Abstract

Archaeosomes are a new generation of stable liposomes composed of natural ether lipids extracted from archaea, or synthetic archaeal lipids. Archaea constitute a domain of single-celled microorganisms that are structurally similar to but evolutionarily distinct from bacteria. They synthesize unique membrane lipids with isoprenoid hydrocarbon side chains attached via an ether linkage to the glycerol-phosphate backbone. Compared to the ester linkages found in the lipids of Eukarya and bacteria, the ether linkages in archaeal lipids are more stable in various environmental conditions such as high/low temperatures, acidic or alkaline pH, bile salts, and enzymatic hydrolysis. This feature has intrigued scientists to use archaeal lipids to prepare archaeosomes with superior physicochemical stability and utilize them as effective carriers to deliver various cargos of biomedical importance such as drugs, proteins, peptides, genes, and antioxidants to the target site. Archaeosomes carrying antigens and/or adjuvants are also proven to be better candidates for stimulating antigen-specific, humoral, and cell-mediated immune responses, which broadens their scope in vaccine delivery. These properties associated with excellent biocompatibility and a safety profile provide numerous advantages to the archaeosomes to function as a versatile delivery system. This mini-review will provide an overview of the unique features of archaeal lipids, preparation and characterization of archaeosomes, and emphasize the prospects related to drug delivery and other biomedical applications.
1. Introduction
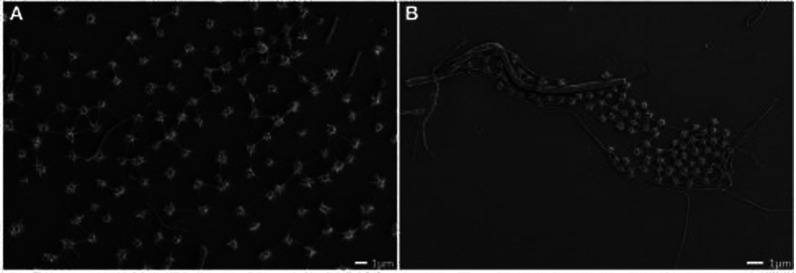
Archaea are single-celled microorganisms with no nucleus or defined organelles. They represent the third domain of life on earth and are evolutionarily distinct from the other two domains, namely bacteria and Eukarya. Archaea are well-known for their ability to grow and thrive in extreme habitats ranging from hot springs and volcanoes to hypersaline environments such as salt lakes and oceans, highly acidic or alkaline conditions, and high pressure that are hostile to most other organisms on earth.1,2 Such organisms, known as extremophiles have been found at depths of 6.7 km inside the earth’s crust, more than 10 km deep inside the ocean at pressures of up to 110 MPa, from extreme acidic (pH 0) to extreme basic conditions (pH 12.8), and from hydrothermal vents at 122 °C to frozen seawater at −20 °C.3 Based on their living habitats, they are classified into different groups such as thermophiles and hyperthermophiles (able to grow at high or very high temperatures, respectively), psychrophiles (adapted to grow best at low temperatures), acidophiles and alkaliphiles (survive at high acidic or basic pH values, respectively), barophiles (able to thrive under pressure), and halophiles (organisms that require NaCl for growth).4 For instance, the hyperthermophilic methanogen Methanopyrus kandleri strain 116 is reported to proliferate even at the highest recorded temperature of 122 °C and produce methane under an in situ high pressure (20 MPa), while the genus Picrophilus (e.g., Picrophilus torridus) belonging to acidophilic archaea grows optimally at a pH of 0.06.5 Archaea exist in different shapes such as rod, spherical, spiral, rectangular, lobed, or irregular. For instance, scanning electron micrographs (Figure 1A,B) indicate the coccoid-shaped archaea, called “SM1 euryarchaeon,” which are generally found to float as biofilms on the water surface of springs.6
Figure 1.
(A and B) Scanning electron micrographs showing the homogeneous archaeal population of small coccoid-shaped and SM1 euryarchaeon cells. Reproduced with permission from ref (6). Copyright 2014, Frontiers Media S.A.
Liposomes are self-assembled, spherical lipid bilayer vesicles enclosing an aqueous core. The first-generation conventional liposomes were composed mainly of natural or synthetic phospholipids (neutral/charged) without modification.7 Owing to their amphiphilic nature, they can simultaneously incorporate both hydrophobic and hydrophilic compounds in the lipid bilayer and aqueous core. They are biocompatible, weakly immunogenic, and biodegradable.8 In addition, easy and economical synthesis renders the liposomes superior over the other delivery systems. Hence, they are widely used to deliver various pharmaceutical compounds, including vaccines, drugs, dyes, and diagnostic agents.7,8 However, the major disadvantage of unmodified or conventional liposomes is their instability in the biological environment, due to changes in the temperature, pH, and enzymes. The unstable liposomes tend to fuse with the nearby liposomes to reduce surface tension. Such fused liposomes with altered morphology are rapidly captured by the reticuloendothelial system (RES) and quickly cleared off from the blood circulation leading to very short lifetimes. Biological half-life refers to the time taken for half of the administered drug dose to be metabolized and eliminated from the bloodstream.8 The captured liposomes preferentially accumulate in organs of the RES such as the liver and spleen, which are later metabolized and eliminated by the target tissues. Overall, this affects the efficiency of conventional liposomes to deliver a drug to the target region and restricts the therapeutic efficacy of the drug.
To overcome this problem, “second-generation liposomes” with improved stability and desirable characteristics for biomedical applications were prepared by altering the lipid composition, size, and surface charge of vesicles.9 Liposomes are usually surface modified with molecules, such as glycolipids or sialic acid, or by the incorporation of higher proportions of cholesterol during their preparation to improve the rigidity of lipid membranes.10 A significant step in liposomal technology was the development of stealth liposomes. These long-circulating liposomes were named stealth liposomes due to their intrinsic ability to evade the immune system and thereby enhance their blood-circulation time.11,12 They are formed by coating the liposomes with synthetic polyethylene glycol (PEG) polymers or by the addition of PEG-conjugated lipids (PEG-lipids) to the lipid composition, which is used to prepare the liposomes. The PEG-lipids are generally formed by the covalent bonding of PEG molecules with a lipid anchor in the form of a phospholipid, ceramide, or cholesterol. PEGylation of the liposome surface increases the stability of liposomes drastically and reduces their uptake by the mononuclear phagocyte system.12
Further research on liposome technology has progressed to the development of a new generation of liposomes with improved features termed “archaeosomes”. As the name indicates, they are derived from a combination of two words, archaea and liposomes. Archaeosomes denote the liposomes that are composed of, completely or partly, one or more natural polar ether lipids extracted from archaea or prepared with synthetic archaeal lipids that mimic natural archaeal lipids.13,14 These new-generation delivery vehicles exhibit significant advantages over the conventional liposomes prepared from the ester lipids found in Eukarya and bacteria, such as extreme stability under various harsh environmental conditions, and reduced membrane permeability.13 These features have aroused great interest to develop them as potential carriers to deliver numerous compounds of pharmacological interest and food supplements.14 Despite the exceptional properties of archaeosomes, limited characterization data are available, partly due to the difficulties in the cultivation and extraction of a sufficient quantity of pure archaeal lipids. As an alternative to pure archaeosomes, hybrid archaeosomes composed of a mixture of archaeal lipids and synthetic phospholipids are developed. These hybrid structures combine the advantages of both archaeal lipids and synthetic phospholipids such as stability, easy availability, low cost, and large-scale production.3 A schematic representation of a hybrid archaeosome prepared with conventional phospholipids and archaeal bipolar lipids is shown in Figure 2.
Figure 2.
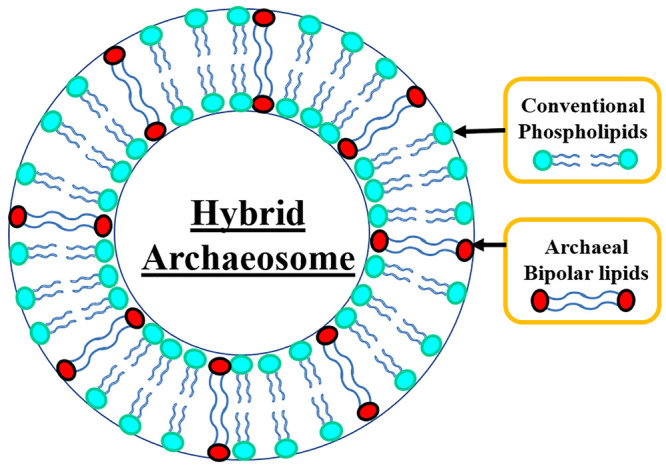
Schematic representation of a hybrid archaeosome composed of a mixture of conventional phospholipids and archaeal bipolar lipids.
2. Unique Features of Archaeal Lipids and Membrane Organization
Numerous reports have shown that the unique polar lipid components and their organization in archaeal membranes play a key role in enabling their survival and growth in extreme habitats.12−17 Unlike bacterial cell walls that contain peptidoglycan, archaeal cell walls contain pseudomurein (or pseudopeptidoglycan), polysaccharide, or protein.15 Archaeal membrane lipids differ from those present in bacteria and Eukarya in two distinct ways. First, the archaeal lipid tails consist of branched isoprenoid (phytanyl) side chains, while unbranched, linear lipid tails are present in the other two domains. Second, the isoprenoid side chains are ether linked to the glycerol-1-phosphate moiety of archaea, whereas the fatty acid tails are ester bound to glycerol-3-phosphate moiety in bacteria and Eukarya,15,16 as shown in Figure 3.
Figure 3.
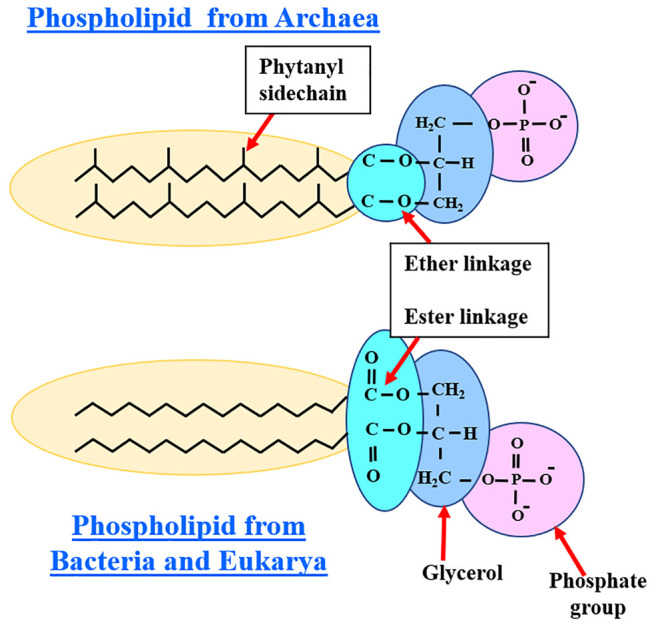
Comparison of differences in the membrane phospholipids found in Archaea and those lipids in bacteria and Eukarya. The phytanyl side chains of Archaea are ether linked to glycerol, whereas the linear fatty acid tails are ester bound to glycerol in bacteria and Eukarya.
Bipolar tetraether lipids (BTLs) are usually abundant in thermoacidophilic archaea (∼90–95%) and are fundamental components in their cell membrane. They usually consist of two different polar head groups at opposite ends of the hydrophobic backbone, leading to asymmetrical lipid structures. These unique structures of BTLs play a crucial role in the adaptation of thermoacidophiles to survive in extreme habitats such as high temperatures and low pH by optimizing membrane organization and properties such as fluidity and transport of various molecules through the cell membrane. The polar lipid fraction E (PLFE) is one of the main BTLs isolated from the plasma membrane of thermoacidophilic archaeon Sulfolobus acidocaldarius, which thrives at temperatures of 65–85 °C at a pH of ∼2–3. PLFE is a mixture of calditolglycerocaldarchaeol (also termed glycerol dialkylcalditol tetraether, or GDNT) and caldarchaeol (also termed glycerol dialkylglycerol tetraether, or GDGT). The GDNT component (∼90% of total PLFE) contains phospho-myo-inositol on the glycerol end and β-d-glucose on the calditol end, whereas the GDGT component (∼10% of total PLFE) has phospho-myo-inositol attached to one glycerol and β-D-galactosyl-d-glucose to the other glycerol skeleton.3 Thus, in PLFE, both GDGT and GDNT components are bisubstituted in the polar headgroup regions. The hydrophobic region of PLFE consists of a pair of 40-carbon biphytanyl chains, and each chain has isoprene units and may include up to four cyclopentane rings. Liposomes prepared with PLFE are extremely stable and have low permeability, compared to liposomes made of diester lipids. These properties of PLFE liposomes are mainly caused by the tight membrane packing and presence of tetraether linkages and cyclopentane rings in the dibiphytanyl chains as well as an extensive hydrogen-bond network by the sugar and phosphate groups of the lipid chains.3
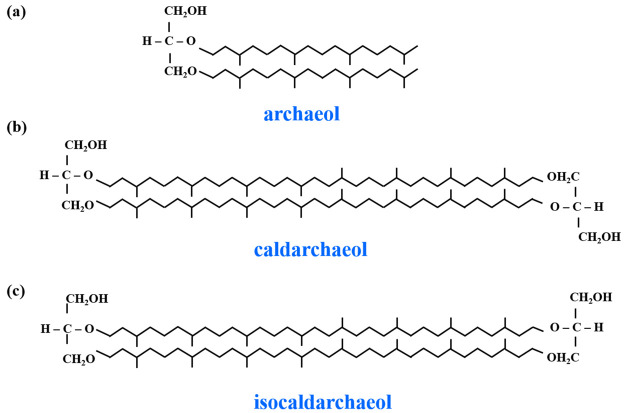
The lipid membrane of archaeosomes can be found in the bilayer form if they are made from monopolar archaeol (diether) lipids, a monolayer if made completely from bipolar caldarchaeol (tetraether) lipids, or a combination of monolayers and bilayers if made from caldarchaeol lipids.17 The two diphytanyl chains linked at both ends to two glycerol residues are oriented in an antiparallel fashion in caldarchaeol and arranged in a parallel fashion in isocaldarchaeol. The lipid structures of archaeol, caldarchaeol, and isocaldarchaeol are shown in Figure 4. Caldarchaeol is a membrane-spanning lipid, commonly present in hyperthermophilic archaea.18 The hydrophobic chains in caldarchaeol are linked together strongly, which offers additional membrane stability and helps the hyperthermophilic archaea to withstand high temperatures. These varied core lipid structures and adaptions enable the archaea to maintain membrane integrity and functions, despite the extremely destabilizing environmental conditions.17,18
Figure 4.
