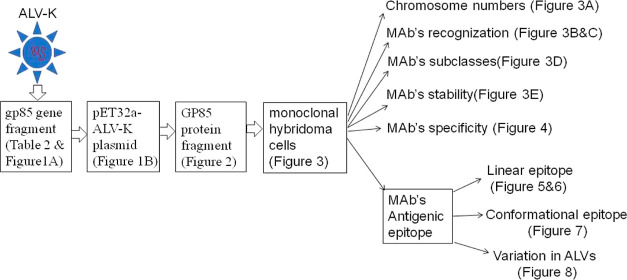
Abstract
This study focused on preparing a new IgG-type monoclonal antibody (MAb) against subgroup K avian leukosis virus (ALV-K) and identifying its biochemical characteristics. A specific gene fragment of ALV-K was amplified by polymerase chain reaction and expressed in E. coli. The purified expressed products were inoculated into BALB/c mice to prepare antibody-secreting spleen lymphocytes, and hybridoma cells were obtained after cell fusion of spleen lymphocytes and myeloma cells. A new hybridoma cell line named 30B9, which stably secreted IgG2b-antibody against ALV-K, was screened and contained 98 chromosomes. The MAb secreted by the 30B9 cells could recognize the ALV-K strain but not the ALV-A/B/J strains in an indirect immunofluorescence assay. Seventeen overlapping truncated ALV-K gp85 protein fragments were expressed, and eight peptides were artificially synthesized to analyze the MAb’s antigen epitope by Western blot or enzyme-linked immunosorbent assay, and the results showed that the linear epitope was located on the 217–RRNYT–221 of ALV-K gp85 protein. A bioinformatics analysis showed that the epitope has a high antigenicity index, hydrophilicity, and surface accessibility and forms a unique linear spatial structure. Its five amino acids are highly conserved in all published ALV-K strains but are very low in ALV-A/B/J/C/D/E strains. This study provides a new biomaterial for developing specific detection methods against ALV-K.
Introduction
Avian leukosis (AL) is an infectious tumor disease of chickens caused by avian leukosis virus (ALV).1 Avian leukemia exists worldwide and can cause immunosuppression, tumors, severe pathological damage, and death in infected chickens, which results in great losses to the poultry industry every year.2 At present, effective vaccines or drugs against ALV are lacking due to its high variability.3 In clinical production, this disease is managed in some countries by culling infected breeding chickens.4
ALV has evolved into many subgroups with different pathogenicities. Eleven subgroups of ALV, named ALV-A/B/C/D/E/F/G/H/I/J/K, have been identified, and seven of these subgroups were from chickens.5 ALV-E, an endogenous virus, is generally not the target of culling detection in clinics due to its weak pathogenicity, but it may interfere with the determination of other exogenous ALVs because its genome has already been embedded into host chickens during evolution.1,6 ALV-K, a new exogenous subgroup, has been reported in local chicken flocks in Asian countries in recent years and causes viremia, immunosuppression, and growth retardation in infected chickens; however, screening for ALV P27 antigen with ELISA methods is difficult due to its slow replication in chickens.5,7−9 Therefore, a more specific and sensitive method is needed to detect ALV-K strains.
In the genome of ALV, the env gene, which contains gp37 and gp85 genes, is an important structural gene with a high mutation frequency. The gp37 gene encodes a transmembrane glycoprotein (TM), which is responsible for the transfer of ALV into host cells and is more conserved than the gp85 protein.10 The gp85 gene encodes a surface glycoprotein (SU), which determines the pathogenicity and virulence of different ALV subgroups,11 is mainly responsible for mediating the binding process between the virus and the corresponding host cell membrane receptors and can also stimulate the body to produce specific antibodies.12,13 Generally, the gp85 gene is used to identify ALV subgroups according to its nucleotide homology and prepare a neutralizing antibody or monoclonal antibody (MAb) against ALV to establish specific detection methods.14,15
MAbs are important biomaterials in the detection and identification of pathogens and have been widely applied for the development of specific and sensitive detection methods.16−19 A monoclonal antibody against ALV-J has been successfully prepared and used to establish an indirect immunofluorescence assay (IFA) and an ultrasensitive electrochemical immunoassay.20−22 In our laboratory, an ALV-A monoclonal antibody was also successfully developed and utilized in a rapid and specific colloidal gold test strip.23,24 Recently, a MAb against ALV-K that can recognize an epitope of the gp85 protein containing 17 amino acid residues (129–AFGPRSIDTLSDWSRPQ–145) has been reported;25 however, it is difficult to apply it clinically due to the lack of reports about its biological characteristics such as specificity, stability, and antigenic epitope structure. In this study, a new MAb against another ALV-K gp85 epitope (217–RRNYT–221) was successfully prepared, and its biological characteristics were clarified, which contributes to the establishment of novel and specific detection methods for ALV-K.
Materials and Methods
Viruses, Cells, Antibodies, and Reagents
ALV-K-JS11C1 (GenBank: KF746200.1), ALV-A-SDAU09C1 (GenBank: HM452339), ALV-B-SDAU09C2 (GenBank: HM446005), and ALV-J-NX0101 (GenBank: AY897227) were gifts from Professor Zhizhong Cui. DF1 cells and SP2/0 cells are maintained in our laboratory. BALB/c mice were purchased from Jinan Pengyue Experimental Animal Breeding Co., Ltd. High Affinity Ni-NTA Resin and Protein G affinity chromatography medium were purchased from GenScript USA Inc., Nanjing, China. Reagents for western blot were purchased from Shanghai Biyuntian Biotechnology Co., Ltd. N-glycosaminidase F (PNGaseF) was purchased from Shanghai Yisheng Biotechnology Co., Ltd (no. 20411ES01). An ELISA kit for the identification of mouse MAb subtypes was purchased from Shanghai Yaji Biotechnology Co., Ltd. ALV P27 antigen detection kits were purchased from Beijing IDEXX Co., Ltd. A BCA protein analysis kit was purchased from Thermo Fisher Scientific (USA), and the plasmid extraction kit and PCR product purification kit were purchased from Beijing Tiangen Biochemical Technology Co., Ltd. Freund’s adjuvant, FITC-labeled goat anti-mouse secondary antibody, PEG-1500, hypoxanthine aminopterin thymidine (HAT) and hypoxanthine thymidine (HT) reagents and a monoclonal antibody subclass identification kit were purchased from Sigma-Aldrich.
