Abstract
Antinucleocapsid (anti-N) immunoglobulin G antibody responses were lower in plasma and oral fluid after severe acute respiratory syndrome coronavirus 2 infection in vaccinated patients compared with patients infected before vaccination or infected without vaccination. This raises questions about the long-term use of anti-N antibodies as a marker for natural infection for surveillance.
Keywords: COVID-19, hybrid immunity, nucleocapsid antibody, surveillance, vaccines
Antispike (anti-S) and antinucleocapsid (anti-N) antibodies are often used to differentiate vaccination responses from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1–3]; all coronavirus infectious disease 2019 (COVID-19) vaccine formulations approved in the United States raise antibodies to the S protein only. In the majority of naturally infected people who are not vaccinated, anti-S and anti-N antibodies are detectable for several months postinfection, with anti-N antibodies having a shorter half-life and lower persistence than anti-S antibodies, using assays that have high sensitivity and specificity [4–7]. In contrast, among people who have been vaccinated against SARS-CoV-2 without having been infected, anti-S, but not anti-N, antibodies are induced and maintained [8, 9]. Antibody responses against the vaccine S are considered correlates of protection against infection [10, 11]. Most studies typically measure antibody responses in serum or plasma, but mucosal immunity to SARS-CoV-2, either in respiratory or oral fluid samples, may provide a better correlate of protection [12]. Using plasma and oral fluid, we measured antibody responses against SARS-CoV-2 S and N in participants with confirmed infection, mild disease, and with or without prior vaccination to determine the reliability of anti-N antibodies for identifying infection postvaccination.
METHODS
Study Design
A total of 182 adult participants who (1) were enrolled into the Outpatient SARS-CoV-2 Mild and Asymptomatic Immune Response and Transmission (OutSMART) cohort [13, 14] after December 9, 2020; (2) had confirmed SARS-CoV-2 infection by nasopharyngeal/saliva RNA test; (3) were ≥18 years old; (4) had at least 1 validated oral/plasma antibody result; and (5) had mild COVID-19 with a reported date of symptom onset were included in the analysis. Oral fluid samples were collected between 1 day and 8 months, and plasma samples were collected from 28 days to 8 months postenrollment. Participants were stratified by self-reported vaccination status (confirmed by vaccine card) at the time of symptom onset. Participants who had their first dose of vaccine (Pfizer-BioNTech/Moderna-NIAID/Johnson & Johnson-Janssen) after their COVID-19 symptom onset date were classified as “infected then vaccinated” (n = 101/182). Participants who had their first dose of vaccine before their COVID-19 symptom onset date were classified as “vaccinated then infected” (n = 28/182). Participants who did not have any vaccine record were classified as “never vaccinated” (n = 53/182) (Table 1). Of 182 participants, 174 had oral fluid median fluorescence intensity (MFI) antibody results (98 in the “infected then vaccinated” group, 26 in the “vaccinated then infected” group, and 50 in the “never vaccinated” group), while 143 had plasma antibody results (88 in the “infected then vaccinated” group, 16 in the “vaccinated then infected” group, and 39 in the “never vaccinated” group).
Table 1.
