Abstract
Corneal transplant is a procedure that aims to replace dysfunctional corneal tissue with a transparent graft and is one of the most widely performed transplant surgeries, but its public and professional awareness is low outside of ophthalmology. Corneal tissue consists of 5 major layers that serve to maintain its structural integrity and refractive shape: the epithelium, Bowman’s layer, the stroma, Descemet’s membrane, and the endothelium. Failure or irreversible damage to any layer of the cornea may be an indication for corneal transplant, and variants of this procedure may be full thickness or selectively lamellar. Complications related to corneal transplantation may occur anywhere from during surgery to years afterward, including rejection, dehiscence, cataract, and glaucoma. Complications should be managed by an ophthalmologist, but other physicians should be aware of prophylactic medications. Topical immunosuppressants and steroids are effective for preventing and treating rejection episodes, whereas there is little evidence to support the use of systemic immunosuppression. Eye protection is recommended for any corneal transplant recipient. Physicians should counsel patients on corneal donation, especially if outside the United States, where donor tissue is in short supply.
INTRODUCTION
The first successful human cornea transplant was performed more than a century ago by Dr Eduard Zirm in the Czech Republic on December 7, 1905.1 He encountered a recipient with bilateral corneal scarring from alkali burns and a blind donor whose eye was enucleated after a cornea-sparing penetrating globe injury. Immediately after donor enucleation, he excised two 5-mm grafts from the donor cornea with a trephine and stored them in gauze moistened with a sterile salt solution. The recipient corneas received matching 5-mm trephinations, into which the donor corneal tissue was inserted and sutured. Although 1 graft failed, the other remained clear and provided a visual acuity of 20/120 at 6 mo.
Prior attempts in corneal grafting included animal grafts, artificial corneas (keratoprosthetics), and substitution with clear tissues such as conjunctiva, but these grafts typically became opaque within a few weeks after surgery.2,3 Zirm’s procedure is known today as a penetrating keratoplasty (PKP) of an allograft cornea.4 Since its inception, corneal transplant has become a domain of highly refined and diverse procedures, although its procedure and indication remain the same: to replace dysfunctional corneal tissue with a transparent and refractive graft.
CURRENT TRENDS
Corneal transplant is now one of the most widely performed solid organ transplant surgeries, but its public and professional awareness is low outside of ophthalmology.5 In 2012, 184 576 corneal transplants were reported in 116 countries.6 In 2019, 51 336 corneal transplants were performed in the United States compared with 32 313 other organ transplants, including kidney, liver, lung, pancreas, heart, and intestine.7,8 Only hematopoietic cell transplantation comes close to matching the volume of corneal tissue allografts, with 48 512 grafts performed in the United States during 2019.9 From 1991 to 2019, the supply of corneal grafts from US eye banks has increased by 116% from 39 515 grafts to 85 601 grafts.8
Recent trends indicate an increased demand for donor corneas worldwide. Data from US eye banks show a substantial increase in exported corneal grafts from 3684 grafts transplanted internationally in 1991 to 28 402 grafts transplanted internationally in 2019.8 Despite this nearly 8-fold increase in corneal graft export from the United States, there is still a worldwide shortage of corneal donor tissue. A global survey in 2016 estimated that‚ for every 70 corneal transplants needed, only 1 is available. Global internet search volume for corneal transplantation also increased by 26.4% from 2010 to 2020.10 However, the global interest in corneal donation is small in relation to its volume and demand. Internet searches regarding corneal transplantation outnumbered searches for corneal donation by 2 to 1, and searches for organ donation were 10-fold that of eye donation during this same period.10
Even within countries with sufficient corneal donors, the high cost of surgery limits access to corneal transplantation. Estimates by US eye banks in 2013 found that the total cost of each corneal transplant, including follow-up and medication‚ ranged from $11 062 to $20 953, depending on the type of transplant.11 Another independent survey in 2017 found that an average bill was $30 200 for each cornea transplant performed in the United States, including cost of hospital admission and excluding cost of follow-up.12 Even for countries with universal health care, corneal transplantation remains expensive. In Quebec, the mean cost of a corneal transplant exceeds $3000 paid by the recipient, including a 3-y follow-up period.13 An estimate of costs from 2001 to 2007 in Singapore for Descemet’s stripping endothelial keratoplasty (DSEK) and PKP, including 3-y follow-up, was $7476 and $7236, respectively.14
However, the resulting lifetime benefits far outweigh the cost of the procedure. In the United States, corneal transplant provides an estimated $118 000 in net lifetime economic benefits, with greater benefits for patients under 65 y old, exceeding $200 000.11 Even in low-income countries, the economic benefit can be substantial. A humanitarian study from 2014 to 2017 in Guyana showed an estimated increase of 4.6 quality-adjusted life years and 4.3 disability-adjusted life years per corneal transplant.15
CORNEAL ANATOMY
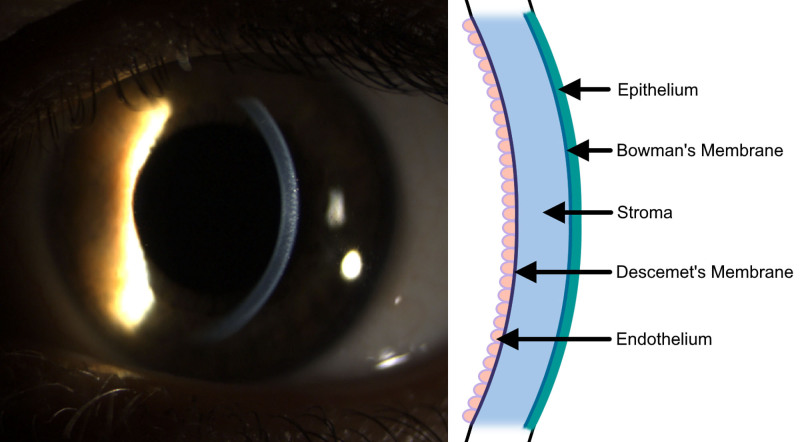
The cornea is the transparent, anterior-most structure of the eye that functions to protect deeper structures and refract light into the eye. The cornea consists of 5 major layers that maintain its structural integrity and refractive shape. In order from anterior to posterior‚ they are the epithelium, Bowman’s layer, the stroma, Descemet’s membrane, and the endothelium (Figure 1).16 The regularity of constituent components of these layers, including collagen, glycosoaminoglycans, and proteoglycans, contributes to the transparency of the cornea. Unrepairable damage to any layer can be an indication for corneal transplant.17 The high success rate of corneal transplantation without immunosuppressive therapy is owed to its relative immune privilege and innate active immunosuppression.18 A healthy cornea is avascular and lacks antigen-presenting cells such as macrophages. Epithelial cells, keratocytes, and endothelial cells also express no major histocompatibility complex-II molecules and only low levels of major histocompatibility complex-I molecules, leading to minimal antigen presentation. Simultaneously, the endothelium constitutively expresses a variety of immunosuppressive factors, including Fas ligand, tumor necrosis factor–related apoptosis-inducing ligand, and B7-H1, which prevent alloimmunization.
