Abstract

Patients with chronic rhinosinusitis (CRS) often show persistent colonization by bacteria in the form of biofilms which are resistant to antibiotic treatment. One of the most commonly isolated bacteria in CRS is Staphylococcus aureus (S. aureus). Nitric oxide (NO) is a potent antimicrobial agent and disperses biofilms efficiently. We hypothesized that S-nitrosoglutathione (GSNO), an endogenous NO carrier/donor, synergizes with gentamicin to disperse and reduce the bacterial biofilm density. We prepared GSNO formulations which are stable up to 12 months at room temperature and show the maximum amount of NO release within 1 h. We examined the effects of this GSNO formulation on the S. aureus biofilm established on the apical surface of the mucociliary-differentiated airway epithelial cell cultures regenerated from airway basal (stem) cells from cystic fibrosis (CF) and CRS patients. We demonstrate that for CF cells, which are defective in producing NO, treatment with GSNO at 100 μM increased the NO levels on the apical surface and reduced the biofilm bacterial density by 2 log units without stimulating pro-inflammatory effects or inducing epithelial cell death. In combination with gentamicin, GSNO further enhanced the killing of biofilm bacteria. Compared to placebo, GSNO significantly increased the ciliary beat frequency (CBF) in both infected and uninfected CF cell cultures. The combination of GSNO and gentamicin also reduced the bacterial density of biofilms grown on sinonasal epithelial cells from CRS patients and improved the CBF. These findings demonstrate that GSNO in combination with gentamicin may effectively reduce the density of biofilm bacteria in CRS patients. GSNO treatment may also enhance the mucociliary clearance by improving the CBF.
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disorder of the sinonasal mucosa. CRS is a highly prevalent disease affecting 13% of the US population or approximately 31 million people each year.1,2 CRS contributes significantly to the socioeconomic burden with overall direct and indirect costs exceeding $33 billion dollars per year in the United States alone.3,4 The availability of effective therapies for CRS is limited, particularly for severe cases, thus warranting a need for new strategies to treat and/or prevent this disease.
The pathogenesis of CRS is poorly understood, but it is proposed that both intrinsic and extrinsic factors contribute to the onset of this disease. Among the various proposed mechanisms, bacterial infections and defective local innate immune defences are thought to play a critical role in CRS disease initiation and perpetuation.5−10 Bacteria are detected in at least 80% of CRS patients, with Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa) being the most prevalent species.6,10−13 Detection of S. aureus biofilms is associated with severe disease presentation,14−19 implying a pathogenic role for this microbe. The primary innate defense against inhaled microorganisms is mucociliary clearance, which, in turn, is linearly dependent on the ciliary beat frequency (CBF).20,21 CRS is associated with a reduction in the mucociliary function22−25 that is attributed to a blunted CBF.26,27 To date, a genetic abnormality that would suggest ciliary dysfunction in CRS has not been found.28 Therefore, ciliary dysfunction in CRS patients may be a result of chronic microbial infection/inflammation. In fact, bacterial products, such as enterotoxin A and β-toxin produced by S. aureus as well as lectins and pyocyanin produced by P. aeruginosa, impair the CBF.29−34 Treatment to reduce the density of biofilm bacteria may therefore also improve the mucociliary clearance, thus preventing disease progression.
Nitric oxide (NO), a key component of the innate immune response system, is produced in the upper respiratory tract by sinus epithelial35,36 as well as innate immune cells, such as macrophages, neutrophils, and other innate immune cells and plays an important role in regulating the CBF.36−38 NO is also a potent antibacterial and antiviral agent39−46 and is capable of dispersing bacterial biofilms.47−49 Thus, any defect in NO production not only reduces the mucociliary clearance but also increases susceptibility to microbial infections and promotes persistence of biofilm bacteria that are refractory to antibiotics. In fact, sinonasal NO levels are reduced in most patients with CRS.50−53 The levels of NO in exhaled breath and NO metabolites in the nasal lavage have been found to increase after surgical or medical intervention, and this correlates negatively with disease severity. Further, measuring the levels of NO in exhaled nasal breath has been proposed as a non-invasive method to monitor the clinical course of CRS patients.54,55 These observations imply that reduced NO levels in the sinus mucosa may contribute to CRS pathogenesis and that enhancing NO levels in the upper airway will likely be beneficial for these patients.
S-Nitrosoglutathione (GSNO) already exists in the airway lining fluid56 due to NO generated by structural and innate immune cells reacting with oxygen to form N2O3, which can form a nitrosonium ion (NO+) to react with glutathione to form GSNO. Cellular GSNO is one of the major sources of biologically stable NO and forms adducts with cysteine-containing proteins. GSNO does not enter the cells and decomposes very slowly outside the cells. Reducing agents, such as ascorbic acid and glutathione (GSH), and certain metal ions (e.g., Cu2+) increase the rate of decomposition of GSNO, thus accelerating the emission of NO, which can diffuse into the cells rapidly.57,58 In this study, we report on the efficacy of a GSNO formulation with added ascorbic acid (to accelerate NO release/generation from GSNO) to reduce the S. aureus biofilm bacterial density on the surface of human bronchial cells in vitro.
Experimental Section
GSNO Synthesis
GSNO was synthesized from reduced l-glutathione (GSH) by a previously described method59 with some modifications. GSH was dissolved in acidified, cold, and deionized water. The acidified GSH solution was continuously sparged with high-purity nitrogen gas to remove the oxygen dissolved in the solution. Equimolar NaNO2 was then added to the GSH solution. GSNO was precipitated from the solution with cold acetone, collected by vacuum filtration, washed with a mixture of ice-cold acetone and deionized water, and dried under vacuum. The GSNO purity was evaluated by measuring the UV–vis absorbance at 334 nm using a UV–vis spectrophotometer (Ocean Insight; Orlando, FL) and was consistently found to be around 98% (98.7 ± 2.1%). The GSNO powder was stored in the dark at −20 °C.
Blending of the GSNO Formulation
The GSNO formulations for the experiments were prepared by blending these components: sodium chloride (NaCl, 0.16 M), sodium bicarbonate (NaHCO3, 0.035 M), ethylenediaminetetraacetic acid, disodium salt (Na2EDTA 10 μM), an equimolar concentration of ascorbic acid, and GSNO (100 μM each). An additional GSNO formulation containing equimolar GSNO and ascorbic acid (1.0 M each) with sodium bicarbonate (to balance the solution pH) was prepared to function as a concentrated spike solution to prepare >100 μM GSNO formulations. We also prepared a placebo formulation which contained NaCl (0.16 M), NaHCO3 (0.035 M) Na2EDTA (10 μM), to which 50, 100, or 250 μM ascorbic acid was added. The GSNO formulations were assessed by alkalinity titrations for homogeneity and assessed for GSNO content using both UV–vis absorbance with a UV–vis spectrometer and chemiluminescence. These GSNO formations were stored in appropriate aliquots in a powder format in amber crimp-sealed vials at −20 °C and reconstituted as needed.
Bronchial and Sinonasal Epithelial Cells
Collection and use of bronchial segments from cystic fibrosis (CF) patients at the time of double lung transplantation or from post-mortem tissue and nasal polyp tissue were approved by the University of Michigan and Temple University Institutional Review Boards. Basal cells, which are specialized airway stem cells, were isolated from the collected tissue samples and grown at the air/liquid interface to promote mucociliary differentiation.60,61 Briefly, airway stem cells were expanded in a bronchial life medium (Lifeline Cell Technology LLC, Frederick, MD). Passage one cells were cultured in transwells for 1 week under submerged conditions using a bronchial life medium until the cells reached 95 to 100% confluency. The cells were then cultured at the air/liquid interface in the differentiation medium for 4 weeks to promote differentiation into a mucociliary phenotype.
Bacteria
S. aureus isolated from human sinonasal samples (SA1696 and SA1692) was purchased from ATCC (Manassas, MD) and stored as glycerol stocks at −80 °C. We also isolated an S. aureus strain from excised polyp tissue (SANP), and the isolate was confirmed as S. aureus by the clinical microbiology laboratory at the University of Michigan. The bacteria were sub-cultured on blood agar plates (Fisher Scientific, Hampton, NH) and incubated for 24 h. A single colony was transferred to BD Bacto Tryptic Soy Broth (Fisher Scientific) and then grown in a 37 °C incubator shaker for 24 h. Bacteria were harvested by centrifugation and suspended in sterile PBS, and the bacterial density was adjusted to 1 × 105 cfu/mL based on OD600 (1 OD600 = 1 × 109 cfu/mL). The inoculum was serially diluted and plated to determine the exact density of the bacteria.
