Abstract

Deep eutectic solvents (DESs) can be used as potential solvents for various applications. However, their recovery depends on both economic and environmental considerations. In this study, the possibilities for the recovery of methyl triphenyl phosphonium bromide/triethylene glycol (MTPPB/TEG 1:4) after the application of combined dearomatization, desulfurization, and denitrogenation of fuels are investigated. The DES was first prepared and characterized for its density, viscosity, and water content. Then, the single-stage liquid–liquid extraction was conducted in addition to testing the repetitive use of the DES. After that, two regeneration methods were studied: the stripping method (with n-heptane) and the washing method (with distilled water or diethyl ether). In addition, a parametric study was conducted to optimize the regeneration methods. The results showed that washing the used DES with distilled water was significantly more effective than stripping the DES with n-heptane. In terms of quinoline reduction, distilled water reduced the quinoline content in the DES from 3.2 to 2.1 wt %, while n-heptane showed a minor reduction in the quinoline content (3.2 to 3 wt %). It was also found that a much more effective recovery could be achieved by (i) increasing the DES-to-regeneration solvent mass ratio and (ii) increasing the number of wash cycles. Furthermore, the regeneration temperature did not have a significant effect on the recyclability of the DES. The results demonstrated that the regenerated DES was as effective in extraction as a fresh batch of DES.
1. Introduction
Deep eutectic solvents (DESs) have attracted attention as promising, effective, and eco-friendly solvents in various processes.1−3 They were first coined by Abbott et al.,4 where a DES composed of choline chloride and urea in a molar ratio of 1:2 was reported. According to Abbott et al.,4 this designer solvent could form a eutectic mixture by mixing one or more hydrogen bond acceptors (HBAs) and one or more hydrogen bond donors (HBDs), which can include hydrogen bonding between its constituents. Nevertheless, the definition of the solvent remains a point of contention. More recently, Martins et al.(5) claimed that a DES could be defined as “a mixture of two or more pure compounds for which the eutectic point temperature is below that of an ideal liquid mixture presenting significant negative deviations from ideality. Additionally, the temperature depression should be such that the mixture is liquid at operating temperature for a specific composition range. Otherwise, a more specific term ‘eutectic solvent’ could describe mixtures that do not fulfill these criteria”. Nevertheless, there is still room for improvement in the definition.6−8
DESs have been actively applied as a promising alternative to volatile organic compounds and ionic liquids.9−12 DESs are attractive for several reasons, including their inexpsensive to moderate cost of synthesis, the absence of purifying steps required before use, their low flammability, and their biodegradability.1,13−15 Since their discovery, the application of DESs in various fields has shown the bright future of DESs as a promising solvent.16−19 Such an example could be found in fuel purification processes, where DESs led their effectiveness in improving the quality of fuels.20−23 One of the most advanced applications of DESs in fuel purification is the simultaneous removal of different aromatics. Choline chloride was used as an HBA in the DESs developed by Larriba et al.(24) to eliminate BTEX (i.e., benzene, toluene, ethylbenzene, and xylene), along with some HBDs (including ethylene glycol, glycerol, levulinic acid, phenylacetic acid, malonic acid, and urea). The results confirmed that DESs are a potential candidate to replace the extraction solvents for the removal of aromatics. Other studies have also investigated the performance of DESs in the simultaneous removal of different fuel impurities (i.e., sulfur- and nitrogen-containing aromatics). They showed the possibility of using DESs in the combined dearomatization, desulfurization, and denitrogenation processes.22,23,25−29
The economic and environmental aspects of the process regarding the amount of solvent utilized increased the importance of solvent recyclability. For example, regenerating the DES reduces costs and prevents the use of substantial amounts of DESs in every purifying startup.30 Therefore, DES recycling has gained importance from different aspects, and many publications have discussed the most effective recyclability methods.31−33
Regeneration methods generally depend on the nature and the extracted impurities of the solvent. For example, in the adsorption method, the DES is recovered by utilizing activated carbons or activated carbon fibers.34−36 In this technique, the adsorption efficiency depends mainly on the nature of the solvent and the adsorbent structure. Activated carbons are widely used because of their porous nature and large surface area. A study by Lee et al.(37) investigated different regeneration techniques for desulfurization. The adsorption method showed extraction efficiencies comparable to those of the fresh DES when extracting thiophene (Th), benzothiophene (BT), and dibenzothiophene (DBT).
A second example is the liquid–liquid back-extraction technique, in which several regeneration solvents demonstrated their viability in efficiently regenerating solvents. Either stripping or anti-solvent washing is used to execute this approach. The most common solvents are ether,38 diethyl ether,21 water,39 and n-heptane.40 Makoś and Boczkaj21 recovered choline chloride/phenol (1:4) containing Th, BT, and DBT using diethyl ether. Their investigation found that the DES could be recycled 15 times without a noticeable decrease in extraction efficiency. Another study by Zhu et al.(41) tested the use of diethyl ether for the recovery of the polyethylene glycol diacid/tetraethylammonium chloride (1:1) DES used for denitrogenation (i.e., removal of indole, pyrrole, quinoline, and pyridine).41 It was found that washing the DES with diethyl ether was effective in regenerating the solvent.
In addition, Ali et al.(40) investigated the feasibility of washing the choline chloride/phenylacetic acid (1:2) DES with water after using the DES in fuel denitrogenation. The results indicated that recycling and reusing the DES after washing with water effectively regenerated the DES. Simultaneously, Yin et al.(42) also reported the effectiveness of cleaning choline chloride/p-toluenesulfonic acid (1:2) with water in desulfurization.
The evaporation method is another example of a regeneration technique in which heating the mixture is the main driving force to recover the DES from the volatile compounds. Here, the operating conditions should be set with caution to avoid solvent degradation. Rogošić and Zagajski Kučan43 investigated the evaporation method where a DES consisting of choline chloride and propylene glycol was recovered in a 1:3 molar ratio after the removal of sulfur and nitrogen compounds from hydrocarbon mixtures. The results showed the success of applying the evaporation method to low-vapor pressure substances: toluene, Th, and pyridine.43
Other studies have investigated combining regeneration methods to maximize the recovery of the DES. In 2018, Lima et al.(44) used the liquid–liquid back-extraction method combined with the evaporation method to recover the tetrabutylammonium chloride/polyethylene glycol 400 (1:2) DES. The selected DES was first used to extract DBT and Th from n-heptane. Then, the regeneration was carried out by washing the rich phase (DES containing sulfur compounds) with water, where the DBT formed a precipitant. After that, the DES was regenerated using the evaporation method. Comparing the fresh and recycled DES confirmed the success of restoring the DES from both Th and water. The same procedure was applied with different DESs in other studies.45,46
To simulate fundamental fuel purification processes, our previous work investigated the use of the methyl triphenyl phosphonium bromide/triethylene glycol (MTPPB/TEG 1:4) DES in simultaneously extracting toluene, Th, and quinoline from n-heptane.28 However, it was found that regenerating the MTPPB/TEG 1:4 DES using evaporation under vacuum resulted in removing only the low-boiling-point impurities Th and toluene, whereas quinoline showed an insignificant reduction. Therefore, in this contribution, we aimed to overcome the deficiency of regenerating the MTPPB/TEG (1:4) DES and to investigate different techniques for recovering the DES in the combined processes in addition to optimizing the key factors for efficient regeneration. To the extent of our knowledge, this is the first work that investigates various regeneration methods and their optimized conditions to maximize DES recyclability from high-boiling-point compounds such as quinoline.
