Abstract
An efficient, regioselective, and environmentally benign approach was established using the multicomponent reaction-based synthesis of novel antioxidant spiroquinoline derivatives such as spiro[dioxolo[4,5-g]quinoline], spiro[dioxino[2,3-g]quinoline], and spiro[pyrazolo[4,3-f]quinoline] by reaction of aryl aldehyde, Meldrum’s acid, and amine derivatives under an additive-free reaction in aqueous ethanol. Here, two asymmetric carbon centers, three new C–C bonds, and one C–N bond are developed in the final motif. This synthetic methodology offers excellent yields with an easy workup procedure, high diastereoselectivity [d.r. >50:1 (cis/trans)], admirable atom economy, and low E-factor values. Synthesized spiro compounds were investigated for their in vitro antioxidant activity by 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assays. In the ABTS radical scavenging assay, compounds 4d, 4f, and 4l exhibit excellent potency, and in the DPPH radical scavenging assay, compounds 4a, 4d, 4f, and 4g, exhibit excellent potency.
1. Introduction
In present days, the development of novel heterocycles through a sustainable synthetic route is of much-growing interest.1−3 Especially, multicomponent reactions provide excellent atom economy (AE) and synthetic efficiency to construct different heterocycles of biological interest via multiple C–C bond formation without isolation of an intermediate. In such cases, the challenging task is maintaining the sustainability of the procedure because of the higher sensitivity of functional groups attached to the substrate at a higher temperature. Due to the operational simplicity and better reaction efficiency, MCRs are more susceptible than conventional multistep synthesis.4−10 The toxicity of some highly volatile and hazardous organic solvents poses a threat to the workers if they pass into the atmosphere. Highly volatile solvents often cause fires and/or detonations, resulting in destruction. Therefore, the attempt made to explore organic synthesis in aqueous solvents, deep eutectic solvents, and ionic liquids are in high demand.11−17 The reactions which lead to successful conversion in aqueous media are gaining much more attention in synthetic organic chemistry not only because water is abundant in nature but due to extensive hydrogen bonding, inexpensive, environmentally benign, high dielectric constant, non-flammability, eco-compatibility, and selectivity in many organic reactions than conventional organic solvents.18−20
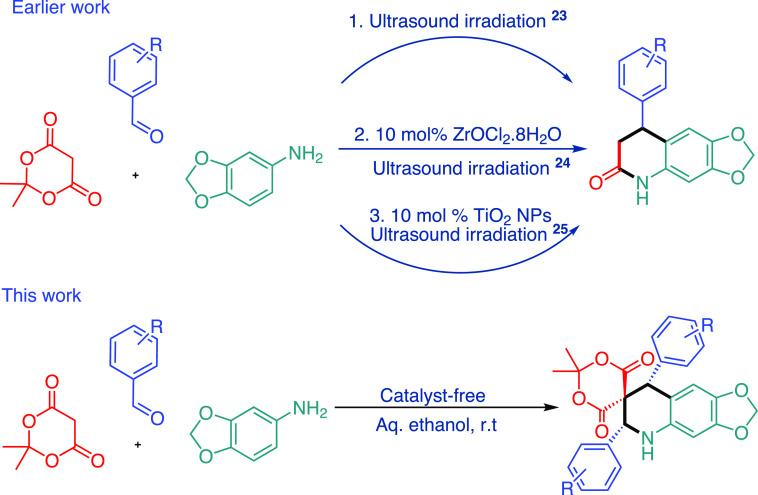
In the past few years, researchers have developed different multicomponent reactions for the synthesis of small heterocycles of biological and chemical interest.21,22 For example, the multicomponent synthesis of 7,8-dihydro-[1,3]dioxolo[4,5-g]quinolin-6(5H)-ones using 5-amino-1,3-benzodioxole, aldehyde, and Meldrum’s acid. In 2012, Azarifar and Sheikh have developed an ultrasound-assisted multicomponent reaction of 5-amino-1,3-benzodioxole, aldehyde, and isopropylidene malonate (Meldrum’s acid) under the neat condition at ambient temperature for the synthesis of 7,8-dihydro-[1,3]dioxolo[4,5-g]quinolin-6(5H)-ones.23 In 2013, the same authors reported an efficient synthesis of 8-aryl-7,8-dihydro[1,3]dioxolo[4,5-g]quinolin-6-(5H)-ones and 4-aryl-3,4-dihydroquinolin-2(1H)ones using Zirconyl chloride octahydrate catalyst.24 In 2019, the same MCR was performed by Bhardwaj et al. using TiO2-based nanoparticles in water and achieved the same motif (Scheme 1).25 These derivatives are formed because Meldrum’s acid commonly holds the exclusive ring-seized malonic acid part, which is generated by the loss of an acetone molecule, while the reaction with nucleophiles can be convoyed by the liberation of carbon dioxide.26−28
Scheme 1. Multicomponent Reaction of Meldrum’s Acid, Aldehyde, and Amine.
Antioxidant activity of dihydro and tetrahydroquinolines were reported rarely; however, quinoline-based alkaloids show excellent activity.29 As the free radical causes harmful effects on biological organs called oxidative stress which is arisen from an imbalance between free radicals and naturally producing antioxidants.30,31 Antioxidant substances that are present in the cell at low concentrations significantly inhibit or eliminate oxidative stress.32 Generally, the human body has created numerous defenses against oxidative stress by manufacturing antioxidants naturally and using antioxidants provided by food.33 Quinoline and its reduced forms show a broad spectrum of biological properties such as an anticancer,34 antibacterial,35 antioxidant,36 anthelmintic,37 antiglaucoma,38 and antimalarial39 agents. Some aromatic, phenolic, and heterocyclic compounds, especially N–H bond-containing heterocyclic compounds have potent antioxidant activity.40 The antioxidant properties of 2-oxo-1,2-dihydroquinoline-4-carboxylates has been examined using radical scavenging [(2,2-diphenyl-1-picryl-hydrazyl-hydrate) (DPPH)] assay, ferric reducing (FRAP) power assay, and β-carotene kinetic blanching assays by Sebbar et al.41 Some quinoline-based compounds that exhibit excellent antioxidant properties are shown in Figure 1.36,43−45
Figure 1.
Quinoline derivatives exhibiting antioxidant activity.
With our continued efforts for the development of novel spiro heterocycles, herein, we wish to explore an unforeseen result obtained from the one-pot reaction of Meldrum’s acid, aldehyde, and 5-amino-1,3-benzodioxole or 1,4-benzodioxin-6-amine or 5-aminoindazole under catalyst-free reaction conditions in aqueous ethanol (Scheme 2). This protocol offers excellent diastereoselectivity and regioselectivity.
Scheme 2. MCRs of Meldrum’s Acid, Amine Derivatives, and Aldehydes.
2. Results and Discussion
2.1. Chemistry
Initially, we choose 3,4-methylenedioxyaniline 1a, Meldrum’s acid 2, and p-chlorobenzaldehyde 3b as our template substrates to investigate the viability of multicomponent reactions. At first, the reaction was performed using water as reaction media at room temperature with no catalyst, the sticky mass formation was seen in this experiment (Table 1, entry 1). To resolve this problem, we replaced water with ethanol and achieved successful transformation 4b. Next, we utilized ethanol:water system in different volumetric ratios (1:9, 3:7, and 1:1 v/v) as the reaction medium. Here, an ethanol:water system with a 1:1 v/v ratio enables excellent reaction transformation (87% yield) (Table 1, entry 4). It is observed that an increase in the volumetric amount of water in the ethanol/water system leads to sticky reaction mixture formation (Table 1, entries 2–3).
Table 1. Optimization of Reaction Parametersa.
| entry | solvent system | temp. (°C) | time (min) | % yieldb |
|---|---|---|---|---|
| 1c | water | RT | 120 | − |
| 2c | ethanol/water(1:9) | RT | 120 | − |
| 3c | ethanol/water(3:7) | RT | 90 | − |
| 4 | ethanol/water(1:1) | RT | 60 | 87 |
| 5 | ethanol | RT | 60 | 80 |
| 6 | butanol | RT | 90 | 75 |
| 7 | DCM | RT | 90 | 50 |
| 8 | methanol | RT | 120 | 68 |
| 9 | acetic acid | RT | 100 | 64 |
| 10 | acetonitrile | RT | 60 | 80 |
| 11c | n-hexane | RT | 75 | − |
Reaction condition: 3,4-methylenedioxyaniline (1a, 1.0 mmol), Meldrum’s acid (2, 1.0 mmol), and p-chloro benzaldehyde (3b, 2.0 mmol), 5 mL solvent, room temperature (25–30 °C).
