Abstract
Schistosoma mansoni masks its surface with adsorbed host proteins including erythrocyte antigens, immunoglobulins, major histocompatibility complex class I, and β2-microglobulin (β2m), presumably as a means of avoiding host immune responses. How this is accomplished has not been explained. To identify surface receptors for host proteins, we biotinylated the tegument of live S. mansoni adults and mechanically transformed schistosomula and then removed the parasite surface with detergent. Incubation of biotinylated schistosome surface extracts with human immunoglobulin G (IgG) Fc-Sepharose resulted in purification of a 97-kDa protein that was subsequently identified as paramyosin (Pmy), using antiserum specific for recombinant Pmy. Fc also bound recombinant S. mansoni Pmy and native S. japonicum Pmy. Antiserum to Pmy decreased the binding of Pmy to Fc-Sepharose, and no proteins bound after removal of Pmy from extracts. Fluoresceinated human Fc bound to the surface, vestigial penetration glands, and nascent oral cavity of mechanically transformed schistosomula, and rabbit anti-Pmy Fab fragments ablated the binding of Fc to the schistosome surface. Pmy coprecipitated with host IgG from parasite surface extracts, indicating that complexes formed on the parasite surface as well as in vitro. Binding of Pmy to Fc was not inhibited by soluble protein A, suggesting that Pmy does not bind to the region between the CH2 and CH3 domains used by many other Fc-binding proteins. β2m did not bind to the schistosome Fc receptor (Pmy), a finding that contradicts reports from earlier workers but did bind to a heteromultimer of labeled schistosomula surface proteins. This is the first report of the molecular identity of a schistosome Fc receptor; moreover it demonstrates an additional aspect of the unusual and multifunctional properties of Pmy from schistosomes and other parasitic flatworms.
Schistosomes establish chronic infections in their hosts despite the presence of specific cellular and humoral immune responses. One of the most striking immunoevasive mechanisms displayed by these parasites is the acquisition of host products onto the tegument of the schistosome to mask its foreign status. Host molecules adsorbed onto the surface include immunoglobulins (20, 40, 44), major histocompatibility complex products (3, 36), β2-microglobulin (β2m) (40), complement components (35, 38), α2-macroglobulin (7, 19), C3 decay-accelerating factor (14), and glycolipids in the form of A, B, H, and Lewis blood group antigens (12).
More than 20 years ago, Schistosoma mansoni schistosomula were shown to selectively bind the Fc but not the Fab fragment of immunoglobulin G (IgG) (40). This was demonstrated by the adhesion of rosetted sheep erythrocytes to transformed schistosomula of S. mansoni in vitro and inhibition of adhesion by free Fc fragments. IgG from rat, mouse, and rabbit also inhibited rosette formation. Whereas most of the host molecules listed above are adsorbed by schistosomula, receptors for Fc and complement C3 have also been identified on the surface of adult S. mansoni (38).
Our understanding of the molecular interactions between schistosomes and their hosts has progressed significantly since these early studies, a situation facilitated by the availability of more than 15,000 schistosome expressed sequence tags deposited in the public databases (15). However, despite this information, schistosome surface receptors for host immune proteins have yet to identified. Here, we describe the surface biotinylation of live schistosomes and the subsequent purification of labeled Fc- and β2m-binding proteins from the surface of S. mansoni. We identify paramyosin (Pmy) as the Fc-binding protein (Fc-Bp) and show that Pmy from the Asian schistosome, Schistosoma japonicum, also binds human Fc. Pmy is a promising vaccine candidate for schistosomiasis; while it has been shown to have an unusual immunomodulatory role by binding C1q of the complement cascade (25), this is the first report of schistosome Pmy binding to Fc, a finding with important implications for the use of this antigen as a vaccine target.
MATERIALS AND METHODS
Parasites.
Adult S. mansoni (Puerto Rican strain; both sexes) were perfused from BALB/c mice 7 to 10 weeks after infection with cercariae and washed thoroughly in phosphate-buffered saline (PBS). The viability of worms was examined microscopically; intact parasites were washed three times in RPMI 1640 supplemented with penicillin (100 U ml−1), streptomycin (100 μg ml−1), amphotericin β (Fungizone; 0.025 μg/ml), and 20 mM HEPES (RPMI-PSF) and then cultured overnight in the same medium at 37°C in an atmosphere of 5% CO2–95% air. Medium was changed the following morning, and parasites were cultured for another 2 h, checked for viability and then surface biotinylated as outlined below. S. japonicum (Sorsogon, Philippines) adult parasites were perfused from mice and washed in PBS, and soluble adult worm protein was produced as previously described (43). S. mansoni schistosomula were obtained by mechanical transformation of cercariae according to published protocols (6) and cultured overnight at 37°C in RPMI-PSF in an atmosphere of 5% CO2–95% air.
Surface biotinylation.
