent COVID‐19 infection and transmission. Our research results will lay the foundation for further meaningful research. Future studies will conduct in‐depth research on the mechanism of response to the COVID‐19 vaccine in obese individuals.
CONFLICT OF INTEREST
Xiaodan Ou, Jialin Jiang, Bingqian Lin, Qinyu Liu, Wei Lin, Gang Chen, and Junping Wen declare that they have no conflict of interest. The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
Xiaodan Ou: Conceptualization; data curation; formal analysis; investigation; methodology; writing‐original draft. Jialin Jiang: Conceptualization; data curation; formal analysis. Bingqian Lin: Conceptualization; data curation; formal analysis. Qinyu Liu: Conceptualization; data curation; formal analysis. Wei LIN: Supervision. Gang Chen: Supervision. Junping Wen: Investigation; supervision; writing‐review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/irv.13078.
Supporting information
Figure S1. Sensitivity analysis of antibody titers.
Figure S2. Sensitivity analysis of change from baseline of antibody titers.
Figure S3. Egger's test of antibody positivity rates compared with overweight population.
Figure S4. Egger's test of antibody positivity rates compared with obese population.
Table S1. Details of the search history through PubMed.
ACKNOWLEDGMENTS
The study was funded by the National Key Research and Development Program of China (2018YFC2001100‐5), Natural Science Foundation of China (No. 81770848 and 82070878), and Natural Science Foundation of Fujian Province (Grant No. 2020J011081 and 2021J01381).
Ou X, Jiang J, Lin B, et al. Antibody responses to COVID‐19 vaccination in people with obesity: A systematic review and meta‐analysis. Influenza Other Respi Viruses. 2023;17(1):e13078. doi: 10.1111/irv.13078
Xiaodan Ou, Jialin Jiang, Bingqian Lin and Qianyu Liu contributed equally to this study.
Contributor Information
Gang Chen, Email: chengangfj@163.com.
Junping Wen, Email: junpingwen@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author (JPW), upon reasonable request.
REFERENCES
- 1. Who . Coronavirus (COVID‐19). https://www.worldometers.info/coronavirus/, 2022: Accessed Mrach 30 2022.
- 2. Aghili SMM, Ebrahimpur M, Arjmand B, et al. Obesity in COVID‐19 era, implications for mechanisms, comorbidities, and prognosis: a review and meta‐analysis. Int J Obes (Lond). 2021;45(5):998‐1016. doi: 10.1038/s41366-021-00776-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett TD, Moffitt RA, Hajagos JG, et al. The national COVID cohort collaborative: clinical characterization and early severity prediction. medRxiv. 2021. [Google Scholar]
- 4. Ho JSY, Fernando DI, Chan MY, Sia CH. Obesity in COVID‐19: a systematic review and meta‐analysis. Ann Acad Med Singapore. 2020;49(12):996‐1008. doi: 10.47102/annals-acadmedsg.2020299 [DOI] [PubMed] [Google Scholar]
- 5. Eastment MC, Berry K, Locke E, et al. BMI and outcomes of SARS‐CoV‐2 among US veterans. Obesity (Silver Spring). 2021;29(5):900‐908. doi: 10.1002/oby.23111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peres KC, Riera R, Martimbianco ALC, Ward LS, Cunha LL. Body mass index and prognosis of COVID‐19 infection. A systematic review. Front Endocrinol. 2020;11:562. doi: 10.3389/fendo.2020.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westheim AJF, Bitorina AV, Theys J, Shiri‐Sverdlov R. COVID‐19 infection, progression, and vaccination: focus on obesity and related metabolic disturbances. Obes Rev. 2021;22(10):e13313. doi: 10.1111/obr.13313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID‐19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. doi: 10.1111/obr.13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383(25):2439‐2450. doi: 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vergnes JN. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2021;384(16):1577. doi: 10.1056/NEJMc2036242 [DOI] [PubMed] [Google Scholar]
- 11. Hyams C, Marlow R, Maseko Z, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV‐19 COVID‐19 vaccination at preventing hospitalisations in people aged at least 80 years: a test‐negative, case‐control study. Lancet Infect Dis. 2021;21(11):1539‐1548. doi: 10.1016/S1473-3099(21)00330-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agency. M H P . Conditions of Authorisation for COVID‐19 Vaccine AstraZeneca (Regulation 174). https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/conditions-of-authorisation-for-covid-19-vaccine-astrazeneca
- 13. Flower DR. Designing immunogenic peptides. Nat Chem Biol. 2013;9(12):749‐753. doi: 10.1038/nchembio.1383 [DOI] [PubMed] [Google Scholar]