The DES was first prepared, characterized, and then used in a single-stage liquid–liquid extraction (LLX) experiment to remove impurities from a fuel model consisting of toluene, Th, and quinoline as impurities and n-heptane representing the fuel. After that, the capacity of the DES was investigated by performing LLX in three cycles. Then, two regeneration methods were studied: the stripping method (using n-heptane) and the washing method (using distilled water or diethyl ether). Likewise, a parametric study was conducted to optimize the regeneration methods: (1) types of regeneration solvents, (2) effect of the DES-to-regeneration solvent mass ratio, (4) regeneration temperature, and (5) the number of washing cycles. Then, using the optimized conditions, a comparison was made between the fresh and recycled DESs. Finally, the HBD was changed from polyol-based (i.e., TEG) to acidic-based [i.e., 3-phenyl propionic acid (PA)] to study the application of regeneration methods on different DESs. The results showed that washing the used DES with distilled water was significantly more effective than stripping the DES with n-heptane. Moreover, it was observed that a much more effective recovery could be achieved through the use of (i) a higher DES-to-regeneration solvent mass ratio and (ii) an increase in the number of washing cycles. Furthermore, it was found that the regeneration temperature had no significant effect on the recyclability of the DES. After determining the optimal regeneration conditions, the recovered DES was re-utilized in the combined extractive process, and the results showed that the extraction efficiencies of the regenerated DES was comparable to that of a fresh batch of DES.
2. Materials and Methods
2.1. Materials
For the preparation of the DES, methyltriphenylphosphonium bromide (≥98.0%), TEG (≥99.0%), PA (≥99.0%), and ethanol (≥99.8%) were purchased from Sigma-Aldrich. As for the oil model, n-heptane (≥99.0%), toluene (≥99.5%), Th (≥99.0%), and quinoline (≥98.0%) were all obtained from Sigma-Aldrich. Diethyl ether (≥99.5%) as a regeneration solvent was supplied by Honeywell. All chemicals were used without further purification.
2.2. DES and Fuel Model Preparation
DESs were prepared using the heating method, as explained in our previous work.28 Using the Shimadzu balance AUX220, pre-weighed amounts with an uncertainty in the measurement of ±0.0002 g HBA (methyl triphenylphosphonium bromide, MTPPBr) and HBDs (TEG or PA) were placed in screw-capped bottles. The mixture was then kept in a temperature-controlled incubated shaker (IKA KS 4000) i-control with a temperature stability of ±0.1 K at a temperature of 338.2 K and stirred at around ∼300 rpm until a clear homogeneous liquid was formed. Similar to our previous work,28 we used a fuel model consisting of 20% toluene, 2% Th, 2% quinoline, and 76% n-heptane, all by weight (wt %).
2.3. DES Characterization
The DES was characterized for its (i) water content using the Karl-Fischer titration (GRS Scientific/Aquamax KF Coulometric) and (ii) and viscosity and density using an SVM 3001 kinematic viscometer (Anton Paar). Fourier transform infrared (FTIR) spectrometry was also conducted on the prepared DESs using a PerkinElmer VERTEX 80v (transmittance mode) in the wavenumber range of 4000–400 cm–1.
2.4. Regeneration
The regeneration experiment comprises three major steps: (1) conducting the single-stage LLX, (2) washing or back-extraction where the regeneration solvents are used to capture the impurities within the DES, and (3) evaporation under the vacuum of the DES-rich phase to recover the pure DES by evaporating the impurities and regeneration solvent from the DES.
The fuel model and the chosen DESs were combined in a 1:3 ratio (DES/F = 1:1) in the first stage of the LLX experiment. The mixtures were then stirred for 4 h at 1000 rpm and 298.2 K using an Eppendorf thermomixer C. Subsequently, the mixtures were maintained for 24 h at a temperature of 298.2 K for the phases to settle and reach equilibrium. The extract phase (DES-rich phase) was then separated from the raffinate phase (n-heptane) using syringes without disturbing the phases coexistence interface in step 2.
In step 2, two methods were applied: either washing the DESs with water/organic solvent or using a low-boiling-point hydrocarbon to back-extract the impurities. In this work, we used distilled water and diethyl ether as anti-solvents to wash the DES, while n-heptane was used in the back-extraction method. At mass ratios of 2:1, 1:1, and 1:1.5, the extract phases of step 1 were mixed with either distilled water, diethyl ether, or n-heptane, respectively. Subsequently, the mixes were put in an Eppendorf thermomixer C at 298.2 K at 1000 rpm for 4 h and then allowed to stand overnight at sustained 298.2 K to settle and achieve equilibrium. The DES-rich phase was then extracted again for use in step 3.
In step 3, a DES-rich phase was placed in a rotary evaporator (BUCHI, Rotavapor R-215) at vacuum pressure (∼35 mbar) and 298.2 K for 12 h. Samples from the upper and bottom phases were taken for gas chromatography (GC; Agilent 6890 N) and FTIR analysis. All samples analyzed via GC were diluted using 1 mL of ethanol as an internal standard. The operating variables with GC specifications used for this study are described in Table 1. The method was validated by measuring known composition samples, and the root mean square deviation (RMSD) was equal to 0.005. Because the DES is a low-volatility compound, its mass concentration was determined by mass balance calculations. Furthermore, all experiments were duplicated, and the mean and maximum standard deviations were ±0.8 and 3.5%, respectively.