Isolated yield.
Sticky reaction mass.
To achieve a higher yield of the product, we optimized this reaction using different polar and non-polar solvents such as water, ethanol, methanol, butanol, acetic acid, acetonitrile, DCM, and n-hexane (Table 1, entry 1, 5–11). We have seen that all solvents provide good isolated yields of product (4b); nevertheless, this required purification of product, noteworthily, the aqueous ethanol (1:1, v/v) is the best solvent candidate for this reaction as it shows complete reaction transformations with respect to aldehyde substrate (87% yield) (Table 1, entry-4) and no sticky reaction mass formation observed. Therefore, we opt for this reaction condition for the synthesis of 6′,8′-bis(4-chlorophenyl)-2,2-dimethyl-5′,8′-dihydro-6′H-spiro[[1,3]dioxane-5,7′-[1,3]dioxolo[4,5-g]quinoline]-4,6-dione 4b. With optimized reaction parameters in hand, we explore a substrate scope using different amine derivatives (3,4-methylenedioxyaniline (1a) and 6-amino-1,4-benzodioxan (1b)) and functionalized aldehydes.
The MCRs of 3,4-methylenedioxyaniline 1a, Meldrum’s acid 2, and various aryl aldehyde 3 flow smoothly under optimal reaction parameters. The results are summarized in Table 2. The aldehyde-bearing ring deactivating functionalities such as p-chlorobenzaldehydes 3b and p-bromo benzaldehyde 3c are tolerated well and form desired product in excellent yields (87% of 4b and 81% 4c). Aldehydes having ring-activating functionalities such as p-methoxy-, p-phenyl- and p-methyl-benzaldehydes are also tolerated well and form corresponding products in excellent yields.
Table 2. Synthesis of Dihydrospiro[dioxolo[4,5-g]quinoline] 4(a–f) and Tetrahydrospiro[dioxino-[2,3-g]quinoline] 4(g–l)a,b.
Reaction conditions: 1(a–b) (1.0 mmol), 2 (1.0 mmol), and 3 (2.0 mmol), in ethanol/water (1:1 v/v) (5 mL) at room temperature (25–30 °C) for 1 h.
Isolated yields of the product; the diastereomeric ratio were determined by 1H NMR analysis.
Encouraged by these results, we replace 3,4-methylenedioxyaniline 1a with 6-amino-1,4-benzodioxan 1b and 5-aminoindazole 5. We performed the reaction of these amines with Meldrum’s acid 2 and aryl aldehyde 3 to construct 2,2-dimethyl-7′,9′-diaryl-2′,3′,6′,9′-tetrahydro-7′H-spiro[[1,3]-dioxane-5,8′-[1,4]dioxino[2,3-g]quinoline]-4,6-diones 4(g–l) and 2′,2′-dimethyl-6,8-diphenyl-1,5,6,8-tetrahydrospiro[pyrazolo[3,4-g]quinoline-7,5′-[1,3]dioxane]-4′,6′-diones 6(a–e), respectively (Tables 2 and 3). To our delight, all these reactions proceed smoothly and produce the desired products with excellent yields (69–85%). All synthesized compounds were purified by washing them with aqueous ethanol.
Table 3. Synthesis of Tetrahydrospiro[pyrazolo[3,4-g]quinolines 6(a–e)a,b.
Reaction conditions: 5 (1.0 mmol), 2 (1.0 mmol), and 3 (2.0 mmol) in ethanol/water (1:1 v/v) (5 mL) at room temperature (25–30 °C) for—1 h.
Isolated yields of the product; the diastereomeric ratio were determined by 1H NMR analysis.
A plausible reaction pathway for the synthesis of dihydrospiro[dioxolo[4,5-g]quinoline], tetrahydrospiro[dioxino[2,3-g]quinoline] and tetrahydrospiro[pyrazolo[4,3-f]quinoline] shown in Figure 2. It involves the initial Knoevenagel condensation reaction of Meldrum’s acid 2 with aldehyde 3 to form the Knoevenagel adduct (K). This adduct (K) undertakes a Michael-type addition reaction with 3,4-methylenedioxyaniline 1a/1,4-benzodioxane-6-amine 1b/5-aminoindazole 5 (C–H activation step) to produce a reaction intermediate (L) and [L′] respectively. Now this intermediate forms benzylidene type derivatives (M) and [M′] by reaction with 2 equiv of aldehyde. Afterward, this derivative endures the intramolecular ring closing step and furnishes the desired products with high diastereoselectivity.
Figure 2.

Plausible reaction pathways for spiroquinolines 4(a–l) and 6(a–e).
Green metrics is a vital tool to evaluate synthetic process from a green chemistry viewpoint. To highlight the present work from a sustainability point of view, we perform the calculation of “Green metrics” such as the E-factor, AE, reaction mass efficiency (RME), and optimum efficiency (OE). Among all green metrics parameters, E-factor is frequently used to highlight the eco-compatibility of the synthetic procedure. The reaction is more eco-friendly when the E-factor is lower. The E-factor value range from 0.54 to 2.22 confirm the same. As shown in Figure 3, the remarkable values of AE, RME, and OE (up to 94.54, 81.42, and 87.00 respectively) also validate the same.
Figure 3.
Green metrics for spiroquinolines 4(a–l) and 6(a–e).
All synthesized compounds were structurally elucidated by 1H NMR, 13C NMR, and mass spectroscopic methods. Furthermore, we developed a single crystal of compound 4b, and it was examined by single-crystal X-ray diffraction (XRD) analysis. The result of the analysis confirms the molecular structure of 4b. It exhibits a triclinic crystal system with a P1 space group. The triclinic crystal system’s unit cell constants are: a = 13.5982(5) Å, b = 13.6868(5) Å, c = 13.6868(5) Å, α = 71.4840(10)°, β = 66.0180(10)°, γ = 64.6920(10)°, and volume = 2511.7 Å3. It is based on refinement, which was carried out with the help of SHELXL-97.46 We deposited XRD data of compound 4b online to Cambridge Crystallographic Data Centre (CCDC) with a CCDC deposition number 1985091, which contains the Supporting Information crystallographic data for this paper. The 3D view of compound 4b is shown in Figure 4, which indicates that 4b was obtained in the dimer form.
Figure 4.
3D view (dimer) of 4b compound (CCDC: 1985091).
The 1H NMR spectrum shows the most downfield signals for two aryl ring protons that appear as a multiplate at δH 7.26–7.34 and 7.08–7.11. Moreover, the two most shielded protons were observed at 0.75 and 0.59 for the two methyl groups. Whereas one doublet of a doublet at δH 5.85 appears for dioxane (-OCH2O- protons), and the molecule also possesses two singlets at δH 6.34 and 6.20 observed aromatic protons for H-16 and H-10, respectively. The spectrum showed the most significant signals at δH 5.05 and 4.90 of two protons of dihydro spiro quinolines moiety Figure 5a.
Figure 5.
(a) 1H NMR chemical shift of compound 4b. (b) 13C NMR chemical shift of compound 4b.
In the 13C NMR spectra of compound 4b, a total of 25 signals appear. Sixteen signals correspond to eighteen aromatic carbons at δC 105.94–147.20 and two carbonyl carbon signals appear at δC 161.69 and 167.96. Two signals were observed at δC 97.86 and 100.97 for C-13 and C-3, respectively. The most significant 13C NMR signals for chiral center carbon (C-7 and C-18) appear at δC 50.80 and 58.46, respectively, while one signal shows at 64.32 for spiro carbon (C-6), as shown in Figure 5b. In the MM-APCI spectrum, molecular ion [M+] peaks observed at m/z 526.20 correlates to the molecular formula (C27H21Cl2NO6) of the 4b compound.
The 2D NMR spectra of the HSQC and COSY correlations are useful in the signal assignment of 4b, and various characteristic signals are shown in Figure 6a,b. In HSQC NMR analysis of compound 4b, two chiral center protons correlate with carbon at δ 50.80 and 64.25 which corresponds to carbon C-7 and C-18, respectively. One CH2 and two CH3 protons correlate with carbon at δ 100.87, 28.02, and 29.18, which corresponds to carbon C-13, C-25, and C-26, respectively. In addition, other characteristic peaks were found at δ 133.16, 131.11, 129.56, 129.35, 129.12, 128.91, 97.78, and 107.99 which correlate to aromatic carbons (Figure S41).47 In COSY NMR analysis of compound 4b, it shows that H-18 is correlated with H-16, H-20, and H-24′ (Figure S42).
Figure 6.
(a) H–C hetero correlation of 4b. (b) H–H homo correlation 4b.
