Abstract
Background
Substance use (SU) contributes to poor health outcomes, yet limited data exist to inform strategies to optimize SU treatment among persons with human immunodeficiency virus (HIV). We describe SU and SU treatment utilization among women with and without HIV in the Women's Interagency HIV Study (WIHS).
Methods
We included data from women enrolled in WIHS from 2013 to 2020. Current SU was self-reported, nonmedical use of drugs in the past year, excluding use of only marijuana. SU treatment utilization was self-reported use of a drug treatment program in the past year. Multivariable regression models were used to investigate associations between participant characteristics and SU treatment.
Results
Among 2559 women (1802 women living with HIV [WWH], 757 women without HIV), 14% reported current SU. Among those with current SU (n = 367), 71% reported crack/cocaine followed by 40% reporting opioids, and 42% reported any treatment in the past year. The most common treatments were methadone (64%), Narcotics Anonymous (29%), inpatient programs (28%), and outpatient programs (16%). Among women using opioids (n = 147), 67% reported methadone use in the past year compared to 5% using buprenorphine/naloxone. Multivariable analysis showed lower odds of treatment utilization among WWH with concurrent alcohol or marijuana use. Visiting a psychiatrist/counselor was associated with higher odds of treatment. Among WWH, SU treatment was not associated with HIV-related clinical outcomes.
Conclusions
Treatment utilization was high, especially for methadone use. Our results highlight opportunities for accessing SU treatment for WWH, such as the need to prioritize buprenorphine and comprehensive, wraparound services in HIV care settings.
Keywords: HIV, addiction, substance use, women
Among a cohort of older women with and without HIV, substance use treatment utilization was high, especially with methadone; however, findings suggest opportunities to increase access to substance use treatment in the context of HIV care for women.
In the United States (US), up to half of persons with human immunodeficiency virus (HIV) report current substance use (SU) or substance use disorder (SUD) [1, 2], which is associated with worse HIV-related outcomes, including decreased antiretroviral therapy (ART) uptake and adherence, retention in HIV care, and virologic suppression [3, 4]. Among persons without HIV, SU increases risk of HIV acquisition [5, 6]. Therefore, addressing SU may facilitate treatment and prevention of HIV and improve HIV care continuum outcomes, as aligned with the goals of the National HIV/AIDS Strategy [7].
Although evidence-based treatments for SUD exist, uptake remains low. A national survey on drug use in 2015 revealed that as little as 10% of persons with SUD in the US received treatment in the past year [8]. Women with HIV (WWH) experience additional barriers to SU treatment compared to men and to women without HIV, including increased stigma, fear of violence, and loss of parental custody [9–11]. Collectively, these barriers emphasize the need for gender-specific interventions to improve SUD care among WWH [9, 12]. However, many studies on SUD treatment uptake in people with HIV are dated or focused on men; thus, the current extent of treatment uptake among WWH remains unknown, limiting our ability to tailor strategies to improve SUD treatment utilization for women.
In this study, we aimed to (1) describe patterns of and identify factors associated with SU among women by HIV status, (2) describe patterns of utilization of different types of SU treatment programs among participants with current SU, and (3) identify factors associated with SU treatment utilization. Given the availability of resources provided by the Ryan White HIV/AIDS Program for WWH, we hypothesized that SU treatment utilization would be higher among WWH compared to demographically similar women without HIV.
METHODS
Study Population
The Women's Interagency HIV Study (WIHS) is a large, prospective cohort study that began in 1993 and includes WWH and demographically similar women living without HIV. Additional details on eligibility criteria and recruitment methods have been published previously [13–15].
The WIHS enrolled participants in 1994–1995, 2001–2002, and 2011–2012 from Bronx, New York; Brooklyn, New York; Chicago, Illinois; Los Angeles, California; San Francisco, California; and Washington, District of Columbia. Because of the growing HIV epidemic among minority populations in the South, 4 additional sites were added in 2013–2015 (Atlanta, Georgia; Chapel Hill, North Carolina; Miami, Florida; and Birmingham, Alabama–Jackson, Mississippi). WIHS participants completed follow-up visits at 6-month intervals during which detailed medical histories were obtained by interviewers and comprehensive physical examinations were conducted.
We included study visits from WWH and women without HIV from all 10 clinical sites from October 2013 to March 2020 to provide contemporary information on SU and treatment utilization. To capture both current and past SU, we limited our sample to participants with at least 2 visits: a baseline visit and at least 1 follow-up visit during our study period. HIV status was determined at the last observed study visit.
Patient Consent Statement
The WIHS protocol [13] was approved by each site's Institutional Review Board (University of Mississippi Medical Center, University of North Carolina at Chapel Hill, University of Alabama at Birmingham, University of Miami, Emory University, State University of New York Downstate Medical Center, Kings County Medical Center, Montefiore Medical Center, Mount Sinai School of Medicine, Cook County Health and Hospital System, Northwestern University, Rush University Medical Center, University of Illinois at Chicago, University of California, San Francisco, Alameda Health System, Sutter Health, Santa Clara Valley Medical Center, San Mateo Medical Center, Georgetown University, Montgomery County Department of Health and Human Services, Inova, Howard University, Whitman-Walker Clinic, University of Southern California Medical Center, Santa Barbara Neighborhood Clinics, University of Hawaii at Manoa), and all participants provided written informed consent.
Substance Use and Substance Use Treatment Utilization
Substance use was self-reported as nonmedical use of drugs, including crack/cocaine, methamphetamines, other amphetamines, opioids, tranquilizers, and other drugs (including hallucinogens, inhalants, and other club drugs). Marijuana use alone was excluded from the primary outcome, as prior studies have not shown its association with worse HIV care continuum outcomes [16, 17]. Marijuana, alcohol, and tobacco were considered covariates. For this study, SU was characterized by time since last use and reported as current use (<1 year, primary outcome), recent use (1–4.9 years), or prior use (≥5 years).
Substance use treatment utilization was self-reported as utilization of any drug treatment and was similarly categorized by time since last reported. We conceptualized drug treatment broadly; types of treatment included inpatient or outpatient detoxification programs, halfway houses, prison- or jail-based programs, Narcotics Anonymous, and medications for opioid use disorder (MOUD, including methadone or buprenorphine/naloxone). Because some persons with SUD utilize Alcoholics Anonymous, we also described use of this program.
For this analysis, the primary outcome was any SU treatment in the past year (yes/no). We assessed SU treatment utilization only among participants with current SU. We also reported utilization of each SU treatment service by type of substance used.