Table 1. GC Specifications.
| operating variable | specification |
|---|---|
| column | Agilent J&W HP-5(30 m × 0.32 mm × 0.25 μm) |
| detector | flame ionization detector (FID) |
| injector temperature | 548.2 K |
| oven temperature profile | 323.2–523.2 K at 20 K·min–1 |
| detector temperature | 473.2 K |
| carrier gas | helium |
| gas flow rate | 2 mL·min–1 |
| injection volume | 1 μL |
| retention time repeatability | 0.003 |
| method verification (max RMSD) | 0.005 |
| method verification (statistical uncertainty) | 0.004 |
3. Results and Discussion
3.1. DES Characterization
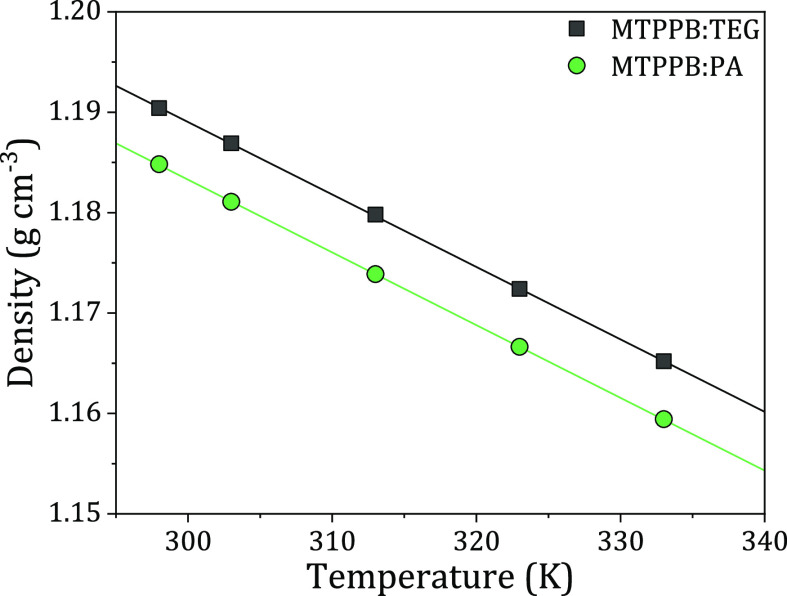
To characterize the new DES reported in this work (i.e., MTPPB/PA), different properties such as density, viscosity, and water content were measured to be compared with the properties of MTPPB/TEG. Starting with the density property, Figure 1 shows that the DES with TEG as an HBD has higher density values than the PA-based DES, with values of 1.190 and 1.185 at 298.2 K, respectively. Both solvents exhibit a high difference compared to the density of fuel, which is advantageous because the larger the difference between the densities of the two phases, the better the separation. The density data were correlated using linear regression with an R2 value of 0.9999, as shown in eqs 1 and 2
| 1 |
| 2 |
where temperature and density are expressed in kelvin and grams per cubic centimeter; respectively.
Figure 1.
Density of MTPPB/TEG (1:4) and MTPPB/PA (1:4) DESs.
Table 2 shows that the MTPPB/TEG (1:4) DES has substantially lower viscosity than the MTPPB/PA (1:4) DES, which may improve the extraction process by allowing the DESs to flow more easily (i.e., lower pumping power). High-viscosity solvents could also limit the mass transfer between phases, resulting in high residence time during extraction. The water content of MTPPB/PA at 298.2 K was also measured to be 0.198 wt %, which was lower than the water content of MTPPB/TEG (0.355 wt %).
Table 2. Viscosity, Water Content, and Density at 298.2 K for MTPPB/TEG (1:4) and MTPPB/PA (1:4) DESs.
| property | MTPPB/TEG(1:4) | MTPPB/PA(1:4) |
|---|---|---|
| viscosity (mPa·s) | 138.66 | 526.48 |
| water content (wt %) | 0.36 | 0.19 |
| density (g·cm–3) | 1.190 | 1.185 |
3.2. Single-Stage LLX
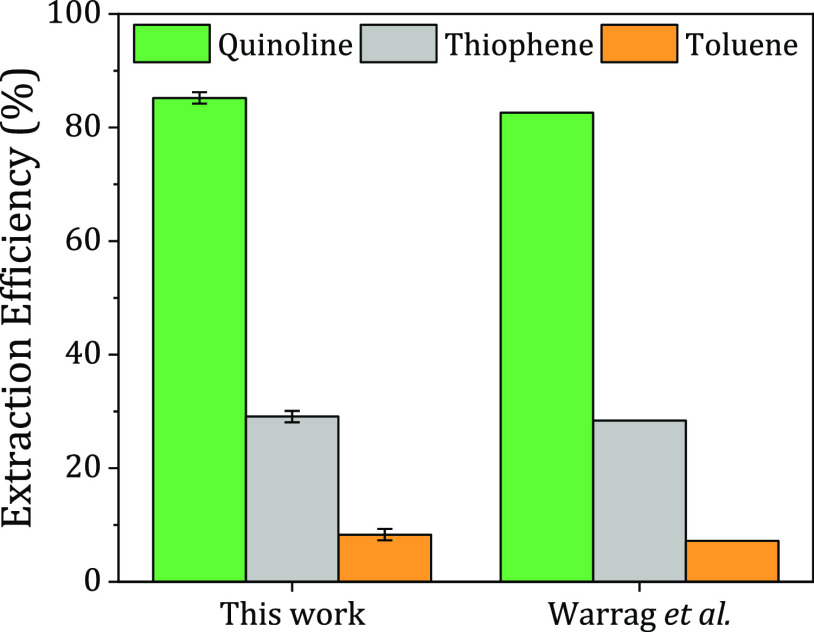
The single-stage LLX experiment was first conducted to prepare the extract phase (bottom phase) to be used in the regeneration step. The experiment was carried out under the same operating conditions as those reported by Warrag et al.(28) except for the mixing and settling times. In this study, the mixture was maintained longer to guarantee a longer contact time between the DES and the fuel model. The fuel model was mixed with the MTPPB/TEG (1:4) DES; the results are illustrated in Figure 2. It can be seen that the MTPPB/TEG (1:4) DES had a similar extraction efficiency for all impurities compared to the reported one,28 so it is safe to conclude that the increase in mixing and settling times has an insignificant effect on the extraction of fuel impurities after 2 h of settling.28
Figure 2.
Comparison of the single-stage extraction efficiency of this work with that of Warrag et al.(28) at DES/F = 1:1, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h. Data adopted from ref (28). Copyright 2020 ACS American Chemical Society.
Another DES was also investigated to examine the reproducibility of the regeneration method. Here, TEG was replaced with PA to form MTPPB/PA at a molar ratio of 1:4. The same operating conditions were applied, and the results are shown in Figure 3. The change in the HBD from a polyol to an acidic HBD slightly increased the extraction efficiency of quinoline and toluene while causing a minor reduction in the extraction of Th. The MTPPB/PA (1:4) DES demonstrated efficiencies of 89.3, 27.3, and 10.0% for the removal of quinoline, Th, and toluene, respectively. This is consistent with the work of Hizaddin et al.,47 who reported that the acidic-based DESs show better performance for the denitrogenation process. This was attributed to the formation of aromatic HBD, which forms a π–π interaction between the C=C bond of toluene and the C=O bond of the acid, resulting in an enhanced removal from the fuel.47 This explains the ability of the MTPPB/PA (1:4) DES to extract toluene, Th, and quinoline simultaneously. Subsequently, the DES was repeatedly used to examine its capacity before saturation.
Figure 3.