The stereochemistry of 4g was established on the basis of NOESY experiments.48 In the NOESY experiment, it is possible to observe signals corresponding to the strong interaction between the C-1 and C-2. The observation is pertinent in that it is consistent with the proton at C-1 and C-2 being in a cis relationship with respect to one another (see Figure 7).
Figure 7.
Characteristic NOEs observed for 4g.
2.2. In Vitro Antioxidant Activity
To establish antioxidant properties of newly synthesized spiroquinolines 4(a–l) and 6(a–e), in vitro 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and DPPH radical scavenging activities of spiroquinolines were performed. Easy operation, rapidity, sensitivity, and utilization of stable radicals, DPPH and ABTS are the best-known and frequently employed techniques for estimating antioxidant activity.49 Results of these activities in IC50 values of 4(a–l) and 6(a–e) are summarized in Table 4. Antioxidant ascorbic acid was used as standard. It displayed IC50 values of 41.84 ± 0.25 and 90.10 ± 0.74 μM for ABTS and DPPH radical scavenging activities, respectively.
Table 4. In Vitro ABTS and DPPH Radical Scavenging Activities of Spiroquinolines 4(a–l) and 6(a–e).
| ABTS radical scavenging activity | DPPH radical scavenging activity | |
|---|---|---|
| code | IC50 ± SEM (μM)a | IC50 ± SEM (μM)a |
| Category “A” | ||
| 4a | 25.22 ± 0.48 | 34.47 ± 0.88 |
| 4b | 21.12 ± 0.79 | 130.97 ± 0.75 |
| 4c | 22.94 ± 0.11 | 108.00 ± 1.11 |
| 4d | 13.16 ± 0.43 | 12.95 ± 0.34 |
| 4e | 14.66 ± 0.39 | 113.07 ± 0.85 |
| 4f | 6.77±0.73 | 23.25 ± 0.53 |
| Category “B” | ||
| 4g | 15.31 ± 0.91 | 77.68 ± 0.19 |
| 4h | 22.13 ± 0.85 | 201.53 ± 0.12 |
| 4i | 24.25 ± 0.21 | 145.79 ± 0.17 |
| 4j | 18.34 ± 0.37 | 266.53 ± 0.18 |
| 4k | 21.54 ± 0.52 | 210.79 ± 0.16 |
| 4l | 11.02 ± 0.88 | 143.02 ± 0.72 |
| Category “C” | ||
| 6a | 27.76 ± 0.88 | 336.08 ± 0.16 |
| 6b | 15.58 ± 1.01 | 224.05 ± 0.79 |
| 6c | 16.88 ± 1.10 | 263.42 ± 0.11 |
| 6d | 27.65 ± 0.69 | 254.28 ± 0.14 |
| 6e | 19.74 ± 0.54 | 280.13 ± 0.09 |
| ascorbic acidb | 41.84 ± 0.25 | 90.10 ± 0.74 |
SEM (standard error mean).
Standard for ABTS and DPPH radical scavenging activity. Bold values show the lowest IC50s.
2.2.1. In Vitro ABTS Radical Scavenging Activity
Results of in vitro ABTS radical scavenging activity of 4(a–l) and 6(a–e) show that all spiroquinoline derivatives show potent activities as compared to standard ascorbic acid. With IC50 of 6.77 ± 0.73 μM displaying the most potent activity among 4(a–l) and 6(a–e). Furthermore, compounds 4l and 4d also displayed excellent activity with IC50 values 11.02 ± 0.88 and 13.16 ± 0.43 μM, respectively.
2.2.2. In Vitro DPPH Radical Scavenging Activity
The result of in vitro DPPH radical scavenging activity of 4(a–l) and 6(a–e) shows that all spiroquinoline derivatives have DPPH radical scavenging activity. 4a, 4d, 4f, and 4g show excellent activity as compared to ascorbic acid. The IC50 of 4d (12.95 ± 0.34 μM) displayed most potent activity among 4(a–l) and 6(a–e). Overall, 4d and 4f have excellent scavenging potential of ABTS and DPPH radicals.
2.3. Structure–Activity Relationship
All the compounds show good to excellent antioxidant activity (ABTS and DPPH). There are three categories: A, B, and C. They were classified into three groups: 1,3-dioxolane, 1,4-dioxane, and pyrazole respectively, which were fused with spiroquinoline derivatives. The lead radical scavenging activity (ABTS and DPPH) of 4a, 4d, 4f, 4g, and 4l compounds are mentioned in Figure 6. From category A, methoxy- and methyl-substituted group at para positions of compound 4d (ABTS, IC50 = 13.16 ± 0.43 μM; DPPH, IC50 = 12.95 ± 0.34 μM) and 4f (ABTS, IC50 = 6.77 ± 0.73 μM; DPPH, IC50 = 23.25 ± 0.53 μM) were the excellent radical scavengers against both DPPH and ABTS as compared to the standard ascorbic acid (ABTS, IC50 = 41.84 ± 0.25 μM; DPPH, IC50 = 90.10 ± 0.74 μM). Without any para substitution, compound 4a shows moderate antioxidant activity (ABTS, IC50 = 25.22 ± 0.48 μM; DPPH, IC50 = 34.47 ± 0.88 μM), while the electron-withdrawing group at para position compound 4b (ABTS, IC50 = 21.12 ± 0.79 μM; DPPH, IC50 = 130.97 ± 0.75 μM), 4c (ABTS, IC50 = 22.94 ± 0.11 μM; DPPH, IC50 = 108.00 ± 1.11 μM), and 4e (ABTS, IC50 = 14.66 ± 0.39 μM; DPPH, IC50 = 113.07 ± 0.85 μM) were weak radical scavengers as compared to compound 4d and 4f. The results of category B shows that the compound bearing the electron-releasing group at the para position exhibited good radical scavenger activity as compared to the electron-withdrawing group at the para position (Table 4). Compounds of category C were the least radical scavenger as compared to categories A and B; compounds 4a, 4g, and 6a showed ABTS (IC50 = 25.22 ± 0.48 μM, 15.31 ± 0.91 μM, and 27.76 ± 0.88 μM, respectively) and DPPH (IC50 = 34.47 ± 0.88 μM, 77.68 ± 0.19 μM, and 336.08 ± 0.16 μM, respectively) radical scavenging activities. Compound 6a belongs to category C (Figure 8).
Figure 8.
Lead radical scavenging activity (ABTS and DPPH) of 4a, 4d, 4f, 4g, and 4l compounds.
3. Conclusion
In conclusion, we successfully designed an eco-compatible and multicomponent reaction-based protocol for spiroquinolines in aq ethanol under catalyst-free conditions. These spiroquinolines show high diastereoselectivity [d.r. >50:1 (cis/trans)] and regioselectivity. This protocol offers several noteworthy benefits such as a simple operating procedure, mild reaction conditions, excellent product yield with purity (HPLC) up to 99%, and good agreements with green metrics parameters. All synthesized spiroquinolines are examined for the radical scavenging (DPPH and ABTS) assay as compared to standard ascorbic acid. The results of in vitro radical scavenging (DPPH and ABTS) assay show that compounds 4d, 4f, and 4l in ABTS radical scavenging assay, and compounds 4a, 4d, 4f, and 4g in DPPH radical scavenging assay were revealed to be the most potent antioxidants. The structure–activity relationship (SAR) highlights that compounds bearing methyl and methoxy groups at the para position are excellent radical scavengers. Altogether, compounds 4d and 4f were discovered as having high scavenging potency with DPPH and ABTS radicals.
4. Experimental Section
4.1. Materials and Apparatus
All reagents used in this synthesis were purchased from commercially available sources and used without any further purification. Melting points were resolute using the open capillary tube method and were uncorrected. NMR spectra (1H NMR & 13C NMR) were recorded on Bruker 500 MHz NMR spectrometer using solvent peak as CDCl3/DMSO-d6 solvent. LCMS analyses were performed on an MS-Agilent 6120 quadrupole spectrometer and HRMS was determined on Waters Micromass Q-Tof Micro 4000 quadrupole spectrometer. TLC analyses were performed on aluminum plates precoated with F254 silica gel 60. Single-crystal was analyzed using a Bruker X8 Kappa APEX II diffractometer.
