Abstract

Polarizable shell-model potentials are widely used for atomic-scale modeling of charged defects in solids using the Mott–Littleton approach and hybrid Quantum Mechanical/Molecular Mechanical (QM/MM) embedded-cluster techniques. However, at the pure MM level of theory, the calculated defect energetics may not satisfy the requirement of quantitative predictions and are limited to only certain charged states. Here, we proposed a novel interatomic potential development scheme that unifies the predictions of all relevant charged defects in CeO2 based on the Mott–Littleton approach and QM/MM electronic-structure calculations. The predicted formation energies of oxygen vacancies accompanied by different excess electron localization patterns at the MM level of theory reach the accuracy of density functional theory (DFT) calculations using hybrid functionals. The new potential also accurately reproduces a wide range of physical properties of CeO2, showing excellent agreement with experimental and other computational studies. These findings provide opportunities for accurate large-scale modeling of the partial reduction and nonstoichiometry in CeO2, as well as a prototype for developing robust interatomic potentials for other defective crystals.
1. Introduction
Ceria (CeO2) is a technologically important rare-earth oxide with broad applications in several areas, including heterogeneous catalysis and solid oxide fuel cells (SOFCs).1−3 It is versatile in catalytic applications because of its unique defect properties. Apart from its critical role in automobile three-way catalysts, ceria also holds great promise in novel catalytic processes such as selective methane oxidation to methanol, CO2 conversion reactions, and hydrogen production through water splitting.4−6 CeO2 has outstanding oxygen storage capacity, which arises from its variable stoichiometry enabling the release or uptake of oxygen to pass through redox cycles.7 As a consequence, reduced ceria (CeO2–x) is widely used as a supporting material for single-atom/nanocluster catalysts with exceptional catalyst-stabilizing and oxygen-releasing capabilities.8−10 In addition, the high ionic conductivity of CeO2 is necessary for its use as an electrolyte in SOFCs and relies to a large extent on the rapid oxygen-vacancy formation and migration processes.11,12 Remarkably, CeO2 remains very stable in the cubic fluorite structure over a wide range of temperatures. The earth-abundant nature of cerium13 further makes it promising for large-scale industrial applications.
Oxygen vacancies are known to have low formation energies in ceria.7 Accompanied by the removal of an oxygen ion to form a doubly ionized vacancy VO••, two excess electrons could localize on two cation sites and form CeCe small polarons14,15 or be compensated by an oxygen interstitial Oi″ to form an anion–Frenkel pair.16 Here, we employ the Kröger–Vink notation17 for a point defect MS, in which M is the defect species, S is the lattice site that the defect occupies, and C is the charge of the defect relative to the original site (dots, primes, and crosses represent positive, negative, and neutral charges, respectively). For the former process, Paier et al.7 proposed that the best estimate of the charge-neutral oxygen vacancy formation energy in bulk ceria could be 4.2 ± 0.3 eV (relative to (1/2)EO2) in O-rich conditions deduced from conductivity and calorimetric titration experiments, close to the heat of reduction from CeO2 to Ce2O3 of 4.0 eV.18,19 Surface vacancies have much lower (1–3 eV) formation energies than those in the bulk, depending on the facets and concentrations.20−24
In recent years, much effort has been devoted to studying the defect chemistry in CeO2 by plane-wave density functional theory (DFT) techniques using periodic supercell approaches.7,25−31 CeO2 is a strongly correlated oxide. The description of the highly localized 4f states becomes problematic in DFT calculations when Ce4+ is reduced to Ce3+. Standard Kohn–Sham DFT calculations using semilocal generalized gradient approximation (GGA) functionals such as Perdew–Burke–Ernzerhof (PBE) predict an unrealistic delocalized spin density for the occupied f states due to the self-interaction error (SIE).25 While the Hubbard U correction scheme32 (DFT+U, UCe4f = 4.5–6.0 eV) can solve the electron localization problem,7 the calculated formation energy of the oxygen vacancy (from 2.84 to 3.27 eV26−29) is underestimated by 1.0–1.5 eV compared with experiment (4.2 ± 0.3 eV7). Moreover, the choice of the U value is not straightforward, as it introduces uncertainty in the description of bulk properties, defect formation, and surface reactivity of ceria.31,33 It has been reported that with the increase in U the lattice constant of CeO2 becomes too large with increasingly underestimated oxygen vacancy formation energy.31 Furthermore, Branda et al.34 reported a variation of the oxidation state of Au on CeO2(111) with the U parameter. Watson et al.25,26 proposed employing the DFT+U correction also to the O2p states, which further properly localizes holes in ceria trapped by native defects and impurities. However, this approach underestimates the formation energy of the oxygen vacancy even more: 2.22 eV based on UCe/O = 5.0/5.5 eV compared with 2.60 eV using UCe/O = 5.0/0 eV. Alternatively, hybrid functionals that include a fraction of the nonlocal Fock exchange, although computationally more demanding, yield results in much closer agreement with experiment. Several hybrid functionals have been used to study the bulk and defect properties of CeO2 based on periodic models.30,31,35−37 Notably, the oxygen vacancy formation energies predicted by hybrid functionals are much closer to experiment than the DFT+U predictions: 3.84 eV using PBE038 and 3.63–4.09 eV using HSE06.7,30,31 Such improvements from hybrid functionals were also seen in other metal oxide systems with accurately predicted defect processes.39
The configurations of oxygen vacancies and the associated CeCe′ sites in reduced CeO2 have been a topic of considerable debate. Early theoretical studies assumed that the two excess electrons are trapped at the nearest neighbor (NN) sites of the vacancy (i.e., NN-NN).20,25,40 Later, DFT+U calculations performed by Wang et al.41 favored the localization of both electrons at the next-nearest neighbor (NNN) sites (i.e., NNN-NNN). In contrast, Murgida et al.29 obtained three configurations (one NN-NNN and two NNN-NNN configurations) sharing the same lowest formation energies. Despite the minor differences, these predictions are generally consistent. Early molecular-mechanical (MM) Mott–Littleton (M-L) calculations on doped ceria also suggested that large trivalent cations prefer to locate at the NNN sites over the NN sites.42 Scanning-tunneling microscopy (STM) imaging over the CeO2(111) surface conducted by Jerratsch et al.43 captured several different configurations, indicating that at least one CeCe is not adjacent to the vacancy site. The polarization energy of VO•• is large due to the high dielectric constants of CeO2, which can stabilize isolated charged species with respect to the neutral lattice, including both VO and CeCe′. Besides, the formation of CeCe requires an expansion of the surrounding lattice, which opposes the location next to the oxygen vacancy site where the neighboring Ce–O bonds shrink. As a result, the binding energy of VO•• with the NN CeCe polaron is very low (ca. 0.1 eV as reported by Sun et al.30), indicating that CeCe′ polarons are not tightly bound to oxygen vacancies. Experimentally, early conductivity measurements by Tuller and Nowick44 on single-crystal CeO2–x samples also revealed that VO is the predominant charge state at a small x in CeO2–x (x < 10–3), with a transition to singly ionized VO• at a larger x in more reducing environments. In addition, a recent cathodoluminescence spectroscopy study by Thajudheen et al.45 demonstrated that the relative concentration of different charge states of oxygen vacancies depends on the oxygen partial pressure. Luo et al.16 observed using neutron scattering that the anion–Frenkel pair is dominant in the bulk, while CeCe polarons tend to aggregate at the surface and form an ordered reduced phase from nanorod samples. Such complexity in the charge compensation mechanism of oxygen vacancies could have a substantial impact on the ionic conductivity in SOFCs12 and surface reactivity when CeO2 serves as a catalyst46 or support.47 To resolve these uncertainties, reliable theoretical techniques are required to study the environmentally dependent formation mechanisms of all the relevant charge states of intrinsic defects in CeO2.
While being well-suited to modeling bulk materials and delocalized states, periodic boundary conditions have inherent limitations in modeling localized states such as isolated defects, polarons, and adsorbed molecules due to spurious image–image interactions. The long-range Coulomb interactions between periodic images originating from the net dipole moment of localized states are non-negligible.48 As a result, periodic DFT calculations on defective solids usually require a large supercell to mitigate these spurious periodic interactions,7,29 and additional correction schemes are usually necessary to achieve a higher level of accuracy.48−50 Also, for some highly charged defects, the finite-size supercells could still be insufficient to fully consider the long-range atomic perturbations. In particular, the formation of highly charged defects in CeO2, such as Cei•••• and VCe, could provide a major perturbation of the surrounding atomic structure. These errors could be magnified when defect clusters are considered. Additionally, it is computationally very demanding with hybrid functionals to employ a sufficiently large supercell to minimize these errors.
A minor change in the formation energy of a point defect could result in several orders of magnitude variations in its calculated concentration, which could strongly affect the predicted electronic and optical properties.39 Therefore, minimizing errors in the modeling method is necessary to ensure the accuracy of defect predictions. Embedded-cluster techniques that naturally avoid periodic image interactions and further consider long-range polarization effects are an effective approach to modeling localized defects in solids at the dilute limit.51−56 The Molecular Mechanical, Mott–Littleton (MM M-L) approach57−59 and hybrid quantum mechanical/molecular mechanical (QM/MM) embedded-cluster techniques55,60,61 are widely used for modeling defect processes in solids. Both methods are based on the embedded-cluster framework: the QM/MM model includes a QM core, an interface, and part of MM atoms in the active region, while the M-L model only has one active MM region, which is allowed to relax fully in response to the formation of charged defects; the outer fixed part reproduces the infinite bulk environment.59 The M-L approach treats the outer part within a harmonic approximation to reproduce the bulk crystal field and linear dielectric response, while our QM/MM model implements a simplified continuous dielectric medium approximation where the response to the defect charge is calculated a posteriori.55 Furthermore, the shell-model62 interatomic potential (IP) is typically used in both techniques, in which the contributions of electronic long-range polarization effects due to the defect formation to the total energy can be computed through shell relaxations. With the rapid development of high-performance computing platforms, embedded-cluster calculations routinely employ over one thousand or more atoms in the active region (including QM and MM atoms) to consider explicitly the atomic displacements caused by defects, ensuring accuracy in predicting the formation energies and spectroscopic features.54,63
M-L calculations of defects in solids are performed at the pure MM level, which relies heavily on the accuracy of the IP. A reliable shell-model IP is a fundamental prerequisite for quantitively predicting the defect structures and formation energies. In contrast, embedded-cluster results commonly use DFT calculations employing hybrid functionals (although higher level theory may be used64−66) and with the IP-predicted dielectric response. Hence, an intrinsically consistent prediction of defect structures and formation energies by both approaches using the same potential is the ultimate goal that demonstrates the robustness of an IP. In previous work, such consistent predictions were achieved for MgO67,68 and ZnO51,69 but were limited to a few charge states of point defects without holes or electrons.
To date, a number of shell-model potentials have been developed for CeO2, which are collected in Table S1.42,70−76 IP-based computational studies of defect chemistry in ceria were first performed by Butler, Catlow, Cormack et al.70,77,78 using the M-L approach; these studies calculated vacancy migration energies in good agreement with experiment and demonstrated the crucial role of the ionic radius of dopants in determining the solution energy and the magnitude of dopant–vacancy interactions. Periodic models were also exploited to investigate the oxygen vacancy formation on surfaces and its role in CO oxidation.71,79,80 Despite these advances, no single IP can offer a consistently accurate description of all the physical and chemical properties of CeO2, as shown in Figure 1. It was possible to reproduce accurately the main bulk properties of ceria, including phonon frequencies and the oxygen vacancy migration barrier based on a more complex potential model (IP9 in Table S1),76 but the defect formation and surface energies proved to be significantly overestimated. One of the critical reasons for the less satisfying performance of previous potentials is the lack of reliable reference data in the IP development that exacerbates the errors in empirical parametrization.
Figure 1.
Performance of the previous (IP1-IP9) and our newly developed (IP10a and IP10b) shell-model potentials for modeling CeO2, compared with experimental and first-principles results. IP10a is developed based on the pure Buckingham potential in short-range interactions, while IP10b is the refined final version with improved performance, using a more complex potential form. The calculated values of each observable based on the corresponding potential are shown in the blocks. The blocks are colored according to the relative errors of the predicted properties compared with these experimental or theoretical references. Lattice constant a0 (extrapolated to 0 K),33,81 elastic constants C11, C12, and C44,82 phonon frequencies F1u(TO), F2g, and F1u(LO),82 static and high-frequency dielectric constants ε0 and ε∞,83 bulk modulus B0,84 and migration barrier of oxygen vacancy EVOmigration(85) are from previous experiments. References for Born effective charges ZCe and ZO* and surface energies E(100), E(110)surf, and E(111) are calculated at the PBE0 level of theory using VASP. References for the defect energies including EVO••, EOi, EVCe⁗, and ECei and defect pair/trio formation energies Ecation–Frenkel, Eanion–Frenkel, ESchottky, and Einterstitial are calculated based on our hybrid QM/MM embedded-cluster model at the PBE0 level.
In this work, we propose a novel methodology for IP development assisted by the QM/MM approach, which allows us to obtain accurate reference data for ionic polarizabilities, structure and formation energies of polarons, and intrinsic defects in dielectric materials. A new shell-model IP has been developed for CeO2 that reproduces accurately the experimental structure, elastic and dielectric constants, defect and surface properties, and phonon dispersion. This approach also provides references for parametrizing localized holes and polarons, endowing powerful capacities for modeling the localization of charge carriers accompanied by defect formation. Based on the new IP, the calculated structure and formation energies of charged defects by the M-L and QM/MM approaches using hybrid functionals achieve a very high level of consistency, which is greater than that achieved in previous work. Our potential is parametrized entirely using the shell-model and pairwise potentials without complex many-body interactions, ensuring excellent computational efficiencies in studying complex defective systems. Moreover, the newly proposed strategies for developing reliable shell-model potentials for accurate defect predictions assisted by QM/MM calculations could be extended to other systems.
2. Methodology
This work combines various computational techniques including plane-wave DFT calculations, IP-based lattice and M-L defect calculations, and the hybrid QM/MM embedded-cluster approach.
2.1. Plane-Wave DFT Calculations
Plane-wave DFT calculations were performed at several levels of theory using the Vienna Ab-initio Simulation Package (VASP)86 to set theoretical references for developing new IPs. Full computational details for calculating the bulk and surface properties of ceria are given in Section S1.1 of the Supporting Information.
2.2. Interatomic-Potential-Based Calculations
The development of IPs and IP-based calculations are performed based on the Born model of ionic solids87 using the General Utility Lattice Package (GULP) code.88,89 In traditional shell-model potentials, pairwise interactions are described by the Buckingham potential:
| 1 |
where rij is the distance between two interacting ions and A, ρ, and C6 are the parameters. Ionic polarizability is treated by the shell model, in which the massless shell is connected to the atomic core by a harmonic spring with a spring constant k2:
| 2 |
The sum of the core and shell charges on an ion equals its formal charge. The ionic polarizability α in vacuum in the shell model is given by
| 3 |
where Y is the shell charge. The electrostatic Coulomb interaction is calculated as
| 4 |
where ke is the dimensional Coulomb constant (14.3996 eV Å e–2).
In this work, our new potential keeps the classical Buckingham form for describing the O–O and Ce–Ce interactions, while taking a complex form combining repulsive and attractive terms for Ce–O interactions parametrized in GULP. This potential can be represented by using the Buckingham, 12-6 Lennard-Jones, Morse-like, and constant offset (0-order polynomial) analytical forms in GULP:
| 5 |
where A, ρ, C6, E, D, a, r0, and c0 are the parameters of the potential.