Core lipid structure of archaea: (a) standard archaeol, (b) caldarchaeol, and (c) isocaldarchaeol.
Some of the salient features of archaeal lipids that contribute to enhanced membrane stability are summarized below:
-
(1)
The ether linkages enhance membrane stability over a wide range of pH.
-
(2)
Steric hindrance caused by branched methyl groups is helpful to reduce the crystallization of membrane lipids and lowering the membrane permeability.
-
(3)
The saturated alkyl chains enhance the stability and protect the membrane against oxidative degradation.
-
(4)
The isoprenoid side chains of archaeal lipids are ether-linked to a glycerol-1-phosphate backbone. The high stability of polar ether lipids is partly attributed to the branched phytanyl chains which are usually fully saturated and linked via stable ether bonds to the glycerol backbone.3 The stereochemistry of these linkages in the glycerophosphate backbone of archaea is opposite to that of their bacterial/eukaryotic counterparts, where the straight-chain fatty acids are linked by ester bonds to the glycerol-3-phosphate backbone. This unusual or opposite stereochemistry of the glycerol backbone of archaeal lipids helps them to resist the attack from enzymes such as phospholipases, which are basically lipolytic enzymes that hydrolyze phospholipid substrates at specific ester bonds.
-
(5)
The membrane-spanning properties of the bipolar lipids enhance membrane rigidity and reduce membrane permeability.
-
(6)
The presence of cyclic structures in the transmembrane portion of the lipids contributes to a thermo-adaptive response, leading to tight membrane packing and reduced membrane fluidity.15,17
3. Archaeosomes: Preparation and Characterization
Several types of archaea such as methanogens (Methanococcus voltae, Methanosaeta concilii, Methanospirillum hungatei, Methanosarcina mazei, Methanococcus jannaschii, Methanobrevibacter smithii, Methanosphaera stadtmanae, Methanobacterium espanolae), halophiles (Halobacterium cutirubrum, Natronobacteriummagadii), and thermoacidophiles (Thermoplasma acidophilum) are usually cultivated in optimal growth conditions in the lab from which the total polar lipids (TPLs), polar lipid fractions, and purified polar lipids are extracted to prepare archaeosomes for therapeutic applications.13−18 TPL is usually obtained by precipitation with acetone from a chloroform/methanol (2:1 v/v) solution.17 The resulting extracts are generally analyzed by thin-layer chromatography (TLC) or mass spectrometry and used as such or further purified by preparative TLC to separate pure polar lipids. When compared with TPLs, the PLFE isolated from the thermoacidophile S. acidocaldarius (growth conditions: 75–80 °C and pH 2.5) and the polar lipid methanol fraction extracted from the hyperthermophile Aeropyrum pernix (growth conditions: 92 °C and pH 7) are widely employed to prepare archaeosomes, due to their simple lipid composition and easy data analysis.19
Similar to the preparation of liposomes, the archaeosomes can also be prepared using various methods such as thin film, freeze/thawing, reverse-phase evaporation, microfluidics, and polycarbonate membrane filter extrusion protocols, at any temperature in the physiological range or lower, which helps to encapsulate a variety of thermally stable compounds.15,16 In the thin-film method, the organic solvent from pure archaeal lipids or a mixture of synthetic phospholipids and archaeal lipids is completely removed under vacuum using a rotary evaporator to form a thin lipid film, which is then hydrated with an aqueous buffer or water. This leads to the spontaneous swelling of lipids to form archaeosomes.16,19 In the freeze–thaw method, the vesicles are initially frozen in dry ice-ethanol (−80 °C) or in liquid nitrogen, followed by a repeated three to eight times of freeze and thaw cycle to form archaeosomes.16 In the reverse-phase evaporation method, the lipids are dissolved in the chloroform-methanol mixture, and the organic solvents are evaporated to form a thin film. The lipid film is then resuspended in chloroform, methanol, and water mixture, and again evaporated under vacuum. The dried lipid film is then hydrated with an aqueous buffer to form archaeosomes.2 Usually, the drug or a biomolecule (e.g., protein) of interest can be either encapsulated (hydrophilic) or entrapped (hydrophobic) in the archaeosomes during their formulation or incubated with the archaeosomes after their formation.17 A variety of microscopic techniques such as cryo-transmission electron microscopy, scanning electron microscopy, fluorescence microscopy, and phase-contrast light microscopy are widely used to analyze the morphology of archaeosomes.16 The size, polydispersity, and ζ potential of the archaeosomes are determined using the dynamic light scattering technique and ζ potential analyzer. High-performance thin-layer chromatography is commonly used to determine the lipid composition of archaeosomes.16
4. Archaeosomes: Biomedical Applications
Archaea express a myriad of cellular and molecular adaptations that ensures the stability of cellular contents, especially proteins and cell membranes under different living conditions. These adaptations to extreme environments have created a huge interest to develop them as carriers to deliver various pharmaceutical compounds of interest, such as drugs, vaccines, proteins, peptides, genes, and natural antioxidant compounds inside the biological systems.13−15 These applications are discussed below in detail and summarized in Table 1.