Comparison between MTPPB/PA (1:4) and MTPPB/TEG (1:4) DESs in terms of the single-stage extraction efficiency. Conditions: DES/F = 1:1, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
3.3. DES Capacity
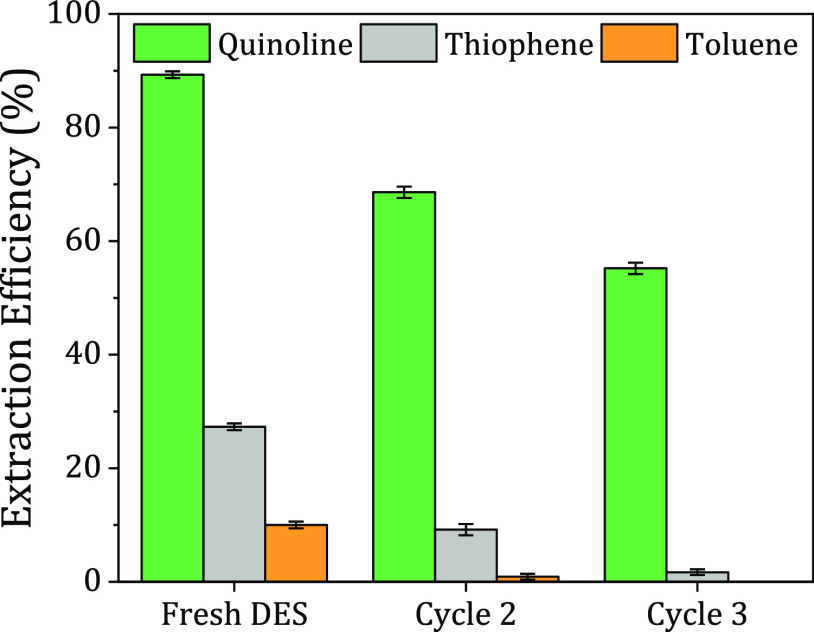
The importance of studying the DES capacity from an economic standpoint stems from the concept of using the solvent multiple times. When a solvent can be reused, it saves time, effort, resources, and money. This experiment was evaluated by performing LLX using the same DES added to a fresh fuel model in every cycle at a 1:1 solvent-to-feed mass ratio, a temperature of 298.2 K, and 1.01 bar. The procedure was repeated until the DES was saturated with impurities. In Figure 4, the results showed that the extraction efficiency generally decreased after each cycle until the DES was saturated with contaminants after three cycles. The toluene extraction efficiency decreased from 8.3 to ≈0%, where the DES could not extract additional toluene, while the extraction efficiency of Th decreased from 29.1 to 1.9%. As for quinoline, it dropped from 85.2 to 49.1%. The trends obtained are attributed to the following; (1) the π-electrons found in the toluene structure generate an electrostatic cloud that may facilitate its interaction with the DES, leading to partial solubility and low extraction efficiency and (2) the existence of the electronegative elements, that is, sulfur and nitrogen, found in Th and quinoline, respectively, increased the attraction forces between the solute and the DES. Therefore, presumably, the high extraction ability of these molecules is due to a combination of electrostatic and hydrogen-bonding interactions. The results conclude that the prospect of solvent regeneration could substantially increase its reusability and improve the purification process.
Figure 4.
Repetitive use of the MTPPB/TEG (1:4) DES under the following conditions: DES/F = 1:1, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
The same experiment was conducted for the MTPPB/PA (1:4) DES, and the same trends were observed where the extraction efficiency decreased when the MTPPB/PA (1:4) DES was reused, proving the importance and necessity of regenerating the DES again. Therefore, the regeneration solvents were tested for their ability to restore the DES (Figure 5).
Figure 5.
LLX using MTPPB/PA (1:4) in multiple cycles. Conditions: DES/F = 1:1, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
3.4. DES Regeneration
In this work, the regeneration method was conducted in two steps: (1) extraction of the impurities by washing the extract phase with either anti-solvent (e.g., diethyl ether or water) or back-extraction with a low-boiling-point hydrocarbon (e.g., n-heptane) and then (2) introducing the extraction phase (bottom phase) into evaporation under vacuum. The following sections summarize the parametric investigation to optimize DES regeneration.
3.4.1. Selection of the Regeneration Method
An initial screening was conducted to select the most suitable solvent for DES regeneration. The extract phase prepared from the single-stage LLX experiment was mixed separately with three regeneration solvents: distilled water, diethyl ether, and n-heptane. Then, the bottom phase was placed in the vacuum evaporator to regenerate the DESs. Of note, the diethyl ether formed a precipitate in the extract phase, as shown in Figure 6. This could be attributed to the incompatibility between the DES constituents and the diethyl ether solvent. The same behavior was observed when testing MTPPB/PA (1:4).
Figure 6.
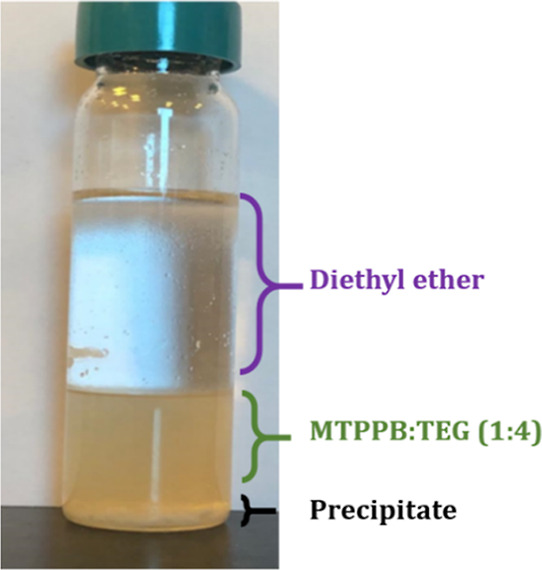
Precipitation formed when diethyl ether as a regeneration solvent was used.
Consequently, diethyl ether was excluded from the rest of the experiments. The results are shown in Figure 7a for selecting regeneration solvents; distilled water and n-heptane. It can be seen that both solvents successfully purified MTPPB/TEG (1:4) from Th and toluene. However, distilled water reduced the quinoline content in the DES from 3.2 to 2.1 wt %, while n-heptane showed a minor reduction in the quinoline content (3.2 to 3 wt %). The same results were obtained when regenerating the MTPPB/PA (1:4) DES (Figure 7b).
Figure 7.
Types of solvents selected and tested for (a) MTPPB/TEG (1:4) DES and (b) MTPPB/PA (1:4) regeneration under the following conditions: DES/F = 1:1, DES/RS = 2:1, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
Although these results encourage the use of distilled water and n-heptane to regenerate the DES used in fuel purification, other concerns should be considered. For instance, distilled water is an environmentally friendly solvent that has shown its effectiveness in purifying different DESs but is still questionable regarding the energy cost required to be separated from the DES. Moreover, diethyl ether and n-heptane are volatile organic solvents that could raise environmental concerns. Therefore, the selection of the regeneration method is a compromise between the effectiveness of the solvent and its greenness.