IP-based defect calculations were undertaken based on the M-L approach.57,58 A point defect or defect cluster is embedded in the central part of the model (region I), where ionic relaxations and polarization are allowed explicitly. The surrounding regions (region IIa and IIb) that model the infinite solid are described within the linear response approximation. Region IIa acts as the interface, in which interactions with region I ions are calculated by explicit summation, but ionic displacements are calculated self-consistently using a harmonic approximation for the defect energy expanded around defect equilibrium configuration, as first proposed by Norgett90 and implemented in the GULP code.88 Region IIb extends to infinity to reproduce the periodic electrostatic environment of the solid, in which the long-range polarization energy of a charged defect outside the active region I is calculated using the macroscopic dielectric response of a perfect crystal (without a defect). The radii of region I and region IIa were set to 25 Å and 40 Å, respectively, which proved to be sufficiently large for considering the long-range polarization effects and provide well-converged defect energies (<0.1 eV).
Details of IP-based surface and vacancy migration calculations are described in Sections S1.2 and S1.3 of the Supporting Information.
2.3. QM/MM Calculations
We employ the hybrid QM/MM embedded-cluster approach to model defect formation in bulk CeO2 as implemented in the ChemShell60,91 code. Our hybrid QM/MM embedded-cluster model is divided into five regions. In the QM region, electronic-structure calculations are performed at the hybrid DFT level using NWChem.92 We have tested the performance of several pseudopotentials and basis sets to balance the computational accuracy and efficiency in large-scale QM/MM calculations of CeO2 (with 100–200 QM atoms). The combination of the Def2-TZVP basis set93 for O and a [4s4p2d3f] basis set developed by Erba et al.94,95 based on the Stuttgart–Dresden quasi-relativistic small-core (28 core electrons) effective core potential (ECP)96 for Ce was determined as the best choice. The differences in the calculated formation energies of defects and ionization potential between the current setting and more complete basis sets are less than 0.1 eV. We utilized three hybrid functionals for defect calculations: hybrid GGA functional B97-297 and PBE098 and hybrid meta-GGA functional BB1K,99 which include 21%, 25%, and 42% HF exact exchange, respectively. For consistency, we selected PBE0 results as the reference data in the IP development, together with those from VASP calculations using the same functional.
The MM calculations are performed using GULP. The MM part of the QM/MM structural model is divided into two regions: the MM-active region, which is allowed to relax during the calculations, and the MM-frozen region, which is fixed to reproduce the effect of the bulk environment. The outermost layer of the entire model includes point charges, which were fitted to eliminate the effects of surface termination and reproduce the Madelung potential of CeO2.
The interface region participates in both QM and MM interactions, serving as the buffer layer to minimize the mismatch of the QM and MM levels of theory. We used a specially designed local pseudopotential on the cationic sites in the form of a linear combination of three Gaussian functions.56 The fundamental idea and detailed procedures of the QM/MM interface treatment are shown in Section S1.4 of the Supporting Information. The parameters were trained with a least mean square procedure for residual gradients on the ions in the active (QM, interface, and MM-active) region and the scatter of deep core levels (in this study, O1s) in the Kohn–Sham spectrum. The fitted pseudopotential for the interface cations has the form of
| 6 |
QM/MM defect calculations were performed with the python-based version of ChemShell (Py-ChemShell)91 on a large embedded-cluster model with ∼10 000 atoms in total and an O-centered 197-atom QM cluster focusing on the formation of oxygen vacancies. The accurately calculated energies of polaron formation at the NN and NNN sites have been used for validation and refinement of the interatomic potentials that we report. A Ce-centered 111 QM-atom model was used for other types of defects, which yields convergence of defect formation energies to ca. 0.1 eV.
2.4. Calculations of Defect Formation Energies
Lattice energy is the energy of the ionic compound with respect to constituent ions in the gas phase. The lattice energy ΔUL can be calculated according to the Born–Haber cycle100 from experimental thermodynamical data, which (for CeO2) is given by
| 7 |
where ΔHsubl(Ce) is the sublimation enthalpy of Ce (4.380 eV101), DO2 is the dissociation energy of O2 (5.136 eV101), ICe1–4 is the sum of the first four ionization potentials of Ce (73.745 eV101), AO is the sum of the first (AO1) and second (AO) electron affinities of O, and ΔHf(CeO2) is the formation enthalpy of CeO2 (−11.28 eV101). While AO1 is experimentally known as 1.461 eV,102AO is dependent on the atomic environment of oxides, adding uncertainty to the IP development and subsequent defect formation calculations. Freeman and Catlow suggested a value of 8.75 eV for SnO2, while Waddington103 obtained an average value of 9.41 eV from several oxides. Previous work has shown that ΔUL is a critical quantity that strongly affects the accuracy of M-L defect calculations.104 However, fitting the potential to reproduce the “experimental” ΔUL according to an arbitrary choice of AO2 may be problematic and could lead to several electronvolt errors in the calculated formation energies. We propose a self-consistent approach to determine the value of AO in CeO2 (8.14 eV, and therefore ΔUL = −107.5 eV according to eq 7) based on QM/MM calculations of defect energies and plane-wave DFT calculations of surface energies, which will be discussed in the following sections. For the defect formation energy calculations using the Born–Haber cycle based on the M-L results, a consistent usage of the AO2 and ΔUL predicted by the IP gives accurate results that are comparable with QM/MM calculations.
It is necessary to clarify the definition of some energy terms in defect calculations. In QM/MM calculations, the formation energy of a defect in the charge state of q is defined as
| 8 |
where E[Xq] and E0 are the calculated total QM/MM energies of the defect and perfect structures, ni is the number of species that have been added (ni > 0) or removed (ni < 0) from the system to form the defect, μi is the chemical potential of the species i, and EF is the Fermi energy relative to the valence band maximum (VBM). In order to account for the long-range polarization effect for charged defects that extends to infinity, an a posteriori correction is applied using the Jost formula,61,105
| 9 |
where R is the radius of the active region (including QM, interface, and MM-active regions) and Q is the net charge of the defect system. High frequency ε∞ and static ε0 dielectric constants are used for vertical and adiabatic processes, respectively.
M-L calculations compute the defect energy E[Xq] taking the gas phase ions (O2–(g) and Ce4+(g)) as the reference, which differs from the formation energy (with respect to O2(g) and Ce(s)) in conventional DFT calculations. The energy of O2(g) and Ce(s) is not defined in IP-based calculations. Instead, such energies can be in turn obtained from the Born–Haber cycle100 and relate E[Xq] to Ef[Xq] by
| 10 |
| 11 |
Therefore, the energy of the added or subtracted species in defect formation can be obtained by
| 12 |
| 13 |
Accordingly, the defect energies EVO••, EOi, EVCe⁗, and ECei can be directly obtained and compared in M-L and QM/MM approaches. Furthermore, the energy changes during the formation of localized electrons or holes trapped by the defects do not include the fourth ionization potential of Ce (ICe4, 36.762 eV101) or AO, which should be subtracted from the M-L-calculated defect energies.100 Defect energies calculated by the M-L approach implemented in GULP have already accounted for the long-range polarization effect, so extra corrections are not needed.
For example, when an oxygen vacancy VOq with a charge state q (q = 0, +1,+2) is formed in CeO2, the excess 2 – q electrons are not trapped at the vacancy site but will localize on the nearby Ce site to form CeCe small polarons. This process can be formed thus:
| 14 |
The formation energy determined using M-L calculations is then calculated as the corresponding reaction energy:
| 15 |
where E[VOq] = E[VO + (2 – q)CeCe′] is the defect energy obtained from an M-L calculation and EF is the Fermi level relative to VBM. Similarly, a cerium vacancy with a charge state q (q = 0, −1, −2, −3, −4) can trap (4 + q) localized holes OO. The formation energy of VCeq can be calculated by
| 16 |
Also, the formation energies of oxygen interstitials Ef[Oiq] are calculated by
| 17 |
Finally, the formation energies of cerium interstitials Ef[Ceiq] are calculated by
| 18 |
The formation energy of a charged defect is also dependent on the growth conditions. In the O-rich/Ce-poor conditions, the upper limit is determined by the formation of O2 molecules, in which μO = 0 eV and μCe = ΔHf(CeO2). In O-poor/Ce-rich conditions, the lower limit of μO is determined not only by CeO2 but also by other CenOm phases, where CeO2 should remain more stable than any other reduced phases. Conventionally, we take Ce2O3 as the limiting phase as employed in previous DFT calculations.27,28,30 Hence,
| 19 |
| 20 |
Therefore, the chemical potentials should satisfy
| 21 |
| 22 |
Thermochemical experimental measurements give ΔHf(CeO2) = −11.28 eV and ΔHf(Ce2O3) = −18.62 eV,101 which allows us to calculate μO min = −3.94 eV and μCe max = −3.40 eV, which were used in our calculations. In QM/MM calculations, the reference energies for an O2 molecule and a single Ce4+ ion are calculated using NWChem with the corresponding basis set and functional. The sum of the first four ionization potentials of Ce (ICe1–4, 73.745 eV101) and sublimation enthalpy of Ce (ΔHsubl(Ce), 4.380 eV101) are then used to obtain the reference energy of Ce(s).
In both M-L and QM/MM calculations, formation energies (per defect) of the unbound cation–Frenkel pair, anion–Frenkel pair, Schottky trio, and interstitial disorder defects were calculated by
| 23 |
| 24 |
| 25 |
| 26 |
3. Results and Discussions
3.1. Strategies for Developing Robust Shell-Model Potentials
In this work, we report a framework for developing a robust shell-model IP for CeO2 assisted by QM/MM calculations, which could also be extended to other ionic solids. First, a classical shell-model IP based on Buckingham potentials was parametrized to reproduce the bulk structure and dielectric constants. As discussed in the previous section, to make the optimal choice, we systematically reviewed the performance of several candidate potential parameter sets based on different values of the ρ parameter in modeling defects and surface properties. Next, the optimal potential, IP10a, was used in hybrid QM/MM embedded-cluster models to obtain accurate references of the structure and formation energies of intrinsic defects in CeO2, including oxygen vacancies, cerium vacancies, oxygen interstitials, cerium interstitials, hole polaron, and electron polaron. Then, these data were used in turn to optimize the Ce4+–O2– interactions using a more complex form that combines the Buckingham, Lennard-Jones, and Morse potentials for correcting the derivatives and an offset constant for correcting the lattice energy, IP10b. Finally, the Ce3+–O2– and Ce4+–O1– potentials were fitted based on the Ce4+–O2– potential according to QM/MM calculations of electron and hole polarons in terms of bond length and formation energy. Based on the newly developed IP10b, the calculated defect structures and formation energies at the MM level of theory using the M-L approach are highly consistent with QM/MM results using hybrid DFT functionals.
3.1.1. Construction of the Reference Data Set
CeO2 is a widely studied material with well-documented experimental measurements of physical and chemical properties. However, due to the easily reducible nature, oxygen vacancies and electron polarons in CeO2 could significantly affect the measurements, resulting in inconsistent reports of several properties. For example, a considerable concentration of Ce3+ (12%–45%) was detected in many experimentally synthesized polycrystalline thin-film samples, which are very difficult to eliminate even with oxygen plasma treatment at high temperatures.106,107 Such a high concentration of Ce3+ in CeO2 could originate not only from surface defects but also from amorphous Ce2O3 formed at the grain boundaries of nanocrystals.106 Hence, we focus on data obtained from single crystal and stoichiometric samples if available. The experimental lattice constant a0 of undoped CeO2 is typically reported to be 5.411 Å at room temperature.108−110 Gupta and Singh reported an a0 of 5.401 Å at 100 K, and no data are available close to 0 K. Based on several thermal expansion experiments, we obtained an estimate for a0 of 5.395 Å when extrapolated to 0 K.81,111−113 This estimate also allows us to obtain an a0 of 5.411 Å at 300 K based on the new IP using free energy calculations within the quasi-harmonic approximation (20 × 20 × 20 k-points on the 12-atom conventional cell of CeO2), in excellent agreement with experiment.
The O2p → Ce4f band gap of CeO2 is typically reported ranging from 3.0 to 3.5 eV3,106,114−116 and is known to decrease with the increase in the concentrations of oxygen vacancies and Ce3+.3,106 However, Pelli Cresi et al.117 recently proposed a revised value of 4 eV for the optical band gap based on steady-state and ultrafast transient absorption spectra measured on stoichiometric samples. They argued that the long absorption tail from 3 to 4 eV in the Tauc plot should be identified as the Urbach tail, further confirmed by the photobleaching in the transient absorbance measurement, which corresponds to the occupied Ce 4f localized state due to the rapid small polaron formation. Such an Urbach tail is also seen in nanoparticle samples with a larger band gap of 4.2–4.3 eV due to the quantum confinement effect.118 This conclusion is also supported by previous high-resolution electron energy loss spectroscopy (EELS) measurements on stoichiometric119 and fully oxidized single-crystal120 samples, which located the unoccupied Ce4f state 4–4.4 eV higher than the O2p state. Hence, we consider that a band gap of 4 eV measured by Pelli Cresi et al.117 could be the best estimate for CeO2 and is further employed in this work. We will compare with experimental data for the elastic constants C11, C12, and C44;(82) zone-center phonon frequencies F1u(TO), F2g, and F1u(LO);82 static and high-frequency dielectric constants, ε0 and ε∞;83 bulk modulus B0;84 and migration barrier of oxygen vacancy EVOmigration.85
Plane-wave DFT calculations using VASP at different levels of theory were performed alongside our semiclassical GULP simulations. The calculated lattice constants, band gaps, Born effective charges, and surface energies of CeO2 are listed in Table 1, where we find that the hybrid functionals in our assessment set show a superior agreement with experiment. Previous work also showed that in general hybrid functionals perform much better than GGAs in describing the formation enthalpy, heat of reduction, and defect formation energy of CeO2.7,30,36 Because the PBE0 functional is implemented in both the plane-wave DFT code (VASP) and atomic-centered basis set code (NWChem), employed in our hybrid QM/MM calculations, we consistently use PBE0 results as the primary reference in the IP development. The Born effective charges and surface energies from VASP calculations, as well as the in-lattice ionic polarizabilities, defect structures, and energies from QM/MM results, were used in refining the IP. Our new IP can easily be reparameterized to reproduce the predictions from any other functional based on the same protocol.
Table 1. Calculated Lattice Constant a0, Band Gap Eg, High-Frequency Dielectric Constant ε∞, Born Effective Charges ZCe* and ZO, and Surface Energies of CeO2 Using VASP Compared with Previous Experimental and Theoretical Results.
| a0 (Å) | Eg (eV) | ε∞ | ZCe* | ZO* | E(100)surf(J m–2) | E(110)surf(J m–2) | E(111)surf(J m–2) | |
|---|---|---|---|---|---|---|---|---|
| PBE+U (UCe 4f = 5 eV)a | 5.490 | 2.29 | 6.59 | 5.53 | –2.77 | 1.45 | 1.06 | 0.69 |
| PBEsol+U (UCe 4f = 5 eV)a | 5.435 | 2.41 | 6.57 | 5.51 | –2.75 | 1.77 | 1.27 | 0.90 |
| PBE0a | 5.397 | 4.38 | 5.67 | 5.67 | –2.84 | 1.61 | 1.21 | 0.85 |
| PBEsol0a | 5.361 | 4.46 | 5.68 | 5.68 | –2.84 | 1.83 | 1.33 | 0.96 |
| HSE06a | 5.396 | 3.63 | 5.71 | 5.67 | –2.84 | 1.59 | 1.20 | 0.84 |
| PBE+U (UCe 4f = 5 eV)b | 5.489 | 1.44 | 1.06 | 0.71 | ||||
| PBEsol+U (UCe 4f = 5 eV)c | 5.431 | 6.55 | 5.53 | –2.76 | ||||
| PBE0d | 5.403 | 4.35 | ||||||
| PBE0e | 5.401 | 1.64 | 1.27 | 0.86 | ||||
| PBEsol0d | 5.368 | 4.40 | ||||||
| HSE06f | 5.40 | 3.50 | ||||||
| GW0g | 3.88 | |||||||
| Experiment | 5.411h | 2.9–3.3k | 5.31q | 1.20 ± 0.2 (averaged)r | ||||
| 5.401i | 4.0l | |||||||
| 5.395j | 3.6–4.11m | |||||||
| 4.0n | ||||||||
| 4.4o | ||||||||
| 4.3–4.4p | ||||||||
Present work.
