Abstract
Introduction
Rectal cancer is common with a 60% 5-year survival rate. Treatment usually involves surgery with or without neoadjuvant chemoradiotherapy or adjuvant chemotherapy. Sphincter saving curative treatment can result in debilitating changes to bowel function known as low anterior resection syndrome (LARS). There are currently no clear guidelines on the management of LARS with only limited evidence for different treatment modalities.
Methods and analysis
Patients who have undergone an anterior resection for rectal cancer in the last 10 years will be approached for the study. The feasibility trial will take place in four centres with a 9-month recruitment window and 12 months follow-up period. The primary objective is to assess the feasibility of recruitment to the POLARiS trial which will be achieved through assessment of recruitment, retainment and follow-up rates as well as the prevalence of major LARS.
Feasibility outcomes will be analysed descriptively through the estimation of proportions with confidence intervals. Longitudinal patient reported outcome measures will be analysed according to scoring manuals and presented descriptively with reporting graphically over time.
Ethics and dissemination
Ethical approval has been granted by Wales REC1; Reference 22/WA/0025. The feasibility study is in the process of set up. The results of the feasibility trial will feed into the design of an expanded, international trial.
Trial registration number
CT05319054.
Keywords: Colorectal surgery, Clinical trials, Gastrointestinal tumours
Strengths and limitations of this study
This feasibility trial is the first step in addressing a National Institute for Clinical Excellence research recommendation to assess the effectiveness of transanal irrigation and sacral neuromodulation in the treatment of major low anterior resection syndrome (LARS).
This trial is pragmatically designed to optimise and assess recruitment and retainment.
This trial aims to add knowledge on the natural progression of LARS over time.
This is a feasibility trial and will not be powered to answer whether transanal irrigation or sacral neuromodulation is more effective in the treatment of major LARS.
Not all patients with debilitating bowel dysfunction may be identified in the study due to the lack of quality of life measures in the current LARS score.
Introduction
Over 10 000 people are diagnosed with rectal cancer each year in the UK1 with a 5-year survival of just over 60%, which has risen by over 35% since the 1970s.2 While the survival rate has vastly improved due to oncological and surgical advances, the adverse consequences of these treatments are now increasingly recognised. One such consequence is low anterior resection syndrome (LARS) which describes a constellation of bowel dysfunction symptoms including urgency, frequency, faecal incontinence (FI), stool clustering and incomplete evacuation which have a significant impact on quality of life (QoL). It is estimated that around 75% of the patients who have undergone an anterior resection, the most common operation for rectal cancer, will be affected by LARS in the first year following surgery.3 Of those patients 25% will have persisting symptoms beyond 1 year, with half having symptoms up to 10 years.4 The severity of LARS is currently calculated using the validated LARS score which defines LARS as ‘no LARS’ (score 0–20), 'minor LARS' (score 21–29) and 'major LARS' (score over 30).5
LARS was defined in 20126 and while it is a widely accepted condition within coloproctology there is limited guidance on management. Patients are often not informed about the likelihood of changes to their bowel function following surgery and the chronicity of these changes.7 Due to the sensitive nature of LARS symptoms there is often a reluctance from patients to discuss their symptoms causing a barrier to treatment and may lead on to a downward spiral with isolation, anxiety and loss of relationships and intimacy.8
The current treatment for LARS is largely based on that of FI, though it is worth noting that FI is only one potential component of LARS. Conservative management treatments including changes to diet, medications such as loperamide and enemas and physiotherapy techniques are the mainstay of management. If these do not adequately improve the symptoms of LARS then transanal irrigation (TAI) or sacral neuromodulation (SNM) can be trialled. A recent systematic review, looking at the impact of TAI on a range of bowel conditions including LARS suggested improved bowel function and a likely improvement in QoL but a lack of high quality evidence limited the review.9 Currently, SNM is only licenced for use in FI, but there is evidence that significant improvements in function might be achieved in patients with LARS as well.10 A systematic review of 21 studies assessing the treatment options for LARS concluded that the existing quality of research was poor with only small studies on single treatments.11 The recent MANUEL project is the first study to address the variability in the treatment of LARS by setting out a clear management pathway.12 The lack of evidence regarding SNM and TAI remains an issue and has led to the National Institute for Clinical Excellence identifying this as a research priority for the treatment options for LARS.13 The recommendation was to assess effectiveness and safety of SNM and TAI compared with symptomatic treatment for people with major LARS following treatment for colorectal cancer.
The prevalence and natural history of LARS and its treatment strategies remain poorly understood. Clinician and patient awareness and compliance with available treatments remains unknown. The POLARiS trial is designed to further characterise LARS and investigate these specific interventions. Developed in parallel, this feasibility trial will describe the prevalence of LARS and test the POLARiS trial design to explore the feasibility of running a definitive, expanded randomised control trial. The POLARiS feasibility trial will invite individuals who have had an anterior resection, high or low, or a sigmoid colectomy to take part. The inclusion of high anterior resection and sigmoid colectomy participants will aid further characterisation of LARS symptoms in these groups which have been shown to also suffer with bowel dysfunction postoperatively.
Objectives
The objectives of the feasibility trial are to establish the prevalence of major LARS in patients up to 10 years following treatment for rectal cancer and to explore the study design of the trial prior to commencing an expanded, definitive trial.
Methods and analysis
Study design
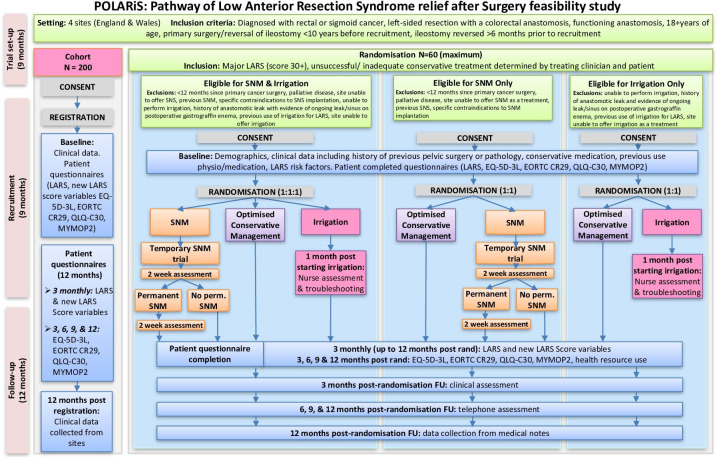
This feasibility trial is a multicentre cohort study with embedded randomised controlled trial (RCT) using the Trials within a Cohort study design.14 This feasibility trial is a multicentre cohort study with embedded open-label, parallel group, RCT, offering two-arm or three-arm randomisation options depending on eligibility criteria. Participating centres include Cardiff & Vale University Health Board, Leeds Teaching Hospitals NHS Trust, University Hospital Southampton NHS Trust and Aneurin Bevan Health Board. Cardiff & Vale University Health Board will act as the trial sponsor. The trial protocol has been developed in line with the 2013 Standard Protocol Items: Recommendations for Interventional Trials statement.15 The study design is demonstrated in figure 1. The trial will primarily establish the prevalence of LARS in the study sites, and then explore the feasibility to recruit, retain and follow-up patients. All study participants will initially be recruited to the cohort during the 9-month recruitment window. All cohort patients will be asked to complete an LARS score and QoL questionnaires on recruitment and every 3 months for 12 months. If a participant within the cohort is identified as having major LARS according to their LARS score (score of 30 or more) they will be invited to the RCT. The trial treatments are optimised conservative management (OCM), TAI and SNM. The trial opened for recruitment on 1 June 2022 and is due to run for 18 months, ending on 1 December 2023.
Figure 1.
Flow diagram to outline the study design for POLARiS feasibility. EORTC, European Organisation for Research and Treatment of Cancer; FU, follow up; LARS, low anterior resection syndrome; MYMOP, Measure Yourself Medical Outcomes Profile; QLQ, quality of life questionnaires; SNM, sacral neuromodulation.
Study population
All patients who have had an anterior resection or sigmoid colectomy in the last 10 years will be screened and a random selection of 50 eligible patients per participating site will be recruited for this feasibility trial.
Eligibility criteria
Inclusion criteria for the cohort:
Diagnosis of rectal or sigmoid cancer.
Low or high anterior resection (colorectal resection with anastomosis to the rectum).
Functioning anastomosis.
18+ years of age.
Primary surgery/reversal of ileostomy less than 10 years before recruitment.
Reversal of ileostomy at least 12 weeks prior to recruitment with at least a further 12 weeks of standard care to manage symptoms following reversal.
Willing and able to provide valid informed consent.
Exclusion criteria for the cohort:
-
Inability to understand and complete study questionnaires independently.
Due to cognitive or intellectual impairment.
Due to insufficient English language skills.
Patients eligible to join the cohort according to the above criteria will then be screened for eligibility to be randomised.
Inclusion criteria for the RCT (all randomisation options):
Recruited to cohort study.
Willing and able to provide valid informed consent for randomisation.
-
Major LARS.
Defined as an LARS score of 30 or more.
Previous unsuccessful conservative treatment as determined by treating clinician and patient.
Exclusion criteria for the RCT (all randomisation options):
Pregnancy.
No previous conservative treatment plan for the management of LARS.
Does not meet any treatment-specific criteria.
Exclusion criteria for RCT TAI-inclusive randomisation options (randomisation options 1 and 3):
Unable to perform TAI.
History of anastomotic leak with evidence of ongoing leak/sinus on postoperative gastrografin enema.
Previous use of TAI for LARS.
Site unable to offer TAI as a treatment.
Any other contraindications advised by the care team, product manufacturer or distributor.
Exclusion criteria for SNM-inclusive randomisation options (randomisation options 1 and 2):
<12 months since primary cancer surgery.
Palliative disease.
Site unable to offer SNM as a treatment.
Previous SNM.
Specific contraindications to implantation.
Any other contraindications advised by the care team, product manufacturer or distributor.
Recruitment
Eligible participants will be identified through local cancer databases, note-screening and outpatient clinics at National Health Service (NHS) hospital sites. Potential cohort participants will be sent a postal invitation which will include a detailed patient information sheet, reply slip and informed consent form. Participants who have an anterior resection in the last 10 years will be randomly approached. To ensure recruitment targets are met the recruitment log will be regularly reviewed and further participants invited when needed. Participants who are invited but do not respond will receive a follow-up phone call.
Informed consent
Valid informed consent will be sought in writing from participants prior to enrolment in the study and before any interventions or data collection can take place. Returned consent forms will be checked to ensure completeness and counter signed remotely by a member of the research team.
Participants who are eligible from the cohort, to the RCT will be approached by telephone. Participants will be informed of their eligibility and offered further information about the RCT which will be explained over the phone and followed by a postal patient information sheet. Interested participants will be asked to return a reply slip. On receipt of this an appointment to discuss the trial and sign the consent form will be made in person with a clinically qualified member of the research team.
Randomisation
Cohort participants with an LARS score of 30 or more will be invited to take part in the RCT. Depending on their eligibility to receive TAI or SNM, patients will be randomised in one of the three randomisation options, all with equal allocation ratio. The trial will use multiple randomisation options such that ineligibility to one treatment does not exclude a patient from the whole trial.
Randomisation option 1: OCM versus SNM versus TAI.
Randomisation option 2: OCM versus SNM.
Randomisation option 3: OCM versus TAI.
Randomisation will be carried out by the person consenting the patient to the RCT. Blocked randomisation using variable block sizes will be performed to produce random treatment allocations. An automated 24-hour, online randomisation system will be developed and maintained by the Clinical Trials Research Unit at the University of Leeds. Due to the nature of the interventions, this is a non-blinded trial.
Interventions
Every patient to be randomised will be given an LARS information booklet which will outline some of the conservative treatments and links to online support. Participants are able to access those treatments and this will be captured on the case report form. Participants who access TAI or SNM outside of the trial will be removed from the study. Participants who wish to stop treatment will be able to do so at their request, a reason for this will be sought.
OCM
The OCM treatment programme has been designed for this feasibility trial using current evidence on the conservative treatment of LARS. The programme will include lifestyle advice, dietary changes, medication and physiotherapy. OCM will be delivered by a suitably qualified healthcare professional with experience in managing bowel dysfunction. All healthcare professionals delivering OCM will undergo training on the POLARiS OCM treatment programme and will be supplied with the guides and patient booklets to use with their patients. Each treatment or management option delivered will be clearly recorded for every participant. The OCM treatments will be tailored to the symptoms and needs of the participant and where available referral on for specialist pelvic floor physiotherapy and dietetics will be encouraged.
TAI
TAI will be commenced by an appropriately trained clinical nurse specialist. The choice and frequency of TAI, including device, volume and frequency of use, will be guided by clinical expertise and evidence-based guidance16 and will be recorded for every participant. Participants will undergo a period of training with their TAI device, during which time the device and volume can be changed to achieve optimal outcome for the patient.
SNM
Participants randomised to SNM will undergo temporary testing according to local protocol (either with temporary testing wire or with the tined lead).17 This testing phase typically lasts 1–3 weeks and seeks to evaluate acceptability and response (using symptoms diaries) prior to a permanent device being fitted. The temporary and permanent devices will be implanted by a qualified surgeon in sites that can offer SNM.
Assessments
The assessments are carried out at recruitment, and then at 3, 6, 9 and 12 months for cohort and RCT participants. The assessments being used are outlined in table 1 and are to be completed by the participant. These will be used to evaluate the interventions for those participants in the RCT and for LARS characterisation for those in the cohort. Participants who do not return completed questionnaires within 1 month of them being sent will be followed-up in writing or by telephone.
Table 1.
Assessment tools
| Assessment/questionnaire | Description |
| LARS score | Internationally validated five-question assessment exploring different bowel dysfunction symptoms and their frequency. The overall score (maximum 42) corresponds to either no LARS (0–20), minor LARS (21–29) or major LARS (over 30).18 |
| EQ-5D-3L | Designed and validated by EuroQol as a health-related quality of life tool that generates a single index value for health status. This score is also valuable in the assessment of healthcare evaluation and economic analysis.19 |
| European Organisation for Research and Treatment of Cancer (EORTC) QLQ-CR29 QLQ-C20 |
Internationally validated cancer specific questionnaires. The EORTC produces cancer specific quality of life questionnaires (QLQ) which focus on the effects of diagnosis and treatments. The QLQ-C3020 focuses on cancer while the CR2921 is specific to colorectal cancer. |
| Measure Yourself Medical Outcomes Profile | Patient specific outcome tool in which the patient identifies two symptoms with the most significant impact on their quality of life. This tool allows for an individualised approach and measure regarding the identified symptoms to assess morbidity/adverse events related to treatment and occupational outcomes.22 |
LARS, low anterior resection syndrome.
In addition to the study questionnaires each participant will have a case report form completed which will collect further information on participant demographics, medical history and LARS therapies. For randomised participants additional information on their randomisation treatments will also be collected on the case report form.
Sample size estimation
Sample size requirement has been determined in terms of number of patients to be recruited to the cohort and number of site-months of recruitment.
A minimum of 200 patients is the target recruitment set across all investigational research sites in this cohort study. This sample size ensures a maximum 95% CI half-width of 0.058 when estimating proportions in this cohort population, such as the prevalence of major LARS and the proportions of cohort patients who are eligible for, and recruited to, the RCT. This is sufficiently precise to inform sample size assumptions and expectations in the definitive POLARiS trial.
The aim is to observe a minimum of 36 site-months (four sites recruiting for 9 months) of recruitment. This will provide sufficient precision of the Poisson parameter estimate of recruitment rate per site per month. With 200 patients recruited to the cohort over 36 site-months, the Poisson parameter estimate would be 5.55 patients recruited per site per month, with a 95% CI half-width of 1.57, that is, 95% CI: (4.0 to 7.1). This is sufficiently precise to inform recruitment rate assumptions and expectations in the POLARiS trial.
We have set a maximum of 60 patients to be recruited to the RCT to allow assessment of acceptability and crossover.
Outcome measures
The objectives of the trial and the outcome measures those objectives will be assessed against are listed in table 2.
Table 2.
Objects and outcome measures
| Objectives | Outcome measures | Endpoints |
|
Primary objective To assess the feasibility of conducting the ‘POLARiS’ trial |
|
|
|
Secondary objectives Clinical and patient reported outcomes |
|
|
EORTC, European Organisation for Research and Treatment of Cancer; LARS, low anterior resection syndrome; MYMOP, Measure Yourself Medical Outcomes Profile; QLQ, quality of life questionnaire.
A screening log will be kept of all the patients who are invited to take part in the trial. Patients who do not wish to participate in the study will be asked if they would like to provide additional information on why they have declined.
The secondary objective of the trial is to characterise and define the patient with LARS population. This will be achieved through longitudinal patient reported outcomes (see Data Collection), specifically calculating the variability (standard deviation(SD)) in these measures, in addition to collecting data on the current standard of care offered to patients with bowel dysfunction after anterior resection.
Adverse events relating to the interventions will be collected and reported in line with Good Clinical Practice. Usability data will be collected for TAI and SNM and analysed along with compliance to treatment and reasons for stopping if applicable.
Data analysis
Feasibility outcomes will be analysed descriptively through the estimation of proportions with confidence intervals (CIs). Patient characteristics will be reported descriptively as either proportions (CI) or mean (SD, CI)/median (interquartile range (IQR)).
Longitudinal patient reported outcome measures (PROMs) will be scored according to scoring manuals and analysed descriptively and reported graphically over time. Standardised area under the curve will be calculated and reported. Hierarchical repeated measures modelling will include covariate adjustment for stratification factors.
Randomised treatment groups will be combined across the three randomisation options to describe variability in PROMs for SNM, TAI and OCM.
As a feasibility trial there will be no statistical testing carried out to compare randomised treatment groups. Rather the variability in measures will inform the statistical design of the definitive trial.
Data collection and management
Data collection
Data collection will be undertaken by an appropriately trained clinical researcher as outlined in the delegation log. Data including basic demographics, medical history and details of their cancer diagnosis and treatment will be collected through health records for all patients recruited to the cohort. A short interview will also be conducted to gather information regarding current and previous treatments they have received for LARS. Participants will be asked to complete the following assessments and questionnaires at recruitment and then every 3 months for 12 months. Assessments can be completed electronically or on paper dependent on patient choice.
Data management
Direct access to data will be granted to authorised representatives from the sponsor and host institution for monitoring and/or audit of the study to ensure compliance with the relevant data protection legislation.
A combination of paper and electronic data will be collected for this study. All data recorded in paper will be handled, transferred and stored securely. Paper data will be stored in the investigator site file for the duration of the study, in a locked cupboard, in a locked room. Data from paper records will be uploaded digitally by a delegated member of the local research team. Electronic data will be captured using Microsoft forms and/or Research Electronic Data Capture. All data collected using third-party software will be stored on NHS servers, or hosted on a secure server in accordance with NHS Information Governance policy. No personal identifiers will be collected on study questionnaires.
Participant’s personal details will be stored on a link database, with corresponding ID and NHS number. This database will remain on-site and will be archived in accordance with local electronic data archiving protocols.
Management and safety
The trial will be managed in accordance with the principles of Good Clinical Practice and the UK Policy Framework for Health and Social Care Research. An internal Trial Management Group (TMG) will meet monthly over the duration of the study and its role is to develop the study documentation, determine the study activities and undertake the study activities. The wider TMG will meet every 2–3 months to support the data interpretation and dissemination. The TMG will ensure that the study is running to time and that recruitment is on target.
Adverse events (AEs) relating to trial specific interventions will be recorded for the purpose of the study as well as reported to the study sponsor (Cardiff & Vale University Health Board) and discussed by the TMG, any AEs related to devices will be reported to the Medicines and Healthcare products Regulatory Agency and product manufacturer. The process for reporting AEs is clearly outlined in the study protocol and will be verbally addressed at site initiation visits.
Confidentiality
Data collected during the course of the research will be kept strictly confidential and accessed only by delegated members of the research team. Personal data will not be kept for longer than is required for the purpose for which it has been acquired. All investigators and study site will comply with the General Data Protection Regulation and Data Protection Act 2018.
Patient and public involvement
Two lay representatives were involved in the protocol design and will sit on the TMG throughout the lifecycle of the trial. The trial protocol and patient-related trial documents including the information sheets, consent forms, case report forms and OCM treatment pathway have all been reviewed by the trial’s lay representatives.
Ethics and dissemination
The trial will be conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki (2013). This study was reviewed and approved by Wales REC1 (ref: 22/WA/0025).
The outcomes of this feasibility trial will be analysed and adjustments made where necessary to the study design ahead of an expanded, definitive trial. The trial outcomes will also be disseminated to participants upon request and published on completion of the trial in a peer-reviewed journal and at international conferences. Authorship for the publication of the results of this study will be based on the principles of the International Committee of Medical Journal Editors Recommendations 2018.
Supplementary Material
Footnotes
Twitter: @coxon_harriet, @NPCorrigan, @jules_cornish
Collaborators: POLARiS Trial Management Group: Julie Cornish, Aaron Quyn, David Jayne, Charles Knowles, Jared Torkington, Deborah Stocken, Julie Croft, Judith White, Neil Corrigan, Alun Meggy, Alexandra Coxon-Meggy, Irene Vogel, Roel Hompes, Deborah Keller, Andrea Warwick, Kheng-Seong Ng, Julie Hepburn (PPI) and Ralph Powell (PPI).
Contributors: JCo, DK and RH conceived and designed the study. IV and JCo secured funding and drafted the initial protocol. AHC-M, JW, JCr, NC, AM, DDS, CHK, AQ and JCo developed the protocol and submitted for sponsorship and ethical approval. All authors have had significant input into the production of this manuscript.
Funding: This study was funded by Bowel Research UK (charity number 1186061).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Cancer Research UK . No Title [Internet]. Bowel Cancer Incidence Statistics, 2017. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence#heading-Zero [Google Scholar]
- 2.Cancer Research UK. Bowel Cancer Survival Statistics [Internet]. Bowel Cancer Survival Statistics, 2017. Available: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/survival#heading-Zero
- 3.Martellucci J, Sturiale A, Bergamini C, et al. Role of transanal irrigation in the treatment of anterior resection syndrome. Tech Coloproctol 2018;22:519–27. 10.1007/s10151-018-1829-7 [DOI] [PubMed] [Google Scholar]
- 4.Battersby NJ, Bouliotis G, Emmertsen KJ, et al. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut 2018;67:688–96. 10.1136/gutjnl-2016-312695 [DOI] [PubMed] [Google Scholar]
- 5.Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 2012;255:922–8 https://journals.lww.com/annalsofsurgery/Fulltext/2012/05000/Low_Anterior_Resection_Syndrome_Score__Development.17.aspx 10.1097/SLA.0b013e31824f1c21 [DOI] [PubMed] [Google Scholar]
- 6.Bryant CLC, Lunniss PJ, Knowles CH, et al. Anterior resection syndrome. Lancet Oncol 2012;13:e403–8. 10.1016/S1470-2045(12)70236-X [DOI] [PubMed] [Google Scholar]
- 7.Ridolfi TJ, Berger N, Ludwig KA. Low anterior resection syndrome: current management and future directions. Clin Colon Rectal Surg 2016;29. 10.1055/s-0036-1584500. [Epub ahead of print: Available from] https://pubmed.ncbi.nlm.nih.gov/27582649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kupsch J, Kuhn M, Matzel KE, et al. To what extent is the low anterior resection syndrome (LARS) associated with quality of life as measured using the EORTC C30 and CR38 quality of life questionnaires? Int J Colorectal Dis 2019;34 10.1007/s00384-019-03249-7. [Epub ahead of print: Available from]. [DOI] [PubMed] [Google Scholar]
- 9.Mekhael M, Kristensen Helle Ø, Larsen HM, et al. Transanal irrigation for neurogenic bowel disease, low anterior resection syndrome, faecal incontinence and chronic constipation: a systematic review. J Clin Med 2021;10. 10.3390/jcm10040753. [Epub ahead of print: 13 02 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Koh CE. Sacral nerve stimulation for bowel dysfunction following low anterior resection: a systematic review and meta-analysis. Colorectal Dis 2019;21:1240–8. 10.1111/codi.14690 [DOI] [PubMed] [Google Scholar]
- 11.Dulskas A, Smolskas E, Kildusiene I, et al. Treatment possibilities for low anterior resection syndrome: a review of the literature. Int J Colorectal Dis 2018;33:251–60. 10.1007/s00384-017-2954-x [DOI] [PubMed] [Google Scholar]
- 12.Christensen P, Im Baeten C, Espín-Basany E, et al. Management guidelines for low anterior resection syndrome - the MANUEL project. Colorectal Dis 2021;23:461–75 https://pubmed.ncbi.nlm.nih.gov/33411977 10.1111/codi.15517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institue for Health and Care Excellence [NICE]. . Colorectal cancer, 2020. Available: https://www.nice.org.uk/guidance/ng151/resources/colorectal-cancer-pdf-66141835244485
- 14.Relton C, Torgerson D, O'Cathain A, et al. Rethinking pragmatic randomised controlled trials: introducing the "cohort multiple randomised controlled trial" design. BMJ 2010;340:c1066 https://www.bmj.com/content/340/bmj.c1066 10.1136/bmj.c1066 [DOI] [PubMed] [Google Scholar]
- 15.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586 https://www.bmj.com/content/346/bmj.e7586 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emmanuel A, Collins B, Henderson M. Decision Guide for the use of rectal/transanal irrigation in adults [Internet].. MacGregor Healthcare; 2020. https://www.macgregorhealthcare.com/wp-content/uploads/2021/05/Decision-Guide_2020_UK_new.pdf [Google Scholar]
- 17.Matzel KE, Chartier-Kastler E, Knowles CH, et al. Sacral neuromodulation: standardized electrode placement technique. Neuromodulation 2017;20:816–24. 10.1111/ner.12695 [DOI] [PubMed] [Google Scholar]
- 18.Juul T, Battersby NJ, Christensen P, et al. Validation of the English translation of the low anterior resection syndrome score. Colorectal Dis 2015;17:908–16. 10.1111/codi.12952 [DOI] [PubMed] [Google Scholar]
- 19.NICE . