Abstract
Purpose.
It is well-established that persistent tobacco use among patients with cancer results in numerous adverse outcomes. However, the assessment and treatment of tobacco use with evidence-based methods has been lacking in cancer care. Our cancer center has established its first tobacco treatment program, a multidisciplinary, evidence-based, clinical program for hematology/oncology patients.
Methods.
We describe the development and implementation of the program, emphasizing lessons learned in treating nicotine addiction among patients who are at very high risk for continuing to use tobacco throughout the survivorship phase.
Results.
We developed a system to assess tobacco use at each outpatient visit, from those recently diagnosed to long-term survivors. For patients who have smoked in the past month, the protocol offers standard behavioral and pharmacological treatments, delivered by tobacco treatment specialists and nurse practitioners over four in-person and/or telephone-based sessions. Partnerships with the Psychosocial Oncology and Cancer Survivorship Programs have provided integrated and comprehensive care for patients during and after their cancer treatment.
Conclusions.
The systematic efforts to reach and engage current smokers have laid the groundwork for maximizing the program’s future effectiveness and impact. Our initial results demonstrate the complexities but also the feasibility of developing a new tobacco treatment program in the oncology setting.
Implications for Cancer Survivors.
The implications for cancer survivors are the significant improvements in treatment outcomes that occur with tobacco abstinence.
Keywords: tobacco use, smoking cessation, program development
Introduction
It is well established that persistent tobacco use among patients with cancer undermines treatment effectiveness, including increased risk of all-cause mortality, cancer-specific mortality, second primary cancers, poorer response to treatment, and treatment-related toxicity [1]. Conversely, tobacco cessation dramatically improves cancer survivors’ prognosis, quality of life, and comorbidity profile [2]. Despite the availability of this information, the assessment and treatment of tobacco use with evidence-based methods has been lacking in oncology [3]. With the growing emphasis on implementing tobacco control interventions that are effective for this population, the NCI established the Cancer Center Cessation Initiative (C3i), and provided support to 42 NCI-designated cancer centers to create or enhance clinically-based tobacco treatment programs [3]. Through this mechanism, our center established its first tobacco treatment program for patients with cancer - the Smoking Treatment and Recovery (STAR) Program. STAR is a multidisciplinary, evidence-based, clinical program providing tobacco treatment to hematology/oncology patients. Based at an academic health center, the program has plans to expand to other regional cancer centers within our hospital network that serve a diverse catchment area.
Herein, we describe the development of the STAR program in an oncology setting, emphasizing lessons learned in treating nicotine addiction among patients who are at very high risk for continuing to use tobacco throughout the survivorship phase. Importantly, we cover essential elements of the program’s successful development, including key personnel, institutional resources, and time to implementation. Finally, we provide a summary of program data collection, tracking efforts, and future plans.
Program Development
STAR Program Members.
The program is led by an administrative team that includes cancer center and hospital administrators, and a clinical team that includes medical oncology, oncology nursing, psycho-oncology, and health information technology. Oncology nurse practitioners (NP) and tobacco treatment specialists (TTS) provide the tobacco treatment. We invited a well-regarded expert on integrating tobacco control in the oncology setting to deliver Oncology Grand Rounds and to consult on a quality improvement plan to determine the prevalence of tobacco use and to deliver and track tobacco treatment.