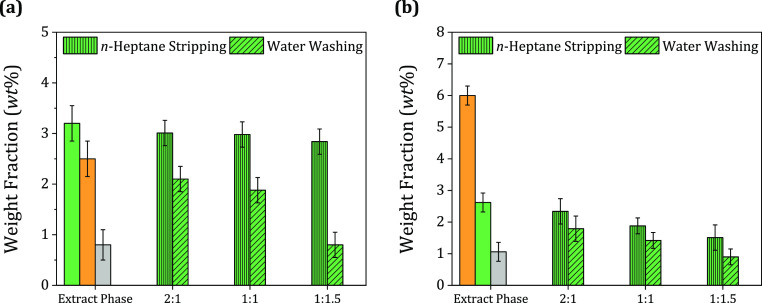
3.4.2. Effect of the DES-To-Regeneration Solvent Mass Ratio
After the regeneration solvents were selected, the rest of the experiments focused on the removal of quinoline. Therefore, in this experiment, the effect of varying the DES-to-regeneration solvent (DES/RS) mass ratio on the reduction of quinoline was studied. The DES/RS mass ratio went from 2:1 to 1:1.5. Figure 8a shows that the increase in the amount of regeneration solvent increases the removal of quinoline, which was expected. In comparison to the performance of the regeneration solvents, distilled water significantly affected the removal of quinoline. The increase in the mass ratio substantially reduced the amount of quinoline content in the regenerated DES from 3.2 to 0.8 wt %. In contrast, n-heptane reduced the quinoline content to 2.8 wt %. The mass ratio of the DES to regeneration solvent is critical to determining the extraction efficiency. Therefore, in all subsequent experiments, a mass ratio of 1:1.5 was maintained between the DES and the regeneration solvent. Furthermore, the DES-to-regeneration solvent mass ratio was also investigated for the MTPPB/PA (1:4) DES, and the results are shown in Figure 8b. The most feasible DES-to-regeneration solvent mass ratio was 1:1.5. Therefore, the DES-to-regeneration solvent mass ratio of 1:1.5 was also selected for the rest of the experiments of the recovery of the MTPPB/PA (1:4) DES.
Figure 8.
Effect of the DES-to-regeneration solvent mass ratio for (a) MTPPB/TEG (1:4) DES and (b) MTPPB/PA (1:4) under the following conditions: DES/F = 1:1, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
3.4.3. Effects of Washing Cycles
The effect of washing cycles was investigated by adding fresh regeneration solvent to the regenerated DES to examine the number of cycles needed to obtain the high quinoline removal. These experiments were carried out in three cycles, adding fresh distilled water or n-heptane in each cycle to the regenerated DES phase from the previous stage. The results are shown in Figure 9a. Generally, both regeneration solvents showed a decrease in quinoline content after every cycle. Figure 9a shows a significant reduction in quinoline content when using distilled water, reaching 0.1 wt % after three washes, suggesting that using distilled water in cycles is highly effective in regenerating. This indicates that using distilled water in cycles is highly effective in restoring the DES. However, the use of n-heptane had a minor effect on regenerating the DES, indicating the ineffectiveness of n-heptane as a regeneration solvent for MTPPB/TEG (1:4). Therefore, washing the DES with distilled water was selected for further investigation.
Figure 9.
Effect of the number of washes when using (a) MTPPB/TEG (1:4) and (b) MTPPB/PA (1:4) under the following conditions: DES/F = 1:1, DES/RS = 1:1.5, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
As for the regenerated MTPPB/PA (1:4) DESs, Figure 9b shows the results obtained. It can be observed that a significant decrease in quinoline content was obtained using distilled water after three washes, suggesting that the use of distilled water in cycles is highly effective in regenerating the DES.
3.4.4. Effects of Regeneration Temperature
The effect of the regeneration temperature on the performance of the regeneration solvent (distilled water) is shown in Figure 10. It can be seen that the increase in regeneration temperature had a minor effect on regenerating MTPPB/TEG (1:4). Thus, the regeneration temperature was maintained at 298.2 K for the rest of the experiments.
Figure 10.
Effect of regeneration temperature on quinoline removal under the following conditions: DES/F = 1:1, DES/RS = 1:1.5, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
3.4.5. Fresh and Regenerated DES Comparison
The degradation of the regenerated DES (after three water washing cycles) compared to that of the fresh DES was examined through their FTIR spectra (Figure 11). As can be seen, the spectra of the regenerated DES and the fresh DES are identical, indicating that the regenerated DES did not degrade after being washed with distilled water and exposed to vacuum conditions. This confirms the effectiveness of the washing method in regenerating the selected DES from the high-boiling-point compound (quinoline).
Figure 11.
Comparison between the fresh and regenerated MTPPB/TEG (1:4) DES FTIR spectra under the following conditions: DES/F = 1:1, DES/RS = 1:1.5, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
Fresh and regenerated DESs were also used for extraction to evaluate the efficiency of the regenerated DES compared to that of the fresh DES. It can be seen in Figure 12 that the extraction efficiency of the three impurities, quinoline, Th, and toluene, showed a minor decrease when using the regenerated DES. The fresh DES showed 85.2, 29.0, and 8.3% removal of quinoline, Th, and toluene, respectively. The regenerated solvent showed 81.3, 28.1, and 7.1% quinoline, Th, and toluene, respectively. This is presumably because of the presence of quinoline traces in the regenerated DES, which could hamper the performance of the DES. This experiment concludes the ability to regenerate the MTPPB/TEG (1:4) DES from quinoline using water and vacuum evaporation for use in combined dearomatization, desulfurization, and denitrogenation.
Figure 12.
Comparison between fresh and regenerated MTPPB/TEG (1:4) DESs under the following conditions: DES/F = 1:1, DES/RS = 1:1.5, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
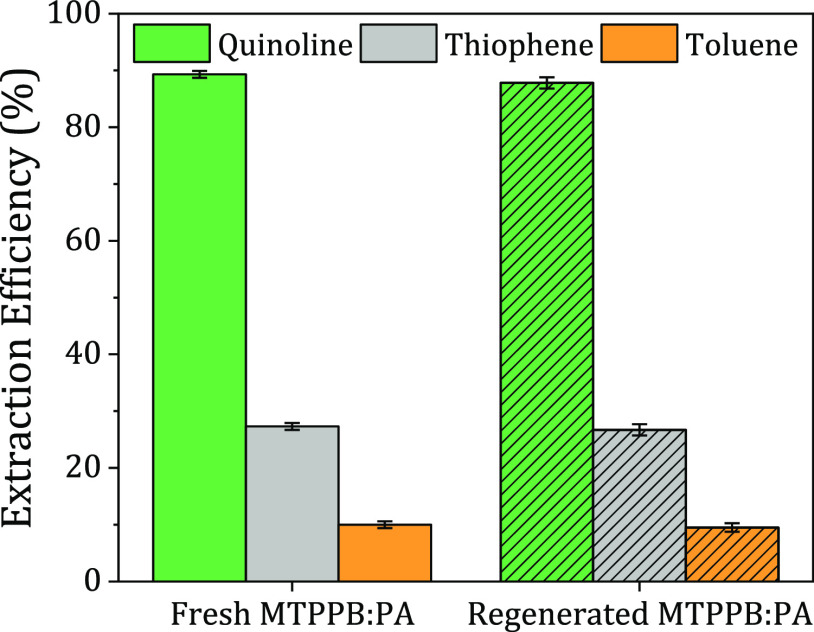
The effectiveness of the regenerated MTPPB/PA (1:4) DES, which underwent three cycles of water regeneration, was compared to that of the fresh DES (Figure 13). It can be observed that the performance of the regenerated DES is almost similar (with a slight decrease) to that of the fresh DES, demonstrating that the regeneration method was successful.