4.2. General Procedure for Synthesis of Spiroquinolines 4(a–l) and 6(a–e)
Amine derivatives (1 & 5, 1.0 mmol), Meldrum’s acid (2, 1.0 mmol), and aldehyde (3, 2.0 mmol) were mixed in 5 mL of aqueous ethanol into an oven-dried round-bottomed flask. After mixing, the reaction mass was stirred at room temperature for 60–90 min (Table 1). The reaction progress was monitored through periodic TLC analysis (using n-hexane/ethyl acetate (7:3) as the mobile phase). After completion of the reaction (monitored by TLC), 5 mL of distilled water was added and stirred at room temperature for complete solidification of the product. The solid mass was filtered off and washed with 5 mL ethanol to yield the pure form. All newly synthesized compounds were characterized by spectral analysis such as 1H NMR, 13C NMR, HRMS or LCMS, and HPLC.
4.2.1. 2,2-Dimethyl-6′,8′-diphenyl-5′,8′-dihydro-6′H-spiro[[1,3]dioxane-5,7′-[1,3]dioxolo[4,5-g]quinoline]-4,6-dione (4a)
Off white solid (80%), % purity (HPLC) = 99.5%, mp 204–206 °C; 1H NMR (500 MHz, CDCl3) (δ, ppm): 7.36 (s, 5H, ArH), 7.26–7.32 (m, 4H, ArH), 7.15–7.18 (m, 1H, ArH), 6.36 (s, 1H, ArH), 6.28 (s, 1H, ArH), 5.85 (dd, J = 2 Hz, J = 12 Hz, 2H, CH2), 5.07 (s, 1H, CH), 4.95 (s, 1H, CH), 4.29 (s, 1H, NH), 0.64 (s, 3H, CH3), 0.47 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3) (δ, ppm): 168.22, 161.90, 147.01, 140.87, 138.63, 138.03, 136.46, 132.00, 129.70, 129.64, 129.31, 128.94, 128.44, 127.75, 114.46, 108.38, 105.73, 100.85, 97.8, 64.80, 58.49, 51.64, 28.95, 27.98; MS (MM-APCI) m/z: [M – H]+ calcd for C27H23NO6, 456.15; found, 456.20.
4.2.2. 6′,8′-Bis(4-chlorophenyl)-2,2-dimethyl-5′,8′-dihydro-6′H-spiro[[1,3]dioxane-5,7′-[1,3]dioxolo[4,5-g]quinoline]-4,6-dione (4b)
White solid (85%), % purity (HPLC) = 98.8% mp 208–210 °C; 1H NMR (500 MHz,CDCl3) (δ, ppm): 7.26–7.34 (m, 7H, ArH), 7.08–7.11 (m, 1H, ArH), 6.34 (s, 1H, ArH), 6.20 (s, 1H, ArH), 5.85 (dd, J = 1.5 Hz, J = 14.5 Hz, 2H, CH2), 5.05 (s, 1H, CH), 4.90 (s, 1H, CH), 4.30 (s, 1H, NH), 0.75 (s, 3H, CH3), 0.59 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3) (δ, ppm): 167.97, 161.69, 147.20, 141.09, 137.86, 137.11, 135.58, 134.79, 134.47, 133.25, 131.17, 129.67, 129.44, 129.20, 129.01, 114.10, 108.09, 105.94, 100.98, 97.86, 64.33, 58.46, 50.81, 29.29, 28.21; MS (MM-APCI) m/z: [M – H]+ calcd for C27H21Cl2NO6, 524.07; found, 524.20.
4.2.3. 6′,8′-Bis(4-bromophenyl)-2,2-dimethyl-5′,8′-dihydro-6′H-spiro[[1,3]dioxane-5,7′-[1,3]dioxolo[4,5-g]quinoline]-4,6-dione (4c)
Light brown solid (84%), % purity (HPLC) = 97.8%, mp 210–212 °C; 1H NMR (500 MHz, CDCl3) (δ, ppm): 7.44–7.53 (m, 4H, ArH), 7.26 (s, 1H, ArH), 7.24 (m, 1H, ArH), 7.21 (dd, J = 2.5 Hz, J = 8.0 Hz, 1H, ArH), 7.06 (dd, J = 2 Hz, J = 8.5 Hz, 1H, ArH), 6.36 (s, 1H, CH), 6.22 (s, 1H, CH), 5.87 (dd, J = 1.5 Hz, J = 13.5 Hz, 2H, CH2), 5.06 (s, 1H, CH), 4.91 (s, 1H, CH), 4.27 (s, 1H, NH), 0.77 (s, 3H, CH3), 0.61 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3) (δ, ppm): 167.73, 161.43, 147.01, 140.90, 137.60, 137.39, 135.07, 133.35, 132.40, 132.23, 131.82, 131.31, 129.25, 123.50, 122.36, 113.77, 107.89, 107.87, 105.76, 100.79, 97.61, 97.58, 64.14, 58.15, 50.67, 29.09, 27.91; MS (MM-APCI) m/z: [M – H]+ calcd for C27H21Br2NO6, 611.97; found, 612.00.
4.2.4. 6′,8′-Bis(4-methoxyphenyl)-2,2-dimethyl-5′,8′-dihydro-6′H-spiro[[1,3]dioxane-5,7′-[1,3]dioxolo[4,5-g]quinoline]-4,6-dione (4d)
White solid (79%), % purity (HPLC) = 96.8%, mp 190–192 °C;1H NMR (500 MHz, CDCl3) (δ, ppm): 7.21–7.28 (m, 3H, ArH), 7.06–7.08 (m, 1H, ArH), 6.80–6.89 (m, 4H ArH) 6.33 (s, 1H, CH), 6.27 (s, 1H, CH), 5.84 (dd, J = 2 Hz, J = 13 Hz, 2H, CH2), 5.00 (s, 1H, CH), 4.88 (s, 1H, CH), 4.23 (s, 1H, NH), 3.76 (d, J = 8.5 Hz, 6H, 2CH3), 0.74 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3) (δ, ppm): 168.47, 162.16, 160.43, 159.55, 146.90, 140.74, 131.14, 133.05, 130.76, 130.55, 128.97, 128.56, 115.09, 114.48, 113.71, 108.34, 105.67, 100.79, 97.65, 64.30, 58.77, 55.45, 50.89, 29.17, 28.17; MS (MM-APCI) m/z: [M – H]+ calcd for C29H27NO8, 516.17; found, 516.20.
4.2.5. 6′,8′-Di([1,1′-biphenyl]-4-yl)-2,2-dimethyl-5′,8′-dihydro-6′H-spiro[[1,3]dioxane-5,7′-[1,3]dioxolo[4,5-g]quinoline]-4,6-dione (4e)
Off white solid (80%), % purity (HPLC) = 99.3%, mp 206–208 °C; 1H NMR (500 MHz, CDCl3) (δ, ppm): 7.61–7.63 (m, 2H, ArH), 7.54–7.57 (m, 6H, ArH), 7.41–7.47 (m, 7H, ArH), 7.26–7.38 (m, 3H, ArH), 6.41 (s, 1H, CH), 6.38 (s, 1H, CH), 5.88 (dd, J = 1.5 Hz, J = 11.2 Hz, 2H, CH2), 5.17 (s, 1H, CH), 5.04 (s, 1H, CH3), 4.36 (s, 1H, NH), 0.70 (s, 3H, CH3), 0.55 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3) (δ, ppm): 168.05, 161.78, 146.89, 142.32, 141.07, 140.77, 140.20, 139.96, 137.85, 137.42, 135.16, 132.21, 129.90, 128.82, 128.76, 128.00, 127.80, 127.68, 127.64, 127.46, 127.21, 126.90, 126.84, 114.46, 108.19, 105.64, 100.68, 97.64, 64.38, 58.40, 51.15, 28.84, 27.86; MS (MM-APCI) m/z: [M – H]+ calcd for C39H31NO6, 608.21; found, 608.20.
4.2.6. 2,2-Dimethyl-6′,8′-di-p-tolyl-5′,8′-dihydro-6′H-spiro[[1,3]dioxane-5,7′-[1,3]dioxolo[4,5-g]quinoline]-4,6-dione (4f)
Off white solid (78%), % purity (HPLC) = 98.5%, mp 190–192 °C; 1H NMR (500 MHz, CDCl3) (δ, ppm): 7.18–7.28 (m, 5H, ArH), 7.13 (d, J = 8.5 Hz, 2H, ArH), 7.06–7.08 (m, 1H, ArH), 6.37 (s, 1H, CH), 6.31 (s, 1H, CH), 5.86 (d, J = 1.5 Hz, J = 13.5 Hz, 2H, CH2), 5.05 (s, 1H, CH), 4.93 (s, 1H, CH), 2.33 (d, J = 15 Hz, 6H, 2CH3), 0.72 (s, 3H, CH3), 0.55 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3) (δ, ppm): 168.24, 161.95, 146.84, 140.74, 139.37, 138.03, 138.01, 135.47, 133.48, 131.80, 129.94, 129.73, 129.40, 129.27, 127.54, 114.99, 108.30, 105.33, 100.69, 97.72, 64.53, 58.44, 51.31, 28.86, 27.96, 21.13, 21.05; MS (MM-APCI) m/z: [M – H]+ calcd for C29H27NO6, 484.18; found, 484.20.