Screening and Amplification of ALV-K gp85-Specific Gene
The gp85 gene sequences of ALV-K strains were compared with those of other published ALV subgroups using the DNAStar software, and the specific sequence of ALV-K containing approximately 310 bp was screened. A pair of specific primers (F, 5′-CGCGGATCCATGGTCCCTATGTTGGATGA GC-3′; R, 5′-CCCAAGCTTTAGCGAGGACCTGTCTGTGA-3′) was designed according to the open reading frame (ORF) sequence of the ALV-K-JS11C1 gp85 gene (GenBank: KF746200.1), and the specific gene was amplified from the cDNA of ALV-K-JS11C1 gp85 gene. The PCR conditions were as follows: an initial step at 95 °C for 3 min, denaturizing at 94 °C for 30 s, annealing at 59 °C for 30 s, and extension at 72 °C for 30 s, followed by an extension at 72 °C for 5 min. The PCR product was observed using electrophoresis in 1% agarose gel.
Construction of a Recombinant Expression Plasmid Containing ALV-K gp85 Gene
PCR products were purified with an agarose gel recovery kit (TianGen) and transformed into a pMD18-T vector and pET-32a vector to obtain recombinant clone vector pMD18-T-gp85 and recombinant expression vector pET-32a-gp85 containing a His-tag. The successful establishment of the recombinant vector pMD-18T-gp85 and recombinant expression vector pET-32a-gp85 was confirmed by enzyme digestion and DNA sequencing (BGI Biotechnology Co., Ltd. Shenzhen, China).
Expression and Purification of Recombinant ALV-K gp85 Protein
The recombinant pET32a-gp85 plasmid was expressed in BL21 (DE3) cells with 1.0 mM isopropyl-β-d-thiogalactoside (IPTG) at 37 °C. The cells were harvested 3 h post-induction and lysed in a lysis buffer (150 mM NaCl, 100 mM Tris-Cl, 1 mM phenylmethanesulfonyl fluoride (PMSF), 1 mg/mL lysozyme, and 1% glycerol; pH 8.0).The soluble fraction was harvested and applied to a high-affinity Ni-NTA column (GenScript USA Inc., Nanjing, China). The eluted proteins were further purified by running the eluate through an SD200 gel filtration column (GenScript USA Inc., Nanjing, China) twice, with and without 1% nadeoxycholate, to remove the endotoxins. The elution product was identified by 12% polyacrylamide gel electrophoresis (SDS–PAGE), and the purity of the protein was analyzed by Western blotting with an anti-His antibody. The total protein content was determined with a BCA protein detection kit.
Immunization of Mice
The purified recombinant ALV-K gp85 protein with complete/incomplete Freund’s adjuvant was intraperitoneally injected into 6-week-old BALB/c mice (200 μg protein antigen per mouse). Booster immunizations were administered every other week; on the 15th day after the third immunization, the titer of the antibody was detected by indirect ELISA with recombinant ALV-K gp85 protein as the antigen and mouse serum as the primary antibody according to a previously reported method.23 Mice with a high antibody titer (>1:107) were used to prepare antibody-secreting splenic lymphocytes.
Preparation of Monoclonal Hybridoma Cells
Monoclonal hybridoma cells were prepared according to published methods.23,26 Logarithmically growing myeloma cells (SP2/0 cells) were fused with antibody-secreting splenic lymphocytes at a ratio of 1:10 using polyethylene glycol (PEG) reagent. The fused cells were first cultured in hypoxanthine-aminopterin-thymidine (HAT) medium for 7 days and then suspended in hypoxanthine-thymidine (HT) medium. The surviving hybridoma cells grew to cover more than 1/3 of the bottom area of each well, and their supernatants were analyzed using indirect ELISA with the purified ALV-K-JS11C1 strain applied as a coating antigen according to a published method.23 Serum from the inoculated mice and the supernatant of SP2/0 cells were used as positive and negative controls, respectively. Hybridoma cells with positive supernatants were cloned five times by limiting the dilution, and their chromosomes in the nucleus were identified using the Giemsa staining method.23 The hybridoma cells were passaged for 20 generations. The stability of the antibody secreted by the hybridoma cell line was detected by indirect ELISA using recombinant ALV-Kgp85 protein as the antigen and hybridoma cell supernatant as the primary antibody.
Analysis of Monoclonal Antibody Subclasses and Specificity
Adult female BALB/c mice were inoculated with positive hybridoma cells to prepare the ascetic fluid containing MAb. The prepared ascetic fluid was purified according to the protein G instructions as follows: the ascite samples and the binding/washing buffer were homogeneously mixed and added to the adsorption column containing the medium of precipitated protein G affinity chromatography. The outflow fluid samples were slowly collected and washed with 30 mL of binding/washing buffer, followed by the addition of 10 mL of elution buffer before collecting the eluted samples; neutralization buffer was added and the pH was adjusted to 7.4; the eluted samples were then placed into a dialysis bag, and the MAb was dialyzed with phosphate buffered saline (PBS) (pH 7.4). The titer of purified ascetic MAb was detected by indirect ELISA with the prepared recombinant ALV-K gp85 protein as the antigen and the diluted MAb as primary antibody. The class and subclass of the MAb were determined using a commercial mouse monoclonal antibody isotype ELISA kit (Proteintech) according to the manufacturer’s protocol.
The purified recombinant ALV-K gp85 protein and the gp85 protein of ALV-K-JS11C1 in DF1 cells were used to identify the MAb binding reaction by western blot as antigens. The protein of ALV-K-JS11C1 strain was collected from DF1 cells inoculated with ALV-K-JS11C1 strain (the S/P value of ALV P27 antigen ELISA ≥1.5) and treated with RIPA lysis solution according to a previously reported method.23 The obtained proteins were digested with PNGase F enzyme to cleave N-glycosylated products for western blotting analysis according to the instruction manual of the kit.