Figure 13.
Comparison of the extraction efficiencies of fresh and regenerated MTPPB/PA (1:4).
The FTIR spectra of fresh and regenerated DESs are also shown in Figure 14, which shows that no noticeable degradation of the DES occurred after the regeneration procedure. From the demonstration experiments, it can be concluded that MTPPB/PA (1:4) can also be regenerated with water and vacuum evaporation when the combination of dearomatization, desulfurization, and denitrification is used.
Figure 14.
Comparison between the fresh and regenerated MTPPB/PA (1:4) DES FTIR spectra under the following conditions: DES/F = 1:1, DES/RS = 1:1.5, T = 298.2 K, P = 1.01 bar, stirring time = 4 h, and settling time = 24 h.
4. Conclusions
Global environmental problems require an improvement in fuel quality. This study investigates the potential of regenerating MTPPB/TEG (1:4) after use in simultaneous dearomatization, desulfurization, and denitrogenation. Two regeneration methods were used: the back-extraction method (using n-heptane) and the washing method (using distilled water or diethyl ether). In addition, several parameters were studied to optimize the regeneration methods: (1) type of the regeneration solvent, (2) effect of the DES-to-regeneration solvent mass ratio, (3) number of washing cycles, and (4) regeneration temperature. It was found that the use of diethyl ether was not practical because it caused precipitation when mixed with MTPPB/TEG (1:4). Therefore, it was excluded from the study, while the other two regeneration solvents, distilled water and n-heptane, were used for further studies. The increase in the regeneration solvent mass ratio increased the removal of quinoline from the regenerated DESs. It was found that a DES-to-regeneration solvent mass ratio of 1:1.5 was effective in regenerating the DES. In addition, variation in regeneration temperature had little effect on regeneration performance and was therefore kept at 298.2 K. The fresh DES was compared with the regenerated DES (after three cycles of water washing). The results showed the effectiveness of the regeneration method in recovering the DES as the extraction efficiencies of fresh and regenerated DESs were almost the same. The same regeneration method was applied to another DES by changing the HBD (from TEG to PA). The results showed that after regeneration with water in three cycles, the performance of the regenerated DES was also almost the same (with a slight decrease) as that of the fresh DES, indicating that the regeneration method was successful for both the polyol-based DES and the acid-based DES.
Acknowledgments
The authors acknowledge the support from the Center of Membranes and Advanced Water Technology (CMAT). The authors acknowledge the support from the Deanship of Research, King Khalid University, Saudi Arabia, for large research groups under the grant number R.G.P.2/59/1443. The authors gratefully acknowledge the Taif University Researchers Supporting Project, number (TURSP-2020/242), Taif University, Taif, Saudi Arabia. The Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant by the Ministry of Trade, Industry and Energy (MOTIE) of South Korean Government (no. 20206410100040) and a support by the Soil Treatment Facility Council of Korea are also acknowledged.
Glossary
List of Abbreviations
- DESs
deep eutectic solvents
- LLE
liquid–liquid equilibrium
- LLX
liquid–liquid extraction
- HBA
hydrogen bond acceptor
- HBD
hydrogen bond donor
- S/F
solvent-to-feed ratio
- MTPPB
methyl triphenyl phosphonium bromide
- TEG
triethylene glycol
- VOCs
volatile organic compounds
- ILs
ionic liquids
- FTIR
Fourier transform infrared
- GC
gas chromatography
- RMSD
root mean square deviation
- Th
thiophene
- BT
benzothiophene
- DBT
dibenzothiophene
- DES/RS
DES-to-regeneration solvent mass ratio
- PA
3-phenyl propionic acid
Author Contributions
○ Shared first authorship between F.A.H. and O.A.Z.I.
The authors declare no competing financial interest.
References
- Darwish A. S.; Warrag S. E. E.; Lemaoui T.; Alseiari M. K.; Hatab F. A.; Rafay R.; Alnashef I.; Rodríguez J.; Alamoodi N. Green Extraction of Volatile Fatty Acids from Fermented Wastewater Using Hydrophobic Deep Eutectic Solvents. Fermentation 2021, 7, 226. 10.3390/fermentation7040226. [DOI] [Google Scholar]
- Salleh M. Z. M.; Hadj-Kali M. K.; Hizaddin H. F.; Ali Hashim M. Extraction of Nitrogen Compounds from Model Fuel Using 1-Ethyl-3-Methylimidazolium Methanesulfonate. Sep. Purif. Technol. 2018, 196, 61–70. 10.1016/j.seppur.2017.07.068. [DOI] [Google Scholar]
- Pan Y.; Liu Y.; Zhang X.; Shi M.; Tu Z.; Hu X.; Wu Y. Design of Deep Eutectic Solvents with Multiple-Active-Sites for HCl Separation and Storage. Sep. Purif. Technol. 2022, 300, 121799. 10.1016/j.seppur.2022.121799. [DOI] [Google Scholar]
- Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. K.; Tambyrajah V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 9, 70–71. 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- Martins M. A. R.; Pinho S. P.; Coutinho J. A. P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solution Chem. 2019, 48, 962–982. 10.1007/s10953-018-0793-1. [DOI] [Google Scholar]
- Smith E. L.; Abbott A. P.; Ryder K. S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- Paiva A.; Craveiro R.; Aroso I.; Martins M.; Reis R. L.; Duarte A. R. C. Natural Deep Eutectic Solvents - Solvents for the 21st Century. ACS Sustainable Chem. Eng. 2014, 2, 1063–1071. 10.1021/sc500096j. [DOI] [Google Scholar]