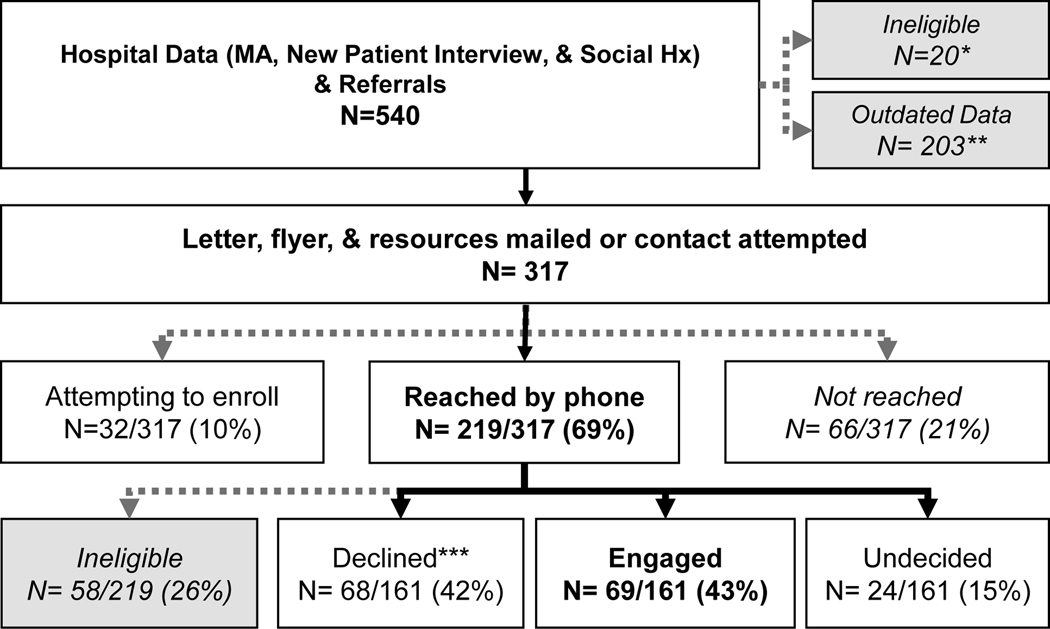
Identification and Assessment of Current Smokers (Figure).
Figure. Workflow and Recruitment.
*no permission from provider, too ill, 2nd opinion visit, not a clinic patient
**data are two months out of date; will not contact (i.e. ineligible) unless updated data received
***active or passive declines
We developed a system to screen hematology/oncology outpatients, relying on medical assistants to assess tobacco use at each patient visit [4]. Currently, 70% of patients are assessed, with the goal of reaching 90%. We piloted the workflow in thoracic oncology, later expanding to all hematology/oncology patients, from those recently diagnosed to long-term survivors. Additional methods to identify tobacco users include: new patient questionnaires, the EMR-based tobacco assessment, and referrals from providers and families. We posted STAR flyers in exam rooms, on the hospital’s website, and medical assistants hand a flyer to current smokers. The hospital recently adopted a tobacco-free policy, central to the expectation that tobacco treatment will be offered to all patients.
Each week, we mail an introductory letter, flyer, and a list of free and low-cost interventions (e.g., telephone quitline, evidence-based Internet resources) to newly-identified patients who report smoking within the past month. We then make up to two calls to describe the treatment program, conduct an intake assessment with interested patients, and schedule an initial counseling session. We recontact those who are initially uninterested and agree to future contact. We manage program-related data in a secure database, separate from the EMR.
Using the RE-AIM framework [5], we evaluate the program’s Reach (percentage of current smokers in hematology/oncology who we successfully contact). We assess patient Engagement at three points: completion of the intake assessment, treatment sessions, and follow-up assessments. We measure program Effectiveness (smoking status and other nicotine/tobacco use variables) at 6 months post-enrollment. We are measuring program Costs to determine requirements for sustainability, and collaborating with the C3i cost assessment initiative to produce metrics including the cost per patient enrolled and cost per quit.
Intervention Development.
We reviewed existing tobacco treatment programs in oncology settings [6,7] and met with cancer center and hospital stakeholders to assess potential barriers for providing tobacco treatment. We determined that a referral system to knowledgeable medical and behavioral specialists was the optimal model for our setting. Due to patient travel and scheduling barriers, our protocol offers both in-person and telephone-based sessions. Patients select the modality and frequency of their treatment sessions, with up to six months to complete four sessions (initially we allowed >4 sessions, but this was unsustainable). At the 6-month assessment, we offer two additional sessions to patients needing further treatment.
The STAR protocol includes standard behavioral intervention components, with a focus on motivational interviewing for those not ready to quit and relapse prevention strategies for newly abstinent patients. Telephone-based treatment is delivered by a TTS and in-person, clinic-based sessions (during a weekly, half-day clinic) are delivered by an NP and a TTS. NPs prescribe both over-the-counter and prescription cessation medications. Nicotine replacement products are provided at no cost by the program or billed to insurance when possible. We continue to recommend additional evidence-based interventions (quitline, smartphone-based cessation apps, text messaging).
Integration of STAR with the Psychosocial-Oncology and Cancer Survivorship Programs.
The Psychosocial Oncology Program provides individual psychotherapy, education, pharmacological evaluation and treatment, support groups, and distress screening to oncology patients. Providers include four social workers, a psychologist and a psychiatrist. This team has referred 6 current smokers to STAR, and 22 STAR patients have been referred to the Psychosocial Oncology Program. Based on patients’ receptivity to connect with available psychosocial services, we recently formalized the process, using the NCCN Distress Thermometer [8] to refer based on patient scores and preferences.
The Cancer Survivorship Program is a new program in two of our cancer hospitals. We are working to increase the referrals to and from STAR, given the importance of tobacco abstinence in maintaining health and cancer-free survival. The development of these programs has highlighted the importance of coordinated survivorship care, particularly vital for survivors with uncertainty about a variety of medical and psychological concerns following treatment completion. This transition is especially difficult when patients are also attempting to stop smoking, particularly when it has been a life-long coping strategy.
Program Evaluation
The Table presents demographic, clinical, and tobacco characteristics of the 540 smokers identified during the program’s first 15-months. We successfully reached 219/317 (69%), and of those eligible, we engaged 69/161 (43%) in treatment. These rates of reach and engagement are comparable to other programs [9].
Table.
Demographic, Clinical,- and Tobacco-Related Characteristics
| All Current Smokers N=540 | Eligible Patients N= 259 | Enrolled Patients N=65 | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 59.9 (13.5) | 59.9 (12.1) | 60.6 (11.3) |
| Median (Range) | 61 (21–98) | 61 (21–84) | 61(28–79) |
| Gender | |||
| Female N (%) | 265 (49%) | 136 (53%) | 40 (62%) |
| Race N (%) | |||
| White | 272 (53%) | 115 (46%) | 22 (34%) |
| African American or Black | 184 (36%) | 103 (42%) | 38 (59%) |
| Other | 58 (11%) | 30 (12%) | 4 (6%) |
| Missing | 26 | 11 | 1 |
| Ethnicity N (%) | |||
| Hispanic | 25 (5%) | 15 (6%) | 4 (6%) |
| Initial Smoking Status N (%) | |||
| Smoked a cigarette today | 326 (60%) | 192 (74%) | 50 (79%) |
| 1 to 7 days ago | 113 (21%) | 33 (13%) | 7 (11%) |
| 8 days to 1 month ago | 99 (18%) | 32 (12%) | 6 (9%) |
| Primary Diagnosis N (%) | |||
| *Hematological (non-cancer; eg, anemia, DVT) | 77 (14%) | 29 (12%) | 9 (14%) |
| Genitourinary Cancer | 71 (13%) | 34 (15%) | 6 (9%) |
| Lung Cancer | 69 (13%) | 42 (18%) | 17 (26%) |
| Breast Cancer | 67 (12%) | 34 (15%) | 9 (14%) |
| Gastrointestinal Cancer | 130 (24%) | 44 (19%) | 10 (15%) |
| Leukemia/Lymphoma | 49 (9%) | 16 (7%) | 5 (8%) |
| Head/Neck Cancer | 41 (8%) | 22 (9%) | 6 (9%) |
| Skin Cancer | 18 (3%) | 3 (1%) | 0 (0%) |
| Other Cancers | 21 (3%) | 10 (4%) | 3 (5%) |
| Referral Method N (%) | |||
| Medical Assistant data | 324 (60%) | 210 (81%) | 40 (62%) |
| EMR Social History | 128 (24%) | 7 (3%) | 4 (6%) |
| Clinician Referred | 23 (4%) | 23 (9%) | 17 (26%) |
| Family Referred | 1 (<1%) | 1 (<1%) | 1 (2%) |
| New patient questionnaire | 64 (12%) | 18 (7%) | 3 (5%) |
| Months since First Cancer Diagnosis | |||
| Mean (SD) | 36 (SD=42) | ||
| Median (Range) | 22 (3–227) | ||
| N/A - Hematological diagnosis | 12 | ||
| Cigarettes Per Day | |||
| Mean (SD) | 12.2 (9.4) | ||
| Median (Range) | 10 (0–40) | ||
| Missing CPD | 5 | ||
| Pack years | |||
| Mean (SD) | 29.4 (17.9) | ||
| Median (Range) | 21.5 (3 – 75) | ||
| Missing Pack years | 3 | ||
| Time to first cigarette N (%) | |||
| Within 5 minutes | 11 (21%) | ||
| 6 to 30 minutes | 21 (40%) | ||
| 31 to 60 minutes | 8 (15%) | ||
| After 60 minutes | 12 (23%) | ||
| Missing | 13 | ||
| Readiness to Quit N (%) | |||
| I have already quit smoking* | 6 (13%) | ||
| I have made changes, but haven’t quit yet. | 15 (31%) | ||
| I plan to quit smoking in the next 30 days. | 11 (23%) | ||
| I plan to quit smoking in the next 6 months. | 6 (13%) | ||
| I think about quitting and I have no plans yet. | 9 (19%) | ||
| I do not think about quitting smoking. | 1 (2%) | ||
| Missing | 17 | ||
| Use of Tobacco-Related Products N (%) | |||
| Cigarettes only | 43 (75%) | ||
| E-cigarettes | 4 (7%) | ||
| Cigars | 2 (4%) | ||
| Marijuana (recreational, medical, smokeless) | 6 (11%) | ||
| More than one product | 2 (4%) | ||
| Missing | 8 | ||
| Prior Cessation Medication Use N (%) | |||
| Nicotine Replacement Therapy only | 10 (17%) | ||
| Chantix only | 5 (8%) | ||
| Zyban only | 3 (5%) | ||
| >1 type of cessation medication | 26 (44%) | ||
| None | 15 (25%) | ||
| Missing | 6 | ||
| Longest Non-smoking Period N (%) | |||
| Less than 1 week | 6 (11%) | ||
| 1 week to 1 month | 5 (9%) | ||
| 1 month to 1 year | 25 (45%) | ||
| More than 1 year | 13 (24%) | ||
| Have not quit | 6 (11%) | ||
| Missing | 10 | ||
Both hematology and oncology patients were included as they are seen in the same clinic within the cancer center.
Compared to the patients identified as current smokers and who were eligible, those who elected to participate in STAR were somewhat more likely to be female, African American, have a primary diagnosis of lung cancer, and have smoked on the day of the clinic visit (vs. past week/past month); see Table. Among STAR participants, we found that patients were a median of 22 months post-diagnosis, smoked a median of 10 cigarettes per day, had a median of 22 pack years, 61% smoked within 30 minutes of waking (suggesting high nicotine dependence), 75% smoked cigarettes exclusively, and 67% were already quit or planned to quit within the next 30 days. The majority of patients had made efforts to quit, with 65% having quit for <1 year, and 75% having used at least one cessation medication. Regarding program engagement, 74% of those enrolled have completed a median of 2 treatment sessions, and patients were equally engaged with phone and in-person sessions (data not shown).
Regarding effectiveness, we are currently calling patients to assess self-reported smoking status six months post-enrollment. The long-term goal is to obtain carbon monoxide readings following self-reported quitting, although this is not feasible until we have a funded intervention trial [6].