FIGURE 1.
Cornea location and anatomy. The cornea is the anterior refractive structure of the eye. A slit-lamp photograph with oblique lighting is shown with a schematic detailing layers of the cornea. In order from anterior to posterior, the layers are the epithelium, Bowman’s membrane, corneal stroma, Descemet’s membrane, and endothelium.
The epithelium is the outermost structure of the cornea and functions as the primary barrier to damage and infection of underlying structures. A smooth corneal epithelium is also critical to good vision. The epithelium consists of approximately 50 µm of stratified nonkeratinized squamous cells bound to a basement membrane. Between these cells, tight junctions chemically seal the eye from the external environment.19 The innermost basal layer of the epithelium consists of a single layer of mitotically active columnar stem cells that serve to regenerate epithelial cells and mediate cell migration in response to minor injury. Basal cells are attached to the basement membrane by hemidesmosomes that are pivotal for preventing separation of the epithelium from the underlying layers of the cornea. Destruction of the basal layer leads to corneal abrasions but reserves limbal stem cells at the circumference of the cornea can migrate and repairs the entire epithelium within a few days.20
Posterior to the epithelial basement membrane are Bowman’s layer and the corneal stroma that make up 90% of the corneal thickness. Bowman’s layer is not a true membrane; it is a 15-µm thick, acellular condensation of proteoglycans and collagen continuous with the anterior stroma. Unlike Bowman’s layer, the stroma is around 500 µm and is inhabited by keratocytes that maintain the extracellular matrix. Collagen fibrils and proteoglycans that comprise the stromal extracellular matrix are regularly arranged in lamellae to maintain transparency and structural strength of the stroma. The anterior stroma contains more highly interwoven lamellae at a higher packing density to provide greater tensile strength and maintain corneal curvature.21 The posterior lamellae of the stroma are less densely packed and easily opacify while absorbing water. Dua et al22 proposed the existence of a sixth layer of the cornea termed the pre-Descemet’s layer, which may serve as a plane of cleavage in deep anterior lamellar keratoplasty (DALK) procedures. Damage to the stroma and Bowman’s layer may lead to vision-limiting scar tissue formation, which may require transplantation.16
The posterior cornea comprises Descemet’s membrane and the endothelium. Descemet’s membrane is composed primarily of type IV collagen and laminin, serving as the basement membrane of the endothelium. Collagen forming Descemet’s membrane is continuously secreted by the endothelium‚ reaching a maximum thickness of around 10 µm late in life.16 The endothelium is a 5-µm monolayer of tightly adhered cuboidal cells that separates the cornea from the anterior chamber. At birth, the endothelium has a cell density of around 6000 cells/mm, but because of minimal mitotic activity, cell density decreases throughout life.23 The endothelium functions to dehydrate the posterior stroma and to supply nutrients from the aqueous humor to the avascular cornea. Tight regulation of water content in the posterior stroma is essential to maintaining the transparency of the cornea because increased swelling would disrupt the crystalline organization and tightly regulated spacing of collagen fibrils. Endothelial cells contain numerous Na+/K+ ATPase channels that actively pump ions and osmotically leach water from the stroma into the anterior chamber. Failure of the endothelium leads to corneal edema and is an indication for transplantation.24
INDICATIONS FOR CORNEAL TRANSPLANT
Broadly, failure or irreversible damage to any layer of the cornea may be an indication for corneal transplant. Superficial injuries such as corneal abrasions are reversible, rapidly healing within days. Regular refractive errors may also be corrected with glasses, intraocular lens, or refractive surgery. Even the mitotically inactive endothelial cells can enlarge and migrate, compensating for a damaged area. A large proportion of indications are not reported (32%) or because of repeat corneal transplant (10%), but there are several recognized primary indications listed in Table 1.8
TABLE 1.
Indications for corneal transplant
| Indication | Transplant variant (%) | |||
|---|---|---|---|---|
| PKP | ALK | EK | Total | |
| Endothelial dystrophy | 1.57 | – | 34.12 | 35.69 |
| Repeat corneal transplant | 6.60 | 0.06 | 6.19 | 12.84 |
| Pseudophakic corneal edema | 1.77 | – | 7.37 | 9.14 |
| Corneal ectasia and thinning | 4.99 | 0.62 | – | 5.61 |
| Noninfectious ulceration and perforation | 2.46 | 0.08 | – | 2.54 |
| Stromal dystrophy | 1.52 | 0.14 | – | 1.66 |
| Trauma | 0.89 | 0.05 | – | 0.94 |
| Infectious keratitis | 0.86 | 0.02 | – | 0.88 |
| Congenital opacity | 0.54 | 0.02 | – | 0.57 |
| Refractive | 0.07 | 0.00 | – | 0.07 |
| Pterygium | 0.01 | 0.00 | – | 0.01 |
| Other endothelial dysfunction | 2.59 | – | 8.95 | 11.53 |
| Other stromal dysfunction | 3.45 | 0.29 | – | 3.74 |
| Unknown or unreported | 8.37 | 0.23 | 6.18 | 14.78 |
| All indications | 35.67 | 1.53 | 62.80 | 100.00 |
This table is adapted from the 2019 Eye Banking Statistical Report8 using data from transplants performed in the United States. Percentages of transplant variants not indicated are marked with a dash. Percentages are rounded to the nearest hundredth percent. Keratoprostheses may be used for any indication, but frequencies are not reported.