Live adults or schistosomula of S. mansoni were surface biotinylated using sulfo-N-hydroxysuccinimide-LC-biotin (Pierce) as previously described (8). After removal of free biotin and washing, parasites were resuspended in Tris-buffered saline (TBS)–1% Triton X-100 and incubated on ice for 30 min with occasional agitation. The supernatants, hereafter referred to as bio-ad-TX (adult worms) or bio-som-TX (schistosomula), were then removed, centrifuged at 10,000 × g and dialyzed overnight against 500 volumes of TBS–10 mM CaCl2. Protease inhibitors (all purchased from Sigma) were added to the extract at the final concentrations given in parentheses: E64 (10 μM), pepstatin A (5 μM), and PMSF (1 mM). The extract was then stored at −80°C until use. Two different surface extracts were prepared from adult worms, the first was from parasites that were perfused from mice and washed thoroughly in RPMI-PSF for 5 min before surface biotinylation (bio-ad-TX-0), and the second was from parasites that had been incubated in RPMI-PSF for 24 h as outlined above (bio-ad-TX-24). Efficacy of the biotinylation process was monitored by precipitating biotinylated proteins from extracts with streptavidin-agarose (8) and by probing bound and unbound proteins with streptavidin-peroxidase or various antisera as follows. Bound and unbound proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose paper (NCP), and the membrane was blocked overnight in 5% skimmed milk in PBS at 4°C. Blots were probed with either horseradish peroxidase (HRP)-conjugated streptavidin (1 μg/ml; Sigma) or a rabbit antiserum (1:2,000) prepared against S. japonicum recombinant Pmy (rSj-Pmy) (18) for 1 h at room temperature (RT). Pmy has been localized to the musculature underlying the tegument of S. mansoni adult worms and has been shown by others not to incorporate biotin during surface labeling (8). Furthermore, S. mansoni and S. japonicum Pmy are 96% identitical at the amino acid level, and an antiserum raised to rSj-Pmy in our laboratory has been shown to cross-react with S. mansoni Pmy (42). Streptavidin blots were then washed with PBS–0.1% Tween 20 and developed using an enhanced chemiluminescence substrate (Amersham). Immunoblots were washed then probed with goat anti-rabbit IgG-HRP (1:2,000; Bio-Rad) for 1 h at RT, washed, and developed using chemiluminescence. Additional monitoring of the biotinylation process was performed using antibodies raised to recombinant tegumental antigen Sj22.6 (41) and recombinant aspartic protease (localized to the gut) (2) of S. japonicum.
Coupling of ligands to Sepharose.
Five milligrams of human IgG Fc (Rockland Immunochemicals) was dialyzed overnight into coupling buffer (0.1 M NaHCO3, 0.5M NaCl, pH 8.3) and bound to 2 ml (packed volume) of cyanogen bromide-activated Sepharose 4B (Pharmacia) according to the manufacturer's instructions. Human β2m (500 μg; Sigma) was coupled to 500 μl of Sepharose as outlined above.
Purification of biotinylated Fc-Bp.
Bio-ad-TX (100 μl) or bio-som-TX (100 μl) was added to Fc-Sepharose (50-μl packed volume) in 200 μl of TBS–0.1% Triton X-100 (TBS-TX) and rotated on an orbital shaker for 2 h at RT. The reactions were centrifuged, and the supernatant was retained as unbound protein. Sepharose was washed three times with TBS-TX (1 ml per wash), and bound proteins were eluted by resuspending the Sepharose in 100 μl of reducing SDS-PAGE sample buffer and boiling. Alternatively, after washing, the following buffers were tested for the ability to elute bound proteins from Fc-Sepharose under native conditions: NaCl (0.6, 1.0, and 2.0 M), 0.1 M glycine (pH 2.5), and TBS–10 mM EDTA. Supernatants and eluted proteins were electrophoresed on 10% polyacrylamide gels and transferred to NCP, nonreactive sites were blocked, and the membranes were probed with HRP-streptavidin as described earlier. We attempted to competitively inhibit binding of Pmy to Fc-Sepharose by pre-incubating biotinylated extracts with either rabbit antiserum to rSj-Pmy (2.5 μl) or soluble protein A (100 μg) for 1 h at RT before adding the extract to Fc-sepharose.
Purification of biotinylated β2-m-binding proteins.
Bio-som-TX (100 μl) was added to β2m-Sepharose (50-μl settled bed volume) in 200 μl of TBS-TX, and bound proteins were identified as outlined above for Fc-Bp. Bound proteins were eluted by boiling in reducing SDS-PAGE loading dye.
Immunoprecipitations.
To observe whether bio-ad-TX contained complexes of Pmy and host IgG, 50 μl (packed volume) of protein G-Sepharose (Sigma) was added to 100 μl of bio-ad-TX-0 and rotated at RT for 1 h. The sepharose was then centrifuged and washed three times with TBS-TX, and bound proteins were eluted by boiling in SDS-PAGE reducing sample buffer. Proteins were electrophoresed, transferred to NCP, and probed with anti-rSj-Pmy serum; this and all subsequent steps were performed as outlined above.
Preparation of Fab fragments.
Rabbit immunoglobulins were purified from either 10 μl of anti-Pmy serum or 10 μl of normal rabbit serum by using protein G-Sepharose. Bound immunoglobulins were eluted in 0.1 M glycine (pH 2.8), and 0.1 M Tris was added until the solution reached pH 8.0. Immunoglobulins were buffer exchanged into 0.1 M sodium acetate (pH 5.5) in Amicon Centricon 10 concentrators (Millipore), and Fab was cleaved from Fc domains by using papain according to published protocols (13). Cleaved Fc regions and entire, uncleaved immunoglobulins were removed using protein G-Sepharose. The remaining, unbound Fab regions were tested for recognition of Pmy by dot blotting.
Microscopy.
S. mansoni mechanically transformed schistosomula were fixed in 100% methanol and stored at −20°C. Worms were transferred to PBS at RT and processed for fluorescence microscopy as follows. All fluorescent media were processed in PBS. Schistosomula were incubated in fluorescein isothiocyanate (FITC)-conjugated Fc (17 μg/ml) for 24 h at 4°C and rinsed in multiple changes of PBS. Other schistosomula were incubated concurrently in the following media for 24 h: (i) unconjugated Fc fragment (17 μg/ml, Rockland), (ii) Cy3-conjugated rabbit anti-human Fc (17 μg/ml, Sigma), (iii) anti-Pmy serum diluted 1:200, (iv) normal rabbit serum diluted 1:200, (v) anti-Pmy Fab fragment, and (vi) normal rabbit Fab. After several rinses in PBS, all samples except sample (ii) were incubated in human Fc-FITC (17 μg/ml) for 24 h at 4°C. In parallel experiments, schistosomula were incubated in a 1:200 dilution of anti-Pmy serum for 24 h at 4°C, followed by incubation in Cy3-conjugated anti-rabbit IgG serum (Jackson Laboratories) diluted 1:300 in PBS. All samples for fluorescence were mounted in DABCO and examined using an Olympus BX60 fluorescence microscope.
