Abstract
Background
Respiratory syncytial virus (RSV) can cause serious lung infections in young children and there is currently no available vaccine.
Methods
We used complementary statistical frameworks to analyze 4 RSV serology measurements in mothers and their infants in South Africa who participated in a phase 3 maternal immunization trial of an RSV F protein nanoparticle vaccine as correlates of risk and of protection against different RSV disease endpoints.
Results
We found evidence to support each antibody measurement—encompassing RSV-neutralizing antibodies and F surface glycoprotein-binding antibodies—as an inverse correlate of risk of RSV-associated acute lower respiratory tract infection with severe hypoxia in at least 1 framework, with vaccine-induced fold-rise from the maternal enrollment to day 14 samples of anti-F immunoglobulin G (IgG) binding antibodies having the most consistent evidence. This evidence includes a significant association of fold-rise anti-F IgG with vaccine efficacy (VE); achieving a baseline covariate-adjusted VE of 75% requires a vaccine-induced maternal anti-F IgG fold-rise of around 16. Neither multivariable logistic regression nor superlearning analyses showed benefit to including multiple time points or assays in the same model, suggesting a parsimonious correlate. Post hoc exploratory analyses supported adherence of vaccine-induced maternal anti-F IgG fold-rise to the Prentice criteria for a valid surrogate endpoint.
Conclusions
Our results suggest that the vaccine induced protective anti-F antibody responses. If this finding is confirmed, VE could potentially be augmented by increasing these responses.
Keywords: F glycoprotein, nonparametric threshold regression, Prentice criteria surrogate endpoint, principal stratification causal inference, superlearning
Respiratory syncytial virus (RSV) is the most common etiological agent of acute lower respiratory tract infection (LRTI) in children aged <5 years [1]. Maternal immunization elicits antibodies that can be transferred transplacentally to the fetus and through breast milk to the infant [2]. Three maternal RSV vaccine candidates are currently in clinical trials [3]. The identification of correlates of protection (CoPs) [4] against infant RSV disease or severe disease could aid vaccine development and licensure. Serum neutralizing antibodies (nAbs) in maternal blood and in infant cord blood are implicated in protection against RSV-associated LRTI and/or severe RSV disease in infants via passive nAb administration/challenge studies in animal models [5–8], prospective and case-control observational studies in infants/young children [9–14], and clinical trials of passively administered high-nAb-titer immune globulin [15, 16] and of palivizumab [17, 18] in high-risk infants/young children. However, maternal vaccination likely elicits a polyfunctional immune response [19], and thus CoPs of maternal immunization may differ from those in natural infection or passive transfer studies.
In the Prepare trial [20], healthy pregnant women were randomized to a single injection of recombinant prefusogenic RSV fusion (F) protein nanoparticle vaccine or placebo. Vaccination elicited maternal anti-F immunoglobulin G (IgG) antibodies that were transferred transplacentally; estimated vaccine efficacy (VE) during the first 90 days of life against the primary endpoint of RSV-associated, medically significant LRTI was modest (39.4% [95% confidence interval [CI], 5.3%–61.2%) [20]. Estimated VE was also modest against the secondary endpoints of RSV-associated LRTI with severe hypoxemia (48.3% [95% CI, −8.2% to 75.3%]) and RSV-associated LRTI hospitalization (44.4% [95% CI, 19.6%–61.5%]).
We assessed maternal and infant antibody markers as correlates of risk and as CoPs against RSV disease endpoints during the first 90 days of life in the Prepare trial. The trial was conducted in both high-income and low- to middle-income countries (HICs and LMICs, respectively). As VE against the various endpoints was variable in HICs but consistently moderate to high in LMICs [20], and 95% of LMIC cases were in South Africa, we restricted analyses to South Africa (South Africa estimated VE, 73.8% [95% CI, 50.4%–86.2%] against RSV-associated LRTI with severe hypoxemia and estimated VE, 76.7% [95% CI, 54.1%–88.2%] against RSV-associated LRTI hospitalization). Focusing on a setting with moderate-to-high VE increases power to detect CoPs and restricting to 1 country reduces the risk of confounding in CoPs analyses.
METHODS
Antibody Assays
Serum nAbs against RSV-A and RSV-B subtypes were measured by the microneutralization assay [21]. Concentrations of anti-F IgG antibodies (enzyme immunoassay [EIA]) and palivizumab-competitive antibodies (PCAs), the latter of which block the binding of palivizumab to antigenic site II on the F protein [22], were measured by enzyme-linked immunosorbent assay [23] (Supplementary Methods).
Approach to Assessing Immune Correlates of Risk and of Protection
All analyses were prespecified in the statistical analysis plan except where noted.
The immune correlates analyses were restricted to South African study sites and follow-up period of infants through 90 days of age. Correlates for each of 3 nested RSV disease endpoints were assessed: Endpoint 1 was medically significant RSV-associated LRTI, RSV-associated LRTI with hospitalization, or RSV-associated LRTI with severe hypoxemia (52 vaccine and 51 placebo endpoints). Endpoint 2 was RSV-associated LRTI with severe hypoxemia (14 vaccine and 27 placebo endpoints). Endpoint 3 was RSV LRTI with severe hypoxemia without cough as an element of the definition of LRTI (12 vaccine and 26 placebo endpoints) (Supplementary Figure 1). We only report results for endpoints 1 and 2 given that 38 of the 41 participants with endpoint 2 also experienced endpoint 3. Based on a reviewer's suggestion, correlates were also assessed against a post hoc–defined endpoint, medically significant RSV-associated LRTI only (endpoint 4; 38 vaccine and 44 placebo endpoints).
Sixteen antibody markers were included in the statistical analysis plan, as defined by the 4 assays (EIA, PCA, RSV-A, RSV-B) cross-classified by measurement time point and whether fold-rise was considered (maternal vaccination blood sample at day 0 [D0], maternal postvaccination blood sample at day 14 [D14], maternal fold-change from D0 to D14 [D14/D0], and infant cord blood [Cord]; we refer to these 4 marker sets as the 4 measurement time point sets, such that D14/D0 is considered to be a single time point). Post hoc analyses of fold-change from D0 to Cord (Cord/D0) of the 4 assay markers were also performed.
Cohort for Correlates Analyses
The correlates analyses were performed on all mother–infant pairs at South African sites whose maternal participants qualified for the per-protocol efficacy cohort for maternal participants, defined as all maternal participants who received the test article and regimen to which they were randomized and had at least 1 posttreatment encounter documented during which active and/or passive surveillance activities for RSV-suspected illness could occur, and had no major protocol deviations affecting the primary efficacy outcomes. Only 2 randomized maternal participants did not qualify for the per-protocol efficacy cohort.
Case and Control Definitions
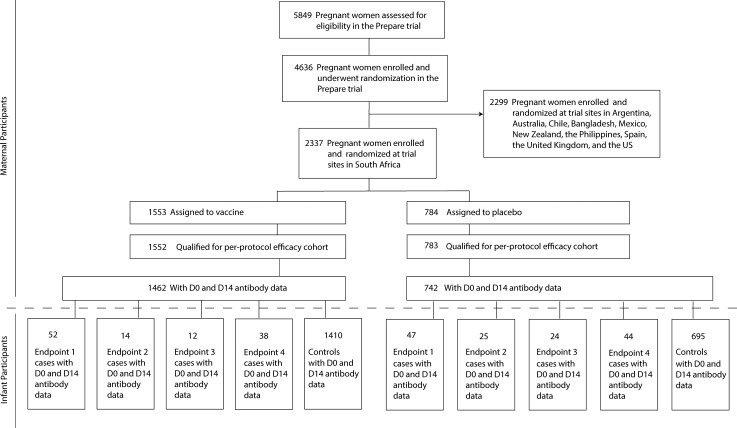
Cases are defined as infants in the correlates analysis cohort with an RSV illness defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the “expanded data set,” which included physical findings, pulse oximetry data, and RSV diagnoses extracted from the medical records of infants hospitalized for respiratory or infectious illness in addition to data from study sites. When studying correlates for RSV disease defined by a given endpoint (1, 2, 3, or 4), controls are defined as infants who did not experience RSV disease defined by that same endpoint through to 90 days of age in the expanded data set. Figure 1 depicts the flow of participants from assessment for eligibility in the overall trial to cases and controls included in the analysis.