Study Cohort and Participant Characteristics by Vaccination Group
| Vaccinated Then Infected | Infected Then Vaccinated | Never Vaccinated | P Value | |
|---|---|---|---|---|
| n = 28 | n = 101 | n = 53 | n = 182 | |
| Age at time of consent, mean (SD), y | 52.0 (15.5) | 50.4 (14.5) | 49.4 (14.2) | .744 |
| Sex assigned at birth, No. (%) | .453 | |||
| Male | 10 (35.7) | 38 (37.6) | 25 (47.2) | |
| Female | 18 (64.3) | 63 (62.4) | 28 (52.8) | |
| BMI, mean (SD), kg/m2 | 28.0 (8.09) | 30.3 (7.2) | 30.5 (7.6) | .351 |
| Cancer, No. (%) | .017 | |||
| Blood-type cancers | 4 (15.38) | 9 (9.57) | 4 (8.16) | |
| Other cancers | 0 (0) | 0 (0) | 5 (10.2) | |
| Autoimmune disease, No. (%) | 6 (26.09) | 7 (7.45) | 4 (8.0) | .057 |
| Hypertension, No. (%) | 9 (36.0) | 40 (42.55) | 18 (36.0) | .675 |
| Race/ethnicity, No. (%) | .107 | |||
| Non-Hispanic White | 19 (67.9) | 46 (45.5) | 18 (34.0) | |
| African American | 7 (25.0) | 32 (31.7) | 25 (47.2) | |
| Non-Hispanic other | 0 (0.00) | 7 (6.9) | 3 (5.7) | |
| Hispanic | 2 (7.1) | 16 (15.8) | 7 (13.2) | |
| Type of 1st dose vaccine, No. (%) | .155 | |||
| Pfizer-BioNTech | 19 (67.9) | 73 (72.3) | 0 (0.0) | |
| Moderna-NIAID | 6 (21.4) | 26 (25.7) | 0 (0.0) | |
| Johnson & Johnson-Janssen | 3 (10.7) | 2 (2.0) | 0 (0.0) | |
| Type of 2nd dose vaccine, No. (%) | 1.00 | |||
| Pfizer-BioNTech | 20 (74.1) | 71 (72.5) | 0 (0.0) | |
| Moderna-NIAID | 7 (25.9) | 27 (27.5) | 0 (0.0) | |
| Type of 3rd dose vaccine, No. (%) | .495 | |||
| Pfizer-BioNTech | 16 (69.6) | 40 (80.0) | 0 (0.0) | |
| Moderna-NIAID | 7 (30.4) | 10 (20.0) | 0 (0.0) | |
| Days from infection to 1st dose vaccine, mean (SD) | −135.0 (100.4) | 226.1 (120.4) | <.001 | |
| Days from infection to 2nd dose vaccine, mean (SD) | −97.5 (124) | 245 (113.8) | <.001 |
Abbreviations: BMI, body mass index; NIAID, National Institute of Allergy and Infectious Diseases.
Patient Consent
Verbal consent was obtained for all participants enrolled into the OutSMART cohort in accordance with the protocol approved by the Johns Hopkins University (JHU) School of Medicine Institutional Review Board.
Antibody Assays on Plasma
SARS-CoV-2 S- and N-specific immunoglobulin (Ig)G antibody responses in plasma samples were measured by a standardized indirect enzyme-linked immunosorbent assay (ELISA) [15, 16]. Recombinant S and nucleocapsid C-terminal domain (CTD) proteins derived from the ancestral strain of SARS-CoV-2 (SeroNet) were used to coat ELISA plates (2 μg/mL for S and 1 μg/mL for N), and IgG antibody responses were measured using secondary antibodies at 1:5000 dilution (catalog A18823, Invitrogen, ThermoFisher Scientific). A SARS-CoV-2 anti-S monoclonal antibody (dilution factor 1:5000, catalog 40150-D001, Sino Biological) and convalescent plasma (dilution factor 1:100) were used as positive controls for anti-S and anti-N ELISAs, respectively. Prepandemic plasma samples (1:100) were used as negative controls. Results were expressed in international units (binding antibody units [BAU]/mL) based on a 3-fold titration starting with 1:20 dilution. Limit of detection (LOD) was determined as half of the lowest BAU for the sample with a detectable titer (ie, titer ≥20), while samples with undetectable titers (ie, <20) received a value that was half the limit of detection [16].