Table 1. Biomedical Applications of Archaeosomes-Based Formulations.
| Drug/biomolecule | Application | Mechanism of action | Inferences | Refs |
|---|---|---|---|---|
| Doxorubicin (DOX) drug | Cancer therapy | Hyperthermia-induced cell death | Sustained drug release and reduced side effects | (3) |
| Plasmid DNA | Gene delivery | Transfection | Cations help to improve the transfection process | (7) |
| BMD drug | Anti-inflammatory | Absorption to the deeper layers | Effective drug penetration and accumulation in the epidermis | (11) |
| A1 peptide | Cancer therapy | Cause rapid leakage of cytoplasmic contents | Cytotoxic to cancer cells | (12) |
| PTX drug | Cancer therapy | Enhance polymerization of tubulin | Reduce side effects and improve its therapeutic index | (14) |
| Phenol (recovered from olive mill waste) | Antioxidant | Inhibit cell damage | High stability, sustained drug release | (21) |
| Insulin | Diabetes treatment | Reduce blood glucose levels | Stable in simulated gastrointestinal tract conditions, poor permeability of the intestinal epithelium | (22) |
| BSA | Vaccine therapy | Stimulated proliferation of CD8+ T cells | Modulate primary, long-term, and/or innate immunity, impacting adjuvant choice for vaccine design | (26) |
| BSA/cholera toxin B subunits | Vaccine delivery | Increased antibody production | Better humoral immune response | (28) |
4.1. Drug and Antioxidant Delivery
Studies have shown that the archaeosomes prepared with either natural, chemically modified, or synthetic archaeal lipids have the potential to function as efficient drug carriers.3,14 The major benefits of employing archaeosomes in drug delivery include protection of the drug from acidic/enzymatic degradation, increased shelf life, and bioavailability. For instance, Alavi et al.14 reported that archaeosomes have the potential to function as an effective carrier of paclitaxel (PTX), a widely used drug to treat breast cancer. The archaeosomes (mean diameter: 521.4 nm) were prepared by hydrating the lipid extract of methanogenic archi bacteria in phosphate-buffered saline buffer containing 10 mg of PTX dissolved in 100 μL of dimethyl sulfoxide. The prepared archaeosomal-PTX formulation was incubated with the MCF-7 human breast cancer cells for 24 h to assess their cytotoxic effects using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide colorimetric assay at different time intervals. The results have shown that the cytotoxicity of the archaeosomal-PTX formulation was significantly higher than the standard PTX formulation. The authors have explained that this effect could be attributed to the stability of archaeal lipids under various conditions (pH, temperature, lipases, etc.) and the lipid coverage enabled sustained drug release, leading to reduced side effects and increased therapeutic index. This report indicates that drug loading in archaeosomes represents an effective method to achieve a better therapeutic response. Another study evaluated the potential of archaeosomes as novel colloidal carriers for the topical delivery of betamethasone dipropionate (BMD), a medication with anti-inflammatory and immunosuppressive properties, which is widely used to treat a variety of skin problems such as eczema, dermatitis, allergies, and rashes.11 In this study, the archaeal lipids were extracted from Halobacterium salinarum and mixed with BMD in chloroform (1 mg/mL) to form archaeosomes using the thin-film method. The in vitro drug permeation studies through full-thickness pig skin was carried out by using Franz diffusion vertical cells. The results indicated that the archaeosomes were able to achieve substantial drug penetration and accumulation in the skin strata, especially in the epidermis layer. These data suggest that the archaeosomes may hold great promise as drug delivery vehicles for topical applications.
Napotnik et al.19 studied the uptake mechanism, stability, drug release rate, and the in vitro cytotoxicity of archaeosomes to analyze their potential as an effective targeted drug delivery system. The archaeosomes were prepared from the lipid extract of A. pernix K1, which is an obligate aerobic hyperthermophilic organism having C25,25-archaeol membrane lipids with head groups containing inositol. The prepared archaeosomes were loaded with a fluorescent model drug, calcein. The interactions and the in vitro cytotoxicity of archaeosomes were tested on five different cell lines: rodent mouse melanoma cells (B16-F1), Chinese hamster ovary (CHO) cells, and three human cell lines namely epithelial colorectal adenocarcinoma cells (CACO-2), liver hepatocellular carcinoma cell line (Hep G2), and endothelial umbilical vein cell line (EA.hy926). Laser scanning confocal microscope images have shown that the archaeosomes were taken up by endocytosis. The cytotoxicity results revealed that the archaeosomes were found to be nontoxic to human Hep G2 and CACO-2 and mildly toxic to rodent CHO and B16-F1 cells, but showed a strong cytotoxic effect on EA.hy926 cells. The uptake of archaeosomes and the subsequent release of calcein were more prominent in EA.hy926 cells, which could most probably be due to the rapid endocytosis and/or intracellular release and action in these specific cells. This study also demonstrated that the archaeosomes prepared from A. pernix were extremely stable in a wide range of pH from 4 to 12 and the temperature range from 0 to 100 °C. Taken together, the results infer that the archaeosomes are capable of encapsulating biologically active compounds and delivering them safely owing to their stability in various environments. Further, they can be effectively taken up by different cell types, and therefore they can be considered a potential drug delivery system.
Since the archaeosomes exhibit high stability, a major obstacle in applying them to hyperthermia cancer treatment is the lack of an effective triggering mechanism to release the entrapped drug in a controlled fashion at the target site. This long-standing problem is tackled by the synthesis of hybrid archaeosomes composed of a mixture of archaeal lipids and synthetic phospholipids. For instance, Ayesa et al.3 prepared thermosensitive “smart archaeosomes” composed of a mixture of the PLFE isolated from the thermoacidophilic archaeon S. acidocaldarius, and the synthetic phospholipid dipalmitoyl phosphatidylcholine (DPPC), in a specific proportion PLFE/DPPC (3:7). They were entrapped with the anticancer drug doxorubicin (DOX) and incubated with the MCF-7 breast cancer cells to evaluate the drug release profile and in vitro cytotoxicity. Due to the excellent membrane stability offered by the archaeal lipids, the spontaneous drug leakage was restricted at and below 37 °C. However, when the temperature is increased to 42–44 °C (mild hyperthermia treatment), a drastic increase in DOX release from the hybrid archaeosomes occurred, leading to an increase of DOX entry into the nucleus of MCF-7 cancer cells causing increased cell death. Phase transition studies using the fluorescent probe 6-lauroyl-1,2-dimethylamino-naphthalene (Laurdan) have made it evident that the PLFE lipids have an ordering effect on fluid DPPC liposomal membranes, probably due to the DPPC lipid domain melting and PLFE lipid flip-flop, causing abrupt changes in the ζ potential values and modulations in their surface properties. These data indicate that the hybrid archaeosomes can be used as thermosensitive liposomes, wherein the temperature range (from 37 to 42–44 °C) clinically used for mild hyperthermia treatment of tumors can be used as a trigger to release the drug specifically in the cancer cells with reduced side effects.