ALK, anterior lamellar keratoplasty; EK, endothelial keratoplasty; PKP, penetrating keratoplasty.
Endothelial dysfunction is the most common indication (42%) for corneal transplantation, encompassing several causes that prevent adequate dehydration of the corneal stroma. In these patients, corneal edema causes corneal haziness and creates bullous disruptions of the epithelium in advanced cases. In longstanding, severe cases, subepithelial fibrosis further opacifies the cornea. Fuchs’ dystrophy encompasses the majority of endothelial dysfunction, leading to progressive loss of endothelial cells for more than decades.25 It can be sporadic or inherited in an autosomal dominant manner. Posterior polymorphous corneal dystrophy is another autosomal dominant disorder in which the endothelial cells differentiate into stratified squamous epithelium with decreased pump function, contributing to a thickened and keratinized Descemet’s membrane within the first year of life.26 Congenital hereditary endothelial dystrophy is a similar but rare autosomal recessive condition presenting at birth with endothelial pump dysfunction.27 Endothelial injury may also result in corneal edema. A common example is pseudophakic bullous keratopathy resulting from exposure to ultrasound energy during cataract surgery, although many of these cases are thought to be undiagnosed Fuchs’ dystrophy.28 Before permanent stromal damage occurs, endothelial transplant rapidly restores corneal transparency and structure within weeks. However, in severe and late-stage dysfunction, stromal and subepithelial scarring often require PKP.
Corneal ectasias and thinning are the next most common primary indication (9.2%) for transplant, despite typically transparent corneas. In ectasia, weakened corneal stroma distorts and creates irregular refractive errors that cannot be corrected with lenses or refractive surgery. Corneal thinning is frequently comorbid and contributes to low structural corneal integrity that may present as ectasias or other irregular refractive errors. In severe cases, flexible deformation of the cornea results in rupture of Bowman’s layer, Descemet’s membrane, and corneal scarring. Among these disorders, the most common is keratoconus, a multifactorial progressive thinning and steepening of the cornea.29 It has associations with connective tissue disorders and atopy, potentially because of chronic microscopic trauma from eye rubbing. Pellucid marginal corneal degeneration is a more uncommon diagnosis in which a band of cornea near the limbus develops thin stroma and may have a disrupted Bowman’s membrane or mucopolysaccharide or lipid deposits.30 Refractive surgeries are another rare cause of ectasia and thinning, resulting from the excision or ablation of corneal stroma, thus weakening it.31 Collagen cross-linking, refractive correction, and contact lenses may stabilize and correct refractive errors in mild cases, but transplant is the definitive treatment for advanced pathology. For these indications, only the stroma requires transplantation because the endothelium is typically functional and intact and DALK procedures may be adequate, although PKP is often used to replace the entire cornea.
Other indications for corneal transplantation include corneal scarring, ulceration, and perforation.4 The epithelium and endothelium may be dysfunctional within these conditions, but it is typically the stroma that is damaged beyond repair and must be replaced. Corneal ulcerations occur when an inflammatory or infectious infiltrate destroys the corneal stroma, often with surrounding corneal edema. Ulcerations may progress to full-thickness perforations that risk destruction of the entire eye. Similar, albeit nonprogressive‚ injuries may occur either by chemical or mechanical trauma. Corneal opacities such as stromal scarring, fibrosis, and neovascularization may result after initial treatment and require transplant. Most transplants are performed for inflammatory conditions (2%), whereas infectious keratitis (1%) and trauma (1%) make up the remainder of corneal transplants worldwide. Notably, viral keratitis, typically from the herpes family, is particularly predisposed to stromal scarring and may recur after transplant in 20% of patients.32
CORNEAL TRANSPLANT PROCEDURES
Surgery is often performed under general anesthesia, which produces the desired analgesia and akinesia. However, it can be done under local anesthetic with retrobulbar (behind the eye), peribulbar (beside the extraocular muscles), and sub-Tenon’s block (in the space immediately outside the sclera) or infrequently under only topical anesthesia.33 Sterile precautions are taken, and some surgeries may require a combination of topical, injected, or systemic antibiotic prophylaxis.34 After surgery, steroid and antibiotic drops are continued to minimize rejection, corneal edema, and infection risk.35
PKP is based on the original procedure performed by Eduard Zirm in 1905, wherein a full-thickness disc of the diseased cornea is excised via trephination, corneal scissors, or femtosecond laser and replaced with a matching full-thickness disc from a donor cornea.36 The grafted tissue consists of all corneal layers, from epithelium to endothelium (Figure 2A), and is sutured onto the donor eye with radial sutures that are serially removed during follow-up. This procedure is relatively straightforward and can be used for any indication, including stromal and endothelial disease. Cases of deep stromal scarring and fungal ulcers resistant to medical therapy are predominantly treated with PKP because the entire corneal stroma must be removed.37 Optical outcomes after PKP are sometimes complicated by astigmatism in 10% of cases. The risk of endothelial cell loss and rejection, however, is high.4 Up to 40% of donor endothelial cells are lost during surgery, and the 5-y risk of acute rejection (stromal or endothelial) is 20%.38 Because of the weakened donor-recipient stromal junction after surgery and the nearly full-thickness sutures used, complications including endophthalmitis, choroidal hemorrhage, and graft dehiscence are more common after PKP than for other corneal transplants.