4.2.7. 2,2-Dimethyl-7′,9′-diphenyl-2′,3′,6′,9′-tetrahydro-7′H-spiro[[1,3]dioxane-5,8′-[1,4]dioxino[2,3-g]quinoline]-4,6-dione (4g)
White solid (82%), % purity (HPLC) = 99.2%, mp 218–220 °C; 1H NMR (500 MHz, CDCl3) (δ, ppm): 7.29–7.40 (m, 9H, ArH), 7.22–7.24 (m, 1H, ArH), 6.38 (s, 2H, ArH), 5.09 (s, 1H, CH), 4.99 (s, 1H, CH), 4.16–4.25 (m, 4H, 2CH2), 0.67 (s, 3H, CH3), 0.51 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3) (δ, ppm): 168.05, 161.84, 142.89, 138.48, 137.65, 136.60, 136.51, 131.97, 129.65, 129.46, 129.19, 129.14, 128.78, 128.26, 127.76, 116.11, 105.58, 103.94, 64.70, 64.65, 64.27, 58.67, 51.20, 28.89, 27.88; MS (MM-APCI) m/z: [M – H]+ calcd for C28H25NO6, 470.17; found, 470.30.
4.2.8. (7′R,9′R)-7′,9′-Bis(4-chlorophenyl)-2,2-dimethyl-2′,3′,6′,9′-tetrahydro-7′H-spiro[[1,3]dioxane-5,8′-[1,4]dioxino[2,3-g]quinoline]-4,6-dione (4h)
White solid (70%), % purity (HPLC) = 97.5%, mp 218–220 °C; 1H NMR (500 MHz, CDCl3): δ 7.36–7.23 (m, 7H, ArH), 7.15 (dd, J = 9.0, 2 Hz, 1H, ArH), 6.34 (s, 1H, ArH), 6.27 (s, 1H, ArH), 5.06 (s, 1H, CH), 4.92 (s, 1H, CH), 4.29–4.10 (m, 4H, 2CH2), 0.77 (s, 3H, CH3), 0.61 (s, 3H, CH3). 13C NMR (126 MHz, CDCl3): δ 167.82, 161.64, 143.07, 137.41, 136.97, 136.73, 135.44, 134.93, 134.32, 133.21, 131.14, 130.92, 129.82, 129.54, 129.29, 129.20, 128.85, 116.63, 115.49, 105.80, 104.05, 64.69, 64.20, 58.64, 50.37, 29.23, 27.99. ESI HRMS: [M + H] + calcd for C28H23Cl2NO6, 540.0975; found, 540.0961.
4.2.9. (7′R,9′R)-7′,9′-Bis(4-bromophenyl)-2,2-dimethyl-2′,3′,6′,9′-tetrahydro-7′H-spiro[[1,3]dioxane-5,8′-[1,4]dioxino[2,3-g]quinoline]-4,6-dione (4i)
White solid (65%), % purity (HPLC) = 98.5%, mp 214–216 °C; 1H NMR (500 MHz, CDCl3): δ 7.54–7.48 (m, 2H), 7.45 (td, J = 8.4, 7.8, 2.2 Hz, 2H), 7.24–7.16 (m, 3H), 7.10 (dd, J = 8.9, 2.3 Hz, 1H), 6.33 (s, 1H), 6.27 (s, 1H), 5.04 (s, 1H), 4.92 (s, 1H), 4.31–4.04 (m, 4H), 0.77 (s, 3H), 0.62 (s, 3H). 13C NMR (126 MHz, CDCl3): δ 167.78, 161.59, 143.10, 137.46, 137.37, 136.74, 135.42, 134.93, 133.53, 132.48, 132.29, 131.87, 131.48, 129.46, 123.56, 122.42, 116.63, 115.37, 105.82, 103.99, 64.70, 64.22, 58.52, 50.43, 29.22, 27.99. ESI HRMS: [M + H] + calcd for C28H23Br2NO6, 627.9965; found, 627.9997.
4.2.10. 7′,9′-Bis(4-fluorophenyl)-2,2-dimethyl-2′,3′,6′,9′-tetrahydro-7′H-spiro[[1,3]dioxane-5,8′-[1,4]dioxino[2,3-g]quinoline]-4,6-dione (4j)
White solid (85%), % purity (HPLC) = 96.1%, mp 202–204 °C; 1H NMR (500 MHz, CDCl3) (δ, ppm): 7.39 (dd, J = 5.5 Hz, J = 10.7 Hz, 2H, ArH), 7.21–7.31 (m, 2H, ArH), 7.01–7.10 (m, 4H, ArH), 6.35 (s, 1H, ArH), 6.30 (s, 1H, CH), 5.09 (s, 1H, CH), 4.97 (s, 1H, CH), 4.16–4.25 (m, 4H, 2CH2), 0.78 (s, 3H, CH3), 0.62 (s, 3H, CH3);13C NMR (125 MHz, CDCl3) (δ, ppm): 167.97, 161.77, 161.77, 143.02, 137.49, 136.64, 134.25, 134.23, 133.50, 132.38, 131.47, 129.62, 116.66, 116.17, 115.00, 115.76, 115.52, 115.35, 105.71, 103.90, 77.24, 64.70, 64.03, 58.92, 50.27, 29.16 27.99; MS (MM-APCI) m/z: [M – H]+ calcd for C28H23F2NO6, 506.14; found, 506.20.
4.2.11. 7′,9′-Di([1,1′-biphenyl]-4-yl)-2,2-dimethyl-2′,3′,6′,9′-tetrahydro-7′H-spiro[[1,3]dioxane-5,8′-[1,4]dioxino[2,3-g]quinoline]-4,6-dione (4k)
Off white solid (79%), % purity (HPLC) = 98.5%, mp 210–212 °C;1H NMR (500 MHz, CDCl3) (δ, ppm): 7.56–7.65 (m, 8H, ArH), 7.33–7.50 (m, 10H, ArH), 6.45 (s, 1H, ArH), 6.41 (s, 1H, ArH), 5.18 (s, 1H, CH), 5.08 (s, 1H, CH), 4.22–4.28 (m, 2H, CH2), 4.19–4.27 (m, 4H, 2CH2), 0.72 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3) (δ, ppm): 168.11, 161.91, 142.98, 142.42, 141.14, 140.43, 140.13, 137.65, 137.46, 136.62, 135.49, 132.38, 130.05, 128.93, 128.85, 128.20, 127.92, 127.77, 127.72, 127.52, 127.29, 127.02, 126.96, 116.88, 116.03, 105.73, 103.98, 77.24, 64.73, 64.43, 64.29, 58.76, 50.89, 28.99, 27.96; MS (MM-APCI) m/z: [M – H]+ calcd for C40H33NO6, 622.22; found, 622.30.
4.2.12. 2,2-Dimethyl-7′,9′-di-p-tolyl-2′,3′,6′,9′-tetrahydro-7′H-spiro[[1,3]dioxane-5,8′-[1,4]dioxino[2,3-g]quinoline]-4,6-dione (4l)
Off white solid (78%), % purity (HPLC) = 99.1%, mp 200–202 °C;1H NMR (500 MHz, CDCl3) (δ, ppm): 7.09–7.27 (m, 8H, ArH), 6.36 (s, 1H, ArH), 6.34 (s, 1H, CH), 5.03 (s, 1H, CH) 4.94 (s, 1H, CH), 4.13–4.27 (m, 4H, 2CH2), 2.33 (d, J = 17.5 Hz, 6H, 2CH3), 0.71 (s, 3H, CH3), 0.56 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3) (δ, ppm): 168.17, 161.99, 142.80, 139.33, 137.92, 137.70, 136.46, 135.35, 133.64, 131.83, 129.93, 129.69, 129.42, 129.21, 127.60, 116.85, 116.41, 105.50, 103.86, 77.25, 64.71, 64.43, 64.26, 58.67, 50.88, 28.90, 27.94, 21.13, 21.05; MS (MM-APCI) m/z: [M – H]+ calcd for C30H29NO6, 498.19; found, 498.20.