Antibody Assays on Oral Fluid
Oral fluid samples were tested using a modified version of a previously described multiplex SARS-CoV-2 IgG immunoassay based on Luminex xMAP technology [17, 18]. The multiplex assay included SARS-CoV-2 nucleocapsid (N) and spike (S) antigens in addition to control antibodies and proteins, including bovine serum albumin (BSA), and antihuman IgG, IgM, and IgA antibodies). Ten microliters of oral fluid supernatant was added to a 96-well microtiter plate containing 40 μL of PBST with 0.1% BSA (assay buffer) and 1000 coupled beads per bead set in each well. Each plate contained a dilution series of SARS-CoV-2 IgG standard, high, and low positive controls, a negative control (pre-COVID-era oral fluid), and a blank with assay buffer instead of sample for subtraction of background fluorescence. Positive controls were created by spiking SARS-CoV-2 IgG–positive oral fluid with high IgG levels to SARS-CoV-2 antigens into prepandemic negative oral fluid. Phycoerythrin-labeled antihuman IgG (dilution factor 1:100) in assay buffer was used to detect the IgG signal in oral fluid. Assay plates were read on a Luminex MAGPIX instrument. Results were expressed in units of MFI.
Statistical Analysis
Oral antibody MFI and plasma antibody data were log10-transformed. Data were separately plotted against days after symptom onset by vaccination group. Loess smoothing curves and 95% confidence intervals were generated using ggplot2 in RStudio. Linear mixed-effects regression models were used to statistically compare antibody titers across groups in Stata.
RESULTS
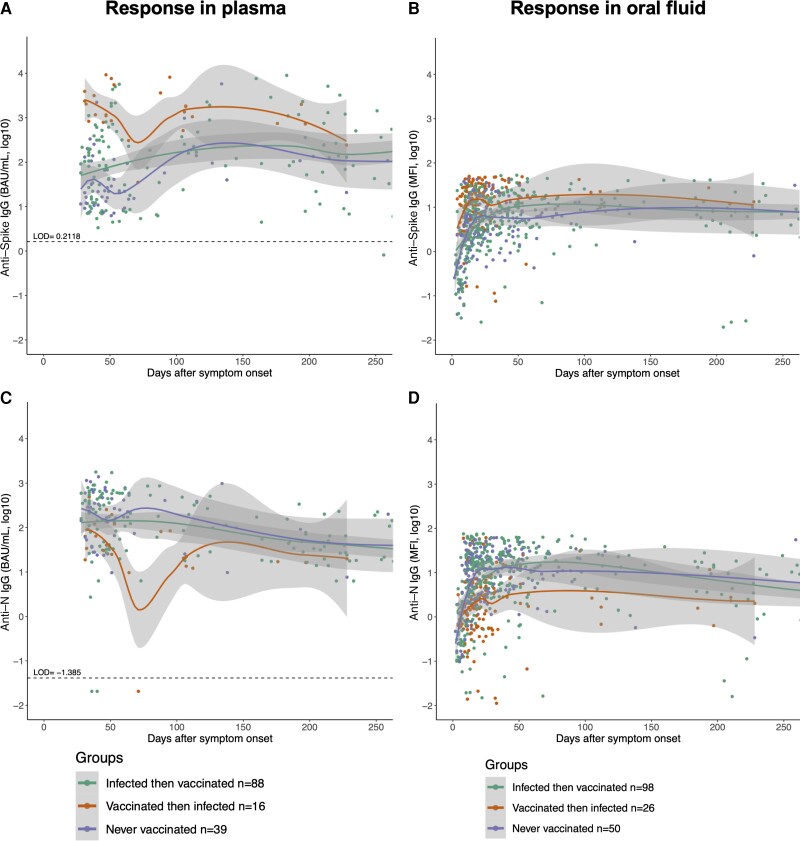
In both plasma and oral fluid, the anti-S antibody responses were greater among patients who were vaccinated before infection or vaccinated after infection than in patients who were never vaccinated (Figure 1A, B). Anti-S IgG titers in groups with hybrid immunity were greatest during the first 100 days after symptom onset. Patients who received vaccination before infection had greater plasma anti-S IgG titers than patients who were infected then vaccinated (P < .001) or never vaccinated (P < .001). Oral anti-S IgG titers were greater in patients who were vaccinated then infected as compared with people who were infected then vaccinated (P < .001) or never vaccinated (P < .001). A different pattern was observed for anti-N IgG antibody responses in both plasma and oral fluid. While patients who were infected before vaccination or were never vaccinated maintained elevated anti-N IgG antibody responses, those who were vaccinated before infection had significantly lower anti-N IgG responses (P < .001 in each case) (Figure 1C). Oral anti-N IgG responses followed a similar pattern, in which responses were significantly greater in patients who were infected but never vaccinated and patients who were infected then vaccinated as compared with patients who were vaccinated then infected (P < .001 in each case) (Figure 1D).