RESULTS AND DISCUSSION
Surface biotinylation of S. mansoni.
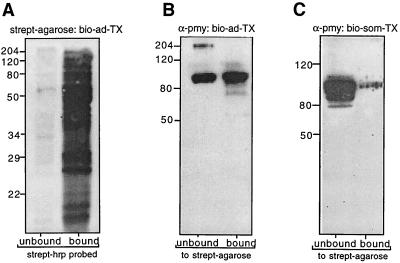
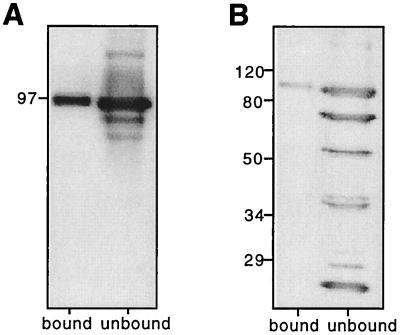
Live S. mansoni adults, determined to be undamaged by microscopic analysis, were subjected to surface biotinylation as previously described (8). In their pioneering studies, Davies and Pearce (8) used Pmy as a marker for determining the efficacy of surface labeling. They probed biotinylated and nonbiotinylated proteins from detergent extracts of adult S. mansoni (separated by precipitating with streptavidin-agarose) with antiserum to Pmy and noted that the protein was found only in the nonbiotinylated extract. They concluded that their labeling was specific for exposed surface proteins only. In a similar experiment probing labeled and nonlabeled detergent extracts (Fig. 1A) with anti-rSj-Pmy serum, we found Pmy to be present in both surface and underlying tissue extracts of bio-ad-TX-24 (Fig. 1B). The exact localization of Pmy in adult schistosomes remains a matter of debate (11, 26, 28). When probing the nonbiotinylated proteins (i.e., those not bound by streptavidin-agarose) from bio-ad-TX-24, we obtained reactivity with proteins with molecular sizes of 97 kDa (Pmy) and approximately 200 kDa (Fig. 1B). The 200-kDa antigen is most likely to be myosin, a related muscle protein with sequence similarity to Pmy (4) but which has not been found on the surface of adult worms (45). We concur with this finding, as our Western blots of biotinylated and underlying, nonbiotinylated extracts using anti-rSj-pmy serum showed a band of about 200 kDa (presumed to be myosin) only in the unlabeled fraction of bio-ad-TX-24 (i.e., subtegumental tissues), while Pmy was present in both labeled and unlabeled detergent extracts. Pmy is found in the tegument of schistosomula, as well as in the musculature and postacetabular glands (11, 31). Antiserum to schistosome cathepsin D, an aspartic protease found in the gut of schistosomes (2), did not bind to surface-labeled proteins but did bind to underlying, nonlabeled proteins (not shown), further accentuating the specificity of the surface biotinylation procedure.
FIG. 1.
(A) Comparison of biotinylated and nonbiotinylated surface proteins of adult S. mansoni solubilized with Triton X-100 and separated using streptavidin (strept)-agarose. Proteins were then probed with peroxidase-labeled streptavidin (A). The left lane (unbound) contains proteins that were not precipitated with streptavidin-agarose, while the right lane (bound) contains biotinylated surface proteins that were precipitated. (B) Western blot of the extracts in panel A probed with antiserum raised to rSj-Pmy (α-pmy), showing that Pmy is exposed to labeling procedures on the surface of live parasites. (C) Western blot of biotinylated and nonbiotinylated surface proteins of S. mansoni schistosomula precipitated with streptavidin-agarose and probed with α-pmy serum. Here and in succeeding figures, positions of size markers are shown in kilodaltons at the left.
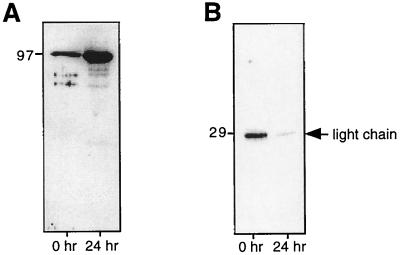
Adult schistosomes that had been cultured at 37°C overnight were still motile after 12 h, but their teguments were sloughing and had a patchy, blistered appearance (not shown). To test whether Pmy was expressed only on the surface due to damage of the tegument induced by overnight in vitro incubation (which allowed worms to shed host IgG before surface labeling), we performed the same experiments with worms that had been labeled with biotin within 1 h of removal from the host, i.e., bio-ad-TX-0 extract. Biotinylated bio-ad-TX-0 proteins were separated from nonbiotinylated, detergent-soluble proteins by the affinity of labeled proteins for streptavidin-agarose and then probed with anti-rSj-Pmy serum. Pmy was detected in the biotinylated extract (Fig. 2A), indicating that it is exposed on the tegumental surface of freshly perfused parasites as well as those cultured in vitro after removal from the host. Mouse IgG was detected in bio-ad-TX-0 and −24 but at a much lower level in the latter, indicating that host IgG is shed from the surface during overnight culturing of the parasites (Fig. 2B), a finding supported by others using microscopic analysis (44).
FIG. 2.