Additionally, ALV-K-JS11C1, ALV-A-SDAU09C1, ALV-B-SDAU09C2, and ALV-J-NX0101 strains with the viral titers not less than 103 TCID50 were inoculated into DF1 cells. Five days after inoculation, the S/P value of the virus in the cell supernatant was detected by an ALV P27 antigen detection kit, and an immunofluorescence test (IFA) mediated by the prepared MAb was established with culture supernatant-positive DF1 cells to evaluate the specificity of the monoclonal antibody.
Determination of the Linear Epitope Recognized by the MAb
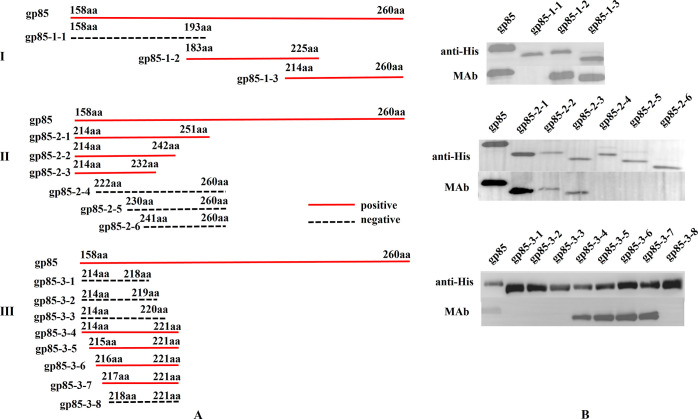
To locate the antigenic epitope recognized by the MAb on the ALV-K gp85 protein antigen, a series of overlapping truncated ALV-K gp85 fragments, shown in Figure 5A, were designed in this study, and their primers are listed in Table 1. These truncated gene fragments were amplified and cloned into pET-32a vectors and expressed in E. coli as fusion proteins containing His-tags. After purification, these expressed protein products were used as the antigens, and the MAb was used as the first antibody. Then, the location of the antigen epitope was determined according to the binding reaction of the antigen and antibody in western blot.
Figure 5.
Analysis of the MAb linear antigen epitope using the expressed protein fragments by western blot. (A) Strategies for expressing different protein fragments of gp85 protein antigen (the red line indicates the positive responses in western blot; the dotted black line indicates the negative ones). (B) Reaction results of 17 overlapping truncated protein fragments in western blot mediated with the prepared MAb.
Table 1. Primers and Locations of 17 Overlapping Truncated ALV-Kgp85 Gene Fragments.
| primer
sequences (5′–3′) |
|||
|---|---|---|---|
| number | forward | reverse | location (aa) |
| gp85-1-1 | GTCCCTATGTTGGATGAGC | AGCTACGCATCCCCCG | 158–193 |
| gp85-1-2 | CAGATCCCTAACGTGGC | GCAATTACACAGAGCCGTTTACG | 183–225 |
| gp85-1-3 | CTCCTCCTCCGCCGC | AGCGAGGACCTGTCTGT | 214–260 |
| gp85-2-1 | CTCCTCCTCCGCCGC | TAGCTTCCAAAATCTGTAACCATA | 214–251 |
| gp85-2-2 | CTCCTCCTCCGCCGC | ACCGCAGTACTCACTCCC | 214–242 |
| gp85-2-3 | CTCCTCCTCCGCCGC | TGTCTATCTGCTGTCACC | 214–232 |
| gp85-2-4 | GAGCCGTTTACGCTGGTGA | AGCGAGGACCTGTCTGT | 222–260 |
| gp85-2-5 | GATAGACACAATCTTTTTAGGG | AGCGAGGACCTGTCTGT | 230–260 |
| gp85-2-6 | TGCGGTACATATGGTTACAGA | AGCGAGGACCTGTCTGT | 241–260 |
| gp85-3-1 | CTCCTCCTCCGCCGC | GCGGCGGAGGAGGAG | 214–218 |
| gp85-3-2 | CTCCTCCTCCGCCGCAAT | ATTGCGGCGGAGGAGGAG | 214–219 |
| gp85-3-3 | CTCCTCCTCCGCCGCAATTAC | GTAATTGCGGCGGAGGAGGAG | 214–220 |
| gp85-3-4 | CTCCTCCTCCGCCGCAATTACACA | TGTGTAATTGCGGCGGAGGAGGAG | 214–221 |
| gp85-3-5 | CTCCTCCGCCGCAATTACACA | TGTGTAATTGCGGCGGAGGAG | 215–221 |
| gp85-3-6 | CTCCGCCGCAATTACACA | TGTGTAATTGCGGCGGAG | 216–221 |
| gp85-3-7 | CGCCGCAATTACACA | TGTGTAATTGCGGCG | 217–221 |
| gp85-3-8 | CGCAATTACACA | TGTGTAATTGCG | 218–221 |
To further verify the accuracy of the epitope, the eight peptides in Figure 6A were artificially synthesized, and their reactions with the prepared MAb were analyzed using ELISA. Their optical density (OD) values at 450 nm were detected and statistically compared.
Figure 6.
Identification on the shortest MAb linear antigen epitope using the synthesized peptides by ELISA. (A) Strategies for synthesizing the peptides. (B) Results of ELISA (MAb, the ascites antibody from the prepared monoclonal hybridoma cells; NC, negative control using the negative mouse’s serum; ** on the bar means significant difference (P < 0.01) compared with NC groups).
