Abstract
Objective Treatment-refractory antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a life-threatening condition without evidence-based treatment options. One emerging treatment option for several antibody-mediated autoimmune diseases is the anti-CD38 antibody daratumumab, which depletes autoantibody-secreting plasma cells.
Methods We treated two patients with severe life-threatening AAV with renal and pulmonary manifestation despite induction therapy with rituximab and cyclophosphamide with four to eight doses of 1800 mg daratumumab. We followed clinical and immunological responses.
Results The first patient with myeloperoxidase-ANCA-positive microscopic polyangiitis had resolution of pneumonitis and pleuritis and stabilisation of kidney function after daratumumab. The second patient with proteinase 3-ANCA-positive granulomatosis with polyangiitis, diffuse alveolar haemorrhage necessitating extracorporeal membrane oxygenation (ECMO) and acute kidney failure, requiring kidney replacement therapy, was weaned off ECMO, mechanical ventilation and dialysis and discharged home after daratumumab. Clinical improvement was paralleled by a strong reduction in serum ANCA levels as well as total IgG, indicating depletion of plasma cells. Apart from the depletion of CD38+ natural killer cells, blood leucocyte levels were not notably influenced by daratumumab. Only mild adverse events, such as hypogammaglobulinaemia and an upper respiratory tract infection occurred.
Conclusion Daratumumab was safe and effective in inducing remission in two patients with severe treatment-refractory AAV, warranting prospective clinical trials to establish safety and efficacy.
Keywords: Treatment, Systemic vasculitis, Autoimmune Diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
Long-lived plasma cells contribute to the pathogenesis of antineutrophil cytoplasmic antibody-associated vasculitis, and the anti-CD38 antibody daratumumab has been used to target long-lived plasma cells in some autoantibody-mediated diseases.
WHAT THIS STUDY ADDS
This study reports two patients with refractory ANCA-associated vasculitis successfully treated with daratumumab.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Daratumumab could be useful in the treatment of ANCA-associated vasculitis, but clinical trials will be needed to establish safety and efficacy.
Background
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of autoimmune diseases mediated by autoantibodies against either myeloperoxidase (MPO) or proteinase 3 (PR3).1 These autoantibodies activate myeloid cells (neutrophils and monocytes) and thus induce small vessel vasculitis, resulting in inflammation and damage of the kidney, lung, skin and other organs.
The standard of care for the treatment of AAV includes induction therapy, a combination of glucocorticoids and either the anti-CD20 antibody rituximab or cyclophosphamide.1 While the induction treatment with rituximab leads to clinical remission in approximately two-thirds of patients,2 some have ongoing disease activity despite complete B cell depletion in the peripheral blood. AAV that shows ongoing active, inflammatory disease despite both first-line induction treatments is associated with significant mortality and persistent morbidity, including chronic kidney failure and pulmonary fibrosis.3 In this situation, no evidence-based treatment options exist.
The failure of standard induction therapies can, in part, be explained by long-lived plasma cells (LLPCs): LLPCs are terminally differentiated, antibody-secreting cells that do not express CD20 and thus are not targeted by rituximab. LLPCs contribute to the pathogenesis of various antibody-mediated autoimmune diseases4 and are a challenging treatment target as they do not proliferate and reside in various tissues and in dedicated survival niches within the bone marrow. Different approaches have been investigated in order to specifically target plasma cells in autoantibody-mediated diseases such as immunoablation followed by autologous stem cell transplantation5 or the proteasome inhibitor bortezomib.6 7 The monoclonal anti-CD38 antibody daratumumab, licensed for use in multiple myeloma, also depletes non-malignant plasma cells and its successful use has been reported in cases of refractory autoimmune haemolytic anaemia,8 autoimmune encephalitis,9 immune thrombocytopenia10 and systemic lupus erythematosus (SLE).11 Here we report two cases of refractory AAV successfully treated with daratumumab.
Methods
Written informed consent for off-label treatment and publication was obtained from the patients or their legal guardian. Daratumumab was administered as a 1800 mg fixed-dose subcutaneous injection once weekly, according to the standard protocol for multiple myeloma treatment and was administered with the recommended co-medication of methylprednisolone, paracetamol and dimentidene. Infection prophylaxis with trimethoprim sulfamethoxazole and acyclovir was initiated or continued, and the previous glucocorticoid treatment was continued. Clinical disease activity was evaluated by the Birmingham Vasculitis Activity Score (BVAS) V.3.
