Abstract
In Escherichia coli, the waaP (rfaP) gene product was recently shown to be responsible for phosphorylation of the first heptose residue of the lipopolysaccharide (LPS) inner core region. WaaP was also shown to be necessary for the formation of a stable outer membrane. These earlier studies were performed with an avirulent rough strain of E. coli (to facilitate the structural chemistry required to properly define waaP function); therefore, we undertook the creation of a waaP mutant of Salmonella enterica serovar Typhimurium to assess the contribution of WaaP and LPS core phosphorylation to the biology of an intracellular pathogen. The S. enterica waaP mutant described here is the first to be both genetically and structurally characterized, and its creation refutes an earlier claim that waaP mutations in S. enterica must be leaky to maintain viability. The mutant was shown to exhibit characteristics of the deep-rough phenotype, despite its ability to produce a full-length core capped with O antigen. Further, phosphoryl modifications in the LPS core region were shown to be required for resistance to polycationic antimicrobials. The waaP mutant was significantly more sensitive to polymyxin in both wild-type and polymyxin-resistant backgrounds, despite the decreased negative charge of the mutant LPSs. In addition, the waaP mutation was shown to cause a complete loss of virulence in mouse infection models. Taken together, these data indicate that WaaP is a potential target for the development of novel therapeutic agents.
The outer membrane of a gram-negative bacterium is a barrier to many antibiotics and host defense factors (36). This barrier function is due in large part to structural features of the lipopolysaccharide (LPS) molecules that make up the outer leaflet of the outer membrane bilayer. In Escherichia coli and Salmonella enterica, the LPS molecule is conceptually divided into three distinct regions: (i) a hydrophobic membrane anchor designated lipid A; (ii) a short chain of sugar residues with multiple phosphoryl substituents, referred to as the core oligosaccharide; and (iii) a structurally diverse polymer composed of oligosaccharide repeats, termed the O antigen (26). The presence of phosphoryl substituents in the heptose region of the LPS core oligosaccharide is a key structural feature required for the formation of a stable outer membrane in E. coli and S. enterica (18, 43). Phosphoryl substituents are postulated to be critical to outer membrane integrity because their negative charge allows neighboring LPS molecules to be cross-linked by divalent cations (24, 36). Mutants of E. coli and S. enterica deficient in core phosphate exhibit a pleiotropic phenotype called deep rough, characteristics of which include (i) hypersensitivity to detergents and hydrophobic antibiotics, (ii) the appearance of phospholipid in the outer leaflet of the outer membrane bilayer, (iii) leakage of periplasmic proteins into the culture medium, and (iv) a marked decrease in the protein content of the outer membrane (reviewed in references 15 and 29). It has also been shown that the LPS from phosphate-deficient deep-rough mutants cannot support the proper folding of some outer membrane proteins (6).
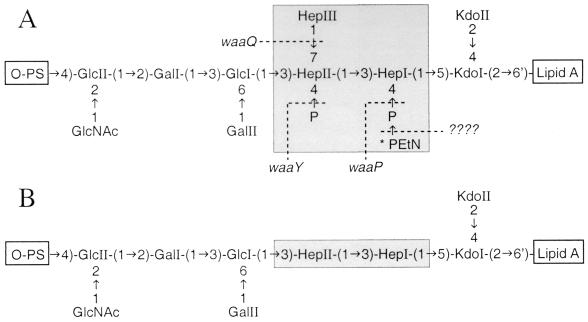
The gene products involved in core phosphorylation have only recently been characterized. In E. coli F470, WaaP was shown to be required for phosphate addition to HepI (43), and this reaction was found to be a prerequisite for the functioning of WaaQ and WaaY, which are responsible for the addition of HepIII and the phosphorylation of HepII, respectively (Fig. 1A). Both WaaP and WaaY share limited similarity with eukaryotic kinases (43) although kinase activity has yet to be proven. Intuitively, the activity of WaaP is also a prerequisite for the functioning of the presently unidentified enzyme responsible for 2-aminoethyl phosphate (PEtN) modification of the core heptose region (Fig. 1A). Since the E. coli and S. enterica waaP, waaQ, and waaY gene products are highly conserved (43), these enzymes likely function the same way in both organisms. Therefore, the LPS core oligosaccharide of an S. enterica waaP mutant is predicted to have the structure shown in Fig. 1B.
FIG. 1.
(A) Structure of the LPS core oligosaccharide from S. enterica serovar Typhimurium (20). The inner core heptose region is highlighted by shading. Genetic determinants for the modification of the inner core heptose region (conserved between E. coli and S. enterica) are shown, and they must function in the following sequence: waaP, waaQ, and then waaY (43). Abbreviations: Hep, l-glycero-d-manno-heptose; Kdo, 3-deoxy-d-manno-oct-2-ulosonic acid; P, phosphate; PS, polysaccharide. ∗, the PEtN substitution is nonstoichiometric but is reportedly present in larger amounts in PM-resistant mutants of S. enterica (16). (B) Predicted structure of the LPS core oligosaccharide from CWG304, based on the function of WaaP in E. coli F470 and its effect on WaaQ and WaaY activities (43).