- Zhang Q.; De Oliveira Vigier K.; Royer S.; Jérôme F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- Mishra D. K.; Gopakumar G.; Pugazhenthi G.; Siva Brahmmananda Rao C. V.; Nagarajan S.; Banerjee T. Molecular and Spectroscopic Insights into a Metal Salt-Based Deep Eutectic Solvent: A Combined Quantum Theory of Atoms in Molecules, Noncovalent Interaction, and Density Functional Theory Study. J. Phys. Chem. A 2021, 125, 9680–9690. 10.1021/acs.jpca.1c07809. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Cai D.; Zhang S.; Wang H.; Guo K.; Zhang L.; Yang L. Effective Removal of Nitrogen Compounds from Model Diesel Fuel by Easy-to-Prepare Ionic Liquids. Sep. Purif. Technol. 2019, 222, 92–98. 10.1016/j.seppur.2019.04.026. [DOI] [Google Scholar]
- Mu M.; Zhang X.; Yu G.; Sun C.; Xu R.; Liu N.; Wang N.; Chen B.; Dai C. Deep Removal of Chlorobenzene Based Volatile Organic Compounds from Exhaust Gas with Ionic Liquids. Sep. Purif. Technol. 2022, 298, 121610. 10.1016/j.seppur.2022.121610. [DOI] [Google Scholar]
- Mishra D. K.; Pugazhenthi G.; Banerjee T. Ionic Liquid-Based Deep Eutectic Solvent as Reaction Media for the Thermal Dehydrogenation of Ethylene Diamine-Bis-Borane. ACS Sustainable Chem. Eng. 2020, 8, 4910–4919. 10.1021/acssuschemeng.0c00220. [DOI] [Google Scholar]
- Dai C.; Chen M.; Mu W.; Peng B.; Yu G.; Liu N.; Xu R.; Wang N.; Chen B. Highly Efficient Toluene Absorption with π-Electron Donor-Based Deep Eutectic Solvents. Sep. Purif. Technol. 2022, 298, 121618. 10.1016/j.seppur.2022.121618. [DOI] [Google Scholar]
- Wazeer I.; Hayyan M.; Hadj-Kali M. K. Deep Eutectic Solvents: Designer Fluids for Chemical Processes. J. Chem. Technol. Biotechnol. 2018, 93, 945–958. 10.1002/jctb.5491. [DOI] [Google Scholar]
- Zhang X.; Wang J.; Shen J.; Wang Y.; Liu G.; Niu Y.; Sheng Q. Highly Efficient Extraction of Indole from Model Wash Oil by Using Environmentally Benign Deep Eutectic Solvents. Sep. Purif. Technol. 2022, 285, 120381. 10.1016/j.seppur.2021.120381. [DOI] [Google Scholar]
- Abo-Hamad A.; Hayyan M.; AlSaadi M. A. H.; Hashim M. A. Potential Applications of Deep Eutectic Solvents in Nanotechnology. Chem. Eng. J. 2015, 273, 551–567. 10.1016/j.cej.2015.03.091. [DOI] [Google Scholar]
- Tomé L. I. N.; Baião V.; da Silva W.; Brett C. M. A. Deep Eutectic Solvents for the Production and Application of New Materials. Appl. Mater. Today 2018, 10, 30–50. 10.1016/j.apmt.2017.11.005. [DOI] [Google Scholar]
- Almustafa G.; Darwish A. S.; Lemaoui T.; O’Conner M. J.; Amin S.; Arafat H. A.; AlNashef I. Liquification of 2,2,4-Trimethyl-1,3-Pentanediol into Hydrophobic Eutectic Mixtures: A Multi-Criteria Design for Eco-Efficient Boron Recovery. Chem. Eng. J. 2021, 426, 131342. 10.1016/j.cej.2021.131342. [DOI] [Google Scholar]
- Shamsaee B. H.; Mehri F.; Rowshanzamir S.; Ghamati M.; Behrouzifar A. Desulfurization of Benzothiophene from Model Diesel Fuel Using Experimental (Dynamic Electroreduction) and Theoretical (DFT) Approaches. Sep. Purif. Technol. 2019, 212, 505–514. 10.1016/j.seppur.2018.11.057. [DOI] [Google Scholar]
- Kumar N.; Banerjee T. Molecular Mechanism and Solubility Performance Evaluation for Separation of Benzothiophene and Model Diesel Compounds through Deep Eutectic Solvents as Extractants. Ind. Eng. Chem. Res. 2022, 61, 1464–1474. 10.1021/acs.iecr.1c04083. [DOI] [Google Scholar]
- Makoś P.; Boczkaj G. Deep Eutectic Solvents Based Highly Efficient Extractive Desulfurization of Fuels—Eco-Friendly Approach. J. Mol. Liq. 2019, 296, 111916. 10.1016/j.molliq.2019.111916. [DOI] [Google Scholar]
- Hatab F. A.; Darwish A. S.; Lemaoui T.; Warrag S. E. E.; Benguerba Y.; Kroon M. C.; AlNashef I. M. Extraction of Thiophene, Pyridine, and Toluene from n-Decane as a Diesel Model Using Betaine-Based Natural Deep Eutectic Solvents. J. Chem. Eng. Data 2020, 65, 5443–5457. 10.1021/acs.jced.0c00579. [DOI] [Google Scholar]
- Lemaoui T.; Benguerba Y.; Darwish A. S.; Hatab F. A.; Warrag S. E. E.; Kroon M. C.; Alnashef I. M. Simultaneous Dearomatization, Desulfurization, and Denitrogenation of Diesel Fuels Using Acidic Deep Eutectic Solvents as Extractive Agents: A Parametric Study. Sep. Purif. Technol. 2021, 256, 117861. 10.1016/j.seppur.2020.117861. [DOI] [Google Scholar]
- Larriba M.; Ayuso M.; Navarro P.; Delgado-Mellado N.; Gonzalez-Miquel M.; García J.; Rodríguez F. Choline Chloride-Based Deep Eutectic Solvents in the Dearomatization of Gasolines. ACS Sustainable Chem. Eng. 2018, 6, 1039–1047. 10.1021/acssuschemeng.7b03362. [DOI] [Google Scholar]
- Rogošić M.; Kučan K. Z. Deep Eutectic Solvents Based on Choline Chloride and Ethylene Glycol as Media for Extractive Denitrification/Desulfurization/Dearomatization of Motor Fuels. J. Ind. Eng. Chem. 2019, 72, 87–99. 10.1016/j.jiec.2018.12.006. [DOI] [Google Scholar]
- Zagajski Kučan K.; Rogošić M. Purification of Motor Fuels by Means of Extraction Using Deep Eutectic Solvent Based on Choline Chloride and Glycerol. J. Chem. Technol. Biotechnol. 2019, 94, 1282–1293. 10.1002/jctb.5885. [DOI] [Google Scholar]
- Kučan K. Z.; Perković M.; Cmrk K.; Načinović D.; Rogošić M. Betaine + (Glycerol or Ethylene Glycol or Propylene Glycol) Deep Eutectic Solvents for Extractive Purification of Gasoline. ChemistrySelect 2018, 3, 12582–12590. 10.1002/slct.201803251. [DOI] [Google Scholar]