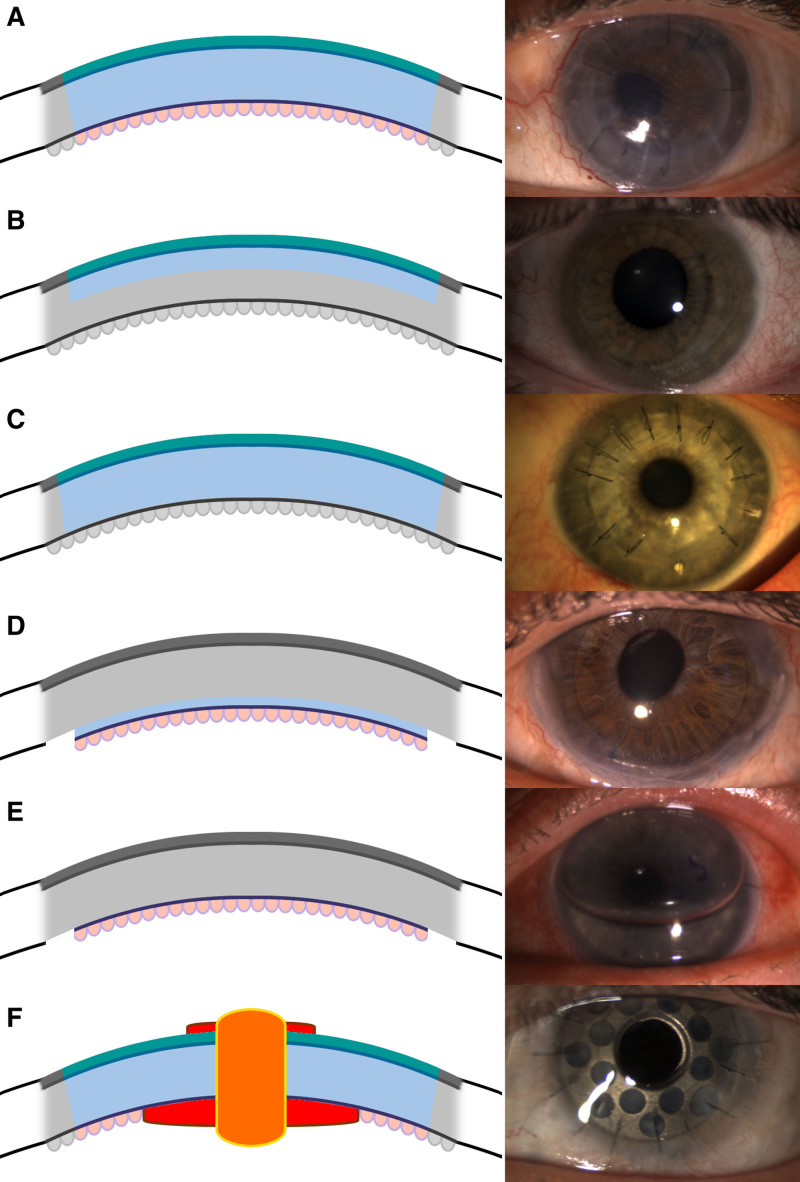
FIGURE 2.
Cross sections and photos of corneal transplant procedures. Transplanted tissue is colored, and the recipient eye is grayed out in schematics. A, PKP replaces the entire corneal thickness, with a photo several months after transplant with several sutures removed. B, ALK replaces the anterior stroma, Bowman’s membrane, and epithelium. Photograph shows an intact graft with all sutures removed several months after transplant. C, DALK replaces the entire stroma, Bowman’s membrane, and epithelium while leaving Descemet’s membrane intact. Photograph shows an intact graft several months after transplant with selected sutures removed to correct astigmatism. D, DSEK replaces Descemet’s membrane and endothelium, supported by a thin layer of donor stroma. E, DMEK replaces Descemet’s membrane and endothelium without any donor stroma. An air bubble is used to flatten the graft onto the cornea, and an “S” is used to orient the graft. F, Keratoprosthesis replaces the entire corneal thickness, with artificial optical surfaces displayed in red on the schematic. ALK, anterior lamellar keratoplasty; DALK, deep anterior lamellar keratoplasty; DMEK, Descemet membrane endothelial keratoplasty; DSEK, Descemet stripping endothelial keratoplasty; PKP, penetrating keratoplasty.
Anterior lamellar keratoplasty (ALK) replaces the anterior layers of the cornea-sparing Descemet’s membrane and the deep stroma (Figure 2B).39 In this procedure, a trephine is used to cut a circle into the anterior stroma, after which the anterior stroma is excised by a spatulated blade or femtosecond laser. The donor cornea must then be cut with a microkeratome to create an equivalent flap that is then placed on the exposed recipient stroma and sutured in place with partial-thickness sutures such that incisions never penetrate Descemet’s membrane. DALK is a variant of ALK in which the entire stroma is replaced, leaving only the recipient Descemet’s membrane and endothelium intact (Figure 2C). With DALK, air bubbles are injected directly between the stroma and Descemet membrane to separate these layers and allow the stroma to be removed without penetrating the Descemet membrane and protecting the endothelium.40 Despite protection, Descemet’s membrane is thin and easily perforated, in which case the surgery must be converted to PKP.41 Indications for ALK include any scenario in which the diseased tissue is limited to the stroma and anterior, such as scarring and ectasia. In ALK, preservation of Descemet’s membrane eliminates endothelial rejection, has minimal risk of posterior fibrous membrane formation, and reduces loss of endothelial cells to ≤15% during surgery. Stromal rejection is also reduced or visually insignificant but still occurs in 1% to 2% of cases. Complications of endophthalmitis and choroidal hemorrhage are rare with ALK and DALK, but astigmatism and graft dehiscence are as common as in PKP because the donor–recipient stromal interface is similarly weakened.42
Another form of lamellar keratoplasty, endothelial keratoplasty (EK), aims to replace the endothelial cells and Descemet’s membrane via a posterior approach.43 PKP was once the most widely used procedure, but since 2012, EKs have become the most common form of corneal transplant.8 This procedure requires special instrumentation that is inserted into the eye through a paracentesis to score and remove Descemet’s membrane. An endothelial graft extracted from the donor by the surgeon or the eye bank can then be inserted into the recipient eye, where it is unfolded and positioned by introducing an air or gas bubble into the anterior chamber, which floats and presses the graft onto the posterior stroma. After surgery, patients must lie supine such that the bubble continues to press against and adhere the graft to the stroma. Grafts include a thin layer of stroma in DSEK (Figures 2D and 3A) or contain no stroma in Descemet membrane EK (Figures 2E and 3C). If a microkeratome or femtosecond laser is used to produce the graft, the procedure is considered automated (Descemet stripping automated EK [DSAEK] and Descemet membrane automated EK). Subcategories of DSAEK are defined by the thickness of graft stroma (Figure 3B), including nanothin DSAEK (15–49 µm), ultrathin DSAEK (50–99 µm), thin DSAEK (100–149 µm), and conventional DSAEK (150–250 µm).43 Descemet membrane automated EK may include a rim of donor stromal tissue but none in the central visual axis (Figure 3D). EK is used only to replace diseased endothelium (eg, in Fuch’s dystrophy) and does not replace diseased stroma. In cases in which the patient has healthy stroma, EK does not result in significant astigmatism, choroidal hemorrhage risk, or globe rupture.44 Small surgical incisions heal quickly with low rates of endophthalmitis, similar to outcomes in cataract surgery. The graft can detach in approximately 10% of cases but can be remedied by injection of another air bubble.45 The risk of rejection is lower than that of PKP and theoretically lower among thinner endothelial grafts.46 Overall, endothelial grafts have become more popular because of faster recovery, lower risks, lower rejection rates, and improved visual outcomes.