It is noteworthy, however, that the structure of LPS appears to be dynamic. For example, the LPSs of Helicobacter, Neisseria, and Haemophilus have all been shown to undergo phase variation (2, 37, 40). Further, both Pseudomonas aeruginosa (7) and S. enterica serovar Typhimurium (9, 11, 12) are able to specifically modify their LPSs in response to environmental cues. One mechanism by which S. enterica modifies its LPS involves the PmrA-PmrB two-component regulatory system, which modulates resistance to numerous cationic antimicrobial compounds, including polymyxin (PM) (28, 32). Resistance to PM is correlated with the addition of aminoarabinose to the 4′ phosphate of lipid A (9), a modification that is proposed to decrease the net negative charge of the LPS molecule and reduce electrostatic interactions between PM and the outer membrane. Previous studies also suggest, however, that there is increased PEtN substitution of phosphate residues in the LPS core heptose region of PM-resistant mutants of S. enterica (16). This PEtN substitution in the LPS core might also play a role in PM resistance by decreasing the negative charge of the bacterial cell surface. The ability of S. enterica and other pathogens to modulate their LPS suggests that LPS plays both structural and functional roles in the biology of these organisms.
Given the predicted lack of phosphate in the LPS of an S. enterica waaP mutant (Fig. 1B), it was expected that such a mutant would exhibit hypersensitivity to hydrophobic antimicrobial agents and other characteristics of the deep-rough phenotype. However, we envisioned two possible outcomes to sensitivity testing with polycationic antimicrobials such as PM. In one predicted scenario, the loss of phosphoryl substituents would increase resistance to PM by decreasing the LPS negative charge, in a similar manner to that described above for aminoarabinose modifications of lipid A. In the second scenario, the previously noted sensitivity of waaP mutants to hydrophobic agents might decrease PM resistance due to PM-outer membrane interactions of a hydrophobic nature—a possibility strongly supported by titration calorimetric studies of the PM-LPS interaction (33). In this paper we address these possibilities and also examine the contribution of core oligosaccharide phosphorylation to the virulence of S. enterica serovar Typhimurium in a mouse infection model. To determine unequivocally the role of WaaP in S. enterica virulence, we required a genetically defined mutant strain. However, earlier “waaP mutants” of S. enterica were obtained using chemical mutagenesis and phage selection, and all are reported to be leaky (18). Therefore, we also report the construction of the first genetically defined S. enterica waaP mutant and the chemical characterization of its LPS defect.
MATERIALS AND METHODS
Bacterial strains.
All strains in this study are derivatives of S. enterica strain ATCC 14028. Strain JSG435 carries the pmrA505 allele (28), conferring a PmrA-constitutive phenotype, and was described previously (10). Strain CWG304 (ATCC 14028 waaP::aacC1) was constructed as follows. Briefly, waaP (and flanking DNA) was PCR amplified (primers 5′-TGGCATCGCTACCCGAATCT-3′ and 5′-TTGGCATAAAGACATGAGAT-3′). The 1.9-kb amplified fragment was cloned into pBluescript II SK(+) (Stratagene), and sequenced to ensure error-free amplification. A 48 bp NruI fragment from the middle of the waaP coding region was then replaced with the aacC1 gene (a nonpolar cassette conferring gentamicin resistance) excised with SmaI from plasmid pUCGM (30). The DNA fragment containing the insertionally inactivated waaP gene was subsequently cloned into the suicide delivery vector pMAK705 (13), and chromosomal gene replacement was performed as described previously (1). JSG778 was derived by P22 transduction of the waaP::aacC1 allele into JSG435.
LPS analysis by SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
LPS was prepared by the method of Hitchcock and Brown (19). Samples were separated on 10–20% Tricine sodium dodecyl sulfate (SDS)-polyacrylamide gels (Novex) or standard SDS–12% polyacrylamide gels (23). Following electrophoresis, the LPS was visualized by silver staining (35).
Purification of core oligosaccharides.
LPS was isolated from strains ATCC 14028 and CWG304 by hot phenol-water extraction (41). The LPS was delipidated by hydrolysis in 2% acetic acid at 100°C to cleave the acid-labile ketosidic linkage between the core oligosaccharide and lipid A. Water-insoluble lipid A was removed by centrifugation, and the polysaccharide-containing supernatant was passed through a column of Bio-Gel P-2 (1 m by 1 cm) with water as the eluent. Fractions eluting first contained large amounts of O polysaccharide, but later fractions contained predominantly core oligosaccharide.
Structural analysis of core oligosaccharides.
31P nuclear magnetic resonance (NMR) spectra of core oligosaccharides were recorded with a Bruker DRX 400-MHz instrument at 161.98 MHz with ortho-phosphoric acid as the external reference (0.0 ppm) and with p1 = 30 in the proton-decoupling mode. Prior to performance of the NMR experiments, the samples were lyophilized three times in 2H2O (99.9%). The p2H was adjusted to approximately 8.0 with triethylamine. Methylation linkage analysis of isolated core oligosaccharides was performed by the procedure of Ciucanu and Kerek (5). The permethylated alditol acetate derivatives of the core-containing samples were characterized by gas-liquid chromatography–mass spectrometry in the electron impact mode using a column of DB-17 operated isothermally at 190°C for 60 min.