4.2.13. 2′,2′-Dimethyl-7,9-diphenyl-3,6,7,9-tetrahydrospiro[pyrazolo[4,3-f]quinoline-8,5′-[1,3]dioxane]-4′,6′-dione (6a)
White solid (83%), % purity (HPLC) = 99.7%, mp 298–300 °C; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 12.69 (s, 1H, NH), 7.42–7.46 (m, 3H, ArH), 7.40 (s, 1H, ArH), 7.29–7.38 (m, 4H, ArH), 7.21–7.24 (m, 1H, ArH), 7.04–7.06 (d, J = 9 Hz, 1H), 6.75–6.77 (m, 1H, ArH), 6.63 (s, 1H, ArH), 6.11 (s, 1H, ArH), 5.22 (s, 1H, CH), 4.85 (s, 1H, CH), 0.62 (s, 3H, CH3), 0.46 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 167.50, 160.48, 138.45, 138.16, 136.23,130.69, 129.92, 129.07, 128.69, 128.53, 128.38, 128.11, 127.85, 121.42, 118.41,108.97, 104.87, 64.50, 59.03, 49.18, 28.98, 28.38; ESI HRMS: [M + H]+ calcd for C27H23N3O4, 454.1767; found, 454.1780.
4.2.14. 7,9-Bis(4-chlorophenyl)-2′,2′-dimethyl-3,6,7,9-tetrahydrospiro[pyrazolo[4,3-f]quinoline-8,5′-[1,3]dioxane]-4′,6′-dione (6b)
White solid (87%), % purity (HPLC) = 99.2%, mp 280–282 °C; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 12.76 (s, 1H, NH), 7.56–7.57 (m, 2H, ArH), 7.31–7.43 (m, 5H, ArH), 7.03 (d, J = 8.5 Hz, 5H, ArH), 6.71–6.73 (m, 2H, ArH), 6.17 (s, 1H, ArH), 5.27 (s, 1H, CH), 4.86 (s, 1H, CH), 0.73 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 167.27, 160.36, 138.11, 137.35, 135.07, 135.00, 133.74, 132.88, 132.47, 131.75, 131.20, 129.73, 128.63, 128.56, 121.25, 118.32, 109.38, 108.57, 105.06, 63.96, 59.09, 48.17, 28.60, 26.99; ESI HRMS: [M + H]+ calcd for C27H21Cl2N3O4, 522.0982; found, 522.1014.
4.2.15. 7,9-Bis(4-bromophenyl)-2′,2′-dimethyl-3,6,7,9-tetrahydrospiro[pyrazolo[4,3-f]quinoline-8,5′-[1,3]dioxane]-4′,6′-dione (6c)
Off white solid (84%), % purity (HPLC) = 99.1%, mp 280–282 °C;1H NMR (500 MHz, DMSO-d6) (δ, ppm): 12.77 (s, 1H, NH), 7.69 (dd, J = 2 Hz, J = 7 Hz, 2H, ArH), 7.46 (dd, J = 2 Hz, J = 8.5 Hz, 1H, ArH), 7.28–7.36 (m, 4H, ArH), 7.03 (d, J = 9 Hz, 1H, ArH), 6.72 (s, 1H, ArH), 6.66 (dd, J = 2 Hz, J = 8.5 Hz, 1H, ArH), 6.17 (s, 1H, ArH), 5.25 (s, 1H, CH), 4.84 (s, 1H, CH), 0.73 (s, 3H, CH3), 0.58 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 167.25, 160.33, 138.11, 137.72, 135.39, 134.37, 132.78, 132.07, 131.64, 131.51, 131.47, 131.38, 130.01, 122.25, 121.30, 121.21, 118.35, 108.46, 105.07, 64.02, 58.97, 48.22, 28.59, 26.97; ESI HRMS: [M + H]+ calcd for C27H21Br2N3O4, 609.9972; found, 609.9996.
4.2.16. 7,9-Bis(4-methoxyphenyl)-2′,2′-dimethyl-3,6,7,9-tetrahydrospiro[pyrazolo[4,3-f]quinoline-8,5′-[1,3]dioxane]-4′,6′-dione (6d)
Off white solid (78%), % purity (HPLC) = 98.8%, mp 268–270 °C; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 12.66 (s, 1H, NH), 7.24–7.28 (m, 3H, ArH), 7.00–7.03 (m, 4H, ArH), 6.81 (dd, J = 3 Hz, J = 9 Hz, 1H, ArH), 6.64–6.67 (m, 1H, ArH), 6.50 (s, 1H, ArH), 6.15 (s, 1H, ArH), 5.14 (s, 1H, CH), 4.76 (s, 1H, CH), 3.74 (d, J = 11 Hz, ArH), 0.71 (s, 3H, CH3), 0.55 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 167.75, 160.74, 159.76, 158.89, 138.16, 134.99, 131.73, 131.46, 130.95, 130.25, 128.98, 128.17, 121.51, 118.33, 114.18, 113.82, 113.53, 109.46, 108.92, 104.81, 64.00, 59.29, 55.18, 55.02, 48.41, 28.55, 27.14; ESI HRMS: [M + H]+ calcd for C29H27N3O6, 514.1973; found, 514.1995.
4.2.17. 2′,2′-Dimethyl-7,9-bis(4-nitrophenyl)-3,6,7,9-tetrahydrospiro[pyrazolo[4,3-f]quinoline-8,5′-[1,3]dioxane]-4′,6′-dione (6e)
Light yellow solid (85%), % purity (HPLC) = 98.3%, mp 294–296 °C; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 12.84 (s, 1H, NH), 8.13–8.38 (m, 3H, ArH), 7.70–7.72 (m, 1H, ArH), 7.64 (d, J = 9 Hz, 2H, ArH), 7.38 (d, J = 9 Hz, 1H, ArH), 7.07 (d, J = 8.5 Hz, 1H, ArH), 6.96–6.99 (m, 2H, ArH), 6.19 (s, 1H, CH), 5.52 (s, 1H, CH), 5.07 (s, 1H, CH), 0.67 (s, 3H, CH3), 0.52 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 166.85, 160.12, 147.99, 147.20, 145.95, 143.06, 138.05, 135.18, 132.16, 131.41, 131.13, 129.53, 124.01, 123.70, 123.51, 121.10, 118.32, 109.81, 108.01, 105.36, 64.15, 59.00, 48.19, 28.8, 26.97; ESI HRMS: [M + H]+ calcd for C27H21N5O8, 544.1463; found, 544.1480.
4.3. ABTS Radical Scavenging Assay
The ABTS free radical cation scavenging activity of the compounds was performed using a standard method.50,51 First, a 7 mM concentrated solution of ABTS was prepared, and then, a 2.45 mM concentrated solution of potassium persulfate was added to the ABTS solution. This mixture was kept in a dark place at room temperature for 14–16 h. The test compound sample solutions were prepared in absolute alcohol at concentrations ranging from 0.01 to 1 mg/mL. The test sample was added to the ABTS solutions and incubated for 30 min at 37 °C. The absorbance was measured at a wavelength of 734 nm, and the procedure was repeated for ascorbic acid as a reference standard. The % inhibition of radical scavenging activity was determined using the given formula.
4.4. DPPH Radical Scavenging Assay
The free radical scavenging activity of DPPH was tested using a standard procedure.50,51 0.3 mM DPPH concentrated solution in ethanol incubated for 30 min at 37 °C with various test samples. The absorption of the sample was taken at 517 nm. The same procedure was followed for ascorbic acid as the standard. The % inhibition of radical scavenging activity was determined using the given formula.
Acknowledgments
P.J.P., R.M.V., D.B.U., S.G.P., and H.M.P. are grateful to the Department of Chemistry, Sardar Patel University, for providing chemicals and lab facilities. P.J.P. is grateful to UGC New Delhi, India, for financial support (file no. 16-9(June 2019)/2019 (NET/CSIR); dated 10/06/2020). All authors are thankful to Manoj Mangukiya, Associate Analytical Scientist, Aether Industries Ltd., Surat, for the LC–MS experiments. This work is dedicated to my adored parents.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05020.