Reports have shown that the archaeosomes can be successfully employed to deliver various antioxidants, which are substances that can prevent or reduce the damage caused to cells by free radicals produced by the body as a reaction to environmental and other pressures.20,21 The sources of antioxidants can be natural or artificial. Plant-based products such as vegetables and fruits are rich in several antioxidants.20 They are also called “free-radical scavengers” and play a key role in protection from various disorders such as heart disease, and cancer. Hence, they have beneficial medical implications. Pedone et al.20 reported that the hyperthermophilic archaea are equipped with a range of antioxidant enzymes, which protect the biomolecules from oxidative damage induced by the reactive oxygen species (ROS). For example, the enzymes such as catalase, superoxide dismutase, and peroxiredoxins present in archaea are actively involved in the scavenging of the most common ROS, such as superoxide radical and hydrogen peroxide. Together with thioredoxin, protein disulfide oxidoreductase, and thioredoxin reductase, these enzymes are involved in redox homeostasis, which represents the core of the antioxidant system. Paredes et al.21 compared the encapsulation efficiency and topical delivery of antioxidants using archaeosomes and conventional liposomes prepared from phosphatidylcholine. For this analysis, unilamellar (size: ∼200 nm) and multilamellar archaeosomes up to a few microns were prepared and encapsulated with natural antioxidant compounds such as phenols, recovered from olive mill waste. Both, the archaeosomes and conventional liposomes showed high encapsulation efficiency and were found to be stable even after a month, without notable changes in the initial characteristics of the suspensions. The possibility of incorporating liposomal suspensions in excipients such as Carbopol-940 and Pluronic-127 for topical administration was also investigated. The release behavior of antioxidants from both the archaeosomes and liposomes was evaluated via in vitro diffusion studies using vertical diffusion Franz cells. The results demonstrated that the archaeosomal gels showed a sustained release of a similar quantity of phenolic compounds for 24 h regardless of the excipient used, whereas significant release differences were found between Carbopol and pluronic excipients in the case of conventional liposomal gels. This confirms the superiority of archaeosomes over conventional liposomes as sustained delivery systems for natural antioxidants.
4.2. Oral Delivery of Proteins and Peptides
Oral delivery of therapeutic proteins, peptides, and small molecules is challenging mainly due to the harsh gastrointestinal (GI) environments such as acidic pH, susceptibility to enzymatic degradation, short plasma half-life, and poor penetration across the intestinal mucosa.22,23 Stable delivery systems are required to protect them from barriers in the GI tract and increase their bioavailability. In this context, archaeosomes are considered an excellent choice for oral delivery because they are stable against acidic pH, digestive enzymes in the GI tract, and bile salts in the intestinal lumen. Li et al.22 demonstrated the success of employing archaeosomes in the oral delivery of insulin by using the PLFE extracts of S. acidocaldarius. Cyanine5.5 near-infrared fluorescent dye-labeled insulin, as a model peptide, was embedded in archaeosomes and in conventional liposomes to compare their potential as a vehicle for oral delivery. The insulin-loaded archaeosomes were orally administered to diabetic mice to evaluate the pharmacodynamics and treated with the human colorectal adenocarcinoma Caco-2 cells in vitro to determine the membrane permeability of insulin. The vesicle stability and release profiles of insulin from vesicles after 4 h were evaluated by treating them with the simulated gastric fluid. The experimental data demonstrated that the archaeosomes were relatively more stable in the simulated GI tract conditions and enable prolonged insulin retention time in the GI tract. The in vivo experiments revealed that the archaeosomes containing insulin were superior in lowering the blood glucose levels in diabetic mice, compared to the conventional liposomes. By combining all these data, it becomes evident that the archaeosomes could be a potential oral delivery platform for peptide drugs, and further in vivo experiments are required to ensure their efficiency and safety profile.
Bioactive peptides are low molecular weight, short protein fragments (usually 2–20 amino acids in length) and serve as excellent candidates for cancer therapeutic applications.23,24 They can easily traverse or disrupt the cell membrane leading to apoptosis or necrosis. Further, with the aid of targeting ligands that can specifically bind with the receptors on tumor cells, peptide-mediated cancer therapy has gained importance. But the free peptides are subjected to rapid elimination from circulation, enzymatic degradation, and accumulation in nontargeted organs and tissues.24 Therefore, the entrapment of peptides into vesicles tagged with targeting ligands that enables specific binding with cancer cells is an efficient strategy to increase the influx and retention of the drug in cancer cells.25 In this context, Jiblaoui et al.12 developed novel folic acid (FA)-conjugated stealth archaeosomes based on egg-PC and a mixture of PEGylated archaeal tetraether lipids and investigated their potential as nanocarriers for the targeted in vitro delivery of antitumoral peptides. The tetraether lipids extracted from a mixture of archaeal sources were tagged with a folate ligand at the PEG5000 terminal end (FA-PEG5000-tetraether) to bind specifically with the folate receptors that are overexpressed on the surface of cancer cells. The therapeutic peptide A1 (2302 Da, 17 amino acids, RRKYGRDFLLRFRYIRS), which exhibits rapid antitumoral activities on different cell lines, was encapsulated inside the folate-PEG bound archaeosomes. Later, they were treated with human cervical carcinoma HeLa cells, and the in vitro biological assays were carried out to study the importance of encapsulating the A1 peptide in the folated archaeosomes. The biological activity of A1peptide-archaeosome formulations was tested at 1.7 mg/mL of lipids corresponding to 50 mM of A1 peptide. The results have indicated that the A1 peptide exhibited a drastic and rapid cytotoxic activity on the HeLa cells by causing rapid leakage of cytoplasmic contents, with cell mortality close to 80% after 20 min of incubation. This study has shown the scope of developing synthetic analogs of natural archaeal lipids to be incorporated in archaeosomes formulations to develop efficient protein/peptide delivery systems.
4.3. Delivery of Vaccines and Adjuvants
Archaeosomes have been widely explored as delivery systems for vaccines and adjuvants. An adjuvant is usually a substance that enhances the body’s immune response to an antigen, and they are generally added to vaccine formulations to stimulate stronger immunogenicity in vivo. Since the lipid carriers are easily targeted by the phagocytic cells, liposomes and archaeosomes are ideal vehicles to carry and deliver antigens or adjuvants.26 In this regard, Krishnan et al.26 evaluated the ability of archaeosomes to facilitate the different facets of immune responses against entrapped antigens, mechanisms of adjuvant action, and compared their efficiency with alum and conventional liposomes prepared with a mixture of synthetic phospholipids (phosphatidylcholine-phosphatidylglycerol-cholesterol). For this purpose, conventional liposomes and archaeosomes of similar size (∼200 nm) were prepared with the ether glycerolipids of several archaea and entrapped with bovine serum albumin (BSA). They were then administered to mice of varying genetic backgrounds via different routes, such as intramuscular, intraperitoneal, and subcutaneous injections at the base of the tail. The results have shown that the archaeosomes entrapped with BSA enhanced serum anti-BSA antibody titers considerably in mice than the alum and conventional liposomes. Moreover, antigen-specific immunoglobulin G1 (IgG1), IgG2a, and IgG2b isotype antibodies were also induced. Immunizations using archaeosomes-antigen formulation also stimulated a strong cell-mediated immune response: antigen-dependent proliferation and substantial production of cytokines gamma interferon and interleukin-4 by spleen cells in vitro. In contrast to alum and Freund’s adjuvant, archaeosomes prepared from lipid extracts of Thermoplasma acidophilum showed a prolonged antibody memory response to the same antigen (at ∼300 days) just after two immunizations (days 0 and 14). These results conclude that the archaeosomes are efficient antigen carriers and adjuvants to boost humoral and cell-mediated immune responses to the entrapped antigen.