Paraffin sections of a kidney biopsy from patient 1, obtained shortly before the initiation of daratumumab treatment, were stained for the presence of B and plasma cells using a commercially available antibody against CD20 (Dako, Glostrup, Denmark) and CD138 (Zytomed, Berlin, Germany, clone B-A38) or antibody cocktails for detection of kappa (Dako, Glostrup, Denmark and Epitomics, Hannover, Germany, Cat. No. AC-0149) and lambda (Dako, Glostrup, Denmark and Monosan, Am Uden, The Netherlands, Cat. No. MONX10620) and routine staining protocols on Ventana BenchMark Ultra stainers (Roche, Switzerland) as described before.12
In order to investigate the immunological changes under daratumumab treatment, we cryopreserved whole blood of patient 1, using the Proteomic Stabilizer (Smart Tube, Las Vegas, USA) and performed mass cytometric immunosurveillance as previously reported.13 We used a multi-epitope CD38 antibody (Cytognos, Salamanca, Spain) to detect CD38 even in the presence of daratumumab. We measured MPO-ANCA and PR3-ANCA by ELISA (EUROIMMUN, Lübeck, Germany). We provide clinical and immunological follow-up data up to 7 months after the initiation of treatment.
Results
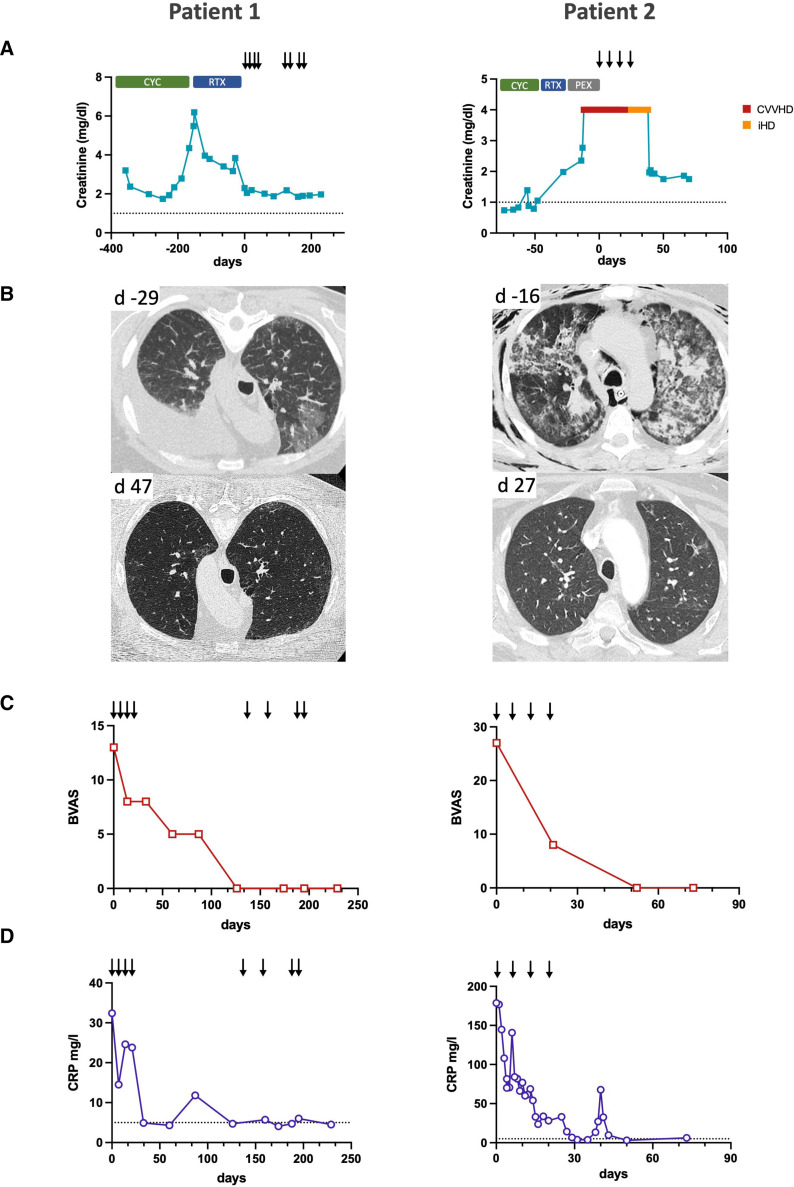
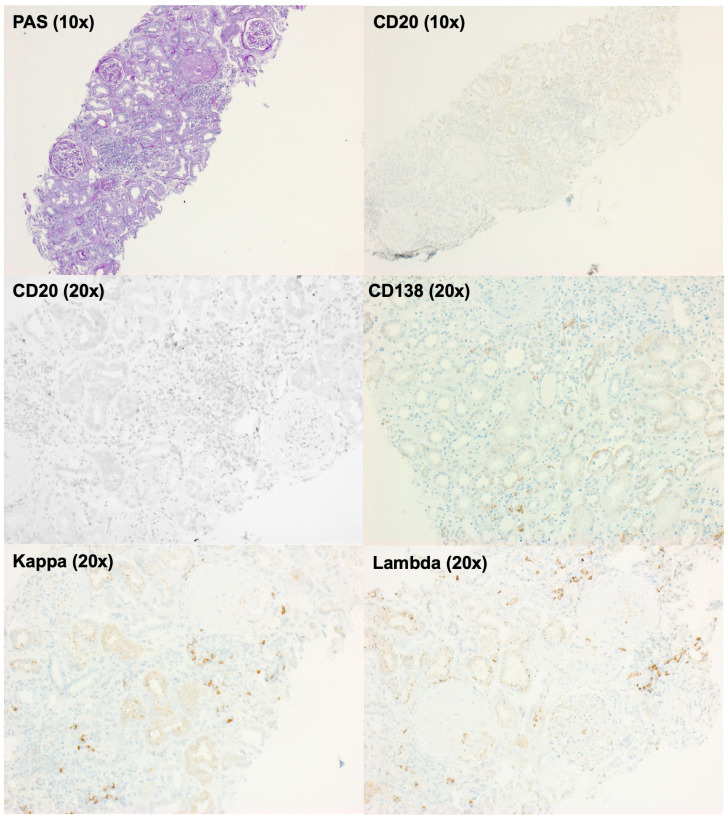
Patient 1 was a 57-year-old male patient with a treatment-refractory MPO-ANCA-positive microscopic polyangiitis. The patient had been diagnosed 1 year prior, when he presented with crescentic glomerulonephritis. Despite treatment with cyclophosphamide and rituximab, he never reached remission. At the time of evaluation for daratumumab treatment, he presented with worsening dyspnoea due to refractory pleural effusions, alveolitis and worsening kidney function with a nephritic urinary microscopy (figure 1A, B). The BVAS initially was 13 (figure 1C). A repeat kidney biopsy revealed ongoing crescentic pauci-immune glomerulonephritis. Immunohistochemistry showed a plasma cell-dominant interstitial infiltrate positive for kappa