MALDI-TOF mass spectrometry.
LPS was isolated by hot phenol-water extraction (41), and lipid A was subsequently obtained by centrifugation after hydrolysis of the LPS in 1% SDS at pH 4.5 (4). Negative-ion matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) mass spectrometry was performed as described previously (7). Lyophilized lipid A was dissolved in 5 μl of 5-chloro-2-mercaptobenzothiazole MALDI matrix in chloroform-methanol (1:1, vol/vol), and 1 μl was then applied to the sample plate. All MALDI-TOF experiments were performed using a BiflexIII mass spectrometer (Bruker Daltonics, Inc., Billerica, Mass.).
Antibiotic and detergent sensitivity testing.
SDS and novobiocin sensitivity testing was performed as described previously (43). MIC testing for PM susceptibility was performed in polypropylene microtiter dishes as described by Steinberg et al. (34).
Mouse virulence studies.
Inbred mouse strains C57BL/6J and A/J were bred and maintained in the Montreal General Hospital Research Institute under conditions specified by the Canadian Council on Animal Care. Mice between 8 and 12 weeks of age were challenged with either ATCC 14028 or CWG304 by inoculation in the caudal vein with 0.2 ml of physiological saline containing 102, 103, or 104 CFU of S. enterica. The inoculum of S. enterica was prepared by growing the bacteria for 2 h at 37°C in tryptic soy broth followed by enumeration of the CFU by incubating serial 10-fold dilutions on tryptic soy agar at 37°C for 16 h (31, 38). The degree of CWG304 virulence was established in vivo by survival analysis and by measuring the numbers of CFU in the spleens and livers of surviving mice 21 days postinoculation. The numbers of viable salmonellae in the spleens and livers of the infected animals were determined by plating serial 10-fold dilutions of organ homogenates in physiological saline on tryptic soy agar (8).
Oral and intraperitoneal infections of 16- to 18-g BALB/c mice (Harlan Sprague-Dawley, Indianapolis, Ind.) were accomplished as follows. For oral infections, mice deprived of food or water for at least 4 h were prefed 20 μl of 10% sodium bicarbonate 30 min prior to oral inoculation with 20 μl of stationary-phase bacteria (∼ 3 × 106 to 6 × 106 CFU) diluted in phosphate-buffered saline. Intraperitoneal infection was performed with 100 μl of stationary-phase bacteria (∼ 60 to 100 CFU) diluted in phosphate-buffered saline. Mouse survival was monitored for three weeks.
RESULTS
Structure of the LPS core oligosaccharide of a waaP mutant.
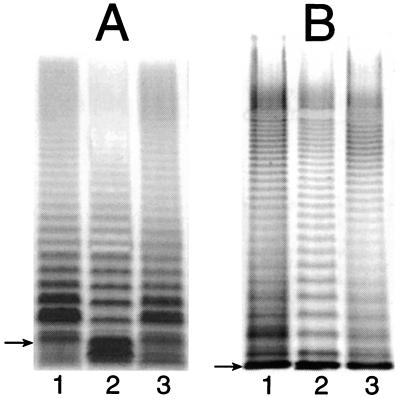
Earlier studies performed with genetically uncharacterized S. enterica “waaP mutants” indicated that the core oligosaccharide of such mutant strains was truncated after the first glucose residue of the outer core (GlcI) (Fig. 1) (18). To determine whether such was the case for our defined mutant strain (CWG304), we examined the CWG304 and parent (ATCC 14028) LPSs by SDS-PAGE (Fig. 2). A typical ladder-like pattern of smooth LPS bands is visible for the parent strain and also (although to a lesser extent) for CWG304 (compare lanes 1 and 2 in Fig. 2). Both strains show the same high-molecular-weight modal cluster of smooth LPS bands (Fig. 2B). However, the CWG304 profile obtained using gradient Tricine SDS-polyacrylamide gels also shows an intense band that migrates slightly faster than any band in the parent profile (Fig. 2A, lane 2). These observations indicate that CWG304 produces a full-length (complete) core capped with O antigen but that a portion of its LPS molecules is prematurely truncated in the core. The wild-type LPS banding pattern could be restored to CWG304 (lane 3 in Fig. 2) by complementation with plasmid pWQ909, carrying waaP from E. coli F470 (43), further confirming the identical functioning of WaaP in both organisms. This was the expected result given that the predicted WaaP proteins of S. enterica and E. coli F470 are 81.5% identical (90.6% similar).
FIG. 2.