Bacterial Infection and Treatment of Cell Cultures with the GSNO Formulation
Transwells with mucociliary-differentiated airway epithelial cell cultures were transferred to a new receiver plate containing a fresh medium. The cell cultures were infected with S. aureus apically at multiplicity of infection from 0.01 to 0.05 and incubated for 24 h to allow bacteria to form the biofilm. The medium in the basolateral chamber was amended with gentamicin at 50 μg/μL to prevent contamination and growth of bacteria in the basolateral surface of the cultures.
A fresh medium containing gentamicin was added to the basolateral chamber, and the apical surface was rinsed with PBS. The GSNO formulation was reconstituted with sterile distilled water, and 30 μL of the 50–250 μM GSNO solution or appropriate placebo formulations was added to the apical surface of the uninfected and infected cultures 3 times (at 0, 8, and 20 h) during a 24 h period to mimic the treatment strategy in patients. The total exposure time to GSNO was 24 h. The apical surface of the cultures was rinsed with PBS prior to adding fresh GSNO. Four hours after the last treatment, the apical surface was washed with PBS, and the cells were lysed in 0.1% Triton X-100, serially diluted, and plated to determine the bacterial density. The basolateral medium was collected to assess IL-8 and LDH concentrations.
Determination of NO Release by GSNO Formulations in Saline and on Cell Cultures
Formulations containing GSNO, and GSNO mixed with ascorbate, or GSH at equimolar ratio were reconstituted with deionized water. Aliquots of each formulation were transferred into an amber glass reaction cell in a 37 °C water bath. NO emission was monitored via chemiluminescence using a Sievers 280i Nitric Oxide Analyzer over an 8.0 h period after reconstitution.62
Four hours after the last treatment with GSNO, 100 μL of sterile PBS was added to the apical surface of the cell cultures and incubated for 15 min in a cell culture incubator. The apical wash was collected, homogenized, and centrifuged, and the supernatant was used for estimating the NO content as nitrite by HPLC combined with chemiluminescence detection.63
Enzyme-Linked Immunosorbent Assay
IL-8 was determined in the basolateral medium using a Duoset ELISA kit (R & D system, Minneapolis, MN). LDH was assessed by using the CytoTox 96 non-radioactive cytotoxicity assay (Promega, Madison, WI).
Scanning Electron Microscopy
After appropriate treatment, the mucociliary-differentiated cell cultures were processed as described previously.64 Briefly, the cell cultures were fixed in 2.5% glutaraldehyde at 4 °C overnight, washed with 0.1 M sodium cacodylate (CAC), and post fixed with 1% osmium tetroxide in CAC buffer for 1 h. The cells were washed with water and then dehydrated in graded ethanol series. Samples were critical-point dried and mounted on carbon-coated stubs. The samples were then sputter-coated with gold and observed under an AMRAY 1910 field emission scanning electron microscope.
Confocal Microscopy
After appropriate treatment, mucociliary-differentiated CF airway epithelial cell cultures were washed, fixed in cold methanol, and incubated with the polyclonal antibody to S. aureus (Abcam, Cambridge, MA) and the monoclonal antibody to ZO-1 (BD Biosciences, Franklin Lakes, NJ). Bound antibodies were detected by using Alexa Flour 488-conjugated anti-rabbit IgG (for detection of S. aureus) and Alexa Flour 594-conjugated anti-mouse IgG (for detection of ZO-1). Cells were counterstained with DAPI (Invitrogen, Carlsbad, CA) and visualized by confocal fluorescent microscopy.
Ciliary Beat Frequency Measurement
Cell cultures were subjected to high-speed video microscopy at 210 frames per second. The cell cultures were maintained at 37 °C while imaging. The video was recorded for 10 s each in 10 random fields per culture, and the CBF was determined by using the publicly available software, CiliarMove.65
Statistical Analysis
Data were expressed as mean ± SD or median with range. Data were analyzed by using the SigmaStat statistical software (Systat Software, San Jose, CA). To compare two groups, we used unpaired t tests or Mann–Whitney analysis as appropriate. To compare three or more groups, one-way ANOVA with Tukey’s post-hoc test, ANOVA on ranks with the Kruskal–Wallis H test were performed. A p value ≤ 0.05 was considered significant.
Results
Ascorbate Accelerates NO Release from GSNO
GSNO is a relatively stable molecule and releases NO at a slow rate (Figure 1A). Therefore, we tested different additives such as ascorbate, reduced glutathione (GSH), and cysteine to accelerate NO release from GSNO. Formulations of GSNO, GSNO + ascorbate, and GSNO + GSH released more than 85–95% of the NO available from the GSNO content with the GSNO + ascorbate mixture releasing the highest (Supporting Information Table S1). The rate of NO release also varied between these formulations. While GSNO and GSNO + GSH released 85% of the total NO content in 4 h, the GSNO + ascorbate formulation released 95% of the total NO content in 53 min (Figure 1A,B). When GSNO and cysteine were mixed at equimolar levels, the rate and the total amount of NO released were similar to those of GSNO + GSH (data not shown). Based on these results, we choose to use the GSNO and ascorbate mixture at a 1:1 proportion in the subsequent studies.
Figure 1.
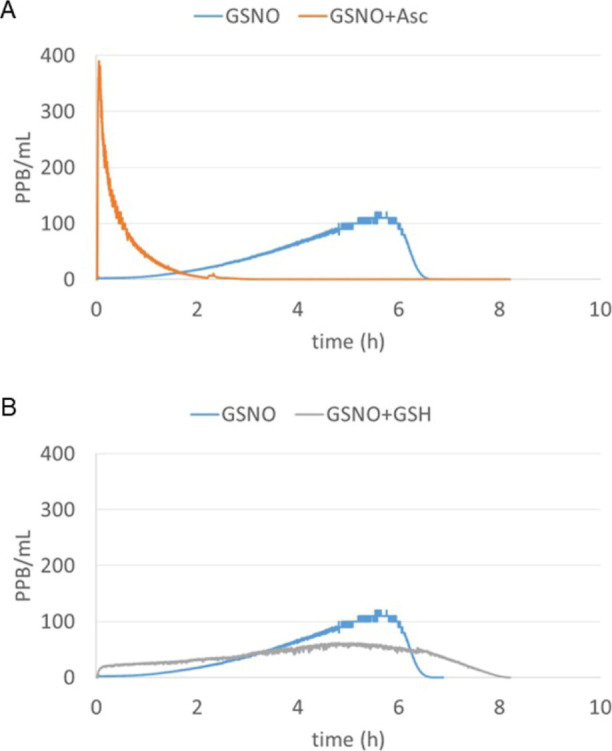
(A) NO release over time from a 100 μM GSNO solution in 0.16 M NaCl/0.03 M NaHCO3 in the absence (blue trace) or presence (orange trace) of 100 μM sodium ascorbate. (B) NO release over time from the 100 μM GSNO solution in 0.16 M NaCl/0.03 M NaHCO3 in the absence (blue trace) or presence (gray trace) of 100 μM GSH. NO release was measured by purging the solution continuously with nitrogen and measuring the NO release in the gas stream by chemiluminescence.
The presence of trace levels of Cu(II) ion can cause instantaneous release of NO from GSNO.58 To prevent the quick decomposition of GSNO, we examined the effect of mixing EDTA (10 μM) into the formulation blend on the release of NO from the ascorbate/GSNO mixture. Addition of EDTA did not have any effect on the NO release and likely resulted due to the high purity of the deionized water and the ACS chemicals used in this study (data not shown). Since commercial sources of distilled water may contain small amounts of divalent cations, we used a GSNO formulation containing ascorbate and EDTA in all our subsequent studies. To confirm that GSNO, ascorbate, and EDTA show the emission of NO similar to that of the GSNO and ascorbate mixture, we determined the NO release from 50, 100, and 250 μM GSNO formulations (Supporting Information Figure S1). We found that 90–95% of the total NO was released within 1 h, as observed with GSNO/ascorbate.