and lambda light chains but without evidence of CD20+ B cells, with complete absence of B cells in the peripheral blood, indicating that rituximab treatment had depleted B cells but not CD20- antibody-secreting cells in the kidney (figure 2). The patient initially received 1800 mg daratumumab in four weekly doses.
Figure 1.
Improvement of antineutrophil cytoplasmic antibody-associated vasculitis (AAV) disease activity after daratumumab treatment. (A) Serum creatinine levels before and after daratumumab treatment. Previous induction therapies with cyclophosphamide (CYC), rituximab (RTX) and plasma exchange (PEX) did not lead to clinical remission or stabilisation of kidney function. Arrows indicate daratumumab doses, the dotted line indicates the upper normal limit for serum creatinine. Patient 2 underwent renal replacement therapy with continuous veno-venous haemodialysis (CVVHD) and intermittent haemodialysis (iHD). (B) CT of lung sections showing pleural effusion and alveolitis (patient 1) and AAV-induced acute respiratory distress syndrome and ventilator-associated soft tissue emphysema (patient 2). Only some fibrotic changes remain after daratumumab treatment. (C) Birmingham Vasculitis Activity Score (BVAS) shows clinical remission after daratumumab treatment. (D) C reactive protein (CRP) levels in both patients decline to normal levels after daratumumab treatment, the dotted line indicates the upper normal limit.
Figure 2.
CD20-negative antibody-secreting cells are present in the kidney after rituximab treatment. Tissue sections of a kidney biopsy from patient 1 24 days before the initiation of daratumumab show ongoing crescentic glomerulonephritis (PAS, periodic acid–Schiff staining (×10)). No CD20-positive cells were detected in the kidney (10×, 20×), consistent with previous rituximab treatment. However, antibody-secreting cells, indicated by their expression of the kappa or lambda immunoglobulin light chains and CD138 (×20), are still found in the kidney.