Silver-stained SDS-polyacrylamide gels showing the LPS profiles of strains ATCC 14028 (lane 1), CWG304 (lane 2), and CWG304 complemented with the waaP open reading frame from E. coli F470 (lane 3). Samples were run on a 10 to 20% Tricine SDS-polyacrylamide gel (A) and on a standard SDS–12% polyacrylamide gel (B). The gel system in panel A gives better resolution of low-molecular-weight LPS (i.e., LPS lacking O antigen), while the standard gel system shows that the modality of O-antigen expression is unaffected in the mutant strain. The migration of Ra-LPS (lipid A and complete core) in each gel system is indicated by an arrow. Note that Ra-LPS comigrates with LPS molecules with one O-antigen repeat in the gel system in panel B, so the amount of free (uncapped) lipid A-core is misleading. The extent of capping is clear in panel A.
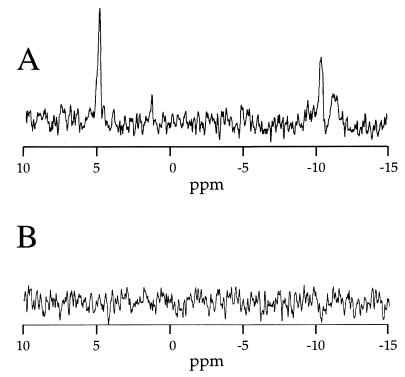
Phosphorylation of the inner core heptose region in CWG304 was then examined by 31P NMR and methylation linkage analysis. The 31P NMR spectra of the core oligosaccharides from the wild-type parent and the waaP-null strain are shown in Fig. 3. The signal at approximately 5 ppm in the parent spectrum corresponds to the phosphate residues on HepI and HepII, while the doublet at approximately −10 ppm corresponds to PPEtN on HepI (16, 17, 25, 43). The complete lack of phosphate in the CWG304 core is shown by the disappearance of all phosphorus signals (compare Fig. 3A and B).
FIG. 3.
31P NMR spectra of the core oligosaccharides from ATCC 14028 (A) and CWG304 (B). The signal at 5 ppm is indicative of a phosphomonoester (P on either HepI or HepII), and the two peaks near −10 ppm are characteristic of a diphosphodiester (PPEtN on HepI) (16).
Methylation linkage analysis of the LPS core oligosaccharides from ATCC 14028 and CWG304 further confirmed the predicted structure of the mutant core (Table 1). Derivatives from HepI [→3)-Hep4P(PEtN)-(1→] or HepII [→3,7)-Hep4P-(1→] were not evident for ATCC 14028 LPS because the phosphoryl substituents attached to these residues make their derivatives too polar to elute from the gas-liquid chromatography column, as established previously with E. coli (43). Analysis of the CWG304 core showed the disappearance of the HepIII [Hep-(1→] derivative and the appearance of a derivative corresponding to 3-substituted heptose [→3)-Hep-(1→] (Table 1). The 3-substituted heptose derivative reflects both nonphosphorylated HepI and nonphosphorylated HepII lacking the branch HepIII residue (Table 1). Together with our 31P NMR results, these data confirm the predicted structural defect caused by the waaP mutation (Fig. 1B).
TABLE 1.
Methylation linkage analysis of the core oligosaccharide heptose regions from the LPSs of ATCC 14028 and CWG304
Influence of the waaP mutation on sensitivity to antimicrobial compounds.
To confirm the predicted sensitivity of CWG304 to hydrophobic compounds (as part of the deep-rough phenotype), the strain's susceptibility to SDS and novobiocin was tested. CWG304 exhibited more than a 125-fold increase in susceptibility to SDS and more than a 30-fold increase in susceptibility to novobiocin compared to the parent strain (Table 2). Complementation with plasmid pWQ909, carrying waaP from E. coli F470, restored wild-type levels of resistance to these compounds. The MIC results for PM susceptibility testing are also summarized in Table 2. In the absence of the waaP defect, the PmrA-constitutive strain shows increased resistance to PM (compare ATCC 14028 and JSG435), as reported previously (9). The waaP mutation, however, causes a clear increase in PM sensitivity. In the wild-type background, the PM MIC is decreased by 100-fold (compare ATCC 14028 and CWG304), while a somewhat smaller decrease (8-fold) is observed in the PmrA-constitutive background (compare JSG435 and JSG778).
TABLE 2.