Biotinylated and nonbiotinylated surface proteins of adult S. mansoni were solubilized with Triton X-100 shortly after removal from the host (0 h) or after 24 h culture of in vitro after removal from the host (24 h). Surface-labeled proteins were precipitated using streptavidin-agarose and probed with antiserum to rSj-Pmy (A) or to murine IgG (B). The position of the light chain of IgG is shown at the right.
S. mansoni and S. japonicum Pmy binds to Fc.
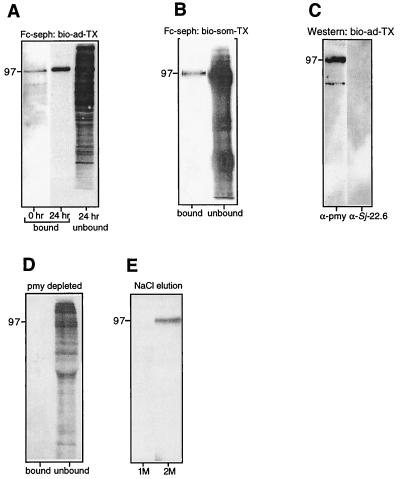
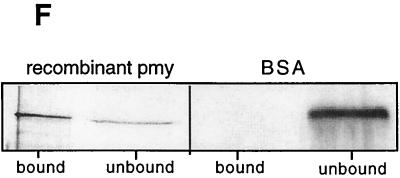
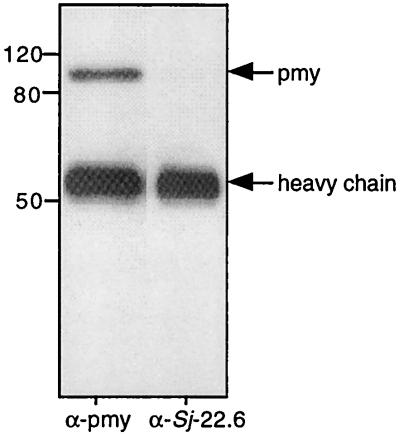
When bio-ad-TX-0, bio-ad-TX-24, and bio-som-TX were incubated with Fc-Sepharose, a single, biotinylated band remained bound after washing (Fig. 3A and B). The band corresponding to the Fc-Bp was less intense in bio-ad-TX-0 than in bio-ad-TX-24, indicating that more of the protein was present in the tegument after overnight incubation of parasites in vitro or that the majority of host IgG had been shed by 24 h, allowing a greater quantity of the Fc-Bp to bind to the resin. The Fc-Bp had the same molecular size as S. mansoni Pmy (97 kDa), and probing of the bound protein with anti-rSj-pmy serum revealed its identity as Pmy (Fig. 3C). In addition, when Pmy was removed from bio-ad-TX by immunoprecipitation with anti-rSj-Pmy serum, no biotinylated proteins bound to Fc-Sepharose (Fig. 3D). Pmy from keyhole limpet (Mollusca) also bound to human Fc (not shown), indicating that the binding process is not restricted to schistosome Pmy. Pmy from molluscs, cestodes, and trematodes has been shown to bind to collagen (22, 25, 34) and to the collagen-like region of the complement protein C1q (25). Pmy can be eluted from collagen using 0.6 M NaCl (22), and so we attempted to elute Pmy from Fc-Sepharose in a similar manner. Partial elution of bound Pmy was achieved with 2.0 M but not with 1.0 M NaCl (Fig. 3E). Recombinant S. mansoni Pmy expressed in bacteria (kindly provided by Chris King, Case Western Reserve University, Cleveland, Ohio) also bound to Fc-Sepharose (Fig. 3F), indicating that Pmy binds directly to Fc and not indirectly via a complex of host and parasite proteins.
FIG. 3.
(A) Detection of a 97-kDa biotinylated surface protein of adult S. mansoni that bound to human Fc-Sepharose, (seph). Extracts were obtained from parasites that were labeled after removal from the host (0 h) or after 24 h of culture in vitro (24 h). The blot was probed with peroxidase-labeled streptavidin. (B) Binding of a 97-kDa biotinylated protein from schistosomula to Fc. (C) Demonstration that the bound protein from panel A (24 h) is recognized by antiserum to rSj-Pmy (α-pmy) but not by antiserum to another tegument protein, Sj22.6 (α-Sj 22.6). Depletion of Pmy from biotinylated surface extracts by immunoprecipitation using anti-Pmy serum and protein G-Sepharose prior to incubation of the extract with Fc-Sepharose resulted in an absence of any Fc-BP detected using streptavidin-peroxidase (D). Pmy eluted from Fc-Sepharose in the presence of 2 M but not 1 M NaCl, as detected using streptavidin peroxidase (E). (F) Binding of recombinant Pmy from S. mansoni (kindly provided by C. King) but not of bovine serum albumin (BSA) control to Fc-Sepharose.
Preincubation of bio-ad-TX-24 with anti-rSj-Pmy serum before addition of the extract to Fc-Sepharose partially ablated binding of Pmy to Fc, although bound Pmy was still detectable (not shown). Pmy is a large molecule that adopts an alpha-helical coiled-coil structure (16). The B-cell epitope of a protective IgE anti-Sj-Pmy monoclonal antibody has been mapped (32), but the epitopes recognized by our polyclonal anti-rSj-Pmy serum have yet to be determined. The antiserum we used clearly binds to and depletes bio-ad-TX of Pmy (Fig. 3D), but it does not appear to block all of the sites on Pmy that bind to Fc.
S. japonicum had not previously been shown to adsorb host ligands. We therefore incubated soluble protein extracts from adult S. japonicum with Fc-Sepharose and showed that Pmy from this parasite also binds to IgG in a similar manner (Fig. 4).
FIG. 4.