Tolson et al.27 have compared the uptake efficiency of liposomes (size ∼200 nm) prepared from the total polar lipids extracted from various methanogenic archaea, such as M. voltae, M. mazei, and M. smithii, and mixed with the conventional phospholipids composed of a mixture of ester lipids dipalmitoylphosphatidylcholine, or dimyristoylphosphatidylcholine: dimyristoylphosphatidyl glycerol: and cholesterol in the molar ratio of 1.8:0.2:1.5, respectively. The results have shown that the archaeosomes prepared from the total polar lipids of several archaea were taken up 3–53 times more efficiently by phagocytic cells than the conventional liposomes. The dramatic uptake of archaeosomes by phagocytic cells suggests their potential for targeting drugs to the reticuloendothelial system and for the delivery of antigens to antigen processing cells. This indicates that the archaeosomes may be considered potential candidates in clinical applications where phagocytic cells are a target site.
In a similar trend, Sprott et al.28 evaluated the ability of archaeosomes as novel antigen delivery systems, by comparing the humoral immune response in BALB/c mice, stimulated by the archaeosomes and conventional liposomes encapsulated with protein antigens such as bovine serum albumin/cholera toxin B subunits. The archaeosomes were prepared from the total polar lipids of several archaea such as Methanospirillum hungatei GP1 (DSM 1101), Methanococcus voltae PS (DSM 1537), Methanosarcina mazei S-6 (DSM 2053), Methanosaeta concilii GP6 (DSM 3671), Methanobrevibacter smithii ALI (DSM 2375), Methanobacterium espanolae GP9 (DSM 5982), and Thermoplasma acidophilum 122-1B3 (ATCC 27658). Conventional liposomes were prepared with mixtures of various synthetic phospholipid/cholesterol formulations. It was found that with only two immunizations, the maximum IgG+IgM antibody titer in sera was achieved in the immunized mice using archaeosomes prepared with the polar lipids from M. smithii, which is an inhabitant of the human colon. Similarly, a positive response to the cholera B subunit protein was achieved using M. smithii archaeosomes to trigger the antibody production in mice. The authors have speculated that apart from the in vivo stability of archaeosomes at the injection site, their superior uptake by antigen processing cells, slow and sustained release effect of the encapsulated antigens might be some of the strong reasons for a drastic rise in the antibody production when compared to the unstable conventional liposomes. Further experiments in this study have also suggested that encapsulation of protein antigens in the archaeosomes is essential for achieving a better humoral immune response than the free antigens.
4.4. Gene Delivery Applications
Cationic lipid-DNA complexes have been widely applied for gene transfection applications, both in vitro and in vivo. Recent studies have found that archaeosomes also can function as efficient carriers of DNA.29 Recently, Attar et al.7 have evaluated the potential of archaeosomes as carriers of plasmid DNA in mammalian cells. The archaeosomes were formed by mixing ether lipids extracted from H. hispanica 2TK2 strain with plasmid DNA encoding a green fluorescent protein or β-galactosidase and incubated with human embryonic kidney (HEK293) cells up to 24 h at room temperature. Owing to their anionic nature, initially, the archaeosomes showed low efficiency in complexing with plasmid DNA and delivery to HEK293 cells. To overcome this issue, the authors have used positively charged ions or molecules, such as 1,2-dioleoyl-3-(trimethylammonium) propane or Ca2+ as helper molecules in the archaeosomes formulation. As expected, cell studies have shown that the presence of cationic molecules/ions contributed to a significant increase in the rate of gene transfection in mammalian cells. In another study, Rethore et al.29 reported that novel cationic hybrid archaeosomes, prepared with the mixtures of neutral/cationic bilayer-forming lipids and synthetic archaebacterial tetraether-type bipolar lipids, exhibited excellent gene transfection features in vitro. Cationic lipid-DNA complexes were prepared by mixing the appropriate amount of aqueous liposomal or archaeosomal suspensions with plasmid DNA (pTG11033, 9.7 kb) expressing the luciferase reporter gene and analyzed after 30 min of incubation at room temperature. These results have demonstrated the advantage of developing hybrid membranes by combining conventional bilayer-forming lipids with monolayer-forming lipids as a new strategy to modulate the membrane fluidity of the complexes they form with DNA.
Similarly, Benvegnu et al.24 prepared an archaeosomal formulation using synthetic cationic archaeal lipid analogs (either a cationic tetraether or a synthetic cationic diester) via lipid film hydration method followed by size reduction using sonication or extrusion through polycarbonate membranes. The plasmid DNA (pCMV-Lluc), which is an expression vector encoding the firefly luciferase gene, was incorporated into the archaeosomal formulation, and conventional liposomes prepared with specific proportions of the synthetic phospholipid dioleoylphosphatidylethanolamine and cholesterol. The prepared archaeosomes were then treated with A549 lung carcinoma epithelial cells in vitro to measure the transfection rate. Results have shown that the transfection efficiencies were tremendously improved with archaeosomes composed of cationic synthetic tetraether archaeal lipids and with neutral tetraether colipid, rather than the conventional liposomes. The data further indicated that the presence of synthetic tetraether archaeal lipid analogs in the lipid vehicle formulations has significantly improved the stability level, similar to those vesicles prepared with the natural lipid extracts of the archaean Thermoplasma acidophilum. These findings pave a new path in the application of synthetic archaeal lipid analogs as stable systems for gene delivery.
4.5. Stealth Archaeosomes
Similar to “stealth liposomes”, archaeosomes stabilized with PEG, known as “stealth archaeosomes”, were developed recently, which have several beneficial features such as long blood circulation time, reduced protein binding, and uptake by phagocytic cells such as macrophages.8 These stealth archaeosomes provide the added advantages of encapsulation and safe in vivo delivery of various biomolecules like proteins/peptides which are highly sensitive to enzymatic degradations and drugs that are prone to immune reactions.10 Further, by synthetic modification of the terminal PEG molecule, stealth archaeosomes can be actively targeted with monoclonal antibodies or ligands. Such vesicles provide added advantages of high targeting and delivery efficacy, which favors the production of a variety of lipid-based formulations with active drug compounds.11
Laine et al.23 demonstrated the synthesis and the superior in vitro transfection efficiency of PEGylated archaeal lipid derivatives. A series of novel di- and tetraether-type archaeal derivatives were incorporated as colipids into glycine betaine-based cationic lipid formulations for gene transfection. These lipid formulations were encapsulated with plasmid DNA (pCMV-Luc). They were then coated with the PEG570 chain to enhance their stability and further conjugated with folic acid, in order to achieve targeted gene transfection. The archaeal derivatives equipped with FA-PEG moieties were then treated with HeLa cells that express high levels of affinity for folate receptors. The transfection efficiency was measured after 48 h by using a chemiluminescent assay. The cell studies have indicated that all the novel colipids used in this study have afforded efficient in vitro gene transfection. Moreover, the FA-equipped derivatives permitted receptor-based targeted transfection, and their activity was inhibited when free FA was added to the transfection medium. These data suggest that the novel archaeal derivatives equipped with FA-PEG moieties may thus be of significant interest for targeted in vivo gene transfection.
