Abstract
To investigate the mechanisms underlying superantigen (SAg) stimulation, we analyzed the effect of SAg on monocyte responses with or without lipopolysaccharide (LPS). Addition of gamma interferon (IFN-γ) to unstimulated cultures induced a marked increase in the number of CD80+ monocytes, which was inhibited by LPS through the action of interleukin-10. However, CD80+ monocytes began to increase before IFN-γ production, observed after 9 h of stimulation with staphylococcal enterotoxin B (SEB). SEB selectively increased the number of apoptotic CD80− monocytes, whereas LPS-treated monocytes were resistant to the apoptotic action of SEB. This SEB-induced killing was abrogated by anti-CD95 monoclonal antibody (MAb) ZB4 and anti-CD95 ligand (CD95L) MAb NOK2, suggesting a CD95-based pathway of apoptosis. Furthermore, the numbers of SEB-induced CD80+ monocytes were partially decreased by anti-CD119 (IFN-γ receptor) MAb and by anti-CD95L (NOK2) MAb. The CD30 expression of CD27high T cells induced by SEB was increased by agonistic anti-CD95 (CH11) MAb. Together, our findings showed that SEB-induced monocyte apoptosis is closely associated with the enrichment of CD80+ monocytes generated before IFN-γ production, followed by up-regulation of CD80 by IFN-γ, and that LPS has negative effects in both cases. These results also suggested that induction of monocyte apoptosis is an important mechanism by which SAg exerts its anti-inflammatory effects.
Activation of T cells is initiated by the interaction between T-cell receptor (TCR) on T cells and bacterial superantigen (SAg) bound to class II major histocompatibility complex on monocytes (13, 16). Previously, we reported evidence for SAg-mediated modulatory effects on the human immune response characterized by signaling molecules (CD27 and CD30) on SAg-activated T cells (12). CD27 and CD30 are lymphocyte-specific members of the tumor necrosis factor (TNF) receptor superfamily (10, 22). Interactions between accessory molecules on T cells and their ligands on monocytes can play critical roles in determining the T-cell response that occurs following TCR stimulation with SAg (17). CD28 and CD152 (CTLA-4) are known to be important for receptors on T cells that bind two related ligands, CD80 and CD86, on monocytes (8, 9). While CD28 can provide important costimulatory signals for T-cell activation, CD152 functions to down-regulate T-cell activities (3). SAg was shown to activate T cells (CD27high CD30+) via mainly CD28-CD80 interaction in addition to TCR-class II major histocompatibility complex interaction (12, 17).
Lipopolysaccharide (LPS) is the major component of the outer membrane of gram-negative bacteria and mediates a number of biological processes, including modulation of T-cell functions (12, 18). We showed that LPS selectively down-regulates CD27high CD30+ T cells induced by SAg in a dose-dependent manner, attributable to failure of cell-cell interaction of responding T cells in the peripheral blood with viable accessory monocytes, indicating a role of costimulatory interactions strongly associated with CD28 interaction with CD80 (12). That is, the decrease in number of SAg-induced CD80+ monocytes 1 day after addition of LPS resulted in insufficient delivery of positive signals to T cells via CD28-CD80 interaction. Subsequently, when CD152 appeared late (2 days) on activated T cells, the number of CD80+ monocytes recovered upon LPS treatment. Therefore, significant levels of expression of CD152 on activated T cells (CD30+) might have resulted in these cells being much more sensitive to CD152-dependent down-regulation of CD27high CD30+ T cells. These results also suggested that the LPS released from the gram-negative flora of the host's gastrointestinal tract or oral cavity under physiological conditions may induce down-regulation of functional receptors expressed by SAg-activated T cells, leading to the inability of T cells to respond to overactivation signals via the CD27-CD27 ligand (CD27L) (CD70) or CD30-CD30L (CD153) interaction (10, 22).
We further investigated the regulation of induction of CD80 molecules on human monocytes by SAg and/or LPS. The induction of CD80 is up-regulated by exogenous gamma interferon (IFN-γ), but the time course of IFN-γ production on staphylococcal enterotoxin B (SEB) stimulation is different from those of the generation of CD80+ monocytes. Furthermore, before IFN-γ production, CD80 is selectively enriched by increased monocyte apoptosis by SEB via a CD95 (Fas)-based pathway, which is inhibited by LPS or cytokines secreted from monocytes upon stimulation with LPS. Their mode of action provides valuable insight into the constant struggle between microbes and the immune system.
MATERIALS AND METHODS
MAbs and reagents.