Reference (121).
Reference (122).
Reference (37).
Reference (38).
Reference (35).
Reference (123).
Low-temperature measurement at 100 K.113
Steady-state and ultrafast transient absorption spectra measurement on stoichiometric CeO2 thin film. A revised optical band gap of 4 eV was proposed for bulk ceria, and the absorption tail below 4 eV was identified as the Urbach tail.117
Optical measurement on polycrystalline CeO2 films with 140–180 nm thicknesses.124
The electron energy loss spectroscopy (EELS) spectrum captured the O2p → Ce4f transition starting at 4 eV on single-crystal thin films, and the intensity between 0 and 3 eV indicates the Ce3+ state.120
High-resolution EELS study on stoichiometric thin films observed a 4.4 eV energy loss from O2p to the empty Ce4f state transition.119
Measurement from the optical absorption spectrum on the CeO2 nanoparticles.118
Transmissivity and reflectivity measurement on CeO2 quasi-single crystal sample.83
3.1.2. Strategies for Improving the Accuracy of Shell-Model Potentials
A shell-model potential can be divided into two parts: the shell model (Y and k2) and other parameters including short-range repulsion and dispersion interactions. The shell-model defines the ionic polarizabilities that significantly affect the dielectric properties of materials, which play a key role in defect formation. Previous parametrizations of interatomic potentials for ceria have in common a somewhat unsatisfactory approach to choosing how the lattice polarizability is distributed between individual ions, which may not correctly reflect the physical nature of the target material. Polarizabilities have often been determined by fitting spring constants and shell charges to experimental data without consideration of the relative polarizabilities of cations and anions; it is possible to obtain a number of candidate potential sets with different shell-model parameters but with similar performances in modeling the bulk properties. An alternative to purely empirical fitting was developed by Lewis and Catlow74 considering in-lattice polarizabilities based on gas-phase Pauling’s polarizabilities of cations, which are known to be less affected by the crystal environment. By contrast, the anion polarizability varies strongly with the oxide structure and chemical composition.74 The in-crystal ionic polarizabilities can be calculated from hybrid QM/MM embedded-cluster models (cf. ref (127)). Here, we have employed ChemShell with NWChem as a QM driver to construct suitable embedded-cluster models and calculated the in-lattice ionic polarizability. The detailed methodology is presented in Section S1.5 of the Supporting Information.
In Table 2, we collected the calculated in-lattice polarizabilities of Ce4+ and O2– ions using the Def2-TZVP93 basis set. It can be seen that the gas-phase cation polarizability is very close to the frozen in-crystal polarizability, as has been pointed out by Lewis and Catlow.74 The gas-phase calculations for O2– are extremely basis-set-dependent, (as expected, since the O2– ion is an unbound species in the gas phase), and increases from 6.293 Bohr3 using the Def2-TZVP basis set to 50.871 Bohr3 using Def2-QZVPPD128 at the PBE0 level of theory. In the limit of a complete basis set, the ground-state solution would include an electron dissociated from the oxide ion to infinity and, therefore, an infinite polarizability. The hybrid DFT predictions are in good agreement with higher-level coupled-cluster results. The intrinsic polarizability of an ion, αi, in the CeO2 crystal environment does not include effects of charge transfer from or to surrounding ions, which plays a significant role in real materials, and the total polarizability of an ion is
| 27 |
where αct is the respective contribution from charge transfer. When fitting IP parameters, we kept the ratio of Ce4+ and O2– polarizabilities (α = Y2/k2) constant according to the QM/MM calculated polarizability data, based on a conjecture that αct is proportional to αi. Under this methodology, a unique set of shell-model parameters can be determined that models more accurately the relative ionic polarizabilities in solids. Our results show that Ce4+ and O2– have similar polarizabilities in CeO2. By calculating the ionic polarizabilities through eq 3, we found that most previous IPs cannot reproduce this feature.
Table 2. Calculated Intrinsic In-Lattice Ionic Polarizabilities (Bohr3) of CeO2 Using the ChemShell Embedded-Cluster Model Compared with Their Counterparts Calculated in the Gas Phase Where Possible (O2– Ions Are Unstable in the Gas Phase).
| B97-2 | PBE0 | BB1K | HF | CCSD | CCSD(T) | |
|---|---|---|---|---|---|---|
| αi (O2– in CeO2) | 5.601 | 5.601 | 5.535 | 5.400 | 5.380 | 5.373 |
| αi (Ce4+ in CeO2) | 5.901 | 5.935 | 5.894 | 5.915 | 5.886 | 5.900 |
| αi (gas-phase Ce4+) | 5.842 | 5.871 | 5.832 | 5.852 | 5.825 | 5.836 |
Polarizability also determines the dispersion (London) interaction, the strength of which is usually described by the C6 coefficient in the Buckingham interatomic potential. The similarly polarizable Ce4+ and O2– in CeO2 suggest that the C6 coefficients for Ce4+–O2–, Ce4+–Ce4+, and O2––O2– should all be considered, whereas most of the previous IPs did not introduce the C6 coefficients for all these interactions. We have calculated C6 according to the Slater–Kirkwood formula129 using the participation numbers reported by Pyper et al.130 and our calculated polarizability data. C6(XY) is given as
| 28 |
where PX is the participation number of ion X (4.455 for O2– and 7.901 for Ce4+ from Pyper et al.130).
In ionic solids, the long-range electrostatic interaction contributes greatly (over 90%) to the total lattice energy. Although the short-range repulsive interaction originating from the overlapping electron densities contributes much less, the pairwise parameters in an IP largely control the bond lengths and structures and, hence, energies and physical properties. Among all the short-range interactions, the cation–anion interaction has the greatest effects due to its directly bonded nature. The cation–cation and anion–anion interactions are much weaker because of the longer interatomic distances. We note that parameter ρ in the previous Ce4+–O2– Buckingham potentials varied from 0.335 Å to 0.429 Å. A small ρ with a large A or a large ρ with a small A may show only minor differences in the calculated bulk properties. However, we found that the choice of ρ is of vital importance in predicting defect and surface properties, which are typically not included in the list of fitted observables. Hence, the “best” IP derived by the fitting procedure may not be appropriate for modeling defects and surfaces, as can be seen in the predicted incorrect relative stabilities of surfaces and defect pairs by several IPs.
To explore the effects of ρ, we parametrized several IPs based on different fixed values of ρ within the traditional Buckingham potential framework (shown in Table S2). These potentials share the same Ce4+–Ce4+ and O2––O2– parameters, while other parameters are fitted to minimize the sum of squares of selected observables. We used the A and ρ parameters of the O2––O2– Buckingham potential from Lewis and Catlow74 since they have remarkable transferability in many ionic oxide systems and can be combined with other potentials to study dopants and solid solutions. We also put the highest fitting weights on the lattice constant (ca. 5.395 Å at 0 K) and static and high-frequency dielectric constants due to their critical importance in determining the accuracy of QM/MM calculations. Therefore, all the IPs we derived give accurate reproductions of the lattice constant and dielectric constants.
Figure S1 shows how the ρ parameter affects the predicted properties of CeO2. The dark gray horizontal lines represent the reference data from experiment or DFT calculations at the PBE0 level of theory. If we only consider the bulk properties that are normally used as fitting observables, a larger value of ρ, such as ρ = 0.37 Å, could be the result obtained by the least-squares procedure. However, if we further consider the defect and surface properties, those IPs with a larger value of ρ produce inaccurate results for ceria. By systematically considering bulk, defect, and surface properties (discussed in Section S1.6 of the Supporting Information), we determined the IP with ρ = 0.349 Å as the best Buckingham potential for CeO2, which was further used in QM/MM defect calculations. We stress that the QM/MM calculations based on IP10a are reliable and consistent with those based on IP10b since the lattice constants, dielectric constants, and ionic polarizabilities predicted by the two potentials are identical. The full set of parameters is provided in Table S2, and the performance in reproducing the properties of ceria has also been shown in Figure 1, denoted as “IP10a”. Within the current model, it is still impossible to reproduce simultaneously all targeted properties because the cation–anion short-range interaction cannot be accurately described by a single Buckingham potential. However, we have shown here that the shell-model potential with the classical Buckingham form can qualitatively and semiquantitatively reproduce the correct atomic structure and properties of CeO2 based on a careful selection of some critical parameters.
3.1.3. Refining the Interatomic Potential According to QM/MM Calculations
Short-range interactions in solids sometimes cannot be exactly described by a single potential adopting a simple analytical form. The Lennard-Jones potential is known to be considerably too repulsive at short bond distances.131 Although the Buckingham (and its constituent Born–Mayer) potential is a good physically justified approximation for the short-range repulsion,132 empirical fitting procedures could cause unwanted problems, as illustrated by the critical choice of ρ described above. Combining different forms, nevertheless, could increase the parametric space and overcome the weakness of each component to improve the accuracy.104,133 Derivatives of an IP determine the structure and elastic, dielectric, and phonon properties of the modeling system. The absolute potential value determines the lattice energy, which further affects the calculated energies of defects and surfaces. To obtain a robust IP, both derivatives and absolute values of the potential should be accurately determined.
We define a new analytic form for the cation–anion pairwise potential, as shown in eq 5. Parameters of the refined potential (IP10b) for CeO2 based on this form are shown in Table 3. The sole Buckingham potential representing the short-range interaction is insufficient for a consistently accurate description of elastic, dielectric, and phonon properties. This weakness can be attributed to inaccurate potential derivatives near the first and second neighbor bond distances. Hence, a Morse-like function is superimposed on the Buckingham potential to correct these derivatives and reproduce these bulk properties accurately. The offset constant c0 shifts down the potential curve by a constant to correct the lattice energy. Weak Lennard-Jones potentials were used to add repulsion to the interactions at very short bond distances to overcome the Coulomb catastrophe that could occur in molecular dynamics or Monte Carlo simulations.133 The overall potential is truncated at 5.278 Å where the potential energy drops to 0 eV, between the second and third Ce–O neighbor bond lengths. The resulting potential curve is shown in Figure S2, compared with previous potentials in the literature. The shell model parameters were slightly optimized for better overall performance, while the ratio of polarizabilities of Ce4+ and O2– remained consistent with IP10a.
Table 3. Optimized Potential IP10b (Defined in Equation 5) for CeO2 Combined with Parameters for Modeling Hole (O1–) and Electron (Ce3+) Polaronsa.
| (a) Short-Range Potential | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Interaction | A (eV) | ρ (Å) | C6(eV Å6) | E(eV Å12) | D (eV) | a (Å–1) | r0 (Å) | c0 (eV) | rmin (Å) | rmax (Å) |
| O2––Ce4+ | 1138.963021 | 0.417578 | 25.082349 | 1.0 | –1.15172262 | 0.4 | 4.53327 | –1.077767 | 0 | 5.278 |
| O2––Ce3+ | 1025.066719 | 0.417578 | 25.082349 | 1.0 | –1.036550358 | 0.4 | 4.53327 | –0.9249903 | 0 | 5.532 |
| O1––Ce4+ | 706.157073 | 0.417578 | 25.082349 | 1.0 | –0.714068 | 0.4 | 4.53327 | –0.7462155 | 0 | 5.278 |
| O2––O2– | 22764.3 | 0.149 | 20.983768 | 1.0 | 0 | 15.0 | ||||
| O1––O2– | 22764.3 | 0.149 | 20.983768 | 1.0 | 0 | 15.0 | ||||
| O1––O1– | 22764.3 | 0.149 | 20.983768 | 1.0 | 0 | 15.0 | ||||
| Ce4+–Ce4+ | 1.0 | 0.1 | 30.481293 | 1.0 | 0 | 15.0 | ||||
| Ce3+–Ce4+ | 1.0 | 0.1 | 30.481293 | 1.0 | 0 | 15.0 | ||||
| Ce3+–Ce3+ | 1.0 | 0.1 | 30.481293 | 1.0 | 0 | 15.0 | ||||
| (b) Shell Model | ||
|---|---|---|
| Species | Y (e) | k2(eV Å–2) |
| Ce4+ shell | 13.85 | 1071.1845 |
| Ce3+ shell | 12.85 | 1071.1845 |
| O2– shell | –2.936345 | 53.022513 |
| O1– shell | –1.936345 | 53.022513 |
Short-range potentials were designed only for shell–shell interactions. A GULP-readable format is also provided in Section S3 of the Supporting Information.
We here also emphasize the critical role of the lattice energy predicted by a shell-model potential in determining the accuracy of defect and surface calculations. The lattice energy ΔUL sets an essential basis for every calculation regarding energy differences such as surface energy, defect formation energy, and migration barrier. Lattice energy is not a direct experimental observable but can be evaluated theoretically through the Born–Haber cycle based on the experimental formation enthalpy, as shown in eq 7. However, the exact value of ΔUL requires the knowledge of the oxygen second electron affinity AO2, which is a crystal-structure-dependent variable134 and not known from experiment. We proposed that the required ΔUL and AO in the IP model can be determined according to DFT-calculated surface and defect energies. As shown in Figure 2, several variants of potentials are derived based on different ΔUL values by only varying the c0 term while keeping all other parameters constant. As a result, these potentials share the same shape (derivatives) but differ in the absolute potential energy, thus predicting the same equilibrium-state structure but different absolute energies for defects and surfaces. The predicted defect and surface energies change considerably despite the minor differences in the predicted ΔUL. Periodic DFT calculations of surface energies and QM/MM calculations of defect energies at the PBE0 level were employed as references to obtain the required lattice energy that gives the most accurate prediction. In IP-based surface calculations, the lattice energy mainly determines the required energy to generate the unrelaxed surfaces rather than the relaxation energy. The most stable (111) surface has only 0.01 (0.05) J m–2 relaxation energy as predicted by periodic DFT (IP-based) calculations, which could be the best reference for the target lattice energy. We found that both defect and surface calculations support that ΔUL = −107.5 eV, corresponding to an AO2 of 8.14 eV, which is employed as the final version of our CeO2 potential (IP10b). We note that the new potential still has certain errors on the calculated surface energies compared with periodic DFT results. To our knowledge, the only experimental measurement of surface energies of CeO2 was based on nanoparticle samples (which should be dominated by the most stable (111) surface) that reported an average value of 1.20 ± 0.2 J m–2,125,126 slightly higher than DFT predictions. The overestimation mainly originates from the cleavage energy before structural relaxation, which is also seen in other IP models. We have checked that the optimized surface structures are consistent with PBE0 predictions with an average error of 0.012 Å for the predicted surface and subsurface Ce–O bond lengths, suggesting a reasonably good description by the IP model.
Figure 2.
Effects of the predicted lattice energy ΔUL on the calculated defect and surface properties of CeO2 based on the new potential formula.
3.1.4. Parameterization for Hole and Electron Polarons in CeO2
Finally, we developed additional parameters for modeling localized electrons (CeCe′) and holes (OO) in CeO2. The electron small polaron has been experimentally observed by STM imaging and confirmed by DFT calculations on the (111) surface.12,30,40,43 Holes also stabilize as small polarons in CeO2,25 which is a common feature of many oxide materials.135 The formation of electrons and holes could be modeled through the M-L approach as described by Freeman and Catlow in SnO2.100 Conventionally, electrons/holes were modeled by adding/subtracting a unit charge from the original shell charge and keeping the same core charge. However, using the same short-range potential to describe interactions between ions in different oxidation states is less accurate and usually results in several electronvolt errors in the predicted ionization potential, electron affinity, and band gap compared with experiment or DFT.100,104 Instead, we propose a correction scheme based on the new O2––Ce4+ potentials but where we target the bond lengths and formation energies of small polarons obtained from our QM/MM calculations.