S. japonicum soluble extracts were incubated with Fc-Sepharose, and bound and unbound proteins were probed with antiserum to rSj-Pmy (A) or antiserum to soluble adult worm protein (B).
Neither the regions of interaction between Pmy and Fc nor the mechanisms by which they bind are understood, but the hinge region of human Fc is somewhat promiscuous, interacting with at least four different natural proteins as well as selected small peptides that bind at a common site (10). Cocrystallization of Fc and a 13-amino-acid peptide showed that this common binding site is highly accessible, adaptive, and hydrophobic, permitting binding of small peptide ligands (10) and large proteins such as rheumatoid factor (5) and protein A (9). We found that preincubation of Fc-Sepharose with an excess of soluble protein A did not affect binding of Pmy to Fc (not shown), suggesting that Pmy binds to a different region(s) on the Fc molecule.
To observe whether Pmy-murine IgG complexes were present on the surface of parasites when removed from the host, mouse IgG was precipitated from bio-ad-TX-0 by using protein G-sepharose, and bound proteins were probed with antisera to Pmy. Pmy was detected in the precipitated complex (Fig. 5), indicating that IgG-Pmy complexes were already present in the extract and binding of the two proteins was not an in vitro artifact. Probing of the precipitated complex with anti-rSj-22.6 serum did not reveal any bands (Fig. 5). The ability of protein G to coprecipitate IgG and Pmy provides further evidence that Pmy binds to a unique site in the Fc molecule, as opposed to the consensus site between the CH2 and CH3 domains.
FIG. 5.
Adsorbed murine immunoglobulins and associated complexes were precipitated from surface extracts of adult S. mansoni by using protein G-Sepharose, and bound proteins were probed by Western blotting with antiserum to recombinant Pmy (α-pmy) or recombinant Sj22.6 (α-Sj22.6) as a control surface protein. Reactivity of the murine immunoglobulin heavy chain with the anti-rabbit IgG secondary antibody is highlighted.
The elution of Pmy from Fc-Sepharose by 2.0 M NaCl indicates that IgG and Pmy bind due to noncovalent, ionic charge interactions. Pmy forms an alpha-helical coiled coil, and analysis of the Caenorhabditis elegans Pmy, encoded by unc-15, shows regular zones of positive and negative charge on the outer surface of the helix (16). These highly charged zones are predicted to be the areas of intermolecular interactions between Pmy molecules and between Pmy and other proteins involved in the packing of thick muscle filaments such as myosin. Such charged zones might also account for the ability of Pmy to bind to ligands such as Fc, collagen, and C1.
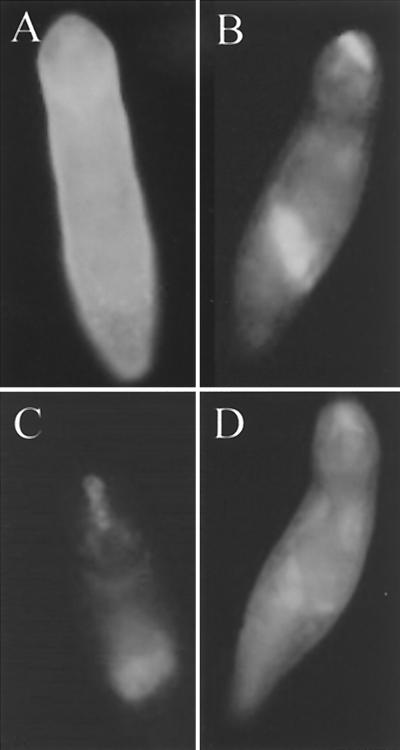
Torpier and colleagues showed the presence of Fc receptors on the surface of mechanically transformed schistosomula using an erythrocyte-antierythrocyte complex (40). Probing of fixed schistosomula with Fc-FITC also showed binding to the parasite surface, but the strongest fluorescence was localized to internal structures (presumed to be the vestigial penetration glands and the nascent oral cavity) (Fig. 6B). Antiserum to Pmy also bound to the surface of parasites but did not bind to internal structures (Fig. 6A), suggesting that there might be more than one Fc-Bp. To confirm these observations, we probed schistosomula with purified Fab domains of both anti-Pmy and normal rabbit serum and then probed parasites with Fc-FITC. Preincubation of parasites with anti-Pmy Fab ablated binding of Fc-FITC to the surface whereas normal rabbit Fab did not (Fig. 6C and D). Furthermore, attempts to block Fc binding with anti-Pmy Fab did not ablate binding to internal glandular material yet did inhibit binding to the surface, again suggesting the presence of distinct, internal Fc-binding proteins that are unrelated to Pmy.
FIG. 6.
Fluorescence micrograph showing binding of rabbit anti-Pmy serum to the surface of an S. mansoni schistosomulum (A). FITC-conjugated human Fc bound to the surface, oral cavity, and vestigial penetration glands of schistosomula (B), while preincubation of parasites with anti-Pmy Fab fragments ablated subsequent binding of Fc-FITC to the surface (C); normal rabbit Fab did not interfere with subsequent binding of Fc-FITC (D).
Antigen B is secreted by a range of cestodes, and it shares high sequence identity with Pmy from schistosomes (23). Antigen B is secreted from the tegumental membrane of Taenia solium metacestodes (24), and a homologous protein from Taenia crassiceps binds to murine Fcγ (17). Though not surprising, this is nevertheless the first definitive proof that Pmy is a receptor for Fc in schistosomes. Furthermore, a smaller, antigen B-like coiled-coil protein from the tapeworm Moniezia expansa binds to lipids via a hydrophobic tryptophan residue (1). We do not know whether schistosome Pmy can bind to lipids, but it might be an additional role for this obviously multifunctional protein.