For surface markers, anti-CD3 (Leu-4) and anti-CD80 (L307.4) monoclonal antibodies (MAbs) were purchased from Becton Dickinson (Mountain View, Calif.). Anti-CD14 (IOM2 [Immunotech, Marseille, France] and MY4 [Coulter, Hialeah, Fla.]) MAbs were purchased from the sources shown. The MAbs against CD86 (IT2.2) and anti-CD95 (DX2) were obtained from PharMingen (San Diego, Calif.). Anti-CD27 (M-T271) and anti-CD30 (Ki-1) MAbs were purchased from Ancell Co. (Bayport, Minn.). Fluorochrome-conjugated goat anti-mouse immunoglobulin G's (IgGs) were obtained from Southern Biotechnology Associates (Birmingham, Ala.). SEB, toxic shock syndrome toxin 1 (TSST-1), and LPS (from Escherichia coli O55:B5) were purchased from Sigma Chemical Co. (St. Louis, Mo.). SEB purified by a combination of ion-exchange chromatography and gel filtration was also used. Recombinant human TNF-α, IFN-γ, interleukin-1β (IL-1β), IL-4, and IL-10 were supplied by R & D systems (Minneapolis, Minn.). Neutralizing or blocking anti-IL-12 (C8.6), anti-CD119 (IFN-γ receptor α chain) (Genzyme, Cambridge, Mass.), anti-CD95 (ZB4; Coulter), and anti-CD95L (NOK2; PharMingen) MAbs and control mouse IgGs (IgG1 and IgG2a) and IgM (PharMingen) were purchased from the sources shown. Agonistic anti-CD95 (CH11) MAb was obtained from Coulter. Purified SPM-2 (Streptococcus pyogenes mitogen 2) was isolated from culture supernatants of S. pyogenes type 12 (ATCC 12353) as described previously (20).
Cell isolation and activation.
Peripheral blood mononuclear cells (PBMC) were isolated by Lympholyte-H (Cedarlane Laboratories, Hornby, Ontario, Canada) density centrifugation of heparinized blood from healthy volunteers. PBMC were plated at 2 × 105 cells/well in 96-well plates (Falcon; Becton Dickinson) in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum. In some cases, monocytes were enriched by 90-min adherence to culture plates and washed twice with phosphate-buffered saline. Cells were then incubated with various stimuli (SEB, TSST-1, SPM-2, CH11 MAb, and/or LPS, each at 0.1 to 1 μg/ml) or various cytokines (TNF-α, 10 ng/ml; IFN-γ, 100 U/ml; IL-1β, 10 ng/ml; IL-4, 10 ng/ml; IL-10, 10 ng/ml) for 18 h before assay of CD80+ monocytes or up to the indicated time points to examine the time course of CD80 induction or for 72 h before assay of CD27 induction on T cells. Blocking antibodies (anti-IL-12, anti-CD95, anti-CD95L, and anti-CD119 MAbs) or control antibody of appropriate IgG isotype or IgM were added at a concentration of 1 to 10 μg/ml 1 h before stimulation.
Monocyte apoptosis.
Staining of monocytes with annexin V and propidium iodide (PI) was performed using an apoptosis detection kit (Trevigen Inc., Gaithersburg, Md.) in accordance with the manufacturer's instructions to quantitatively determine the percentage of cells undergoing apoptosis. Briefly, cells cultured for 3 h were washed with phosphate-buffered saline and resuspended in the binding buffer provided. Phycoerythrin (PE)-conjugated anti-CD14 (IOM2) MAb (30 min) and fluorescein isothiocyanate (FITC)-conjugated annexin V (15 min) were incubated with monocytes in the dark. The monocyte population was selected by gating of CD14-positive cells and analyzed for apoptosis by flow cytometry. Monocyte death induced by different agents (after 18 h) was determined by PI uptake of cells stained with FITC-conjugated anti-CD14 (MY4) MAb.
Measurement of cytokines.
IL-1β, IL-4, IL-10, TNF-α, and IFN-γ were determined in supernatants of cultured cells for defined periods using enzyme-linked immunosorbent assays (ELISAs) developed by Endogen Inc. (Woburn, Mass.). Assays were performed according to the manufacturer's specifications. All samples were assayed in triplicate. The lower limits of detection, as determined using standard curves, were set as follows: for IFN-γ, IL-1β, IL-10, and TNF-α, <20 pg/ml; for IL-4, <10 pg/ml. Standard cytokine preparations (defined concentration of recombinant cytokine) were used as internal controls in all tests.
Flow cytometry.
The methods used for immunofluorescence staining in conjunction with single- or two-color flow cytometry and for counting of fluorescein-positive cells were described previously (1). For triple staining, cells were incubated with FITC-, PE-, and peridinin chlorophyll protein-conjugated MAbs at saturating concentrations at 4°C for 30 min and then washed twice. Cells were then analyzed using a FACScalibur (Becton Dickinson). Fluorochrome-conjugated murine IgG isotypes of unrelated specificities were used as controls. Gates were set around the monocyte or lymphocyte populations, and a total of 20,000 cells/sample were analyzed.
Statistical analysis.
Data are given as means ± standard deviations (SD). Significance between the control group and a treated group was examined with the unpaired Student's t test. P values less than 0.05 were regarded as significant.
RESULTS
Time course of induction of CD80+ monocytes by SEB.