Already at completion of the initial four daratumumab doses, the patient showed an improvement of kidney function and resolution of alveolitis and pleural effusion (figure 1B). Four months after the last dose, as ANCA levels were still elevated and ongoing haematuria present, the patient received a second cycle of four doses of daratumumab. After dose 5, treatment was halted for 4 weeks due to an upper respiratory tract infection that did not require specific treatment. At the most recent follow-up 8 months after the initiation of daratumumab treatment, the patient remained in complete remission with stable kidney function and no sign of active pulmonary disease. During the course of the treatment, no serious adverse events occurred.
Patient 2 was a 41-year-old male patient with refractory PR3-ANCA-positive granulomatosis with polyangiitis, who had been diagnosed 4 months prior with renal, pulmonary and upper respiratory tract manifestations. Despite induction therapy with cyclophosphamide and prednisolone, he presented with diffuse alveolar haemorrhage requiring mechanical ventilation and acute kidney injury. Even with additional therapy with methylprednisolone pulses, rituximab and plasma exchange, his illness further progressed and he developed severe acute respiratory distress syndrome requiring extracorporeal membrane oxygenation (ECMO) and progressive kidney failure with need for continuous kidney replacement therapy, despite complete depletion of B cells in the peripheral blood (figure 1A, B). Furthermore, he developed a severe aortic stenosis. He received four doses of daratumumab. Within 2 weeks following initiation of daratumumab treatment, the patient was weaned from ECMO and lung and kidney function improved substantially. Eight weeks after the start of daratumumab treatment, he was discharged home without the need for oxygen supplementation or kidney replacement therapy. He remained in complete remission 4 months later and is currently being evaluated for an aortic valve replacement. Apart from hypogammaglobulinaemia without signs of infection requiring prophylactic intravenous supplementation twice, no adverse events occurred.
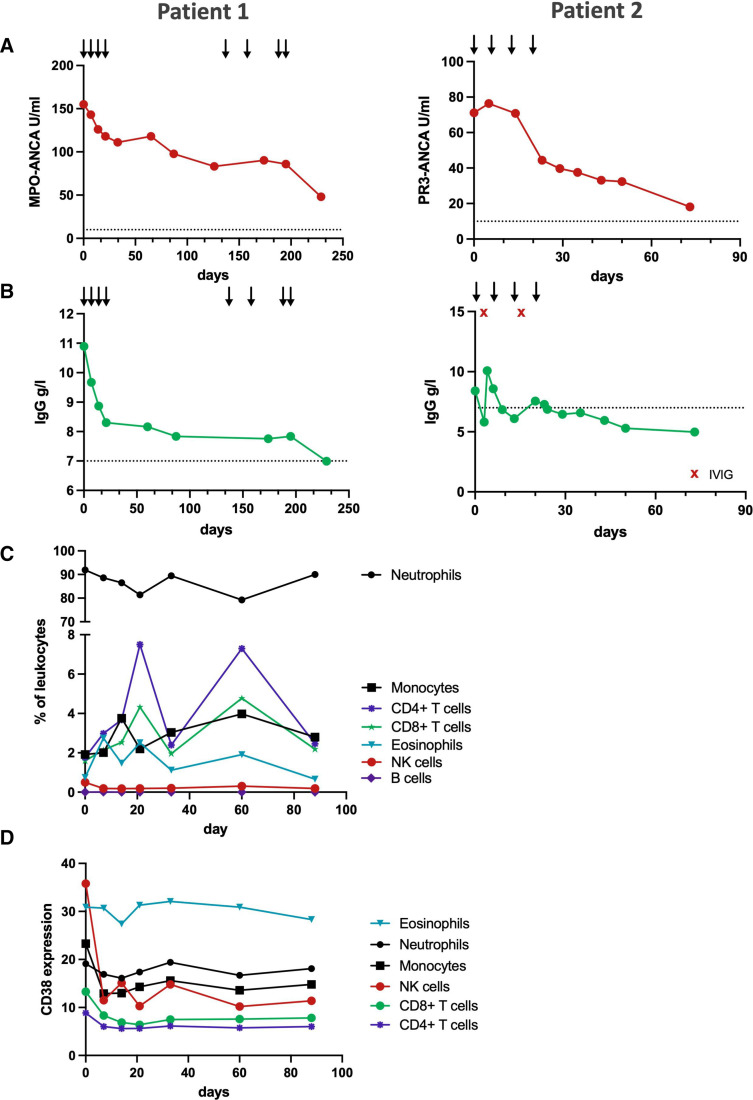
Both patients showed a significant reduction in ANCA levels after initiation of daratumumab (patient 1: 69%, patient 2: 74%), consistent with the depletion of autoantibody-secreting plasma cells (figure 3A). Total immunoglobulin levels similarly initially declined, but remained stable after the initial treatment phase (figure 3B).