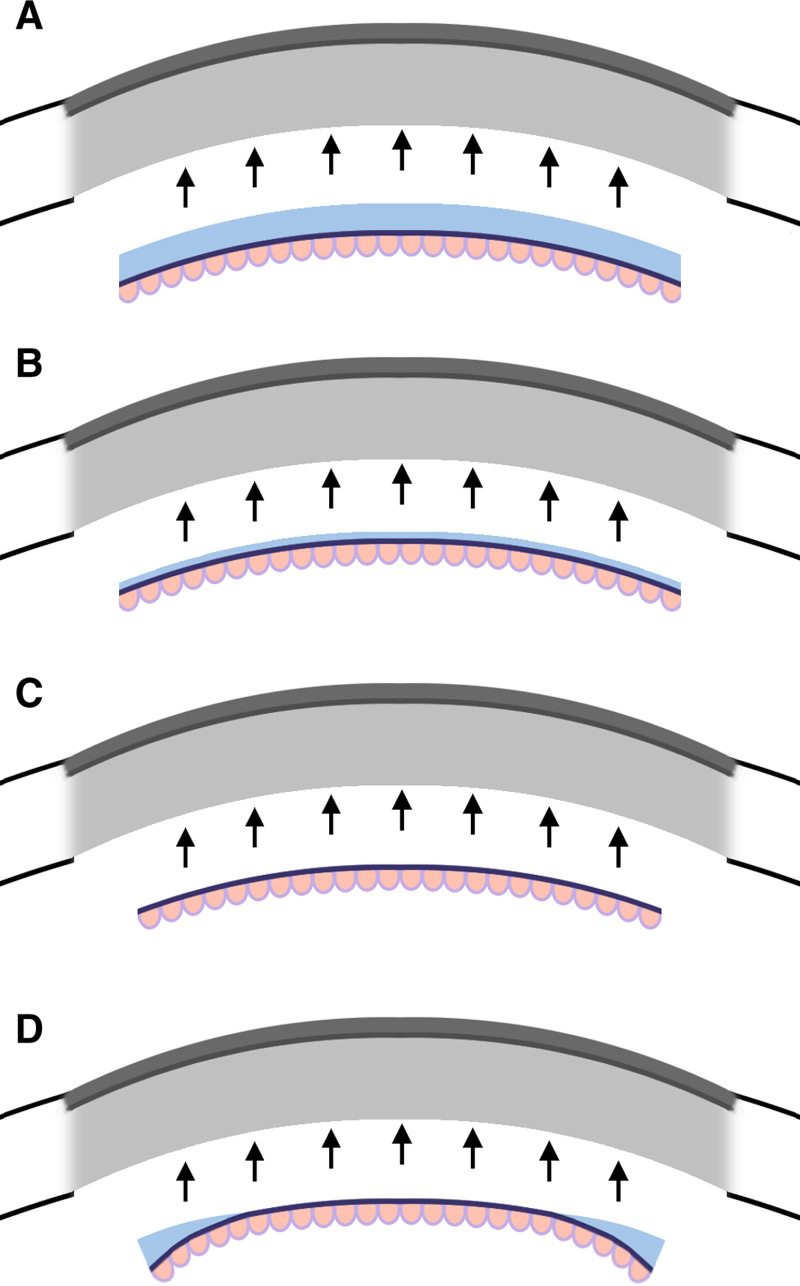
FIGURE 3.
Cross sections comparing automated and traditional endothelial keratoplasty. Transplanted tissue is colored, and the recipient eye is grayed out. A, Traditional DSEK grafts include manually resected layers of stroma, Descemet’s membrane, and endothelium. B, DSAEK grafts are produced by microkeratome or femtolaser incisions and may reduce the stromal thickness compared with DSEK. C, Traditional DMEK grafts include manually resected Descemet’s membrane and endothelium with no stromal tissue. D, DMAEK grafts are produced by microkeratome or femtolaser incisions and may include a peripheral rim of stromal tissue not included in DMEK. DMEK, Descemet membrane endothelial keratoplasty; DMAEK, Descemet membrane automated endothelial keratoplasty; DMEK, Descemet membrane endothelial keratoplasty; DSAEK, Descemet stripping automated endothelial keratoplasty; DSEK, Descemet stripping endothelial keratoplasty.