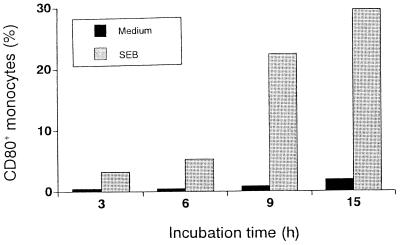
To investigate the time course of CD80 induction, PBMC were stimulated with SEB. At the indicated time points, cells were harvested and stained with FITC-conjugated anti-CD14 and PE-conjugated anti-CD80 MAbs. Representative results of surface expression analysis are shown in Fig. 1. The expression of CD80 was induced after 3 h of stimulation and peaked after 15 h of stimulation. The increase in number of CD80+ monocytes was accompanied by an increase in fluorescence intensity (data not shown). A slight increase in medium alone was observed after 15 h of culture.
FIG. 1.
Time course of induction of CD80+ monocytes by SEB. PBMC (106/ml) were cultured with SEB (1 μg/ml) for 3, 6, 9, or 15 h, and harvested at the indicated time points. After staining with FITC-conjugated anti-CD14 MAb and PE-conjugated anti-CD80 MAb, a total of 2 × 104 monocytes were analyzed to determine the CD80+ population. A representative result from three different donors is shown.
Effects of LPS on SAg-induced CD80+ monocytes.
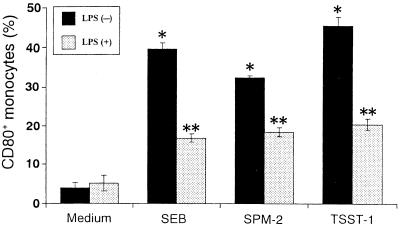
Variations by SAg and/or LPS in the percentages of CD80+ monocytes were assessed by evaluating approximately 2 × 104 monocytes. Figure 2 shows the effects of SAg and LPS on the induction of CD80+ monocytes. After 18-h exposure of PBMC to 1 μg of SEB, SPM-2, or TSST-1 per ml, the percentages of CD80+ monocytes in CD14+ populations increased to 39.7% (SEB), 32.5% (SPM-2), or 45.6% (TSST-1) compared with the untreated control (4.0%). However, LPS at 1 μg/ml in cultures with SAg decreased CD80+ monocytes to 42% (SEB), 56% (SPM-2), or 44% (TSST-1) of the value with SAg alone, whereas no significant effect was observed in monocytes treated with LPS alone (Fig. 2).
FIG. 2.
Effects of LPS on SAg-induced CD80+ monocytes. PBMC were stimulated with SEB, SPM-2, or TSST-1 (1 μg/ml) with or without LPS (1 μg/ml) for 18 h. After staining with FITC-conjugated anti-CD14 MAb and PE-conjugated anti-CD80 MAb, cells were analyzed to determine the CD80+ population. Percentages of CD80+ monocytes are expressed as means ± SD of three different donors. ∗, P < 0.01 versus medium control; ∗∗, P < 0.01 versus SAg treatment without LPS.
Cytokine production after treatment with SAg or LPS.
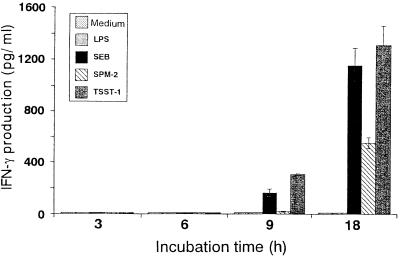
To examine the influence of SAg or LPS on cytokine production, we performed ELISAs at intervals of 3 h in culture supernatants from the same donor as in Fig. 1. PBMC were incubated with SAg or LPS for up to 18 h. As shown in Fig. 3, no increase in IFN-γ production was evident at early incubation time points in cultures treated with SAg, and an increase was observed at 9 h of stimulation. In SPM-2-stimulated cultures, low levels of IFN-γ were observed at 18 h of stimulation compared with SEB and TSST-1 stimulation. Significant levels of IL-1β, IL-10, and TNF-α production were observed in cultures stimulated with LPS, but IL-4 under these conditions was only minimally secreted in response to SEB after 18 h of stimulation (Table 1).
FIG. 3.
Time course of IFN-γ production by SAg. PBMC from the same donor as in Fig. 1 were cultured with SAg (1 μg/ml) or LPS (1 μg/ml) for 3, 6, 9, or 18 h. IFN-γ contents of the supernatants were determined by ELISA. All samples were assayed in triplicate, and the results are expressed as means ± SD.
TABLE 1.
Effects of SEB and LPS on cytokine production
| Treatmenta | Cytokine production (pg/ml)b
|
|||
|---|---|---|---|---|
| IL-4 | IL-1β | IL-10 | TNF-α | |
| None | <10 | <20 | <20 | <20 |
| LPS | <10 | 1,013 ± 28 | 549 ± 55 | 1,534 ± 74 |
| SEB | 16 ± 1 | 49 ± 2 | 171 ± 11 | 132 ± 4 |
PBMC (106/ml) were cultured with SEB (1 μg/ml) or LPS (1 μg/ml) for defined periods.