1H NMR and 13C NMR spectral data for all synthesized compounds and 2D NMR of 4b (HSQC and COSY), 4g, and 6a (NOESY); LCMS spectral data for 4a–g and 4j–l and HRMS spectral data for 4h–I and 6a–e; ORTEP diagram of compound 4b; and green matrix factors, E-factor, AE, RME, and OE calculation, for representative all the synthesized (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sarkar A.; Santra S.; Kundu S. K.; Hajra A.; Zyryanov G. V.; Chupakhin O. N.; Charushin V. N.; Majee A. A decade update on solvent and catalyst-free neat organic reactions: a step forward towards sustainability. Green Chem. 2016, 18, 4475–4525. 10.1039/c6gc01279e. [DOI] [Google Scholar]
- Vlaminck L.; Van de Voorde B.; Du Prez F. E. Sustainable synthesis routes towards urazole compounds. Green Chem. 2017, 19, 5659–5664. 10.1039/c7gc02027a. [DOI] [Google Scholar]
- Maji M.; Panja D.; Borthakur I.; Kundu S. Recent advances in sustainable synthesis of N-heterocycles following acceptorless dehydrogenative coupling protocol using alcohols. Org. Chem. Front. 2021, 8, 2673–2709. 10.1039/d0qo01577f. [DOI] [Google Scholar]
- Patel D. M.; Sharma M. G.; Vala R. M.; Lagunes I.; Puerta A.; Padrón J. M.; Rajani D. P.; Patel H. M. Hydroxyl alkyl ammonium ionic liquid assisted green and one-pot regioselective access to functionalized pyrazolodihydropyridine core and their pharmacological evaluation. Bioorg. Chem. 2019, 86, 137–150. 10.1016/j.bioorg.2019.01.029. [DOI] [PubMed] [Google Scholar]
- Brahmachari G.; Nurjamal K.; Karmakar I.; Begam S.; Nayek N.; Mandal B. Development of a Water-Mediated and Catalyst-Free Green Protocol for Easy Access to a Huge Array of Diverse and Densely Functionalized Pyrido[2,3-d:6,5-d′]dipyrimidines via One-Pot Multicomponent Reaction under Ambient Conditions. ACS Sustainable Chem. Eng. 2017, 5, 9494–9505. 10.1021/acssuschemeng.7b02696. [DOI] [Google Scholar]
- Patel D. M.; Patel H. J.; Padrón J. M.; Patel H. M. A novel substrate directed multicomponent reaction for the syntheses of tetrahydro-spiro[pyrazolo[4,3-f]quinoline]-8,5′-pyrimidines and tetrahydro-pyrazolo[4,3-f]pyrimido[4,5-b]quinolines via selective multiple C–C bond formation under metal-free conditions. RSC Adv. 2020, 10, 19600–19609. 10.1039/d0ra02990d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. M.; Patel H. J.; Padrón J. M.; Patel H. M. J. R. a. A novel substrate directed multicomponent reaction for the syntheses of tetrahydro-spiro [pyrazolo [4, 3-f] quinoline]-8, 5′-pyrimidines and tetrahydro-pyrazolo [4, 3-f] pyrimido [4, 5-b] quinolines via selective multiple C–C bond formation under metal-free conditions. RSC Adv. 2020, 10, 19600–19609. 10.1039/d0ra02990d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M. G.; Pandya J.; Patel D. M.; Vala R. M.; Ramkumar V.; Subramanian R.; Gupta V. K.; Gardas R. L.; Dhanasekaran A.; Patel H. M. One-Pot Assembly for Synthesis of 1,4-Dihydropyridine Scaffold and Their Biological Applications. Polycyclic Aromat. Compd. 2021, 41, 1495–1505. 10.1080/10406638.2019.1686401. [DOI] [Google Scholar]
- Vala R. M.; Patel D. M.; Sharma M. G.; Patel H. M. J. R. a. Impact of an aryl bulky group on a one-pot reaction of aldehyde with malononitrile and N-substituted 2-cyanoacetamide. RSC Adv. 2019, 9, 28886–28893. 10.1039/c9ra05975j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. G.; Vala R. M.; Patel P. J.; Upadhyay D. B.; Ramkumar V.; Gardas R. L.; Patel H. M. J. R. a. Synthesis, crystal structure and in silico studies of novel 2, 4-dimethoxy-tetrahydropyrimido [4, 5-b] quinolin-6 (7 H)-ones. RSC Adv. 2022, 12, 18806–18820. 10.1039/d2ra02694e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba Reddy B. V.; Gopal Reddy S.; Durgaprasad M.; Bhadra M. P.; Sridhar B. Domino Prins/pinacol reaction for the stereoselective synthesis of spiro[pyran-4,4′-quinoline]-2′,3′-dione derivatives. Org. Biomol. Chem. 2015, 13, 8729–8733. 10.1039/c5ob01077b. [DOI] [PubMed] [Google Scholar]
- Patel D. M.; Patel H. M. Trimethylglycine-Betaine-Based-Catalyst-Promoted Novel and Ecocompatible Pseudo-Four-Component Reaction for Regioselective Synthesis of Functionalized 6,8-Dihydro-1′H,5H-spiro[[1,3]dioxolo[4,5-g]quinoline-7,5′-pyrimidine]-2′,4′,6′(3′H)-trione Derivatives. ACS Sustainable Chem. Eng. 2019, 7, 18667–18676. 10.1021/acssuschemeng.9b05184. [DOI] [Google Scholar]
- Patel H. M.; Rajani D. P.; Sharma M. G.; Bhatt H. G. Synthesis, molecular docking and biological evaluation of mannich products based on thiophene nucleus using ionic liquid. Lett. Drug Des. Discovery 2019, 16, 119–126. 10.2174/1570180815666180502123743. [DOI] [Google Scholar]
- Patel H. M. Synthesis, characterizations and microbial studies of novel mannich products using multicomponent reactions. Curr. Bioact. Compd. 2018, 14, 278–288. 10.2174/1573407213666170424164716. [DOI] [Google Scholar]
- Sharma M. G.; Rajani D. P.; Patel H. M. Green approach for synthesis of bioactive Hantzsch 1,4-dihydropyridine derivatives based on thiophene moiety via multicomponent reaction. R. Soc. Open Sci. 2017, 4, 170006. 10.1098/rsos.170006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H. M.; Patel K. D.; Patel H. D. Facile Synthesis and Biological Evaluation of New Mannich Products as Potential Antibacterial, Antifungal and Antituberculosis Agents: Molecular Docking Study. Curr. Bioact. Compd. 2017, 13, 47–58. 10.2174/1573407212666160517145130. [DOI] [Google Scholar]
- Patel H. M. Synthesis of New Mannich Products Bearing Quinoline Nucleous Using Reusable Ionic Liquid and Antitubercular Evaluation. Green Sustainable Chem. 2015, 05, 137–144. 10.4236/gsc.2015.54017. [DOI] [Google Scholar]
- Lei J.; Li Y.; Xu J.; Tang D.-Y.; Shao J.-W.; Li H.-y.; Chen Z.-Z.; Xu Z.-G. An acid-catalyzed 1,4-addition isocyanide-based multicomponent reaction in neat water. Green Chem. 2020, 22, 3716–3720. 10.1039/d0gc00652a. [DOI] [Google Scholar]
- Hu Z.; Men Y.; Xu Z.; Wu T.; Xu X.; Tang B. A catalyst-free aqueous mediated multicomponent reaction of isocyanide: expeditious synthesis of polyfunctionalized cyclo[b]fused mono-, di- and tricarbazoles. Org. Chem. Front. 2020, 7, 3720–3726. 10.1039/d0qo01095b. [DOI] [Google Scholar]
- He Y.; Li X.; Zheng Y.; Wang Z.; Ma Z.; Yang Q.; Yao B.; Zhao Y.; Zhang H. A green approach for synthesizing silver nanoparticles, and their antibacterial and cytotoxic activities. New J. Chem. 2018, 42, 2882–2888. 10.1039/c7nj04224h. [DOI] [Google Scholar]
- Wiemann J.; Fischer L.; Kessler J.; Ströhl D.; Csuk R. Ugi multicomponent-reaction: Syntheses of cytotoxic dehydroabietylamine derivatives. Bioorg. Chem. 2018, 81, 567–576. 10.1016/j.bioorg.2018.09.014. [DOI] [PubMed] [Google Scholar]
- McKeown M. R.; Shaw D. L.; Fu H.; Liu S.; Xu X.; Marineau J. J.; Huang Y.; Zhang X.; Buckley D. L.; Kadam A.; Zhang Z.; Blacklow S. C.; Qi J.; Zhang W.; Bradner J. E. Biased Multicomponent Reactions to Develop Novel Bromodomain Inhibitors. J. Med. Chem. 2014, 57, 9019–9027. 10.1021/jm501120z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarifar D.; Sheikh D. Ultrasound-promoted One-pot Synthesis of 8-Aryl-7, 8-dihydro-[1, 3]-dioxolo [4, 5-g] quinolin-6 (5H)-one Derivatives under Catalyst-free and Solvent-free Conditions. Acta Chim. Slov. 2012, 59, 664. [PubMed] [Google Scholar]