Stability of the GSNO Formulation
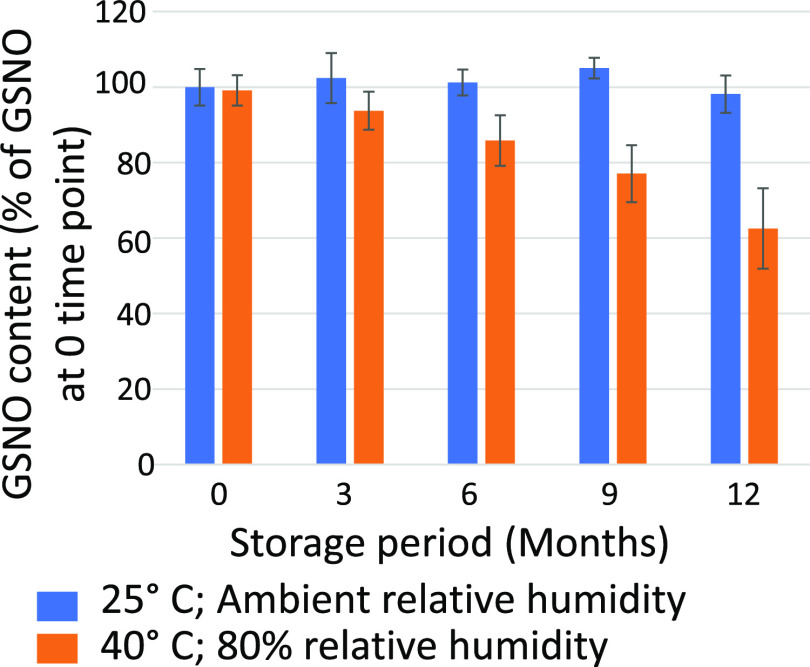
A stability study was performed on a large batch of the 100 μM GSNO/100 μM ascorbic acid formulation. Samples were sealed into polymer-lined aluminum foil pouches to shield the powder from moisture and light exposure. The overall study design evaluated the exposure of the formulations during both packaging and shelf storage conditions. The GSNO formulations were packaged under low (8–11% RH) and high (42–45% RH) humidity conditions using a controlled atmosphere glovebox (Terra Universal). The sealed foil packets of each formulation were then stored at 25 °C and at ambient humidity (25–55% RH) and elevated temperature (40 °C) and high humidity (80% RH). Samples were evaluated immediately after packaging and then at months 3, 6, 9, and 12 for the GSNO content by measuring the A334 and comparing to the initial A334 values. The dry powder formulation stored at 25 °C and ambient humidity (25–55% RH) retained 98 ± 5% of the initial GSNO content when assessed at 12 months. In contrast, when stored at 40 °C and high relative humidity (80%), the GSNO formulation samples experienced degradation and lost an average of 13.3% of their initial GSNO content at the 6 month assessment point, an average of 22.1% at the 9 month assessment, and 36.6% at the 12 month assessment (Figure 2). These results indicate that the dry powder can tolerate short periods of storage at higher than typical room temperature, when protected from moisture and light exposure, but are preferentially stored at typical ambient room temperature and humidity conditions.
Figure 2.
Stability study of the GSNO/Asc (1:1) formulation (in 0.16 M NaCl/0.035 M NaHCO3/10 μM Na2EDTA). Samples were packaged under 8–11% RH in a controlled atmosphere glovebox. Samples were stored at 25 °C and ambient humidity (25–55% RH) and at elevated 40 °C and high humidity (80% RH). The GSNO content was determined by measuring the UV–vis absorbance at 334.
GSNO Formulation Reduces the Density of S. aureus Biofilms Grown on Mucociliary-Differentiated Airway Epithelial Cells
In the initial studies, airway basal cells isolated from the bronchial tissue of a CF patient were used. These cells are defective in generating NO66 and, therefore, NO in these cultures likely represent NO generated from the added GSNO formulation. The mucociliary-differentiated cell cultures regenerated from basal cells were infected with S. aureus apically and incubated for 24 h. By scanning electron microscopy, we observed bacterial biofilms on the apical surface of the cultures (Figure 3A,B).
Figure 3.
S. aureus forms biofilms on the apical surface of mucociliary-differentiated bronchial epithelial cells. The apical surface of the mucociliary-differentiated CF bronchial epithelial cell culture was infected with S. aureus SA1692 and incubated for 24 h. The cell culture was fixed and subjected to SEM. (A) SEM showing the SA biofilm, mucus, and cilia on the apical surface of the cell culture. (B) Magnified view of the SA biofilm. The images are representative of three experiments.
To evaluate the effect of GSNO in reducing the biofilm bacterial density, the GSNO formulation was added to the apical surface of S. aureus-infected cell cultures at 50, 100, or 250 μM or respective placebos 3 times during a 24 h period, and the cells were harvested 4 h after the last treatment. The cell cultures were briefly rinsed with PBS, and the bacterial density associated with the cells was determined by a colony counting method. Compared to cultures treated with placebo, the cells treated with GSNO exhibited <0.5 to 1.5 log reduction in viable bacterial density depending on the GSNO concentration (Figure 4A). A maximum effect was observed at 100 μM, which did not improve when GSNO concentration was increased to 250 μM.
Figure 4.
Treatment with GSNO reduces the biofilm bacterial density on the apical surface of CF bronchial epithelial cells. Mucociliary-differentiated CF bronchial epithelial cells were infected apically with S. aureus isolates SA1696, SA1692, SANP, or a mixture of all three isolates and incubated for 24 h. The cells were then treated apically with GSNO (50, 100, or 250 μM) or appropriate placebo formulations 3 times during 24 h. Four hours after the last treatment, the apical surface was gently rinsed with PBS, and then the cells were lysed in 0.1% Triton X-100. The viable bacterial density in the cell lysates was determined by dilution plating. (A–D) The data represent the range with the median calculated from two or three independent experiments with two–three replicates (*p = ≤ 0.05; Mann–Whitney test, different from respective placebo-treated cultures). (E,F) Cell cultures were infected with a mixture of three S. aureus isolates and treated with placebo or 100 μM GSNO as above. The cultures were immunostained with antibodies to S. aureus (green) and ZO-1 (red) and counterstained with DAPI (blue) (E) and observed under a confocal microscope. In some experiments, the cell cultures were fixed in glutaraldehyde and processed for SEM (F). Images are representative of three experiments.
Next, we measured the NO content in the apical washes of cell cultures infected with S. aureus and treated with GSNO to determine whether NO released by GSNO reaches the maximum at 100 μM. We found that the NO content in the apical wash increased with the increasing concentration of GSNO (Table 1). However, the NO content on the apical wash accounted only for 29–65% of the total NO content of the added GSNO. This is not surprising because the NO emitted by GSNO diffuses into both bacteria and airway epithelial cells, reducing the NO content in the apical surface. These results indicate that increasing NO beyond a certain threshold may not improve the efficacy of NO in reducing biofilm density.
Table 1. NO Levels Measured as Nitrite on the Apical Surface of the S. aureus-Infected Airway Epithelial Cell Cultures in Relation to the Total NO Content of the Added GSNO.
| GSNO (μM) | GSNO added over 24 h(nmol) | total NO content in added GSNO (nmol) | nitrite in apical surface (nmol) ± SD |
|---|---|---|---|
| placebo | 0 | 0.0 | 0.69 ± 0.089 |
| 50 | 6 | 5.88 | 1.73 ± 0.525 |
| 100 | 12 | 11.76 | 5.50 ± 1.995a |
| 250 | 30 | 29.40 | 19.34 ± 6.843a |
Statistically different from cells treated with placebo.
A total of 120 μL of varying concentrations of GSNO was added to the cell cultures over a 24 h period. The second column represents the total nanomoles of GSNO added to the cell culture over 24 h.
To confirm that the efficacy of NO in reducing the bacterial biofilm density is not strain-dependent, we repeated the experiment with two more S. aureus strains (Figure 4B,C). Since CRS patients are often colonized with more than one S. aureus strain, we tested the effect of the GSNO formulation on epithelial cell cultures infected with a mixture of all three S. aureus strains. Again, GSNO at 100 μM was optimal in reducing the bacterial density (Figure 4D).
Confocal microscopy of infected cell cultures immuno-stained with anti-S. aureus antibodies revealed a reduction in the overall abundance of biofilm bacteria (green) in GSNO formulation-treated cultures compared to placebo-treated cultures (Figure 4E). We also observed an increased integrity of tight junctions in GSNO-treated cultures as revealed by ZO-1 staining (red). By SEM, S. aureus-infected cultures treated with GSNO show a dramatic reduction in biofilms compared to placebo-treated cells (Figure 4F). Interestingly, there was also a reduction in the accumulated mucus on the cell surface. Together these results indicate that GSNO may reduce the bacterial density by dispersing the biofilm and enhancing the hydration and removal of mucus along with bacteria.
Synergistic Effect of GSNO with Gentamicin
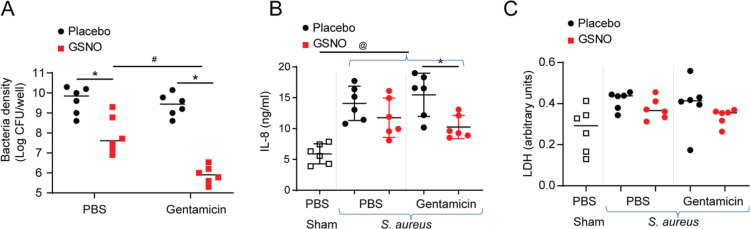
Unlike biofilms, planktonic S. aureus is more susceptible to gentamicin.67 NO is known to disperse biofilm bacteria and release planktonic bacteria, which are sensitive to gentamicin. To examine whether GSNO increases the susceptibility of biofilm bacteria to gentamicin, cultures infected with a mixture of S. aureus were exposed to a combination of 100 μM GSNO formulation and 5 μg of gentamicin as above, and the bacterial density was determined. Gentamicin alone did not reduce the viable bacterial burden (Figure 5A). As observed above, GSNO alone reduced the bacteria density by 2 log units. Interestingly, GSNO in combination with gentamicin decreased the bacterial density by 4 log units, indicating a synergy between GSNO and gentamicin in reducing the burden of biofilm bacteria.