IL-4 (18 h), IL-1β (6 h), IL-10 (9 h), and TNF-α (6 h) contents of the supernatants were determined by ELISA. All samples were assayed in triplicate, and the results are expressed as means ± SD.
Effects of IFN-γ on induction of CD80+ monocytes.
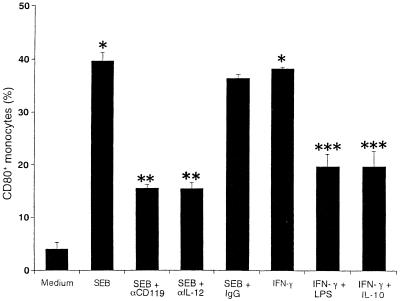
We investigated the roles of cytokines in induction of CD80 on monocytes. PBMC were cultured with IFN-γ, IL-4, IL-10, IL-1β, or TNF-α for 18 h. As shown in Fig. 4, IFN-γ showed significant enhancement of CD80 induction on unstimulated monocytes, and addition of anti-CD119 and anti-IL-12 MAbs 1 h before stimulation partially inhibited SEB-induced CD80+ monocytes. Addition of LPS or IL-10 to PBMC treated with IFN-γ caused a marked decrease in CD80 induction. CD80 inhibition was significantly observed when culture was performed in the presence of IL-4 (24.6% ± 3.1%) but not IL-1β or TNF-α with IFN-γ. CD86 was used as a control, and its expression was not significantly altered by these treatments (data not shown).
FIG. 4.
Effects of IFN-γ on induction of CD80+ monocytes. PBMC were cultured with anti-CD119 MAb (10 μg/ml), anti-IL-12 MAb (10 μg/ml), or control mouse IgG1 (10 μg/ml) before 1 h of SEB stimulation (1 μg/ml) or with IFN-γ (100 U/ml) in combination with LPS (1 μg/ml) or IL-10 (10 ng/ml). After 18 h of stimulation, cells were stained with FITC-conjugated anti-CD14 MAb and PE-conjugated anti-CD80 MAb. Percentages of CD80+ monocytes are expressed as means ± SD of three different donors. ∗, P < 0.01 versus medium control; ∗∗, P < 0.01 versus SEB treatment; ∗∗∗, P < 0.01 versus IFN-γ treatment.
SEB-induced monocyte apoptosis.
We observed that exogenous IFN-γ up-regulates CD80+ monocytes. However, there was a discrepancy in the time courses of IFN-γ production and CD80 induction by SEB. Monocytes undergo apoptosis when cultured in vitro, and addition of LPS prevents apoptosis for maintenance of viability of these cells (15). We investigated whether SEB enhances monocyte apoptosis, accompanied by enrichment of CD80+ monocytes, and LPS has negative effects on the action of SEB. When analyzed after 6 h of culture without any stimulation, monocytes were consistently found to be positive for CD95 and CD95L expression. We reasoned that if SEB treatment enhances monocyte apoptosis via the CD95-mediated pathway, antagonistic MAb against CD95 or CD95L would inhibit SEB-induced monocyte death.
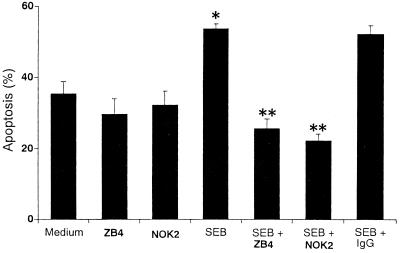
PBMC were pretreated with anti-CD95 (ZB4) or anti-CD95L (NOK2) MAb for 1 h before treatment with SEB. After 3 h of cell culture, monocyte apoptosis was determined by annexin V binding with phosphatidylserine, whereas after longer (1-day) culture, cells with morphological features of apoptosis such as cytoplasm hypervacuolization showed PI staining in the monocyte cultures (15). T cells were resistant to apoptosis 1 day after SEB stimulation (data not shown). However, in the absence of any exogenous stimuli, the percentage of annexin V+ monocytes was 35.5%. The incidence of SEB-induced apoptosis, 53.7%, was reduced to 25.6 or 22.3% in monocytes treated with anti-CD95 or anti-CD95L MAb, respectively (Fig. 5). Although cells could express phosphatidylserine without being committed to die, SEB increased the number of PI+ monocytes after 18 h. LPS abrogated the apoptotic effect of SEB, whereas no significant changes in PI staining were observed in monocytes treated with LPS compared with untreated controls (data not shown).
FIG. 5.