MIC testing for susceptibility to novobiocin, SDS, and PM
| Strain | MIC
|
||
|---|---|---|---|
| Novobiocin (μg/ml) | SDS (mg/ml) | PM (μg/ml) | |
| ATCC 14028 (parent) | 200 | 200 | 1.0 |
| CWG304 (waaP::aacC1) | 6.3 | 1.6 | 0.01 |
| CWG304(pWQ909) | 200 | 100 | 0.4 |
| JSG435 (pmrA505) | 4.0 | ||
| JSG778 (pmrA505 waaP::aacC1) | 0.5 | ||
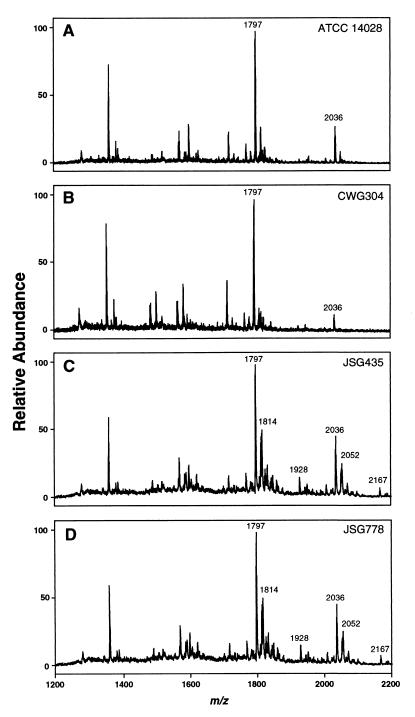
To address whether the changes in PM susceptibility were related to or independent of lipid A aminoarabinose substitution, the lipid A of each LPS was analyzed by MALDI-TOF mass spectrometry (Fig. 4). The mass spectra of the lipids A from ATCC 14028 and CWG304 show no significant differences in hexa- and hepta-acylated forms (Fig. 4). Peaks for aminoarabinose-substituted lipid A are not evident in the wild-type background because this substitution occurs only at very low levels (11). The spectra of the parent and waaP mutant strains in the PmrA-constitutive background (JSG435 and JSG778) are also essentially identical (Fig. 4). However, of particular note in the PmrA-constitutive background, mutation of waaP does not affect lipid A aminoarabinose substitution. Peaks corresponding to aminoarabinose-substituted hexa- and hepta-acylated lipid A (m/z 1928 and 2167) are present in the same ratios in both the JSG435 and JSG778 spectra. It was therefore concluded that the contribution of core phosphate residues to PM resistance is distinct from the effects of lipid A aminoarabinose modification.
FIG. 4.
Characterization of structural modifications of S. enterica lipid A by negative-ion MALDI-TOF mass spectrometry. All values given are average masses rounded to the nearest whole number for singly charged, deprotonated molecules [M − H]−. (A) ATCC 14028 (parent) lipid A, with the major signal representing the hexa-acylated form (at m/z 1797). The hepta-acylated form containing palmitate (at m/z 2036) is indicated. (B) CWG304 (waaP::aacC1) lipid A, showing ions at m/z 1797 and 2036 as described above. (C) JSG435 (pmrA505) lipid A, showing the hexa- and hepta-acylated forms (as described above), as well as modification by the addition of aminoarabinose (m/z 1928 and 2167, respectively) or by the addition of a hydroxyl group (m/z 1814 and 2052, respectively). (D) JSG778 (pmrA505 waaP::aacC1) lipid A, showing ions at m/z 1797, 1814, 1928, 2036, 2052, and 2167 as described above.
Effect of the waaP mutation on growth and virulence.
Given the profound changes in outer membrane composition caused by the waaP mutation, we performed growth curve determinations for ATCC 14028 and CWG304 to determine if mutation of waaP affected basic growth characteristics. Somewhat surprisingly, the growth curves of the wild type and the waaP mutant (in Luria-Bertani broth at 37°C) were identical (data not shown).
To then assess whether the virulence of CWG304 was altered in vivo, three different mouse strains (C57BL/6J, A/J, and BALB/c) were used. Both C57BL/6J and BALB/c are extremely susceptible to S. enterica infection due to a mutation in the Nramp1 gene (38), while the wild-type strain A/J is naturally more resistant to infection. The resistant A/J mice were unaffected by challenge with either 102 or 103 CFU of ATCC 14028 (administered by injection in the caudal vein). At 104 CFU, three of five mice died after 15 days. The remaining mice survived over the 21-day course of the experiment. All of the highly susceptible C57BL/6J mice died within 5 days at 103 or 104 CFU and within 6 days at 102 CFU (as expected). By contrast, all of the mice (both strains) challenged with the waaP mutant strain survived (at all doses and for the duration of the experiment).
The surviving A/J mice were euthanized on day 21, and spleen and liver homogenates were plated to determine the extent of persisting S. enterica infection (Table 3). In mice challenged with ATCC 14028, the bacteria could still be isolated from the liver and spleen homogenates in significant numbers. However, CWG304 bacteria were virtually cleared by day 21 and could be detected only in very small numbers in the spleen at the highest dose administered.
TABLE 3.
Average numbers of CFU in the spleens and livers of A/J mice challenged intravenously with the indicated doses of ATCC 14028 or CWG304a
| Dose (CFU) | Avg CFUb per:
|
|||
|---|---|---|---|---|
| Spleen
|
Liver
|
|||
| ATCC 14028 | CWG304 | ATCC 14028 | CWG304 | |
| 104 | 3,468 ± 3,352c | 9 ± 5 | 142 ± 46 | 0 |
| 103 | 56 ± 13 | 0 | 32 ± 17 | 0 |
| 102 | 58 ± 9 | 0 | 31 ± 3 | 0 |
Surviving mice were sacrificed at 21 days postinfection.
Results are averages and standard deviations from five mice, except as indicated.
Only two of five mice survived until day 21 at this dose.