Figure 5.
Gentamicin synergizes with GSNO to reduce the bacterial density (A) and IL-8 (B). CF bronchial epithelial cells were infected with a mixture of three S. aureus isolates or sham-infected with PBS and incubated for 24 h. Then, the cultures were apically treated with placebo, 5 μg of gentamicin, 100 μM GSNO, or a combination of GSNO and gentamicin 3 times over a 24 h period. Four hours after the last treatment, the basolateral medium was collected, the apical surface was gently rinsed with PBS, and then the cells were lysed in 0.1% Triton X-100. (A) The viable bacterial density in the cell lysates was determined by dilution plating. IL-8 (B) and LDH (C) were determined in the basolateral medium. The data represent the range with the median calculated from three independent experiments with two replicates. Statistical significance was pinpointed by ANOVA on ranks with the Kruskal–Wallis H test (*p = ≤ 0.05; different from the respective placebo-treated cultures, #p = ≤ 0.05; different from the GSNO-treated cultures, @p = ≤ 0.05; different from the sham-infected cultures).
GSNO Does Not Induce IL-8 Production or Cell Death
To test whether GSNO stimulates pro-inflammatory effects or cell death, the levels of IL-8 protein and LDH activity in the basolateral medium were measured. Uninfected cultures treated with placebo, GSNO alone, or GSNO + gentamicin showed similar levels of IL-8 and LDH, indicating that 100 μM GSNO or the combination of GSNO and gentamicin does not induce pro-inflammatory effects or cell death (Figure 5B,C). As expected, infection with bacteria increased IL-8 levels with no significant difference in LDH levels (Figure 5C). However, infected cells treated with the GSNO formulation or GSNO formulation + gentamicin showed a significant reduction in IL-8. These results indicate that treatment with GSNO or GSNO + gentamicin reduces bacteria-induced IL-8.
GSNO Improves the CBF of Bronchial Epithelial Cell Cultures
NO regulates the CBF, and NO donors, including GSNO, have been shown to improve the CBF in airway epithelial cells.68 We examined the effect of the GSNO formulation on infected and uninfected CF airway epithelial cells. Compared to uninfected cells, infected cells showed a significant reduction in the CBF (Figure 6). Treatment with either GSNO or the combination of GSNO and gentamicin reversed the bacteria-induced reduction in the CBF. Gentamicin alone had no effect on the CBF.
Figure 6.
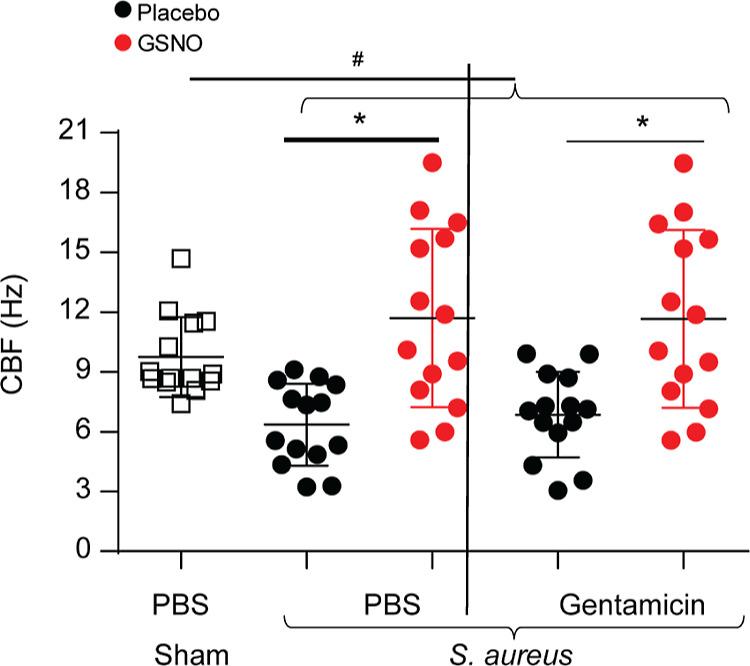
GSNO enhances the CBF in CF bronchial epithelial cell cultures. Mucociliary-differentiated CF bronchial epithelial cells were infected with a mixture of three S. aureus isolates or sham. After 24 h of incubation, the cultures were apically treated with PBS, gentamicin, GSNO, and a combination of GSNO and gentamicin 3 times over a 24 h period. Four hours after the last treatment, high-speed video microscopy was performed, and the CBF was analyzed. The data represent the range with the median calculated from three independent experiments with 3–6 replicates (*p = ≤ 0.05; ANOVA, different from respective placebo treated cultures, #p = ≤0.05; ANOVA on ranks with the Kruskal–Wallis H test, different from sham-infected cultures).
GSNO Reduces Biofilm Bacteria Grown on CRS Nasal Epithelial Cell Cultures and Improves the CBF
Next, we examined whether the GSNO formulation similarly affects S. aureus biofilms grown on the apical surface of nasal epithelial cell cultures established from two CRS patients. The mucociliary-differentiated sinonasal epithelial cell cultures were apically infected with a mixture of three S. aureus isolates and treated with a100 μM GSNO formulation with or without gentamicin as above and assessed for bacterial density, IL-8, and CBF. As observed with CF cell cultures, the GSNO formulation alone reduced the bacterial density by 1.5–2 log units (Figure 7A,B). In contrast, the combination of the GSNO formulation with gentamicin reduced the bacterial density by 4–5 log units, indicating a significant synergy between GSNO and gentamicin. As anticipated, the cell cultures infected with S. aureus showed an increase in IL-8 levels (Figure 7C,D). Treatment with a combination of the GSNO formulation and gentamicin but not gentamicin or GSNO alone significantly reduced IL-8, and this may be due to a reduction in the bacterial density by 4–5 log units. S. aureus-infected cultures treated with GSNO or the combination of GSNO and gentamicin also showed an improved CBF compared to those treated with placebo or gentamicin alone (Figure 7E,F). Together, these results indicate that the GSNO efficiently disperses biofilms, potentiates bacterial killing by gentamicin to decrease the viable bacterial density in S. aureus biofilms, and improves the CBF in airway epithelial cell cultures.
Figure 7.
Effect of gentamicin and GSNO in reducing the biofilm bacterial density, IL-8, and CBF of sinonasal epithelial cells. Mucociliary-differentiated sinonasal epithelial cell cultures from two CRS patients were infected with a mixture of three S. aureus isolates or sham. After 24 h of incubation, the cultures were apically treated with placebo, gentamicin, GSNO, or a combination of GSNO and gentamicin 3 times over a 24 h period. Three hours after the last treatment, the basolateral medium was collected, and IL-8 was analyzed by ELISA. The cells were subjected to high-speed video microscopy, and the CBF was analyzed. The cells were then washed with PBS and lysed in 0.1% Triton X-100, and the cell lysates were dilution-plated to determine the bacterial density. (A,C,E) Cells from patient 1 and (B,D,E) cells from patient 2. (A,B) Bacterial density determined in cell lysates. (C,D) IL-8 levels in the basolateral medium. (E,F) CBF. Data in (A–D) represent the range with the median from three replicate wells and in (E,F) represent the mean with SD from triplicates (*p = ≤ 0.05; ANOVA, different from respective placebo-treated cultures, #p = ≤ 0.05; ANOVA, different from GSNO-treated cultures, @p = ≤ 0.05; ANOVA, different from sham-infected cultures).
Discussion
Bacterial biofilms are refractory to antibiotics and often persist in the sinonasal cavity of infected CRS patients. Colonization with S. aureus and P. aeruginosa biofilms has been associated with chronic inflammation of the sinonasal mucosa in some CRS patients.14,69,70 Often, these persisting biofilm bacteria bloom and cause acute exacerbations. Short- and long-term antibiotic therapy to eradicate bacteria even for acute exacerbations has produced equivocal benefits of symptom or endoscopic scores.71,72 Therefore, there is a need for alternative or adjuvant therapies to treat bacterial infections in CRS patients. In this study, we report that treatment with GSNO, a naturally occurring NO donor, functions synergistically with gentamicin to reduce the density of biofilm bacteria and improve the CBF in mucociliary-differentiated upper airway epithelial cell cultures.