Figure 1.
Participant flow, from assessment for eligibility in the Prepare trial through to cases and controls included in the analysis. Participant flow into endpoint 1, 2, 3, and/or 4 case boxes is not mutually exclusive; that is, an infant participant could be classified in multiple endpoint boxes. Abbreviations: D0, day 0; D14, day 14; US, United States.
Two-Phase Sampling Design for Measuring Antibody Markers
EIA and PCA antibody concentrations were measured at all available time points from
all participants. A 2-phase “cumulative case-control” sampling design was used for determining the subset of participants from whom to measure microneutralization antibody titers (Supplementary Methods). As the study outcomes were rare, a cumulative case-control design was used to ensure that all controls were “pure controls” who did not experience the outcome over the whole follow-up period.
Exploratory Methods for Comparing Antibody Markers Between Cases and Controls
Boxplots are used to show the distributions of antibody markers for cases and controls separately. The midline of the box denotes the median and ends of the box denote the 25th and 75th percentiles, with whiskers extending to the most extreme data points. CIs for the geometric mean concentrations of antibody markers accounting for the 2-phase sampling weights are computed using the R package survey [24].
Risk Scores
Before the correlates analyses, superlearning was used to develop a maternal enrollment/baseline RSV risk score based on maternal characteristics at enrollment and an infant birth/delivery RSV risk score based on infant characteristics at birth/delivery (Supplementary Methods, Supplementary Figure 2).
Correlates Analyses
The correlates analysis methods are summarized below (see Supplementary Methods). All markers were on the log10 scale.
Univariable Correlates of Risk Analyses
Univariable correlates of risk analysis addresses whether an antibody marker associates with an RSV disease outcome for infants of vaccinated mothers, where the finding of an inverse correlation provides the first step of evidence that the antibody marker may be predictive of the degree of protection conferred by the vaccine. Because an antibody marker may be an inverse correlate of risk but not predictive of protection due to confounding of the effect of the marker on RSV disease or other factors (eg, [25]), a correlate of risk is not necessarily a CoP.
Logistic Regression Correlates of Risk Modeling
We assessed the association of each of the 16 antibody markers with each endpoint within each study arm. All models adjusted for maternal risk score. Time from vaccination to birth was adjusted for in analyses of maternal markers but not in analyses of infant markers. For the logistic regression analyses, family-wise error rate (Holm-Bonferroni) and false-discovery rate (q values; Benjamini-Hochberg) adjustment was applied, separately for the vaccine and placebo groups (see Supplementary Materials for details).
Nonparametric Threshold Correlates of Risk Modeling
Targeted maximum likelihood estimation with adjustment for the same covariates as with logistic regression was applied to each D14, D14/D0 fold-change, Cord, and Cord/D0 fold-change antibody marker (the last of these markers was post hoc), for each endpoint [26] (see Supplementary Materials for details).
Machine Learning Multivariable Correlates of Risk Analysis
The next statistical framework—machine learning of multiple antibody marker sets to build best models that classify whether infants of vaccinated mothers will acquire an RSV disease endpoint of interest—is a type of correlates of risk analysis, not a CoP analysis. Its new features compared to the univariable framework summarized above are that (1) it focuses on the analysis of many input variables seeking to discover the best way to aggregate information across these variables (including baseline factors, antibody markers at multiple time points, and combinations and interactions of these variables) to predict RSV disease occurrence; and (2) the evaluation of model performance is based on metrics that quantify how well individual infants can be classified by RSV disease outcome, which, being about prediction, involves cross-validation to quantify prediction accuracy for infants not included in the model building. See Supplementary Figure 2.
For this analysis, superlearning [27, 28] was conducted to build models most predictive of individual-level risk of each endpoint (see learner–screen combinations in Supplementary Table 1), within each treatment arm, based on each input variable set (Supplementary Table 2) using different combinations of the 16 antibody markers, always controlling for maternal risk factors.
Principal Stratification Correlate of VE CoP Analysis
Principal stratification correlates of VE analyses directly assess a CoP, by estimating whether and how the level of VE varies over vaccinated subgroups defined by the level of their antibody marker. For each endpoint, VE-by-marker-level curves were estimated using the pseudo-score estimator [29, 30], for D14/D0 fold-change EIA and PCA and for D14 RSV-A and RSV-B. Family-wise error rate (Holm-Bonferroni) and false-discovery rate (q values; Benjamini-Hochberg) adjustment was applied (see Supplementary Materials for details).
Prentice Surrogate Endpoint Evaluation CoP Analysis
Prentice surrogate endpoint analysis—distinct from principal stratification analysis—is another approach to directly assess a CoP, which evaluates whether the data are consistent with the Prentice criteria for whether an antibody marker qualifies as a valid surrogate endpoint for an RSV disease endpoint. If an antibody marker is a valid surrogate endpoint as defined by Prentice [31], then a hypothesis test for whether the vaccine increases the antibody marker level compared to placebo is a valid test of whether the VE against the RSV disease endpoint is positive. In post hoc analyses, logistic regression with adjustment for the same covariates noted above was used to assess the Prentice criteria for a valid surrogate endpoint for endpoint 2 [31]. The models for RSV-A and RSV-B were fit to case/control (phase 2) data; the models for PCA and EIA were fit to cohort (phase 1) data.
Patient Consent Statement
The protocol of the Prepare trial [20] was reviewed and approved by regulatory authorities in all countries and by ethical review committees at all trial sites. All maternal participants provided written informed consent, and parental consent for the participation of infants was obtained according to the standards at each trial site.
RESULTS
Characterization of Vaccine-Elicited Antibody Measurements
Vaccination elicited high levels of maternal RSV antibodies on D14, particularly anti-F binding antibodies, with slightly less effective induction of nAbs; cord blood levels of each of the 4 antibody markers were only slightly lower than the maternal D14 levels (Supplementary Figure 3). Table 1 (vaccine recipients) and Table 2 (placebo recipients) present RSV antibody levels in mothers and their infants by the severe RSV disease composite endpoint (endpoint 1) case, RSV-associated LRTI with severe hypoxemia (endpoint 2) case, and control status. The EIA and PCA markers were highly correlated in both treatment arms (Spearman ρ ≥ 0.8 for all time points), whereas the RSV-A and RSV-B markers were less correlated (Spearman ρ = 0.44–0.61 across time points) (Supplementary Figure 4).
Table 1.
