Abstract
The main purpose of this study was to study the changes in growth, root system, and tissue anatomical structure of Pinus sylvestris var. mongolica under soil drought conditions. In this study, the growth indexes and photosynthesis of P. sylvestris var. mongolica seedlings under soil drought stress were studied by pot cultivation. Continuous pot water control experiment of the indoor culture of P. sylvestris var. mongolica was carried out, ensuring that the soil water content of each treatment reached 80%, 40%, and 20% of the field moisture capacity as control, moderate drought and severe drought, respectively. The submicroscopic structures of the needles and roots were observed using a scanning electron microscope and a transmission electron microscope. The response of soil roots to drought stress was studied by root scanning. Moderate drought stress increased needle stomatal density, while under severe drought stress, stomatal density decreased. At the same time, the total number of root tips, total root length, root surface area, and root volume of seedlings decreased with the deepening of the drought. Furthermore, moderate drought and severe drought stress significantly reduced the chlorophyll a and chlorophyll b content in P. sylvestris var. mongolica seedlings compared to the control group. The needle cells were deformed and damaged, and chloroplasts and mitochondria were damaged, gradually disintegrated, and the number of osmiophiles increased. There was also an increase in nuclear vacuolation.
Keywords: Physiological response, Drought tolerance, Morphological structure, Growing status
Introduction
Drought is a major environmental factor that restricts plant growth and development and even affects the distribution pattern of the world’s forests (Sherwood et al., 2013). There will be more severe and frequent droughts in many parts of the world as a result of climate change (IPCC, 2013; Trenberth et al., 2014; Okunlola et al., 2017). Drought is a climate disaster that occurs under most climatic conditions and can have considerable economic, social, and environmental impacts. In recent decades, drought caused by rapid warming has deeply affected the global forest ecosystem (Allen, Macalady & Chenchouni, 2010; Vurukonda et al., 2016; Nolan et al., 2018; Anderegg William et al., 2019).
Pinus sylvestris var. mongolica is an important geographical variety of P. sylvestris in eastern Asia. It has the biological characteristics of strong resistance to stress, rapid growth, and rapid lumber. Therefore, due to these properties, it has become one of the main afforestation tree species for vegetation restoration in mountainous, grassland and sandy land in arid and semi-arid areas of northern China. This plant species plays an important role in ecological construction and environmental restoration, such as soil and water conservation, wind prevention, and sand fixation (Zhu et al., 2003; Song et al., 2018; Guo et al., 2019; Kong et al., 2019). However, since the 1990s, the artificial sand fixing pure forest of P. sylvestris var. mongolica has shown a recession phenomenon. The decline was manifested mainly in withered and yellow branches, reduced growth, the occurrence of diseases and pests, leading to the death of the whole plant and could not be natural regeneration (Meng et al., 2010; Song, Zhu & Zheng, 2017). Thus, considering the recession mechanism of P. sylvestris var. mongolica, the current study focuses mainly on water factors (Song, Zhu & Kang, 2013), and it was found that soil water and drought stress are the main factors limiting its development and growth in sandy soil (Song, Zhu & Yan, 2015).
Drought stress has many effects on plant growth and metabolism. Initially, drought stress directly affects the germination of plant seeds and reduces the survival rate of seedlings (Shi, Ding & Yuan, 2004; Wei, 2005). Secondly, drought stress directly leads to a water deficit in plant cells, making them unable to divide and normally increase, thus inhibiting plant growth and development (Long & Deng, 2019). The study found that severe drought caused obvious drought damage symptoms to forest trees in northern Finland (Muukkonen et al., 2015). Drought also restricted the growth and development of Scots pine trees (Zang, Pretzsch & Rothe, 2012) and seriously reduced the radial growth of Picea crassifolia, Pinus tabuliformis, Larix decidua Mill. and Picea meyeri (Vitasse et al., 2019; Gao, Yang & Qin, 2021; Xue et al., 2022a; Xue et al., 2022b). At the same time, previous studies have shown that similar larch species (such as Larix principis-rupprechtii Mayr) are at high risk of growth stagnation in large central and northern China areas during extreme drought (Zhang et al., 2021).
The root structure determines the efficiency of water absorption and transport of plants, which can help alleviate the damage caused by drought stress. Under drought stress, the root tip first perceives the signal and transmits it to the aboveground part (Jia & Zhang, 2008; Yin, Wang & Qi, 2021). After drought, phenotypic characteristics such as total surface area, total volume, average diameter, total length, and biomass of plant roots change (Zhang & Sun, 2016; Zhang et al., 2019). In addition, the morphology and number of mitochondria and other organelles in root cells will also change (Liu, Yue & Chen, 2010). Under mild drought, plants can improve their tolerance to drought by increasing the length of the main roots and the number of lateral roots and root hairs (Salazar-Henao, Vélez-Bermúdez & Schmidt, 2016). However, with the deepening of drought, plant root respiration is reduced, resulting in an insufficient supply of ATP (adenosine triphosphate) and a significant decrease in root activity that leads to slowing or even stops the growth (Kim, Chae & Choi, 2020; Nikolova et al., 2020). The water balance in plants also alters, causing irreversible damage to the plants (Ma et al., 2012; Isaji et al., 2018). Some studies found that under short-term drought stress, Robinia pseudoacacia absorbs more water by promoting the relative growth of fine roots. However, under long-term drought stress, the root growth of Robinia pseudoacacia is inhibited (Gao, Wang & Zhang, 2010). At the same time, moderate drought treatment will increase the root biomass of Platycladus orientalis and Pinus tabulaeformis while decreasing under severe drought stress (Chen & Zhao, 2011). And different degrees of water loss also affect the root water content and root activity of Larix principis-rupprechtii and Pinus tabuliformis (Chen, Gao & Shi, 2017). Similarly, changes in root microstructure can also reflect the adaptability of plants to drought stress. The structure and properties of the root cortical tissue, the diameter, and the number of xylem vessels changed according to the degree of drought stress (Konijnendijk & Randrup, 2002). Furthermore, some studies have also found that drought stress reduced the average root diameter and the diameter of the root vessel (Wang, Zhang & Liu, 2005; Lee et al., 2016; Wang et al., 2018). Previous studies showed that long-term drought stress reduced the root length and root volume of Pinus sylvestris var. mongolica, and summer drought would limit the establishment of the Scot pine (Pinus sylvestris L.) forest by reducing growth and increasing seedling mortality (Castro et al., 2005; Qian et al., 2021).
The leaf structure is the most intuitive embodiment of the adaptability of plants to arid habitats. It determines the functions of plant carbon assimilation, water loss, and retention and can also be used to evaluate biomass accumulation capacity (Marron, Dillen & Ceulemans, 2007). To adapt to drought, plant needles tend to increase mesophyll palisade tissue and the number of cell layers and reduce spongy tissue, cell volume, and cell space (Chartzoulakis et al., 2002; Burling et al., 2013; Scoffoni et al., 2014; Bhusal et al., 2020). In addition, the self-regulation ability of the stomata in the needles can also reflect the drought resistance of plants to some extent (Guo & Wu, 2015). Plants resistant to drought can regulate the stomata to a greater degree to reduce water loss (Fiorin, 2016). Under drought stress, the relative water content of plants decreases, and the stomatal aperture decreases or even closes to reduce the water loss from needles, which facilitates the recovery of leaf water potential (Casson & Hetherington, 2009; Wang et al., 2010). Similarly, the reduction of stomatal conductance or stomatal closure of plant needles in an arid environment affects the absorption of CO2 and reduces the photosynthetic rate (Reddy, Chaitanya & Vivekanandan, 2004; Pagter, Bragato & Brix, 2005). In the context of chlorophyll, the main pigment responsible for photosynthesis, drought stress can cause chlorophyll decomposition and decrease chlorophyll content. It has been shown that drought stress causes chlorophyll decomposition and chlorophyll content decreases, leading to changes in photosynthetic function (Zhao et al., 2006; Jafari, Hashemi Garmdareh & Azadegan, 2019).
To reveal the decline mechanism of P. sylvestris var. mongolica, explaining the internal mechanism and the water influence mechanism on the plantation are key factors and provide a theoretical basis for the management of P. sylvestris var. mongolica plantation (Song, Zhu & Zheng, 2017). In this study, the potted water control experiments of P. sylvestris var. mongolica seedlings were conducted, and the growth characteristics of P. sylvestris var. mongolica seedlings were clarified by measuring changes in growth indexes, photosynthetic pigment indexes, root indexes, and submicroscopic structure under different levels of drought stress. The physiological mechanism of drought resistance in P. sylvestris var. mongolica seedlings was briefly explained, which provided a theoretical basis and practical reference for further study of the physiological mechanism under the drought-induced decline of P. sylvestris var. mongolica plantation.
Material and Methods
Plant materials and experimental design
The seeds of P. sylvestris var. mongolica were collected from Zhanggutai Experimental Forest Farm, Fuxin City, Liaoning Province. The seeds used in the experiment were disinfected and sterilized with potassium permanganate (0.5%, v/v) for 30 min, followed by washing with distilled water three times. In addition, seeds were wrapped with sterile gauze for moisturizing and kept under 25 °C for germination, sprayed with sterile water every day until growth. Seeds were transferred to plastic pots filled with sterilized vermiculite: soil: sand mixture (1:2:1) and kept under the corresponding controlled greenhouse conditions (Yin, Wang & Qi, 2021). After the seedlings were unearthed, fix the seedlings at 8 in each pot. Furthermore, three months after emergence, 30 pots of seedlings with stable and similar growth were selected and divided into three groups containing 10 pots in each group. In this experiment, three different treatments of drought were adopted; control (80% of field water capacity; CK), moderate drought (40% of field water capacity; MD), and heavy drought (20% of field water capacity; HD) (Yan et al., 2003; Zhu, Kang & Li, 2006; Shan et al., 2007). After emergence, each group of P. sylvestris var. mongolica seedlings was subjected to the corresponding drought stress treatment. The soil water content was maintained by weighing and replenishing water, i.e., each pot was weighed every day to supplement the lost water to maintain the stability of the corresponding soil water content.
Growth of seedlings
In the process of seedling drought treatment, the height and ground diameter of five random P. sylvestris var. mongolica seedlings per treatment were measured with a ruler and a Vernier caliper and recorded every week. After two months of drought treatment, the seedlings were dug out and cleaned with water. The fresh weight of the primary roots, lateral roots, stems, and needles of the seedlings was weighed, followed by drying in the oven to a constant weight. The dry weight of each part was measured using balance.