Given the somewhat decreased amount of O-antigen expression in strain CWG304, as observed by SDS-PAGE (Fig. 2), it was possible that the mutant's loss of virulence could be attributed to an increase in susceptibility to complement-mediated serum killing. O antigen is known to be an important factor in the ability of many bacteria to evade complement (21). To determine whether bacteria injected into the bloodstream were simply cleared by complement-mediated cell lysis, the parent and waaP mutant strains were incubated with fresh mouse serum for 2 h at 37°C, and then serial dilutions were plated on Luria-Bertani agar plates. No difference in CFU was observed between the two strains (data not shown), indicating that complement-mediated killing is likely not a factor in inhibiting CWG304 infection.
Finally, to determine whether the loss of virulence in CWG304 was influenced by the route of infection, BALB/c mice were challenged with ATCC 14028 and CWG304, both orally and by intraperitoneal injection. These models are the most widely accepted for S. enterica virulence studies, and 50% lethal doses (LD50s) have been determined for both methods (6.5 × 104 and <10 for oral and intraperitoneal infection, respectively) (3). The results from the oral and intraperitoneal infection experiments show that the virulence defect of CWG304 does not depend on how the inoculum is administered: all of the mice infected with CWG304 (n = 5 to 10) survived at a dose of 10 times the LD50 (intraperitoneally) or 100 times the LD50 (orally), while all of the mice challenged with the wild-type ATCC 14028 died at these doses.
DISCUSSION
Strain CWG304 is the first S. enterica waaP mutant to be characterized both genetically and structurally. Earlier “waaP mutants” of S. enterica serovar Typhimurium were obtained using chemical mutagenesis and phage selection, and are all reported to be leaky, with small amounts of phosphate still detectable in the core (18). CWG304, however, is known to be nonleaky because of the nature of the mutation (see Materials and Methods) and the complete lack of phosphate in its core region as observed by 31P NMR (Fig. 3), the most sensitive detection method available. As such, the creation of our waaP mutant of S. enterica refutes the earlier conclusion that such mutations in S. enterica must be leaky to maintain viability. Further, the waaP::aacC1 mutation could be transduced by P22 into a clean S. enterica serovar Typhimurium background, giving a strain with the same deep-rough phenotype (J. A. Yethon, J. S. Gunn, and C. Whitfield, unpublished data). This argues against the possibility of unlinked secondary, compensating mutations. Strain CWG304 therefore represents a valuable tool for dissection of the various factors involved in antibiotic sensitivities (i.e., changes in core phosphorylation versus core truncation and the presence or absence of O antigen). Interestingly, our waaP mutant strain shows a slightly decreased efficiency of core completion and capping with O antigen (Fig. 2), but this difference is not enough to cause any increase in susceptibility to complement-mediated serum killing. There is no obvious change in maximal O-chain length or modal distribution of O antigen. Therefore, the waaP mutant is essentially a smooth strain that expresses characteristics of the deep-rough phenotype, clearly demonstrating that it is the lack of core phosphate and not core truncation that causes this phenotype.
The effects of various LPS core defects on antibiotic susceptibilities in S. enterica have been examined previously (27), but the genes mutated were not precisely defined. Therefore, it is difficult to compare our results directly with those of these earlier studies. However, S. enterica mutants with highly truncated cores were previously shown to be more susceptible to numerous antibiotics, including PM (27). In light of the findings reported here, and given the observation that E. coli mutants lacking the outer core glycoses are affected in their degree of heptose phosphorylation (J. A. Yethon, E. V. Vinogradov, M. B. Perry, and C. Whitfield, submitted for publication), it is concluded that LPS core phosphate residues are essential to the outer membrane barrier function of S. enterica and in particular to PM resistance.
The interaction between cationic antimicrobial peptides and the gram-negative outer membrane is complex (reviewed in references 14, 24, and 36). Indeed, there appear to be distinct modifications required for resistance to different cationic antimicrobial compounds (10, 12). The increased PM susceptibility of S. enterica waaP mutants brings into question the current dogma that PM resistance is mediated by LPS charge modulation alone. It appears instead, at least in the case of a waaP mutation, that detrimental hydrophobic interactions between PM and the outer membrane can outweigh the benefits associated with a decrease in LPS net negative charge. This does not mean that electrostatic interactions are not important. It has been proposed, for example, that PM functions by a detergent-like mechanism, requiring numerous PM molecules to aggregate into clusters at the outer membrane surface (42). Clearly, electrostatic interactions would favor the accumulation of PM molecules at the outer membrane surface and thus facilitate the formation of such clusters. However, our studies and the work of others (33) suggest that hydrophobic interactions may play a critical role in the PM-LPS interaction.
Our data must be interpreted with caution, however, because there is evidence that other defects (not related to LPS structure) in the outer membrane of deep-rough mutants might influence the membrane barrier function in unexpected ways. For example, there are regions of phospholipid bilayer in the outer membranes of deep-rough mutants (22), although the extent of these regions is not known. One hypothesis, proposed by Nikaido and Vaara (24), suggests that these regions of phospholipid bilayer are responsible for the increased susceptibility of deep-rough mutants to hydrophobic agents. Therefore, the increased PM sensitivity of our S. enterica waaP mutant might be the result of PM simply passing through phospholipid-enriched domains in the outer membrane and not interacting with LPS at all. However, the surface area occupied by these phospholipid domains is estimated to be small (24), and given the fact that a PmrA-constitutive strain can still upregulate PM resistance despite carrying a waaP mutation (compare CWG304 and JSG778 in Table 1), we feel that this is likely not the case.