In both M-L and QM/MM calculations, vertical processes were modeled through electronic (shell) relaxation, while adiabatic processes required explicit structural optimization of the active regions. We performed QM/MM calculations to obtain the vertical and adiabatic ionization potentials and electron affinities. In the adiabatic processes, electrons and holes are self-trapped, forming small polarons in CeO2. Before fitting the potentials for modeling the polarons, the shell charges were first corrected in line with the formal charges of Ce3+ and O1–. Then, the coefficients in the Ce4+–O2– potentials (A and D) are multiplied by a factor to correct the derivatives of the potential and reproduce the polaron structures. This factor was determined by targeting the average Ce3+–O2–/O1––Ce4+ first-neighbor bond length around the ionized species calculated by the M-L approach to correspond to PBE0 QM/MM results. Finally, the potential is offset by a c0 constant to reproduce the polaron formation energy calculated by QM/MM. A similar procedure was used to parametrize the Ce4+–O1– potential for describing localized holes, while the vertical ionization potential was used as the reference for correcting c0, ensuring the alignment of the positions of VBM in both techniques for accurate formation energy calculations of charged defects. Full details of the additional parameters have been given in Table 3.
In summary, we have provided some new strategies to develop shell-model potentials with improved performance in modeling ionic materials in Section 3.1. Implementation of in-lattice ionic polarizabilities, lattice energy, defect energies, and surface energies in the potential fitting improves the description of various physical properties, while consideration of QM/MM results of charge carriers further makes it possible to model various charged defects at the MM level of theory. Based on this approach, the formation of native defects in different charge states can be reasonably described through the M-L approach, assuming that the charge carrier is well localized and trapped by defects, as is the case in CeO2. Such an approximation gives reasonable accuracy with results comparable with DFT calculations using hybrid functionals, as will be shown in Section 3.2.
3.2. Performance of the New Potential in Modeling Defects in CeO2
The structure and properties of CeO2 predicted by the revised potential have been shown in Figure 1, denoted as IP10b. Compared with IP10a, the revised version significantly improves the performance in many aspects: the predicted bulk modulus, elastic constants, and phonon frequencies are in excellent agreement with experiment, and the calculated defect energies are also more consistent with QM/MM predictions. Based on the new potential, we also calculated the phonon dispersion of CeO2 at 0 K (Figure 3). Experimental results are presented in markers for comparison, derived from reflectivity by inelastic neutron scattering by Clausen et al.136 and Marabelli and Wachter137 and Raman scattering by Nakajima et al.82 and Kourouklis et al.138 Our potential shows excellent agreement with the experimental measurements in terms of the phonon modes at the high symmetry points and acoustic mode dispersion, ensuring good accuracy for future study of thermodynamic properties of defective CeO2 using free-energy calculations.
Figure 3.
Calculated phonon dispersion for CeO2 based on the new potential compared with experimental data from Clausen et al.,136 Marabelli and Wachter,137 Nakajima et al.,82 and Kourouklis et al.138
A detailed comparison of QM/MM and M-L calculations of the vertical and adiabatic formation energies of holes and electrons in CeO2 is given in Table 4. Full computational details are given in Section S1.7 of the Supporting Information. For comparison, if we directly employ the Ce4+–O2– short-range potential to model the localized electrons and holes without any correction (listed in the first column), as originally proposed by Freeman and Catlow,100 the bond lengths around the polarons and the band gap of CeO2 are significantly overestimated. Our corrected potential accurately reproduces the formation mechanisms of localized electrons and holes in CeO2 and gives a more reasonable band gap. This strategy could be a general approach and extended to other systems with localized charge carriers. The self-trapping energy of the localized electron is predicted to be −0.92 eV, compared with −0.61, −0.69, and −1.39 eV by the QM/MM approach using B97-2, PBE0, and BB1K functionals, respectively. Previous periodic DFT calculations reported −0.30 eV and −0.54 eV by HSE06 and PBE+U.30
Table 4. Vertical and Adiabatic Ionization Potentials (Ivertical and Iadiabatic), Vertical and Adiabatic Electron Affinities (Avertical and Aadiabatic), Self-Trapping Energies (EholeST and Eelectron) of a Hole or an Electron, the Average First-Neighbor Bond Length around the Hole or Electron (rCe4+–O1– and rCe3+–O2–), and the Band Gap (Eg) of CeO2 Calculated by the M-L Approach and QM/MM Calculationsa.
| M-L (with modified shell charges)b | M-L (with modified shell charges and potentials)b | QM/MMB97-2b | QM/MM PBE0b | QM/MM BB1Kb | Periodic HSE06c | Periodic PBE+Uc | |
|---|---|---|---|---|---|---|---|
| Ivertical (eV) | 9.64 | 5.10 | 4.92 | 5.10 | 5.38 | ||
| Iadiabatic (eV) | 7.11 | 3.78 | 4.35 | 4.41 | 3.80 | ||
| EholeST (eV) | –2.53 | –1.32 | –0.57 | –0.69 | –1.58 | ||
| rCe4+–O1– (Å) | 2.533 | 2.451 | 2.447 | 2.451 | 2.452 | ||
| Avertical (eV) | 0.18 | 0.28 | 0.70 | 0.51 | 0.11 | ||
| Aadiabatic (eV) | 1.44 | 1.20 | 1.31 | 1.20 | 1.50 | ||
| EelectronST (eV) | –1.26 | –0.92 | –0.61 | –0.69 | –1.39 | –0.30 | –0.54 |
| rCe3+–O2– (Å) | 2.474 | 2.444 | 2.448 | 2.442 | 2.435 | 2.42 | |
| Eg (eV) | 8.08 | 4.82 | 4.22 | 4.59 | 5.27 |
The predicted band gaps by the embedded-cluster approaches are higher than the experimental values for two reasons. First, despite the advantages in predicting bulk properties and formation energies of defects, most hybrid functionals significantly overestimate the band gap of CeO2.36,37 CeO2 is a strongly correlated f electron oxide, and the noncorrelated HF method predicts the band gap as 14.5 eV.37 As a result, the hybrid functional that includes a higher percentage of HF exchange usually provides a worse description of the band gap of CeO2. However, a certain percentage of HF exchange (usually 25%) is required to ensure the predictions of localized small polarons in CeO2, or both electrons and holes will become more delocalized even when trapped by defects, which is however contrary to experiment.7,25,43 Second, the overestimation of the band gap is partly due to the poor description of delocalized states and quantum confinement effects by the hybrid QM/MM embedded-cluster approach. Hence, it can be seen that the predicted “band gap” through Ivertical – Avertical by the QM/MM method using the PBE0 functional (4.59 eV) is slightly higher than plane-wave periodic DFT calculations using VASP (4.38 eV) and the experimental measurement (4 eV). To counter these problems, we typically use the experimental band gap and calculated vertical ionization potential to determine the position of conduction band minimum (CBM) in QM/MM calculations.56
3.2.1. Formation of Charged Defects in CeO2
Using the new IP, we modeled the formation of intrinsic point defects and defect pairs in CeO2 with the M-L and QM/MM approaches. The optimized structures of some typical defects in ceria obtained by the M-L approach are shown in Figure 4. The arrows indicate the directions and magnitudes (six times the actual length) of atomic displacements following the defect formation. We first consider the formation of atomic defects without trapped charge carriers. The formation of VO•• results in an inward displacement of the six nearest O2– ions by 0.275 Å, compared with 0.223–0.245 Å in the QM/MM calculations. We note that the long-range polarization effect of VO is quite large, which causes the fourth, fifth, and sixth neighboring O2– ions to move inward by 0.06 Å, 0.04 Å, and 0.02 Å, respectively. Unless a supercell with hundreds of atoms is used, periodic DFT calculations may have difficulties modeling such a long-range perturbation effect. Our embedded-cluster models explicitly considered this effect because sufficiently large active regions are used. The four neighboring Ce4+ ions displaced outward by 0.166 Å (corresponding to 7.1% of the equilibrium bond length), which agrees well with previous DFT calculations by Sun et al. (6.8% in HSE06 and 7.1% in PBE+U).30 For VCe⁗, the largest displacement was on the eight coordinating O2– ions, which move away from the defect center by 0.431 Å, while the surrounding Ce4+ ions are barely perturbed. The Oi sits symmetrically in the center of the octahedral site. The O2–-O2– bond length is calculated to be 2.50 Å (107.1% of the equilibrium interoxygen separation distance), which agrees well with the PBE+U prediction by Zacherle et al.27 (2.52 Å and 105.9% of the PBE+U bond length). Finally, the introduction of a Cei•••• results in a strong repulsion to the nearest Ce4+ ions, forcing them to move away by 0.357 Å (0.32–0.324 Å by previous DFT+U predictions25,28 and 0.337–0.349 Å by our QM/MM calculations). Cei has much less influence on the O2– ions (displaced only by 0.096 Å). Among these types of defects, O2– ions are more easily affected by defect formation than Ce4+ ions (except Cei••••, which is less common in CeO2 due to the relatively high formation energy). A detailed comparison of the structural relaxation due to defect formation is made in Table S3. Overall, M-L calculations show excellent agreement with previous periodic DFT and our QM/MM predictions of the defect structures.
Figure 4.
Defect-induced structural relaxation in CeO2 predicted by the Mott–Littleton approach. (a) Electron polaron CeCe′. (b) Hole polaron OO. (c–e) Oxygen vacancies VOq, q = +2, +1, 0. (f–h) Oxygen interstitials Oi, q = −2, −1, 0. (i) Bound anion–Frenkel pair [VO••–Oi]×. (j) Bound Schottky trio [VO••–VCe–VO••]×. (k–o) Cerium vacancies VCe, q = −4, −3, −2, −1, 0. (p–t) Cerium interstitials Ceiq, q = +4, +3, +2, +1, 0. Arrows indicate directions and magnitudes of atomic displacements (magnified 6-fold to improve the clarity of images) upon relaxation.
We also studied the formation energies of different charge states of native defects using the new IP and the embedded-cluster approaches. We started from the various possible configurations of polarons trapped by oxygen vacancies in bulk ceria. Previous DFT calculations have predicted that the oxygen vacancy becomes less stable when the two polarons locate beyond the second-neighbor sites.29,42 This conclusion is confirmed by our M-L calculations. The calculated formation energies of [VO••–CeCe]• in O-rich conditions when the Fermi level is at VBM are 0.049 eV, 0.046 eV, 0.306 eV, 0.267 eV, and 0.303 eV for the localized electrons at the first-, second-, third-, fourth-, and fifth-nearest neighbor sites, respectively. Hence, we only focus on the possible configurations within the second neighbor radius. For the charge-neutral oxygen vacancy [VO••–2CeCe]× formed in bulk ceria, the excess electrons will localize on two Ce sites (CeCe′). As shown in Figure 5 and Table 5, there are one NN-NN, three NN-NNN, and five NNN-NNN symmetrically unique configurations on which the two CeCe polarons can locate.41
Figure 5.

Electron polaron configurations in the charge-neutral oxygen vacancy [VO••–2CeCe]× in CeO2.
Table 5. Calculated Formation Energies (eV) in O-Rich Conditions of Charge-Neutral Oxygen Vacancies Associated with Electron Polarons in Differing Configurations.
| Configuration | dCe3+–Ce3+ | M-La | QM/MM PBE0a | QM/MM BB1Ka | Periodic PBE+Ub | Periodic PBE+Uc | Periodic HSE06c | Periodic PBE+Ud | Periodic PBE+Ue | Periodic HSE06e | Periodic PBE0f |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NN1-NN2 | 1N | 3.657 | 4.04 | 2.91 | 2.50 | 3.73 | 2.22 | 2.70 | 4.09 | 3.84 | |
| NN1-NNN6 | 2N | 3.681 | 2.95 | ||||||||
| NN1-NNN1 | 1N | 3.673 | 2.93 | ||||||||
| NN1-NNN2 | 3N | 3.564 | 3.70 | 2.92 | 2.85 | ||||||
| NNN1-NNN2 | 5N | 3.596 | 2.92 | ||||||||
| NNN1-NNN3 | 4N | 3.604 | 2.85 | ||||||||
| NNN1-NNN4 | 1N | 3.578 | 3.77 | 3.02 | 2.85 | 2.43 | 3.63 | ||||
| NNN1-NNN5 | 3N | 3.579 | 2.86 | ||||||||
| NNN1-NNN6 | 1N | 3.604 | 2.92 |
Present work.
Plane-wave PBE+U (UCe 4f = 4.5 eV) calculations using the 96-atom supercell.29
Plane-wave PBE+U (UCe 4f = 5.0 eV) and HSE06 (25% HF) calculations using the 192-atom supercell.31
Plane-wave PBE+U (UCe 4f = 5.0 eV and UO 2p = 5.5 eV) calculations using the 96-atom supercell.143
Plane-wave PBE+U (UCe 4f = 5.0 eV) and HSE06 (25% HF) calculations using the 96-atom supercell.30
Plane-wave PBE0 (25% HF) calculations using the 96-atom supercell.38
The calculated formation energies of all nine configurations of [VO••–2CeCe]× in O-rich conditions are summarized in Table 5. In general, our simulations confirm that the excess electrons have lower energies when localized on the NNN site, consistent with other DFT predictions.29,41,139 The most stable configuration is the NN1-NNN2 with a formation energy of 3.564 eV, and the second stable NNN1-NNN4 configuration is only 0.014 eV higher in the formation energy. Our QM/MM calculations also favor the stabilization of the NN1-NNN2 configuration against the NNN1-NNN4 configuration, consistent with the M-L predictions. The tiny energetic deviations (<0.12 eV) among the formation energies of all the nine configurations suggest very close stabilities of different configurations, consistent with the previous periodic DFT+U study by Murgida et al..29 Because the adiabatic hopping barrier for CeCe′ in CeO2 is very low (0.15–0.2 eV12,140,141), the positions of polarons could change rapidly at elevated temperatures. Previous DFT calculations predict lower formation energy at the PBE+U level (2.2–2.95 eV)26,29−31 and slightly higher formation energy using HSE06 (3.63–4.09 eV7,30,31). Brugnoli et al.38 reported 3.84 eV for the formation energy of the NN1-NN2 configuration based on the periodic model using PBE0, compared with 4.04 eV from our QM/MM model using the same functional. Burow et al.142 conducted the periodic electrostatic embedded-cluster method (PEECM) using PBE0 but obtained a much lower formation energy (3.0 eV) than our QM/MM predictions and periodic calculations from Brugnoli et al.38 Our predictions at the PBE0 level of theory are also in line with the experimental heat of reduction of ceria, which is ca. 4.0 eV.18,19
The same approach was used to investigate other charged defects with localized electrons and holes. Table 6 summarizes the calculated formation energies of all charge states of point defects studied by the M-L and QM/MM methods using our new IP. Figure 6 shows a direct comparison of the calculated formation energies under different conditions using the M-L approach and QM/MM calculations. These two embedded-cluster approaches present a high level of consistency in predicting the formation energies, confirming the success of our newly proposed fitting strategy for localized charge carriers. For the charge-neutral point defects compensated by electrons and holes, our predictions are in good agreement with periodic calculations. For highly charged defects, the long-range polarization effects should greatly contribute to the calculated formation energies as evaluated using the Jost correction in eq 9, which is −0.46 eV, −1.84 eV, −4.14 eV, and −7.37 eV for the ±1, ±2, ±3, and ±4 charge states, respectively. These effects were typically not explicitly considered in previous supercell models. Recently, there has been increasing awareness of the application of long-range electrostatic corrections toward more quantitative predictions of defect formation energies using supercell approaches.50 Apart from the long-range effect, it should also be noted that there are significant differences between the PBE+U and HSE06 predictions on VO•• and VO using the standard PAW method based on the same setting.30 Therefore, for these defects in higher charge states such as VCe⁗ and Cei, it is not surprising that PBE+U calculations might have several electron volts difference compared with hybrid functional predictions, although there is no corresponding reference data available.