Stomatal density of needles
The needles of P. sylvestris var. mongolica treated with different treatments were sliced, and their slides were observed and photographed under the electron scanning microscope (Hitachi s-3400n). The number of pores was observed in five visual fields, and the number of pores in each visual field was counted using Image J software, and the density of the pores was calculated.
Chlorophyll content in seedlings
The chlorophyll content of the seedlings was measured as described by William & Paul (1985). Fresh leaf samples were cleaned with deionized water. After the surface pollution was removed, 0.5 g of needles were added to 10 ml of acetone for grinding. In addition, the samples were centrifuged at 10,000 rpm for 5 min and the supernatant was collected, followed by spectrophotometer analysis at 663 nm and 645 nm. The experiment was repeated three times.
Root morphological indexes of seedlings
The entire root system was carefully separated from the soil, cleaned with tap water and deionized water, and then scanned with a root scanner (Epson Expression 1640XL scanner, Epson, Quebec, Canada). The length, surface area, volume, and the number of roots at the tip were analyzed by WinRhizo Reg software.
Microscopic observation of seedlings
Fresh samples of roots, stems, and needles from P. sylvestris var. mongolica seedlings were rinsed and cut into small samples of one cm, then immediately fixed in 4% glutaraldehyde solution. The samples were put under vacuum suction until completely immersed in the fixative and kept at 4 °C overnight. Furthermore, the samples were rinsed with phosphate buffer (0.1M, pH 6.8) 3–5 times, 10-15 min each time. The samples were then fixed in 1% osmic acid for 2 h and then transferred to phosphate buffer (0.1 M, pH 6.8) for 1 h. A gradient of acetone solution (30%, 50%, 70%, 80%, 90%, and 100% v/v) was used to dehydrate twice for 15–30 min each time. The samples were then treated twice with isoamyl acetate for 30 min and 20 min, respectively, and shaken to replace acetone in the sample. Furthermore, the samples were collected in a sample cage and placed in a critical point dryer (HCP-2, Hitachi, Tokyo, Japan) for drying. Finally, the stick and spray gold were performed with the JSM-6360LV scanning electron microscope (SEM) observation.
In each treatment, three groups of fresh needle samples of P. sylvestris var. mongolica seedlings were taken and rinsed. Then they were prefixed with 2.5% glutaraldehyde overnight at 4 °C. After being washed three times with phosphate buffer saline for 15 min each time, 1% osmic acid fixative was used for fixation and was kept for 2 h. Furthermore, after being fully washed with PBS and ethanol (30%, 50%, 70%, 90%, and 100%), samples were successively used for dehydration. Then the ethanol was replaced with 25%, 50%, 75%, and 100% propylene oxide (dissolved in ethanol). A gradient of resin embedding agent (10%, 30%, 70%, and 100%, dissolved in propylene oxide) was used for penetration and, finally, 100% resin embedding agent was used to polymerize at 70 °C for 12 h (Wei, Wang & Zhang, 2010). A Leica EM UC slicer was used to slice ultrathin sections, and uranyl acetate-lead citrate double staining was performed. Finally, the H-7650 transmission electron microscope (TEM) was used to observe and photograph the samples. A total of nine slices were observed from three samples in each group.
Statistical analysis
SPSS software was used for the one-way analysis of variance (ANOVA). To illustrate the graph, Excel 2010 and GraphPad Prism 7.0 software was used for chart making.
Results
Analysis of plant growth parameters
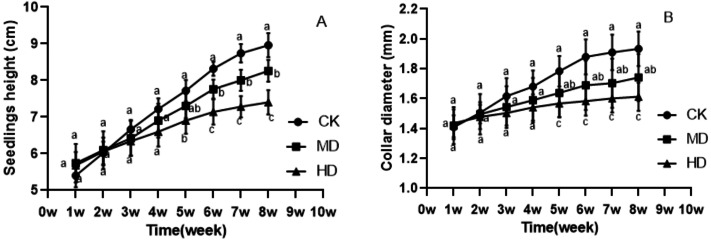
The growth of P. sylvestris var. mongolica seedlings under drought treatment was inhibited both during and after the experiment (Fig. 1). It is clear from Fig. 2 that under different degrees of drought stress, the difference in seedling height and ground diameter between CK and MD as well as HD gradually increased with time. During the fifth week of drought treatment, the seedling height and ground diameter in the CK increased by 42.96% and 26.7%, respectively, compared to those in the initial state. Similarly, the seedling height and ground diameter ratio of MD treatment increased by 27.18% and 15.35%, respectively; however, in the HD treatment, these parameters increased only by 21.48% and 9.19%, respectively. At the end of the drought treatment, which was the eighth week, the seedling height and ground diameter in the CK increased by 65.93% and 37.36%, respectively, compared to the initial value. These parameters increased by 43.9% and 22.68% in MD treatment, respectively. However, in HD treatment, they increased only by 30.28% and 12.56%, respectively (Figs. 2A and 2B). These findings indicate that drought inhibited the growth of plant height and ground diameter and the degree of inhibition of seedling height and ground diameter increased with the increase in water deficit and time.
Figure 1. Growth of P. sylvestris var. mongolica under different drought conditions.
CK, non-drought stress; MD, moderate drought; HD, heavy drought.
Figure 2. Changes in the seedling height and ground diameter of P. sylvestris var. mongolica under different drought stress; ((A) Seedlings height; (B) Ground diameter; CK, non-drought stress; MD, moderate drought; HD, heavy drought).
The lowercase letters indicate significant (p < 0.05) differences between different treatments at the same time.
Biomass analysis of different tissue
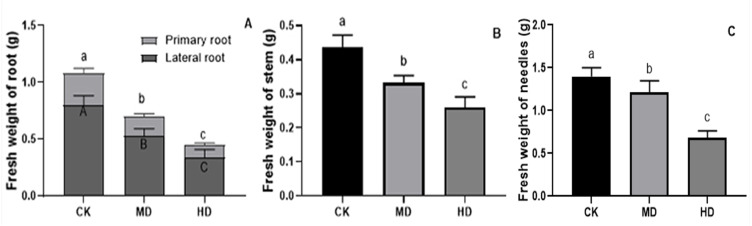
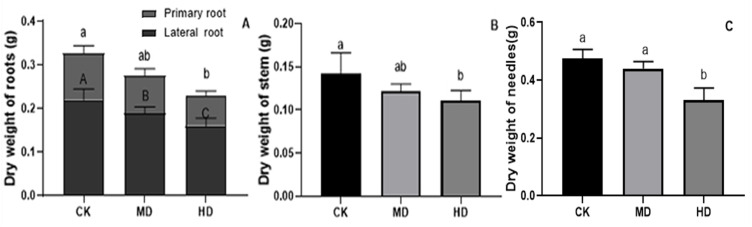
The effects of various degrees of drought stress on the biomass of P. sylvestris var. mongolica seedlings are shown in Figs. 3 and 4. For MD and HD-treated seedlings, the fresh weight of each part was significantly different from CK, which showed that the fresh weight of the primary root, the lateral root, the stem, and the leaf decreased significantly after drought treatment (P < 0.05). Compared to CK, the primary root and lateral root of P. sylvestris var. mongolica seedlings decreased by 39.29% and 33.75% under MD treatment, 60.71%, and 57.50% under HD treatment, respectively (Fig. 3A). The decrease in the stem was 25% and 40.91% under MD treatment, and HD treatment, respectively (Fig. 3B), and leaf decreased by 13.57% and 51.43% under MD treatment and HD treatment, respectively (Fig. 3C). In case of the dry weight of each tissue after drought treatment, the lateral roots of the plant both under MD treatment and HD treatment were significantly lower than CK, while, for primary roots, stems, and needles, only under HD treatment had significant differences compared to CK (P < 0.05). The dry weights of the roots (primary roots and lateral roots), stems, and needles of the seedlings were significantly decreased under HD treatment. There was a decrease of 18.18% and 13.64% under MD treatment and 36.36% and 27.27% under HD treatment, respectively, in the dry weight of the primary root and the lateral root (Fig. 4A). Furthermore, the dry weight of the stem decreased by 14.29% and 21.43% compared to CK under MD treatment and HD treatment (Fig. 4B), and the leaf decreased by 8.33% and 31.25% compared to CK under MD treatment and HD treatment, respectively (Fig. 4C). This indicated that MD and HD treatment reduced the dry and fresh weight of plants and severely inhibited the growth and accumulation of dry matter in seedlings.
Figure 3. Fresh weight of each tissue of P. sylvestris var. mongolica seedlings after drought stress treatment.
(A) Root; (B) Stem; (C) Leaves; CK, non-drought stress; MD, moderate drought; HD, heavy drought. The lowercase letters indicate significant (p < 0.05) differences among different treatment times subjected to the same species. Error bars are ±SD (n = 6).
Figure 4. Dry weight of each tissue of P. sylvestris var. mongolica seedlings after drought stress treatment.
(A) Root; (B) Stem; (C) Leaves; CK, non-drought stress; MD, moderate drought; HD, heavy drought. The lowercase letters indicate significant (p < 0.05) differences among different treatment times subjected to the same species. Error bars are ± SD (n = 6).
Analysis of photosynthetic pigment content
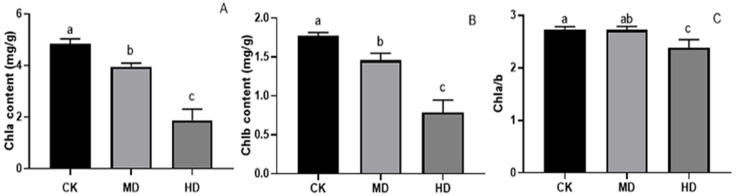
Under various degrees of drought stress, the contents of chlorophyll a and chlorophyll b in the needles of P. sylvestris var. The mongolica seedlings showed a downward trend (Fig. 5). The chlorophyll a and chlorophyll b content in MD or HD treatment was significantly different from CK (P < 0.05). Under MD and HD treatment, chlorophyll a decreased by 18.31% and 61.11%, respectively, compared to CK (Fig. 5A), while chlorophyll b in seedlings decreased by 17.70% and 55.37%, respectively, in MD and HD (Fig. 5B). Furthermore, the chlorophyll a/b value under MD treatment decreased compared to CK, but there was no significant difference (P > 0.05). There was a significant difference between HD and CK (P < 0.05) (Fig. 5C).