Antibody Levels in Mothers (Vaccine Recipients) and Their Infants by Case/Control Outcome Status
| Marker | Controlsa | Cases, Endpoint 1b | Cases, Endpoint 2c | Case/Control Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison for Endpoint 1 Cases | Comparison for Endpoint 2 Cases | ||||||||||||
| No. | Absolute Level GMT or GMC (95% CI) | GM Fold-Rise (95% CI) | No. | Absolute Level GMT or GMC (95% CI) | GM Fold-Rise (95% CI) | No. | Absolute Level GMT or GMC (95% CI) | GM Fold-Rise (95% CI) | GMT or GMC Ratio (95% CI) | GM Fold-Rise Ratio (95% CI) | GMT or GMC Ratio (95% CI) | GM Fold-Rise Ratio (95% CI) | |
| EIA D0 | 1485 | 534 (104–2734) | NA | 52 | 608 (103–3602) | NA | 14 | 798 (142–4476) | … | 1.1 (.1–12.7) | NA | 1.5 (.1–16.1) | NA |
| PCA D0 | 1485 | 13 (3–46) | NA | 52 | 14 (3–58) | NA | 14 | 16 (5–58) | … | 1.1 (.2–7.5) | NA | 1.3 (.2–7.9) | NA |
| RSV-A D0 | 210 | 693 (126–3815) | NA | 52 | 730 (121–4416) | NA | 14 | 888 (124–6352) | … | 1.1 (.1–12.6) | NA | 1.3 (.1–17.3) | NA |
| RSV-B D0 | 210 | 567 (78–4136) | NA | 52 | 684 (66–7096) | NA | 14 | 771 (132–4517) | … | 1.2 (.1–26.0) | NA | 1.4 (.1–19.4) | NA |
| EIA D14 | 1419 | 10 667 (2260–50 356) | 20 (3–158) | 52 | 10 112 (2787–36 691) | 17 (3–95) | 14 | 7277 (1617–32 747) | 9 (3–31) | 0.9 (.1–7.1) | 0.8 (.1–12.3) | 0.7 (.1–5.9) | 0.5 (.0–5.0) |
| PCA D14 | 1419 | 162 (46–575) | 13 (2–70) | 52 | 168 (52–545) | 12 (3–53) | 14 | 127 (31–516) | 8 (2–30) | 1.0 (.2–5.8) | 0.9 (.1–8.9) | 0.8 (.1–5.2) | 0.6 (.1–5.3) |
| RSV-A D14 | 210 | 1522 (273–8472) | 2 (0–11) | 52 | 1582 (326–7687) | 2 (0–13) | 14 | 1232 (207–7335) | 1 (0–11) | 1.0 (.1–10.7) | 1.0 (.1–11.4) | 0.8 (.1–9.6) | 0.6 (.0–8.7) |
| RSV-B D14 | 210 | 1619 (209–12 525) | 3 (1–15) | 52 | 2017 (214–19 017) | 3 (1–17) | 14 | 1426 (176–11 542) | 2 (0–8) | 1.2 (.1–25.9) | 1.0 (.1–11.4) | 0.9 (.0–16.4) | 0.6 (.1–6.1) |
| EIA Cord | 1391 | 8450 (1667–42 837) | 16 (2–130) | 50 | 9035 (2876–28 379) | 14 (3–74) | 14 | 6843 (2190–21 377) | 9 (2–31) | 1.1 (.1–7.8) | 0.9 (.1–12.8) | 0.8 (.1–5.9) | 0.5 (.0–6.3) |
| PCA Cord | 1379 | 126 (33–491) | 10 (2–56) | 50 | 131 (50–344) | 9 (2–38) | 14 | 100 (38–261) | 6 (2–20) | 1.0 (.2–5.5) | 0.9 (.1–8.6) | 0.8 (.1–4.2) | 0.6 (.1–5.0) |
| RSV-A Cord | 210 | 1467 (246–8760) | 2 (0–10) | 52 | 1689 (430–6626) | 2 (0–11) | 14 | 1325 (435–4035) | 1 (0–5) | 1.2 (.1–10.9) | 1.1 (.1–9.9) | 0.9 (.1–7.4) | 0.7 (.1–4.6) |
| RSV-B Cord | 210 | 1148 (152–8663) | 2 (0–10) | 52 | 1522 (207–11 169) | 2 (0–10) | 14 | 1194 (220–6481) | 2 (1–5) | 1.3 (.1–22.7) | 1.1 (.1–10.2) | 1.0 (.1–14.5) | 0.8 (.1–5.4) |
Abbreviations: CI, confidence interval; Cord, infant cord blood; D0, day 0; D14, day 14; EIA, enzyme immunoassay; GMC, geometric mean concentration; GMT, geometric mean titer, NA, not applicable; PCA, palivizumab-competitive antibody; RSV-A, respiratory syncytial virus subtype A; RSV-B, respiratory syncytial virus subtype B.
Infants in the correlates analysis cohort who did not experience RSV disease defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set.
Infants in the correlates analysis cohort with medically significant RSV-associated lower respiratory tract infection (LRTI), RSV-associated LRTI with hospitalization, or RSV-associated LRTI with severe hypoxemia through 90 days of age in the expanded data set.
Infants in the correlates analysis cohort with RSV-associated LRTI with severe hypoxemia through 90 days of age in the expanded data set.
Table 2.
Antibody Levels in Mothers (Placebo Recipients) and Their Infants by Case/Control Outcome Status
| Marker | Controlsa | Cases, Endpoint 1b | Cases, Endpoint 2c | Case/Control Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison for Endpoint 1 Cases | Comparison for Endpoint 1 Cases | ||||||||||||
| No. | Absolute Level GMT or GMC (95% CI) | GM Fold-Rise (95% CI) | No. | Absolute Level GMT or GMC (95% CI) | GM Fold-Rise (95% CI) | No. | Absolute Level GMT or GMC (95% CI) | GM Fold-Rise (95% CI) | GMT or GMC Ratio (95% CI) | GM Fold-Rise Ratio (95% CI) | GMT or GMC Ratio (95% CI) | GM Fold-Rise Ratio (95% CI) | |
| EIA D0 | 724 | 549 (108–2783) | NA | 51 | 532 (122–2312) | NA | 27 | 565 (151–2119) | … | 1.0 (.1–8.7) | NA | 1.0 (.1–8.4) | NA |
| PCA D0 | 724 | 13 (4–48) | NA | 51 | 14 (4–49) | NA | 27 | 16 (5–54) | … | 1.1 (.2–6.4) | NA | 1.2 (.2–7.1) | NA |
| RSV-A D0 | 104 | 792 (167–3752) | NA | 51 | 681 (123–3760) | NA | 27 | 740 (130–4206) | … | 0.9 (.1–8.7) | NA | 0.9 (.1–9.6) | NA |
| RSV-B D0 | 104 | 583 (97–3503) | NA | 51 | 517 (61–4412) | NA | 27 | 509 (64–4040) | … | 0.9 (.1–14.5) | NA | 0.9 (.1–13.5) | NA |
| EIA D14 | 702 | 569 (114–2847) | 1 (0–3) | 47 | 527 (131–2122) | 1 (0–2) | 25 | 562 (154–2051) | 1 (0–2) | 0.9 (.1–7.8) | 1.0 (.3–3.5) | 1.0 (.1–7.8) | 1.0 (.3–3.4) |
| PCA D14 | 702 | 12 (3–45) | 1 (0–2) | 47 | 12 (4–36) | 1 (0–2) | 25 | 12 (4–34) | 1 (0–2) | 1.0 (.2–5.3) | 0.9 (.3–2.7) | 1.0 (.2–5.1) | 0.8 (.2–2.8) |
| RSV-A D14 | 104 | 772 (161–3692) | 1 (0–4) | 51 | 668 (133–3367) | 1 (0–3) | 27 | 660 (149–2925) | 1 (0–3) | 0.9 (.1–8.2) | 1.0 (.2–6.4) | 0.9 (.1–7.4) | 0.9 (.2–5.3) |
| RSV-B D14 | 104 | 556 (76–4063) | 1 (0–3) | 51 | 465 (70–3115) | 1 (0–3) | 27 | 430 (69–2665) | 1 (0–2) | 0.8 (.1–13.1) | 0.9 (.2–5.0) | 0.8 (.1–11.5) | 0.9 (.2–4.1) |
| EIA Cord | 686 | 625 (127–3071) | 1 (0–4) | 48 | 648 (153–2755) | 1 (0–4) | 25 | 664 (166–2646) | 1 (0–3) | 1.0 (.1–8.9) | 1.1 (.2–5.6) | 1.1 (.1–8.8) | 1.0 (.2–4.8) |
| PCA Cord | 680 | 13 (3–48) | 1 (0–3) | 48 | 14 (5–41) | 1 (0–2) | 25 | 14 (5–40) | 1 (0–2) | 1.1 (.2–6.0) | 1.0 (.3–3.8) | 1.1 (.2–6.0) | 0.9 (.2–3.8) |
| RSV-A Cord | 104 | 694 (151–3186) | 1 (0–3) | 51 | 614 (121–3111) | 1 (0–3) | 27 | 663 (142–3104) | 1 (0–3) | 0.9 (.1–8.2) | 1.0 (.2–5.1) | 1.0 (.1–8.4) | 1.0 (.2–5.3) |
| RSV-B Cord | 104 | 582 (83–4083) | 1 (0–4) | 51 | 506 (58–4435) | 1 (0–4) | 27 | 510 (50–5178) | 1 (0–3) | 0.9 (.0–16.1) | 1.0 (.1–7.3) | 0.9 (.0–18.1) | 1.0 (.2–6.5) |
Abbreviations: CI, confidence interval; Cord, infant cord blood; D0, day 0; D14, day 14; EIA, enzyme immunoassay; GMC, geometric mean concentration; GMT, geometric mean titer, NA, not applicable; PCA, palivizumab-competitive antibody; RSV-A, respiratory syncytial virus subtype A; RSV-B, respiratory syncytial virus subtype B.