Figure 1.
Ancestral SARS-CoV-2 S- and N-specific IgG antibody responses were measured in plasma (A, C) and oral fluid samples (B, D) of SARS-CoV-2-infected patients at different days after onset of symptoms and compared based on their vaccination status at the time of symptom onset. Loess curves were plotted with 95% CIs, shown in shading, using ggplot2 in RStudio. Statistical tests comparing groups were performed using linear mixed-effects regression models in Stata. Antispike IgG and anti-N IgG titers expressed in log10-transformed units of BAU/mL or MFI were plotted against continuous days after symptom onset based on participant self-reporting. Abbreviations: BAU, binding antibody units; IgG, immunoglobulin G; MFI, mean fluorescence intensity; N, nucleocapsid, S, spike, SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
While infection after vaccination causes the greatest sustained rise in anti-S IgG responses in both plasma and oral fluid, these same participants had the lowest anti-N IgG responses. The N protein in SARS-CoV-2 variants is conserved, has a lower mutation rate than S, and is highly immunogenic [19]. After SARS-CoV-2 infection, N-specific antibodies are induced at a high level, but wane over time [4]. The observed faster waning of anti-N antibodies, however, may be associated with lower sensitivity of the assay being used rather than the actual biological waning of antibodies [20]. Commercial assays used to detect anti-N antibodies have highly variable performances with significant loss of sensitivity with time postinfection [20–22]. Such variability in anti-N antibody assay performance raises concern over their widespread use during surveillance and highlights the importance of standardized, sensitive assays that maintain high sensitivity over time. When infection occurs after vaccination, it is likely that vaccine-induced S-specific antibodies bind virus particles and prevent their efficient replication and persistence at the mucosal surfaces, causing lower relative induction of anti-N antibodies. Though COVID-19 vaccines are administered through intramuscular routes, they can elicit detectable immune responses in the upper and lower respiratory mucosa through antibody transcytosis [23, 24]. Receipt of COVID-19 vaccines not only lowers infectious virus shedding after subsequent infection but also results in faster virus clearance [25–27].
Hybrid immunity, induced by SARS-CoV-2 infection and vaccination, confers more effective immune responses and reduces the risk of reinfection [28, 29]. Data on hybrid immunity are mostly limited to responses in plasma, with no indication of whether this is reflective of mucosal immunity. The consistency in antibody responses between plasma and oral fluid observed in this study illustrates that oral fluid IgG antibody responses are likely a surrogate of IgG antibody responses in plasma during hybrid immunity. Further, in cases where oral fluid is more readily obtained, as in the current study, this compartment is comparable to published data on systemic antibody responses [30]. While hybrid immunity boosted S-specific antibody responses, the same was not observed for N-specific antibodies in plasma or oral fluid samples. These data highlight that measuring N-specific antibody seropositivity may not be useful long term to distinguish vaccine-induced from infection-induced immunity in global COVID-19 surveillance efforts as more people develop hybrid immunity. A seroprevalence study from Ireland, for example, showed that only 26% of individuals who were vaccinated (BNT162b2) then infected had detectable anti-N antibodies, as compared with 82% in individuals who were only infected [31]. A randomized, placebo-controlled mRNA-1273 vaccine efficacy trial also showed that seroconversion to anti-N antibodies after infection was observed in only 40% of vaccinated then infected individuals as compared with 93% of individuals who were infected and never vaccinated [32]. Subsequent studies have also reported the induction of stronger anti-S- but not N-specific antibodies in individuals with hybrid immunity [33, 34]. Our findings in participants who received at least 1 dose of COVID-19 vaccine also raise concern over the use of anti-N antibody assays in identifying recent infections in the vaccinated population. Larger studies will be required to segregate and analyze data based on vaccine types, partial and full vaccination status, and infecting variants of concern. Furthermore, whether anti-N IgG antibody responses are reflective of IgA or sIgA antibody responses in plasma and oral fluid should be further investigated.