Figure 5. Chlorophyll content of P. sylvestris var. mongolica seedlings after different drought stress treatments.
(A) Chla; (B) Chlb; (C) Chla/b; CK, non-drought stress; MD, moderate drought; HD, heavy drought. The lowercase letters indicate significant (p < 0.05) differences among different treatment times subjected to the same species. Error bars are ±SD (n = 6).
Analysis of stomatal density
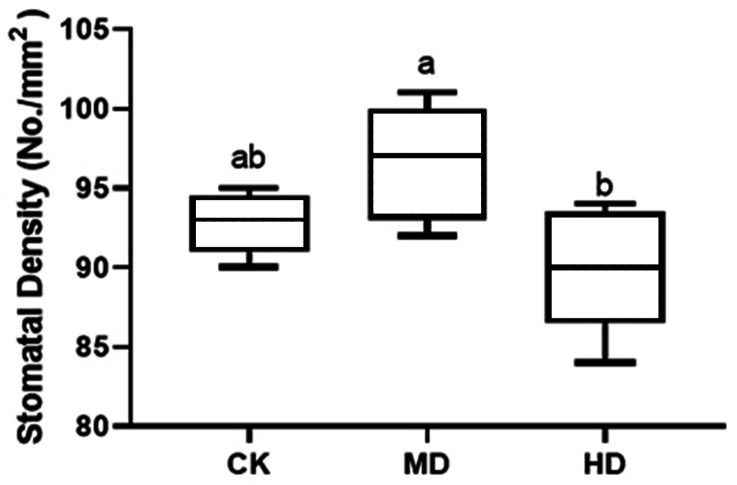
The stomatal density of P. sylvestris var. mongolica needles under different drought stresses is shown in Fig. 6. Compared to CK, MD treatment showed an increasing trend. The stomatal density increased by 4.09% under MD treatment and decreased by 3.02% under HD treatment compared to CK. However, these alterations were not prominent, and there were no significant differences in MD and HD treatment compared to CK by analysis of variance (P > 0.05).
Figure 6. Change of stomatal density in leaves of P. sylvestris var. mongolica under different drought stress.
CK, non-drought stress; MD, moderate drought; HD, heavy drought. The lowercase letters indicate the significant (p < 0.05) differences among different treatment times subjected to the same species. Error bars are ±SD (n = 5).
Analysis of root structure of seedlings
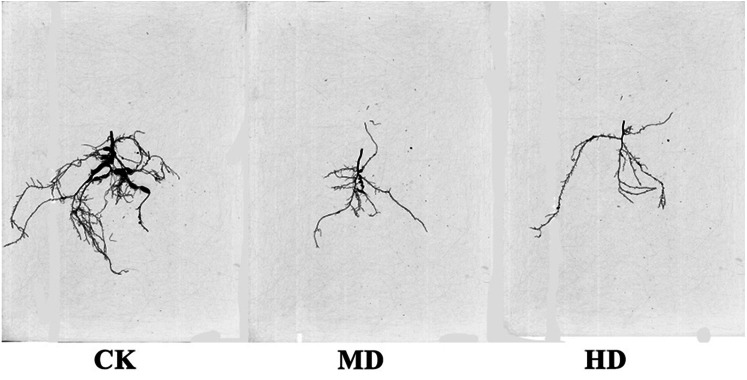
The root scanning diagram of P. sylvestris var. mongolica seedlings under various degrees of drought are shown in Fig. 7. The measurements of root growth parameters under different drought treatments are shown in Table 1. It is clear from Fig. 7 and Table 1 that HD treatment significantly reduced total root length, surface area, root volume, average root diameter, total root length per unit of soil volume (LenPerVol), root tips number, root fork number, and crossing number of P. sylvestris var. mongolica (P < 0.05). Similarly, MD treatment significantly reduced root surface area, root volume, total root length per unit of soil volume, root fork number, and crossing number of P. sylvestris var. mongolica (P < 0.05). Compared to CK, the total root length of MD and HD treatment decreased by 9.79% and 27.88%, respectively; the root surface area decreased by 6.62% and 8.89%, respectively; the root volume decreased by 13.07% and 38.34%, respectively; the total root length per unit of soil volume decreased by 14.10% and 18.42%, respectively; the number of root tips decreased by 8.40% and 18.42%, respectively; the root forks number decreased by 15.07% and 22.87%, respectively, and the number of crossings decreased by 18.28% and 29.66%, respectively.
Figure 7. Effects of drought stress on the root structure of P. sylvestris var. mongolica seedlings.
Table 1. Root system index of P. sylvestris var. mongolica seedlings after different drought stress treatments. (LenPerVol: total root length per unit soil volume).
| Drought intensity Treatments (T) | Length (cm) | Surface area (cm2) | Root volume (cm3) | Average diameter (mm) | LenPerVol (cm/m3) | Tips | Forks | Crossings |
|---|---|---|---|---|---|---|---|---|
| CK | 898.19 ± 11.72a | 575.41 ± 9.18a | 34.06 ± 0.88a | 2.88 ± 0.3a | 888.85 ± 17.17a | 4,332 ± 105a | 9,324 ± 250a | 1,116 ± 64a |
| MD | 810.17 ± 30.26a | 537.33 ± 7.81b | 29.61 ± 0.85b | 2.53 ± 0.12a | 763.50 ± 27.32b | 3,968 ± 126a | 7,919 ± 186b | 912 ± 36b |
| HD | 647.75 ± 35.62b | 524.27 ± 2.03b | 21 ± 0.32c | 1.90 ± 0.18b | 677.42 ± 16.08b | 3,534 ± 160b | 7,192 ± 121b | 785 ± 14b |
Notes.
The lowercase letters indicate the significant (p < 0.05) differences among different treatment times subjected to the same species. Error bars are ±SD (n = 3).
Analysis of the microstructure under the scanning electron microscope
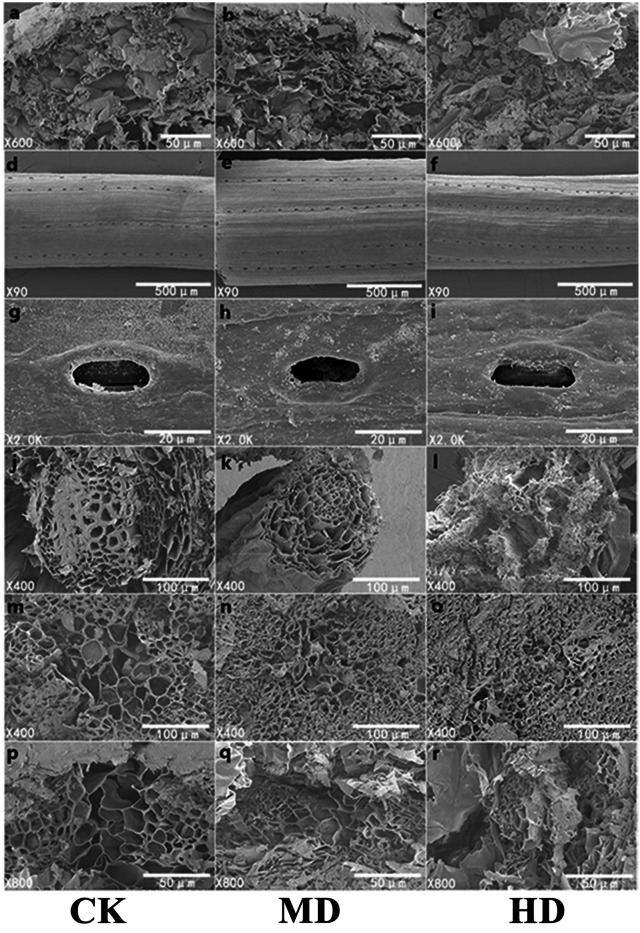
The microscopic study of plant needles can reveal the effect of drought stress more intuitively on the morphology of P. sylvestris var. mongolica needles. Adaptive changes in leaf structural characteristics are an important manifestation of the plant response to drought. With increasing drought stress, starch grains in the needles of P. sylvestris var. mongolica gradually decreased (Figs. 8A–8C); the elevated drought stress forced the stomatal opening to decrease (Figs. 8G–8I). Furthermore, with increasing drought stress, the epidermis of the needles appeared to fold and with distortion (Figs. 8D–8F). In the CK, the cross-sectional shape of the needle showed a relatively full state as a whole, and the structure of each part was clear. MD treatment caused a slight shrinkage of transmission tissue cells, and the needles were deformed. On the other hand, under HD treatment, the needles were severely shrunk and deformed, the shrinkage of the transmission tissue cells intensified, and the area decreased significantly (Figs. 8P–8R). Simultaneously, with increasing drought stress, the tracheid diameter of the root of P. sylvestris var. mongolica shrinks and gets injured (Figs. 8J–8L). Similarly, the tracheid diameter of the stem becomes smaller and damaged (Figs. 8M–8O).
Figure 8. SEM observation of P. sylvestris var. mongolica under different drought stress (CK, MD, and HD in each column from left to right).
(A–C) starch granule morphology in needles; (D–F) needle epidermis morphology; (G–I) stomatal morphology of needles; (J–L) transverse anatomical structure of root; (M–O) transverse anatomical structure of stem; (P–R) anatomical structure of needle transverse section).