Effects of antagonistic MAbs on SEB-induced apoptosis of monocytes. PBMC were pretreated with anti-CD95 MAb (ZB4, 1 μg/ml), anti-CD95L MAb (NOK2, 1 μg/ml) or control mouse IgG (IgG1 plus IgG2a, 1 μg/ml) before 1 h of stimulation with SEB (1 μg/ml). For binding with annexin V, cells were harvested after 3 h of stimulation, washed, and then stained for 30 min with PE-conjugated anti-CD14 MAb and for 15 min with FITC-conjugated annexin V. The percentage of apoptotic monocytes was then measured by flow cytometry. The results represent means ± SD of three different donors. ∗, P < 0.01 versus medium control; ∗∗, P < 0.01 versus SEB treatment.
Effects of LPS and cytokines on monocyte apoptosis.
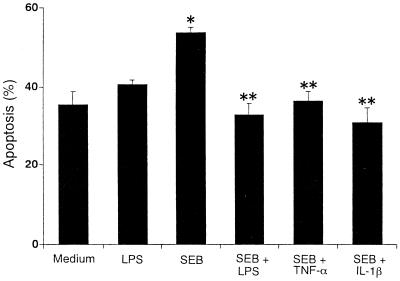
The functional roles of cytokines in monocyte apoptosis during the first 3 h of culture were investigated. PBMC were cultured in the presence of LPS or IL-1β, TNF-α, IFN-γ, IL-4, or IL-10 with SEB. As shown in Fig. 6, LPS had an inhibitory effect on SEB-induced apoptosis. With IL-1β and TNF-α, we also observed an inhibitory effect in SEB-treated monocytes. When IFN-γ, IL-4, and IL-10 were added, no inhibitory changes were seen in the numbers of annexin V+ monocytes compared with SEB-treated cultures (data not shown). These results suggested that LPS and the derived cytokines (IL-1β and TNF-α), when added from the start of culture, have inhibitory effects on monocyte apoptosis caused by SEB.
FIG. 6.
Effects of LPS and cytokines on monocyte apoptosis induced by SEB. PBMC were stimulated with SEB (1 μg/ml) in medium alone or in medium containing IL-1β (10 ng/ml), TNF-α (10 ng/ml), or LPS (1 μg/ml) for 3 h. After staining with PE-conjugated anti-CD14 MAb followed by addition of FITC-conjugated annexin V, the percentage of apoptotic monocytes was measured by flow cytometry. The results represent means ± SD of three different donors. ∗, P < 0.05 versus medium control; ∗∗, P < 0.05 versus SEB treatment.
Enrichment of CD80+ monocytes by apoptosis.
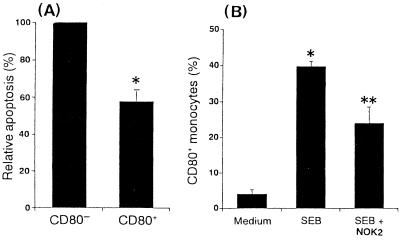
The differences in apoptosis between CD80− and CD80+ monocytes were evaluated by flow cytometry using annexin V, although surface CD95 was expressed at similar levels on CD80− and CD80+ monocytes (mean fluorescence intensity [MFI] ranging from 11.7 to 14.1). Figure 7A shows that CD80− monocytes expressed annexin V binding at high frequency when PBMC were cultured with SEB for 6 h, whereas low binding was observed in the CD80+ monocytes, suggesting that the amount of surface CD95 on monocytes may not have contributed to the occurrence of apoptosis.
FIG. 7.
Enrichment of CD80+ monocytes by apoptosis. (A) PBMC were stimulated with SEB (1 μg/ml). After 6 h, cells were stained with PE-conjugated anti-CD80 MAb and with FITC-conjugated annexin V. The gate was set around the monocyte population. The annexin V+ CD80− monocytes (25.1 to 27.3%) are expressed as 100%. The results represent means ± SD of three different donors. ∗, P < 0.05 versus CD80− cells. (B) After addition of anti-CD95L MAb (NOK2, 1 μg/ml) before 1 h of stimulation with SEB (1 μg/ml), PBMC were stimulated for 18 h and stained with FITC-conjugated anti-CD14 MAb and PE-conjugated anti-CD80 MAb. Percentages of CD80+ monocytes are expressed as means ± SD of three different donors. ∗, P < 0.01 versus medium control; ∗∗, P < 0.01 versus SEB treatment.
To further investigate the effects of incubation with an antiapoptotic MAb on induction of CD80+ monocytes by SEB, PBMC were cultured with SEB and anti-CD95L (NOK2) MAb either alone or in combination. After treatment with anti-CD95L MAb, the level of induction of CD80+ monocytes by SEB was significantly lower than in cells treated with SEB alone (Fig. 7B).
Effects of apoptosis on SEB-activated T cells.