Covariates
Our primary independent variable was HIV serostatus. Gender was not included as a variable, as only 1 participant was transgender. Other demographic covariates included age (continuous); race/ethnicity (White/non-Hispanic, Black/non-Hispanic, Hispanic, other/non-Hispanic); WIHS study region (New York [Bronx, Brooklyn], Washington, District of Columbia, California [San Francisco, Los Angeles], Illinois [Chicago], and South [Chapel Hill, Atlanta, Miami, Birmingham-Jackson]); highest level of education (≤high school, >high school); marital status (married/partner, unmarried/no partner); current employment status (unemployed, employed full-time/part-time); median household income (≤$24000, >$24 000); and insurance status, defined as private health insurance, Ryan White program, or AIDS Drug Assistance Program (insurance, no insurance). Sociobehavioral covariates included alcohol use (none, 1–7 drinks/week, >7 drinks/week), tobacco use, defined as cigarette smoking (current, former, never), history of incarceration (yes, no), history of reported physical abuse (yes, no), history of reported sexual abuse (yes, no), history of transactional sex (yes, no), and depressive symptoms (yes/no). Presence of depressive symptoms was defined as Center for Epidemiologic Studies–Depression (CESD) score of ≥16. Degree of alcohol use was based on the National Institute on Alcohol Abuse and Alcoholism, which defines >7 drinks/week as heavy drinking in women [18]. Clinical covariates among participants with HIV included HIV CD4+ lymphocyte count >200 cells/μL (yes, no), viral suppression, defined as HIV RNA level <200 copies/mL (yes, no), and current use of ART (yes, no).
Statistical Analysis
We compared participant characteristics by HIV serostatus using frequencies, χ2 tests for categorical variables, Wilcoxon rank-sum tests for non–normally distributed continuous variables, and t tests for normally distributed continuous variables. HIV-specific clinical characteristics were described for WWH only.
We performed bivariate analyses between current SU and HIV serostatus, as well as each of the other covariates of interest using χ2 tests, t tests, and Wilcoxon rank-sum tests. We then used multivariable logistic regression models to assess the association of demographic, sociobehavioral, and clinical factors with current SU. For our regression models, we included variables with P < .05 in bivariate analyses or variables selected based on literature review; HIV status and HIV-related clinical outcomes, as well as WIHS study region, were included in the model a priori based on the empirical literature. To assess association between current SU and HIV-related clinical outcomes, we used the same methods to conduct a subset analysis among WWH only. Model fit was assessed by Hosmer-Lemeshow goodness-of-fit tests.
Among those with current SU, we used descriptive statistics to report utilization of types of SU treatment programs, stratified by type of current SU.
Finally, we repeated the same methods above to assess factors associated with SU treatment utilization in the past year among those reporting current SU. Again, we conducted a subset analysis among WWH only to assess associations between SU treatment and HIV-related outcomes.
RESULTS
Baseline Characteristics
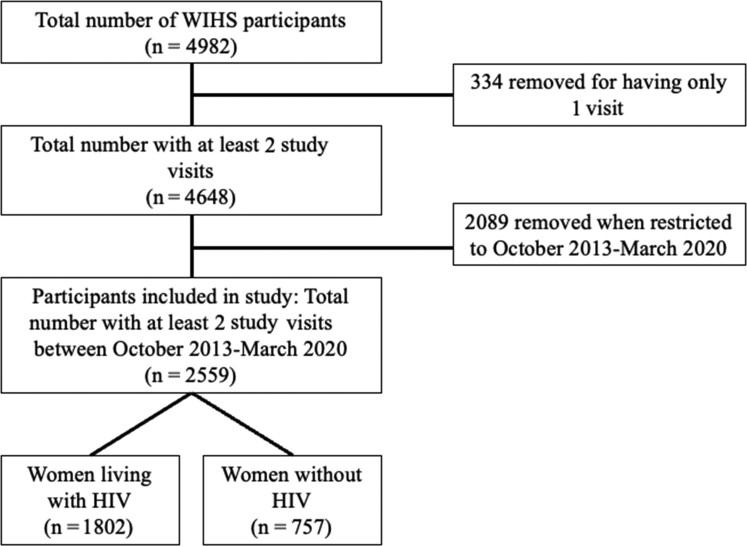
Our study included 2559 women (1802 WWH, 757 women without HIV) (Figure 1). The mean age was 51.7 (standard deviation, 9.5) years, and 71.7% self-identified as non-Hispanic Black race/ethnicity. Most were unemployed (63.0%), had annual household incomes ≤$24 000/year (72.1%), had completed high school (65.1%), and had health insurance (95.3%). Nearly half (43.5%) had history of incarceration. High proportions of women had experienced physical abuse (37.6%), depressive symptoms (30.3%), or sexual abuse (26.5%). In this cohort, 39.3% reported current tobacco use, 27.1% reported current marijuana use, and 10.1% reported drinking >7 drinks/week. Regarding healthcare utilization, 85.0% reported seeing any healthcare provider since their last study visit, and of those, 30.0% saw a psychiatrist or counselor. Among WWH, 89.3% saw an HIV provider in the past 6 months. The majority of WWH in this cohort were virologically suppressed (84.9%) and taking ART (91.8%). Baseline characteristics stratified by HIV serostatus are shown in Table 1.
Figure 1.
Flow diagram showing Women's Interagency HIV Study participant selection for this study. Abbreviations: HIV, human immunodeficiency virus; WIHS, Women's Interagency HIV Study.
Table 1.