- Azarifar D.; Sheikh D. J. S. C. ZrOCl2·8H2O: An Efficient, Ecofriendly, and Recyclable Catalyst for Ultrasound-Accelerated, One-Pot, Solvent-Free Synthesis of 8-Aryl-7, 8-dihydro-[1, 3] dioxolo [4, 5-g] quinolin-6-(5 H)-one and 4-Aryl-3, 4-dihydroquinolin-2 (1 H)-one Derivatives. Synth. Commun. 2013, 43, 2517–2526. 10.1080/00397911.2012.718026. [DOI] [Google Scholar]
- Bhardwaj D.; Singh A.; Singh R. Eco-compatible sonochemical synthesis of 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-ones using green TiO2. Heliyon 2019, 5, e01256 10.1016/j.heliyon.2019.e01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury S. K.; Kumar D.; Kamal A.; Singh H. K.; Kumari S.; Singh S. A facile and efficient multicomponent ultrasound-assisted ″on water″ synthesis of benzodiazepine ring. Mol. Diversity 2021, 25, 131–142. 10.1007/s11030-019-10031-y. [DOI] [PubMed] [Google Scholar]
- Vala R. M.; Sharma M. G.; Patel D. M.; Puerta A.; Padrón J. M.; Ramkumar V.; Gardas R. L.; Patel H. M. Synthesis and in vitro study of antiproliferative benzyloxy dihydropyrimidinones. Arch. Pharm. 2021, 354, 2000466. 10.1002/ardp.202000466. [DOI] [PubMed] [Google Scholar]
- Tankov I.; Yankova R.; Genieva S.; Mitkova M.; Stratiev D. Density functional theory study on the ionic liquid pyridinium hydrogen sulfate. J. Mol. Struct. 2017, 1139, 400–406. 10.1016/j.molstruc.2017.03.040. [DOI] [Google Scholar]
- Chung H. S.; Woo W. S. A Quinolone Alkaloid with Antioxidant Activity from the Aleurone Layer of Anthocyanin-Pigmented Rice. J. Nat. Prod. 2001, 64, 1579–1580. 10.1021/np010324g. [DOI] [PubMed] [Google Scholar]
- Lushchak V. I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.Biol. Interact. 2014, 224, 164–175. 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Khajeh Dangolani S.; Panahi F.; Tavaf Z.; Nourisefat M.; Yousefi R.; Khalafi-Nezhad A. Synthesis and Antioxidant Activity Evaluation of Some Novel Aminocarbonitrile Derivatives Incorporating Carbohydrate Moieties. ACS Omega 2018, 3, 10341–10350. 10.1021/acsomega.8b01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurutas E. B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 2016, 15, 71. 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo V.; Patil A.; Phatak A.; Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobeiri N.; Rashedi M.; Mosaffa F.; Zarghi A.; Ghandadi M.; Ghasemi A.; Ghodsi R. Synthesis and biological evaluation of quinoline analogues of flavones as potential anticancer agents and tubulin polymerization inhibitors. Eur. J. Med. Chem. 2016, 114, 14–23. 10.1016/j.ejmech.2016.02.069. [DOI] [PubMed] [Google Scholar]
- Narender P.; Srinivas U.; Ravinder M.; Ananda Rao B.; Ramesh C.; Harakishore K.; Gangadasu B.; Murthy U. S. N.; Jayathirtha Rao V. Synthesis of multisubstituted quinolines from Baylis–Hillman adducts obtained from substituted 2-chloronicotinaldehydes and their antimicrobial activity. Bioorg. Med. Chem. 2006, 14, 4600–4609. 10.1016/j.bmc.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Puskullu M. O.; Shirinzadeh H.; Nenni M.; Gurer-Orhan H.; Suzen S. Synthesis and evaluation of antioxidant activity of new quinoline-2-carbaldehyde hydrazone derivatives: bioisosteric melatonin analogues. J. Enzyme Inhib. Med. Chem. 2016, 31, 121–125. 10.3109/14756366.2015.1005012. [DOI] [PubMed] [Google Scholar]
- Rossiter S.; Péron J.-M.; Whitfield P. J.; Jones K. Synthesis and anthelmintic properties of arylquinolines with activity against drug-resistant nematodes. Bioorg. Med. Chem. Lett. 2005, 15, 4806–4808. 10.1016/j.bmcl.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Langlois M.; Brémont B.; Rousselle D.; Gaudy F. Structural analysis by the comparative molecular field analysis method of the affinity of β-adrenoreceptor blocking agents for 5-HT1A and 5-HT1B receptors. Eur. J. Pharmacol. Mol. Pharmacol. 1993, 244, 77–87. 10.1016/0922-4106(93)90061-d. [DOI] [PubMed] [Google Scholar]
- Kaur K.; Jain M.; Reddy R. P.; Jain R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010, 45, 3245–3264. 10.1016/j.ejmech.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Sonmez F.; Gunesli Z.; Kurt B. Z.; Gazioglu I.; Avci D.; Kucukislamoglu M. Synthesis, antioxidant activity and SAR study of novel spiro-isatin-based Schiff bases. Mol. Diversity 2019, 23, 829–844. 10.1007/s11030-018-09910-7. [DOI] [PubMed] [Google Scholar]
- Filali Baba Y.; Sert Y.; Kandri Rodi Y.; Hayani S.; Mague J. T.; Prim D.; Marrot J.; Ouazzani Chahdi F.; Sebbar N. K.; Essassi E. M. Synthesis, crystal structure, spectroscopic characterization, Hirshfeld surface analysis, molecular docking studies and DFT calculations, and antioxidant activity of 2-oxo-1,2-dihydroquinoline-4-carboxylate derivatives. J. Mol. Struct. 2019, 1188, 255–268. 10.1016/j.molstruc.2019.03.103. [DOI] [Google Scholar]
- Wilhelm E. A.; Ferreira A. T.; Pinz M. P.; Reis A. S.; Vogt A. G.; Stein A. L.; Zeni G.; Luchese C. Antioxidant effect of quinoline derivatives containing or not selenium: Relationship with antinociceptive action quinolines are antioxidant and antinociceptive. An. Acad. Bras. Cienc. 2017, 89, 457–467. 10.1590/0001-3765201720160668. [DOI] [PubMed] [Google Scholar]
- Manjunatha J. R.; Bettadaiah B. K.; Negi P. S.; Srinivas P. Synthesis of quinoline derivatives of tetrahydrocurcumin and zingerone and evaluation of their antioxidant and antibacterial attributes. Food Chem. 2013, 136, 650–658. 10.1016/j.foodchem.2012.08.052. [DOI] [PubMed] [Google Scholar]
- Song D.; Cao X.; Wang J.; Ke S. Discovery of γ-lactam derivatives containing 1,3-benzodioxole unit as potential anti-phytopathogenic fungus agents. Bioorg. Med. Chem. Lett. 2020, 30, 126826. 10.1016/j.bmcl.2019.126826. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Adv. 2008, 64, 112–122. 10.1107/s0108767307043930. [DOI] [PubMed] [Google Scholar]
- Akhavan M.; Bekhradnia A. Stereoselective synthesis of spirocyclic pyrrolidines/pyrrolizidines/pyrrolothiazolidines using l-proline functionalized manganese ferrite nanorods as a novel heterogeneous catalyst. RSC Adv. 2021, 11, 14755–14768. 10.1039/d1ra00841b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano R.; Andrés J. M.; Muruzábal M. D.; Pedrosa R. Stereocontrolled Construction of Quaternary Stereocenters by Inter- and Intramolecular Nitro-Michael Additions Catalyzed by Bifunctional Thioureas. Adv. Synth. Catal. 2010, 352, 3364–3372. 10.1002/adsc.201000612. [DOI] [Google Scholar]
- Olszowy M.; Dawidowicz A. L. Is it possible to use the DPPH and ABTS methods for reliable estimation of antioxidant power of colored compounds?. Chem. Pap. 2018, 72, 393–400. 10.1007/s11696-017-0288-3. [DOI] [Google Scholar]
- Salar U.; Khan K. M.; Chigurupati S.; Taha M.; Wadood A.; Vijayabalan S.; Ghufran M.; Perveen S. New Hybrid Hydrazinyl Thiazole Substituted Chromones: As Potential α-Amylase Inhibitors and Radical (DPPH & ABTS) Scavengers. Sci. Rep. 2017, 7, 16980. 10.1038/s41598-017-17261-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafique R.; Khan K. M.; Arshia; Chigurupati S.; Wadood A.; Rehman A. U.; Salar U.; Venugopal V.; Shamim S.; Taha M.; Perveen S. Synthesis, in vitro α-amylase inhibitory, and radicals (DPPH & ABTS) scavenging potentials of new N-sulfonohydrazide substituted indazoles. Bioorg. Chem. 2020, 94, 103410. 10.1016/j.bioorg.2019.103410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.