Microscopic tissue analysis under transmission electron microscopy
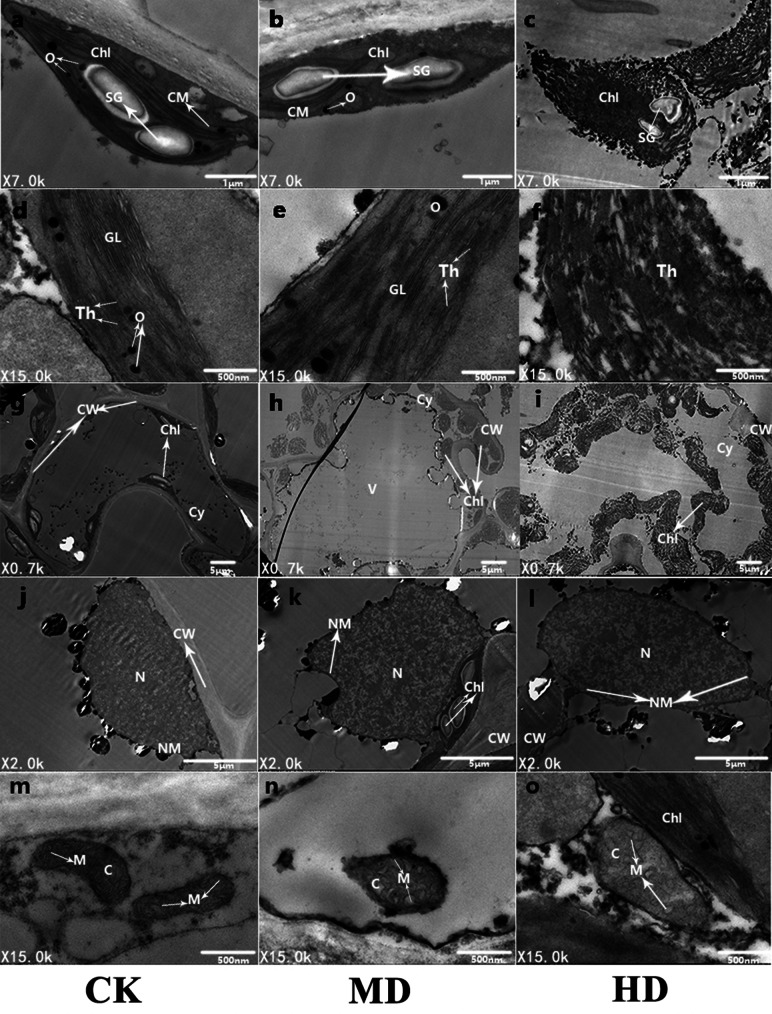
To further observe the ultrastructure of P. sylvestris var. mongolica needle cells, the submicroscopic structure of the needle cells was analyzed using a transmission electron microscope (Fig. 9). It is clear from Fig. 9 that under CK, the mesophyll cells of P. sylvestris var. mongolica were filled and closely arranged; the morphology of mesophyll cells was normal (Fig. 9G); the chloroplast structure in the cells was spindle-shaped; the chloroplasts of the cells were attached to the inner wall of the mesophyll cells in a spindle shape and were arranged neatly; and starch granules, as well as a small amount of osmiophilic globule on the chloroplasts, can be observed (Fig. 9A). The distribution of the chloroplast stroma was compact, the chloroplast bilayer membrane was visible, and the thylakoid lamellar structure was developed, clearly visible, and arranged in parallel (Fig. 9D). The structure of mitochondria in cells was complete and abundant, and the matrix was evenly distributed in the mitochondria. The inner and outer membranes of the mitochondria were intact, and the cristae of the mitochondria could also obviously be observed (Fig. 9M). The nuclear structure was complete, and the nucleoplasm compact (Fig. 9J). Under MD treatment, the structure of the chloroplast bilayer membrane was destroyed, and the thylakoid lamella was reduced and blurred. Starch granules were still observed in the chloroplasts, and the number of osmiophilic globules increased (Figs. 9B, 9E and 9H). The outer membrane of the mitochondria showed that the cristae of the mitochondria began to expand and that the whole mitochondria swelled and decreased in number (Fig. 9N). The nucleoplasm of the nucleus was loose (Fig. 9K). Furthermore, under HD treatment, the chloroplast bilayer membrane in the needle cells of P. sylvestris var. mongolica was destroyed, the starch granules wrinkled, and the outer wall blurred. The osmiophilic globule can no longer be identified, the chloroplast stroma flows out, the thylakoid disintegrates, and the lamellar structure of the thylakoid cannot be observed at all (Figs. 9C, 9F and 9I). The mitochondrial bilayer membrane was severely damaged, vacuolized, the mitochondrial matrix flowed out, and the cristae in the mitochondria had disappeared (Fig. 9O). The nuclear cytoplasm of the nucleus was severely vacuolated (Fig. 9L).
Figure 9. Ultrastructure of P. sylvestris var. mongolica needles under different drought stress (CK, MD, and HD in each column from left to right).
Chl, chloroplast; CM, chloroplast membrane; Th, thylakoid; CW, cell wall; N, nucleus; SG, starch.
Discussion
Water is a key limiting factor for seed germination and seedling growth and plant survival (Han, Cheng & Li, 2016). Drought stress is one of the common stresses in plant growth, and its impact on plants has been widely concerned (Yang, Miao & Xu, 2007).
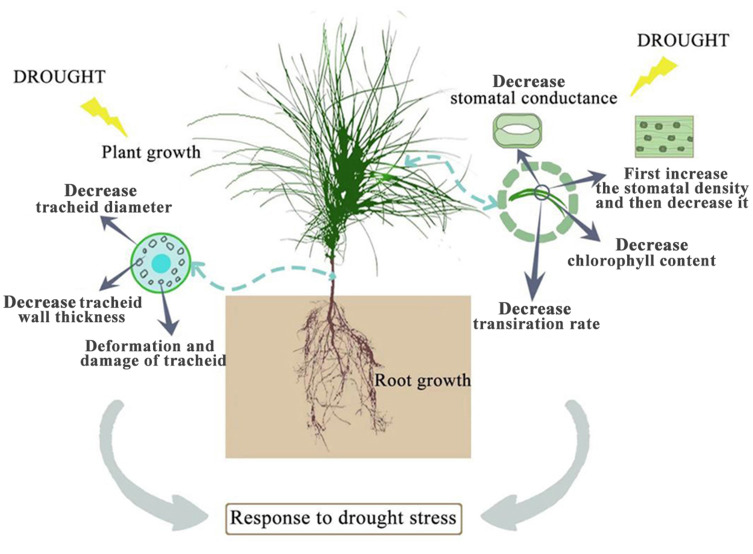
Drought stress has many adverse effects on plants. Severe drought will even lead to the death of plants (Zhang et al., 2018). The effect of drought on the plant growth index is the most intuitive expression affected by drought stress. In this study, among the seedlings of CK, P. sylvestris var. mongolica seedlings have a higher degree of increase in plant height and ground diameter, indicating that under relatively good water conditions, plants maintained a high photosynthetic rate and thus ensured a relatively high carbon acquisition capacity (Fig. 10). However, under moderate or severe drought conditions, the photosynthetic rate, transpiration rate, and water use efficiency of plants decreased, which reduced the carbon acquisition ability of plants and inhibited the growth of plant height and ground diameter (Zhu et al., 2005). Overall, the growth of plant height and ground diameter in P. sylvestris var. mongolica seedlings gradually slow down with increasing drought stress, indicating that drought stress limits seedling growth, and these findings are consistent with previous research studies in Larix principis-rupprechtii, Pinus tabuliformis, Picea meyeri, and some other plant species (He, 2001; Xu, Guo & Xu, 2010; Xue et al., 2022a; Wang et al., 2022; Xue et al., 2022b). This also demonstrates that when plants are faced with drought stress, they normally limit their growth rate to reduce water consumption and maintain plant survival (Wu & Li, 2014; Poorter, Niklas & Reich, 2012). The dry weight and the fresh weight of the plant tissues declined as the drought deepened, especially under the condition of severe drought, and this effect was more pronounced. These results were consistent with the response of Larix gmelinii and many other plant seedlings to drought stress (Wang et al., 2020; Nadia et al., 2021; Park et al., 2021). In terms of biomass measured after drought stress, the root system was the first to be subjected to drought stress due to the different environments where the aboveground part and underground parts of the plant are located, and their ecological indicators, as well as their physiological and biochemical functions, directly affect the drought resistance of the plant. In an arid environment, the growth and development of plants, as well as their adaptability to the external environment, can be reflected by the structural characteristics of the roots (Pan, Zhang & Shao, 2017). The characteristic indexes of root growth of this study suggest that drought stress inhibited the indexes such as total root length, root surface area, root volume, total root length per unit of soil volume, bifurcation number, and crossing number (Fig. 10). Especially under severe drought, these indexes were significantly different from those of the control group. A reduction in total root length was observed, which was consistent with the results of tall fescue (Wang, Bughrara & Nelson, 2008). However, in moderate drought, there was no significant difference between total root length and root tip number with the control group, indicating that P. sylvestris var. mongolica can resist certain drought stress, but beyond that, changes appear in its morphological structure due to drought stress, thus affecting some physiological and biochemical functions (Fig. 10).
Figure 10. Effects of drought stress on growth of P. sylvestris var. mongolica.
Photosynthesis is essential for the growth and development of green plants, and chlorophyll is a primary substance involved in photosynthesis and the main pigment that drives photosynthesis. Therefore, to some extent, the rate of plant photosynthesis can be reflected by the chlorophyll content (Huang et al., 2012; Li et al., 2016; Zheng et al., 2018; Zhang et al., 2018). Chlorophylls a and b are the most common pigments involved in photosynthesis. In this process, chlorophyll a is mainly responsible for the conversion of light energy to chemical energy. In addition to absorption and conversion, chlorophyll b also has the responsibility of adjusting the size of the antenna for the photosynthetic mechanism (LaRoche et al., 1996; Green & Dumford, 1996; Barber, Morris & Büchel, 2000). When plants are subjected to drought or low-temperature stress, chlorophyll a decomposes faster than chlorophyll b, and the content ratio of both pigments changes, which can also reflect the rate of photosynthesis (Yamasato et al., 2005). Therefore, as the main participant in photosynthesis, the determination of its content is an important means to understand the rate of photosynthesis under drought stress (Fig. 10). The results of this study showed that the chlorophyll a and chlorophyll b content of P. sylvestris var. mongolica needles decreased significantly under drought stress. However, under severe drought stress, compared to moderate stress, the contents of chlorophyll a and chlorophyll b in the needles of P. sylvestris var. mongolica decreased greatly, which may be the compensation effect under moderate drought, or it may be because the plants were in the growing season and contain part of the undivided chlorophyll, so the reduction in chlorophyll content was relatively small. This conclusion is consistent with previous experimental studies on Mongolian pine, Scots pine, and some other plants (Zlobin et al., 2019; Xia et al., 2019; Zhang et al., 2020; Qian et al., 2021). Additionally, chlorophyll a and chlorophyll b of P. sylvestris var. mongolica seedlings gradually decreased with the aggravation of drought stress. In severe drought, the chlorophyll content was significantly different from that of the control group. Previous studies have shown that there is a negative correlation between chlorophyll a/b change and drought resistance (Zhao et al., 2019). This indicates the drought resistance of P. sylvestris var. mongolica plants gradually weakened under drought stress. The plant needles remain with the most contact with the outside world. In addition to serving as a crucial location for photosynthetic physiological processes, needles are not only an important part to ensure the normal growth and development of plants but are also a sensitive part of drought stress. Their morphological and structural changes are closely related to plant drought resistance (Li et al., 2013; Ren et al., 2015; Nina & Meruert, 2017). The size, opening, and density of the stomata on the needles can affect the rate of plant transpiration. Among them, stomatal density significantly affects plant drought tolerance, water use efficiency, and stomatal conductance. Increased stomatal density is also a typical feature of the plant response to drought stress (Fig. 10). A small and dense stoma will reduce transpiration and improve plant resistance to drought stress (Ferris et al., 1996; Drake, Froend & Franks, 2013; Guo & Wu, 2018). Furthermore, under mild drought, plant leaf cells elongate slowly, and leaf growth slows, decreasing leaf area and increasing stomatal density. In case of severe drought, the stomatal development of plant needles becomes slow, manifested in the decrease in stomatal density (Sam et al., 2000; Xu, Zou & Zhao, 2003; Xu & Zhou, 2008; Fraser et al., 2009). The findings of this study were also consistent with previous studies, as described. Furthermore, in moderate drought, the stomatal density increases, which may be due to the decrease in individual and leaf area and the increase in the number of stomata per unit area, resulting in the increase in the stomatal density (Xie, Song & Cao, 2015). This is consistent with previous research results by Larix kaempferi (Bhusal et al., 2020). The larger stomatal density is conducive to making full use of available water for photosynthesis and better control of respiration in a short time, contributes to heat dissipation, and reduces the degeneration of chloroplasts and protoplasts caused by drought. Under severe drought stress, the stomatal density decreases, which may be due to the needles being seriously affected, which inhibits the occurrence of stomata, significantly reduces the number of stomata, and finally shows the decrease of stomatal density (Wang, Ren & Lou, 1992).