β2m does not bind to Pmy but does bind to other S. mansoni surface proteins.
To control for nonspecific binding of Pmy to indiscriminate ligands or to Sepharose, we conjugated human β2m to cyanogen bromide-activated Sepharose and repeated the binding assays described for Fc-Sepharose using bio-som-TX. Previously Torpier et al. (40) reported that β2m binds to the surface of S. mansoni mechanically transformed schistosomula and inhibits Fc receptor-mediated rosette formation, prompting these authors to suggest that both Fc and β2m bound to the same receptor on the surface of the parasite. Furthermore, β2m associates with the intestinal Fc receptor in neonatal rats (37). We incubated bio-som-TX with β2m-Sepharose and examined the biotinylated, bound proteins. Pmy did not bind to β2m-Sepharose, but four bands with molecular sizes of approximately 38, 42, 110, and 200 kDa were eluted (Fig. 7). Bound proteins were probed with anti-rSj-pmy but were not recognized by the antibody (not shown), indicating that β2m binds to a receptor other than Pmy on the parasite tegument and that this receptor appears to be a heteromultimer. The nature of this receptor awaits elucidation.
FIG. 7.
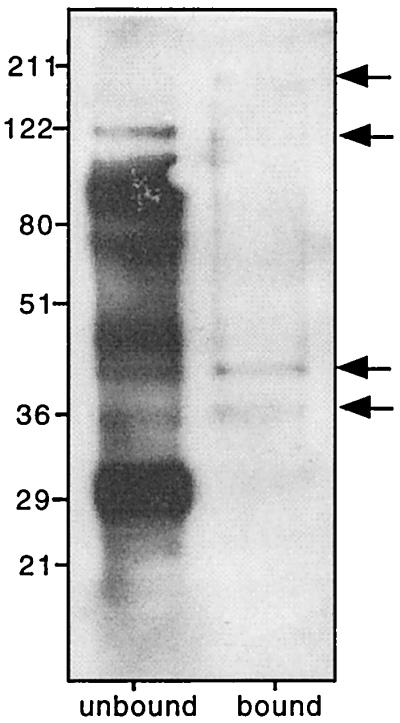
Biotinylated β2m-binding proteins from the surface of S. mansoni schistosomula. Bound proteins and unbound proteins were detected using HRP-streptavidin. Bound proteins are highlighted by arrows.
Concluding remarks.
It has long been known that schistosomes adsorb host Fc onto their tegument, but the molecular mechanisms by which they achieve this process had not been elucidated. Here we have shown that Pmy, a vaccine candidate antigen that elicits protection against schistosomiasis in laboratory and domestic animals (27, 30, 33), is an Fc-Bp. Furthermore, we have shown that host β2m does not bind to Pmy but does bind to other proteins on the surface of schistosomula. If schistosomes mask themselves from immune recognition by adsorbing host proteins such as immunoglobulins, antibodies directed at the surface receptors for these host proteins might interfere with adsorption and thus masking. Specific antiserum to Pmy interfered with binding of Fc to Pmy in vitro and to the tegument of live parasites, offering a plausible explanation for the mechanism of protection against S. mansoni infection induced by anti-Pmy immunization of mice (33) and against S. japonicum in natural reservoir hosts such as sheep (39) and buffalo (29). Protective immunization with Pmy resulting in decreased worm burdens in the S. mansoni murine model appears to be primarily cell mediated and is not transferred in serum (33). However, transfer of an IgE monoclonal antibody against Pmy conferred partial protection in mice challenged with S. japonicum (21), suggesting that specific antibodies might have blocked adsorption of nonspecific Fc domains and thus at least partially interfered with masking.
Pmy is an intriguing schistosome molecule that has received considerable attention mainly due to its efficacy as a protective vaccine candidate. However, investigations by others and that reported here suggest multiple functions for this protein including an involvement in immune evasion. This is unexpected given that myosins and related proteins including Pmy were thought to be primarily structural proteins located in muscle layers. By binding to host proteins such as Fc and C1q, Pmy can potentially interfere with immune processes such as engagement of Fc receptors on cells and complement activation. Interactions between Pmy and the immune and other physiological systems need to be explored given that schistosome Pmy is considered a prime candidate antigen for a vaccine against schistosomiasis (29).
ACKNOWLEDGMENTS
This work was funded by the National Health and Medical Research Council of Australia. A.L. is a Howard Florey Centenary Research Fellow.
We are grateful to Chris King, Case Western Reserve University, Cleveland, Ohio, for provision of recombinant S. mansoni Pmy and to Mary Duke for maintenance of the schistosome life cycles. Additional thanks go to Michael Smout, Christiana Verity, Danielle Smyth, and Yeusheng Li for technical assistance, provision of reagents, and helpful discussions.