Discussion
The STAR program provides an example of the multidisciplinary collaboration required to initiate a tobacco treatment program in the cancer setting. The development of regular screening of tobacco use by medical assistants, followed by tobacco treatment staff reaching and engaging current smokers in treatment, may be a useful model for other de novo programs. Further, by increasing the identification and referral of patients in need of psychological assistance and survivorship care, the program has provided opportunities for more comprehensive care during and after cancer treatment.
There are important lessons learned that are relevant to program development and sustainability for cancer centers planning new tobacco treatment programs. First, the importance of the administrative and financial support pledged by cancer center and hospital leadership for STAR’s expansion within our hospital network is central to the program’s long-term sustainability. Second, regarding treatment modality, although both telephone-based and in-person counseling options were equally utilized, phone-based counseling (and telemedicine in the future) will be vital for the program’s sustainability. We expect that this will be particularly true when the program is expanded to larger cancer centers. Further, the transition of the Oncology EMR to Cerner will automate tobacco use assessments and increase provider referrals. These modifications will also improve the identification of patients closer to the initial cancer diagnosis and aid the program’s expansion to surgical and radiation oncology clinics.
The limitations of the STAR program center mainly on the fact that this is a new program, and therefore still relatively small and lacking effectiveness data. Compared to data from the BRFSS on smoking among cancer survivors [10], our sample of current smokers was older and more likely to be African American, but was similar to BRFSS regarding ethnicity, gender, and e-cigarette use. These differences may be due to the cancer center’s catchment area and the types of cancers that are most often treated here, but suggest that our data are not fully representative of national data. Finally, we are in the process of collecting abstinence data, but will not know the effectiveness of the program until quit rates can be demonstrated.
Tobacco treatment is an essential component of high-quality cancer treatment and survivorship care [1]. The systematic efforts to reach and engage current smokers have laid the groundwork for maximizing the program’s effectiveness and impact. Our initial results demonstrate the complexities but also the feasibility of developing a new tobacco treatment program in the oncology setting.
Funding Source:
The program described was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA051008-26S1 (PI: Weiner) and by the Survey, Recruitment and Biospecimen Shared Resource which is partially supported by P30CA051008. The content is solely the responsibility of the authors and does not necessarily represent the National Institutes of Health.”
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress: a report of the surgeon general. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Gallaway M, Glover-Kudon R, Momin B, Puckett M, Lunsford NB, Ragan K, et al. Smoking cessation attitudes and practices among cancer survivors-United States, 2015. J Ca Surviv. 2019;13(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croyle RT, Morgan GD, Fiore MC. Addressing a core gap in cancer care- The NCI Moonshot Program to help oncology patients stop smoking. N Engl J Med. 2019;380(6):512515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Land SR, Toll BA, Moinpour CM, Mitchell SA, Ostroff JS, Hatsukami DK, et al. Research priorities, measures, and recommendations for assessment of tobacco use in clinical cancer research. Clin Cancer Res. 2016;22(8):1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health 2013;103(6):e38–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karam-Hage M, Oughli HA, Rabius V, Beneventi D, Wippold RC, Blalock JA, et al. Tobacco cessation treatment pathways for patients with cancer: 10 years in the making. J Natl Compr Canc Netw. 2016;14(11):1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren GW, Ward KD. Integration of tobacco cessation services into multidisciplinary lung cancer care: Rationale, state of the art, and future directions. Transl. Lung Cancer Res. 2015;4(4):339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Distress Management. Version 2.2018. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf Accessed 10/12/19. [DOI] [PMC free article] [PubMed]
- 9.Warren GW, Marshall JR, Cummings KM, Toll B, Gritz ER, Hysert P, et al. Automated tobacco assessment and cessation support for cancer patients. Cancer. 2014;120(4):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antwi GO, Lohrmann DK, Jayawardene W, Chow A, Obeng CS, Sayegh AM. Associations between e-cigarette and combustible cigarette use among U.S. cancer survivors: implications for research and practice. J Cancer Surviv. 2019. Apr;13(2):316–325. [DOI] [PubMed] [Google Scholar]