When optically clear donor cornea is unavailable or likely to result in graft failure, artificial implants such as keratoprostheses can be used.47 The most common design is the Boston type 1 keratoprosthesis consisting of donor cornea sandwiched between 2 plates of inert material and an optical surface replacing the central cornea (Figure 2F).48 This prosthesis then replaces the recipient cornea using a similar method as used in PKP. It carries all the risks and complications of PKP, but the optical surfaces are unaffected by graft rejection. Infections and autoimmune disorders such as herpes simplex keratitis and ocular cicatricial pemphigoid are also indications for keratoprosthesis because donor grafts are likely to fail in these diseases.49 Good visual outcomes can be achieved quickly, but long-term outcomes of keratoprosthetics are complicated by glaucoma, corneal melts, and microbial infection, which can colonize the abiotic prosthetic surfaces. Bioengineered corneas in development aim to resolve these disadvantages of keratoprosthesis and have already been tested in select patients.50
COMPLICATIONS AND REJECTION
Complications related to corneal transplant may occur anywhere from during surgery to years afterward and are listed in Table 2. In the perioperative period, expulsive choroidal hemorrhage is the most catastrophic complication of corneal transplantation, which is thought to occur secondary to sudden decreases in intraocular pressure after globe incision. Most cases occur secondary to PKP (up to 1%) but can occur from any intraocular surgery (0.16%).51 Other risk factors for choroidal hemorrhage include anticoagulant therapy, advanced age, glaucoma, elevated intraocular pressure, infection, and hypertension. Although incidence is low, vision outcomes are devastating and cannot be repaired, often leading to total blindness of the hemorrhaged eye. Endophthalmitis, retinal detachments, and macular edema are other serious postsurgical complications but have decreased in incidence because of the advent of new treatments, such as prophylactic topical antibiotics for preventing endophthalmitis and corneal ulcer.4
TABLE 2.
Complications of corneal transplant
| Complication | Transplant variant | |||
|---|---|---|---|---|
| PKP | ALK | EK | Keratoprosthesis a | |
| Glaucomab | +++ | +++ | +++ | ++++ |
| Cataractb | +++ | +++ | +++ | NA |
| Macular edema | +++ | ++ | ++ | +++ |
| Infectious keratitis | ++ | ++ | + | ++ |
| Astigmatism | +++ | +++ | + | NA |
| Primary graft failurec | + | + | ++ | + |
| Wound dehiscence | ++ | + | + | ++ |
| Graft rejection | +++ | + | +++ | NA |
| Retrocorneal membrane | ++ | – | + | +++ |
| Endophthalmitis | + | – | – | +++ |
| Noninfectious keratitis | + | + | – | ++ |
| Corneal epithelialization | + | + | – | ++ |
| Retinal detachment | + | – | – | ++ |
| Endophthalmitis | + | – | – | ++ |
| Choroidal hemorrhage | + | – | – | + |
Data are compiled from studies cited in this article or estimated by the authors when data are unavailable. Symbols for 10-y incidences are as follows: rarely reported cases (–), <1% (+), 1%–10% (++), 10%–50% (+++), and >50% (++++).
aKeratoprostheses are abiotic and always preceded by cataract extraction, leading to no incidence of cataract, astigmatism, or graft rejection.
bGlaucoma and cataracts are thought to be caused by prolonged corticosteroid treatment after surgery, not as a direct consequence of transplantation.
cRates of primary graft failure after Descemet membrane EK may be higher among inexperienced surgeons.
ALK, anterior lamellar keratoplasty; EK, endothelial keratoplasty; NA, not available; PKP, penetrating keratoplasty.
The most common complication after corneal transplant is wound dehiscence, and cataract or glaucoma also frequently follows longstanding postoperative corticosteroid use in these patients. After PKP, ALK, or DALK, the integrity of the cornea never fully recovers. Traumatic wound dehiscence can occur any time postoperatively, often between 18 wk and 17.5 y, in up to 3% of keratoplasties and may require resuturing or retransplant.52 Traumatic dehiscence is preventable, and recipients are highly encouraged to wear protective eyewear at all times. Cataracts are the second most common complication, occurring in up to 25% of all patients after PKP and to a significantly lesser degree in ALK and EK.53 These can be remedied by cataract extraction and lens implantation, but cataract surgery may damage the endothelium and increase risk of graft opacification. Elevated intraocular pressure and glaucoma are another group of common complications, particularly exacerbated by steroid use after keratoplasty. They are the leading cause of irreversible blindness after transplant, estimated to have an incidence from 11% to 50%, especially after keratoprosthesis.54 Therefore, close follow-up and intraocular pressure monitoring by an ophthalmologist after surgery are required.
Aside from these surgical complications, graft failure can still occur anywhere from immediately postoperatively to years later, whereby the recipient cornea is permanently opacified. The most common causes of failure for each type of corneal transplant are immunologic graft rejection after PKP (34.0%), ocular surface diseases after ALK or DALK (37.8%), nonimmune endothelial decompensation after DSEK (31.9%), and primary graft failure immediately after Descemet membrane EK (64.1%).55
Overall, the most common cause of corneal allograft failure is graft rejection, except for ALK and DALK‚ for which rejection is rarer.56 Although the eye is conventionally thought to demonstrate immune privilege, surgery and surgical complications may introduce intraocular antigens and lead to allograft rejection. Trauma, inflammation, or infection can cause neovascularization of the cornea that further increases antigen presentation and risk for rejection. Even when the cornea is not acutely rejected, chronic rejection is thought to lead to the loss of donor endothelial cells and eventual endothelial decompensation. Each ocular surgery increases this risk, and therefore‚ graft failure rate is higher for retransplants (17%) than for the first transplant (6%) within 2 y.57 For this reason, postoperative topical steroid treatment is commonly used to suppress immune responses despite increased risk of cataracts, glaucoma, and infection. In repeat transplants, other medications, such as topical cyclosporine, may be used to prevent rejection. Steroids may eventually be discontinued, with low rejection rates among low-risk transplants such as those for keratoconus.58 However, systemic and topical immunosuppressive therapy after failure of steroid treatment has shown mixed results‚ and there is no consensus on optimal therapy. Instead, these secondary treatments are used as a last resort as an alternative to retransplant.