Demographic, Sociobehavioral, and Clinical Characteristics Among Women's Interagency HIV Study Participants Enrolled in All Study Sites, 2013–2020, by HIV Serostatus (N = 2559)
| Participant Characteristics | Total (N = 2559), No. (%) | Women Without HIV (n = 757), No. (%)a | Women With HIV (n = 1802), No. (%)a | P Valueb |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 51.7 (9.5) | 50.6 (9.9) | 52.2 (9.2) | <.001 |
| Race | ||||
| Non-Hispanic Black | 1835 (71.7) | 544 (71.9) | 1291 (71.6) | .91 |
| Other | 724 (28.3) | 213 (28.1) | 511 (28.4) | |
| WIHS region | ||||
| New York | 728 (28.5) | 227 (30.0) | 501 (27.8) | .58 |
| Washington, D.C. | 315 (12.3) | 94 (12.4) | 221 (12.3) | |
| California | 354 (13.8) | 111 (14.7) | 243 (13.5) | |
| Illinois | 316 (12.4) | 88 (11.6) | 228 (12.7) | |
| South | 846 (33.1) | 237 (31.3) | 609 (33.8) | |
| Marital status | ||||
| Married/partner | 623 (27.4) | 196 (30.0) | 427 (26.3) | .08 |
| Unmarried/no partner | 1654 (72.6) | 458 (70.0) | 1196 (73.7) | |
| Highest level of education | ||||
| ≤ High school graduation | 1523 (65.1) | 423 (63.0) | 1100 (66.0) | .18 |
| >High school graduation | 816 (34.9) | 248 (36.7) | 568 (34.1) | |
| Employed (full- or part-time) | ||||
| No | 1473 (63.0) | 386 (57.4) | 1087 (65.3) | <.001 |
| Yes | 864 (37.0) | 286 (42.6) | 578 (34.7) | |
| Annual household income | ||||
| ≤$24 000 | 1613 (72.1) | 434 (67.4) | 1179 (74.1) | .002 |
| >$24 000 | 623 (27.9) | 210 (32.6) | 413 (25.9) | |
| Health insurancec | ||||
| No | 108 (4.7) | 88 (13.2) | 20 (1.2) | <.001 |
| Yes | 2212 (95.3) | 580 (86.8) | 1632 (98.8) | |
| Ever jailed/incarcerated | ||||
| No | 1447 (56.6) | 393 (51.9) | 1054 (58.5) | .002 |
| Yes | 1112 (43.5) | 364 (48.1) | 748 (41.5) | |
| Ever reported physical abuse | ||||
| No | 1597 (62.4) | 436 (57.6) | 1161 (64.4) | .001 |
| Yes | 962 (37.6) | 321 (42.4) | 641 (35.6) | |
| Ever reported sex abuse | ||||
| No | 1881 (73.5) | 536 (70.8) | 1345 (74.6) | .04 |
| Yes | 678 (26.5) | 221 (29.2) | 457 (25.4) | |
| Ever had sex for drugs, money, shelter (baseline visits) | ||||
| No | 1508 (64.5) | 409 (60.9) | 1099 (65.9) | .02 |
| Yes | 831 (35.5) | 263 (39.1) | 568 (34.1) | |
| Depressive symptomsd | ||||
| No | 1613 (69.7) | 471 (70.7) | 1142 (69.3) | .51 |
| Yes | 700 (30.3) | 195 (29.3) | 505 (30.7) | |
| Alcohol use | ||||
| Abstain | 1242 (53.5) | 297 (44.4) | 945 (57.1) | <.001 |
| 0–7 drinks/wk | 846 (36.4) | 274 (41.0) | 572 (34.6) | |
| >7 drinks/wk | 235 (10.1) | 98 (14.7) | 137 (8.3) | |
| Tobacco use | ||||
| Never | 703 (30.0) | 168 (25.0) | 535 (32.1) | <.001 |
| Former | 718 (30.7) | 200 (29.8) | 518 (31.0) | |
| Current | 920 (39.3) | 304 (45.2) | 616 (36.9) | |
| Marijuana use in last year | ||||
| No | 1865 (72.9) | 517 (68.3) | 1348 (74.8) | <.001 |
| Yes | 694 (27.1) | 240 (31.7) | 454 (25.2) | |
| Injection of drugs in last year | ||||
| No | 2516 (98.3) | 743 (98.2) | 1773 (98.3) | .67 |
| Yes | 43 (1.7) | 14 (1.9) | 29 (1.6) | |
| Seen healthcare provider since last visit | ||||
| No | 349 (15.0) | 180 (27.0) | 169 (10.2) | <.001 |
| Yes | 1971 (85.0) | 488 (73.1) | 1483 (89.8) | |
| Seen psychiatrist or counselor since last visit | ||||
| No | 1379 (70.0) | 340 (69.7) | 1039 (70.1) | .87 |
| Yes | 592 (30.0) | 148 (30.3) | 444 (29.9) | |
| HIV care in last 6 moe | ||||
| No | 177 (10.8) | NA | 177 (10.8) | |
| Yes | 1470 (89.3) | NA | 1470 (89.3) | |
| HIV RNA <200 copies/mLe | ||||
| No | 244 (15.2) | NA | 244 (15.2) | |
| Yes | 1358 (84.8) | NA | 1358 (84.8) | |
| CD4 >200 cells/µLe | ||||
| No | 112 (6.9) | NA | 112 (6.9) | |
| Yes | 1502 (93.1) | NA | 1502 (93.1) | |
| ART usee | ||||
| No | 147 (8.2) | NA | 147 (8.2) | |
| Yes | 1655 (91.8) | NA | 1655 (91.8) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; NA, not applicable; OR, odds ratio; SD, standard deviation; WIHS, Women’s Interagency HIV Study.
Values in bold indicate statistical significance, defined as p < 0.05.
Percentages are column percentages unless otherwise noted and may not total 100 due to rounding.
χ2 test performed for categorical variables, Wilcoxon rank-sum test for non–normally distributed continuous variables, and t tests for normally distributed continuous variables.
Health insurance, AIDS Drug Assistance Program, and/or Ryan White Program.
As defined as Center for Epidemiologic Studies–Depression score ≥16.
Among women with HIV only.
Current Substance Use and Associated Factors
In this cohort of women, 14.3% (12.8% WWH, 18.1% women without HIV) reported current SU. An additional 9.8% reported recent use, and an additional 41.9% reported prior use. Lifetime SU in this cohort was 66.0%.
Types of substances used currently, recently, or previously are shown in Supplementary Figure 1. Among women with current SU (n = 367), 71.4% reported using crack/cocaine, 52.0% marijuana, 40.1% opioids, 6.5% methamphetamines, 6.5% tranquilizers, and 1.9% other amphetamines; 11.7% reported injecting drugs. Additionally, among women with current SU, 77.1% reported current tobacco use, 52.0% reported current marijuana use, and 27.0% reported >7 drinks/week.
Regarding polysubstance use, among those with current SU, crack/cocaine and opioids were the most frequently co-utilized substances (15.5%). Supplementary Table 1 shows other patterns of polysubstance use. When assessing the number of substances used, half (50.1%) of women with current SU used 2 substances when including marijuana. The proportion of women who utilized SU treatment, by number of substances used is shown in Supplementary Table 2.
In an adjusted model (Table 2), unemployment (odds ratio [OR], 1.96 [95% confidence interval {CI}, 1.34–2.85]), history of incarceration (OR, 2.50 [95% CI, 1.81–3.45]), history of trading sex for drugs/money/shelter (OR, 2.35 [95% CI, 1.74–3.16]), depressive symptoms (OR, 1.40 [95% CI, 1.05–1.86]), consuming >7 drinks/week (OR, 3.79 [95% CI, 2.59–5.55]), and current tobacco use (OR, 3.84 [95% CI, 2.42–6.10]) were associated with higher odds of current SU, while non-Hispanic Black race/ethnicity was associated with lower odds (OR, 0.61 [95% CI, .45–.83]). In a separate model including only WWH, current SU was associated with viral nonsuppression (OR, 2.25 [95% CI, 1.32–3.84]), but not other HIV outcomes.
Table 2.
Association Between HIV Status, Participant Characteristics, and Current Substance Use Among Women's Interagency HIV Study Participants in Crude and Adjusted Regression Models (N = 2559)
| Participant Characteristics | Current Substance Use (n = 367), No. (%)a | No Current Substance Use (n = 2192), No. (%)a | Odds of Current Substance Use (Within Past Year) |
|
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |||
| HIV status | ||||
| Negative | 137 (37.3) | 620 (28.3) | REF | REF |
| Positive | 230 (62.7) | 1572 (71.7) | 0.66 (.53–.83) | 0.75 (.56–.995) |
| Age, y | ||||
| Mean (SD) | 53.0 (8.5) | 51.5 (9.6) | 1.18 (1.05–1.33) b | 1.13 (.95–1.34)b |
| Race | ||||
| Non-Hispanic Black | 247 (67.3) | 1588 (72.5) | 0.78 (.62–.99) | 0.61 (.45–.83) |
| Other | 120 (32.7) | 604 (27.6) | REF | REF |
| WIHS region | ||||
| New York | 83 (22.6) | 645 (29.4) | 0.76 (.57–1.03) | 1.30 (.87–1.94) |
| Washington, D.C. | 26 (7.1) | 289 (13.2) | 0.53 (.34–.83) | 0.82 (.48–1.41) |
| California | 84 (22.9) | 270 (12.3) | 1.85 (1.35–2.52) | 2.23 (1.48–3.36) |
| Illinois | 52 (14.2) | 264 (12.0) | 1.17 (.82–1.67) | 1.37 (.89–2.11) |
| South | 122 (33.2) | 724 (33.0) | REF | REF |
| Marital status | ||||
| Married/partner | 93 (26.4) | 530 (27.5) | 0.95 (.73–1.22) | … |
| Not married/no partner | 259 (73.6) | 1395 (72.5) | REF | … |
| Highest level of education | ||||
| ≤High school graduation | 250 (69.8) | 1273 (64.3) | 1.29 (1.01–1.64) | 0.67 (.49–.92) |
| >High school graduation | 108 (30.2) | 708 (35.7) | REF | REF |
| Employed (full- or part-time) | ||||
| No | 300 (84.0) | 1173 (59.2) | 3.62 (2.69–4.87) | 1.96 (1.34–2.85) |
| Yes | 57 (16.0) | 807 (40.8) | REF | REF |
| Annual household income | ||||
| ≤$24 000 | 302 (88.1) | 1311 (69.3) | 3.27 (2.33–4.60) | 1.51 (.998–2.29) |
| >$24 000 | 41 (12.0) | 582 (30.7) | REF | REF |
| Health insurancec | ||||
| No | 28 (8.0) | 80 (4.1) | 2.05 (1.31–3.20) | … |
| Yes | 323 (92.0) | 1889 (95.9) | REF | … |
| Ever jailed or incarcerated | ||||
| No | 86 (23.4) | 1361 (62.1) | REF | REF |
| Yes | 281 (76.6) | 831 (37.9) | 5.35 (4.14–6.92) | 2.50 (1.81–3.45) |
| Ever reported physical abuse | ||||
| No | 176 (48.0) | 1421 (64.8) | REF | … |
| Yes | 191 (52.0) | 771 (35.2) | 2.0 (1.60–2.50) | … |
| Ever reported sexual abuse | ||||
| No | 235 (64.0) | 1646 (75.1) | REF | REF |
| Yes | 132 (36.0) | 546 (24.9) | 1.69 (1.34–2.14) | 0.92 (.68–1.26) |
| Ever had sex for drugs, money, shelter | ||||
| No | 129 (36.0) | 1379 (69.6) | REF | REF |
| Yes | 229 (64.0) | 602 (30.4) | 4.07 (3.21–5.15) | 2.35 (1.74–3.16) |
| Depressive symptomsd | ||||
| No | 188 (53.9) | 1425 (72.6) | REF | REF |
| Yes | 161 (46.1) | 539 (27.4) | 2.26 (1.79–2.86) | 1.40 (1.05–1.86) |
| Alcohol use | ||||
| Abstain | 152 (43.2) | 1090 (55.3) | REF | REF |
| 0–7 drinks/wk | 105 (29.8) | 741 (37.6) | 1.02 (.78–1.33) | 1.29 (.95–1.77) |
| >7 drinks/wk | 95 (27.0) | 140 (7.1) | 4.87 (3.57–6.64) | 3.79 (2.59–5.55) |
| Tobacco use | ||||
| Never | 28 (7.8) | 675 (34.0) | REF | REF |
| Former | 54 (15.1) | 664 (33.5) | 1.96 (1.23–3.13) | 0.81 (.48–1.37) |
| Current | 276 (77.1) | 644 (32.5) | 10.33 (6.90–15.47) | 3.84 (2.42–6.10) |
| Marijuana use in past year | ||||
| No | 176 (48.0) | 503 (23.0) | REF | … |
| Yes | 191 (52.0) | 1689 (77.1) | 3.64 (2.90–4.58) | … |
| Seen healthcare provider since last visit | ||||
| No | 67 (19.1) | 282 (14.3) | REF | … |
| Yes | 284 (80.9) | 1687 (85.7) | 0.71 (.53–.95) | … |
| Seen psychiatrist or counselor since last visit | ||||
| No | 240 (68.4) | 1488 (75.6) | REF | … |
| Yes | 111 (31.6) | 481 (24.4) | 1.43 (1.12–1.83) | … |
| HIV care in last 6 moe | ||||
| No | 36 (16.5) | 141 (9.9) | REF | … |
| Yes | 182 (83.5) | 1288 (90.1) | 0.55 (.37–.82) | … |
| HIV RNA <200 copies/mLe | ||||
| No | 56 (27.2) | 185 (13.3) | 2.43 (1.72–3.43) | … |
| Yes | 150 (72.8) | 1205 (86.7) | REF | … |
| CD4 >200 cells/µLe | ||||
| No | 21 (9.9) | 91 (6.5) | 1.58 (.96–2.61) | … |
| Yes | 191 (90.1) | 1311 (93.5) | REF | … |
| ART usee | ||||
| No | 26 (11.3) | 121 (7.7) | 1.53 (.98–2.39) | … |
| Yes | 204 (88.7) | 1451 (92.3) | REF | … |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; SD, standard deviation; WIHS, Women’s Interagency HIV Study.
Values in bold indicate statistical significance, defined as p < 0.05.
Percentages are column percentages unless otherwise noted and may not total 100 due to rounding.
Ten-year increments.
Health insurance, AIDS Drug Assistance Program, and/or Ryan White Program.
As defined as Center for Epidemiologic Studies–Depression score ≥16.
Among women with HIV only. A separate adjusted model among women with HIV only was also performed, and these results are reported in the text.
Patterns of Utilization of Types of Substance Use Treatment Programs
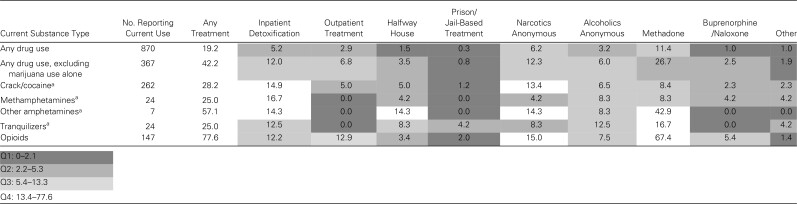
The proportion of women with current SU utilizing each SU treatment program, by substance type, is shown in Table 3 as a heat map. The proportion of women reporting any SU treatment in the past year was 77.6% among those who used opioids, 28.2% among those who used crack/cocaine, and 25.0% among those who used methamphetamines or tranquilizers. Among women with current opioid use (n = 147), 67.4% reported methadone treatment in the past year, and 5.4% received buprenorphine/naloxone treatment. Outpatient detoxification programs were underutilized compared with inpatient programs. Except for use of tranquilizers, <2% of women with other types of SU reported jail-/prison-based treatment programs.
Table 3.
Types of Substance Use Treatment Programs Utilized in the Past Year Among Women's Interagency HIV Study Participants, by Substance Type
Values are reported as row percentages unless otherwise indicated and shaded by quartile, with darker cells representing the lowest quartile of utilization and lighter cells representing the highest quartile.
Number of participants reporting concurrent opioid use: crack/cocaine (n = 57), methamphetamines (n = 9), other amphetamines (n = 5), tranquilizers (n = 9).
Substance Use Treatment Utilization and Associated Factors
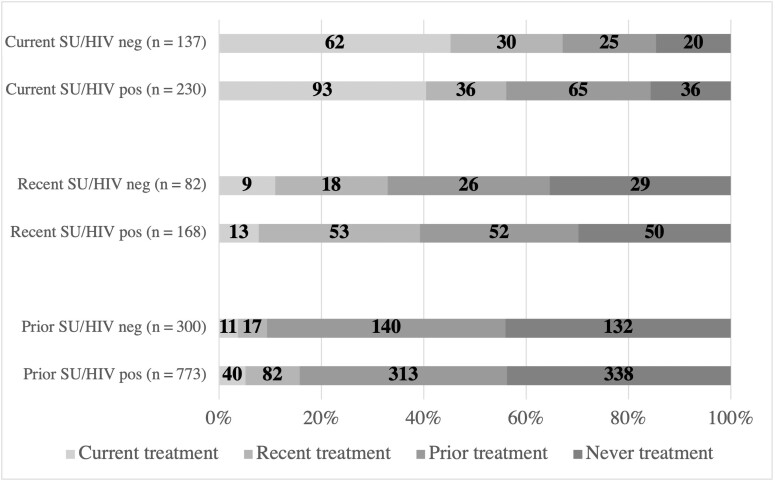
Among women reporting current SU (n = 367), 42.2% (40.4% WWH, 45.3% women without HIV) reported utilization of any SU treatment program in the past year. The most common SU treatment types among those who utilized treatment in the past year were methadone (64%), Narcotics Anonymous (29%), and inpatient detoxification programs (28%). Current, recent, and prior treatment among women with current, recent, and prior SU are shown in Figure 2.
Figure 2.
Current, recent, and prior substance use (SU) treatment among women with current, recent, and prior SU, stratified by human immunodeficiency virus (HIV) serostatus. Current SU or treatment is within the past year. Recent SU or treatment is in the past 1–4.9 years. Prior SU or treatment is ≥5 years ago. Counts are reported with bar graphs showing proportion of current, recent, prior, or never treatment among those reporting substance use.
In an adjusted model, HIV seropositivity was associated with lower odds of SU treatment utilization, although this association did not reach statistical significance (OR, 0.57 [95% CI, .31–1.04]; Table 4). Co-utilization of alcohol was associated with lower odds of treatment (OR, 0.24 [95% CI, .12–.48] for >7 drinks/week), as was use of marijuana (OR, 0.31 [95% CI, .18–.54]). In contrast, current tobacco use was associated with higher odds of treatment (OR, 3.35 [95% CI, 1.07–10.45]). Regarding healthcare utilization, seeing a psychiatrist or counselor since their last visit was associated with higher odds of treatment (OR, 2.46 [95% CI, 1.34–4.50]); however, seeing any healthcare provider since last study visit was not associated with treatment.
Table 4.
Association Between HIV Status, Participant Characteristics, and Substance Use Treatment Utilization in the Past Year Among Women's Interagency HIV Study Participants With Current Substance Use in Crude and Adjusted Regression Models (n = 367)
| Participant Characteristics | Substance Use Treatment Utilization (Within the Past Year) | Odds of Substance Use Treatment Utilization (Within the Past Year) | ||
|---|---|---|---|---|
| SU Treatment (n = 155), No. (%)a | No SU Treatment (n = 212), No. (%)a | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| HIV status | ||||
| Negative | 62 (40.0) | 75 (35.4) | REF | REF |
| Positive | 93 (60.0) | 137 (64.6) | 0.82 (.54–1.26) | 0.57 (.31–1.04) |
| Age, y | ||||
| Mean (SD) | 54.2 (8.1) | 52.1 (8.7) | 1.34 (1.05–1.73) b | 0.90 (.64–1.29)b |
| Race | ||||
| Non-Hispanic Black | 90 (58.1) | 157 (74.1) | 0.49 (.31–.76) | 0.66 (.37–1.18) |
| Other | 65 (41.9) | 55 (25.9) | REF | REF |
| WIHS region | ||||
| New York | 57 (36.8) | 26 (12.3) | 9.44 (4.93–18.05) | 4.65 (2.00–10.79) |
| Washington, D.C. | 7 (4.5) | 19 (9.0) | 1.59 (.60–4.22) | 1.55 (.49–4.92) |
| California | 33 (21.3) | 51 (24.1) | 2.79 (1.48–5.23) | 2.56 (1.19–5.52) |
| Illinois | 35 (22.6) | 17 (8.0) | 8.86 (4.25–18.50) | 6.41 (2.75–14.93) |
| South | 23 (14.8) | 99 (46.7) | REF | REF |
| Marital status | ||||
| Married/partner | 40 (27.2) | 53 (25.9) | 1.07 (.66–1.73) | … |
| Not married/no partner | 107 (72.8) | 152 (74.2) | REF | … |
| Highest level of education | ||||
| ≤High school | 111 (74.0) | 139 (66.8) | 1.41 (.89–2.25) | … |
| >High school | 39 (26.0) | 69 (33.2) | REF | … |
| Employment (full- or part-time) | ||||
| No | 132 (88.0) | 168 (81.2) | 1.70 (.93–3.11) | … |
| Yes | 18 (12.0) | 39 (18.8) | REF | … |
| Annual household income | ||||
| ≤$24 000 | 126 (86.9) | 176 (88.9) | 0.83 (.43–1.60) | … |
| >$24 000 | 19 (13.1) | 22 (11.1) | REF | … |
| Health insurancec | ||||
| No | 6 (4.1) | 22 (10.7) | 0.36 (.14–.90) | 0.43 (.13–1.38) |
| Yes | 140 (95.9) | 183 (89.3) | REF | REF |
| Ever jailed or incarcerated | ||||
| No | 36 (23.2) | 50 (23.6) | REF | … |
| Yes | 119 (76.8) | 162 (76.4) | 1.02 (.63–1.66) | … |
| Ever reported physical abuse | ||||
| No | 78 (50.3) | 98 (46.2) | REF | … |
| Yes | 77 (49.7) | 114 (53.8) | 0.85 (.56–1.29) | … |
| Ever reported sexual abuse | ||||
| No | 105 (67.7) | 130 (61.3) | REF | … |
| Yes | 50 (32.3) | 82 (38.7) | 0.76 (.49–1.17) | … |
| Ever had sex for drugs, money, shelter | ||||
| No | 54 (36.0) | 75 (36.1) | REF | … |