Figure 3.
Immunological effects of daratumumab treatment. (A) Antibody levels of myeloperoxidase (MPO)-antineutrophil cytoplasmic antibody (ANCA) and proteinase-3 (PR3)-ANCA after daratumumab treatment. Arrows indicate daratumumab doses, the dotted line indicates the upper normal limit for ANCA. (B) Total serum IgG levels decline under daratumumab treatment. The dotted line indicates the lower limit of normal IgG levels, red x indicates intravenous IgG (IVIG) supplementation. (C) Relative levels of major leucocyte subsets in the peripheral blood remain stable under daratumumab treatment of patient 1. (D) Mean signal intensity of surface CD38 expression of major leucocyte subsets in the peripheral blood of patient 1 as measured with a polyclonal antibody that binds independent of daratumumab presence. NK, natural killer.
In our analysis of peripheral blood leucocytes of patient 1, we observed mostly stable counts of leucocyte subsets with the exception of natural killer cells, which were depleted after daratumumab administration due to their high CD38 expression.14 CD19+ B cells were absent in the peripheral blood, consistent with the previous rituximab treatment (figure 3C).
Measurement of leucocyte CD38 expression after daratumumab showed a sustained suppression of CD38 levels on long-lived lymphoid cells (figure 3D).
Discussion
We report two cases of severe treatment-refractory AAV successfully treated with the anti-CD38 antibody daratumumab. Both patients had persistently high ANCA levels despite induction treatment with both rituximab and cyclophosphamide. As ANCAs are usually depleted after B cell-targeted therapies, most of ANCA production in most cases is likely attributable to CD20+ B cells. Refractory cases might represent those, where long-lived plasma cells (LLPCs) are contributing more to the ANCA production. LLPCs are refractory to both CD20-targeted treatments and antiproliferative drugs, and thus represent a promising new treatment target.7 15 It will thus be important to investigate the relative contributions of plasma cells and B cell to AAV, in order to identify patients who would benefit from a plasma cell-targeted therapy. In patient 1, a kidney biopsy after rituximab showed an abundance of infiltrating antibody-secreting cells negative for CD20, which may suggest the need for plasma cell-targeted therapies. However, future investigation should be conducted to investigate the role of kidney-infiltrating plasma cells and if their detection should necessitate plasma cell-targeted therapies.
Our findings complement previous reports of the successful use of the anti-CD38 antibody daratumumab for plasma cell depletion in other autoantibody-mediated diseases, such as SLE11 and autoimmune haemolytic anaemia.8
In the two patients reported here, daratumumab treatment was associated with fast clinical and immunological response with only mild adverse effects (uncomplicated upper respiratory tract infection, hypogammaglobulinaemia). However, we cannot exclude a confounding delayed treatment effect of previous therapies. Rituximab is known to induce a delayed response, and time between rituximab and daratumumab was short in patient 2 due to the dynamic worsening of his disease. While we did not observe significant adverse events, daratumumab treatment was associated with an increased risk of infection in systematic studies for multiple myeloma16 and in case reports in autoimmune diseases.9 17
Prospective clinical trials will be needed to investigate the safety and efficacy of daratumumab in AAV and to identify patients who could benefit from a plasma cell-targeting drug.
Acknowledgments
We thank our patients and their families for trusting us with their care and allowing us to publish this report. We thank the many physicians of the Department of Nephrology and Medical Intensive Care at Charité-Universitätsmedizin Berlin involved in the care of the patients. We also thank Alexander Kühnl (EUROIMMUN) for the analysis of ANCA levels and Heike Hirseland for technical assistance.
Footnotes
Contributors: LO and AS conceptualised the study, LO, MB, DLW, PE, KA, HM and ES collected data and performed experiments. K-UE and AS supervised the work. LO wrote the first draft of the manuscript. All authors contributed to the revision of the manuscript.