Analysis of the Spatial Epitope Recognized by the MAb
According to the previously reported methods,23,27 the secondary structural domains (e.g., α-helix, β-folder/turn, and random coil) and the biochemical characteristics (e.g., hydrophilicity, amphipathic regions, flexible regions, surface probability, and antigenic index) of ALV-K gp85 protein fragments containing the antigenic epitope were predicted using Biological Information Analysis software; additionally, a partial gp85 protein amino acid sequence of the ALV-K-JS11C1 strain containing the antigenic epitope was sent to Swiss-Model (http://swissmodel.expasy.org/) to construct a three-dimensional (3D) model online and simultaneously used to predict the spatial conformation of the antigen epitope.27
Biological Information Analysis of MAb’s Antigenic Epitope
The gp85 gene sequences of 43 representative ALV strains, namely, 17 strains of ALV-K, 8 strains of ALV-A, 5 strains of ALV-B, 6 strains of ALV-J, 1 strain of ALV-C, 1 strain of ALV-D, and 5 strains of ALV-E, were obtained from GenBank to identify the existence of the antigenic epitope in these ALV strains. The differences in the antigenic epitope were compared with the Megalign software.23
Immunofluorescence Assay
DF1 cells were fixed for 30 min in precooled methanol at 4 °C and then washed three times with PBS. The cells were blocked with 10% fetal bovine serum, incubated with the prepared MAb at a dilution of 200% for 2 h, and then washed three times with phosphate-buffered saline with Tween (PBST). Sheep anti-mouse antibodies coupled with fluorescein isothiocyanate (FITC) were incubated at 37 °C for 1 h at a ratio of 1–100 and washed three times with PBST. The images were collected by fluorescence microscopy, and the fluorescence signals in the whole imaging area were analyzed using Image J software.
Western Blot
The expressed products including 14 overlapping truncated protein fragments were mixed with SDS–PAGE sample buffer, boiled for 10 min, and separated by 12% SDS–PAGE. The proteins on SDS–PAGE protein gels were transferred to polyvinylidene fluoride (PVDF) membranes by rapid transmembrane solution. The PVDF film was blocked in tris-buffered saline with Tween (TBST) solution for 2 h with 5% skimmed milk powder at room temperature and washed three times with PBST. The diluted MAb was used as the primary antibody and incubated at 4 °C overnight. After washing with PBST, the PVDF membrane was treated with horseradish peroxidase (HRP)-labeled sheep anti-mouse IgG as a secondary antibody at room temperature for 1 h. After washing three times with TBST, the results were observed on a Tanon 5200 chemiluminescence imaging system with electrochemiluminescence (ECL) reagent.
Ethics Statement
The animals in this study were approved by the Animal Ethics Committee of Shandong Animal Protection and Welfare Institute (no. SDAU2020-044). All animal-related procedures and care were in line with the internationally accepted principles in the government-issued guidelines for the feeding of experimental animals.
Results and Discussion
Construction of the Recombinant Expression Plasmid Containing a Specific ALV-K gp85 Gene Fragment
The gp85 gene is the key gene for distinguishing different avian leukemia virus subgroups12,13 and has been used to successfully prepare ALV-A/J monoclonal antibodies as discussed in previous reports.20,23 In this study, the gp85 sequences of ALV-A/B/J/K strains were first compared with DNASTAR software, and the specific gene fragment, which is located in the 283–592 bp of ALV-K gp85 gene (Table 2), was screened as the target gene due to its low homology with ALV-A/B/J strains and a high antigenic index (data not shown); then, the target gene with the size of 310 bp was amplified from the cDNA of ALV-K-JS11C1 by PCR (Figure 1A, lane 5). The sequencing result shows that its sequence is completely consistent with that of the screened ALV-K-JS11C1 gene fragment in Table 2. Compared with the sequence obtained using the whole gp85 gene as a target gene in the previous report,25 this strategy greatly improved the accuracy and efficiency of preparing a specific monoclonal antibody.
Table 2. Sequence of ALV-A/B/J/K Strains gp85 Gene Fragment (472–781 bp).
| ALV subgroup strains (GenBank No) | the sequence of gp85 fragment (472–781 bp) |
|---|---|
| ALV-K-JS11C1 (KF746200.1) | 5′-gtccctatgttggatgagccaccggaactacagctgctaggttcccagtctctccctaacattactaacattactcagatccctaacgtggccgggggatgcgtagctttcggcccccggagcattgacaagctttcagattggtccaggccgcaactcacgaggtggctcctcctccgccgcaattacacagagccgtttacgctggtgacagcagatagacacaatctttttagggggagtgagtactgcggtacatatggttacagattttggaagctatataattgttcacagacaggtcctcgct-3′ |
| ALV-A-SDAU09C1 (HM452339) | 5′-gccacctccccttttcaaacatgtttgataggtatcccgtcccctatttccgaaggtgattttaagggatacgtctctgataattgcaccaccttgggaactgaccggttagtctcgtcagccagcattactggcggccctgacaacagcaccaccctcacttatcgaaaggtttcatgcttgctgttaaagctgaatgtctctatgtgggatgagccaccggaactacagctgctaggttcccagtctctccctaacattactgatattactcagatttctggtgtagctgggggatgcgtaggcttca-3′ |
| ALV-B-SDAU09C2 (HM446005) | 5′-aatgtttccctgttggacgagccatcagaactacaactgttaggttcccagtctctccctaatataactaatattactcagatccctagtgtggctgggggatgcatcggcttcaccccgtacggcagtccggcgggcgtttacggatgggaccggagacaagttacacacatcctcttgactgacccagggagcaatcctttctttaataaggcctctaactcctcgaaaccgtttacagtagtgacagcagataggcacaatctttttatgggaagtgaatattgcggtgcatatggctacagatttt-3′ |
| ALV-J-NX0101 (AY897227) | 5′-acctgcttgataggcattccacagtatcctctgaacacctttgagggatatgtcactaatgttactgcttgcgataacaacgccgatttagccagccaaacagcatgcttgataaaggctcttaacacaaacctcccttgggacccccaaaaattggatattttagggtctcagatgatcaagaacggaacaacacgtacgtgtgttacctttggttcgatgtgctataaagagaacaatcacagcagagtctgccacaattttgatgggaattttaatgggactggtggggtggaagcagaattgcgtg-3′ |
Figure 1.
Amplification of specific ALV-K gp85 gene fragment (A) and construction of the recombinant expression plasmid (B). (A) Lane M, DNA marker; Lane 1, DF1 cell control; Lane 2, ALV-A-SDAU09C1; Lane 3, ALV-B-SDAU09C2; Lane 4, ALV-J-NX0101; and Lane 5, ALV-K-JS11C1. (B) Lane M1/M2/M3, DNA marker; Lane 1, double enzyme digestion of pMD18T-ALV-K; Lane 2, single enzyme digestion of pMD18T-ALV-K; Lane 3, double enzyme digestion of pET-32a-ALV-K; Lane 4, single enzyme digestion of pET32a-ALV-K; and Lane 5, single enzyme digestion of pET32a.
The target gene was ligated into the pMD18-T vector to construct the recombinant amplification plasmid (pMD18T-ALV-K) and prepare enough target gene products. Then, these target gene products were ligated into the pET32a plasmid to construct the recombinant expression plasmid (pET32a-ALV-K). The two recombinant plasmids including pMD18T-ALV-K and pET32a-ALV-K (Figure 1B, lane 2 and lane 4/5) were confirmed by agarose gel electrophoresis, and the results of double enzyme digestion (Figure 1B, lane 1 and lane 3) and sequencing showed that the recombinant plasmids containing the target gene were successfully constructed.
Expression and Purification of the Specific ALV-K gp85 Protein Products
The recombinant pET-32a-gp85 plasmid containing a His-tag was transferred into E. coli cells and successfully expressed with IPTG induction (Figure 2A, lane 3). The recombinant expression product containing target ALV-K gp85 protein and His-tag was approximately 30 kDa and purified by high-affinity chromatography gel filtration (GenScript USA Inc., Nanjing, China). The purified product was confirmed by SDS–PAGE (Figure 2A, lane 4) and western blot mediated by anti-His MAb (Figure 2B).
Figure 2.

Expression and purification of the recombinant ALV-K gp85 protein fragment (A) and identification of western blot (B). (A) Lane M, protein Marker; Lane 1, pET32a empty plasmid; Lane 2, pET32a-ALV-K plasmid without IPTG; Lane 3, pET32a-ALV-K plasmid with IPTG; and Lane 4, purified expressed product of pET32a-ALV-K. (B) Lane M, protein Marker and Lane 1, western blot mediated by anti-His MAb.
Preparation of the Antibody-Secreting Hybridoma Cells
In many reports, IgG antibody is considered to be more suitable for the establishment of specific detection methods because it has better stability and higher antibody titers.28−30 To prepare the IgG-secreting hybridoma cells in this study, the expressed protein products were purified and inoculated into Balb/c mice, and booster inoculations were administered three times to obtain more IgG-secreting spleen lymphocytes. Finally, dozens of antibody-secreting hybridoma cells were successfully screened using ELISA and IFA with ALV-K-JS11C1 strain coating. Ultimately, a monoclonal antibody-secreting hybridoma cell line named 30B9 was obtained after repeatedly subcloning these hybridoma cells.
In general, MAb hybridoma cells prepared by the cell-fusion method with mouse myeloma cells and spleen lymphocytes in vitro have specific biological characteristics, such as increased nuclear chromosomes, antibody-secreting performance, and permanent proliferation ability, and these characteristics are also used to identify monoclonal hybridoma cells.31,32 The results show that the hybridoma cell line prepared in this study contains approximately 98 chromosomes in its nucleus (Figure 3A) and can stably secrete the MAb, binding to the ALV-K gp85 protein, as confirmed by western blotting (Figure 3B,C); the subclass of the secreted MAb was identified as IgG2b using a mouse antibody homotype ELISA kit (Figure 3D), and the titers of the MAb reached 1:214 (Figure 3E). Moreover, it was also confirmed that antibody secretion was not significantly affected after 20 passages of hybridoma cells (data not shown). These results suggest that the antibody secretion of the prepared hybridoma cell line is superior to that described in previous reports.25
Figure 3.
Analysis of the monoclonal hybridoma cell’s chromosomes (A) and identification of the secreted MAb by western blot (B,C) and determination of the antibody subclasses (D) and the titers of ascites MAb (E). (A) Chromosomes in the nucleus were identified by Giemsa-staining method under a microscope (10 × 100). (B) Western blot with the expressed ALV-K gp85 protein as antigen and the ascites antibody as primary antibody. (C) Western blot with ALV-K-JS11C1 protein as antigen and the ascites antibody as primary antibody; Lane M, protein marker; lane 1, positive reactions to the viral proteins after being digested with PNGase F enzyme; and lane 2, positive reactions to the viral proteins before being digested with PNGase F enzyme. (D) Subclass of the MAb was identified using mouse antibody homotype ELISA kit; **, P < 0.01. (E) Titers of ascites MAb were determined by indirect ELISA using recombinant ALV-K gp85 protein as the antigen and the ascites MAb as the primary antibody.
It can be shown from the results in Figure 3B,C that the prepared MAb can react both with the expressed products of the pET32a-ALV-K plasmid (Figure 3B) and with the extracted gp85 protein from the ALV-K-JS11C1 strain (Figure 3C). In Figure 3C, the extracted viral proteins were digested with PNGase F enzyme (an amidohydrolase, which can cleave high mannose, heterozygous, and complex oligosaccharide from the glycoprotein linked by asparagine), and the digested products were analyzed by the western blot mediated with the prepared MAb; the results show a positive reaction at the electrophoretic band (39 kDa) of the digested protein. It further confirms that the prepared MAb can also bind to the N-glycosylated envelope protein of ALV-K-JS11C1 strain in DF1 cells. Additionally, because the gp85 protein obtained from ALV-K-JS11C1-infected DF1 cells is part of the envelope membrane protein encoded by the env gene, and its molecular weight in Figure 3C is larger than that of the prepared recombinant ALV-K gp85 protein in Figure 3B, therefore these proteins exhibited different positive reaction positions in the western blotting assay.
Specificity of the MAb
To analyze the specificity of the prepared MAb, the ALV-A-SDAU09C1, ALV-B-SDAU09C2, ALV-J-NX0101, and ALV-K-JS11C1 strains with no less than 103TCID50/0.1 mL were inoculated into DF1 cells and detected using IFA mediated with the prepared MAb. The results in Figure 4 show that green fluorescence was present in the DF1 cells inoculated with the ALV-K-JS11C1 strain (Figure 4A) but not in the cells inoculated with the ALV-A-SDAU09C1 strain (Figure 4B), ALV-B-SDAU09C2 strain (Figure 4C), or ALV-J-NX0101 strain (Figure 4D). The statistical results also show that the relative fluorescence intensity in the DF1 cells infected with ALV-K strain is significantly higher than that in the cells infected with other subgroup strains (Figure 4E). These results suggest that the prepared MAb can recognize only the ALV-K-JS11C1 strain but not the ALV-A-SDAU09C1 strain, ALV-B-SDAU09C2 strain, or ALV-J-NX0101 strain.
Figure 4.
IFA mediated by the prepared MAb in the DF1 cells containing different ALV strains. (A) ALV-K-JS11C1 strain; (B) ALV-A-SDAU09C1 strain; (C) ALV-B-SDAU09C2 strain; and (D) ALV-J-NX0101 strain. (E) Relative fluorescence intensity in the DF1 cells infected with different ALV subgroup strains was quantified using image J software. ** on the bar means significant difference (P < 0.01) compared with ALV-A/B/J strain. The S/P value of ALV P27 antigen in the cells’ supernatant of each sample used in IFA is not less than 1.0.
Identification of the Linear Epitope Recognized by the MAb
Antigenic epitope, including linear epitope (a small linear peptide amino acid sequence) and conformational epitope (a large region formed by protein folding) is an important structural basis for the specific recognition of an antigen by a MAb33,34 and is usually developed as diagnostic markers in clinics.27,29 Therefore, identifying epitopes is very important to assess the innovation of a MAb. In this study, the linear epitope of the prepared MAb was first identified. In Figure 5A(I), three overlapping truncated ALV-K gp85 protein fragments with His-tags, namely, gp85-1-1 (158–193 aa), gp85-1-2 (183–225 aa), and gp85-1-3 (214–260 aa), were designed and expressed in E. coli for a western blot; the recombinant ALV-K gp85 protein (158–260 aa) containing His-tags on the expressed products were used as positive controls. In Figure 5B(I), the western blotting results show that the gp85-1-2 and gp85-1-3 fragments, but not the gp85-1-1 fragment, could react with the MAb, indicating that the epitope is located near the repeating region (214–225 aa) of gp85-1-2 and gp85-1-3.
According to the above western blotting results, six overlapping truncated protein fragments, including gp85-2-1 (214–251 aa), gp85-2-2 (214–242 aa), gp85-2-3 (214–232 aa), gp85-2-4 (222–260 aa), gp85-2-5 (230–260 aa), and gp85-2-6 (241–260 aa), were designed [Figure 5A(II)] and expressed. The results in Figure 5B(II) show that the gp85-2-1, gp85-2-2, and gp85-2-3 protein fragments, but not the gp85-2-4, gp85-2-5, and gp85-2-6 fragments, could react with the MAb, suggesting that the MAb epitope exists from aa 214 to 221 on the whole gp85 protein.
To locate the amino acids of the antigenic epitope, eight protein fragments, gp85-3-1 (214–218 aa), gp85-3-2 (214–219 aa), gp85-3-3 (214–220 aa), gp85-3-4 (214–221 aa), gp85-3-5 (215–221 aa), gp85-3-6 (216–221 aa), gp85-3-7 (217–221 aa), and gp85-3-8 (218–221 aa), from the 214–221 aa fragment were truncated [Figure 5A(III)] and analyzed by western blot. The results in Figure 5B(III) show that only the gp85-3-4, gp85-3-5, gp85-3-6, and gp85-3-7 protein fragments reacted with the MAb, which suggests that the MAb’s antigenic epitope contains five consecutive amino acids, namely, 217–RRNYT–221.
To further confirm the above result, eight peptides that included different amino acids of the antigenic epitope in the table of Figure 6A were artificially synthesized, and the ELISA results in Figure 6B show that the optical densities at 450 nm (OD450) of peptides containing the complete 217–RRNYT–221 sequence are much higher than those of the peptides containing the less antigenic epitope amino acids (P < 0.01). These results confirm that 217–RRNYT–221 is an optimum linear epitope of the MAb and differs from that reported by Chen et al.,25 which also proves that the prepared monoclonal hybridoma cell line is a new cell line.
Prediction and Analysis of the Spatial Structure of the MAb Epitope
Some studies have shown that the conformational epitope of the MAb also plays an important role in immune recognition,28,31 but it is often ignored in many studies due to the need for more sophisticated methods.25,33 To analyze the epitope’s spatial structure and biochemical characteristics, the secondary structural domains (e.g., α-helix, β-folder/turn, and random coil) and spatial conformation of ALV-K gp85 protein fragments were predicted by the biological software Protean and the online website Swiss-Model. The results predicted by Chou-Fasman and Garnier-Robson show that the MAb epitope contains a turning angle with high hydrophilicity, antigen index, and surface accessibility (Figure 7A). The three-dimensional spatial structure model shows that five amino acids of the antigenic epitope form a curved linear spatial structure and are exposed to the surface of the ALV-K gp85 protein (Figure 7B,C), which benefits binding to the MAb. These findings provide more evidence to better understand the immune recognition between the ALV-K gp85 protein and the MAb.
Figure 7.
Prediction of the biochemical characteristics and spatial conformation of the MAb’s antigen epitope. (A) Predicted secondary structures and biological characteristics of the MAb’s antigen epitope. (B) Predicted three-dimensional structure of the MAb’s antigen epitope on ALV-K gp85 protein. (C) Predicted spatial conformation of the MAb’s antigen epitope on ALV-K gp85 protein. The gene sequence was obtained from ALV-K-JS11C1 (GenBank: KF746200.1) in Genbank. The model was constructed using Swiss-Model Online Prediction Software.
Comparisons of the Antigenic Epitope in Different ALV Strains
In Figure 8, the gp85 amino acid sequences of 43 representative ALV strains, including 17 ALV-K strains, 8 ALV-A strains, 5 ALV-B strains, 6 ALV-J strains, 1 ALV-C strain, 1 ALV-D strain, and 5 ALV-E strains, were used to analyze the variation of the antigenic epitope in different ALV strains. The results in Figure 8 show that 5 amino acids of the antigenic epitopes are highly conserved in 17 ALV-K strains including ALV-K-JS11C1 strain used in IFA but are very low (≤40%) in other ALV subgroup strains, including ALV-A-SDAU09C1 strain, ALV-B-SDAU09C2 strain, and ALV-J-NX0101 strain, which can explain why the developed MAb can only recognize ALV-K-JS11C1 strain but not the ALV-A-SDAU09C1 strain/ALV-B-SDAU09C2 strain/ALV-J-NX0101 strain in IFA. Correspondingly, it can be deduced that the MAb (30B9) can also recognize other 16 ALV-K strains but not ALV-A/B/C/D/E/J strains among the published 41 ALV subgroups strains. These results provide important data for the MAb’s future application in clinics.
Figure 8.
Analysis on the variation of the antigen epitope recognized by the MAb (30B9) in different ALV subgroups’ strains. Number of representative strains: 17 ALV-K STRAINS; 8 ALV-A strains; 5 ALV-B strains; 6 ALV-J strains; 1 ALV-C strain; 1 ALV-D strain; and 5 ALV-E strain. The ALV strains used in IFA are marked with a box.
Conclusions
A new hybridoma cell line (30B9), which can stably secrete IgG antibody against ALV-K, was successfully prepared, and the secreted MAb can recognize ALV-K strains but not ALV-A/B/J strains in IFA. The MAb’s antigen epitope contains five consecutive amino acids (217–RRNYT–221) and forms a hydrophilic and curved linear spatial structure in the 217–RRNYT–221 region; these five amino acids have complete homology in all 17 published representative ALV-K strains but few in other ALV subgroup strains. This study supplies a new biomaterial for developing new specific diagnostic methods for the ALV-K subgroup.
Acknowledgments
This study was funded by the National Key Research and Development Program of China (2016YFD0500800), by the Project of Shandong Provincial Natural Science Foundation (ZR2020MC179), by Funds of Shandong Student Research Training (S202110434005), and by Funds of Shandong “Double Tops” Program.
Author Contributions
X.Z., H.L., and C.W. contributed equally. H.G. and J.Q. conceived and designed the study; X.Z., C.W., and H.L. performed the experiments, analyzed the results, and prepared the manuscript; Y.D., Y.L., L.Z., and M.H. assisted in analyzing the results and manuscript preparation.
The authors declare no competing financial interest.
References
- Payne L. N.; Nair V. The long view: 40 years of avian leukosis research. Avian Pathol. 2012, 41, 11–19. 10.1080/03079457.2011.646237. [DOI] [PubMed] [Google Scholar]
- Payne L. N.; Gillespie A. M.; Howes K. Induction of myeloid leukosis and other tumours with the HPRS-103 strain of ALV. Vet. Rec. 1991, 129, 447–448. 10.1136/vr.129.20.447. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Hu W.; Li B.; Chen R.; Shen W.; Guo H.; Guo H.; Li H. Comparison of Viremia, Cloacal Virus Shedding, Antibody Responses and Pathological Lesions in Adult Chickens, Quails, and Pigeons Infected with ALV-A. Sci. Rep. 2019, 9, 3027. 10.1038/s41598-019-39980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z. Z.; Guo H. J.; Sun S. H. Prevalence Situation and Prevention and Control of Avian Leukosis. Chin. J. Vet. Drug 2009, 43, 37–41. [Google Scholar]
- Wang X.; Zhao P.; Cui Z. Identification of a new subgroup of avian leukemia virus isolated from local chicken breeds in China. J. Virus 2012, 28, 609–614. [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc. Natl. Acad. Sci. U.S.A. 1972, 69, 576–580. 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Lin W.; Chang S.; Zhao P.; Zhang X.; Liu Y.; Chen W.; Li B.; Shu D.; Zhang H.; Chen F.; Xie Q. Isolation, identification and evolution analysis of a novel subgroup of avian leukosis virus isolated from a local Chinese yellow broiler in South China. Arch. Virol. 2016, 161, 2717–2725. 10.1007/s00705-016-2965-x. [DOI] [PubMed] [Google Scholar]
- Li X.; Yu Y.; Ma M.; Chang F.; Muhammad F.; Yu M.; Ren C.; Bao Y.; Zhang Z.; Liu A.; Pan Q.; Gao L.; Qi X.; Li K.; Liu C.; Zhang Y.; Cui H.; Wang X.; Gao Y. Molecular characteristic and pathogenicity analysis of a novel multiple recombinant ALV-K strain. Vet. Microbiol. 2021, 260, 109184. 10.1016/j.vetmic.2021.109184. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Rao M.; Chen J.; Zhang J.; Yuan L.; Liao M.; Cao W. Analysis of 5′LTR sequence and initiating activity of subgroup K Avian Leukosis virus. J. Anim. Husb. Vet. Med. 2018, 49, 754–760. [Google Scholar]
- Li T.; Yao X.; Li C.; Zhang J.; Ye J. Gp37 Regulates the Pathogenesis of Avian Leukosis Virus Subgroup J via Its C Terminus. J. Virol. 2020, 94, e02180 10.1128/jvi.02180-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R.; Graham W. D.; Zhou S. Sequencing the Biology of Entry: The Retroviral env Gene. Curr. Top. Microbiol. Immunol. 2017, 407, 65–82. 10.1007/82_2017_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal K.; Howes K.; Barron G. S.; Payne L. N. Recombinant env-gp85 of HPRS-103 (subgroup J) avian leukosis virus: antigenic characteristics and usefulness as a diagnostic reagent. Avian Dis. 1997, 41, 283–288. 10.2307/1592179. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Cui Z.; Zhang Z.; Wu Y. The mutation tendency of the gp85 gene of Chinese field strains of ALV-J from 1999 to 2003. Virol. Sin. 2005, 20, 393–398. [Google Scholar]
- Bova C. A.; Olsen J. C.; Swanstrom R. The avian retrovirus env gene family: molecular analysis of host range and antigenic variants. J. Virol. 1988, 62, 75–83. 10.1128/jvi.62.1.75-83.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Meng F. W.; Li Y.; Wang S.; Chang P.; Zhao Z.; Cui Z. Characterization of avian leukosis virus subgroup J isolated between 1999 and 2013 in China. Poult. Sci. 2018, 97, 3532–3539. 10.3382/ps/pey241. [DOI] [PubMed] [Google Scholar]
- Nanbu A.; Hayakawa M.; Takada K.; Shinozaki N.; Abiko Y.; Fukushima K. Production, characterization, and application of monoclonal antibodies which distinguish four glucosyltransferases fromStreptococcus sobrinus. FEMS Immunol. Med. Microbiol. 2000, 27, 9–15. 10.1111/j.1574-695x.2000.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Lv L.; Sun H.; Li S.; Fan H.; Wang X.; Bai J.; Jiang P. Identification of linear B cell epitope on gB, gC, and gE proteins of porcine pseudorabies virus using monoclonal antibodies. Vet. Microbiol. 2019, 234, 83–91. 10.1016/j.vetmic.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Pan X.; Wu Y.; Wang W.; Zhang L.; Xiao G. Novel neutralizing monoclonal antibodies against Junin virus. Antiviral Res. 2018, 156, 21–28. 10.1016/j.antiviral.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Petrovan V.; Yuan F.; Li Y.; Shang P.; Murgia M. V.; Misra S.; Rowland R. R. R.; Fang Y. Development and characterization of monoclonal antibodies against p30 protein of African swine fever virus. Virus Res. 2019, 269, 197632. 10.1016/j.virusres.2019.05.010. [DOI] [PubMed] [Google Scholar]
- Qin A.; Lee L. F.; Fadly A.; Hunt H.; Cui Z. Development and characterization of monoclonal antibodies to subgroup J avian leukosis virus. Avian Dis. 2001, 45, 938–945. 10.2307/1592872. [DOI] [PubMed] [Google Scholar]
- Shang K.; Zhu J.; Meng X.; Cheng Z.; Ai S. Multifunctional Fe3O4 core/Ni-Al layered double hydroxides shell nanospheres as labels for ultrasensitive electrochemical immunoassay of subgroup J of avian leukosis virus. Biosens. Bioelectron. 2012, 37, 107–111. 10.1016/j.bios.2012.04.035. [DOI] [PubMed] [Google Scholar]
- Liu C.; Dong J.; Waterhouse G. I. N.; Cheng Z.; Ai S. Electrochemical immunosensor with nanocellulose-Au composite assisted multiple signal amplification for detection of avian leukosis virus subgroup J. J. Biosens. Bioelectron. 2018, 101, 110–115. 10.1016/j.bios.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Yan Z. Y.; Li H. M.; Wang C. C.; Qiu J.; Pan Y.; Zhang D.; Hu W.; Guo H. J. Preparation of a new monoclonal antibody against subgroup A of avian leukosis virus and identifying its antigenic epitope. Int. J. Biol. Macromol. 2020, 156, 1234–1242. 10.1016/j.ijbiomac.2019.11.161. [DOI] [PubMed] [Google Scholar]
- Hu W. G.; Yan Z. Y.; Li H. M.; Qiu J. H.; Zhang D. D.; Li P.; Pan Y.; Guo H. J. Development of a new colloidal gold immunochromatographic strip for rapid detecting subgroup A of avian leukosis virus using colloidal gold nanoparticles. Biochem. Eng. J. 2019, 148, 16–23. 10.1016/j.bej.2019.04.015. [DOI] [Google Scholar]
- Chen X.; Wang H.; Fang X.; Gao K.; Fang C.; Gu Y.; Gao Y.; Wang X.; Huang H.; Liang X.; Yang Y. Identification of a novel epitope specific for Gp85 protein of avian leukosis virus subgroup K. Vet. Immunol. Immunopathol. 2020, 230, 110143. 10.1016/j.vetimm.2020.110143. [DOI] [PubMed] [Google Scholar]
- Zhang P. P.; Lv L.; Sun H. F.; Li S. H.; Fan H.; Wang X. W.; Bai J.; Jiang P. Identification of linear B cell epitope on gB, gC, and gE proteins of porcine pseudorabies virus using monoclonal antibodies. Vet. Microbiol. 2019, 234, 83–91. 10.1016/j.vetmic.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Arnold K.; Bordoli L.; Kopp J.; Schwede T. The swiss-model workspace: a web-based environment for protein structure homology modeling. Bioinformatics 2006, 22, 195–201. [DOI] [PubMed] [Google Scholar]
- Saleem M.; Kamal M. Monoclonal antibodies in clinical diagnsis: A brief review application. Afr. J. Biotechnol. 2008, 7, 923–925. [Google Scholar]
- Seyfi Abad Shapouri M. R.; Rashno M.; Mahmoodi P.; Aria M. Production of a monoclonal antibody against chicken immunoglobulin G: A valuable molecule with research and diagnostic applications. Vet. Res. Forum 2018, 9, 67–72. [PMC free article] [PubMed] [Google Scholar]
- Yang M.; van Bruggen R.; Xu W. Generation and diagnostic application of monoclonal antibodies against Seneca Valley virus. J. Vet. Diagn. Invest. 2012, 24, 42–50. 10.1177/1040638711426323. [DOI] [PubMed] [Google Scholar]
- Kennett R. H. Hybridomas: a new dimension in biological analyses. Vitro 1981, 17, 1036–1050. 10.1007/bf02618601. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Ji R.; Wang J.; Han C.; Yu X.; Dong X.; Tao H. Establishment and preliminary characterization of hybridoma cell lines secreting monoclonal antibodies against prion proteins. Chin. J. Exp. Clin. Virol. 2003, 17, 133–136. [PubMed] [Google Scholar]
- Zhu R. L.; Cui Z. Z.; Zhao J. Establishment and biological properties of hybridoma cell lines secreting anti-IBDV idiotypic antibodies. Sheng Wu Gong Cheng Xue Bao 2003, 19, 462–466. [PubMed] [Google Scholar]
- Ma X.; Li R.; Hou B. Advent and rise of monoclonal antibodies. Sci. Bull. 2020, 65, 3078–3084. 10.1360/tb-2020-0492. [DOI] [Google Scholar]