The study of leaf microstructure is helpful in understanding the response of plants to drought stress and provides the basis for further research. The results of the scanning electron microscope showed that the size of stomata and opening of the needles were significantly reduced, which was consistent with the results of some previous studies on Cryptomeria japonica, Larix kaempferi and some other species of forest trees (Bhusal et al., 2020; Kenzo et al., 2021; Nadia et al., 2021). Furthermore, this study found that the tracheid diameter and tracheid wall thickness of roots, stems, and needles gradually decreased with the deepening of the drought. Previous studies have demonstrated that the tracheid diameter is positively correlated with water conveyance efficiency (Sperry, Hacke & Pittermann, 2006; Schuldt et al., 2016). Furthermore, the thickness of the root tracheids was closely related to embolism resistance. The thickening of the wall of the root tracheids can strengthen the fragile pipes of the roots and improve the mechanical strength of the roots, to improve the embolism resistance of roots (Fichot et al., 2010). This phenomenon shows that when subjected to drought stress, the water-carrying capacity and anti-embolism capacity of plants are inhibited. Meanwhile, in this study, with increasing degree of stress, phloem cells become denser and narrower, which is not conducive to the transportation and distribution of water and organic matter in plants, leading to plant growth restriction or even death due to the decline or unreasonable distribution of water, sugar and other organic matter. Transport tissue is primarily responsible for material transport between mesophyll cells and vascular bundles (Qi, 2006). Therefore, dehydration and deformation of cells in seedlings will also lead to the obstruction of material exchange between the two parts of tissues, which is not conducive to stable growth and metabolic activities of plants and could be one of the reasons why drought restricts the growth of P. sylvestris var. mongolica. Through the study of Picea mariana and Abies balsamea, it was found that the tracheid lumen size of trees was smaller and the cell wall was thicker under drought conditions, which was consistent with the results of this study (Belien et al., 2012; D’Orangeville et al., 2013).
Chloroplasts and mitochondria, important organelles performing more physiological functions, are sensitive to environmental changes. Their morphological structure and physiological functions often change under stress conditions (Vain, Pardha & Saradhi, 2001). The change in plant ultrastructure under drought stress is also an important index to measure its resistance to drought (Zhang, Li & Chen, 2014). According to the TEM analysis of this study, the number of chloroplasts and mitochondria in P. sylvestris var. mongolica needle leaf cells not only decreased with the aggravation of drought stress, but their morphological structure also changed to varying degrees. The bilayer membrane of chloroplasts and mitochondria was destroyed, the content flowed out, the lumen was hollowed out, the number of osmiophilic globules in chloroplasts increased under moderate drought, and the cristae in mitochondria also changed. Several studies have shown that drought stress can lead to the disordered arrangement and structural damage to chloroplasts, expansion, and rounding of mitochondria, membrane damage, dissolution of internal cristae, etc. (Yu et al., 2011; Wang, Zhou & Mao, 2020; Das, 2021; Li et al., 2022). Similarly, as an important site for photosynthesis, chloroplasts are destroyed under drought, which shows that the photosynthesis of the plant itself has been inhibited, resulting in a decrease in photosynthetic capacity. The osmiophiles are droplets formed by aggregation of some lipid substances, which are used as storage of lipid substances in plant chloroplasts (Huan et al., 2014). These osmiophiles are supposed to act as an electron carrier protecting the vesicle from free radical damage caused by adversity stress (Grigorova et al., 2012). In addition, it is also considered to be the product of polymerization after thylakoid degrades the membrane lipid. In this study, the number of osmiophiles increased under moderate drought, which also showed that the coniferous chloroplasts of P. sylvestris var. mongolica seedlings had been damaged under moderate drought. The results of mitochondrial research were consistent with the previous studies on Fraxinus mandshurica, Ormosia hosiei, and Cyclocarya paliurus seedlings (Wei, Wang & Zhang, 2010; Liu & Wei, 2019; Li et al., 2022). As the main site of the tricarboxylic acid cycle and oxidative phosphorylation, mitochondria were destroyed under drought stress, showing that needle cells struggle to maintain normal physiological functions, resulting in changes in needle structure.
Conclusions
The plant height, ground diameter, biomass, and photosynthetic pigment indexes of P. sylvestris var. mongolica seedlings decreased with increasing degrees of drought stress. Drought stress inhibited total root length, root surface area, root volume, total root length per unit of soil volume, bifurcation number, and crossing number of P. sylvestris var. mongolica seedlings. Furthermore, the deepening of the drought caused the stomatal density to increase first, followed by a decrease. The drought stress also led to changes in the structure of roots, stems, needles, needle cell structure, and organelle structure of P. sylvestris var. mongolica seedlings, thus, destroying the normal metabolic pathway and physiological function of cells. Morphological changes in organelles also provide cytological evidence for the study of drought resistance in P. sylvestris var. mongolica. In summary, P. sylvestris var. mongolica is affected by the increasing drought pressure caused by current climate change, making its risk of recession under drought higher. Therefore, special attention should be paid to the effect of environment on Mongolian pine when afforestation is carried out in arid and semi-arid areas.
Supplemental Information
Funding Statement
This work was supported by the National Natural Science Foundation of China (31800542). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Fanjun Meng conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Tianze Zhang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Dachuan Yin performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.
References
- Allen, Macalady & Chenchouni (2010).Allen CD, Macalady AK, Chenchouni H. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management. 2010;259(4):660–684. [Google Scholar]
- Anderegg William et al. (2019).Anderegg William RL, Anderegg Leander DL, Kerr Kelly L, Trugman Anna T. Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Global Change Biology. 2019;25(11):3793–3802. doi: 10.1111/gcb.14771. [DOI] [PubMed] [Google Scholar]
- Barber, Morris & Büchel (2000).Barber J, Morris E, Büchel C. Revealing the structure of the photosystem II chlorophyll binding proteins, CP43 and CP47. BBA-Bioenergetics. 2000;1459(2):239–247. doi: 10.1016/S0005-2728(00)00158-4. [DOI] [PubMed] [Google Scholar]
- Belien et al. (2012).Belien E, Rossi S, Morin H, Deslauriers A. Xylogenesis in black spruce subjected to rain exclusion in the field. Canadian Journal of Forest Research. 2012;42(7):1306–1315. doi: 10.1139/x2012-095. [DOI] [Google Scholar]
- Bhusal et al. (2020).Bhusal N, Lee M, Han R, Han A, Kim H. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. Forest Ecology and Management. 2020;465:118099. doi: 10.1016/j.foreco.2020.118099. [DOI] [Google Scholar]
- Burling et al. (2013).Burling K, Cerovic ZG, Cornic G, Ducruet JM, Noga G, Hunsche M. Fluorescence-based sensing of drought-induced stress in the vegetative phase of four contrasting wheat genotypes. Environmental and Experimental Botany. 2013;89:51–59. doi: 10.1016/j.envexpbot.2013.01.003. [DOI] [Google Scholar]
- Casson & Hetherington (2009).Casson SA, Hetherington AM. Environmental regulation of stomatal development. Current Opinion in Plant Biology. 2009;13(1):90–95. doi: 10.1016/j.pbi.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Castro et al. (2005).Castro J, Zamora R, Hódar JA, Gómez JM. Alleviation of summer drought boosts establishment success of Pinus sylvestris in a Mediterranean mountain: an experimental approach. Plant Ecology. 2005;181(2):191–202. doi: 10.1007/s11258-005-6626-5. [DOI] [Google Scholar]
- Chartzoulakis et al. (2002).Chartzoulakis K, Patakas A, Kofidis G, Bosabalidis A, Nastou A. Water stress affects leaf anatomy, gas exchange, water relations, and growth of two avocado cultivars. Scientia Horticulturae. 2002;95(1-2):39–50. doi: 10.1016/S0304-4238(02)00016-X. [DOI] [Google Scholar]
- Chen, Gao & Shi (2017).Chen LT, Gao RM, Shi XD. Drought stress on chlorophyll content and root activity in seedlings of Larix principis-rupprechtii and Pinus tabuliformis. Journal of Agricultural and Food Chemistry. 2017;7(03):67–72. [Google Scholar]
- Chen & Zhao (2011).Chen MT, Zhao Z. Effects of drought on root characteristics and mass allocation in each part of seedlings of four tree species. Journal of Beijing University of Chemical Technology. 2011;33(1):16–22. [Google Scholar]
- Das (2021).Das J. Interactive effect of elevated CO2 and drought stress on leaf anatomy in brassica species. International Journal of Environment and Climate Change. 2021;11(2):97–108. [Google Scholar]
- D’Orangeville et al. (2013).D’Orangeville L, Côté B, Houle D, Morin H. The effects of throughfall exclusion on xylogenesis of balsam fir. Tree Physiology. 2013;33(5):516–526. doi: 10.1093/treephys/tpt027. [DOI] [PubMed] [Google Scholar]
- Drake, Froend & Franks (2013).Drake PL, Froend RH, Franks PJ. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. The Journal of Experimental Botany. 2013;64(2):495–505. doi: 10.1093/jxb/ers347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris et al. (1996).Ferris R, Nijs I, Behaeghe T, Impens I. Elevated CO2 and temperature have different effects on leaf anatomy of perennial ryegrass in spring and summer. Annals of Botany. 1996;78(4):489–497. doi: 10.1006/anbo.1996.0146. [DOI] [Google Scholar]
- Fichot et al. (2010).Fichot R, Barigah TS, Chamaillard S, Le Thiec D, Laurans F, Cochard H, Brignolas F. Common trade-offs between xylem resistance to cavitation and other physiological traits do not hold among unrelated Populus deltoides × Populus nigra hybrids. Plant, Cell & Environment. 2010;33:1553–1568. doi: 10.1111/j.1365-3040.2010.02164.x. [DOI] [PubMed] [Google Scholar]
- Fiorin (2016).Fiorin L. Transport efficiency through uniformity: organization of veins and stomata angiosperm leaves. New Phytologist. 2016;209:216–227. doi: 10.1111/nph.13577. [DOI] [PubMed] [Google Scholar]
- Fraser et al. (2009).Fraser LH, Greenall A, Carlyle C, Turkington R, Friedman CR. Adaptive phenotypic plasticity of Pseudoroegneria spicata: response of stomatal density, leaf area and biomass to changes in water supply and increased temperature. Annals of Botany. 2009;103(5):769–775. doi: 10.1093/aob/mcn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Yang & Qin (2021).Gao JN, Yang B, Qin C. Response of intra-annual stem radial growth to drought events: a case study of Pinus tabuliformis in the Helan Mountains, China. Chinese Journal of Applied Ecology. 2021;32(10):3505–3511. doi: 10.13287/j.1001-9332.202110.015. [DOI] [PubMed] [Google Scholar]
- Gao, Wang & Zhang (2010).Gao XF, Wang JX, Zhang B. Effects of drought stress on dry matter partitioning of young Robinia pseudoacacia at its different growth stages. Chinese Journal of Ecology. 2010;29(6):1103–1108. [Google Scholar]
- Green & Dumford (1996).Green BR, Dumford DG. The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:685–714. doi: 10.1146/annurev.arplant.47.1.685. [DOI] [PubMed] [Google Scholar]
- Grigorova et al. (2012).Grigorova B, Vassileva V, Klimchuk D, Feller U. Drought, high temperature, and their combination affect ultrastructure of chloroplasts and mitochondria in wheat (Triticum aestivum L.) leaves. Journal of Plant Interactions. 2012;7(3):204–213. doi: 10.1080/17429145.2011.654134. [DOI] [Google Scholar]
- Guo & Wu (2018).Guo SJ, Wu YQ. Leaf anatomical structure characteristics and drought resistance of Chinese chestnut. Northwest Agricultural Science and Technology University. 2018;46(9):51–59. [Google Scholar]
- Guo et al. (2019).Guo YJ, Niu QH, Cui Y, Fan HT, Peng B, Gu JC, Lu GQ. Soil improvement benefits of Pinus sylvestris var. Mongolica forests of different ages in bashang area. Journal of Northeast Agricultural University. 2019;47(10):64–69. [Google Scholar]
- Guo & Wu (2015).Guo Y, Wu JH. Effect of water stress on stomatal characteristics of leaves of Potentilla sericea. Jilin Agricultural Science and Technology University. 2015;24(4):13–16. [Google Scholar]
- Han, Cheng & Li (2016).Han ZJ, Cheng L, Li ZJ. Response of germination on ten chenopodiaceae seeds to drought stress and resistances evaluation in Tarim Basin. Bulletin of Botanical Research. 2016;36(2):266–273. [Google Scholar]
- He (2001).He WM. Effects of water stress factor on hydraulic and growth characteristics of Sabina vulgaris seedling. Chinese Journal of Plant Ecology. 2001;25(1):11–16. [Google Scholar]
- Huan et al. (2014).Huan JJ, Lu ZG, Han LW, Wang ZY, Yin YL. Effects of mixed salts solution stress on chloroplast submicroscopic structure of Taxodium ‘Zhongshanshan 405’ and its parents. International Journal of Forest Engineering. 2014;28(4):58–62. [Google Scholar]
- Huang et al. (2012).Huang CJ, Zhao SY, Wang LC, Shu ZX, Yang Y, Fang CM. Effect of drought stress on chlorophyll contents in ramie. Chinese Journal of Plant Ecology. 2012;34(5):208–212. [Google Scholar]
- IPCC (2013).IPCC . In: Climate change 2013: the physical science basis. Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Cambridge Univ Press; Cambridge, United Kingdom and New York, NY, USA: 2013. pp. 1–1535. [Google Scholar]
- Isaji et al. (2018).Isaji S, Yoshinaga N, Teraishi M, Ogawa D, Kato E, Okumoto Y, Habu Y, Mori N. Biosynthesis and accumulation of GABA in rice plants treated with acetic acid. Journal of Pesticide Science. 2018;43(3):214–219. doi: 10.1584/jpestics.D18-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari, Hashemi Garmdareh & Azadegan (2019).Jafari S, Hashemi Garmdareh SEH, Azadegan B. Effects of drought stress on morphological, physiological, and biochemical characteristics of stock plant (Matthiola incana L.) Scientia Horticulturae. 2019;253:128–133. doi: 10.1016/j.scienta.2019.04.033. [DOI] [Google Scholar]
- Jia & Zhang (2008).Jia WS, Zhang JH. Stomatal movements and long-distance signaling in plants. Plant Signaling & Behavior. 2008;3(10):772–777. doi: 10.4161/psb.3.10.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzo et al. (2021).Kenzo T, Inoue Y, Araki MG, Kawasaki T, Kitaoka S, Tsurita T, Sakata T, Saito S. Effects of throughfall exclusion on photosynthetic traits in mature Japanese cedar (Cryptomeria japonica (L.f.) D. Don.) Forests. 2021;12(8):971–971. doi: 10.3390/f12080971. [DOI] [Google Scholar]
- Kim, Chae & Choi (2020).Kim I, Chae HM, Choi B. Effects of experimental throughfall exclusion on soil respiration in a continental coniferous stand, South Korea. Forests. 2020;11(9):972–972. doi: 10.3390/f11090972. [DOI] [Google Scholar]
- Kong et al. (2019).Kong T, Zhang Y, Lei ZY, Wang DL, Liu Y, Yu J, Wu D. Soil nitrogen mineralization under pinus sylvestris var. mongolica plantation on sandy soil. Central Arid Zone Research Institute. 2019;36(02):296–306. [Google Scholar]
- Konijnendijk & Randrup (2002).Konijnendijk CC, Randrup TB. Urban Forestry & Urban Greening. Urban For Urban Green. 2002;47:1–478. [Google Scholar]
- LaRoche et al. (1996).LaRoche J, VanderStaay G, Partensky F, Ducret A, Aebersold R, Li R, Golden SS, Hiller RG, Wrench PM, Larkum AW, Green BR. Independent evolution of the prochlorophyte and green plant chlorophyll a/b light-harvesting proteins. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(26):15244–15248. doi: 10.1073/pnas.93.26.15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2016).Lee DK, Jung H, Jang G, Jeong JS, Kim YS, Ha SH, Do YK, Kim JK. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiology. 2016;172(1):575–588. doi: 10.1104/pp.16.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2022).Li CH, Wan YF, Shang XL, Fang SZ. Responses of microstructure, ultrastructure and antioxidant enzyme activity to PEG-induced drought stress in Cyclocarya paliurus seedlings. Forests. 2022;13(6):836–836. doi: 10.3390/f13060836. [DOI] [Google Scholar]
- Li et al. (2016).Li HX, Du JM, GuoY Z, Zhao JH. Effects of drought stress and rewatering on the growth and chlorophyll contents of wolfberry seedlings. Ningxia Journal of Agriculture and Forestry Science. 2016;57(08):5–8. [Google Scholar]
- Li et al. (2013).Li H, Fan JF, Gao JS, Zhou YX. Comparison on drought resistance on anatomical structures of black poplar leaf. Journal of Northwest University. 2013;28(3):113–118. [Google Scholar]
- Liu & Wei (2019).Liu Y, Wei XL. Dark septate endophyte improves drought tolerance of Ormosia hosiei Hemsley & E. H. Wilson by modulating root morphology, ultrastructure, and the ratio of root hormones. Forests. 2019;10(10):830–830. doi: 10.3390/f10100830. [DOI] [Google Scholar]
- Liu, Yue & Chen (2010).Liu Y, Yue X, Chen GL. Effects of water stress on ultrastructure and membrane lipid peroxidation of leaf and root cells of Glycyrrhiza uralensis. Acta Prataculturae Sinica. 2010;19(6):79–86. [Google Scholar]
- Long & Deng (2019).Long HY, Deng LX. Response and adaptation of plant morphology to drought stress. Hubei Agricultural Sciences. 2019;58(8):5–7. [Google Scholar]
- Ma et al. (2012).Ma FJ, Li DD, Cai J, Jiang D, Cao WX, Dai TB. Responses of wheat seedlings root growth and leaf photosynthesis to drought stress. Chinese Journal of Applied Ecology. 2012;23(3):724–730. [PubMed] [Google Scholar]
- Marron, Dillen & Ceulemans (2007).Marron N, Dillen SY, Ceulemans R. Evaluation of leaf traits for indirect selection of high yielding poplar hybrids. Environmental and Experimental Botany. 2007;61(2):103–116. doi: 10.1016/j.envexpbot.2007.04.002. [DOI] [Google Scholar]
- Meng et al. (2010).Meng P, Li YL, Zhang BX, Zhang XL, Lei ZY, Song XD. A comparative study on physiological characteristics of drought resistance of Pinus densiflora var. zhangwuensis and P. sylvestris var. mongolica in Sandy Soil. Sci Silv Sin. 2010;46(12):56–63. [Google Scholar]
- Muukkonen et al. (2015).Muukkonen P, Nevalainen S, Lindgren M, Peltoniemi M. Spatial occurrence of drought-associated damages in Finnish boreal forests: results from forest condition monitoring and GIS analysis. Boreal Environment Research. 2015;20:172–180. [Google Scholar]
- Nadia et al. (2021).Nadia S, Luc EP, Guillaume B, Singh AP, Gierlinger N, Rosner S, Brendel O. Physiological and anatomical responses to drought stress differ between two larch species and their hybrid. Trees. 2021;35(5):1–18. doi: 10.1007/s00468-020-02001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova et al. (2020).Nikolova PS, Bauerle TL, Häberle K, Blaschke H, Brunner I, Matyssek R. Fine-root traits reveal contrasting ecological strategies in european beech and norway spruce during extreme drought. Frontiers in Plant Science. 2020;11:1211–1211. doi: 10.3389/fpls.2020.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nina & Meruert (2017).Nina T, Meruert K. Change in leaf anatomical parameters of seedlings of different wheat species under conditions of drought and salt stress. Pakistan Journal of Botany. 2017;49(3):857–865. [Google Scholar]
- Nolan et al. (2018).Nolan C, Overpeck JT, Allen JRM, Anderson PM, Betancourt JL, Binney HA, Brewer S, Bush MB, Chase BM, Cheddadi R, Djamali M, Dodson J, Edwards ME, Gosling WD, Haberle S, Hotchkiss SC, Huntley B, Ivory SJ, Kershaw AP, Kim S-H, Latorre C, Leydet M, Lézine A-M, Liu K-B, Liu Y, Lozhkin AV, McGlone MS, Marchant RA, Momohara A, Moreno PI, Müller S, Otto-Bliesner BL, Shen C, Stevenson J, Takahara H, Tarasov PE, Tipton J, Vincens A, Weng C, Xu Q, Zheng Z, Jackson ST. Past and future global transformation of terrestrial ecosystems under climate change. Science. 2018;361(6405):920–923. doi: 10.1126/science.aan5360. [DOI] [PubMed] [Google Scholar]
- Okunlola et al. (2017).Okunlola GO, Olatunji OA, Akinwale RO, Tariq A, Adelusi AA. Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Scientia Horticulturae. 2017;224:198–205. doi: 10.1016/j.scienta.2017.06.020. [DOI] [Google Scholar]
- Pagter, Bragato & Brix (2005).Pagter M, Bragato C, Brix H. Tolerance and physiological responses of Phragmites australis to water deficit. Aquatic Botany. 2005;81(4):285–299. doi: 10.1016/j.aquabot.2005.01.002. [DOI] [Google Scholar]
- Pan, Zhang & Shao (2017).Pan XD, Zhang Y, Shao M. Research progress on adaptive responses of crop root structure to drought stress. Journal of Agriculture, Science and Technology. 2017;19(2):51–58. [Google Scholar]
- Park et al. (2021).Park JE, Kim J, Purevdorj E, Son YJ, Nho CW, Yoo G. Effects of long light exposure and drought stress on plant growth and glucosinolate production in pakchoi (Brassica rapasubsp. chinensis) Food Chemistry. 2021;340:128167. doi: 10.1016/j.foodchem.2020.128167. [DOI] [PubMed] [Google Scholar]
- Poorter, Niklas & Reich (2012).Poorter H, Niklas KJ, Reich PB. Biomass allocation to leaves, stems, and roots: meta-analyses of interspecific variation and environment control. New Phytologist. 2012;193(1):30–50. doi: 10.1111/j.1469-8137.2011.03952.x. [DOI] [PubMed] [Google Scholar]
- Qi (2006).Qi WC. Comparative studies on anatomic structures of four species of gymnosperm growing in different habitat. Northeast Normal Univ; Changchun, China: 2006. [Google Scholar]
- Qian et al. (2021).Qian H, Dong AM, Roitto M, Xiang D-Y, Zhang G, Repo T, Wang A-F. Timing of drought affected the growth, physiology, and mortality of mongolian pine saplings. Forests. 2021;12(11):1491–1491. doi: 10.3390/f12111491. [DOI] [Google Scholar]
- Reddy, Chaitanya & Vivekanandan (2004).Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology. 2004;161(11):1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Ren et al. (2015).Ren L, Zhao XL, Xu J, Zhang HY, Guo YH, Guo FL, Zhang CL, Lv JH. Varied morphological and physiological responses to drought stress among four tea Chrysanthemum cultivars. Acta Ecologica Sinica. 2015;35(15):3–12. [Google Scholar]
- Salazar-Henao, Vélez-Bermúdez & Schmidt (2016).Salazar-Henao JE, Vélez-Bermúdez IC, Schmidt W. The regulation and plasticity of root hair patterning and morphogenesis. Development. 2016;143(11):1848–1858. doi: 10.1242/dev.132845. [DOI] [PubMed] [Google Scholar]
- Sam et al. (2000).Sam O, Jerez E, Dellamico J, Ruiz-sanchez MC. Water stress-induced changes in anatomy of tomato leaf epidermis. Biologia Plantarum. 2000;43(2):275–277. doi: 10.1023/A:1002716629802. [DOI] [Google Scholar]
- Schuldt et al. (2016).Schuldt B, Knutzen F, Delzon S, Jansen S, Müller-Haubold H, Burlett R, Clough Y, Leuschner C. How adaptable is the hydraulic system of European beech in the face of climate change-related precipitation reduction? New Phytologist. 2016;210:443–458. doi: 10.1111/nph.13798. [DOI] [PubMed] [Google Scholar]
- Scoffoni et al. (2014).Scoffoni C, Vuong C, Diep S, Cochard H, Sack L. Leaf shrinkage with dehydration: coordination with hydraulic vulnerability and drought tolerance. Plant Physiology. 2014;164(4):1772–1788. doi: 10.1104/pp.113.221424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan et al. (2007).Shan CJ, Xu XJ, Wang GY, Yue Y. Physiological adaption of winter wheat seedling’s root to soil drought. Bulletin of Botanical Research. 2007;27(1):55–58. [Google Scholar]
- Sherwood et al. (2013).Sherwood SC, Alexander MJ, Brown AR, McFarlane NA, Gerber EP, Feingold G, Scaife AA, Grabowsk WW. Climate Science for Serving Society. Springer; Dordrecht: 2013. Climate processes: clouds, aerosols, and dynamics; pp. 73–103. [Google Scholar]
- Shi, Ding & Yuan (2004).Shi JY, Ding GJ, Yuan XF. Studies on water parameters in Pinus massoniana seedlings of different families. Sci Silv Sin. 2004;3:51–55. [Google Scholar]
- Song, Zhu & Kang (2013).Song LN, Zhu JJ, Kang HZ. Response of Hydraulic Structure Parameters and Growth of Pinus sylvestris var. mongolica Seedling to Simulated Precipitation Gradient. Arid Zone Research. 2013;30(6):1021–1027. [Google Scholar]
- Song et al. (2018).Song LN, Zhu JJ, Li MC, JinXin Z, DaBo L. Water use strategies of natural Pinus sylvestris var. mongolica trees of different ages in Hulunbuir sandy land of inner Mongolia, China, based on stable isotope analysis. Trees. 2018;32(4):1001–1011. doi: 10.1007/s00468-018-1691-2. [DOI] [Google Scholar]
- Song, Zhu & Yan (2015).Song LN, Zhu JJ, Yan QL. Comparison of intrinsic water use efficiency between different aged Pinus sylvestris var. mongolica wide windbreaks in semi-arid sandy land of northern China. Agroforestry Systems. 2015;89:477–489. doi: 10.1007/s10457-014-9784-4. [DOI] [Google Scholar]
- Song, Zhu & Zheng (2017).Song LN, Zhu JJ, Zheng X. Forestation and management scheme of Pinus sylvestris var. mongolica plantations in sandy lands based on their decline mechanisms. Chinese Journal of Ecology. 2017;36(11):3249–3256. [Google Scholar]
- Sperry, Hacke & Pittermann (2006).Sperry JS, Hacke UG, Pittermann J. Size and function in conifer tracheids and angiosperm vessels. American Journal of Botany. 2006;93(10):1490–1500. doi: 10.3732/ajb.93.10.1490. [DOI] [PubMed] [Google Scholar]
- Trenberth et al. (2014).Trenberth KE, Dai A, Schrier GVanDer, Jones PD, Barichivich J, Briffa KR, Sheffield J. Global warming and changes in drought. Nature Climate Change. 2014;4(1):17–22. doi: 10.1038/nclimate2067. [DOI] [Google Scholar]
- Vain, Pardha & Saradhi (2001).Vain B, Pardha P, Saradhi PM. Alteration in chloroplast structure and thylakoid membrane composition due to in vivo heat treatment of rice seedlings: correlation with the function change. Plant Physiology. 2001;158(5):583–592. doi: 10.1078/0176-1617-00260. [DOI] [Google Scholar]
- Vitasse et al. (2019).Vitasse Y, Bottero A, Cailleret M, Bigler C, Fonti P, Gessler A, Lévesque M, Rohner B, Weber P, Rigling A, Wohlgemuth T. Contrasting resistance and resilience to extreme drought and late spring frost infive major European tree species. Global Change Biology. 2019;25:3781–3792. doi: 10.1111/gcb.14803. [DOI] [PubMed] [Google Scholar]
- Vurukonda et al. (2016).Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A. Enhancement of drought stress tolerance in crops by plant growth-promoting rhizobacteria. Microbiological Research. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2020).Wang DL, Zhang RS, Fang X, Wang K, Wu YL, Qin SY, Long DD, Shen HO. Seed germination and seedling growth response to drought stress and resistance evaluation for introduced Pinus sylvestris var. mongolica sandy-fixation plantation. Zhejiang Agriculture and Forestry University. 2020;37(01):60–68. [Google Scholar]
- Wang, Zhou & Mao (2020).Wang H, Zhou QP, Mao PS. Ultrastructural and photosynthetic responses of pod walls in alfalfa to drought stress. International Journal of Molecular Sciences. 2020;21(12):4457. doi: 10.3390/ijms21124457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Bughrara & Nelson (2008).Wang JP, Bughrara SS, Nelson CJ. Morpho-physiological responses of several fescue grasses to drought stress. HortScience. 2008;43(3):161–173. [Google Scholar]
- Wang et al. (2022).Wang JC, Li SH, YLi Guo, Yang Q, Ren R, Han YJ. Responses of Larix principis-rupprechtii radial growth to climatic factors at different elevations on Guancen Mountain, North-Central China. Forests. 2022;13(1):99–99. doi: 10.3390/f13010099. [DOI] [Google Scholar]
- Wang et al. (2018).Wang JH, Zhang XM, Chen A, Zhou YW, Chen P, Jiang YF. Response of physiological characteristics and anatomical structure of roots in Amorpha fruticosa seedlings exposed to simulated drought with PEG-6000. Acta Ecologica Sinica. 2018;38(2):511–517. [Google Scholar]
- Wang, Ren & Lou (1992).Wang XC, Ren HY, Lou CH. Root to shoot communication in the responses to drought. Journal of Plant Physiology. 1992;28(6):397–402. [Google Scholar]
- Wang et al. (2010).Wang YJ, Fu QS, Zheng H, Wen CL, Cheng L, Zhao B, Guo YD. Effects of drought stress on growth, photosynthetic physiological features and stomata characters of cucumber seedlings. China Agricultural University. 2010;15(5):12–18. [Google Scholar]
- Wang, Zhang & Liu (2005).Wang ZF, Zhang SQ, Liu XF. Root system hydraulic conductivity of different genotype maize and its relationship with root anatomy. Chinese Journal of Applied Ecology. 2005;16(12):2349–2352. [PubMed] [Google Scholar]
- Wei (2005).Wei XL. Studies oil whole plant drought resistance of three Ulmus tree species in Karst region. NanJing Forestry University; Nanjing: 2005. pp. 27–29. [Google Scholar]
- Wei, Wang & Zhang (2010).Wei X, Wang ZQ, Zhang GZ. Morphological and activity variation of mitochondria in fine roots of Fraxinus mandshurica seedling under drought stress. Chinese Journal of Plant Ecology. 2010;34(12):1454–1462. [Google Scholar]
- William & Paul (1985).William PI, Paul RB. Extinction coefficients of chlorophyll a and b in N, N-dimethyl formamide and 80% acetone. The Journal of Plant Physiology. 1985;77(2):483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu & Li (2014).Wu LJ, Li ZH. Response of growth and physiological characteristics of Cyclobalanopsis gilva seedlings from different provenances to drought stress. Chinese Journal of Ecology. 2014;33(4):996–1003. [Google Scholar]
- Xia et al. (2019).Xia J, Zhang JM, Shi R, Li Q, Zhao YH. Physiological and biochemical responses of Moringa oleifera seedling under the drought stress. West China Medical Journal. 2019;48(1):106–113. [Google Scholar]
- Xie, Song & Cao (2015).Xie ZS, Song SX, Cao HM. Comparisons of leaf anatomical structure and stoma characteristic among three different blueberry varieties. North Hortic. 2015;4:5–8. [Google Scholar]
- Xu, Guo & Xu (2010).Xu F, Guo WH, Xu WH. Effects of water stress on morphology, biomass allocation, and photosynthesis in Robinia pseudoacacia seedlings. Journal of Beijing Forestry University. 2010;32(1):24–30. [Google Scholar]
- Xu, Zou & Zhao (2003).Xu K, Zou Q, Zhao Y. Effects of soil water stress and shading on growth characteristics of ginger. Chinese Journal of Applied Ecology. 2003;14(10):1645–1648. [PubMed] [Google Scholar]
- Xu & Zhou (2008).Xu ZZ, Zhou GS. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. The Journal of Experimental Botany. 2008;59(12):3317–3325. doi: 10.1093/jxb/ern185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue et al. (2022a).Xue F, Jiang Y, Dong MY, Wang M, Ding X, Yang X, Cui M, Xu H, Kang M. Different drought responses of stem water relations and radial increments in Larix principis-rupprechtii and Picea meyeri in a montane mixed forest. Agricultural and Forest Meteorology. 2022a;315:108817. doi: 10.1016/j.agrformet.2022.108817. [DOI] [Google Scholar]
- Xue et al. (2022b).Xue RH, Jiao L, Qi CL, Chen K, Liu XP, Du DS, Wu X. Growth and response patterns of Picea crassifolia and Pinus tabuliformis to climate factors in the Qilian Mountains, northwest China. Dendrochronologia. 2022b;71:125905. doi: 10.1016/j.dendro.2021.125905. [DOI] [Google Scholar]
- Yamasato et al. (2005).Yamasato A, Nagata N, Tanaka R, Tanaka A. The N-terminal domain of chlorophyllide a oxygenase confers protein instability inresponse to chlorophyll b accumulation in Arabidopsis. Plant Cell. 2005;17(5):1585–1597. doi: 10.1105/tpc.105.031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. (2003).Yan JQ, Wang J, Tissue D, Holaday AScott, Allen R, Zhang H. Photosynthesis and seed production under water-deficit conditions in transgenic tobacco plants that overexpress an Arabidopsis ascorbate peroxidase gene. Crop Science. 2003;43:1477–1483. doi: 10.2135/cropsci2003.1477. [DOI] [Google Scholar]
- Yang, Miao & Xu (2007).Yang F, Miao LF, Xu X. Progress in research of plant responses to drought stress. The Chinese Journal of Applied and Environmental Biology. 2007;13(4):586–591. [Google Scholar]
- Yin, Wang & Qi (2021).Yin DC, Wang HL, Qi JY. The enhancement effect of calcium ions on ectomycorrhizal fungi-mediated drought resistance in Pinus sylvestris var. mongolica. Journal of Plant Growth Regulation. 2021;40(4):1389–1399. doi: 10.1007/s00344-020-10197-y. [DOI] [Google Scholar]
- Yu et al. (2011).Yu H, Liu ZL, Hu HL, Guan QW, Wan FX. Effect of drought stress on the ultra microstructures of chloroplasts and mitochondria of five plants. Bulletin of Botanical Research. 2011;31(02):152–158. [Google Scholar]
- Zang, Pretzsch & Rothe (2012).Zang C, Pretzsch H, Rothe A. Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees. 2012;26:557–569. doi: 10.1007/s00468-011-0617-z. [DOI] [Google Scholar]
- Zhang et al. (2019).Zhang CM, Shi SL, Liu Z, Yang F, Zhang Z-K. Effects of drought stress on the root morphology and anatomical structure of alfalfa (Medicago sativa) varieties with differing drought tolerance. Acta Prataculturae Sinica. 2019;28(5):79–89. [Google Scholar]
- Zhang & Sun (2016).Zhang GF, Sun HY. Effects of drought stress with PEG6000 on morphology and structure in Camellia oleifera seedlings. Hubei Agricultural Sciences. 2016;55(11):2830–2833. [Google Scholar]
- Zhang, Li & Chen (2014).Zhang J, Li XP, Chen XH. Biochemical response of winter wheat to long-term soil drought at flowering stage and drought resistance. Journal of Triticeae Crops. 2014;34(6):765–773. [Google Scholar]
- Zhang et al. (2018).Zhang LL, Yu HX, Meng HT, Sun H, Lv L, Zuo XX, Yu HX, Zhang YY. Physiological and biochemical response of Panax quinquefolius to drought stress. Special Wild Economic Animal and Plant Research. 2018;40(4):16–20. [Google Scholar]
- Zhang et al. (2020).Zhang M, Wang Y, Yang L, Hao RQ, Bai XX, Yu X. Effects of plant growth-promoting rhizobacteria on growth and physiological characteristics of Zizyphus jujuba seedlings under drought stress. The Journal of Southwest Jiaotong University. 2020;40(5):48–55. [Google Scholar]
- Zhang et al. (2021).Zhang X, Li X, Manzanedo RD, D’Orangeville L, Lv P, Wang C, Xu C, Hou M, Huang X, Rademacher T. High risk of growth cessation of planted larch under extreme drought. Environmental Research Letters. 2021;16(1):014040. doi: 10.1088/1748-9326/abd214. [DOI] [Google Scholar]
- Zhao et al. (2019).Zhao J, Li WG, Wei GR, Kang HB, Wang DX, Lin Q. Comprehensive study and evaluation on drought resistance of five native tree species in Baoji. The Journal of Southwest Jiaotong University. 2019;34(06):74–81. [Google Scholar]
- Zhao et al. (2006).Zhao TJ, Sun S, Liu Y, Liu JM, Liu Q, Yan YB, Zhou HM. Regulating the Drought-responsive Element (DRE)-mediated Signaling Pathway by Synergic Functions of Trans-active and Trans-inactive DRE Binding Factors in Brassica napus. Journal of Biological Chemistry. 2006;281(16):10752–10759. doi: 10.1074/jbc.M510535200. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2018).Zheng Y, Zhao YW, Fei WQ, Zheng WL. Comparison of chlorophyll content and photosynthetic characteristics of 2 species of plateau Paeonia seedlings under drought stress. Journal of Plateau Agriculture. 2018;2(05):453–461+557. [Google Scholar]
- Zhu et al. (2003).Zhu JJ, Fan ZP, Zeng DH, Jiang F-Q, Matsuzaki T. Comparison of stand structure and growth between plantation and natural forests of Pinus sylvestris var. mongolica on sandy land. Journal of Forestry Research. 2003;14(2):103–111. doi: 10.1007/BF02856774. [DOI] [Google Scholar]
- Zhu, Kang & Li (2006).Zhu JJ, Kang HZ, Li ZH. Comparison of different types of drought stresses affecting photosynthesis of Mongolian pine seedlings on sandy soils. Journal of Beijing Forestry University. 2006;28(2):57–63. [Google Scholar]
- Zhu et al. (2005).Zhu JJ, Kang HZ, Li ZH, Wang GC, Zhang RS. Impact of water stress on survival and photosynthesis of Mongolian pine seedlings on sandy land. Acta Ecologica Sinica. 2005;25(10):2527–2533. [Google Scholar]
- Zlobin et al. (2019).Zlobin IE, Kartashov AV, Pashkovskiy PP, Ivanov YV, Kreslavski VD, Kuznetsov VV. Comparative photosynthetic responses of Norway spruce and Scots pine seedlings to prolonged water deficiency. Journal of Photochemistry and Photobiology B. 2019;201:111659. doi: 10.1016/j.jphotobiol.2019.111659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.