Some studies have shown that the inclusion of archaeal lipids in conventional liposomes greatly enhances their stability and help to achieve sustained drug release.10,12,30 To assess this feature, Barbeau et al.30 prepared stealth archaeosomes with 90 wt % of a classical lipid, egg-phosphatidylcholine (egg-PC), and 10 wt % of a PEGylated tetraether archaeal lipid (PEG(45)-tetraether), and conventional liposomes with 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE) phospholipid. Both, the archaeosomes (egg-PC/PEG(45)-tetraether) and conventional liposomes (egg-PC/PEG(45)-DSPE) were encapsulated with a fluorescent dye, carboxyfluorescein as a marker to assess their drug encapsulation efficiency and release profile. The experimental data have shown that the stability of PEGylated archaeosomes is significantly higher than PEGylated conventional liposomes, as indicated by the slower and sustained release of the encapsulated dye at 37 °C. This enhanced stability could be attributed to the membrane-spanning properties of the archaeal bipolar lipids. The data ensure that the addition of even smaller proportions of PEGylated archaeal lipids to the standard liposomal formulations tremendously improves the vesicle stability and they can be formulated as superior in vivo gene/drug carriers.
5. Conclusion and Future Perspectives
In this review, we have discussed the unique features of archaeal membrane lipids and explored the potential of archaeosomes as a new generation delivery system, capable of carrying both hydrophilic and hydrophobic materials of pharmacological importance. The extreme stability of archaeosomes in various harsh conditions enables them to overcome the current biomedical challenges faced by conventional lipid-based formulations in the delivery of drugs, genes, and therapeutically significant peptides. Furthermore, the ability of archaeosomes to safely deliver the antigens and adjuvants opens up new avenues in vaccine therapy. Finally, we conclude that these promising carrier systems have an enormous potential to be surface modified to bind with specific targeting ligands to deliver various biomolecules and drugs precisely to the target cells. Since the archaeosomes are biocompatible, biodegradable, and proven to be safe via various in vitro and in vivo studies, there is a great interest to widen their scope and commercialize them. It is encouraging to note the increasing amount of ongoing clinical trials on archaeosomes toward their approval in biological applications. Designing long-circulating stealth archaeosomes bound with PEG and smart hybrid archaeosomes bound with targeting moieties, anticancer drugs, and fluorescent dyes that can bind specifically with the cancer cells and release the drug in a sustained manner will have a huge impact on cancer diagnosis and therapeutics. Future research and development should focus on designing cost-effective, safe, novel archaeosomes with multifunctional abilities and improved characteristics for early diagnosis and targeted delivery with controlled drug release features to treat several diseases.
Acknowledgments
We gratefully acknowledge the financial support from the project KP-06-DB8/01.12.2020 with the Bulgarian National Science Fund under the National Scientific Program P. Beron 2020.
Biographies
Dr. Poornima Budime Santhosh is a postdoctoral researcher at the Institute of Solid State Physics, Bulgarian Academy of Sciences, since 2021. Previously, she worked as a postdoctoral researcher at the Indian Institute of Technology Madras and the Indian Institute of Science, Bengaluru. She completed her Ph.D. in Biosciences from the University of Ljubljana, Slovenia, in 2015. She completed her Bachelor’s and Master’s in Biochemistry from Bharathidasan University, India. Most of her research works have been associated with multidisciplinary projects involving the preparation, biophysical characterization of liposomes entrapped with gold and other types of nanomaterials, and cell studies for various biomedical applications.
Dr. Julia Genova is a Full Professor at the Institute of Solid State Physics, Bulgarian Academy of Sciences (ISSP-BAS). She completed her Ph.D. in Condensed Matter Physics from ISSP-BAS in 2008. She completed her Master’s in Physics from Sofia University St. Kliment Ohridski, Bulgaria in 1999. She has more than 50 works in the field of physicochemical properties of model lipid systems. Her scientific interests include also the influence of impurities on properties of lipid systems, membrane biophysics, lyotropic liquid crystals, protein-lipid interactions, cell biophysics, microfluidity, elasticity, morphology, deformability, dynamics, phase behavior, optical microscopy; drug transport and delivery systems, differential scanning calorimetry, ultra and nanofiltration, and nanotechnology.
Author Contributions
P.B.S: Conceived the study design and prepared the original draft of the manuscript. J.G: Project guidance and manuscript revisions.
The authors declare no competing financial interest.
References
- Charo N.; Jerez H.; Tatti S.; Romero E. L.; Schattner M. The Anti-Inflammatory Effect of Nanoarchaeosomes on Human Endothelial Cells. Pharmaceutics 2022, 14 (4), 736. 10.3390/pharmaceutics14040736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamiak N.; Krawczyk K. T.; Locht C.; Kowalewicz-Kulbat M. Archaeosomes and Gas Vesicles as Tools for Vaccine Development. Frontiers in Immunology 2021, 12, 746235. 10.3389/fimmu.2021.746235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayesa U.; Chong P. L. G. Polar Lipid Fraction E from Sulfolobus Acidocaldarius and Dipalmitoylphosphatidylcholine Can Form Stable yet Thermo-Sensitive Tetraether/Diester Hybrid Archaeosomes with Controlled Release Capability. Int. J. Mol. Sci. 2020, 21 (21), 8388. 10.3390/ijms21218388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampelotto P. H. Extremophiles, and extreme environments. Life (Basel) 2013, 3 (3), 482–485. 10.3390/life3030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K.; Nakamura K.; Toki T.; Tsunogai U.; Miyazaki M.; Miyazaki J.; Hirayama H.; Nakagawa S.; Nunoura T.; Horikoshi K. Cell Proliferation at 122 Degrees C and Isotopically Heavy CH4 Production by a Hyperthermophilic Methanogen under High-Pressure Cultivation. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (31), 10949–10954. 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perras A. K.; Wanner G.; Klingl A.; Mora M.; Auerbach A. K.; Heinz V.; Probst A. J.; Huber H.; Rachel R.; Meck S.; Moissl-Eichinger C. Grappling archaea: ultrastructural analyses of an uncultivated, cold-loving archaeon, and its biofilm. Front Microbiol. 2014, 5, 397. 10.3389/fmicb.2014.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attar A.; Ogan A.; Yucel S.; Turan K. The Potential of Archaeosomes as Carriers of PDNA into Mammalian Cells. Artif. Cells Nanomed. Biotechnol. 2016, 44 (2), 710–716. 10.3109/21691401.2014.982800. [DOI] [PubMed] [Google Scholar]