Table 6. Calculated Formation Energies (eV) of Intrinsic Point Defects in CeO2 in O-Rich Conditionsa.
| Defect reactions | M-Lb | QM/MMB97-2b | QM/MM PBE0b | QM/MM BB1Kb | Periodic HSE06c | Periodic PBE+Uc | Periodic PBE+Ud | Periodic PBE+Ue | |
|---|---|---|---|---|---|---|---|---|---|
| (a) Oxygen vacancy | |||||||||
| 4.57 | 4.59 | 4.61 | 3.45 | 5.0 | 4.3 | 4.7 | 4.43 | ||
| 4.05 | 3.52 | 3.84 | 2.89 | 4.5 | 3.4 | 4.13 | 3.66 | ||
| 3.56 | 3.11 | 3.70 | 2.92 | 4.09 | 2.7 | 3.16 | 2.8 | ||
| (b) Oxygen interstitial | |||||||||
| 7.72 | 7.86 | 7.87 | 8.08 | 7.40 | 4.41 | ||||
| 4.04 | 5.28 | 5.14 | 4.81 | 4.49 | 3.60 | ||||
| 2.54 | 3.39 | 3.17 | 2.51 | 1.82 | 2.79 | ||||
| (c) Cerium vacancy | |||||||||
| 16.60 | 14.87 | 16.01 | 17.44 | 9.0 | 7.39 | ||||
| 14.05 | 12.46 | 13.43 | 14.35 | 8.0 | 6.32 | ||||
| 11.58 | 11.00 | 11.81 | 12.24 | 7.1 | 6.52 | ||||
| 9.20 | 8.71 | 9.31 | 9.20 | 5.8 | 6.81 | ||||
| 6.91 | 7.35 | 7.77 | 7.14 | 5.0 | 7.39 | ||||
| (d) Cerium interstitial | |||||||||
| 16.63 | 17.60 | 16.94 | 15.48 | 13.45 | 14.46 | ||||
| 14.68 | 15.86 | 15.41 | 14.02 | 12.95 | 13.55 | ||||
| 13.8 | 14.62 | 14.43 | 13.17 | 12.75 | 12.57 | ||||
| 13.05 | 13.44 | 13.71 | 12.61 | 12.05 | 11.69 | ||||
| 12.44 | 12.64 | 13.00 | 12.19 | 11.69 | 10.9 | ||||
Reaction energies are reported assuming holes formed at the VBM and electrons at the CBM (using the calculated VBM at each level of theory and experimental 4 eV band gap for obtaining CBM).
Present work.
Plane-wave PBE+U (UCe 4f = 5.0 eV) and HSE06 (25% HF) calculations using the 96-atom supercell.30
Plane-wave PBE+U (UCe 4f = 4.0 eV and UO 2p = 4.0 eV) calculations using the 96-atom supercell with a nonlinear core-corrected (NLCC) norm-conserving pseudopotential for Ce.28
Plane-wave PBE+U (UCe 4f = 5.0 eV) calculations using the 96-atom supercell with PAW.27
Figure 6.
Calculated formation energies of intrinsic defects in CeO2 under different growth conditions using the M-L approach. Only the most stable charge states for each type of defect are shown. Solid circles indicate the thermodynamic transition levels. The lighter regions were determined by the minimum and maximum values obtained from B97-2, PBE0, and BB1K QM/MM calculations. The vertical solid lines show the position of the CBM according to the experimental band gap of 4 eV. The vertical dashed lines indicate the calculated self-consistent Fermi energy in each growth condition at 100 K, i.e., the low-temperature regime. (a) In O-rich/Ce-poor conditions. (b) In O-poor/Ce-rich conditions.
Our M-L predictions of the formation energies of charge-neutral defect pairs and trios are also in good agreement with QM/MM predictions. When an anion–Frenkel pair is formed, a lattice oxygen ion occupies an octahedral interstitial site, leaving an oxygen vacancy. The formation energy is predicted to be 2.15 eV per defect (4.30 eV for the total defect reaction) by our potential model at infinite separation (2.23 eV, 2.24 eV, and 1.77 eV at the QM/MM B97-2, PBE0, and BB1K levels of theory, respectively, 2.07–2.08 eV by periodic PBE+U calculations,27,28 and 1.83 eV by experiment144). In our M-L calculations, we observed the recombination behavior of bound anion–Frenkel pairs in the nearest (2.34 Å) and next-nearest (4.47 Å) separations. The first stable anion–Frenkel pair has a separation distance of 5.88 Å with a total formation energy of 3.85 eV (viewed as a defect cluster), yielding a binding energy of 0.45 eV. The second stable pair has a slightly higher formation energy of 3.98 eV due to the larger separation (7.01 Å). Our M-L calculations predict a similar critical stabilization distance of the bound anion–Frenkel pair as reported by Huang et al.28 using periodic PBE+U calculations (5.95 Å). The M-L calculated formation energy of the unbound Schottky trio [VCe⁗] + 2[VO]) is of 3.25 eV per defect, compared with 2.69 eV by B97-2, 3.08 eV by PBE0, and 2.78 eV by BB1K functionals in QM/MM. Previous periodic PBE+U studies reported 2.29 eV by Zacherle et al.27 According to our M-L calculations, Schottky trios can form stable bound complexes with formation energies of 5.18 eV, 5.22 eV, and 5.86 eV for two oxygen vacancies aligned along the ⟨111⟩, ⟨110⟩, and ⟨100⟩ directions, respectively, with respect to the cation vacancy. The corresponding binding energies are quite high due to the high charges and close proximity of the cation and anion vacancies, whose distances are 4.58 Å, 4.53 Å, and 3.89 Å, respectively. Our predicted formation energy for the bound Schottky trio is slightly higher than other PBE+U calculations of 3.66 eV by Keating et al.25 and 3.86 eV by Huang et al.28 The calculated formation energy for the interstitial disorder ([Cei••••] + 2[Oi]) is 5.36 eV by the M-L approach, also in good agreement with the predictions of 5.77 eV, 5.56 eV, and 5.21 eV at the QM/MM B97-2, PBE0, and BB1K levels of theory. We also predicted a higher formation energy for the cation–Frenkel pair ([VCe⁗] + [Cei]) than other periodic PBE+U calculations (8.62 eV compared with 5.8–6.3 eV26−28). However, the M-L prediction agrees well with our QM/MM results (8.24 eV, 8.48 eV, and 8.46 eV at the B97-2, PBE0, and BB1K levels of theory, respectively).
The anion–Frenkel pair ([VO••] + [Oi]) is predicted to be the dominant defect pair in bulk ceria, consistent with a recent neutron scattering study.16 This behavior is similar to other cubic fluorite crystals such as UO2 and cubic ZrO2.72,145 Oxygen vacancy formation is of crucial importance in bulk ceria, which can repeatedly absorb and release oxygen in catalytic reactions through the Mars–van Krevelen mechanism.9,10 Furthermore, the VO•• migration barrier is very low (0.53 eV predicted by our potential, 0.5–0.6 eV by previous DFT and experimental studies30,85,146−148), which could be further reduced by the surrounding CeCe polarons on the surfaces,12,140 ensuring good ionic conductivity in SOFC applications. All these features make this material versatile in energy and catalytic applications.
The energetic difference in predictions between the embedded-cluster approaches and periodic PBE+U results could mainly originate from the differences in DFT functional and long-range polarization effects. As illustrated before, the periodic PBE+U approach may not be accurate enough for the formation energy of highly charged defects, while our QM/MM calculations that employ hybrid functionals and consider long-range polarization should be more reliable and target the defect formation at the dilute limit. Overall, IP10b accurately describes defect structures and formation mechanisms in ceria, which could be useful for investigating more complex defect clusters and partially reduced phases that are computationally too expensive for first-principles calculations. Moreover, our potential model for modeling holes and electron polarons is highly transferable. Using the same correction approach, one can reproduce the predictions of other hybrid functionals.
3.2.2. Thermodynamic Defect Processes in Bulk CeO2
The formation energy of a point defect depends on the position of the Fermi level (electronic chemical potential) and chemical potentials of exchanged species according to eq 8, which will be further affected by conditions, especially oxygen partial pressure P and temperature.107 As summarized by Reuter and Scheffler,149 the relationship between the oxygen chemical potential μO(T, P) and oxygen partial pressure P can be obtained by
| 29 |
| 30 |
where P0 = 1 atm
is typically defined as the zero state, 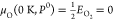 eV, H is the enthalpy,
and S is entropy. Using the experimental enthalpy
and entropy data,150 we calculated the
value of μO at various reaction conditions. As shown
in Figure 7a, μO decreases with increase in temperature or decrease in P. In the case of CeO2, an extreme reducing condition
(e.g., P = 2.3 × 10–10 atm
at 1800 K) is required to reach the O-poor limit of μO min = −3.94 eV at which Ce2O3 becomes more
stable than CeO2. In realistic systems, the formation energies
of defects always lie between the two limits shown in Figure 6.
eV, H is the enthalpy,
and S is entropy. Using the experimental enthalpy
and entropy data,150 we calculated the
value of μO at various reaction conditions. As shown
in Figure 7a, μO decreases with increase in temperature or decrease in P. In the case of CeO2, an extreme reducing condition
(e.g., P = 2.3 × 10–10 atm
at 1800 K) is required to reach the O-poor limit of μO min = −3.94 eV at which Ce2O3 becomes more
stable than CeO2. In realistic systems, the formation energies
of defects always lie between the two limits shown in Figure 6.
Figure 7.

Variations of the (a) oxygen chemical potential and (b) calculated self-consistent Fermi level relative to VBM with temperature and oxygen partial pressure.
The Fermi level of CeO2 is in turn also affected by temperature and oxygen partial pressure.107 Variation of the Fermi level influences further the dominant defect type, energetics of charge states, and concentrations of defects in solids. The self-consistent Fermi energy (shown in Figure 7b) was determined using the SC-FERMI code151 based on the M-L defect formation energies and density of states of the pristine CeO2 (obtained using VASP with PBE0 functional, where the unoccupied states were shifted downward to reproduce the experimental band gap of 4 eV). In the O-rich limit, the Fermi level lies ca. 2.5 eV above the VBM and favors the formation of doubly ionized VO••. These vacancies could be compensated by acceptor defects such as Oi and Oi′ or electrons that are not trapped at the vacancy sites. With the increase in temperature or decrease in oxygen partial pressure, the Fermi level rises toward the CBM, which could further support the stabilization of VO and VO× with trapped electron polarons. An electrical conductivity study by Tuller and Nowick44 on single-crystal CeO2–x samples also concluded that VO is dominant at small x in CeO2–x (x < 10–3), with a transition to singly ionised VO• at greater x under reduced P, confirming our predictions. The thermodynamic transition level ε(q1/q2) is defined as the energy of an adiabatic ionization process changing the charge state q1 of the defect to another charge state q2. It can be obtained as the electron Fermi level where the formation energies of two charge states of a point defect are equal or using the following formula:39
| 31 |
where Ef(Xq, EF = 0) is the formation energy of the defect X with the charge state q when the Fermi level is at the valence band maximum (VBM, EF = 0). The thermodynamic transition level indicates the relative stability of the two charge states. When EF < ε(q1/q2), the charge state q1 is stable and vice versa. The calculated thermodynamic transition levels of oxygen vacancy are shown as dashed lines in Figure 7b. In our M-L calculations, ε(+2/+1) = 3.48 eV and ε(+1/0) = 3.51 eV above the VBM. Sun et al.30 have compared DFT PBE+U and HSE06 predictions using the supercell approach with an appropriate correction scheme on charged defects. They also obtained high transition levels for oxygen vacancies using the HSE06 functional (ε(+2/+1) = 3.1 eV and ε(+1/0) = 3.2 eV), but their PBE+U calculations yielded much lower values of ε(+2/+1) = 1.45 eV and ε(+1/0) = 1.65 eV. This difference could originate from the accuracy of the predicted band gap from the DFT functionals.
Our previous discussion focused on the equilibrium-state defect formation in bulk CeO2 at the dilute limit, without considering the extrinsic doping and surface effects. Even trace dopants, or impurities can considerably affect the position of the Fermi level and defect formation.26,70,152 Further, we should note that the formation energies of VO× on or near the surfaces are much lower than their counterparts in bulk, which could be rationalized by a decrease in the coordination of atoms exposed at the topmost surface layer. Previous PBE+U calculations reported an energetically favorable formation of VO in the subsurface layer of CeO2(111) with a formation energy of ca. 1.8 eV compared with 2.85 eV in bulk.24,153 The formation energies of VO× on less stable CeO2(110) and CeO2(100) surfaces are predicted to be even lower by Ganduglia-Pirovano et al.24 and are 1.06 and 1.35 eV, respectively. Such low formation energies of VO should result in nonnegligible shifts of transition levels toward the VBM and would favor the trapping of excess electrons near the surfaces, as experimentally captured by STM images.40,43 This observation is, in fact, quite general. For example, recently, Wang and Yin154 also found that iodine vacancies in CH3NH3PbI3 formed in the bulk and on the surface have significant differences in the transition level and effects on photovoltaic performance. Returning to CeO2, reduction in practice always starts from the near-surface layers, resulting in the localization and segregation of excess electrons, which could lead to further reconstruction into alternative ordered phases such as CeO1.68, Ce7O12, Ce11O20, and Ce3O5.16,110,155,156 Trace dopants or unintended impurities including divalent157 and trivalent158 metal ions and surface absorbates such as fluorine159 and hydroxyls160 could also become the charge-compensating species for VO•• and have a large impact on the Fermi level and defect concentrations. Our calculations show that, with the increase in temperature or decrease in oxygen partial pressure, the Fermi level rises in bulk CeO2, and singly ionized VO and charge-neutral VO× become dominant before the formation of ordered reduced phases. A complete understanding of the partial reduction behavior of ceria requires further investigation using, e.g., free energy calculations, Monte Carlo, or molecular dynamics techniques161−164 to consider explicitly entropic effects of defect formation and defect–defect interactions, which will be investigated in future research based on our new potential.
4. Conclusions
We have proposed a new strategy for deriving shell-model interatomic potentials based on hybrid QM/MM embedded-cluster calculations of ionic polarizabilities, defect structures, and formation energies. A new potential has been developed for CeO2, with a distinctive performance in predicting the structure, elastic, dielectric, defect, surface, phonon, and thermodynamic properties compared with experimental measurements and ab initio calculations. In particular, the calculated structures and formation energies of polarons, various charged states of native defects, and defect pairs in CeO2 achieved a high level of consistency between the M-L calculations and QM/MM results. Benefiting from our new potential, one could provide more insights into the complex defect chemistry of CeO2. These developments will enable future work on defect formation at elevated temperatures using free energy calculations; partial reduction and nonstoichiometry based on molecular dynamics and Monte Carlo simulations; and the role of surface defects in catalytic reactions using QM/MM techniques. This novel strategy for developing robust shell-model potentials capable of predicting accurate defect properties could be further extended to other ionic systems.
Acknowledgments
The authors acknowledge the use of the THOMAS, YOUNG, and ARCHER2 U.K. National Supercomputing Service (http://www.archer2.ac.uk) via membership of U.K.’s HEC Materials Chemistry Consortium, which is funded by EPSRC (Grant Nos. EP/P020194, EP/T022213, and EP/R029431). We also acknowledge funding provided by EPSRC under Grant Nos. EP/W014580, EP/R001847, EP/K038419, and EP/I030662. Q.H. acknowledges funding from the Shanghai Pujiang Program (Grant 22PJ1411300). X.Z. acknowledges the support from the China Scholarship Council (CSC) and Han Yu for useful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemmater.2c03019.