Infants in the correlates analysis cohort who did not experience RSV disease defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set.
Infants in the correlates analysis cohort with medically significant RSV-associated lower respiratory tract infection (LRTI), RSV-associated LRTI with hospitalization, or RSV-associated LRTI with severe hypoxemia through 90 days of age in the expanded data set.
Infants in the correlates analysis cohort with RSV-associated LRTI with severe hypoxemia through 90 days of age in the expanded data set.
Inverse Association of Vaccine-Elicited Maternal Antibody With Infant Severe RSV Disease
We first report results of the markers measured in cord blood as correlates of risk, as cord blood levels of neutralizing antibodies against RSV-A or RSV-B are hypothesized to be CoPs. Moreover, a reviewer recommended first assessing cord blood markers as correlates of risk of a post hoc endpoint, medically significant RSV-associated LRTI alone, based on the rationale that this was the primary endpoint of the Prepare trial [20]. We performed this assessment against medically significant RSV-associated LRTI alone (endpoint 4), which only differed from the primary efficacy endpoint of the Prepare trial in that endpoint 4 used the expanded data set instead of only the site data (estimated VE against endpoint 4 was 40.9% [95% CI, 15.9%–58.5%], which is very close to that estimated using only the site data, 39.4% [95% CI, 5.3%–61.2%], but with a narrower CI). The results did not provide evidence that higher cord blood levels of the RSV-A or RSV-B markers correlated with a lower rate of endpoint 4 (Supplementary Table 3).
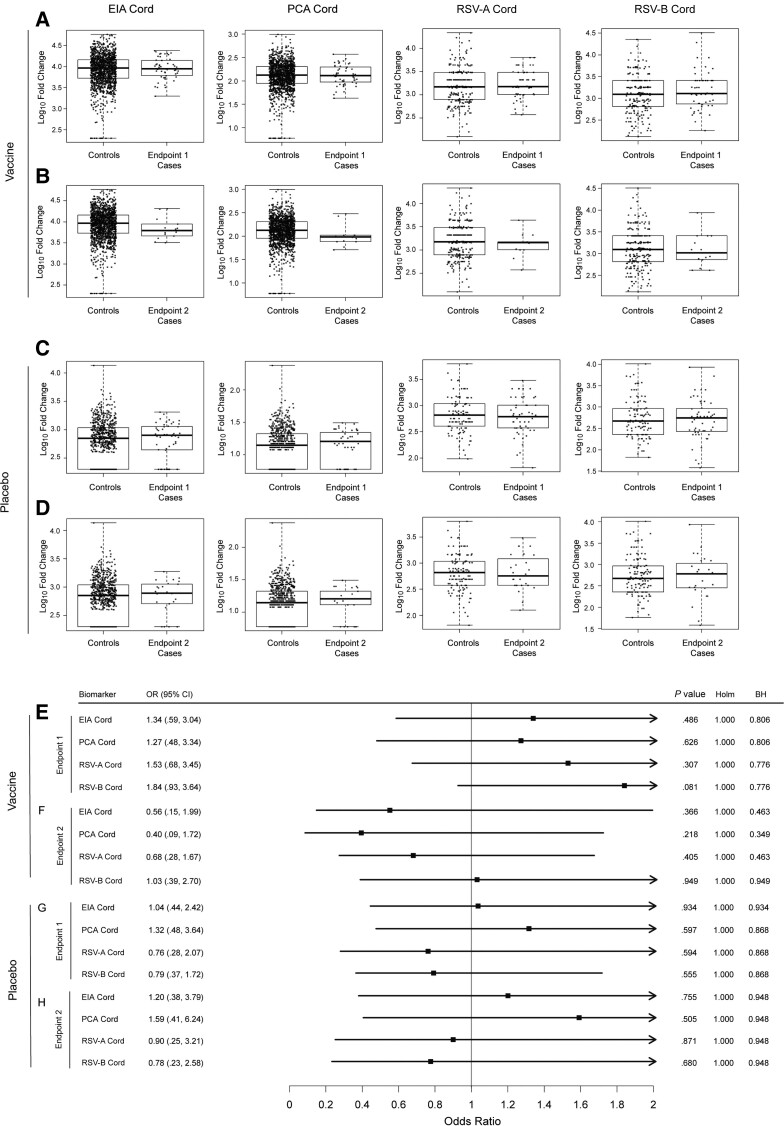
For the prespecified endpoints 1 and 2, the data also did not provide evidence that higher cord blood levels of any of the 4 antibody markers correlated with a lower endpoint rate, for either treatment arm (Figure 2). Specifically, based on logistic regression modeling, for endpoint 1 the point estimate of the odds ratio (OR) per 10-fold higher cord blood marker value exceeded 1 for each of the markers in vaccine recipients, and these point estimates were near 1 (ranging from 0.76 to 1.32) in placebo recipients. For endpoint 2 the point estimates suggested potential inverse correlates of risk in vaccine recipients (0.56, 0.40, 0.68, and 1.03 for EIA, PCA, RSV-A, and RSV-B, respectively), but the 95% CIs overlapped 1; for placebo recipients the point estimates were near 1.
Figure 2.
A–D, Boxplots of enzyme immunoassay, palivizumab-competitive antibody, and respiratory syncytial virus (RSV) subtypes A and B infant cord blood (Cord) levels by case/control status (A and C, endpoint 1 cases; B and D, endpoint 2 cases) in the vaccine arm (A and B) or the placebo arm (C and D). E–H, Odds ratios in the vaccine arm (E and F) or the placebo arm (G and H) of experiencing RSV disease defined by endpoint 1 (E and G) or endpoint 2 (F and H) per 10-fold increase in the designated immunologic biomarker, based on logistic regression modeling adjusting for maternal risk score and number of days from vaccination to birth. Cases were defined as infants in the correlates analysis cohort with an RSV illness defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set. Controls were defined as infants who did not experience RSV disease defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set. Endpoint 1 was defined as medically significant RSV-associated lower respiratory tract infection (LRTI), RSV-associated LRTI with hospitalization, or RSV-associated LRTI with severe hypoxemia (52 vaccine endpoints). Endpoint 2 was defined as RSV-associated LRTI with severe hypoxemia (14 vaccine endpoints). Abbreviations: BH, false-discovery rate (q value, Benjamini-Hochberg) adjustment applied separately for the vaccine arm; CI, confidence interval; Cord, infant cord blood; EIA, enzyme immunoassay; Holm, family-wise error rate (Holm-Bonferroni); OR, odds ratio; PCA, palivizumab-competitive antibody; RSV-A, respiratory syncytial virus subtype A; RSV-B, respiratory syncytial virus subtype B.