Although NO has been recognized as an emerging novel approach to treat antibiotic-resistant biofilm bacterial infections, due to limitations in the methods of delivery to target sites and off-target effects, NO is not currently being used to treat biofilm infections (reviewed in73). There are also some controversial reports based on the NO-specific donor used, the method of delivery, and the model systems employed. In most of the studies, bacterial biofilms grown on abiotic surfaces were used for testing the efficacy of the NO donor, which may not predict the amounts of NO released by the donor and the outcomes in vivo. In addition, based on the type of NO donor used, the amount of NO may vary, leading to variable results. In this study, we focused on a naturally occurring NO donor, such as GSNO, which is stable and can release NO in a reproducible manner when mixed with reducing agents.57,58 GSNO is already present as an NO carrier in the human body.74 For example, levels of GSNO in the blood are estimated to be in the 10 μM range.75 GSNO is also present in appreciable amounts in the upper airways inside the cells and on the surface of the airway epithelium.76 Other NO donors such as diazeniumdiolates,77 which are not endogenous, may have the risk of forming carcinogenic nitrosamines.
GSNO is relatively stable and does not generate NO readily. In this study, we used physiologically relevant reducing agents such as ascorbate or GSH to accelerate NO release from GSNO. We found that compared to GSH, ascorbate had a higher capacity to release NO from GSNO. When ascorbate was present at equimolar concentrations with GSNO, the maximum amount of NO was released from GSNO within 1 h. Moreover, ascorbic acid is consumed every day and has not been shown to have any cytotoxicity at concentrations far higher than those contained in the treatment formulations. Therefore, we choose to test the efficacy of the formulation containing equimolar amounts of GSNO and ascorbate in reducing S. aureus biofilms using physiologically relevant mucociliary-differentiated bronchial epithelial cells from CF patients and sinonasal epithelial cells from CRS patients.
The mucociliary-differentiated cell cultures structurally and functionally resemble the upper airway epithelium in vivo and hence may better predict the outcomes of NO treatment in vivo. Unlike in undifferentiated cell monolayers, S. aureus did not induce cytotoxicity and primarily persisted in the mucus layer that is present on the apical surface of mucociliary-differentiated cells. In addition, all three clinical S. aureus isolates tested formed biofilms on the apical surface of mucociliary-differentiated epithelial cell cultures as assessed by SEM or confocal microscopy, similar to that observed in CRS patients,78,79 indicating the suitability of the model to examine the effects of GSNO. In the initial experiments, we used airway epithelial cells from CF patients since these cells are defective in producing NO66 and therefore the effects of NO on biofilms could be primarily attributed to the NO released from GSNO. Using this model, GSNO at 100 μM was found to reduce viable bacterial density in a biofilm by 1.5 to 2 log units, which did not improve further when GSNO was used at 250 μM. This was not due to the limitation of NO released by GSNO in the presence of bacteria but may suggest that levels of NO above a given concentration may not have any beneficial effects. By confocal microscopy, there was a dramatic reduction in the overall biofilm mass. SEM indicated removal of mucus along with bacteria. These observations indicate that NO may reduce viable bacteria by dispersing the biofilm mass and removal of the bacteria along with mucus. A similar reduction in viable biofilm bacteria was observed when sinonasal epithelial cell cultures were used instead of CF airway epithelial cell cultures, indicating that the effect of GSNO on biofilm bacteria is not specific to CF cells.
Previously, we have demonstrated that controlled delivery of NO potentiates the efficacy of antibiotics against P. aeruginosa biofilm bacteria.80 In this study, we observed that gentamicin had no effect on S. aureus biofilm bacteria. However, when gentamicin was used in combination with GSNO, there was a 3–4 log reduction in viable bacterial density, indicating that GSNO greatly enhances the efficacy of gentamicin against S. aureus. This may be due to the ability of NO to actively disperse bacterial biofilms, thereby reversing bacteria from the biofilms to the planktonic state. S. aureus in the planktonic state is more sensitive than in the biofilm state and readily killed by gentamicin.67 Consistent with this, we observed that all three S. aureus isolates used in the present study are amenable to gentamicin in their planktonic state.
Mupirocin is more commonly used to treat sinus infections in CRS patients. In this study, gentamicin was used as a proof of concept to differentiate between killing of planktonic versus biofilm bacteria. We plan to examine the synergy between the GSNO formulation and mupirocin and other antibiotics that are used to treat CRS patients in reducing the S. aureus biofilm density in the future.
NO regulates the physiological functions of the airway epithelium including the mucociliary function, epithelial ion transport, and airway barrier function.81 We found that GSNO significantly enhanced the CBF in both CF and CRS epithelial cell cultures. In addition, we also observed dramatically reduced mucus on CF cell cultures treated with the GSNO formulation. These observations may indicate that NO released from GSNO not only disperses biofilm bacteria on the cell surface but may also diffuse into underlying epithelial cells to improve the mucociliary clearance.
GSNO added exogenously does not enter the cells but decomposes to NO and GSH. The latter is present in abundant amounts and is one of the major anti-oxidants in the lungs.82 NO at greater than physiological levels may increase nitrosative stress and activate signaling mechanisms to increase inflammatory responses and induce cell death. Therefore, we examined whether the NO released by the GSNO formulation had an effect on cell death and pro-inflammatory responses in both CF and sinonasal epithelial cell cultures. Herein, we clearly demonstrate that a GSNO formulation can release sufficient NO to reduce the density of biofilms without stimulating a pro-inflammatory response or inducing cell death (as determined by LDH). Further, GSNO reduced the IL-8 levels in both infected and uninfected epithelial cell cultures, indicating that the low levels of NO gas released from GSNO may act as an anti-inflammatory agent. It is noteworthy that even though NO measured as nitrite was much higher in the cells treated with 250 μM, it did not cause cytotoxicity or increase the pro-inflammatory effects. In addition, we observed increased localization of ZO-1 at the periphery of cells in GSNO-treated S. aureus-infected cell cultures, indicating that NO may improve the barrier function. Therefore, GSNO treatment, in addition to reducing the bacterial biofilm density, may also reduce inflammation and improve the innate immune function of the sinus epithelium.
In the present study, we used a unique treatment strategy that is utilized 3 times over a 24 h period to determine the efficacy of NO in reducing bacterial biofilms due to the following reasons. The GSNO formulation used in this study has a high release rate of NO, with the majority of the NO released from the sample within 2 h, and repeated treatment was used to maintain the NO levels in the cell cultures. This is necessary to prevent the formation of new biofilms by planktonic bacteria released from the existing biofilm with the initial burst of NO from the added GSNO. Additionally, CRS patients with flare-ups are usually treated two or three times over 24 h, and the strategy used here somewhat mimics the real-world treatment regime.
Although S. aureus infects CF patients at an early age, the majority of CF patients become colonized with mucoid P. aeruginosa by the age of 5 years. Mucoid P. aeruginosa exists as a biofilm in the airways of CF patients. Since CF patients have reduced levels of NO in their lungs, it is possible that treatment with the GSNO formulation may reduce biofilm bacteria in CF patients. In fact, a small study showed that treatment with aerosolized GSNO improves the gas exchange and increases the exhaled NO in CF patients for up to 30 min.83 Recently, it was shown that treatment with 10 ppm NO gas for 8 h a day for 5–7 days significantly reduced the bacterial load in patients admitted to the hospital and who were on intravenous antibiotics.84 Even though GSNO may be effective in killing biofilm bacteria in vitro on the CF airway epithelial cell cultures, it may not be efficacious in CF patients. This is because the CF airways are blocked with viscous sputum, which may affect the distribution of aerosolized or inhaled GSNO and therefore may not effectively disperse bacterial biofilms that are embedded in the viscous sputum. At present, we are investigating the effect of photochemically derived NO on reducing mucoid P. aeruginosa and S. aureus bacterial biofilms established on CF cells, and this is a subject for future publication.
Interestingly, we observed more variability in the bacterial density in the GSNO formulation- or GSNO formulation + gentamicin-treated cell cultures. This may be due to the fact that in placebo-treated cultures, the biofilm is not dispersed and therefore the bacterial density may not change considerably, leading to a more consistent bacterial load. However, in GSNO-treated cells, the bacterial biofilm is disrupted, leading to the release and killing of planktonic bacteria released from the biofilm, and this can vary widely based on the amount of mucus on the cell surface and also the action of antibacterial factors released from the cells, which can kill planktonic bacteria.
In summary, we show that the GSNO formulation containing ascorbic acid in addition to dispersing biofilms potentiates the efficacy of gentamicin to effectively reduce the viable bacterial density in the biofilm established on the surface of mucociliary-differentiated sinonasal epithelial cells without inducing a pro-inflammatory response or cell death. CRS patients usually have chronic sinonasal inflammation, which may be reduced by GSNO given the anti-inflammatory effects of NO under physiological conditions.85,86 Further, GSNO treatment may improve the mucociliary clearance function of the sinonasal epithelium, thus preventing the acute exacerbations and disease perpetuation in these patients. Since GSNO is already present in our bodies and we consume ascorbic acid daily at much higher concentrations, we envision that this formulation may be developed into therapy to treat flare-ups, prevent biofilm formation, and enhance mucociliary clearance in CRS patients.