REFERENCES
- 1.Barrett J, Saghir N, Timanova A, Clarke K, Brophy P M. Characterisation and properties of an intracellular lipid-binding protein from the tapeworm Moniezia expansa. Eur J Biochem. 1997;250:269–275. doi: 10.1111/j.1432-1033.1997.0269a.x. [DOI] [PubMed] [Google Scholar]
- 2.Brindley P J, Kalinna B H, Wong J Y, Bogitsh B J, King L T, Smyth D J, Verity C K, Abbenante G, Brinkworth R I, Fairlie D P, Smythe M L, Milburn P J, Bielefeldt-Ohmann H, Zheng Y, McManus D P. Proteolysis of human hemoglobin by schistosome cathepsin D. Mol Biochem Parasitol. 2001;112:103–112. doi: 10.1016/s0166-6851(00)00351-0. [DOI] [PubMed] [Google Scholar]
- 3.Clegg J A, Smithers S R, Terry R J. Acquisition of human antigens by Schistosoma mansoni during cultivation in vitro. Nature. 1971;232:653–654. doi: 10.1038/232653a0. [DOI] [PubMed] [Google Scholar]
- 4.Cohen C, Lanar D E, Parry D A. Amino acid sequence and structural repeats in schistosome paramyosin match those of myosin. Biosci Rep. 1987;7:11–16. doi: 10.1007/BF01122722. [DOI] [PubMed] [Google Scholar]
- 5.Corper A L, Sohi M K, Bonagura V R, Steinitz M, Jefferis R, Feinstein A, Beale D, Taussig M J, Sutton B J. Structure of human IgM rheumatoid factor Fab bound to its autoantigen IgG Fc reveals a novel topology of antibody-antigen interaction. Nat Struct Biol. 1997;4:374–381. doi: 10.1038/nsb0597-374. [DOI] [PubMed] [Google Scholar]
- 6.Dalton J P, Strand M, Mangold B L, Dean D A. Identification of Schistosoma mansoni glycoproteins recognized by protective antibodies from mice immunized with irradiated cercariae. J Immunol. 1986;136:4689–4694. [PubMed] [Google Scholar]
- 7.Damian R T, Greene N D, Hubbard W J. Occurrence of mouse alpha-2-macroglobulin antigenic determinants on Schistosoma mansoni adults, with evidence on their nature. J Parasitol. 1973;59:64–73. [PubMed] [Google Scholar]
- 8.Davies S J, Pearce E J. Surface-associated serine-threonine kinase in Schistosoma mansoni. Mol Biochem Parasitol. 1995;70:33–44. doi: 10.1016/0166-6851(95)00002-i. [DOI] [PubMed] [Google Scholar]
- 9.Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-Å resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- 10.DeLano W L, Ultsch M H, de Vos A M, Wells J A. Convergent solutions to binding at a protein-protein interface. Science. 2000;287:1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]
- 11.Gobert G N, Stenzel D J, Jones M K, Allen D E, McManus D P. Schistosoma japonicum: immunolocalization of paramyosin during development. Parasitology. 1997;114:45–52. doi: 10.1017/s0031182096008001. [DOI] [PubMed] [Google Scholar]
- 12.Goldring O L, Clegg J A, Smithers S R, Terry R J. Acquisition of human blood group antigens by Schistosoma mansoni. Clin Exp Immunol. 1976;26:181–187. [PMC free article] [PubMed] [Google Scholar]
- 13.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 14.Horta M F, Ramalho-Pinto F J, Fatima M. Role of human decay-accelerating factor in the evasion of Schistosoma mansoni from the complement-mediated killing in vitro. J Exp Med. 1991;174:1399–1406. doi: 10.1084/jem.174.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston D A, Blaxter M L, Degrave W M, Foster J, Ivens A C, Melville S E. Genomics and the biology of parasites. Bioessays. 1999;21:131–147. doi: 10.1002/(SICI)1521-1878(199902)21:2<131::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Kagawa H, Gengyo K, McLachlan A D, Brenner S, Karn J. Paramyosin gene (unc-15) of Caenorhabditis elegans. Molecular cloning, nucleotide sequence and models for thick filament structure. J Mol Biol. 1989;207:311–333. doi: 10.1016/0022-2836(89)90257-x. [DOI] [PubMed] [Google Scholar]
- 17.Kalinna B, McManus D P. An IgG (Fc gamma)-binding protein of Taenia crassiceps (Cestoda) exhibits sequence homology and antigenic similarity with schistosome paramyosin. Parasitology. 1993;106:289–296. doi: 10.1017/s0031182000075119. [DOI] [PubMed] [Google Scholar]
- 18.Kalinna B H, Becker M M, McManus D P. Engineering and expression of a full length cDNA encoding Schistosoma japonicum paramyosin. Purification of the recombinant protein and its recognition by infected patient sera. Acta Trop. 1997;65:111–115. doi: 10.1016/s0001-706x(97)00657-8. [DOI] [PubMed] [Google Scholar]
- 19.Kemp W M, Damian R T, Greene N D, Lushbaugh W B. Immunocytochemical localization of mouse alpha 2-macroglobulin like antigenic determinants on Schistosoma mansoni adults. J Parasitol. 1976;62:413–419. [PubMed] [Google Scholar]
- 20.Kemp W M, Merritt S C, Bogucki M S, Rosier J G, Seed J R. Evidence for adsorption of heterospecific host immunoglobulin on the tegument of Schistosoma mansoni. J Immunol. 1977;119:1849–1854. [PubMed] [Google Scholar]
- 21.Kojima S, Niimura M, Kanazawa T. Production and properties of a mouse monoclonal IgE antibody to Schistosoma japonicum. J Immunol. 1987;139:2044–2049. [PubMed] [Google Scholar]
- 22.Laclette J P, Alagon A, Willms K, Torre-Blanco A. Purification of antigen B from Taenia solium cysticerci by affinity to mammalian collagen. J Parasitol. 1990;76:273–275. [PubMed] [Google Scholar]
- 23.Laclette J P, Landa A, Arcos L, Willms K, Davis A E, Shoemaker C B. Paramyosin is the Schistosoma mansoni (Trematoda) homologue of antigen B from Taenia solium (Cestoda) Mol Biochem Parasitol. 1991;44:287–295. doi: 10.1016/0166-6851(91)90015-x. [DOI] [PubMed] [Google Scholar]