- 14. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S3‐S23. doi: 10.1016/j.jaci.2009.12.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang C, Wang Y, Zhu Y, et al. Development and structural basis of a two‐MAb cocktail for treating SARS‐CoV‐2 infections. Nat Commun. 2021;12(1):264. doi: 10.1038/s41467-020-20465-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID‐19 vaccines in older people. Age Ageing. 2021;50(2):279‐283. doi: 10.1093/ageing/afaa274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Demonbreun AR, Sancilio A, Velez ME, et al. COVID‐19 mRNA vaccination generates greater immunoglobulin G levels in women compared to men. J Infect Dis. 2021;224(5):793‐797. doi: 10.1093/infdis/jiab314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mattiuzzi C, Lippi G. Primary COVID‐19 vaccine cycle and booster doses efficacy: analysis of Italian nationwide vaccination campaign. Eur J Public Health. 2022;32(2):328‐330. doi: 10.1093/eurpub/ckab220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harder T, Koch J, Vygen‐Bonnet S, et al. Efficacy and effectiveness of COVID‐19 vaccines against SARS‐CoV‐2 infection: interim results of a living systematic review, 1 January to 14 may 2021. Euro Surveill. 2021;26(28). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harder T, Külper‐Schiek W, Reda S, et al. Effectiveness of COVID‐19 vaccines against SARS‐CoV‐2 infection with the Delta (B.1.617.2) variant: second interim results of a living systematic review and meta‐analysis, 1 January to 25 August 2021. Euro Surveill. 2021;26(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kow CS, Hasan SS. Real‐world effectiveness of BNT162b2 mRNA vaccine: a meta‐analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075‐1090. doi: 10.1007/s10787-021-00839-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pormohammad A, Zarei M, Ghorbani S, et al. Effectiveness of COVID‐19 vaccines against Delta (B.1.617.2) variant: a systematic review and meta‐analysis of clinical studies. Vaccines (Basel). 2021;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wells GA, Shea B, O'connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses 2021. 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 25. Yamamoto S, Mizoue T, Tanaka A, et al. Sex‐associated differences between BMI and SARS‐CoV‐2 antibody titers following the BNT162b2 vaccine. Obesity (Silver Spring). 2022;30(5):999‐1003. doi: 10.1002/oby.23417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pellini R, Venuti A, Pimpinelli F, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS‐CoV‐2 BNT162b2 vaccine. EClinicalMedicine. 2021;36:100928. doi: 10.1016/j.eclinm.2021.100928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malavazos AE, Milani V, Cardani R, et al. Antibody responses to BNT162b2 mRNA vaccine: infection‐naïve individuals with abdominal obesity warrant attention. Obesity. 2022;30(3):606‐613. doi: 10.1002/oby.23353 [DOI] [PubMed] [Google Scholar]
- 28. Singh AK, Phatak SR, Singh R, et al. Antibody response after first and second‐dose of ChAdOx1‐nCOV (Covishield (TM)®) and BBV‐152 (Covaxin (TM)®) among health care workers in India: the final results of cross‐sectional coronavirus vaccine‐induced antibody titre (COVAT) study. Vaccine. 2021;39(44):6492‐6509. doi: 10.1016/j.vaccine.2021.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balkan İİ, Dinc HO, Can G, et al. Waning immunity to inactive SARS‐CoV‐2 vaccine in healthcare workers: booster required. Ir J Med Sci. 2022;1‐7. doi: 10.1007/s11845-022-02984-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang H, Liu X, Liu Q, et al. Serological reactivity of inactivated SARS‐CoV‐2 vaccine based on an S‐RBD neutralizing antibody assay. Int J Infect Dis. 2022;117:169‐173. doi: 10.1016/j.ijid.2022.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X, Liang C, Xiao X. SARS‐CoV‐2 neutralizing antibody levels post COVID‐19 vaccination based on ELISA method—a small real‐world sample exploration. Vaccine. 2021;9(10):1139. doi: 10.3390/vaccines9101139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alqassieh R, Suleiman A, Abu‐Halaweh S, et al. Pfizer‐biontech and sinopharm: a comparative study on post‐vaccination antibody titers. Vaccine. 2021;9(11): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El‐Ghitany EM, Hashish MH, Farag S, et al. Determinants of the development of SARS‐CoV‐2 anti‐spike immune‐response after vaccination among healthcare Workers in Egypt. Vaccine. 2022;10(2):174. doi: 10.3390/vaccines10020174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lombardi A, Consonni D, Oggioni M, et al. SARS‐CoV‐2 anti‐spike antibody titres after vaccination with BNT162b2 in naïve and previously infected individuals. J Infect Public Health. 2021;14(8):1120‐1122. doi: 10.1016/j.jiph.2021.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang JH, Chiou JF, Hung CS, et al. Humoral immunogenicity and Reactogenicity of the standard ChAdOx1 nCoV‐19 vaccination in Taiwan. Vaccine. 2022;10(2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee A, Wong SY, Chai LYA, et al. Efficacy of covid‐19 vaccines in immunocompromised patients: systematic review and meta‐analysis. BMJ. 2022;376:e068632. doi: 10.1136/bmj-2021-068632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu W, Tang J, Chen C, et al. Safety and efficacy of the COVID‐19 vaccine in children and/or adolescents: a meta‐analysis. J Infect. 2022;84(5):722‐746. doi: 10.1016/j.jinf.2022.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J, Tong Y, Li D, Li J, Li Y. The impact of age difference on the efficacy and safety of COVID‐19 vaccines: a systematic review and meta‐analysis. Front Immunol. 2021;12:758294. doi: 10.3389/fimmu.2021.758294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prasad S, Kalafat E, Blakeway H, et al. Systematic review and meta‐analysis of the effectiveness and perinatal outcomes of COVID‐19 vaccination in pregnancy. Nat Commun. 2022;13(1):2414. doi: 10.1038/s41467-022-30052-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rayalam S, Della‐Fera MA, Baile CA. Phytochemicals and regulation of the adipocyte life cycle. J Nutr Biochem. 2008;19(11):717‐726. doi: 10.1016/j.jnutbio.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 41. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. doi: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Földi M, Farkas N, Kiss S, et al. Visceral adiposity elevates the risk of critical condition in COVID‐19: a systematic review and meta‐analysis. Obesity (Silver Spring). 2021;29(3):521‐528. doi: 10.1002/oby.23096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Green J, Cairns BJ, Casabonne D, et al. Height and cancer incidence in the million women study: prospective cohort, and meta‐analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12(8):785‐794. doi: 10.1016/S1470-2045(11)70154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo CA, Kogan S, Amano SU, et al. CD40 deficiency in mice exacerbates obesity‐induced adipose tissue inflammation, hepatic steatosis, and insulin resistance. Am J Physiol Endocrinol Metab. 2013;304(9):E951‐E963. doi: 10.1152/ajpendo.00514.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26, and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy. 2020;75(11):2829‐2845. doi: 10.1111/all.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Favre G, Legueult K, Pradier C, et al. Visceral fat is associated to the severity of COVID‐19. Metabolism. 2021;115:154440. doi: 10.1016/j.metabol.2020.154440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li L, Spranger L, Soll D, et al. Metabolic impact of weight loss induced reduction of adipose ACE‐2 ‐ potential implication in COVID‐19 infections?. Metabolism. 2020;113:154401. doi: 10.1016/j.metabol.2020.154401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beacon TH, Delcuve GP, Davie JR. Epigenetic regulation of ACE2, the receptor of the SARS‐CoV‐2 virus(1). Genome. 2021;64(4):386‐399. doi: 10.1139/gen-2020-0124 [DOI] [PubMed] [Google Scholar]
- 49. Liu F, Guo Z, Dong C. Influences of obesity on the immunogenicity of hepatitis B vaccine. Hum Vaccin Immunother. 2017;13(5):1014‐1017. doi: 10.1080/21645515.2016.1274475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eliakim A, Schwindt C, Zaldivar F, et al. Reduced tetanus antibody titers in overweight children. Autoimmunity. 2006;39(2):137‐141. doi: 10.1080/08916930600597326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Honce R, Schultz‐Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. doi: 10.3389/fimmu.2019.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jagielska AM, Brydak LB, Nitsch‐Osuch AS. Immunogenicity of Split inactivated quadrivalent influenza vaccine in adults with obesity in the 2017/2018 season. Med Sci Monit. 2021;27:e929572. doi: 10.12659/MSM.929572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park HL, Shim SH, Lee EY, et al. Obesity‐induced chronic inflammation is associated with the reduced efficacy of influenza vaccine. Hum Vaccin Immunother. 2014;10(5):1181‐1186. doi: 10.4161/hv.28332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pellini RVA, Pimpinelli F, et al. Obesity may hamper SARS‐CoV‐2 vaccine immunogenicity. medRxiv. 2021. doi: 10.1101/2021.02.24.21251664 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sensitivity analysis of antibody titers.
Figure S2. Sensitivity analysis of change from baseline of antibody titers.
Figure S3. Egger's test of antibody positivity rates compared with overweight population.
Figure S4. Egger's test of antibody positivity rates compared with obese population.
Table S1. Details of the search history through PubMed.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (JPW), upon reasonable request.