Acknowledgments
This work was funded by NIH R44AI120443.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c06212.
Total amount of NO release from GSNO formulations and NO release from different concentrations of GSNO (PDF)
Author Contributions
A.W. and G.M. prepared and analyzed the GSNO formulations; M.T. analyzed the data and prepared the first draft of the manuscript; M.Z. and M.B.H. participated in designing the studies; M.E.M. conceptualized the overall study and guided the development of GSNO formulations; U.S. is a corresponding author and designed the overall study, conducted experiments, finalized the data analysis, and the manuscript.
The authors declare the following competing financial interest(s): AW and GM are employees of the NOTA Labs MEM serves as Chief Technology Officer for NOTA Labs MZ, MBH, and US are scientific officers for NOTA Labs
Supplementary Material
References
- Pleis J. R.; Ward B. W.; Lucas J. W. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Statistics 2010, 249, 1–207. [PubMed] [Google Scholar]
- Schiller J. S.; Lucas J. W.; Ward B. W.; Peregoy J. A. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Statistics 2012, 252, 1–207. [PubMed] [Google Scholar]
- Caulley L.; Thavorn K.; Rudmik L.; Cameron C.; Kilty S. J. Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: Results of the US Medical Expenditure Panel Survey. J. Allergy Clin. Immunol. 2015, 136, 1517–1522. 10.1016/j.jaci.2015.08.037. [DOI] [PubMed] [Google Scholar]
- Rudmik L. Economics of Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2017, 17, 20. 10.1007/s11882-017-0690-5. [DOI] [PubMed] [Google Scholar]
- Kern R. C.; Conley D. B.; Walsh W.; Chandra R.; Kato A.; Tripathi-Peters A.; Grammer L. C.; Schleimer R. P. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am. J. Rhinol. 2008, 22, 549–559. 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. The role of bacteria in chronic rhinosinusitis. Otolaryngol. Clin. 2005, 38, 1171–1192. 10.1016/j.otc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hamilos D. L. Host-microbial interactions in patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2013, 131, 1263–1264. 10.1016/j.jaci.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Meltzer E. O.; Hamilos D. L.; Hadley J. A.; Lanza D. C.; Marple B. F.; Nicklas R. A.; Bachert C.; Baraniuk J.; Baroody F. M.; Benninger M. S.; Brook I.; Chowdhury B. A.; Druce H. M.; Durham S.; Ferguson B.; Gwaltney J. M.; Kaliner M.; Kennedy D. W.; Lund V.; Naclerio R.; Pawankar R.; Piccirillo J. F.; Rohane P.; Simon R.; Slavin R. G.; Togias A.; Wald E. R.; Zinreich S. J. Rhinosinusitis: establishing definitions for clinical research and patient care. J. Allergy Clin. Immunol. 2004, 114, 155–212. 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M.; Flynn M. J.; Rose F. V.; Jousimies-Somer H.; Jakielaszek C.; McTeague M.; Wexler H. M.; Berkowitz E.; Wynne B. Bacteriologic findings associated with chronic bacterial maxillary sinusitis in adults. Clin. Infect. Dis. 2002, 35, 428–433. 10.1086/341899. [DOI] [PubMed] [Google Scholar]
- Stephenson M. F.; Mfuna L.; Dowd S. E.; Wolcott R. D.; Barbeau J.; Poisson M.; James G.; Desrosiers M. Molecular characterization of the polymicrobial flora in chronic rhinosinusitis. J. Otolaryngol. Head Neck Surg 2010, 39, 182–187. [PubMed] [Google Scholar]
- Psaltis A. J.; Ha K. R.; Beule A. G.; Tan L. W.; Wormald P. J. Confocal scanning laser microscopy evidence of biofilms in patients with chronic rhinosinusitis. Laryngoscope 2007, 117, 1302–1306. 10.1097/mlg.0b013e31806009b0. [DOI] [PubMed] [Google Scholar]
- Oncel S.; Pinar E.; Sener G.; Calli C.; Karagoz U. Evaluation of bacterial biofilms in chronic rhinosinusitis. J. Otolaryngol. Head Neck Surg 2010, 39, 52–55. [PubMed] [Google Scholar]
- Uhliarova B.; Karnisova R.; Svec M.; Calkovska A. Correlation between culture-identified bacteria in the middle nasal meatus and CT score in patients with chronic rhinosinusitis. J. Med. Microbiol. 2014, 63, 28–33. 10.1099/jmm.0.068320-0. [DOI] [PubMed] [Google Scholar]
- Bendouah Z.; Barbeau J.; Hamad W. A.; Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol. Head Neck Surg. 2006, 134, 991–996. 10.1016/j.otohns.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Foreman A.; Wormald P. J. Different biofilms, different disease? A clinical outcomes study. Laryngoscope 2010, 120, 1701–1706. 10.1002/lary.21024. [DOI] [PubMed] [Google Scholar]
- Singhal D.; Foreman A.; Bardy J.; Wormald P. J. Staphylococcus aureus biofilms: Nemesis of endoscopic sinus surgery. Laryngoscope 2011, 121, 1578–1583. 10.1002/lary.21805. [DOI] [PubMed] [Google Scholar]
- Drilling A.; Coombs G. W.; Tan H.-L.; Pearson J. C.; Boase S.; Psaltis A.; Speck P.; Vreugde S.; Wormald P.-J. Cousins, siblings, or copies: the genomics of recurrent Staphylococcus aureus infections in chronic rhinosinusitis. Int. Forum. Allergy Rhinol. 2014, 4, 953. 10.1002/alr.21423. [DOI] [PubMed] [Google Scholar]
- Tan N. C.; Foreman A.; Jardeleza C.; Douglas R.; Tran H.; Wormald P. J. The multiplicity of Staphylococcus aureus in chronic rhinosinusitis: correlating surface biofilm and intracellular residence. Laryngoscope 2012, 122, 1655–1660. 10.1002/lary.23317. [DOI] [PubMed] [Google Scholar]
- Tan N. C.; Foreman A.; Jardeleza C.; Douglas R.; Vreugde S.; Wormald P. J. Intracellular Staphylococcus aureus: the Trojan horse of recalcitrant chronic rhinosinusitis?. Int. Forum. Allergy Rhinol. 2013, 3, 261–266. 10.1002/alr.21154. [DOI] [PubMed] [Google Scholar]
- Teff Z.; Priel Z.; Gheber L. A. The forces applied by cilia depend linearly on their frequency due to constant geometry of the effective stroke. Biophys. J. 2008, 94, 298–305. 10.1529/biophysj.107.111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman A.; Priel Z. Efficient mucociliary transport relies on efficient regulation of ciliary beating. Respir. Physiol. Neurobiol. 2008, 163, 202–207. 10.1016/j.resp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Elwany S.; Hisham M.; Gamaee R. The effect of endoscopic sinus surgery on mucociliary clearance in patients with chronic sinusitis. Eur. Arch. Otorhinolaryngol. 1998, 255, 511–514. 10.1007/s004050050109. [DOI] [PubMed] [Google Scholar]
- Passàli D.; Ferri R.; Becchini G.; Passàli G. C.; Bellussi L. Alterations of nasal mucociliary transport in patients with hypertrophy of the inferior turbinates, deviations of the nasal septum and chronic sinusitis. Eur. Arch. Otorhinolaryngol. 1999, 256, 335–337. 10.1007/s004050050158. [DOI] [PubMed] [Google Scholar]
- Dal T.; Önerci M.; Çaĝlar M. Mucociliary function of the maxillary sinuses after restoring ventilation: a radioisotopic study of the maxillary sinus. Eur. Arch. Otorhinolaryngol. 1997, 254, 205–207. 10.1007/bf00879275. [DOI] [PubMed] [Google Scholar]
- Alexander N. S.; Hatch N.; Zhang S.; Skinner D.; Fortenberry J.; Sorscher E. J.; Woodworth B. A. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. Laryngoscope 2011, 121, 1313–1319. 10.1002/lary.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.; Shaari J.; Claire S. E.; Palmer J. N.; Chiu A. G.; Kennedy D. W.; Cohen N. A. Altered sinonasal ciliary dynamics in chronic rhinosinusitis. Am. J. Rhinol. 2006, 20, 325–329. 10.2500/ajr.2006.20.2870. [DOI] [PubMed] [Google Scholar]
- Chen B.; Antunes M. B.; Claire S. E.; Palmer J. N.; Chiu A. G.; Kennedy D. W.; Cohen N. A. Reversal of chronic rhinosinusitis-associated sinonasal ciliary dysfunction. Am. J. Rhinol. 2007, 21, 346–353. 10.2500/ajr.2007.21.3029. [DOI] [PubMed] [Google Scholar]
- Mfuna-Endam L.; Zhang Y.; Desrosiers M. Y. Genetics of rhinosinusitis. Curr. Allergy Asthma Rep. 2011, 11, 236–246. 10.1007/s11882-011-0189-4. [DOI] [PubMed] [Google Scholar]