| Yes | 96 (64.0) | 133 (63.9) | 1.00 (.65–1.55) | … |
| Depressive symptomsd | ||||
| No | 84 (57.5) | 104 (51.2) | REF | REF |
| Yes | 62 (42.5) | 99 (48.8) | 0.78 (.51–1.19) | 0.95 (.55–1.64) |
| Alcohol use | ||||
| Abstain | 94 (64.0) | 58 (28.3) | REF | REF |
| 0–7 drinks/week | 31 (21.1) | 74 (36.1) | 0.26 (.15–.44) | 0.35 (.19–.66) |
| >7 drinks/week | 22 (15.0) | 73 (35.6) | 0.19 (.10–.33) | 0.24 (.12–.48) |
| Tobacco use | ||||
| Never | 6 (4.0) | 22 (10.6) | REF | REF |
| Former | 23 (15.3) | 31 (14.9) | 2.72 (.95–7.79) | 2.40 (.66–8.37) |
| Current | 121 (80.7) | 155 (74.5) | 2.86 (1.13–7.28) | 3.35 (1.07–10.45) |
| Marijuana use in past year | ||||
| No | 104 (67.1) | 72 (34.0) | REF | REF |
| Yes | 51 (32.9) | 140 (66.0) | 0.25 (.16–.39) | 0.31 (.18–.54) |
| Seen healthcare provider since last visit | ||||
| No | 23 (15.8) | 44 (21.5) | REF | REF |
| Yes | 123 (84.3) | 161 (78.5) | 1.46 (.84–2.55) | 1.10 (.52–2.33) |
| Seen psychiatrist or counselor since last visit | ||||
| No | 89 (61.0) | 151 (73.7) | REF | REF |
| Yes | 57 (39.0) | 54 (26.3) | 1.79 (1.14–2.82) | 2.46 (1.34–4.50) |
| HIV care in last 6 moe | ||||
| No | 9 (10.6) | 27 (20.3) | REF | … |
| Yes | 76 (89.4) | 106 (79.7) | 2.15 (.96–4.83) | … |
| HIV RNA <200 copies/mLe | ||||
| No | 18 (22.5) | 38 (30.2) | 0.67 (.35–1.29) | … |
| Yes | 62 (77.5) | 88 (69.8) | REF | … |
| CD4 >200 cells/µLe | ||||
| No | 7 (8.5) | 14 (10.8) | 0.77 (.30–2.01) | … |
| Yes | 75 (91.5) | 116 (89.2) | REF | … |
| ART usee | ||||
| No | 9 (9.7) | 17 (12.4) | 0.76 (.32–1.78) | … |
| Yes | 84 (90.3) | 120 (87.6) | REF | … |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; SD, standard deviation; SU, substance use; WIHS, Women’s Interagency HIV Study.
Values in bold indicate statistical significance, defined as p < 0.05.
Percentages are column percentages unless otherwise noted and may not total 100 due to rounding.
Ten-year increments.
Health insurance, AIDS Drug Assistance Program, and/or Ryan White Program.
As defined as Center for Epidemiologic Studies–Depression score ≥16.
Among women with HIV only. A separate adjusted model among women with HIV only was also performed, and these results are reported in the text.
In a separate model among WWH only, we included age, race, WIHS region, health insurance, depressive symptoms, alcohol use, current tobacco use, current marijuana use, seeing a healthcare provider, seeing a psychiatrist/counselor, HIV care, viral suppression, CD4 count >200 cells/μL, and ART use. Only alcohol use was associated with lower odds of SU treatment (OR, 0.20 [95% CI, .08–.48] for 0–7 drinks/week and OR, 0.24 [95% CI, .09–.65] for >7 drinks/week). SU treatment utilization in the past year was not associated with any HIV care continuum outcomes, including engagement in HIV care, ART use, or viral suppression. In a subanalysis of each SU treatment type among WWH, no individual SU treatment type was associated with HIV-related outcomes in adjusted models.
DISCUSSION
Among women in the WIHS cohort, 14% reported past-year SU, with crack/cocaine and opioids being the most frequently used substances. Notably, HIV serostatus was not significantly associated with current SU, and non-Hispanic Black race was associated with lower odds of current SU. Women with current SU had a high degree of concomitant health needs and social vulnerabilities, including depression, transactional sex, history of incarceration, alcohol/tobacco use, and unemployment. These factors should be considered as part of comprehensive, wraparound services in SU treatment programs, especially for women. The National Institute on Drug Abuse recommends wraparound services, which are comprehensive services that address co-occurring needs of individuals with SUD, including medical/HIV care, mental health, child care, housing, transportation, financial, and legal issues [19]. Studies have shown that wraparound services improve access to healthcare and social services, address social determinants of health, and improve child welfare [19, 20].
Regarding SU treatment, among WIHS participants with current SU, 42% utilized any treatment in the past year. This indicates a high level of treatment involvement, especially among a cohort with a majority of Black women aged 50 years and older, a population who historically had low levels of treatment engagement and higher barriers to accessing care in the setting of stigma or discrimination [21, 22]. This level of treatment engagement exceeds national estimates of 10%–30% lifetime SU treatment utilization among US adults [8, 23]. However, when excluding methadone treatment, utilization of other SU treatment programs was lower and mostly <15%, underscoring the need to understand acceptability and barriers to accessing different types of treatment services among women.
The most utilized treatment was methadone, with two-thirds of women with current opioid use reporting methadone treatment. This is substantially higher than recent estimates of <30% past-year utilization of MOUD among those needing opioid treatment [24]. In the general population, MOUD uptake was even lower among older adults, with 13% in the past year among adults ≥50 years [24]. Reasons for our findings of high methadone utilization are unclear. Women with HIV in this cohort may be recruited from Ryan White clinics that have more opportunities for linkage to care with local methadone clinics. It is also possible that older women are more engaged in methadone care, in part because methadone has been used as MOUD since the 1960s, and further research is needed in younger women.