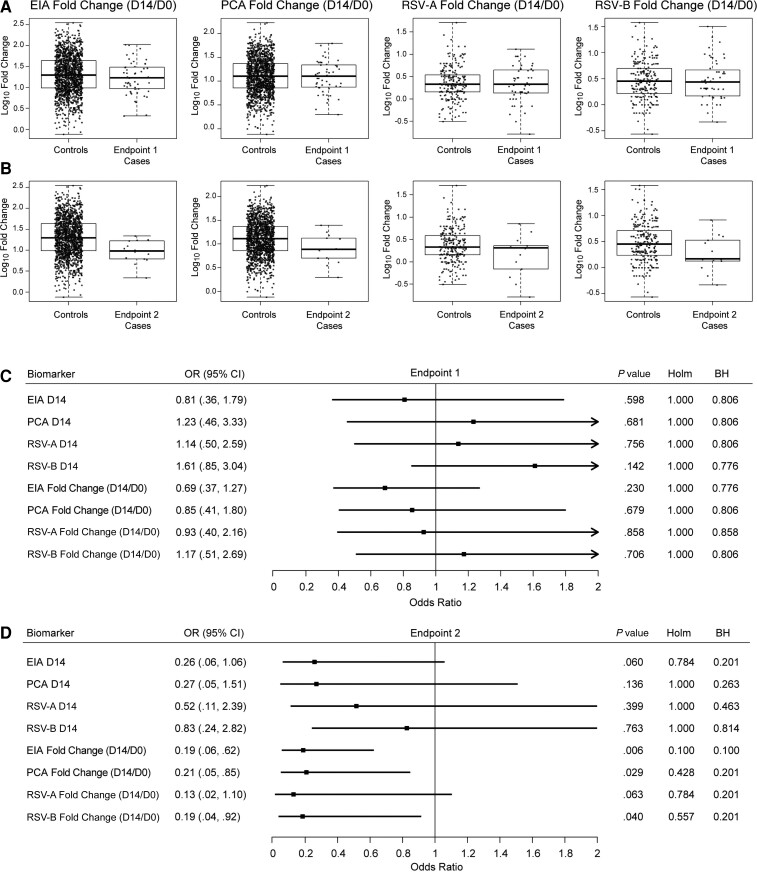
Next, we assessed the antibody markers at D14 and D14/D0 fold-change as correlates of risk. In vaccine recipients, D14/D0 fold-change was similar between endpoint 1 cases versus controls (Figure 3A); however, D14/D0 fold-change tended to be lower for each of the 4 antibody markers in endpoint 2 cases versus controls (Figure 3B). For endpoint 1, the OR estimates were near 1 with no evidence of marker associations with risk (Figure 3C). In contrast, for endpoint 2 the OR estimates supported inverse correlations of D14/D0 fold-change of each antibody marker with risk (Figure 3D), with estimates of 0.19, 0.21, 0.13, and 0.19 per 10-fold increase of EIA, PCA, RSV-A, and RSV-B D14/D0 fold-change, respectively (Figure 3D). The EIA fold-change result was significant after prespecified multiple hypothesis testing adjustment (Holm-Bonferroni–adjusted P ≤ .10). Point estimates for the D14 (Figure 3D) markers also indicated inverse associations with endpoint 2, but with OR estimates closer to 1 and wider CIs that all overlapped 1. In placebo recipients, many of the corresponding D14 and D14/D0 results for each antibody marker also indicated inverse correlations with endpoint 2, with estimated ORs per 10-fold increase of EIA, PCA, RSV-A, and RSV-B D14/D0 fold-change of 0.95, 0.15, 0.62, and 0.53, respectively (Supplementary Figure 5).
Figure 3.
A and B, Boxplots of enzyme immunoassay, palivizumab-competitive antibody, and respiratory syncytial virus (RSV) subtypes A and B day 14/day 0 fold-change by case/control status (A, endpoint 1 cases; B, endpoint 2 cases) in the vaccine arm. C and D, Odds ratios in the vaccine arm of experiencing RSV disease defined by endpoint 1 (C) or endpoint 2 (D) per 10-fold increase in the designated immunologic biomarker, based on logistic regression modeling adjusting for maternal risk score and number of days from vaccination to birth. Cases were defined as infants in the correlates analysis cohort with an RSV illness defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set. Controls were defined as infants who did not experience RSV disease defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set. Endpoint 1 was defined as medically significant RSV-associated lower respiratory tract infection (LRTI), RSV-associated LRTI with hospitalization, or RSV-associated LRTI with severe hypoxemia (52 vaccine endpoints). Endpoint 2 was defined as RSV-associated LRTI with severe hypoxemia (14 vaccine endpoints). Abbreviations: BH, false-discovery rate (q value, Benjamini-Hochberg) adjustment applied separately for the vaccine arm; CI, confidence interval; Cord, infant cord blood; D0, day 0; D14, day 14; EIA, enzyme immunoassay; Holm, family-wise error rate (Holm-Bonferroni); OR, odds ratio; PCA, palivizumab-competitive antibody; RSV-A, respiratory syncytial virus subtype A; RSV-B, respiratory syncytial virus subtype B.
In post hoc analyses assessing Cord/D0 fold-change of each antibody marker as a correlate of risk for endpoint 2 using logistic regression modeling, there was no evidence of correlates of risk in placebo recipients (P > .20 for each marker) (Supplementary Table 4A). In contrast, each Cord/D0 fold-change antibody marker was an inverse correlate of risk in vaccine recipients (P values ranging from .014 to .058), with point estimates of ORs per 10-fold increase in marker value 0.36, 0.29, 0.22, and 0.37 for EIA, PCA, RSV-A, and RSV-B, respectively (Supplementary Table 4A). These Cord/D0 fold-change results were consistent with the D14/D0 fold-change results (Figure 3D), except with weaker estimated correlations (ORs per standard deviation increase closer to 1) (Supplementary Table4B). We next assessed whether multivariable logistic regression models improved risk prediction. None of the considered models provided any evidence, even before multiplicity adjustment, that multiple variables were independently predictive of either endpoint 1 or endpoint 2, in either treatment arm (Supplementary Table 5).
Vaccine-Elicited Maternal Antibody Thresholds Above Which Risk of Infant Severe RSV Disease Is Near Zero
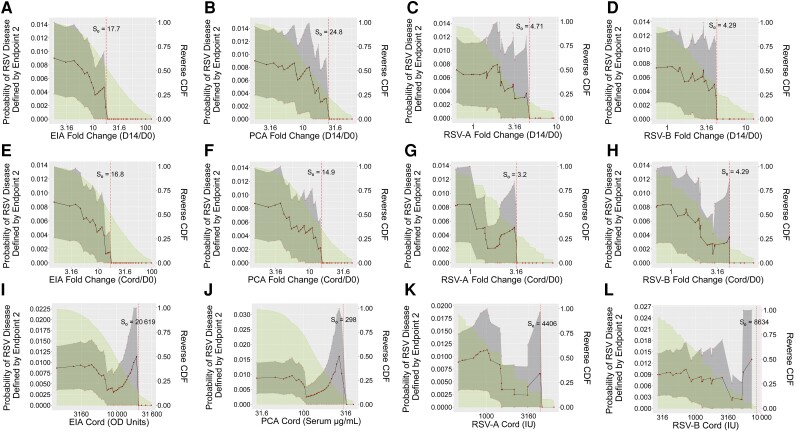
Nonparametric threshold correlates of risk analyses were applied to each D14, D14/D0 fold-change, and Cord antibody marker. A post hoc analysis also assessed fold-change from the enrollment sample to the infant cord blood sample (Cord/D0 fold-change). For endpoint 2, for all 4 antibody markers risk of RSV disease sharply decreased in the vaccine arm across D14/D0 fold-change subgroups and across Cord/D0 fold-change subgroups defined by increasing thresholds, approaching zero risk at a D14/D0 fold-change threshold of approximately 18 (EIA), 25 (PCA), 5 (RSV-A), and 4 (RSV-B) (Figure 4A–D); results were similar for Cord/D0 fold-change (Figure 4E–H) (Supplementary Table 6). (The estimates in Figure 4 have nonsmooth jumps due to the use of nonparametric estimation; the true curve is likely smoother, though with a small number of events the shape cannot be precisely estimated. Moreover, while the point estimates suggest absolute CoPs, the available precision is not sufficient to draw this conclusion; additional verification would be needed.) In contrast, risk remained largely similar across vaccinated subgroups defined by Cord (Figure 4I–L) or D14 (Supplementary Figure 6) marker threshold. For endpoint 1, risk of RSV disease in the vaccine arm generally did not vary markedly depending on threshold value of any antibody marker (Supplementary Figures 7 and 8), consistent with the logistic regression results.