- Kanthakumar K.; Cundell D. R.; Johnson M.; Wills P. J.; Taylor G. W.; Cole P. J.; Wilson R. Effect of salmeterol on human nasal epithelial cell ciliary beating: inhibition of the ciliotoxin, pyocyanin. Br. J. Pharmacol. 1994, 112, 493–498. 10.1111/j.1476-5381.1994.tb13100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustke H.; Kleene R.; Loers G.; Nehmann N.; Jaehne M.; Bartels K. M.; Jaeger K. E.; Schachner M.; Schumacher U. Inhibition of the bacterial lectins of Pseudomonas aeruginosa with monosaccharides and peptides. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 207–215. 10.1007/s10096-011-1295-x. [DOI] [PubMed] [Google Scholar]
- Kim C. S.; Jeon S. Y.; Min Y. G.; Rhyoo C.; Kim J. W.; Yun J. B.; Park S. W.; Kwon T. Y. Effects of beta-toxin of Staphylococcus aureus on ciliary activity of nasal epithelial cells. Laryngoscope 2000, 110, 2085–2088. 10.1097/00005537-200012000-00021. [DOI] [PubMed] [Google Scholar]
- Min Y. G.; Jun Oh S. J.; Won T. B.; Kim Y. M.; Shim W. S.; Rhee C. S.; Min J. Y.; Dhong H. J. Effects of staphylococcal enterotoxin on ciliary activity and histology of the sinus mucosa. Acta Otolaryngol. 2006, 126, 941–947. 10.1080/00016480500469016. [DOI] [PubMed] [Google Scholar]
- Shen J. C.; Cope E.; Chen B.; Leid J. G.; Cohen N. A. Regulation of murine sinonasal cilia function by microbial secreted factors. Int. Forum. Allergy Rhinol. 2012, 2, 104–110. 10.1002/alr.21002. [DOI] [PubMed] [Google Scholar]
- Zhao K. Q.; Goldstein N.; Yang H.; Cowan A. T.; Chen B.; Zheng C.; Palmer J. N.; Kreindler J. L.; Cohen N. A. Inherent differences in nasal and tracheal ciliary function in response to Pseudomonas aeruginosa challenge. Am. J. Rhinol. Allergy 2011, 25, 209–213. 10.2500/ajra.2011.25.3614. [DOI] [PubMed] [Google Scholar]
- Lundberg J. O.; Weitzberg E. Nasal nitric oxide in man. Thorax 1999, 54, 947–952. 10.1136/thx.54.10.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W.; Min Y. G.; Rhee C. S.; Lee C. H.; Koh Y. Y.; Rhyoo C.; Kwon T. Y.; Park S. W. Regulation of mucociliary motility by nitric oxide and expression of nitric oxide synthase in the human sinus epithelial cells. Laryngoscope 2001, 111, 246–250. 10.1097/00005537-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Jiao J.; Wang H.; Lou W.; Jin S.; Fan E.; Li Y.; Han D.; Zhang L. Regulation of ciliary beat frequency by the nitric oxide signaling pathway in mouse nasal and tracheal epithelial cells. Exp. Cell Res. 2011, 317, 2548–2553. 10.1016/j.yexcr.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Fowler C. J.; Olivier K. N.; Leung J. M.; Smith C. C.; Huth A. G.; Root H.; Kuhns D. B.; Logun C.; Zelazny A.; Frein C. A.; Daub J.; Haney C.; Shelhamer J. H.; Bryant C. E.; Holland S. M. Abnormal nasal nitric oxide production, ciliary beat frequency, and Toll-like receptor response in pulmonary nontuberculous mycobacterial disease epithelium. Am. J. Respir. Crit. Care Med. 2013, 187, 1374–1381. 10.1164/rccm.201212-2197oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari A.; Miller C. C.; McMullin B.; Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 2006, 14, 21–29. 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Webert K. E.; Vanderzwan J.; Duggan M.; Scott J. A.; McCormack D. G.; Lewis J. F.; Mehta S. Effects of inhaled nitric oxide in a rat model of Pseudomonas aeruginosa pneumonia. Crit. Care Med. 2000, 28, 2397–2405. 10.1097/00003246-200007000-00035. [DOI] [PubMed] [Google Scholar]
- Long R.; Light B.; Talbot J. A. Mycobacteriocidal action of exogenous nitric oxide. Antimicrob. Agents Chemother. 1999, 43, 403–405. 10.1128/aac.43.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn T.; Huebner J.; Paboura E.; Krause M.; Leititis J. U. Effect of therapeutic concentrations of nitric oxide on bacterial growth in vitro. Crit. Care Med. 1998, 26, 1857–1862. 10.1097/00003246-199811000-00028. [DOI] [PubMed] [Google Scholar]
- Sanders S. P.; Kim J.; Ryan Connolly K. R.; Porter J. D.; Siekierski E. S.; Proud D. Nitric oxide inhibits rhinovirus-induced granulocyte macrophage colony-stimulating factor production in bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001, 24, 317–325. 10.1165/ajrcmb.24.3.4131. [DOI] [PubMed] [Google Scholar]
- Guidotti L. G.; McClary H.; Loudis J. M.; Chisari F. V. Nitric oxide inhibits hepatitis B virus replication in the livers of transgenic mice. J. Exp. Med. 2000, 191, 1247–1252. 10.1084/jem.191.7.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D. R.; Ashkar A. A.; Mossman K. L. The nitric oxide pathway provides innate antiviral protection in conjunction with the type I interferon pathway in fibroblasts. PLoS One 2012, 7, e31688 10.1371/journal.pone.0031688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. C.; Hergott C. A.; Rohan M.; Arsenault-Mehta K.; Döring G.; Mehta S. Inhaled nitric oxide decreases the bacterial load in a rat model of Pseudomonas aeruginosa pneumonia. J. Cyst. Fibros. 2013, 12, 817–820. 10.1016/j.jcf.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Jardeleza C.; Foreman A.; Baker L.; Paramasivan S.; Field J.; Tan L. W.; Wormald P. J. The effects of nitric oxide on Staphylococcus aureus biofilm growth and its implications in chronic rhinosinusitis. Int. Forum. Allergy Rhinol. 2011, 1, 438–444. 10.1002/alr.20083. [DOI] [PubMed] [Google Scholar]
- Sulemankhil I.; Ganopolsky J. G.; Dieni C. A.; Dan A. F.; Jones M. L.; Prakash S. Prevention and treatment of virulent bacterial biofilms with an enzymatic nitric oxide-releasing dressing. Antimicrob. Agents Chemother. 2012, 56, 6095–6103. 10.1128/aac.01173-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud N.; Storey M. V.; Moore Z. P.; Webb J. S.; Rice S. A.; Kjelleberg S. Nitric oxide-mediated dispersal in single- and multi-species biofilms of clinically and industrially relevant microorganisms. Microb. Biotechnol. 2009, 2, 370–378. 10.1111/j.1751-7915.2009.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg S.; And A.; Runer T. Nitric oxide (NO) production in the upper airways is decreased in chronic sinusitis. Acta Otolaryngol. 1997, 117, 113–117. 10.3109/00016489709118001. [DOI] [PubMed] [Google Scholar]
- Deja M.; Busch T.; Bachmann S.; Riskowski K.; Câmpean V.; Wiedmann B.; Schwabe M.; Hell B.; Pfeilschifter J.; Falke K. J.; Lewandowski K. Reduced nitric oxide in sinus epithelium of patients with radiologic maxillary sinusitis and sepsis. Am. J. Respir. Crit. Care Med. 2003, 168, 281–286. 10.1164/rccm.200207-640oc. [DOI] [PubMed] [Google Scholar]
- Colantonio D.; Brouillette L.; Parikh A.; Scadding G. K. Paradoxical low nasal nitric oxide in nasal polyposis. Clin. Exp. Allergy 2002, 32, 698–701. 10.1046/j.1365-2222.2002.01379.x. [DOI] [PubMed] [Google Scholar]
- Gilain L.; Bedu M.; Jouaville L.; Guichard C.; Advenier D.; Mom T.; Laurent S.; Caillaud D. [Analysis of nasal and exhaled nitric oxide concentration in nasal polyposis]. Ann Otolaryngol Chir Cervicofac 2002, 119, 234–242. [PubMed] [Google Scholar]
- Ragab S. M.; Lund V. J.; Saleh H. A.; Scadding G. Nasal nitric oxide in objective evaluation of chronic rhinosinusitis therapy. Allergy 2006, 61, 717–724. 10.1111/j.1398-9995.2006.01044.x. [DOI] [PubMed] [Google Scholar]
- Noda N.; Takeno S.; Fukuiri T.; Hirakawa K. Monitoring of oral and nasal exhaled nitric oxide in eosinophilic chronic rhinosinusitis: a prospective study. Am. J. Rhinol. Allergy 2012, 26, 255–259. 10.2500/ajra.2012.26.3772. [DOI] [PubMed] [Google Scholar]