In contrast, compared with high rates of methadone treatment, buprenorphine was considerably underutilized in this cohort of predominantly non-Hispanic Black women, and racial/ethnic disparities in buprenorphine access have been observed in prior studies [25–27]. Disproportionately low buprenorphine use compared with methadone use has been shown in other studies, with 1 study reporting 27% past-year treatment with methadone versus <5% reporting buprenorphine among persons who inject drugs [28, 29]. This may be because buprenorphine was only approved for opioid use disorder (OUD) treatment in 2002. Both methadone and buprenorphine are first-line, evidence-based treatments for OUD and are effective in reducing overdose deaths and opioid craving [30]. Whereas methadone remains highly regulated and requires frequent clinic visits, buprenorphine can be prescribed by any qualified provider with a waiver, making it an ideal treatment in ambulatory settings, including HIV primary care settings. However, the requirement to obtain a waiver to prescribe buprenorphine remains a barrier to treating OUD [31, 32]. Increasing buprenorphine prescribing, including elimination of the waiver requirement, is an opportunity to increase access to OUD treatment for WWH.
In this analysis, WWH had lower odds of SU treatment utilization compared with women without HIV despite similar sociodemographic characteristics, and treatment was not associated with improved HIV care continuum outcomes. Multiple studies have shown that integration of OUD treatment into HIV care settings is feasible and improves both HIV and OUD outcomes [33–37]. Our findings may reflect heterogenous approaches to treating various types of SU within HIV and non-HIV care settings. We do not know the level of integration of HIV/SU services or availability of wraparound services at most clinical sites where WIHS participants received care. We assessed individual-level factors associated with SU treatment, but further research is needed to better understand systems and structural factors that may contribute to our findings, including understanding the landscape of SU and wraparound services offered to women at HIV clinics.
Notably, there were regional differences in SU treatment, with women in Southern WIHS sites having the lowest odds of receiving SU treatment. Prior studies have found similar geographic disparities to accessing SU treatment. For example, 1 study showed that the Southeastern US had the largest gaps in county-level OUD rates and capacity for treatment at opioid treatment programs that accept Medicaid [38]. Similarly, Southern states have lower rates of counties with at least 1 outpatient SUD facility that accepts Medicaid compared with other regions of the country [39]. These geographic disparities in access to SU treatment may be explained by the lack of Medicaid expansion in many Southern states and have important policy implications. Together, our findings call for policies that support increased funding and infrastructure for SU treatment programs in Southern states, especially for Medicaid enrollees.
At the individual level, alcohol use was associated with lower odds of SU treatment, potentially reflecting a group of women at risk for poor health outcomes, especially if they are co-utilizing alcohol and opioids. Finally, we found that despite nearly 90% of women visiting any healthcare provider and/or their HIV provider, these were not associated with SU treatment, highlighting opportunities for improved linkage to SU care or integration of HIV/SU care for women.
Our study has limitations. SU and treatment utilization were self-reported in questionnaires, which may lead to response or desirability bias, as well as potential misclassification. We were unable to distinguish SUD, as defined by Diagnostic and Statistical Manual of Mental Disorders criteria, limiting our ability to identify the true denominator of women who need treatment. Our study did not capture the extent of housing instability or sexual practices, which could influence ongoing SU and HIV transmission. Finally, the mean age of this cohort was >50 years, so our findings may not be generalizable to younger WWH, and since the WIHS recruits from mostly urban settings, our findings may not be generalizable to other parts of the US.
In 2019, WIHS merged with the Multicenter AIDS Cohort Study (MACS) to form the MACS/WIHS Combined Cohort Study (MWCCS) [40], offering the ability to analyze sex/gender-disaggregated data from multiple sites in a population of individuals aging with HIV. A substudy called the Study of Treatment and Reproduction Outcomes (STAR), focused on reproductive-aged women with and without HIV, is also ongoing and will provide data from younger women [41]. In future analyses, we will leverage MWCCS data to understand population-specific factors associated with SU treatment utilization in men and women with/without HIV.
CONCLUSIONS
In the WIHS cohort, SU treatment utilization was higher than previously reported, suggesting the resilience of a population of older, Black women known to face stigma and barriers to healthcare. However, disproportionately low uptake of SU treatment despite recent engagement with a healthcare provider in a medically and socially complex population provides an opportunity to invest in the integration of wraparound services and buprenorphine into HIV and primary care settings.
Supplementary Material
Contributor Information
Ayako W Fujita, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Aditi Ramakrishnan, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Division of Infectious Diseases, School of Medicine, Washington University in St Louis, St Louis, Missouri, USA.
C Christina Mehta, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Oyindamola B Yusuf, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Tracey Wilson, Department of Community Health Sciences, School of Public Health, State University of New York Downstate Health Sciences University, Brooklyn, New York, USA.
Steven Shoptaw, Department of Family Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA.
Adam W Carrico, Department of Public Health Sciences, University of Miami Miller School of Medicine, Miami, Florida, USA.
Adaora A Adimora, Division of Infectious Diseases, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Ellen Eaton, Division of Infectious Diseases, Department of Medicine, University of Alabama School of Medicine, Birmingham, Alabama, USA.
Mardge H Cohen, Department of Medicine, Stroger Hospital of Cook County, Chicago, Illinois, USA.
Jennifer Cohen, Department of Medicine, Infectious Diseases, University of California, San Francisco, San Francisco, California, USA.
Adebola Adedimeji, Division of Health Behavior Research and Implementation Science, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA.
Michael Plankey, Division of General Internal Medicine, Department of Medicine, Georgetown University Medical Center, Washington, District of Columbia, USA.
Deborah Jones, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, Florida, USA.
Aruna Chandran, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Jonathan A Colasanti, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Infectious Disease Program, Grady Health System, Atlanta, Georgia, USA.
Anandi N Sheth, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS), now the Multicenter AIDS Cohort Study/WIHS Combined Cohort Study (MWCCS). The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites. We would also like to thank the WIHS site co-investigators for serving as site liaisons for data collaboration.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. MWCCS (Principal Investigators): Atlanta Clinical Research Site (CRS) (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos, David Hanna, and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D'Souza, Stephen Gange and Elizabeth Topper), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; University of North Carolina (UNC) CRS (Adaora Adimora and Michelle Floris-Moore), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute, with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute on Aging, National Institute of Dental and Craniofacial Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Institute on Drug Abuse (NIDA), National Institute of Nursing Research, National Cancer Institute, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Minority Health and Health Disparities, and in coordination and alignment with the research priorities of the NIH, Office of AIDS Research. MWCCS data collection is also supported by UL1-TR000004 (University of California, San Francisco Clinical and Translational Science Award), UL1-TR003098 (Johns Hopkins University Institute for Clinical and Translational Research), UL1-TR001881 (University of California, Los Angeles Clinical and Translational Science Institute), P30-AI-050409 (Atlanta Center for AIDS Research [CFAR]), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami Center for HIV and Research in Mental Health). A. W. F. is supported by the National Center for Advancing Translational Sciences of the NIH (UL1TR002378 and TL1TR002382) and NIAID (T32AI157855), and also received support from NIDA (R25DA013582). The authors gratefully acknowledge services provided by the Emory CFAR funded through NIAID (P30-AI-050409).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hartzler B, Dombrowski JC, Crane HM, et al. Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS Behav 2017; 21:1138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs 2006; 66:769–89. [DOI] [PubMed] [Google Scholar]
- 3. Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med 2002; 17:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr 2001; 27:251–9. [DOI] [PubMed] [Google Scholar]
- 5. Strathdee SA, Sherman SG. The role of sexual transmission of HIV infection among injection and non-injection drug users. J Urban Health 2003; 80(4 Suppl 3):iii7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 7. The White House . National HIV/AIDS strategy for the United States 2022–2025.https://files.hiv.gov/s3fs-public/NHAS-2022-2025.pdf. Accessed 13 July 2022.
- 8. Lipari RN, Park-Lee E, Van Horn S. America’s need for and receipt of substance use treatment in 2015. The CBHSQ report. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013:1–7. [PubMed]
- 9. El-Bassel N, Terlikbaeva A, Pinkham S. HIV and women who use drugs: double neglect, double risk. Lancet 2010; 376:312–4. [DOI] [PubMed] [Google Scholar]
- 10. Guerrero EG, Marsh JC, Cao D, Shin HC, Andrews C. Gender disparities in utilization and outcome of comprehensive substance abuse treatment among racial/ethnic groups. J Subst Abuse Treat 2014; 46:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenfield SF, Brooks AJ, Gordon SM, et al. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend 2007; 86:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoff E, Marcus R, Bojko MJ, et al. The effects of opioid-agonist treatments on HIV risk and social stability: a mixed methods study of women with opioid use disorder in Ukraine. J Subst Abuse Treat 2017; 83:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adimora AA, Ramirez C, Benning L, et al. Cohort profile: the Women's Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47:393–4i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998; 9:117–25. [PubMed] [Google Scholar]
- 16. Montgomery L, Bagot K, Brown JL, Haeny AM. The association between marijuana use and HIV continuum of care outcomes: a systematic review. Curr HIV/AIDS Rep 2019; 16:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinha S, McCaul ME, Hutton HE, et al. Marijuana use and HIV treatment outcomes among PWH receiving care at an urban HIV clinic. J Subst Abuse Treat 2017; 82:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institute on Alcohol Abuse and Alcoholism . Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking#:~:text=The%20Substance%20Abuse%20and%20Mental, or%20within%20a%20couple%20of. Accessed 29 September 2022.
- 19. Paino M, Aletraris L, Roman P. The relationship between client characteristics and wraparound services in substance use disorder treatment centers. J Stud Alcohol Drugs 2016; 77:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hubberstey C, Rutman D, Van Bibber M, Poole N. Wraparound programmes for pregnant and parenting women with substance use concerns in Canada: partnerships are essential. Health Soc Care Community 2021; 30:e2264–76. [DOI] [PubMed] [Google Scholar]
- 21. Pinedo M, Zemore S, Beltran-Giron J, Gilbert P, Castro Y. Women's barriers to specialty substance abuse treatment: a qualitative exploration of racial/ethnic differences. J Immigr Minor Health 2020; 22:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacMaster SA. Experiences with and perceptions of, barriers to substance abuse and HIV services among African American women who use crack cocaine. J Ethn Subst Abuse 2005; 4:53–75. [DOI] [PubMed] [Google Scholar]
- 23. Boden MT, Hoggatt KJ. Substance use disorders among veterans in a nationally representative sample: prevalence and associated functioning and treatment utilization. J Stud Alcohol Drugs 2018; 79:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mauro PM, Gutkind S, Annunziato EM. Use of medication for opioid use disorder among US adolescents and adults with need for opioid treatment, 2019. JAMA Netw Open 2022; 5:e223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dunphy CC, Zhang K, Xu L, Guy GP Jr. Racial‒ethnic disparities of buprenorphine and vivitrol receipt in Medicaid. Am J Prev Med 2022; 63:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujita AW, Loughry N, Moore DE, et al. Prevalence, distribution, and characteristics associated with possession of buprenorphine waivers among infectious diseases physicians in the United States [manuscript published online ahead of print 24 November 2022]. Clin Infect Dis 2022. doi: 10.1093/cid/ciac909 [DOI] [PubMed] [Google Scholar]
- 27. Schuler MS, Dick AW, Stein BD. Growing racial/ethnic disparities in buprenorphine distribution in the United States, 2007–2017. Drug Alcohol Depend 2021; 223:108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsui JI, Burt R, Thiede H, Glick SN. Utilization of buprenorphine and methadone among opioid users who inject drugs. Subst Abus 2018; 39:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alderks CE. Trends in the use of methadone, buprenorphine, and extended-release naltrexone at substance abuse treatment facilities: 2003–2015 (update). The CBHSQ report. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013:1–8. [PubMed]
- 30. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. D'Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the X-waiver requirement now. Ann Emerg Med 2021; 78:220–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haffajee RL, Bohnert ASB, Lagisetty PA. Policy pathways to address provider workforce barriers to buprenorphine treatment. Am J Prev Med 2018; 54(6 Suppl 3):S230–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Altice FL, Bruce RD, Lucas GM, et al. HIV Treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr 2011; 56(Suppl 1):S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fiellin DA, Weiss L, Botsko M, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr 2011; 56(Suppl 1):S33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lucas GM, Chaudhry A, Hsu J, et al. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: a randomized trial. Ann Intern Med 2010; 152:704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weiss L, Netherland J, Egan JE, et al. Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J Acquir Immune Defic Syndr 2011; 56(Suppl 1):S68–75. [DOI] [PubMed] [Google Scholar]
- 37. Eaton EF, Tamhane A, Turner W, Raper JL, Saag MS, Cropsey KL. Safer in care: a pandemic-tested model of integrated HIV/OUD care. Drug Alcohol Depend 2022; 231:109241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abraham AJ, Andrews CM, Yingling ME, Shannon J. Geographic disparities in availability of opioid use disorder treatment for medicaid enrollees. Health Serv Res 2018; 53:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cummings JR, Wen H, Ko M, Druss BG. Race/ethnicity and geographic access to Medicaid substance use disorder treatment facilities in the United States. JAMA Psychiatry 2014; 71:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D'Souza G, Bhondoekhan F, Benning L, et al. Characteristics of the MACS/WIHS combined cohort study: opportunities for research on aging with HIV in the longest US observational study of HIV. Am J Epidemiol 2021; 190:1457–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sheth AN, Adimora AA, Golub ET, et al. Study of treatment and reproductive outcomes among reproductive-age women with HIV infection in the southern United States: protocol for a longitudinal cohort study. JMIR Res Protoc 2021; 10:e30398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.