- Adepu S.; Ramakrishna S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules. 2021, 26 (19), 5905. 10.3390/molecules26195905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarzadeh A.; Rezaei-Sadabady R.; Davaran S.; Joo S. W.; Zarghami N.; Hanifehpour Y.; Samiei M.; Kouhi M.; Nejati-Koshki K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8 (1), 102. 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino M. L.; Dosio F.; Cattel L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomedicine 2006, 1 (3), 297–315. [PMC free article] [PubMed] [Google Scholar]
- Paredes A. G.; Manconi M.; Caddeo C.; Ramos-Cormenzana A.; Monteoliva-Sanchez M.; Fadda A. M. Archaeosomes as carriers for topical delivery of betamethasone dipropionate: in vitro skin permeation study. J. Liposome. Res. 2010, 20, 269–276. 10.3109/08982100903402962. [DOI] [PubMed] [Google Scholar]
- Jiblaoui A.; Barbeau J.; Vives T.; Cormier P.; Glippa V.; Cosson B.; Benvegnu T. Folate-Conjugated Stealth Archaeosomes for the Targeted Delivery of Novel Antitumoral Peptides. RSC Adv. 2016, 6 (79), 75234–75241. 10.1039/C6RA15713K. [DOI] [Google Scholar]
- Kashyap K. Archaeosomes: Revolutionary Technique for Both Cell-Based and Drug-Based Delivery Applications. International Journal of Pharmaceutical Sciences and Medicine 2021, 6 (7), 102–127. 10.47760/ijpsm.2021.v06i07.008. [DOI] [Google Scholar]
- Alavi S. E.; Mansouri H.; Esfahani M. K. M.; Movahedi F.; Akbarzadeh A.; Chiani M. Archaeosome: As New Drug Carrier for Delivery of Paclitaxel to Breast Cancer. Indian J. Clin. Biochem. 2014, 29 (2), 150–153. 10.1007/s12291-013-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G.; Garg T.; Rath G.; Goyal A. K. Archaeosomes: An Excellent Carrier for Drug and Cell Delivery. Drug Delivery 2016, 23 (7), 2497–2512. 10.3109/10717544.2015.1019653. [DOI] [PubMed] [Google Scholar]
- Moghimipour E.; Kargar M.; Handali S. Archaeosomes as Means of Nano-Drug Delivery. Rev. Med. Microbiol. 2014, 25 (2), 40–45. 10.1097/MRM.0000000000000000. [DOI] [Google Scholar]
- Benvegnu T.; Lemiegre L.; Cammas-Marion S. New Generation of Liposomes Called Archaeosomes Based on Natural or Synthetic Archaeal Lipids as Innovative Formulations for Drug Delivery. Recent Pat. Drug Delivery Formul. 2009, 3 (3), 206–220. 10.2174/187221109789105630. [DOI] [PubMed] [Google Scholar]
- Andringa R. L. H.; de Kok N. A. W.; Driessen A. J. M.; Minnaard A. J. A Unified Approach for the Total Synthesis of Cyclo-Archaeol, Iso-Caldarchaeol, Caldarchaeol, and Mycoketide. Angew. Chem., Int. Ed. Engl. 2021, 60 (32), 17497–17503. 10.1002/anie.202104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napotnik T. B.; Valant J.; Gmajner D.; Passamonti S.; Miklavcic D.; Ulrih N. P. Cytotoxicity and Uptake of Archaeosomes Prepared from Aeropyrum Pernix Lipids. Hum. Exp. Toxicol. 2013, 32 (9), 950–959. 10.1177/0960327113477875. [DOI] [PubMed] [Google Scholar]
- Pedone E.; Fiorentino G.; Bartolucci S.; Limauro D. Enzymatic Antioxidant Signatures in Hyperthermophilic Archaea. Antioxidants (Basel) 2020, 9 (8), 703. 10.3390/antiox9080703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes A. G.; Clares-Naveros B.; Ruiz-Martinez M. A.; Durban-Fornieles J. J.; Ramos-Cormenzana A.; Monteoliva-Sanchez M. Delivery Systems for Natural Antioxidant Compounds: Archaeosomes and Archaeosomal Hydrogels Characterization and Release Study. Int. J. Pharm. 2011, 421 (2), 321–331. 10.1016/j.ijpharm.2011.09.042. [DOI] [PubMed] [Google Scholar]
- Li Z.; Chen J.; Sun W.; Xu Y. Investigation of Archaeosomes as Carriers for Oral Delivery of Peptides. Biochem. Biophys. Res. Commun. 2010, 394 (2), 412–417. 10.1016/j.bbrc.2010.03.041. [DOI] [PubMed] [Google Scholar]
- Laine C.; Mornet E.; Lemiegre L.; Montier T.; Cammas-Marion S.; Neveu C.; Carmoy N.; Lehn P.; Benvegnu T. Folate-equipped pegylated archaeal lipid derivatives: synthesis and transfection properties. Chemistry. 2008, 14 (27), 8330–40. 10.1002/chem.200800950. [DOI] [PubMed] [Google Scholar]
- Benvegnu T.; Plusquellec D.; Rethore G.; Sachet M.; Ferec C.; Montier T.; Delepine P.; Lehn P.. Compounds analogous to membrane lipids in archaebacteria and liposomal compositions including said compounds. Patent WO 2006061396 A1, 2006.
- Wang L.; Dong C.; Li X.; Han W.; Su X. Anticancer Potential of Bioactive Peptides from Animal Sources (Review). Oncol. Rep. 2017, 38 (2), 637–651. 10.3892/or.2017.5778. [DOI] [PubMed] [Google Scholar]
- Krishnan L.; Dicaire C. J.; Patel G. B.; Sprott G. D. Archaeosome Vaccine Adjuvants Induce Strong Humoral, Cell-Mediated, and Memory Responses: Comparison to Conventional Liposomes and Alum. Infect. Immun. 2000, 68 (1), 54–63. 10.1128/IAI.68.1.54-63.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson D. L.; Latta R. K.; Patel G. B.; Sprott G. D. Uptake of archaeobacterial liposomes by phagocytic cells. J. Liposome Res. 1996, 6, 755–776. 10.3109/08982109609039925. [DOI] [Google Scholar]
- Sprott G. D.; Tolson D. L.; Patel G. B. Archaeosomes as Novel Antigen Delivery Systems. FEMS Microbiol. Lett. 1997, 154 (1), 17–22. 10.1111/j.1574-6968.1997.tb12618.x. [DOI] [PubMed] [Google Scholar]
- Rethore G.; Montier T.; Le Gall T.; Delepine P.; Cammas-Marion S.; Lemiegre L.; Lehn P.; Benvegnu T. Archaeosomes Based on Synthetic Tetraether-like Lipids as Novel Versatile Gene Delivery Systems. Chem. Commun. (Camb.) 2007, 20, 2054–2056. 10.1039/B618568A. [DOI] [PubMed] [Google Scholar]
- Barbeau J.; Cammas-Marion S.; Auvray P.; Benvegnu T. Preparation and Characterization of Stealth Archaeosomes Based on a Synthetic PEGylated Archaeal Tetraether Lipid. J. Drug Delivery 2011, 2011, 396068. 10.1155/2011/396068. [DOI] [PMC free article] [PubMed] [Google Scholar]