Additional information on the methodological details of plane-wave DFT calculations, interatomic-potential-based calculations, QM/MM interface treatment, Mott–Littleton and QM/MM calculations on several properties of ceria; supplementary data on variants of Buckingham potentials with different ρ values and their performances, shell-model potential parameters in the literature, comparison of atomic displacements due to defect formation, and the new potential IP10b in GULP-readable format (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Montini T.; Melchionna M.; Monai M.; Fornasiero P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116 (10), 5987–6041. 10.1021/acs.chemrev.5b00603. [DOI] [PubMed] [Google Scholar]
- Schmitt R.; Nenning A.; Kraynis O.; Korobko R.; Frenkel A. I.; Lubomirsky I.; Haile S. M.; Rupp J. L. A review of defect structure and chemistry in ceria and its solid solutions. Chem. Soc. Rev. 2020, 49 (2), 554–592. 10.1039/C9CS00588A. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Mofarah S. S.; Mehmood R.; Cazorla C.; Koshy P.; Sorrell C. C. Design strategies for ceria nanomaterials: untangling key mechanistic concepts. Mater. Horiz. 2021, 8 (1), 102–123. 10.1039/D0MH00654H. [DOI] [PubMed] [Google Scholar]
- Chueh W. C.; Falter C.; Abbott M.; Scipio D.; Furler P.; Haile S. M.; Steinfeld A. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 2010, 330 (6012), 1797–1801. 10.1126/science.1197834. [DOI] [PubMed] [Google Scholar]
- Chang K.; Zhang H.; Cheng M.-j.; Lu Q. Application of Ceria in CO2 Conversion Catalysis. ACS Catal. 2020, 10 (1), 613–631. 10.1021/acscatal.9b03935. [DOI] [Google Scholar]
- Liu Z.; Huang E.; Orozco I.; Liao W.; Palomino R. M.; Rui N.; Duchoň T.; Nemšák S.; Grinter D. C.; Mahapatra M.; et al. Water-promoted interfacial pathways in methane oxidation to methanol on a CeO2-Cu2O catalyst. Science 2020, 368 (6490), 513–517. 10.1126/science.aba5005. [DOI] [PubMed] [Google Scholar]
- Paier J.; Penschke C.; Sauer J. Oxygen defects and surface chemistry of ceria: quantum chemical studies compared to experiment. Chem. Rev. 2013, 113 (6), 3949–3985. 10.1021/cr3004949. [DOI] [PubMed] [Google Scholar]
- Maurer F.; Jelic J.; Wang J.; Gänzler A.; Dolcet P.; Wöll C.; Wang Y.; Studt F.; Casapu M.; Grunwaldt J.-D. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nature Catal. 2020, 3 (10), 824–833. 10.1038/s41929-020-00508-7. [DOI] [Google Scholar]
- Muravev V.; Spezzati G.; Su Y.-Q.; Parastaev A.; Chiang F.-K.; Longo A.; Escudero C.; Kosinov N.; Hensen E. J. Interface dynamics of Pd-CeO2 single-atom catalysts during CO oxidation. Nature Catal. 2021, 4 (6), 469–478. 10.1038/s41929-021-00621-1. [DOI] [Google Scholar]
- Xing F.; Nakaya Y.; Yasumura S.; Shimizu K.-i.; Furukawa S. Ternary platinum-cobalt-indium nanoalloy on ceria as a highly efficient catalyst for the oxidative dehydrogenation of propane using CO2. Nature Catal. 2022, 5 (1), 55–65. 10.1038/s41929-021-00730-x. [DOI] [Google Scholar]
- Andersson D. A.; Simak S. I.; Skorodumova N. V.; Abrikosov I. A.; Johansson B. Optimization of ionic conductivity in doped ceria. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (10), 3518–3521. 10.1073/pnas.0509537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Han Z.-K.; Murgida G. E.; Ganduglia-Pirovano M. V.; Gao Y. Oxygen-vacancy dynamics and entanglement with polaron hopping at the reduced CeO2(111) surface. Phys. Rev. Lett. 2019, 122 (9), 096101. 10.1103/PhysRevLett.122.096101. [DOI] [PubMed] [Google Scholar]
- Cheisson T.; Schelter E. J. Rare earth elements: Mendeleev’s bane, modern marvels. Science 2019, 363 (6426), 489–493. 10.1126/science.aau7628. [DOI] [PubMed] [Google Scholar]
- Skorodumova N.; Simak S.; Lundqvist B. I.; Abrikosov I.; Johansson B. Quantum origin of the oxygen storage capability of ceria. Phys. Rev. Lett. 2002, 89 (16), 166601. 10.1103/PhysRevLett.89.166601. [DOI] [PubMed] [Google Scholar]
- Franchini C.; Reticcioli M.; Setvin M.; Diebold U. Polarons in materials. Nat. Rev. Mater. 2021, 6 (7), 560–586. 10.1038/s41578-021-00289-w. [DOI] [Google Scholar]
- Luo S.; Li M.; Fung V.; Sumpter B. G.; Liu J.; Wu Z.; Page K. New Insights into the Bulk and Surface Defect Structures of Ceria Nanocrystals from Neutron Scattering Study. Chem. Mater. 2021, 33 (11), 3959–3970. 10.1021/acs.chemmater.1c00156. [DOI] [Google Scholar]
- Kröger F.; Vink H.. Relations between the concentrations of imperfections in crystalline solids. In Solid state physics; Elsevier: 1956; Vol. 3, pp 307–435. [Google Scholar]
- Zinkevich M.; Djurovic D.; Aldinger F. Thermodynamic modelling of the cerium-oxygen system. Solid State Ionics 2006, 177 (11–12), 989–1001. 10.1016/j.ssi.2006.02.044. [DOI] [Google Scholar]
- Trovarelli A.; Fornasiero P.. Catalysis by ceria and related materials; World Scientific: 2013; Vol. 12, 10.1142/p870. [DOI] [Google Scholar]
- Nolan M.; Parker S. C.; Watson G. W. The electronic structure of oxygen vacancy defects at the low index surfaces of ceria. Surf. Sci. 2005, 595 (1–3), 223–232. 10.1016/j.susc.2005.08.015. [DOI] [Google Scholar]
- Migani A.; Vayssilov G. N.; Bromley S. T.; Illas F.; Neyman K. M. Dramatic reduction of the oxygen vacancy formation energy in ceria particles: a possible key to their remarkable reactivity at the nanoscale. J. Mater. Chem. 2010, 20 (46), 10535–10546. 10.1039/c0jm01908a. [DOI] [Google Scholar]
- Murgida G. E.; Ganduglia-Pirovano M. V. Evidence for subsurface ordering of oxygen vacancies on the reduced CeO2(111) surface using density-functional and statistical calculations. Phys. Rev. Lett. 2013, 110 (24), 246101. 10.1103/PhysRevLett.110.246101. [DOI] [PubMed] [Google Scholar]
- Trovarelli A.; Llorca J. Ceria catalysts at nanoscale: how do crystal shapes shape catalysis?. ACS Catal. 2017, 7 (7), 4716–4735. 10.1021/acscatal.7b01246. [DOI] [Google Scholar]
- Pérez-Bailac P.; Lustemberg P. G.; Ganduglia-Pirovano M. V. Facet-dependent stability of near-surface oxygen vacancies and excess charge localization at CeO2 surfaces. J. Phys.: Condens. Matter 2021, 33 (50), 504003. 10.1088/1361-648X/ac238b. [DOI] [PubMed] [Google Scholar]
- Keating P. R.; Scanlon D. O.; Morgan B. J.; Galea N. M.; Watson G. W. Analysis of intrinsic defects in CeO2 using a Koopmans-like GGA+ U approach. J. Phys. Chem. C 2012, 116 (3), 2443–2452. 10.1021/jp2080034. [DOI] [Google Scholar]
- Keating P. R.; Scanlon D. O.; Watson G. W. Computational testing of trivalent dopants in CeO2 for improved high-κ dielectric behaviour. J. Mater. Chem. C 2013, 1 (6), 1093–1098. 10.1039/C2TC00385F. [DOI] [Google Scholar]
- Zacherle T.; Schriever A.; De Souza R.; Martin M. Ab initio analysis of the defect structure of ceria. Phys. Rev. B 2013, 87 (13), 134104. 10.1103/PhysRevB.87.134104. [DOI] [Google Scholar]
- Huang B.; Gillen R.; Robertson J. Study of CeO2 and its native defects by density functional theory with repulsive potential. J. Phys. Chem. C 2014, 118 (42), 24248–24256. 10.1021/jp506625h. [DOI] [Google Scholar]
- Murgida G. E.; Ferrari V.; Ganduglia-Pirovano M. V.; Llois A. M. Ordering of oxygen vacancies and excess charge localization in bulk ceria: a DFT+ U study. Phys. Rev. B 2014, 90 (11), 115120. 10.1103/PhysRevB.90.115120. [DOI] [Google Scholar]
- Sun L.; Huang X.; Wang L.; Janotti A. Disentangling the role of small polarons and oxygen vacancies in CeO2. Phys. Rev. B 2017, 95 (24), 245101. 10.1103/PhysRevB.95.245101. [DOI] [Google Scholar]
- Du D.; Wolf M. J.; Hermansson K.; Broqvist P. Screened hybrid functionals applied to ceria: Effect of Fock exchange. Phys. Rev. B 2018, 97 (23), 235203. 10.1103/PhysRevB.97.235203. [DOI] [Google Scholar]
- Plata J. J.; Márquez A. M.; Sanz J. F. Communication: Improving the density functional theory+U description of CeO2 by including the contribution of the O 2p electrons. J. Chem. Phys. 2012, 136 (4), 041101. 10.1063/1.3678309. [DOI] [PubMed] [Google Scholar]
- Schäfer T.; Daelman N.; López N. Cerium oxides without U: The role of many-electron correlation. J. Phys. Chem. Lett. 2021, 12 (27), 6277–6283. 10.1021/acs.jpclett.1c01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda M. M.; Castellani N. J.; Grau-Crespo R.; de Leeuw N. H.; Hernandez N. C.; Sanz J. F.; Neyman K. M.; Illas F. On the difficulties of present theoretical models to predict the oxidation state of atomic Au adsorbed on regular sites of CeO2(111). J. Chem. Phys. 2009, 131 (9), 094702. 10.1063/1.3216102. [DOI] [PubMed] [Google Scholar]
- Da Silva J. L.; Ganduglia-Pirovano M. V.; Sauer J.; Bayer V.; Kresse G. Hybrid functionals applied to rare-earth oxides: The example of ceria. Phys. Rev. B 2007, 75 (4), 045121. 10.1103/PhysRevB.75.045121. [DOI] [Google Scholar]
- Graciani J.; Márquez A. M.; Plata J. J.; Ortega Y.; Hernández N. C.; Meyer A.; Zicovich-Wilson C. M.; Sanz J. F. Comparative study on the performance of hybrid DFT functionals in highly correlated oxides: the case of CeO2 and Ce2O3. J. Chem. Theory Comput. 2011, 7 (1), 56–65. 10.1021/ct100430q. [DOI] [PubMed] [Google Scholar]
- Brugnoli L.; Ferrari A. M.; Civalleri B.; Pedone A.; Menziani M. C. Assessment of density functional approximations for highly correlated oxides: the case of CeO2 and Ce2O3. J. Chem. Theory Comput. 2018, 14 (9), 4914–4927. 10.1021/acs.jctc.8b00600. [DOI] [PubMed] [Google Scholar]
- Brugnoli L.; Menziani M. C.; Urata S.; Pedone A. Development and application of a ReaxFF reactive force field for cerium oxide/water interfaces. J. Phys. Chem. A 2021, 125 (25), 5693–5708. 10.1021/acs.jpca.1c04078. [DOI] [PubMed] [Google Scholar]
- Freysoldt C.; Grabowski B.; Hickel T.; Neugebauer J.; Kresse G.; Janotti A.; Van de Walle C. G. First-principles calculations for point defects in solids. Rev. Mod. Phys. 2014, 86 (1), 253. 10.1103/RevModPhys.86.253. [DOI] [Google Scholar]
- Esch F.; Fabris S.; Zhou L.; Montini T.; Africh C.; Fornasiero P.; Comelli G.; Rosei R. Electron localization determines defect formation on ceria substrates. Science 2005, 309 (5735), 752–755. 10.1126/science.1111568. [DOI] [PubMed] [Google Scholar]
- Wang B.; Xi X.; Cormack A. N. Chemical strain and point defect configurations in reduced ceria. Chem. Mater. 2014, 26 (12), 3687–3692. 10.1021/cm500946s. [DOI] [Google Scholar]
- Minervini L.; Zacate M. O.; Grimes R. W. Defect cluster formation in M2O3-doped CeO2. Solid State Ionics 1999, 116 (3–4), 339–349. 10.1016/S0167-2738(98)00359-2. [DOI] [Google Scholar]
- Jerratsch J.-F.; Shao X.; Nilius N.; Freund H.-J.; Popa C.; Ganduglia-Pirovano M. V.; Burow A. M.; Sauer J. Electron localization in defective ceria films: A study with scanning-tunneling microscopy and density-functional theory. Phys. Rev. Lett. 2011, 106 (24), 246801. 10.1103/PhysRevLett.106.246801. [DOI] [PubMed] [Google Scholar]
- Tuller H.; Nowick A. Defect structure and electrical properties of nonstoichiometric CeO2 single crystals. J. Electrochem. Soc. 1979, 126 (2), 209. 10.1149/1.2129007. [DOI] [Google Scholar]
- Thajudheen T.; Dixon A. G.; Gardonio S.; Arčon I.; Valant M. Oxygen vacancy-related cathodoluminescence quenching and polarons in CeO2. J. Phys. Chem. C 2020, 124 (37), 19929–19936. 10.1021/acs.jpcc.0c04631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J.; Borrás-Morell J.; García-Baños B.; Balaguer M.; Plaza-González P.; Santos-Blasco J.; Catalán-Martínez D.; Navarrete L.; Catalá-Civera J. M. Hydrogen production via microwave-induced water splitting at low temperature. Nat. Energy 2020, 5 (11), 910–919. 10.1038/s41560-020-00720-6. [DOI] [Google Scholar]
- Zhang Q.; Bu J.; Wang J.; Sun C.; Zhao D.; Sheng G.; Xie X.; Sun M.; Yu L. Highly efficient hydrogenation of nitrobenzene to aniline over Pt/CeO2 catalysts: The shape effect of the support and key role of additional Ce3+ sites. ACS Catal. 2020, 10 (18), 10350–10363. 10.1021/acscatal.0c02730. [DOI] [Google Scholar]
- Kantorovich L. N. Elimination of the long-range dipole interaction in calculations with periodic boundary conditions. Phys. Rev. B 1999, 60 (23), 15476–15479. 10.1103/PhysRevB.60.15476. [DOI] [Google Scholar]
- Lany S.; Zunger A. Assessment of correction methods for the band-gap problem and for finite-size effects in supercell defect calculations: Case studies for ZnO and GaAs. Phys. Rev. B 2008, 78 (23), 235104. 10.1103/PhysRevB.78.235104. [DOI] [Google Scholar]
- Walsh A. Correcting the corrections for charged defects in crystals. npj Comput. Mater. 2021, 7 (1), 72. 10.1038/s41524-021-00546-0. [DOI] [Google Scholar]
- Sokol A. A.; French S. A.; Bromley S. T.; Catlow C. R. A.; van Dam H. J.; Sherwood P. Point defects in ZnO. Faraday Discuss. 2007, 134, 267–282. 10.1039/B607406E. [DOI] [PubMed] [Google Scholar]
- Walsh A.; Buckeridge J.; Catlow C. R. A.; Jackson A. J.; Keal T. W.; Miskufova M.; Sherwood P.; Shevlin S. A.; Watkins M. B.; Woodley S. M.; Sokol A. A. Limits to Doping of Wide Band Gap Semiconductors. Chem. Mater. 2013, 25 (15), 2924–2926. 10.1021/cm402237s. [DOI] [Google Scholar]
- Richter N. A.; Sicolo S.; Levchenko S. V.; Sauer J.; Scheffler M. Concentration of vacancies at metal-oxide surfaces: Case study of MgO(100). Phys. Rev. Lett. 2013, 111 (4), 045502. 10.1103/PhysRevLett.111.045502. [DOI] [PubMed] [Google Scholar]
- Buckeridge J.; Catlow C. R. A.; Scanlon D. O.; Keal T. W.; Sherwood P.; Miskufova M.; Walsh A.; Woodley S. M.; Sokol A. A. Determination of the Nitrogen Vacancy as a Shallow Compensating Center in GaN Doped with Divalent Metals. Phys. Rev. Lett. 2015, 114 (1), 016405. 10.1103/PhysRevLett.114.016405. [DOI] [PubMed] [Google Scholar]
- Catlow C. R. A.; Buckeridge J.; Farrow M. R.; Logsdail A. J.; Sokol A. A. Quantum Mechanical/Molecular Mechanical (QM/MM) Approaches. Handbook of Solid State Chemistry 2017, 647–680. 10.1002/9783527691036.hsscvol5012. [DOI] [Google Scholar]
- Buckeridge J.; Catlow C. R. A.; Farrow M.; Logsdail A. J.; Scanlon D.; Keal T.; Sherwood P.; Woodley S.; Sokol A.; Walsh A. Deep vs shallow nature of oxygen vacancies and consequent n-type carrier concentrations in transparent conducting oxides. Phys. Rev. Mater. 2018, 2 (5), 054604. 10.1103/PhysRevMaterials.2.054604. [DOI] [Google Scholar]
- Mott N.; Littleton M. Conduction in polar crystals. I. Electrolytic conduction in solid salts. Trans. Faraday Soc. 1938, 34, 485–499. 10.1039/tf9383400485. [DOI] [Google Scholar]
- Catlow C. R. A. Mott-Littleton calculations in solid-state chemistry and physics. J. Chem. Soc., Faraday Trans. 2 1989, 85 (5), 335–340. 10.1039/F29898500335. [DOI] [Google Scholar]
- Lidiard A. B. The Mott-Littleton method: an introductory survey. J. Chem. Soc., Faraday Trans. 2 1989, 85 (5), 341–349. 10.1039/F29898500341. [DOI] [Google Scholar]
- Sherwood P.; de Vries A. H.; Guest M. F.; Schreckenbach G.; Catlow C. R. A.; French S. A.; Sokol A. A.; Bromley S. T.; Thiel W.; Turner A. J.; et al. QUASI: A general purpose implementation of the QM/MM approach and its application to problems in catalysis. J. Mol. Struct.: THEOCHEM 2003, 632 (1–3), 1–28. 10.1016/S0166-1280(03)00285-9. [DOI] [Google Scholar]
- Sokol A. A.; Bromley S. T.; French S. A.; Catlow C. R. A.; Sherwood P. Hybrid QM/MM embedding approach for the treatment of localized surface states in ionic materials. Int. J. Quantum Chem. 2004, 99 (5), 695–712. 10.1002/qua.20032. [DOI] [Google Scholar]
- Dick B. G.; Overhauser A. W. Theory of the Dielectric Constants of Alkali Halide Crystals. Phys. Rev. 1958, 112 (1), 90–103. 10.1103/PhysRev.112.90. [DOI] [Google Scholar]
- Xie Z.; Sui Y.; Buckeridge J.; Catlow C. R. A.; Keal T. W.; Sherwood P.; Walsh A.; Farrow M. R.; Scanlon D. O.; Woodley S. M.; et al. Donor and acceptor characteristics of native point defects in GaN. J. Phys. D: Appl. Phys. 2019, 52 (33), 335104. 10.1088/1361-6463/ab2033. [DOI] [Google Scholar]
- Sauer J. Ab Initio Calculations for Molecule-Surface Interactions with Chemical Accuracy. Acc. Chem. Res. 2019, 52 (12), 3502–3510. 10.1021/acs.accounts.9b00506. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Zhang X.; Martirez J. M. P.; Carter E. A. Benchmarking an embedded adaptive sampling configuration interaction method for surface reactions: H2 desorption from and CH4 dissociation on Cu(111). J. Chem. Theory Comput. 2020, 16 (11), 7078–7088. 10.1021/acs.jctc.0c00341. [DOI] [PubMed] [Google Scholar]
- Shi B. X.; Kapil V.; Zen A.; Chen J.; Alavi A.; Michaelides A. General embedded cluster protocol for accurate modeling of oxygen vacancies in metal-oxides. J. Chem. Phys. 2022, 156 (12), 124704. 10.1063/5.0087031. [DOI] [PubMed] [Google Scholar]
- Grimes R. W.; Catlow C. R. A. Modeling Localized Defects in Ionic Materials Using Mott-Littleton and Embedded Quantum Cluster Methodology. J. Am. Ceram. Soc. 1990, 73 (11), 3251–3256. 10.1111/j.1151-2916.1990.tb06446.x. [DOI] [Google Scholar]
- Grimes R. W.; Catlow C. R. A.; Stoneham A. M. Quantum-mechanical cluster calculations and the Mott-Littleton methodology. J. Chem. Soc., Faraday Trans. 2 1989, 85 (5), 485–495. 10.1039/F29898500485. [DOI] [Google Scholar]
- Catlow C. R. A.; French S. A.; Sokol A. A.; Al-Sunaidi A. A.; Woodley S. M. Zinc oxide: A case study in contemporary computational solid state chemistry. J. Comput. Chem. 2008, 29 (13), 2234–2249. 10.1002/jcc.21051. [DOI] [PubMed] [Google Scholar]
- Butler V.; Catlow C.; Fender B.; Harding J. Dopant ion radius and ionic conductivity in cerium dioxide. Solid State Ionics 1983, 8 (2), 109–113. 10.1016/0167-2738(83)90070-X. [DOI] [Google Scholar]
- Sayle T. X. T.; Parker S. C.; Catlow C. R. A. The role of oxygen vacancies on ceria surfaces in the oxidation of carbon monoxide. Surf. Sci. 1994, 316 (3), 329–336. 10.1016/0039-6028(94)91225-4. [DOI] [Google Scholar]
- Grimes R.; Catlow C. R. A. The stability of fission products in uranium dioxide. Philos. Trans. R. Soc., A 1991, 335 (1639), 609–634. 10.1098/rsta.1991.0062. [DOI] [Google Scholar]
- Vyas S.; Grimes R. W.; Gay D. H.; Rohl A. L. Structure, stability and morphology of stoichiometric ceria crystallites. J. Chem. Soc., Faraday Trans. 1998, 94 (3), 427–434. 10.1039/a707052g. [DOI] [Google Scholar]
- Lewis G.; Catlow C. Potential models for ionic oxides. J. Phys. C: Solid State Phys. 1985, 18 (6), 1149. 10.1088/0022-3719/18/6/010. [DOI] [Google Scholar]
- Gotte A.; Spångberg D.; Hermansson K.; Baudin M. Molecular dynamics study of oxygen self-diffusion in reduced CeO2. Solid State Ionics 2007, 178 (25–26), 1421–1427. 10.1016/j.ssi.2007.08.003. [DOI] [Google Scholar]
- Walsh A.; Woodley S. M.; Catlow C. R. A.; Sokol A. A. Potential energy landscapes for anion Frenkel-pair formation in ceria and india. Solid State Ionics 2011, 184 (1), 52–56. 10.1016/j.ssi.2010.08.010. [DOI] [Google Scholar]
- Gerhardt-Anderson R.; Zamani-Noor F.; Nowick A.; Catlow C.; Cormack A. Study of Sc2O3-doped ceria by anelastic relaxation. Solid State Ionics 1983, 9, 931–936. 10.1016/0167-2738(83)90113-3. [DOI] [Google Scholar]
- Cormack A.; Catlow C.; Nowick A. Theoretical studies of off-centre Sc3+ impurities in CeO2. J. Phys. Chem. Solids 1989, 50 (2), 177–181. 10.1016/0022-3697(89)90415-0. [DOI] [Google Scholar]
- Sayle T. X.; Parker S. C.; Catlow C. R. A. Surface oxygen vacancy formation on CeO2 and its role in the oxidation of carbon monoxide. J. Chem. Soc., Chem. Commun. 1992, (14), 977–978. 10.1039/c39920000977. [DOI] [Google Scholar]
- Sayle T. X. T.; Caddeo F.; Zhang X.; Sakthivel T.; Das S.; Seal S.; Ptasinska S.; Sayle D. C. Structure-Activity Map of Ceria Nanoparticles, Nanocubes, and Mesoporous Architectures. Chem. Mater. 2016, 28 (20), 7287–7295. 10.1021/acs.chemmater.6b02536. [DOI] [Google Scholar]
- Stecura S.; Campbell W. J.. Thermal expansion and phase inversion of rare-earth oxides; U.S. Department of the Interior, Bureau of Mines: 1961; Vol. 5847. [Google Scholar]
- Nakajima A.; Yoshihara A.; Ishigame M. Defect-induced Raman spectra in doped CeO2. Phys. Rev. B 1994, 50 (18), 13297. 10.1103/PhysRevB.50.13297. [DOI] [PubMed] [Google Scholar]
- Mochizuki S. Infrared optical properties of cerium dioxide. Phys. Status Solidi B 1982, 114 (1), 189–199. 10.1002/pssb.2221140122. [DOI] [Google Scholar]
- Gerward L.; Olsen J. S.; Petit L.; Vaitheeswaran G.; Kanchana V.; Svane A. Bulk modulus of CeO2 and PrO2—An experimental and theoretical study. J. Alloys Compd. 2005, 400 (1–2), 56–61. 10.1016/j.jallcom.2005.04.008. [DOI] [Google Scholar]
- Steele B. C. H.; Floyd J. M. Oxygen Self-Diffusion and Electrical Transport Properties of Nonstoichiometric Ceria and Ceria Solid Solutions. Proc. Brit. Ceramic Soc. 1971, 55–76. [Google Scholar]
- Kresse G.; Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54 (16), 11169. 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- Born M.; Huang K.. Dynamical Theory of Crystal Lattices; Clarendon Press: 1954. [Google Scholar]
- Gale J. D.; Rohl A. L. The general utility lattice program (GULP). Mol. Simul. 2003, 29 (5), 291–341. 10.1080/0892702031000104887. [DOI] [Google Scholar]
- Gale J. D. GULP: A computer program for the symmetry-adapted simulation of solids. J. Chem. Soc., Faraday Trans. 1997, 93 (4), 629–637. 10.1039/a606455h. [DOI] [Google Scholar]
- Norgett M.A General formulation of the problem of calculating the energies of lattice defects in ionic crystals; Atomic Energy Research Establishment: 1974; Harwell Technical Report R.7650 HL74/335.
- Lu Y.; Farrow M. R.; Fayon P.; Logsdail A. J.; Sokol A. A.; Catlow C. R. A.; Sherwood P.; Keal T. W. Open-source, python-based redevelopment of the chemshell multiscale QM/MM environment. J. Chem. Theory Comput. 2019, 15 (2), 1317–1328. 10.1021/acs.jctc.8b01036. [DOI] [PubMed] [Google Scholar]
- Valiev M.; Bylaska E. J.; Govind N.; Kowalski K.; Straatsma T. P.; Van Dam H. J.; Wang D.; Nieplocha J.; Apra E.; Windus T. L.; et al. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 2010, 181 (9), 1477–1489. 10.1016/j.cpc.2010.04.018. [DOI] [Google Scholar]
- Weigend F.; Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7 (18), 3297–3305. 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- Desmarais J. K.; Erba A.; Dovesi R. Generalization of the periodic LCAO approach in the CRYSTAL code to g-type orbitals. Theor. Chem. Acc. 2018, 137 (2), 28. 10.1007/s00214-018-2200-9. [DOI] [Google Scholar]
- El-Kelany K. E.; Ravoux C.; Desmarais J.; Cortona P.; Pan Y.; Tse J.; Erba A. Spin localization, magnetic ordering, and electronic properties of strongly correlated Ln2O3 sesquioxides (Ln= La, Ce, Pr, Nd). Phys. Rev. B 2018, 97 (24), 245118. 10.1103/PhysRevB.97.245118. [DOI] [Google Scholar]
- Dolg M.; Stoll H.; Preuss H. Energy-adjusted ab initio pseudopotentials for the rare earth elements. J. Chem. Phys. 1989, 90 (3), 1730–1734. 10.1063/1.456066. [DOI] [Google Scholar]
- Wilson P. J.; Bradley T. J.; Tozer D. J. Hybrid exchange-correlation functional determined from thermochemical data and ab initio potentials. J. Chem. Phys. 2001, 115 (20), 9233–9242. 10.1063/1.1412605. [DOI] [Google Scholar]
- Adamo C.; Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110 (13), 6158–6170. 10.1063/1.478522. [DOI] [Google Scholar]
- Zhao Y.; Lynch B. J.; Truhlar D. G. Development and assessment of a new hybrid density functional model for thermochemical kinetics. J. Phys. Chem. A 2004, 108 (14), 2715–2719. 10.1021/jp049908s. [DOI] [Google Scholar]
- Freeman C.; Catlow C. A computer modeling study of defect and dopant states in SnO2. J. Solid State Chem. 1990, 85 (1), 65–75. 10.1016/S0022-4596(05)80061-1. [DOI] [Google Scholar]
- Haynes W. M.CRC handbook of chemistry and physics; CRC Press: 2014. [Google Scholar]
- Chaibi W.; Peláez R.; Blondel C.; Drag C.; Delsart C. Effect of a magnetic field in photodetachment microscopy. Eur. Phys. J. D 2010, 58 (1), 29–37. 10.1140/epjd/e2010-00086-7. [DOI] [Google Scholar]
- Waddington T. Lattice energies and their significance in inorganic chemistry. Adv. Inorg. Chem. Radiochem. 1959, 1, 157–221. 10.1016/S0065-2792(08)60254-X. [DOI] [Google Scholar]
- Hou Q.; Buckeridge J.; Lazauskas T.; Mora-Fonz D.; Sokol A. A.; Woodley S. M.; Catlow C. R. A. Defect formation in In2O3 and SnO2: A new atomistic approach based on accurate lattice energies. J. Mater. Chem. C 2018, 6 (45), 12386–12395. 10.1039/C8TC04760J. [DOI] [Google Scholar]
- Jost W. Diffusion and electrolytic conduction in crystals (ionic semiconductors). J. Chem. Phys. 1933, 1 (7), 466–475. 10.1063/1.1749319. [DOI] [Google Scholar]
- Patsalas P.; Logothetidis S.; Sygellou L.; Kennou S. Structure-dependent electronic properties of nanocrystalline cerium oxide films. Phys. Rev. B 2003, 68 (3), 035104. 10.1103/PhysRevB.68.035104. [DOI] [Google Scholar]
- Wardenga H. F.; Klein A. Surface potentials of (111),(110) and (100) oriented CeO2-x thin films. Appl. Surf. Sci. 2016, 377, 1–8. 10.1016/j.apsusc.2016.03.091. [DOI] [Google Scholar]
- McCullough J. D. An X-ray study of the rare-earth oxide systems: CeIV—NdIII, CrIV—PrIII, CeIV—PrIV and PrIV—NdIII1. J. Am. Chem. Soc. 1950, 72 (3), 1386–1390. 10.1021/ja01159a085. [DOI] [Google Scholar]
- Bevan D.; Kordis J. Mixed oxides of the type MO2 (fluorite)—M2O3—I oxygen dissociation pressures and phase relationships in the system CeO2— Ce2O3 at high temperatures. J. Inorg. Nucl. Chem. 1964, 26 (9), 1509–1523. 10.1016/0022-1902(64)80038-5. [DOI] [Google Scholar]
- Kümmerle E.; Heger G. The structures of C-Ce2O3+ δ, Ce7O12, and Ce11O20. J. Solid State Chem. 1999, 147 (2), 485–500. 10.1006/jssc.1999.8403. [DOI] [Google Scholar]
- Rossignol S.; Gérard F.; Mesnard D.; Kappenstein C.; Duprez D. Structural changes of Ce-Pr-O oxides in hydrogen: a study by in situ X-ray diffraction and Raman spectroscopy. J. Mater. Chem. 2003, 13 (12), 3017–3020. 10.1039/B306726B. [DOI] [Google Scholar]
- Yashima M.; Kobayashi S.; Yasui T. Crystal structure and the structural disorder of ceria from 40 to 1497 °C. Solid State Ionics 2006, 177 (3–4), 211–215. 10.1016/j.ssi.2005.10.033. [DOI] [Google Scholar]
- GUPTA M. L.; Singh S. Thermal Expansion of CeO2, Ho2O3, and Lu2O3 from 100° to 300° K by an X-Ray Method. J. Am. Ceram. Soc. 1970, 53 (12), 663–665. 10.1111/j.1151-2916.1970.tb12037.x. [DOI] [Google Scholar]
- Wuilloud E.; Delley B.; Schneider W.-D.; Baer Y. Spectroscopic Evidence for Localized and Extended f-Symmetry States in CeO2. Phys. Rev. Lett. 1984, 53 (2), 202. 10.1103/PhysRevLett.53.202. [DOI] [Google Scholar]
- Orel Z. C.; Orel B. Optical properties of pure CeO2 and mixed CeO2/SnO2 thin film coatings. Phys. Status Solidi B 1994, 186 (1), K33–K36. 10.1002/pssb.2221860135. [DOI] [Google Scholar]
- Guo S.; Arwin H.; Jacobsen S.; Järrendahl K.; Helmersson U. A spectroscopic ellipsometry study of cerium dioxide thin films grown on sapphire by rf magnetron sputtering. J. Appl. Phys. 1995, 77 (10), 5369–5376. 10.1063/1.359225. [DOI] [Google Scholar]
- Pelli Cresi J. S.; Di Mario L.; Catone D.; Martelli F.; Paladini A.; Turchini S.; D’Addato S.; Luches P.; O’Keeffe P. Ultrafast Formation of Small Polarons and the Optical Gap in CeO2. J. Phys. Chem. Lett. 2020, 11 (14), 5686–5691. 10.1021/acs.jpclett.0c01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno O. P.; Pérez R. G.; Merino R. P.; Portillo M. C.; Tellez G. H.; Rosas E. R.; Tototzintle M. Z. CeO2 nanoparticles growth by chemical bath and its thermal annealing treatment in air atmosphere. Optik 2017, 148, 142–150. 10.1016/j.ijleo.2017.08.133. [DOI] [Google Scholar]
- Pfau A.; Schierbaum K. The electronic structure of stoichiometric and reduced CeO2 surfaces: an XPS, UPS and HREELS study. Surf. Sci. 1994, 321 (1–2), 71–80. 10.1016/0039-6028(94)90027-2. [DOI] [Google Scholar]
- Mullins D.; Overbury S.; Huntley D. Electron spectroscopy of single crystal and polycrystalline cerium oxide surfaces. Surf. Sci. 1998, 409 (2), 307–319. 10.1016/S0039-6028(98)00257-X. [DOI] [Google Scholar]
- Molinari M.; Parker S. C.; Sayle D. C.; Islam M. S. Water adsorption and its effect on the stability of low index stoichiometric and reduced surfaces of ceria. J. Phys. Chem. C 2012, 116 (12), 7073–7082. 10.1021/jp300576b. [DOI] [Google Scholar]
- Buckeridge J.; Scanlon D.; Walsh A.; Catlow C.; Sokol A. Dynamical response and instability in ceria under lattice expansion. Phys. Rev. B 2013, 87 (21), 214304. 10.1103/PhysRevB.87.214304. [DOI] [Google Scholar]
- Jiang H. Revisiting the GW approach to d-and f-electron oxides. Phys. Rev. B 2018, 97 (24), 245132. 10.1103/PhysRevB.97.245132. [DOI] [Google Scholar]
- Debnath S.; Islam M.; Khan M. Optical properties of CeO2 thin films. Bull. Mater. Sci. 2007, 30 (4), 315–319. 10.1007/s12034-007-0052-3. [DOI] [Google Scholar]
- Hayun S.; Ushakov S. V.; Navrotsky A. Direct measurement of surface energy of CeO2 by differential scanning calorimetry. J. Am. Ceram. Soc. 2011, 94 (11), 3679–3682. 10.1111/j.1551-2916.2011.04843.x. [DOI] [Google Scholar]
- Hayun S.; Shvareva T. Y.; Navrotsky A. Nanoceria-Energetics of surfaces, interfaces and water adsorption. J. Am. Ceram. Soc. 2011, 94 (11), 3992–3999. 10.1111/j.1551-2916.2011.04648.x. [DOI] [Google Scholar]
- Fowler P. W.; Pyper N. In-crystal ionic polarizabilities derived by combining experimental and ab initio results. Proc. R. Soc. London, Ser. A 1985, 398 (1815), 377–393. 10.1098/rspa.1985.0040. [DOI] [Google Scholar]
- Rappoport D.; Furche F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133 (13), 134105. 10.1063/1.3484283. [DOI] [PubMed] [Google Scholar]
- Slater J. C.; Kirkwood J. G. The van der Waals forces in gases. Phys. Rev. 1931, 37 (6), 682. 10.1103/PhysRev.37.682. [DOI] [Google Scholar]
- Fowler P.; Harding J.; Pyper N. The polarizabilities and dispersion coefficients for ions in the solid group IV oxides. J. Phys.: Condens. Matter 1994, 6 (48), 10593. 10.1088/0953-8984/6/48/018. [DOI] [Google Scholar]
- Van Vleet M. J.; Misquitta A. J.; Stone A. J.; Schmidt J. R. Beyond born-mayer: Improved models for short-range repulsion in ab initio force fields. J. Chem. Theory Comput. 2016, 12 (8), 3851–3870. 10.1021/acs.jctc.6b00209. [DOI] [PubMed] [Google Scholar]
- Buckingham R. A. The classical equation of state of gaseous helium, neon and argon. Proc. R. Soc. A 1938, 168 (933), 264–283. 10.1098/rspa.1938.0173. [DOI] [Google Scholar]
- Mora-Fonz D.; Schön J. C.; Prehl J.; Woodley S. M.; Catlow C. R. A.; Shluger A.; Sokol A. A. Real and Virtual Polymorphism of Titanium Selenide with Robust Interatomic Potentials. J. Mater. Chem. A 2020, 8, 14054. 10.1039/D0TA03667F. [DOI] [Google Scholar]
- Walsh A.; Butler K. T. Prediction of electron energies in metal oxides. Acc. Chem. Res. 2014, 47 (2), 364–372. 10.1021/ar400115x. [DOI] [PubMed] [Google Scholar]
- Schirmer O. F. O- bound small polarons in oxide materials. J. Phys.: Condens. Matter 2006, 18 (43), R667. 10.1088/0953-8984/18/43/R01. [DOI] [PubMed] [Google Scholar]
- Clausen K.; Hayes W.; Macdonald J. E.; Osborn R.; Schnabel P. G.; Hutchings M. T.; Magerl A. Inelastic neutron scattering investigation of the lattice dynamics of ThO2 and CeO2. J. Chem. Soc., Faraday Trans. 2 1987, 83 (7), 1109–1112. 10.1039/f29878301109. [DOI] [Google Scholar]
- Marabelli F.; Wachter P. Covalent insulator CeO2: optical reflectivity measurements. Phys. Rev. B 1987, 36 (2), 1238. 10.1103/PhysRevB.36.1238. [DOI] [PubMed] [Google Scholar]
- Kourouklis G.; Jayaraman A.; Espinosa G. High-pressure Raman study of CeO2 to 35 GPa and pressure-induced phase transformation from the fluorite structure. Phys. Rev. B 1988, 37 (8), 4250. 10.1103/PhysRevB.37.4250. [DOI] [PubMed] [Google Scholar]
- Ganduglia-Pirovano M. V.; Da Silva J. L. F.; Sauer J. Density-Functional Calculations of the Structure of Near-Surface Oxygen Vacancies and Electron Localization on CeO2(111). Phys. Rev. Lett. 2009, 102 (2), 026101. 10.1103/PhysRevLett.102.026101. [DOI] [PubMed] [Google Scholar]
- Su Y.-Q.; Filot I. A.; Liu J.-X.; Tranca I.; Hensen E. J. Charge transport over the defective CeO2(111) surface. Chem. Mater. 2016, 28 (16), 5652–5658. 10.1021/acs.chemmater.6b01548. [DOI] [Google Scholar]
- Castleton C. W.; Lee A.; Kullgren J. Benchmarking Density Functional Theory Functionals for Polarons in Oxides: Properties of CeO2. J. Phys. Chem. C 2019, 123 (9), 5164–5175. 10.1021/acs.jpcc.8b09134. [DOI] [Google Scholar]
- Burow A. M.; Sierka M.; Döbler J.; Sauer J. Point defects in CaF2 and CeO2 investigated by the periodic electrostatic embedded cluster method. J. Chem. Phys. 2009, 130 (17), 174710. 10.1063/1.3123527. [DOI] [PubMed] [Google Scholar]
- Ganduglia-Pirovano M. V.; Murgida G. E.; Ferrari V.; Llois A. M. a. Comment on “Oxygen Vacancy Ordering and Electron Localization in CeO2: Hybrid Functional Study”. J. Phys. Chem. C 2017, 121 (38), 21080–21083. 10.1021/acs.jpcc.7b01800. [DOI] [Google Scholar]
- Stratton T. G.; Tuller H. L. Thermodynamic and transport studies of mixed oxides. The CeO2-UO2 system. J. Chem. Soc., Faraday Trans. 2 1987, 83 (7), 1143–1156. 10.1039/F29878301143. [DOI] [Google Scholar]
- Liu X.-Y.; Sickafus K. E. Lanthanum energetics in cubic ZrO2 and UO2 from DFT and DFT+ U studies. J. Nucl. Mater. 2011, 414 (2), 217–220. 10.1016/j.jnucmat.2011.03.042. [DOI] [Google Scholar]
- Fuda K.; Kishio K.; Yamauchi S.; Fueki K.; Onoda Y. 17O NMR study of Y2O3-doped CeO2. J. Phys. Chem. Solids 1984, 45 (11–12), 1253–1257. 10.1016/0022-3697(84)90024-6. [DOI] [Google Scholar]
- Nolan M.; Fearon J. E.; Watson G. W. Oxygen vacancy formation and migration in ceria. Solid State Ionics 2006, 177 (35–36), 3069–3074. 10.1016/j.ssi.2006.07.045. [DOI] [Google Scholar]
- Wang D; Park D; Griffith J; Nowick A Oxygen-ion conductivity and defect interactions in yttria-doped ceria. Solid State Ionics 1981, 2 (2), 95–105. 10.1016/0167-2738(81)90005-9. [DOI] [Google Scholar]
- Reuter K.; Scheffler M. Composition, structure, and stability of RuO2(110) as a function of oxygen pressure. Phys. Rev. B 2001, 65 (3), 035406. 10.1103/PhysRevB.65.035406. [DOI] [Google Scholar]
- Stull D. R.JANAF Thermochemical Tables; Clearinghouse: 1965; Vol. 1. [Google Scholar]
- Buckeridge J. Equilibrium point defect and charge carrier concentrations in a material determined through calculation of the self-consistent Fermi energy. Comput. Phys. Commun. 2019, 244, 329–342. 10.1016/j.cpc.2019.06.017. [DOI] [Google Scholar]
- Hu Z.; Metiu H. Effect of dopants on the energy of oxygen-vacancy formation at the surface of ceria: Local or global?. J. Phys. Chem. C 2011, 115 (36), 17898–17909. 10.1021/jp205432r. [DOI] [Google Scholar]
- Ganduglia-Pirovano M. V.; Da Silva J. L.; Sauer J. Density-functional calculations of the structure of near-surface oxygen vacancies and electron localization on CeO2(111). Phys. Rev. Lett. 2009, 102 (2), 026101. 10.1103/PhysRevLett.102.026101. [DOI] [PubMed] [Google Scholar]
- Wang J.; Yin W.-J. Revisiting the Iodine Vacancy Surface Defects to Rationalize Passivation Strategies in Perovskite Solar Cells. J. Phys. Chem. Lett. 2022, 13, 6694–6700. 10.1021/acs.jpclett.2c01815. [DOI] [PubMed] [Google Scholar]
- Murgida G. E.; Ferrari V.; Llois A. M.; Ganduglia-Pirovano M. V. Reduced CeO2(111) ordered phases as bulk terminations: Introducing the structure of Ce3O5. Phys. Rev. Mater. 2018, 2 (8), 083609. 10.1103/PhysRevMaterials.2.083609. [DOI] [Google Scholar]
- Olbrich R.; Murgida G. E.; Ferrari V.; Barth C.; Llois A. M.; Reichling M.; Ganduglia-Pirovano M. V. Surface stabilizes ceria in unexpected stoichiometry. J. Phys. Chem. C 2017, 121 (12), 6844–6851. 10.1021/acs.jpcc.7b00956. [DOI] [Google Scholar]
- Kehoe A. B.; Scanlon D. O.; Watson G. W. Role of lattice distortions in the oxygen storage capacity of divalently doped CeO2. Chem. Mater. 2011, 23 (20), 4464–4468. 10.1021/cm201617d. [DOI] [Google Scholar]
- Nolan M. Charge compensation and Ce3+ formation in trivalent doping of the CeO2(110) surface: the key role of dopant ionic radius. J. Phys. Chem. C 2011, 115 (14), 6671–6681. 10.1021/jp112112u. [DOI] [Google Scholar]
- Kullgren J.; Wolf M. J.; Castleton C.; Mitev P.; Briels W. J.; Hermansson K. Oxygen vacancies versus fluorine at CeO2(111): A case of mistaken identity?. Phys. Rev. Lett. 2014, 112 (15), 156102. 10.1103/PhysRevLett.112.156102. [DOI] [PubMed] [Google Scholar]
- Wu X.-P.; Gong X.-Q. Clustering of oxygen vacancies at CeO2(111): critical role of hydroxyls. Phys. Rev. Lett. 2016, 116 (8), 086102. 10.1103/PhysRevLett.116.086102. [DOI] [PubMed] [Google Scholar]
- Walsh A.; Sokol A. A.; Catlow C. R. A. Free energy of defect formation: Thermodynamics of anion Frenkel pairs in indium oxide. Phys. Rev. B 2011, 83 (22), 224105. 10.1103/PhysRevB.83.224105. [DOI] [Google Scholar]
- Zhang X.; Fu C.; Xia Y.; Duan Y.; Li Y.; Wang Z.; Jiang Y.; Li H. Atomistic Origin of the Complex Morphological Evolution of Aluminum Nanoparticles during Oxidation: A Chain-like Oxide Nucleation and Growth Mechanism. ACS Nano 2019, 13 (3), 3005–3014. 10.1021/acsnano.8b07633. [DOI] [PubMed] [Google Scholar]
- Mora-Fonz D.; Lazauskas T.; Farrow M. R.; Catlow C. R. A.; Woodley S. M.; Sokol A. A. Why are polar surfaces of ZnO stable?. Chem. Mater. 2017, 29 (12), 5306–5320. 10.1021/acs.chemmater.7b01487. [DOI] [Google Scholar]
- Zhang X.; Zheng P.; Ma Y.; Jiang Y.; Li H. Atomic-scale understanding of oxidation mechanisms of materials by computational approaches: A review. Materials & Design 2022, 217, 110605. 10.1016/j.matdes.2022.110605. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