Finally, regardless of the exact mechanism by which mutation of waaP affects the outer membrane, we have shown that such a mutation leads to avirulence in vivo. Loss of virulence was demonstrated by both the survival of C57BL/6J and BALB/c mice and the decrease in CFU in the spleens and livers of infected A/J mice (Table 3). In addition, the virulence defect was shown for intravenous, intraperitoneal, and oral administration of the inoculum. Given the greatly compromised barrier function of the mutant outer membrane and the avirulence of the mutant in our mouse infection model, we believe that the waaP gene product represents a valid potential target for the development of novel therapeutics. Interestingly, a WaaP homolog was recently identified in P. aeruginosa, an important opportunistic pathogen in the lungs of individuals with cystic fibrosis. The initial identification of WaaP in P. aeruginosa was made based on homology (>60% similarity) to WaaP from E. coli and S. enterica, and the available evidence suggests that WaaP is essential for the viability of P. aeruginosa in vitro (39). Therefore, an inhibitor targeted against WaaP would be active against a range of bacteria extending beyond members of the family Enterobacteriaceae.
ACKNOWLEDGMENTS
We thank M. A. Monteiro and M. B. Perry (Institute for Biological Sciences, National Research Council, Ottawa, Ontario, Canada) for assistance with the chemical analyses of the LPS core oligosaccharides and W. Woodward (University of Guelph, Guelph, Ontario, Canada) for mouse serum.
This work was supported in part through funding to C.W. and D.M. by the Canadian Bacterial Diseases Network (Network of Centres of Excellence). J.A.Y. is the recipient of graduate scholarships from the Natural Sciences and Engineering Research Council and from the Medical Research Council of Canada. J.S.G. was supported by a grant from the National Institutes of Health (AI43521). D.M. is a Scholar of Fonds de la Recherche en Santé du Québec (FRSQ).
REFERENCES
- 1.Amor P A, Whitfield C. Molecular and functional analysis of genes required for expression of group IB K antigens in Escherichia coli: characterization of the his-region containing gene clusters for multiple cell-surface polysaccharides. Mol Microbiol. 1997;26:145–161. doi: 10.1046/j.1365-2958.1997.5631930.x. [DOI] [PubMed] [Google Scholar]
- 2.Appelmelk B J, Shiberu B, Trinks C, Tapsi N, Zheng P Y, Verboom T, Maaskant J, Hokke C H, Schiphorst W E, Blanchard D, Simoons-Smit I M, van den Eijnden D H, Vandenbroucke-Grauls C M. Phase variation in Helicobacter pylori lipopolysaccharide. Infect Immun. 1998;66:70–76. doi: 10.1128/iai.66.1.70-76.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caroff M, Tacken A, Szabo L. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr Res. 1988;175:273–282. doi: 10.1016/0008-6215(88)84149-1. [DOI] [PubMed] [Google Scholar]
- 5.Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 1984;131:209–217. [Google Scholar]
- 6.de Cock H, Brandenburg K, Wiese A, Holst O, Seydel U. Non-lamellar structure and negative charges of lipopolysaccharides required for efficient folding of outer membrane protein PhoE of Escherichia coli. J Biol Chem. 1999;274:5114–5119. doi: 10.1074/jbc.274.8.5114. [DOI] [PubMed] [Google Scholar]
- 7.Ernst R K, Yi E C, Guo L, Lim K B, Burns J L, Hackett M, Miller S I. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 8.Govoni G, Canonne-Hergaux F, Pfeifer C G, Marcus S L, Mills S D, Hackman D J, Grinstein S, Malo D, Finlay B B, Gros P. Functional expression of Nramp1 in vitro in the murine macrophage line RAW264.7. Infect Immun. 1999;67:2225–2232. doi: 10.1128/iai.67.5.2225-2232.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunn J S, Lim K B, Krueger J, Kim K, Guo L, Hackett M, Miller S I. PmrA—PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol Microbiol. 1998;27:1171–1182. doi: 10.1046/j.1365-2958.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 10.Gunn J S, Miller S I. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996;178:6857–6864. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L, Lim K B, Gunn J S, Bainbridge B, Darveau R P, Hackett M, Miller S I. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 12.Guo L, Lim K B, Poduje C M, Daniel M, Gunn J S, Hackett M, Miller S I. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95:189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton C A, Aldea M, Washburn B K, Babtizke P, Kushner S R. New method of generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock R E, Chapple D S. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrichs D E, Yethon J A, Whitfield C. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol Microbiol. 1998;30:221–232. doi: 10.1046/j.1365-2958.1998.01063.x. [DOI] [PubMed] [Google Scholar]