CONCLUSIONS
Among mild COVID-19 patients, those with prior vaccination do not reliably induce robust anti-N IgG responses in plasma or oral fluid. Hence, the use of anti-N antibody responses as a surrogate for recent infection may not be reliable for COVID-19 surveillance in the era of expanding hybrid immunity.
Acknowledgments
Financial support. This work was funded by NIH/NCI U54CA260492 to S.L.K. and A.L.C., NIH/NIAID R21AI139784 and R43AI141265 to C.D.H, NIH/NIEHS R01ES026973 to C.D.H, and NIH 3U54EB007958 to Y.C.M. The cohort was supported by the JHU COVID-19 Research and Response Program Fund, the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases Discovery Program, and the Henry Jackson Foundation (W911QY-20-90004).
Contributor Information
Santosh Dhakal, W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Tong Yu, Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Anna Yin, W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Nora Pisanic, Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Zoe O Demko, Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Annukka A R Antar, Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Andrea L Cox, Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Christopher D Heaney, Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Yukari C Manabe, W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Sabra L Klein, W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
References
- 1. Dorschug A, Frickmann H, Schwanbeck J, et al. Comparative assessment of sera from individuals after S-gene RNA-based SARS-CoV-2 vaccination with spike-protein-based and nucleocapsid-based serological assays. Diagnostics (Basel) 2021; 11:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hiramoto S, Miyashita D, Kimura T, et al. Serological screening of immunoglobulin G against SARS-CoV-2 nucleocapsid and spike protein before and after two vaccine doses among healthcare workers in Japan. Tohoku J Exp Med 2022; 257:57–64. [DOI] [PubMed] [Google Scholar]
- 3. Huergo LF, Paula NM, Goncalves ACA, et al. SARS-CoV-2 seroconversion in response to infection and vaccination: a time series local study in Brazil. Microbiol Spectr. 2022; 10:e0102622. Available at: 10.1128/spectrum.01026-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Elslande J, Oyaert M, Ailliet S, et al. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol 2021; 136:104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallais F, Gantner P, Bruel T, et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine 2021; 71:103561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Elslande J, Gruwier L, Godderis L, Vermeersch P. Estimated half-life of SARS-CoV-2 anti-spike antibodies more than double the half-life of anti-nucleocapsid antibodies in healthcare workers. Clin Infect Dis 2021; 73:2366–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jalkanen P, Kolehmainen P, Hakkinen HK, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun 2021; 12:3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pegu A, O'Connell SE, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021; 373:1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021; 39:4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matuchansky C. Mucosal immunity to SARS-CoV-2: a clinically relevant key to deciphering natural and vaccine-induced defences. Clin Microbiol Infect 2021; 27:1724–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demko ZO, Antar AAR, Blair PW, et al. Clustering of SARS-CoV-2 infections in households of patients diagnosed in the outpatient setting in Baltimore, Maryland. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antar AAR, Yu T, Pisanic N, et al. Delayed rise of oral fluid antibodies, elevated BMI, and absence of early fever correlate with longer time to SARS-CoV-2 RNA clearance in a longitudinally sampled cohort of COVID-19 outpatients. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein SL, Pekosz A, Park HS, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest 2020; 130:6141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shapiro JR, Sitaras I, Park HS, et al. Association of frailty, age, and biological sex with SARS-CoV-2 mRNA vaccine-induced immunity in older adults. Clin Infect Dis 2022; 75:S61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pisanic N, Randad PR, Kruczynski K, et al. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J Clin Microbiol 2020; 59:e02204–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heaney CD, Pisanic N, Randad PR, et al. Comparative performance of multiplex salivary and commercially available serologic assays to detect SARS-CoV-2 IgG and neutralization titers. J Clin Virol 2021; 145:104997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng W, Xiang Y, Wu L, et al. Nucleocapsid protein of SARS-CoV-2 is a potential target for developing new generation of vaccine. J Clin Lab Anal 2022; 36:e24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muecksch F, Wise H, Batchelor B, et al. Longitudinal serological analysis and neutralizing antibody levels in coronavirus disease 2019 convalescent patients. J Infect Dis 2021; 223:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karger AB, Brien JD, Christen JM, et al. The serological sciences network (SeroNet) for COVID-19: depth and breadth of serology assays and plans for assay harmonization. mSphere 2022; 7:e0019322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Owusu-Boaitey N, Russell TW, Meyerowitz-Katz G, Levin AT, Herrera-Esposito D. Dynamics of SARS-CoV-2 seroassay sensitivity: a systematic review and modelling study. medRxiv 2022.09.08.22279731 [Preprint]. September 9, 2022. Available at: 10.1101/2022.09.08.22279731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guerrieri M, Francavilla B, Fiorelli D, et al. Nasal and salivary mucosal humoral immune response elicited by mRNA BNT162b2 COVID-19 vaccine compared to SARS-CoV-2 natural infection. Vaccines (Basel) 2021; 9:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang J, Zeng C, Cox TM, et al. Respiratory mucosal immunity against SARS-CoV-2 following mRNA vaccination. Sci Immunol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adamson PC, Pfeffer MA, Arboleda VA, et al. Lower severe acute respiratory syndrome coronavirus 2 viral shedding following coronavirus disease 2019 vaccination among healthcare workers in Los Angeles, California. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puhach O, Adea K, Hulo N, et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med 2022; 28:1491–500. [DOI] [PubMed] [Google Scholar]
- 27. Jung J, Kim JY, Park H, et al. Transmission and infectious SARS-CoV-2 shedding kinetics in vaccinated and unvaccinated individuals. JAMA Netw Open 2022; 5:e2213606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med 2022; 386:2201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suryawanshi R, Ott M. SARS-CoV-2 hybrid immunity: silver bullet or silver lining? Nat Rev Immunol 2022; 22:591–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoschler K, Ijaz S, Andrews N, et al. SARS antibody testing in children: development of oral fluid assays for IgG measurements. Microbiol Spectr 2022; 10:e0078621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allen N, Brady M, Carrion Martin AI, et al. Serological markers of SARS-CoV-2 infection; anti-nucleocapsid antibody positivity may not be the ideal marker of natural infection in vaccinated individuals. J Infect 2021; 83:e9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Follmann D, Janes HE, Buhule OD, et al. Antinucleocapsid antibodies after SARS-CoV-2 infection in the blinded phase of the randomized, placebo-controlled mRNA-1273 COVID-19 vaccine efficacy clinical trial. Ann Intern Med 2022; 175:1258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navaratnam AMD, Shrotri M, Nguyen V, et al. Nucleocapsid and spike antibody responses post virologically confirmed SARS-CoV-2 infection: an observational analysis in the virus watch community cohort. Int J Infect Dis 2022; 123:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qaqish A, Abbas MM, Al-Tamimi M, Abbas MA, Al-Omari M, Alqassieh R. SARS-CoV-2 antinucleocapsid antibody response of mRNA and inactivated virus vaccines compared to unvaccinated individuals. Vaccines (Basel) 2022; 10:643. [DOI] [PMC free article] [PubMed] [Google Scholar]