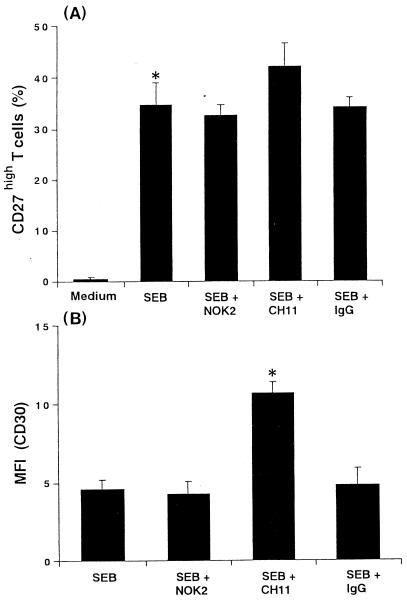
The ability of monocyte apoptosis to regulate SEB-activated T cells that expressed CD27high and CD30 was confirmed by stimulating PBMC with SEB for 72 h in the presence of blocking antibody (NOK2) or stimulating antibody (CH11) and by measuring the cytometric profiles (Fig. 8). Although significant changes in the percentages of CD27high T cells were not observed by treatment with NOK2, T cells treated with CH11 showed a slight increase of CD27high (Fig. 8A). Similarly, there was significant effect of CH11 MAb on the induction of CD30 expression on CD27high T cells, suggesting enhancing effects of monocyte apoptosis on activation of T cells by SEB (Fig. 8B).
FIG. 8.
Effects of apoptosis on SEB-activated T cells. PBMC were pretreated with anti-CD95L MAb (NOK2, 1 μg/ml), anti-CD95 MAb (CH11, 500 ng/ml), or control mouse IgG (IgG2a plus IgM, 1 μg/ml) before 1 h of stimulation with SEB (1 μg/ml). (A) After 72 h, cells were stained with FITC-conjugated anti-CD27 MAb and PE-conjugated anti-CD3 MAb. Percentages of CD27high T cells are expressed as means ± SD of three different donors. ∗, P < 0.01 versus medium control. (B) After triple staining, cells were analyzed on a FACScalibur for CD3, CD27, and CD30 expression. Data represent the MFI of CD30 in the CD27high T cells. The MFI of the negative control antibodies was subtracted, and the results are expressed as means ± SD of three different donors. ∗, P < 0.01 versus SEB treatment.
DISCUSSION
In addition to cytokine induction, stimulation with SEB can lead to monocyte activation, as detected by enhanced induction of surface CD80 expression, and monocyte apoptosis, as detected by enhanced induction of annexin V- and PI-positive cells. Since these responses were very poor in the absence of T cells, purified monocytes were unable to fully substitute for the PBMC population when stimulated with SEB (data not shown). However, our recent findings (12) along with the results presented here indicate that LPS has a negative effect on T-cell activation by decreasing CD80+ monocytes and that LPS suppresses directly or indirectly monocyte apoptosis through intracellular signals that bring about blockade of the activation of caspase-3 (7). Moreover, antiapoptotic MAb (NOK2) influenced the ability of SEB to induce CD80+ monocytes together with inhibition of monocyte apoptosis (Fig. 7B). This apparent coupling of monocyte apoptosis and generation of CD80+ monocytes led to the hypothesis that the early effect exerted by SEB involves selective induction of monocyte apoptosis (Fig. 7A), allowing the enrichment of CD80+ monocytes generated before IFN-γ production, and SEB action proceeds to up-regulation of CD80 by IFN-γ. This is supported by our observation that CD80 expression of THP-1 cells was associated with the selective apoptosis when induced by etoposide (H. Rikiishi and K. Kudo, unpublished observation).
In the presence of SEB, a progressive increase in the number of annexin V-positive monocytes was observed, leading to the appearance of phosphatidylserine on around 50% of the total monocyte population (Fig. 5), suggesting a proper ratio for interaction of residual monocytes with responding T cells bearing appropriate Vβ elements of TCR via CD28 and CD80. We demonstrated diminished induction of SEB-mediated monocyte apoptosis after pretreatment with antagonistic anti-CD95 or anti-CD95L MAb. Therefore, we concluded that there is a CD95-mediated effect of SEB on monocyte apoptosis (11, 26). The lack of complete inhibition by these MAbs may explain why caspase activation appears to be initiated even during cell isolation. Although T cells have been shown to up-regulate CD95L in response to SEB and to be able to delete monocytes through a CD95-mediated mechanism (2, 27), the levels of CD95L on T cells were low even when cultured with SEB. Instead, soluble CD95L (sCD95L) could be efficiently secreted from SEB-activated T cells or monocytes (data not shown). However, sCD95L is thought to be much less cytotoxic than membrane-bound CD95L (19). These results indicated that in addition to the cytotoxicity of sCD95L, the interaction between CD95 and CD95L on monocytes is largely responsible for the SEB induction of apoptosis that occurs upon monocytes. Moreover, we investigated whether these events are affected by the actions of various cytokines induced by SEB or LPS (6, 14, 21). Significant antiapoptotic effects of exogenous IL-1β or TNF-α on SEB-treated monocytes (Fig. 6) implied that LPS inhibition of apoptosis is required for apoptosis-associated modulation via intracellular signals mediated by these cytokines in monocytes (7). It is also possible that LPS directly induces expression of the cytoprotective proteins via a CD14-dependent pathway requiring activation of NF-κB.