- Gaston B.; Reilly J.; Drazen J. M.; Fackler J.; Ramdev P.; Arnelle D.; Mullins M. E.; Sugarbaker D. J.; Chee C.; Singel D. J.; Loscalzo J.; Stamler J. S. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc. Natl. Acad. Sci. U.S.A 1993, 90, 10957–10961. 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. J.; Hogg N.; Joseph J.; Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J. Biol. Chem. 1996, 271, 18596–18603. 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- Smith J. N.; Dasgupta T. P. Kinetics and mechanism of the decomposition of S-nitrosoglutathione by l-ascorbic acid and copper ions in aqueous solution to produce nitric oxide. Nitric Oxide 2000, 4, 57–66. 10.1006/niox.2000.0272. [DOI] [PubMed] [Google Scholar]
- Hart T. W. Some Observations Concerning the S-Nitroso and S-Phenylsulfonyl Derivatives of L-Cysteine and Glutathione. Tetrahedron Lett. 1985, 26, 2013–2016. 10.1016/s0040-4039(00)98368-0. [DOI] [Google Scholar]
- Jing Y.; Gimenes J. A.; Mishra R.; Pham D.; Comstock A. T.; Yu D.; Sajjan U. NOTCH3 contributes to rhinovirus-induced goblet cell hyperplasia in COPD airway epithelial cells. Thorax 2019, 74, 18–32. 10.1136/thoraxjnl-2017-210593. [DOI] [PubMed] [Google Scholar]
- Xander N.; Reddy Vari H.; Eskandar R.; Li W.; Bolla S.; Marchetti N.; Sajjan U. S. Rhinovirus-Induced SIRT-1 via TLR2 Regulates subsequent type I and type III IFN responses in airway epithelial cells. J. Immunol. 2019, 203, 2508–2519. 10.4049/jimmunol.1900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Chug M. K.; Brisbois E. J. Long-term storage stability and nitric oxide release behavior of (N-Acetyl-S-nitrosopenicillaminyl)-S-nitrosopenicillamine-incorporated silicone rubber coatings. ACS Appl. Mater. Interfaces 2022, 14, 30595–30606. 10.1021/acsami.2c06712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan N. S.; Grisham M. B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj S. S.; Ganesan S.; Jones A. M.; Helm J. M.; Comstock A. T.; Bright-Thomas R.; LiPuma J. J.; Hershenson M. B.; Sajjan U. S. Rhinovirus infection liberates planktonic bacteria from biofilm and increases chemokine responses in cystic fibrosis airway epithelial cells. Thorax 2011, 66, 333–339. 10.1136/thx.2010.151431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio P.; da Silva M. F.; Vale I.; Roxo-Rosa M.; Pinto A.; Constant C.; Pereira L.; Quintão C. M.; Lopes S. S. CiliarMove: new software for evaluating ciliary beat frequency helps find novel mutations by a Portuguese multidisciplinary team on primary ciliary dyskinesia. ERJ Open Res. 2021, 7, 00792-2020. 10.1183/23120541.00792-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley T. J.; Drumm M. L. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J. Clin. Invest. 1998, 102, 1200–1207. 10.1172/jci2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino H. A. C.; Valera F. C. P.; Santos D. V.; Fantucci M. Z.; Titoneli C. C.; Martinez R.; Anselmo-Lima W. T.; Tamashiro E. Biofilm and planktonic antibiotic resistance in patients with acute exacerbation of chronic rhinosinusitis. Front. Cell. Infect. Microbiol. 2021, 11, 813076. 10.3389/fcimb.2021.813076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.; Shirakami G.; Zhan X.; Johns R. A. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2000, 23, 175–181. 10.1165/ajrcmb.23.2.4022. [DOI] [PubMed] [Google Scholar]
- Karunasagar A.; Garag S. S.; Appannavar S. B.; Kulkarni R. D.; Naik A. S. Bacterial Biofilms in Chronic Rhinosinusitis and their implications for clinical management. Indian J. Otolaryngol. Head Neck Surg.: Off. Publ. Assoc. Otolaryngologists India 2018, 70, 43–48. 10.1007/s12070-017-1208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina I. W.; Patel N. N.; Cohen N. A. Understanding the role of biofilms and superantigens in chronic rhinosinusitis. Curr. Otorhinolaryngol. Rep. 2018, 6, 253–262. 10.1007/s40136-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J.; Bassler B. L. Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 2019, 26, 15–21. 10.1016/j.chom.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino H. A.; Valera F. C.; Aragon D. C.; Fantucci M. Z.; Titoneli C. C.; Martinez R.; Anselmo-Lima W. T.; Tamashiro E. Amoxicillin-clavulanate for patients with acute exacerbation of chronic rhinosinusitis: a prospective, double-blinded, placebo-controlled trial. Int. Forum. Allergy Rhinol. 2017, 7, 135–142. 10.1002/alr.21846. [DOI] [PubMed] [Google Scholar]
- Poh W. H.; Rice S. A. Recent Developments in nitric oxide donors and delivery for antimicrobial and anti-biofilm applications. Molecules 2022, 27, 674. 10.3390/molecules27030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath N.; Morinaga O.; Singh I. S-nitrosoglutathione a physiologic nitric oxide carrier attenuates experimental autoimmune encephalomyelitis. J. Neuroimmune Pharmacol. 2010, 5, 240–251. 10.1007/s11481-009-9187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustarini D.; Milzani A.; Dalle-Donne I.; Rossi R. Detection of S-nitrosothiols in biological fluids: a comparison among the most widely applied methodologies. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2007, 851, 124–139. 10.1016/j.jchromb.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Gaston B.; Sears S.; Woods J.; Hunt J.; Ponaman M.; McMahon T.; Stamler J. S. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet 1998, 351, 1317–1319. 10.1016/s0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J.; Napoli C.; Loscalzo J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ. Res. 2002, 90, 21–28. 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- Fastenberg J. H.; Hsueh W. D.; Mustafa A.; Akbar N. A.; Abuzeid W. M. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 219–229. 10.1016/j.wjorl.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J. H.; Cha H. E.; Kang I. G.; Kim S. T. Clinical characteristics of biofilms in patients with chronic rhinosinusitis: a prospective case-control study. Indian J. Otolaryngol. Head Neck Surg.: Off. Publ. Assoc. Otolaryngologists India 2015, 67, 1–6. 10.1007/s12070-014-0707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H.; Wu J.; Colletta A.; Meyerhoff M. E.; Xi C. Efficient eradication of mature Pseudomonas aeruginosa biofilm via controlled delivery of nitric oxide combined with antimicrobial peptide and antibiotics. Front. Microbiol. 2016, 7, 1260. 10.3389/fmicb.2016.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarri M. A.; Milara J.; Estornut C.; Cortijo J. Nitric oxide system and bronchial epithelium: More than a barrier. Front. Physiol. 2021, 12, 687381. 10.3389/fphys.2021.687381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski A. V.; Liu Q.; Liu J.; Redlich C. A. Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin. Exp. Allergy 2005, 35, 352–357. 10.1111/j.1365-2222.2005.02185.x. [DOI] [PubMed] [Google Scholar]
- Snyder A. H.; McPherson M. E.; Hunt J. F.; Johnson M.; Stamler J. S.; Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002, 165, 922–926. 10.1164/ajrccm.165.7.2105032. [DOI] [PubMed] [Google Scholar]
- Howlin R. P.; Cathie K.; Hall-Stoodley L.; Cornelius V.; Duignan C.; Allan R. N.; Fernandez B. O.; Barraud N.; Bruce K. D.; Jefferies J.; Kelso M.; Kjelleberg S.; Rice S. A.; Rogers G. B.; Pink S.; Smith C.; Sukhtankar P. S.; Salib R.; Legg J.; Carroll M.; Daniels T.; Feelisch M.; Stoodley P.; Clarke S. C.; Connett G.; Faust S. N.; Webb J. S. Low-Dose Nitric Oxide as Targeted Anti-biofilm Adjunctive Therapy to Treat Chronic Pseudomonas aeruginosa Infection in Cystic Fibrosis. Mol. Ther.: J. Am. Soc. Gene Ther. 2017, 25, 2104–2116. 10.1016/j.ymthe.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri T.; Conti I.; Cerri C.; Tavanti L.; Paggiaro P.; Celi A. Divergent effects of nitric oxide on airway epithelial cell activation. Biol. Res. 2010, 43, 467–473. 10.4067/s0716-97602010000400012. [DOI] [PubMed] [Google Scholar]
- Sharma J. N.; Al-Omran A.; Parvathy S. S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.