- 16.Helander I M, Kilpeläinen I, Vaara M. Increased substitution of phosphate groups in lipopolysaccharides and lipid A of the polymyxin-resistant pmrA mutants of Salmonella typhimurium: a 31P-NMR study. Mol Microbiol. 1994;11:481–487. doi: 10.1111/j.1365-2958.1994.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 17.Helander I M, Kilpeläinen I, Vaara M. Phosphate groups in lipopolysaccharides of Salmonella typhimurium rfaP mutants. FEBS Lett. 1997;409:457–460. doi: 10.1016/s0014-5793(97)00572-3. [DOI] [PubMed] [Google Scholar]
- 18.Helander I M, Vaara M, Sukupolvi S, Rhen M, Saarela S, Zähringer U, Mäkelä P H. rfaP mutants of Salmonella typhimurium. Eur J Biochem. 1989;185:541–546. doi: 10.1111/j.1432-1033.1989.tb15147.x. [DOI] [PubMed] [Google Scholar]
- 19.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holst O, Brade H. Chemical structure of the core region of lipopolysaccharides. In: Morrison D C, Ryan J L, editors. Bacterial endotoxic lipopolysaccharides. I. Boca Raton, Fla: CRC Press; 1992. pp. 134–170. [Google Scholar]
- 21.Joiner K A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- 22.Kamio Y, Nikaido H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase C and cyanogen bromide activated dextran in the external medium. Biochemistry. 1976;15:2561–2570. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsthoorn M M A, Petersen B O, Schlecht S, Haverkamp J, Bock K, Thomas-Oates J E, Holst O. Identification of a novel core type in Salmonella lipopolysaccharide. Complete structural analysis of the core region of the lipopolysaccharide from Salmonella enterica sv. Arizonae O62. J Biol Chem. 1998;273:3817–3829. doi: 10.1074/jbc.273.7.3817. [DOI] [PubMed] [Google Scholar]
- 26.Raetz C R H. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1035–1063. [Google Scholar]
- 27.Roantree R J, Kuo T-T, MacPhee D G. The effect of defined lipopolysaccharide core defects upon antibiotic resistances of Salmonella typhimurium. J Gen Microbiol. 1977;103:223–234. doi: 10.1099/00221287-103-2-223. [DOI] [PubMed] [Google Scholar]
- 28.Roland K L, Martin L E, Esther C R, Spitznagel J K. Spontaneous pmrA mutants of Salmonella typhimurium LT2 define a new two-component regulatory system with a possible role in virulence. J Bacteriol. 1993;175:4154–4164. doi: 10.1128/jb.175.13.4154-4164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnaitman C A, Klena J D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweizer H P. Small broad-host-range gentamycin resistance cassettes for site-specific insertion and deletion mutagenesis. BioTechniques. 1993;15:831–833. [PubMed] [Google Scholar]
- 31.Sebastiani G, Olien L, Gauthier S, Skamene E, Morgan K, Gros P, Malo D. Mapping of genetic modulators of natural resistance to infection with Salmonella typhimurium in wild-derived mice. Genomics. 1998;47:180–186. doi: 10.1006/geno.1997.5116. [DOI] [PubMed] [Google Scholar]
- 32.Shafer W M, Martin L E, Spitznagel J K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984;45:29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srimal S, Surolia N, Balasubramanian S, Surolia A. Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A. Biochem J. 1996;315:679–686. doi: 10.1042/bj3150679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberg D A, Hurst M A, Fujii C A, Kung A H, Ho J F, Cheng F C, Loury D J, Fiddes J C. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai G M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 36.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Putten J P M, Robertson B D. Molecular mechanisms and implications for infection of lipopolysaccharide variation in Neisseria. Mol Microbiol. 1995;16:847–853. doi: 10.1111/j.1365-2958.1995.tb02312.x. [DOI] [PubMed] [Google Scholar]
- 38.Vidal S, Tremblay M L, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh A G, Matewish M J, Burrows L L, Monteiro M A, Perry M B, Lam J S. Lipopolysaccharide core phosphates are required for viability and intrinsic drug resistance in Pseudomonas aeruginosa. Mol Microbiol. 2000;35:718–727. doi: 10.1046/j.1365-2958.2000.01741.x. [DOI] [PubMed] [Google Scholar]
- 40.Weiser J N, Pan N. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol Microbiol. 1998;30:767–775. doi: 10.1046/j.1365-2958.1998.01108.x. [DOI] [PubMed] [Google Scholar]
- 41.Westphal O, Jann K. Bacterial lipopolysaccharide extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 42.Wiese A, Münstermann M, Gutsmann T, Lindner B, Kawahara K, Zähringer U, Seydel U. Molecular mechanisms of polymyxin B-membrane interactions: direct correlation between surface charge density and self-promoted transport. J Membr Biol. 1998;162:127–138. doi: 10.1007/s002329900350. [DOI] [PubMed] [Google Scholar]
- 43.Yethon J A, Heinrichs D E, Monteiro M A, Perry M B, Whitfield C. Involvement of waaY, waaQ, and waaP in the modification of Escherichia coli lipopolysaccharide and their role in the formation of a stable outer membrane. J Biol Chem. 1998;273:26310–26316. doi: 10.1074/jbc.273.41.26310. [DOI] [PubMed] [Google Scholar]