We demonstrated that IFN-γ exerts regulatory effects on CD80 induction in monocytes, using anti-CD119 MAb and exogenous IFN-γ (28). Large amounts of IFN-γ were produced from stimulated T cells as a result of CD28-CD80 costimulation (9), showing that CD80+ monocytes generated before IFN-γ production contribute to IFN-γ production via CD28-mediated signals. Since the negative regulation of IFN-γ responses by LPS and IL-10 occurred at least via down-modulation of CD80 expression, it is likely that stimulation with LPS or IL-10 induced by LPS inhibited the induction of CD80+ monocytes through suppression of Stat-1 activation and transcriptional activity in response to IFN-γ (4, 24, 28). IL-4 has antagonistic effects on IFN-γ functions via molecular mechanisms differing from those of IL-10 (5, 25). More recently, it was shown that CD80 expression on T cells is inhibited by IL-4 and enhanced when IL-4 is neutralized or when T cells are unable to respond to the differentiating signals of IL-4 (23). IL-4 showed no modulatory effect on CD80 expression in untreated monocytes, but IL-4 affected induction of CD80 by IFN-γ, supporting the antagonistic effect of IL-4 on IFN-γ function in influencing CD80 expression. However, the modulatory effect of endogenous IL-4 is unclear because IL-4 is only minimally secreted in response to SEB (Table 1).
In summary, T cells become activated by SEB treatment and produce IFN-γ. SEB-mediated signals (sCD95L secretion or modulation of proapoptotic or antiapoptotic factors) enhance selective monocyte apoptosis, which enriches CD80+ monocytes generated before IFN-γ production. Thereafter, IFN-γ can act on monocytes to up-regulate CD80, further enhancing T-cell stimulation and leading to the induction of CD27high CD30+ T cells by SEB. LPS induces production of IL-1β, TNF-α, and IL-10 from monocytes. The intracellular signals mediated by LPS or IL-1β and TNF-α block SEB-mediated apoptosis, and IL-10 induces transient reduction of CD80 that increases after 48 h (12), lending credence to the hypothesis that modulation of apoptosis and CD80+ phenotype in monocytes observed following SEB and/or LPS treatment are tightly regulated by a complex network of signals provided by several factors. Our results also suggested that induction of apoptosis in human monocytes appears to be an important mechanism by which SAg exerts its anti-inflammatory effects and SAg promotes persistence of the organism during colonization and in the early stages of infection. In the absence of LPS, the monocyte apoptosis induced by SAg may regulate cell number at inflammatory sites and reduce the release of toxic mediators into the systemic circulation.
ACKNOWLEDGMENTS
We thank Hideo Igarashi for kindly providing purified SEB, Seijiro Shindo and Tomako Horiguchi for technical assistance, Daniel Mrozek for English editing of the manuscript, and Yuri Togashi for expert editorial assistance.
This work was supported in part by Grant-in-Aid for Scientific Research 12671759 from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- 1.Abo T, Sugawara S, Seki S, Fujii M, Rikiishi H, Takeda K, Kumagai K. Induction of human TCRγδ+ and TCRγδ− CD2+ CD3− double negative lymphocytes by bacterial stimulation. Int Immunol. 1990;2:775–785. doi: 10.1093/intimm/2.8.775. [DOI] [PubMed] [Google Scholar]
- 2.Ashany D, Song X, Lacy E, Nicolic-Zugic J, Friedman S M, Elkon K B. Th1 CD4+ lymphocytes delete activated macrophages through the Fas/APO-1 antigen pathway. Proc Natl Acad Sci USA. 1995;92:11225–11229. doi: 10.1073/pnas.92.24.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulougouris G, Mcleod J D, Patel Y I, Ellwood C N, Walker L S K, Sansom D M. Positive and negative regulation of human T cell activation mediated by the CTLA-4/CD28 ligand CD80. J Immunol. 1998;161:3919–3924. [PubMed] [Google Scholar]
- 4.Ding L, Linsley P S, Huang L Y, Germain R N, Shevach E M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 5.Donnelly R P, Fenton M J, Finbloom D S, Gerrard T L. Differential regulation of IL-1 production in human monocytes by IFN-γ and IL-4. J Immunol. 1990;145:569–575. [PubMed] [Google Scholar]
- 6.Estaquire J, Ameisen J C. A role for T-helper type-1 and type-2 cytokines in the regulation of human monocyte apoptosis. Blood. 1997;90:1618–1625. [PubMed] [Google Scholar]
- 7.Fahy R J, Doseff A I, Wewers M D. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol. 1999;163:1755–1762. [PubMed] [Google Scholar]