Figure 4.
Risk of respiratory syncytial virus (RSV) disease (defined by endpoint 2) in vaccine arm subgroups defined by antibody marker exceeding thresholds with antibody marker defined by fold-change (day 14/day 0 [D0]) in enzyme immunoassay (EIA) (A), palivizumab-competitive antibody (PCA) (B), RSV-A (C), or RSV-B (D); fold-change (infant cord blood [Cord]/D0) in EIA (E), PCA (F), RSV-A (G), or RSV-A (H); or Cord levels of EIA (I), PCA (J), RSV-A (K), or RSV-B (L), with adjustment for covariates. The Cord/D0 fold-change analyses were post hoc. The gray-shaded region indicates pointwise 95% confidence intervals and the green shaded region is the area under the reverse cumulative distribution function. The vertical dashed red line marks the threshold of estimated zero risk. Endpoint 2 was defined as RSV-associated lower respiratory tract infection with severe hypoxemia (14 vaccine endpoints). Abbreviations: CDF, cumulative distribution function; Cord, infant cord blood; D0, day 0; D14, day 14; EIA, enzyme immunoassay; OD, optical density; PCA, palivizumab-competitive antibody; RSV-A, respiratory syncytial virus subtype A; RSV-B, respiratory syncytial virus subtype B.
Multivariable Machine Learning Suggests a Parsimonious Correlate
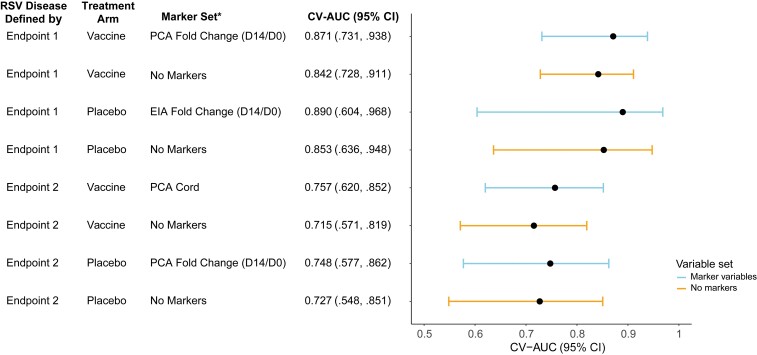
Using SuperLearner to predict individual case/control status defined by each endpoint, the maternal baseline variables alone provided reasonably good classification accuracy; for example, estimated cross-validated area under the receiver operating characteristic curve (CV-AUC) was 0.853 (95% CI, .636–.948) for predicting endpoint 1 in the placebo arm and 0.727 (95% CI, .548–.851) for predicting endpoint 2 in the placebo arm (Figure 5). Antibody markers improved prediction accuracy (ie, higher CV-AUC) compared to only using maternal baseline variables, within each treatment arm and for each endpoint (Figure 5, Supplementary Tables 7–10), albeit with overlapping 95% CIs. The greatest improvement across both endpoints and treatment arms was seen with the addition of PCA Cord to maternal baseline variables for predicting endpoint 2 in the vaccine arm (CV-AUC using maternal baseline variables alone: 0.715 [95% CI, .571–.819] versus CV-AUC using maternal baseline variables and PCA Cord: 0.757 [95% CI, .620–.852], an increase of 0.042) (Figure 5, Supplementary Table 9).
Figure 5.
Performance by treatment arm of SuperLearner for predicting respiratory syncytial virus (RSV) disease case/control status defined by endpoint 1 or by endpoint 2. Cases were defined as infants in the correlates analysis cohort with an RSV illness defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set. Controls were defined as infants who did not experience RSV disease defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set. Endpoint 1 was defined as medically significant RSV-associated lower respiratory tract infection (LRTI), RSV-associated LRTI with hospitalization, or RSV-associated LRTI with severe hypoxemia (52 vaccine and 51 placebo endpoints). Endpoint 2 was defined as RSV-associated LRTI with severe hypoxemia (14 vaccine and 27 placebo endpoints). *All models include maternal baseline covariates. Abbreviations: CI, confidence interval; Cord, infant cord blood; CV-AUC, cross-validated area under the receiver operating characteristic curve; D0, day 0; D14, day 14; EIA, enzyme immunoassay; PCA, palivizumab-competitive antibody; RSV, respiratory syncytial virus.
D14/D0 fold-change EIA and D14/D0 fold-change PCA were generally the most predictive markers, across endpoints and treatment arms. For example, the SuperLearner model with D14/D0 fold-change PCA and maternal baseline variables yielded estimated CV-AUCs of 0.871 (95% CI, .731–.938) for predicting endpoint 1 in the vaccine arm and 0.748 (95% CI, .577–.862) for predicting endpoint 2 in the placebo arm, while the SuperLearner model with D14/D0 EIA fold-change yielded an estimated CV-AUC of 0.890 (95% CI, .604–.968) for predicting endpoint 1 in the placebo arm (Figure 5). The D14 markers were generally weaker predictors, and RSV-A and RSV-B markers were inferior predictors to EIA and PCA (Supplementary Tables 7–10). The modeling did not support any improved performance, measured by the CV-AUC point estimate, gained by including multiple time points (where D14/D0 fold-rise is considered to be a single time point) or multiple assays/markers in the same predictive model. Thus, the data support that a single assay/biomarker may provide the best correlate.
Higher VE Against Infant Severe RSV Disease With Higher Vaccine-Elicited Maternal Anti-F Antibody
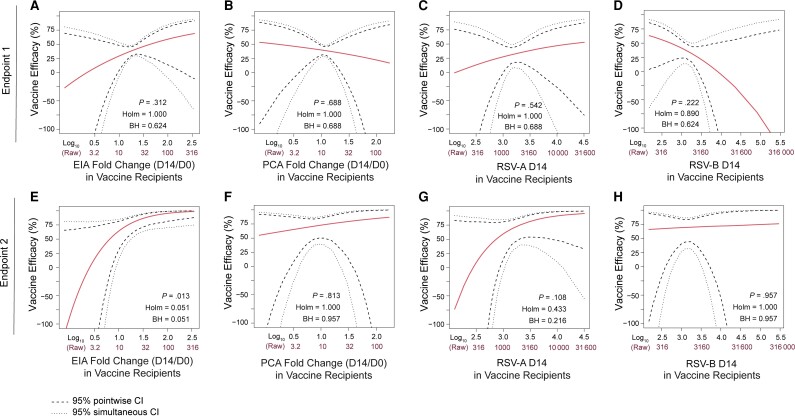
Principal stratification causal inference analyses were next conducted to assess how VE varied over vaccinated subgroups defined by each of 4 antibody markers: EIA D14/D0 fold-change, PCA D14/D0 fold-change, RSV-A D14, and RSV-B D14. EIA D14/D0 fold-change was positively correlated with VE against endpoint 2 (P = .013; Figure 6). For vaccine recipients with EIA D14/D0 fold-change less than around 3, estimated VE was less than 50% and the 95% CIs were wide. The CIs were more precise for larger values of EIA D14/D0 fold-change, with estimated VE exceeding 75% for values of EIA D14/D0 fold-change above about 16 and approaching 90% and greater for higher fold-change values. These results imply that a vaccine-induced boost (from baseline) of around 15- to 20-fold of anti-F IgG binding antibodies is associated with high-level VE. None of the other examined antibody markers had evidence for correlation with VE against RSV disease (all P >.10; Figure 6). The model adjusted for baseline marker level in addition to maternal baseline variables showed a trend of VE correlating with EIA D14/D0 fold-change (P = .10, Supplementary Figure 9).