CONCLUSIONS
Corneal transplant is a common transplant procedure with many thousands of grafts transplanted each year. Professional and public knowledge of corneal transplants is low outside of ophthalmic specialties, which may contribute to the worldwide shortage of donor corneas. Physicians and patients may benefit from a basic introduction to various corneal transplants and an understanding of their risks and benefits. Most patients with corneal blindness may benefit from PKP, but lamellar transplantation may offer superior outcomes depending on patient pathology. Potential postoperative complications should be monitored by an ophthalmologist, but other physicians should be aware of prophylactic medications. Topical immunosuppressants and steroids are the most common and effective choice for preventing and treating rejection episodes, whereas there is little evidence to support the use of systemic immunosuppression. Eye protection is recommended for any corneal transplant recipient. In the future, gene therapy and bioengineered corneas may be useful to reduce the need for transplants and improve rejection outcomes. Physicians should counsel patients on corneal donation, especially if outside the United States, where donor tissue is in short supply.
Footnotes
The authors declare no funding or conflicts of interest.
Y.Z. and T.W. contributed to the literature review and writing of this article. S.S.T. and W.A.S. edited the article. A.A.S. conceived the original idea, edited the article, and supervised this project.
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this article.
REFERENCES
- 1.Zirm E. Successful total keratoplasty. Graefes Arch Klin Exp Ophthalmol. 1906;64:581. [Google Scholar]
- 2.Albert DM, Edwards DD. The History of Ophthalmology. Vol 7. Blackwell Science Cambridge; 1996. [Google Scholar]
- 3.Lee SH, Cortina MS, Cruz Jdl. History of the artificial cornea. In: Keratoprostheses and Artificial Corneas. Springer; 2015:13–16. [Google Scholar]
- 4.Tan DTH, Dart JKG, Holland EJ, et al. Corneal transplantation. Lancet. 2012;379:1749–1761. [DOI] [PubMed] [Google Scholar]
- 5.George AJT, Larkin DFP. Corneal transplantation: the forgotten graft. Am J Transplant. 2004;4:678–685. [DOI] [PubMed] [Google Scholar]
- 6.Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167–173. [DOI] [PubMed] [Google Scholar]
- 7.Israni AK, Zaun D, Rosendale JD, et al. OPTN/SRTR 2019 annual data report: deceased organ donors. Am J Transplant. 2021;21:567–604. [DOI] [PubMed] [Google Scholar]
- 8.2019 Eye Banking Statistical Report. 2020. Available at https://restoresight.org/wp-content/uploads/2020/04/2019-EBAA-Stat-Report-FINAL.pdf. Accessed May 8, 2022.
- 9.Passweg JR, Baldomero H, Chabannon C, et al. ; European Society for Blood and Marrow Transplantation (EBMT). Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021;56:1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodge C, Miles K, Chan A, et al. Organ and tissue donation: a review of Google trends. Int J Eye Bank. 2021;9;1–7. [Google Scholar]
- 11.Cost-benefit analysis of corneal transplant. 2013. Available at https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/EBAARpt.pdf. Accessed May 17, 2022.
- 12.Bentley TS, Hanson SG. 2017 US organ and tissue transplant cost estimates and discussion. Milliman Research Report; 2017:1–20. [Google Scholar]
- 13.Roussy JPF, Aubin MJ, Brunette I, et al. Cost of corneal transplantation for the Quebec health care system. Can J Ophthalmol. 2009;44:36–41. [DOI] [PubMed] [Google Scholar]
- 14.Bose S, Ang M, Mehta JS, et al. Cost-effectiveness of Descemet’s stripping endothelial keratoplasty versus penetrating keratoplasty. Ophthalmology. 2013;120:464–470. [DOI] [PubMed] [Google Scholar]
- 15.Jindal RM, Waller SG, Sugrim S, et al. Microeconomic benefit of corneal transplantation in a developing country via public–private partnership model. World J Surg. 2018;42:3482–3492. [DOI] [PubMed] [Google Scholar]
- 16.DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–598. [DOI] [PubMed] [Google Scholar]
- 17.Mathews PM, Lindsley K, Aldave AJ, et al. Etiology of global corneal blindness and current practices of corneal transplantation: a focused review. Cornea. 2018;37:1198–1203. [DOI] [PubMed] [Google Scholar]
- 18.Hori J. Mechanisms of immune privilege in the anterior segment of the eye: what we learn from corneal transplantation. J Ocul Bio Dis Info. 2008;1:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masterton S, Ahearne M. Mechanobiology of the corneal epithelium. Exp Eye Res. 2018;177:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanoff M, Duker JS. Ophthalmology E-Book. Elsevier Health Sciences; 2018. [Google Scholar]
- 21.Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66:190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dua HS, Faraj LA, Said DG, et al. Human corneal anatomy redefined: a novel pre-Descemet’s layer (Dua’s layer). Ophthalmology. 2013;120:1778–1785. [DOI] [PubMed] [Google Scholar]
- 23.Sherrard ES, Novakovic P, Speedwell L. Age-related changes of the corneal endothelium and stroma as seen in vivo by specular microscopy. Eye. 1987;1:197–203. [DOI] [PubMed] [Google Scholar]
- 24.Meek KM, Knupp C. Corneal structure and transparency. Prog Retin Eye Res. 2015;49:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea. 1988;7:2–18. [PubMed] [Google Scholar]