- 8.Fargeas C A, Truneh A, Reddy M, Hurle M, Sweet R, Sekaly R P. Identification of residues in the V domain of CD80 (B7–1) implicated in functional interactions with CD28 and CTLA4. J Exp Med. 1995;182:667–675. doi: 10.1084/jem.182.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleisher J, Soeth E, Reiling N, Grage-Griebenow E, Flad H D, Ernst M. Differential expression and function of CD80 (B7–1) and CD86 (B7–2) on human peripheral blood monocytes. Immunology. 1996;89:592–598. doi: 10.1046/j.1365-2567.1996.d01-785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hintzen R Q, de Jong R, Lens S M A, van Lier R A W. CD27: marker and mediator of T-cell activation? Immunol Today. 1994;15:307–311. doi: 10.1016/0167-5699(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 11.Iwai K, Miyawaki T, Takizawa T, Kanno A, Ohta K, Yachie A, Seki H, Taniguchi N. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84:1201–1208. [PubMed] [Google Scholar]
- 12.Kai K, Rikiishi H, Sugawara S, Takahashi M, Takada H, Kumagai K. Lipopolysaccharide-dependent down-regulation of CD27 expression on T cells activated with superantigen. Immunology. 1999;98:289–295. doi: 10.1046/j.1365-2567.1999.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kappler J, Kotzin B, Herron L, Gelfand E, Bigler R D, Boylston A, Carrel S, Posneit C D, Choi Y, Marrack P. Vβ-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244:811–814. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 14.Mangan D F, Robertson B, Wahl S M. IL-4 enhances programmed cell death (apoptosis) in stimulated human monocytes. J Immunol. 1992;148:1812–1816. [PubMed] [Google Scholar]
- 15.Mangan D F, Welch G R, Wahl S M. Lipopolysaccharide, tumor necrosis factor-α, and IL-1β prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991;146:1541–1546. [PubMed] [Google Scholar]
- 16.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 17.Mittrucker H W, Shahinian A, Bouchard D, Kundig T M, Mak T W. Induction of unresponsiveness and impaired T cell expansion by staphylococcal enterotoxin B in CD28-deficient mice. J Exp Med. 1996;183:2481–2488. doi: 10.1084/jem.183.6.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison D C, Ryan J L. Endotoxin and disease mechanism. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 19.Oyaizu N, Kayagaki N, Yagita H, Pahwa S, Ikawa Y. Requirement of cell-cell contact in the induction of Jurkat T cell apoptosis: the membrane-anchored but not soluble form of FasL can trigger anti-CD3-induced apoptosis in Jurkat T cells. Biochem Biophys Res Commun. 1997;238:670–675. doi: 10.1006/bbrc.1997.7357. [DOI] [PubMed] [Google Scholar]
- 20.Rikiishi H, Okamoto S, Sugawara S, Tamura K, Liu Z X, Kumagai K. Superantigenicity of helper T-cell mitogen (SPM-2) isolated from culture supernatants of Streptococcus pyogenes. Immunology. 1997;91:406–413. doi: 10.1046/j.1365-2567.1997.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rink L, Luhm J, Koester M, Kirchner H. Induction of a cytokine network by superantigens with parallel Th1 and Th2 stimulation. J Interferon Cytokine Res. 1996;16:41–47. doi: 10.1089/jir.1996.16.41. [DOI] [PubMed] [Google Scholar]
- 22.Romagnani P, Annunziato F, Romagnani S. Pleiotropic biologic functions of CD30/CD30L does it contribute to negative selection in thymus? Immunologist. 1998;6:137–141. [Google Scholar]
- 23.Schweitzer A N, Sparpe A H. Mutual regulation between B7–1 (CD80) expressed on T cells and IL-4. J Immunol. 1999;163:4819–4825. [PubMed] [Google Scholar]
- 24.Stoiber D, Kovarik P, Cohney S, Johnston J A, Steinlein P, Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-γ. J Immunol. 1999;163:2640–2647. [PubMed] [Google Scholar]
- 25.Takeshita S, Gage J R, Kishimoto T, Vredevoe D L, Martinez-Maza O. Differential regulation of IL-6 gene transcription and expression by IL-4 and IL-10 in human monocytic cell lines. J Immunol. 1996;156:2591–2598. [PubMed] [Google Scholar]
- 26.Um H D, Orenstein J M, Wahl S M. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol. 1996;156:3469–3477. [PubMed] [Google Scholar]
- 27.Wang J K M, Zhu B, Ju S T, Tschopp J, Marshak-Rothstein A. CD4+ T cells reactivated with superantigen are both more sensitive to FasL-mediated killing and express a higher level of FasL. Cell Immunol. 1997;179:153–164. doi: 10.1006/cimm.1997.1159. [DOI] [PubMed] [Google Scholar]
- 28.Willems F, Marchant A, Delville J P, Gerard C, Delvaux A, Velu T, de Boer M, Goldman M. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24:1007–1009. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]