Figure 6.
Point and 95% confidence interval estimates of vaccine efficacy against respiratory syncytial virus (RSV) disease defined by endpoint 1 (top row) or endpoint 2 (bottom row) as a function of enzyme immunoassay day 14 (D14)/day 0 (D0) fold-change (A and E), palivizumab-competitive antibody (PCA) D14/D0 fold-change (B and F), RSV-A D14 (C and G), and RSV-B D14 (D and H) in vaccine recipients, denoted S(1). P indicates 2-sided P values based on the Wald test for interaction between treatment and S(1) in the risk model, which evaluates whether vaccine efficacy changes over subgroups defined by S(1). Endpoint 1 was defined as medically significant RSV-associated lower respiratory tract infection (LRTI), RSV-associated LRTI with hospitalization, or RSV-associated LRTI with severe hypoxemia (52 vaccine endpoints). Endpoint 2 was defined as RSV-associated LRTI with severe hypoxemia (14 vaccine endpoints). All P values are with covariate adjustment. Abbreviations: BH, false-discovery rate (q value, Benjamini-Hochberg) adjustment; CI, confidence interval; D0, day 0; D14, day 14; EIA, enzyme immunoassay; Holm, family-wise error rate (Holm-Bonferroni); PCA, palivizumab-competitive antibody; RSV-A, respiratory syncytial virus subtype A; RSV-B, respiratory syncytial virus subtype B.
Exploratory Analyses of Surrogate Endpoint Evaluation
We applied causal mediation methods to assess each D14/D0 fold-change and D14 antibody marker as a mediator of VE against each endpoint. The methods did not provide reliable answers, likely due to violations of assumptions of these methods. Therefore, we conducted post hoc exploratory analyses using a standard approach to surrogate endpoint evaluation [31] developed before the causal mediation framework. If an antibody marker is a valid surrogate endpoint as defined by Prentice [30], then a hypothesis test for whether the vaccine increases the antibody marker level compared to placebo is a valid test of whether the VE against the RSV disease endpoint is positive. Based on the logistic regression modeling, an antibody response marker is supported to meet the Prentice criteria if all of the following conditions are met, all in the context of adjusting for baseline potential confounding variables: (1) there is no evidence of an interaction between treatment and the marker; (2) in a main effects model taking out the interaction term, there is an association of the antibody marker with outcome; (3) in this main effects model, there is no evidence of an association of the treatment/randomization assignment with outcome (ie, after accounting for the marker, treatment contains no additional information about risk). All models that included an interaction term for treatment and the antibody marker gave no evidence for interaction (P > .20), satisfying (1). The results from the main effects models containing the fold-change markers are shown in Table 3. For EIA D14/D0 fold-change and PCA D14/D0 fold-change, the results aligned with Prentice surrogate endpoint criteria (2) and (3). For RSV-A and RSV-B, treatment arm still predicted RSV disease (endpoint 2) after accounting for the marker in the model, implying violation of criterion (3). Similar results were seen for the D14 analysis (not shown).
Table 3.
Logistic Regression Models for Each of the 4 Day 4/Day 0 Fold-Change Markers With Treatment Arm and Adjustment for Covariates
| Variable | EIA | PCA | RSV-A | RSV-B |
|---|---|---|---|---|
| Treatment | 1.24 (.35–4.41, P = .743) | 1.68 (.49–5.80, P = .410) | 0.34 (.17–.67, P = .002) | 0.39 (.19–.79, P = .009) |
| D14/D0 fold-change | 0.28 (.10–.78, P = .014) | 0.18 (.06–.54, P = .002) | 0.37 (.13–1.07, P = .068) | 0.36 (.14–.91, P = .031) |
| Maternal risk score for endpoint 2 | 1.20 (.97–1.47, P = .095) | 1.21 (.98–1.49, P = .081) | 1.39 (1.00–1.94, P = .051) | 1.38 (1.00–1.90, P = .052) |
| Days between vaccination and birth | 0.99 (.97–1.00, P = .100) | 0.99 (.97–1.00, P = .103) | 0.99 (.97–1.00, P = .072) | 0.99 (.97–1.00, P = .099) |
Values are presented as odds ratios (95% confidence intervals and 2-sided P values), where for D14/D0 fold-change odds ratios are per 10-fold increase. All models that included an interaction term for treatment and the antibody marker gave no evidence for interaction (P > .20); thus, the interaction terms were removed from the models. Endpoint 2 was defined as RSV-associated lower respiratory tract infection with severe hypoxemia.
Abbreviations: D0, day 0; D14, day 14; EIA, enzyme immunoassay; PCA, palivizumab-competitive antibody; RSV-A, respiratory syncytial virus subtype A; RSV-B, respiratory syncytial virus subtype B.
DISCUSSION
The maternal fold-rise markers showed, in general, stronger evidence as inverse correlates of risk for RSV-associated acute LRTI with severe hypoxemia than the D14 or infant cord blood markers. These results suggest that the ability of the F nanoparticle vaccine to boost preexisting maternal levels of neutralizing antibodies against either RSV subtype, IgG antibodies that bind to the RSV fusion glycoprotein, or antibodies that compete for binding with palivizumab to the RSV fusion glycoprotein is more relevant to the prediction of risk or protection than the absolute levels of these markers 2 weeks after vaccination or in infants at birth. One potential explanation for our findings is that antibody fold-change is a nonmechanistic CoP [25], in that a higher fold-change reflects acquisition of some qualitative feature of RSV F-specific antibodies important for conferring protection, such as having undergone appropriate affinity maturation [32]. Alternatively, fold-change might be a marker for the acquisition of enhanced Fc receptor binding or complement activating capacity by the maternal F protein–specific antibody pool.
Among the fold-change antibody markers, anti-F glycoprotein-binding antibodies and PCAs appeared to be better correlates than neutralizing antibodies against either RSV subtype. We interpret this finding as suggestive of a potential role for Fc-mediated antibody effector functions not captured by neutralization assays in the clearance of extracellular virus and/or infected cells [33]. In addition, the binding antibody markers appeared to have less technical measurement error than the neutralizing antibody markers, which led to narrower CIs about association parameters based on these markers.
Between the anti-F binding antibody and PCA enrollment to D14 fold-change markers, we observed the most consistent evidence for fold-change anti-F binding antibody as a correlate of risk and CoP against RSV-associated acute LRTI with severe hypoxemia. This marker was the only antibody marker that significantly inversely correlated with risk in the logistic regression analysis after the prespecified multiple hypothesis testing adjustment and the only marker that associated with VE; moreover, it inversely correlated with risk in nonparametric threshold regression and adhered to the Prentice criteria for a valid surrogate endpoint (caveated that this last analysis was post hoc exploratory). A conclusion that the marker satisfies the Prentice criteria relies on there being no unmeasured maternal factors that predict both RSV disease and the antibody marker, an assumption that cannot be verified from the data. The results for fold-change PCA were generally comparable, except that this marker was not found to associate with VE. However, the small numbers of vaccine breakthrough endpoints and the fact that neutralizing antibodies were only measured in a subset of the controls limit the level of confidence in any conclusions related to the relative merit of the 4 antibody markers as correlates.