Funding: This work was supported by grants from the Deutsche Forschungsgemeinschaft with grant SCHR 771/8-1 to AS, grant 394046635–SFB 1365 to AS, grant 387509280, SFB 1350 to KA, and grant Me3644/8-1 to HM and ECRC grants to AS, as well as by funding from the European Union Innovative Medicines Initiative 2 Joint Undertaking under grant agreements no. 777357 (RTCure) and no. 831434 (3TR).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study involves human participants and was approved by the ethics committee of Charité-Universitätsmedizin Berlin (EA2/103/17) and of Friedrich-Alexander-Universität Erlangen-Nürnberg (reference number 4415). Participants gave informed consent to participate in the study before taking part.
References
- 1.Kitching AR, Anders H-J, Basu N, et al. ANCA-associated vasculitis. Nat Rev Dis Primers 2020;6:71. 10.1038/s41572-020-0204-y [DOI] [PubMed] [Google Scholar]
- 2.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. 10.1056/NEJMoa0909905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488–94. 10.1136/ard.2010.137778 [DOI] [PubMed] [Google Scholar]
- 4.Maschmeyer P, Chang H-D, Cheng Q, et al. Immunological memory in rheumatic inflammation — a roadblock to tolerance induction. Nat Rev Rheumatol 2021;17:291–305. 10.1038/s41584-021-00601-6 [DOI] [PubMed] [Google Scholar]
- 5.Alexander T, Thiel A, Rosen O, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 2009;113:214–23. 10.1182/blood-2008-07-168286 [DOI] [PubMed] [Google Scholar]
- 6.Alexander T, Sarfert R, Klotsche J, et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis 2015;74:1474–8. 10.1136/annrheumdis-2014-206016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novikov P, Moiseev S, Bulanov N, et al. Bortezomib in refractory ANCA-associated vasculitis: a new option? Ann Rheum Dis 2016;75:e9. 10.1136/annrheumdis-2015-207947 [DOI] [PubMed] [Google Scholar]
- 8.Schuetz C, Hoenig M, Moshous D, et al. Daratumumab in life-threatening autoimmune hemolytic anemia following hematopoietic stem cell transplantation. Blood Adv 2018;2:2550–3. 10.1182/bloodadvances.2018020883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheibe F, Ostendorf L, Prüss H, et al. Daratumumab for treatment-refractory antibody-mediated diseases in neurology. Eur J Neurol 2022;29:1847–54. 10.1111/ene.15266 [DOI] [PubMed] [Google Scholar]
- 10.Canales-Herrerias P, Crickx E, Broketa M, et al. High-affinity autoreactive plasma cells disseminate through multiple organs in patients with immune thrombocytopenic purpura. J Clin Invest 2022;132:e153580. 10.1172/JCI153580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostendorf L, Burns M, Durek P, et al. Targeting CD38 with Daratumumab in refractory systemic lupus erythematosus. N Engl J Med 2020;383:1149–55. 10.1056/NEJMoa2023325 [DOI] [PubMed] [Google Scholar]
- 12.Büttner-Herold M, Krieglstein N, Chuva T, et al. Light chain restriction in proximal Tubules—Implications for light chain proximal Tubulopathy. Front. Med. 2022;9:723758. 10.3389/fmed.2022.723758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns M, Ostendorf L, Biesen R, et al. Dysregulated CD38 expression on peripheral blood immune cell subsets in SLE. Int J Mol Sci 2021;22:2424. 10.3390/ijms22052424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casneuf T, Xu XS, Adams HC, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv 2017;1:2105–14. 10.1182/bloodadvances.2017006866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bontscho J, Schreiber A, Manz RA, et al. Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic Autoantibodies–Induced glomerulonephritis. JASN 2011;22:336–48. 10.1681/ASN.2010010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnsrud JJ, Susanibar S, Jo-Kamimoto J, et al. Infection risk associated with Daratumumab. Open Forum Infect Dis 2017;4:S702–3. 10.1093/ofid/ofx163.1884 [DOI] [Google Scholar]
- 17.Scheibe F, Ostendorf L, Reincke SM, et al. Daratumumab treatment for therapy-refractory anti-CASPR2 encephalitis. J Neurol 2020;267:317–23. 10.1007/s00415-019-09585-6 [DOI] [PubMed] [Google Scholar]