- Warrag S. E. E.; Darwish A. S.; Abuhatab F. O. S.; Adeyemi I. A.; Kroon M. C.; AlNashef I. M. Combined Extractive Dearomatization, Desulfurization, and Denitrogenation of Oil Fuels Using Deep Eutectic Solvents: A Parametric Study. Ind. Eng. Chem. Res. 2020, 59, 11723–11733. 10.1021/acs.iecr.0c01360. [DOI] [Google Scholar]
- Darwish A. S.; Abu Hatab F.; Lemaoui T.; Ibrahim O. A. Z.; Almustafa G.; Zhuman B.; Warrag S. E. E.; Hadj-Kali M. K.; Benguerba Y.; Alnashef I. M. Multicomponent Extraction of Aromatics and Heteroaromatics from Diesel Using Acidic Eutectic Solvents: Experimental and COSMO-RS Predictions. J. Mol. Liq. 2021, 336, 116575. 10.1016/j.molliq.2021.116575. [DOI] [Google Scholar]
- Cheng H.; Qi Z. Applications of Deep Eutectic Solvents for Hard-to-Separate Liquid Systems. Sep. Purif. Technol. 2021, 274, 119027. 10.1016/j.seppur.2021.119027. [DOI] [Google Scholar]
- Shu C.; Sun T. Extractive Desulfurisation of Gasoline with Tetrabutyl Ammonium Chloride-Based Deep Eutectic Solvents. Sep. Sci. Technol. 2016, 51, 1336–1343. 10.1080/01496395.2016.1155602. [DOI] [Google Scholar]
- Jiang W.; Li H.; Wang C.; Liu W.; Guo T.; Liu H.; Zhu W.; Li H. Synthesis of Ionic-Liquid-Based Deep Eutectic Solvents for Extractive Desulfurization of Fuel. Energy Fuels 2016, 30, 8164–8170. 10.1021/acs.energyfuels.6b01976. [DOI] [Google Scholar]
- Makoś P.; Słupek E.; Gębicki J. Extractive Detoxification of Feedstocks for the Production of Biofuels Using New Hydrophobic Deep Eutectic Solvents—Experimental and Theoretical Studies. J. Mol. Liq. 2020, 308, 113101. 10.1016/j.molliq.2020.113101. [DOI] [Google Scholar]
- Han X.; Lin H.; Zheng Y. Understanding Capacity Loss of Activated Carbons in the Adsorption and Regeneration Process for Denitrogenation and Desulfurization of Diesel Fuels. Sep. Purif. Technol. 2014, 133, 194–203. 10.1016/j.seppur.2014.06.020. [DOI] [Google Scholar]
- Alhamed Y. A.; Bamufleh H. S. Sulfur Removal from Model Diesel Fuel Using Granular Activated Carbon from Dates’ Stones Activated by ZnCl2. Fuel 2009, 88, 87–94. 10.1016/j.fuel.2008.07.019. [DOI] [Google Scholar]
- Kianpour E.; Azizian S. Polyethylene Glycol as a Green Solvent for Effective Extractive Desulfurization of Liquid Fuel at Ambient Conditions. Fuel 2014, 137, 36–40. 10.1016/j.fuel.2014.07.096. [DOI] [Google Scholar]
- Lee H.; Kang S.; Jin Y.; Jung D.; Park K.; Li K.; Lee J. Systematic Investigation of the Extractive Desulfurization of Fuel Using Deep Eutectic Solvents from Multifarious Aspects. Fuel 2020, 264, 116848. 10.1016/j.fuel.2019.116848. [DOI] [Google Scholar]
- Li C.; Zhang J.; Li Z.; Yin J.; Cui Y.; Liu Y.; Yang G. Extraction Desulfurization of Fuels with “metal Ions” Based Deep Eutectic Solvents (MDESs). Green Chem. 2016, 18, 3789–3795. 10.1039/c6gc00366d. [DOI] [Google Scholar]
- Shu C.; Sun T. Extractive Desulfurisation of Gasoline with Tetrabutyl Ammonium Chloride-Based Deep Eutectic Solvents. Sep. Sci. Technol. 2016, 51, 1336–1343. 10.1080/01496395.2016.1155602. [DOI] [Google Scholar]
- Ali M. C.; Yang Q.; Fine A. A.; Jin W.; Zhang Z.; Xing H.; Ren Q. Efficient Removal of Both Basic and Non-Basic Nitrogen Compounds from Fuels by Deep Eutectic Solvents. Green Chem. 2015, 18, 157–164. 10.1039/c5gc01823d. [DOI] [Google Scholar]
- Zhu S.; Xu J.; Cheng H.; Gao J.; Jiang X.; Li C.; Yang W. Poly(Ethylene Glycol) Diacid-Based Deep Eutectic Solvent with Excellent Denitrogenation Performance and Distinctive Extractive Behavior. Energy Fuels 2019, 33, 10380–10388. 10.1021/acs.energyfuels.9b02463. [DOI] [Google Scholar]
- Yin J.; Wang J.; Li Z.; Li D.; Yang G.; Cui Y.; Wang A.; Li C. Deep Desulfurization of Fuels Based on an Oxidation/Extraction Process with Acidic Deep Eutectic Solvents. Green Chem. 2015, 17, 4552–4559. 10.1039/c5gc00709g. [DOI] [Google Scholar]
- Rogošić M.; Zagajski Kučan K. Deep Eutectic Solvent Based on Choline Chloride and Propylene Glycol as a Potential Medium for Extraction Denitrification of Hydrocarbon Fuels. Chem. Eng. Res. Des. 2020, 161, 45–57. 10.1016/j.cherd.2020.06.012. [DOI] [Google Scholar]
- Lima F.; Gouvenaux J.; Branco L. C.; Silvestre A. J. D.; Marrucho I. M. Towards a Sulfur Clean Fuel: Deep Extraction of Thiophene and Dibenzothiophene Using Polyethylene Glycol-Based Deep Eutectic Solvents. Fuel 2018, 234, 414–421. 10.1016/j.fuel.2018.07.043. [DOI] [Google Scholar]
- Lima F.; Dave M.; Silvestre A. J. D.; Branco L. C.; Marrucho I. M. Concurrent Desulfurization and Denitrogenation of Fuels Using Deep Eutectic Solvents. ACS Sustainable Chem. Eng. 2019, 7, 11341–11349. 10.1021/acssuschemeng.9b00877. [DOI] [Google Scholar]
- Gano Z. S.; Mjalli F. S.; Al-Wahaibi T.; Al-Wahaibi Y.; AlNashef I. M. Extractive Desulfurization of Liquid Fuel with FeCl3-Based Deep Eutectic Solvents: Experimental Design and Optimization by Central-Composite Design. Chem. Eng. Process. 2015, 93, 10–20. 10.1016/j.cep.2015.04.001. [DOI] [Google Scholar]
- Hizaddin H. F.; Ramalingam A.; Hashim M. A.; Hadj-Kali M. K. O. Evaluating the Performance of Deep Eutectic Solvents for Use in Extractive Denitrification of Liquid Fuels by the Conductor-like Screening Model for Real Solvents. J. Chem. Eng. Data 2014, 59, 3470–3487. 10.1021/je5004302. [DOI] [Google Scholar]