Among the RSV severe disease endpoints considered in the analyses, the majority of antibody correlates were against RSV-associated acute LRTI with severe hypoxemia (endpoint 2). This suggests that severe hypoxemia may be the critical aspect of the endpoint that defines a correlate of F nanoparticle vaccine–induced protection. To our knowledge, previous studies reporting on neutralizing antibody cutoffs for protection against RSV disease have not included severe hypoxemia in their disease definitions, instead considering protection against, for example, RSV polymerase chain reaction–positive pneumonia or influenza-like illness [14], 2 potentially subjective definitions. Our results thus suggest that severe hypoxemia should be an important parameter to include in future RSV disease correlates analyses, perhaps by providing a key quantitative element of severity.
Overall, our results are encouraging for the RSV vaccine field and suggest that—if the F nanoparticle vaccine is confirmed to have induced protective responses—efficacy could be enhanced by augmenting the vaccine's ability to boost preexisting binding and/or neutralizing antibody responses, particularly anti-F IgG binding antibodies. Given that the F nanoparticle vaccine induced anti-F binding antibodies more effectively than it did neutralizing antibodies, it is perhaps not surprising that protection associated with this vaccine might be associated with induction of anti-F antibodies. However, a challenge posed to the RSV vaccine field is that it is not known whether the antibody correlates identified here apply to other vaccine candidates/platforms in development [3]. A further limitation of this analysis was the restriction to South Africa, which was necessitated due to the lack of available antibody data from other countries. Additional immune correlates studies would need to be performed using efficacy trial data from other vaccine candidates/platforms/countries to answer this question. Another conclusion is that measurement of neutralization alone may not capture all the protective features of the vaccine-induced antibody response. However, given that the correlates results are based on only 27 cases in the placebo arm and 14 cases in the vaccine arm (for endpoint 2), future studies with higher case numbers are needed.
Supplementary Material
Contributor Information
Youyi Fong, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Public Health Sciences, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Ying Huang, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Public Health Sciences, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Bhavesh Borate, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Lars W P van der Laan, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Wenbo Zhang, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Lindsay N Carpp, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Iksung Cho, Novavax, Inc, Gaithersburg, Maryland, USA.
Greg Glenn, Novavax, Inc, Gaithersburg, Maryland, USA.
Louis Fries, Novavax, Inc, Gaithersburg, Maryland, USA.
Raphael Gottardo, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Peter B Gilbert, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Public Health Sciences, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the participants, site investigators, and staff involved in the Prepare trial. We particularly thank Khatija Ahmed, Mark Cotton, Lee Fairlie, Leon Fouche, Johannes Lombaard, Ayman Osman, and Elana van Brakel.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Data availability. The de-identified data underlying the findings of this study and the code used in the analysis are publicly available in the two following repositories: https://github.com/FredHutch/RSVcorr and https://github.com/FredHutch/RSV_correlates_analysis, respectively.
Financial support. This study was supported by the Bill & Melinda Gates Foundation (award number INV-008576/OPP1154739 [Vaccine Statistical Support] to R. G.) and the National Institute of Allergy and Infectious Diseases of the NIH (grant number R37AI054165 to P. B. G.). Under the grant conditions of the Bill & Melinda Gates Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munoz FM, Jamieson DJ. Maternal immunization. Obstet Gynecol 2019; 133:739–53. [DOI] [PubMed] [Google Scholar]
- 3. PATH . RSV vaccine and mAb snapshot. March 2020. https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/. Accessed 18 January 2021.
- 4. Plotkin SA, Orenstein WA, Offit PA, Edwards KM. Plotkin's vaccines. In: Plotkin SA, Gilbert P, eds. Chapter 3: correlates of protection. 7th ed. Amsterdam: Elsevier, 2018:35–40. [Google Scholar]
- 5. Prince GA, Horswood RL, Camargo E, Koenig D, Chanock RM. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun 1983; 42:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prince GA, Horswood RL, Chanock RM. Quantitative aspects of passive-immunity to respiratory syncytial virus-infection in infant cotton rats. J Virol 1985; 55:517–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prince GA, Hemming VG, Horswood RL, Chanock RM. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res 1985; 3:193–206. [DOI] [PubMed] [Google Scholar]
- 8. Walsh EE, Schlesinger JJ, Brandriss MW. Protection from respiratory syncytial virus-infection in cotton rats by passive transfer of monoclonal-antibodies. Infect Immun 1984; 43:756–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. [DOI] [PubMed] [Google Scholar]
- 10. Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98:708–15. [DOI] [PubMed] [Google Scholar]
- 11. Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol 1991; 133:1135–51. [DOI] [PubMed] [Google Scholar]
- 12. Stensballe LG, Ravn H, Kristensen K, et al. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol 2009; 123:398–403. [DOI] [PubMed] [Google Scholar]
- 13. Capella C, Chaiwatpongsakorn S, Gorrell E, et al. Disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis 2017; 216:1398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchwald AG, Graham BS, Traore A, et al. RSV neutralizing antibodies at birth predict protection from RSV illness in infants in the first three months of life. Clin Infect Dis 2020; 73:e4421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Groothuis JR, Simoes EA, Levin MJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med 1993; 329:1524–30. [DOI] [PubMed] [Google Scholar]
- 16. Connor E, Top F, Kramer A, et al. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 1997; 99:93–9. [DOI] [PubMed] [Google Scholar]
- 17. Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003; 143:532–40. [DOI] [PubMed] [Google Scholar]
- 18. The IMpact-RSV Study Group . Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. [PubMed] [Google Scholar]
- 19. Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity 2010; 33:516–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med 2020; 383:426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 2003; 21:3479–82. [DOI] [PubMed] [Google Scholar]
- 22. Rossey I, McLellan JS, Saelens X, Schepens B. Clinical potential of prefusion RSV F-specific antibodies. Trends Microbiol 2018; 26:209–19. [DOI] [PubMed] [Google Scholar]
- 23. August A, Glenn GM, Kpamegan E, et al. A phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine 2017; 35:3749–59. [DOI] [PubMed] [Google Scholar]
- 24. Lumley T. Complex surveys: a guide to analysis using R. New York: John Wiley & Sons; 2011. [Google Scholar]
- 25. Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 2012; 54:1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Laan L, Zhang W, Gilbert PB. Nonparametric estimation of the causal effect of a stochastic threshold-based intervention [manuscript published online ahead of print 8 May 2022]. Biometrics 2022. May 8. [DOI] [PMC free article] [PubMed]
- 27. van der Laan MJ, Polley EC, Hubbard AE. Super learner. Stat Appl Genet Mol Biol 2007; 6:Article25. [DOI] [PubMed] [Google Scholar]
- 28. Polley EC, Van der Laan MJ. “SuperLearner in prediction” (Working Paper 266). U.C. Berkeley Division of Biostatistics Working Paper Series. 2010. https://biostats.bepress.com/ucbbiostat/paper266. Accessed September 3, 2020.
- 29. Huang Y, Gilbert PB, Wolfson J. Design and estimation for evaluating principal surrogate markers in vaccine trials. Biometrics 2013; 69:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang Y. Evaluating principal surrogate markers in vaccine trials in the presence of multiphase sampling. Biometrics 2018; 74:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8:431–40. [DOI] [PubMed] [Google Scholar]
- 32. Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 2009; 15:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol 2019; 10:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.