- 24.Laclette J P, Merchant M T, Willms K. Histological and ultrastructural localization of antigen B in the metacestode of Taenia solium. J Parasitol. 1987;73:121–129. [PubMed] [Google Scholar]
- 25.Laclette J P, Shoemaker C B, Richter D, Arcos L, Pante N, Cohen C, Bing D, Nicholson-Weller A. Paramyosin inhibits complement C1. J Immunol. 1992;148:124–128. [PubMed] [Google Scholar]
- 26.Laclette J P, Skelly P J, Merchant M T, Shoemaker C B. Aldehyde fixation dramatically alters the immunolocalization pattern of paramyosin in platyhelminth parasites. Exp Parasitol. 1995;81:140–143. doi: 10.1006/expr.1995.1102. [DOI] [PubMed] [Google Scholar]
- 27.Lanar D E, Pearce E J, James S L, Sher A. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science. 1986;234:593–596. doi: 10.1126/science.3094144. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto Y, Perry G, Levine R J, Blanton R, Mahmoud A A, Aikawa M. Paramyosin and actin in schistosomal teguments. Nature. 1988;333:76–78. doi: 10.1038/333076a0. [DOI] [PubMed] [Google Scholar]
- 29.McManus D P. The search for a vaccine against schistosomiasis—a difficult path but an achievable goal. Immunol Rev. 1999;171:149–161. doi: 10.1111/j.1600-065x.1999.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 30.McManus D P, Liu S, Song G, Xu Y, Wong J M. The vaccine efficacy of native paramyosin (Sj-97) against Chinese Schistosoma japonicum. Int J Parasitol. 1998;28:1739–1742. doi: 10.1016/s0020-7519(98)00151-9. [DOI] [PubMed] [Google Scholar]
- 31.Nara T, Matsumoto N, Janecharut T, Matsuda H, Yamamoto K, Irimura T, Nakamura K, Aikawa M, Oswald I, Sher A, et al. Demonstration of the target molecule of a protective IgE antibody in secretory glands of Schistosoma japonicum larvae. Int Immunol. 1994;6:963–971. doi: 10.1093/intimm/6.7.963. [DOI] [PubMed] [Google Scholar]
- 32.Nara T, Tanabe K, Mahakunkijcharoen Y, Osada Y, Matsumoto N, Kita K, Kojima S. The B cell epitope of paramyosin recognized by a protective monoclonal IgE antibody to Schistosoma japonicum. Vaccine. 1997;15:79–84. doi: 10.1016/s0264-410x(96)00100-4. [DOI] [PubMed] [Google Scholar]
- 33.Pearce E J, James S L, Hieny S, Lanar D E, Sher A. Induction of protective immunity against Schistosoma mansoni by vaccination with schistosome paramyosin (Sm97), a nonsurface parasite antigen. Proc Natl Acad Sci USA. 1988;85:5678–5682. doi: 10.1073/pnas.85.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plancarte A, Flisser A, Larralde C. Fibronectin-like properties in antigen B from the cysticercus of Taenia solium. Cytobios. 1983;36:83–93. [PubMed] [Google Scholar]
- 35.Santoro F, Ouaissi M A, Pestel J, Capron A. Interaction between Schistosoma mansoni and the complement system: binding of C1q to schistosomula. J Immunol. 1980;124:2886–2891. [PubMed] [Google Scholar]
- 36.Sher A, Hall B F, Vadas M A. Acquisition of murine major histocompatibility complex gene products by schistosomula of Schistosoma mansoni. J Exp Med. 1978;148:46–57. doi: 10.1084/jem.148.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simister N E, Mostov K E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 38.Tarleton R L, Kemp W M. Demonstration of IgG-Fc and C3 receptors on adult Schistosoma mansoni. J Immunol. 1981;126:379–384. [PubMed] [Google Scholar]
- 39.Taylor M G, Huggins M C, Shi F, Lin J, Tian E, Ye P, Shen W, Qian C G, Lin B F, Bickle Q D. Production and testing of Schistosoma japonicum candidate vaccine antigens in the natural ovine host. Vaccine. 1998;16:1290–1298. doi: 10.1016/s0264-410x(98)00055-3. [DOI] [PubMed] [Google Scholar]
- 40.Torpier G, Capron A, Ouaissi M A. Receptor for IgG(Fc) and human β2-microglobulin on S. mansoni schistosomula. Nature. 1979;278:447–449. doi: 10.1038/278447a0. [DOI] [PubMed] [Google Scholar]
- 41.Waine G J, Becker M M, Scott J C, Kalinna B H, Yang W, McManus D P. Purification of a recombinant Schistosoma japonicum antigen homologous to the 22-kDa membrane-associated antigen of S. mansoni, a putative vaccine candidate against schistosomiasis. Gene. 1994;142:259–263. doi: 10.1016/0378-1119(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, Jackson D C, Zeng Q, McManus D P. Multiepitope schistosome vaccine candidates tested for protective immunogenicity in mice. Vaccine. 2001;19:103–113. doi: 10.1016/s0264-410x(00)00165-1. [DOI] [PubMed] [Google Scholar]
- 43.Yang W, Waine G J, Sculley D G, Liu X, McManus D P. Cloning and partial nucleotide sequence of Schistosoma japonicum paramyosin: a potential vaccine candidate against schistosomiasis. Int J Parasitol. 1992;22:1187–1191. doi: 10.1016/0020-7519(92)90041-i. [DOI] [PubMed] [Google Scholar]
- 44.Yong W K, Das P K. Acquisition of host proteins by the tegument of Schistosoma mansoni recovered from rats. Z Parasitenkd. 1983;69:53–60. doi: 10.1007/BF00934010. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Taylor M G, Bickle Q D. Schistosoma japonicum myosin: cloning, expression and vaccination studies with the homologue of the S. mansoni myosin fragment IrV-5. Parasite Immunol. 1998;20:583–594. doi: 10.1046/j.1365-3024.1998.00189.x. [DOI] [PubMed] [Google Scholar]