- 26.Krachmer JH. Posterior polymorphous corneal dystrophy: a disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans Am Ophthalmol Soc. 1985;83:413–475. [PMC free article] [PubMed] [Google Scholar]
- 27.Ehlers N, Módis L, Møller-Pedersen T. A morphological and functional study of congenital hereditary endothelial dystrophy. Acta Ophthalmol Scand. 1998;76:314–318. [DOI] [PubMed] [Google Scholar]
- 28.Narayanan R, Gaster RN, Kenney MC. Pseudophakic corneal edema: a review of mechanisms and treatments. Cornea. 2006;25:993–1004. [DOI] [PubMed] [Google Scholar]
- 29.Rabinowitz Y.Keratoconus. Surv Ophthalmol. 1998;42:297–319. [DOI] [PubMed] [Google Scholar]
- 30.Krachmer JH. Pellucid marginal corneal degeneration. Arch Ophthalmol. 1978;96:1217–1221. [DOI] [PubMed] [Google Scholar]
- 31.Santhiago MR, Giacomin NT, Smadja D, et al. Ectasia risk factors in refractive surgery. Clin Ophthalmol. 2016;10:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen EJ, Laibson PR, Arentsen JJ. Corneal transplantation for herpes simplex keratitis. Am J Ophthalmol. 1983;95:645–650. [DOI] [PubMed] [Google Scholar]
- 33.Mavrakanas NA, Stathopoulos C, Schutz JS. Are ocular injection anesthetic blocks obsolete? Indications and guidelines. Curr Opin Ophthalmol. 2011;22:58–63. [DOI] [PubMed] [Google Scholar]
- 34.Keyhani K, Seedor JA, Shah MK, et al. The incidence of fungal keratitis and endophthalmitis following penetrating keratoplasty. Cornea. 2005;24:288–291. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen NX, Seitz B, Martus P, et al. Long-term topical steroid treatment improves graft survival following normal-risk penetrating keratoplasty. Am J Ophthalmol. 2007;144:318–319. [DOI] [PubMed] [Google Scholar]
- 36.Rahman I, Carley F, Hillarby C, et al. Penetrating keratoplasty: indications, outcomes, and complications. Eye. 2009;23:1288–1294. [DOI] [PubMed] [Google Scholar]
- 37.Ti S-E, Scott JA, Janardhanan P, et al. Therapeutic keratoplasty for advanced suppurative keratitis. Am J Ophthalmol. 2007;143:755–762. [DOI] [PubMed] [Google Scholar]
- 38.Williams KA, Lowe M, Bartlett C, et al. ; All Contributors. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation. 2008;86:1720–1724. [DOI] [PubMed] [Google Scholar]
- 39.Tan DT, Anshu A. Anterior lamellar keratoplasty: ‘Back to the Future’—a review. Clin Exp Ophthalmol. 2010;38:118–127. [DOI] [PubMed] [Google Scholar]
- 40.Anwar M, Teichmann KD. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet’s membrane. Cornea. 2002;21:374–383. [DOI] [PubMed] [Google Scholar]
- 41.Reinhart WJ, Musch DC, Jacobs DS, et al. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:209–218. [DOI] [PubMed] [Google Scholar]
- 42.Luengo-Gimeno F, Tan DT, Mehta JS. Evolution of deep anterior lamellar keratoplasty (DALK). Ocul Surf. 2011;9:98–110. [DOI] [PubMed] [Google Scholar]
- 43.Dapena I, Ham L, Melles GR. Endothelial keratoplasty: DSEK/DSAEK or DMEK—the thinner the better? Curr Opin Ophthalmol. 2009;20:299–307. [DOI] [PubMed] [Google Scholar]
- 44.Price MO, Gorovoy M, Benetz BA, et al. Descemet’s stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the cornea donor study. Ophthalmology. 2010;117:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terry MA. Endothelial keratoplasty: history, current state, and future directions. Cornea. 2006;25:873–878. [DOI] [PubMed] [Google Scholar]
- 46.Woo JH, Ang M, Htoon HM, et al. Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2019;207:288–303. [DOI] [PubMed] [Google Scholar]
- 47.Chen M, Ng SM, Akpek EK, et al. Artificial corneas versus donor corneas for repeat corneal transplants. Cochrane Database Syst Rev. 2020; 5:CD009561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dohlman CH, Harissi-Dagher M, Graney J. The Boston keratoprosthesis: a new threadless design. Digit J Ophthalmol. 2007;13. [Google Scholar]
- 49.Colby KA, Koo EB. Expanding indications for the Boston keratoprosthesis. Curr Opin Ophthalmol. 2011;22:267–273. [DOI] [PubMed] [Google Scholar]
- 50.Ruberti JW, Zieske JD. Prelude to corneal tissue engineering—gaining control of collagen organization. Prog Retin Eye Res. 2008;27:549–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bandivadekar P, Gupta S, Sharma N. Intraoperative suprachoroidal hemorrhage after penetrating keratoplasty: case series and review of literature. Cont Lens. 2016;42:206–210. [DOI] [PubMed] [Google Scholar]
- 52.Jafarinasab M-R, Feizi S, Esfandiari H, et al. Traumatic wound dehiscence following corneal transplantation. J Ophthalmic Vis Res. 2012;7:214–218. [PMC free article] [PubMed] [Google Scholar]
- 53.Rathi VM, Krishnamachary M, Gupta S. Cataract formation after penetrating keratoplasty. J Refract Surg. 1997;23:562–564. [DOI] [PubMed] [Google Scholar]
- 54.Haddadin RI, Chodosh J. Corneal transplantation and glaucoma. Semin Ophthalmol. 2014;29:380–396. [DOI] [PubMed] [Google Scholar]
- 55.Gómez-Benlloch A, Montesel A, Pareja-Aricò L, et al. Causes of corneal transplant failure: a multicentric study. Acta Ophthalmol. 2021;99:e922–e928. [DOI] [PubMed] [Google Scholar]
- 56.Mahabadi N, Czyz CN, Tariq M, et al. Corneal Graft Rejection. 2018. [PubMed] [Google Scholar]
- 57.Armitage WJ, Goodchild C, Griffin MD, et al. High-risk corneal transplantation: recent developments and future possibilities. Transplantation. 2019;103:2468–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klebe S, Coster DJ, Williams KA. Rejection and acceptance of corneal allografts. Curr Opin Organ Transplant. 2009;14:4–9. [DOI] [PubMed] [Google Scholar]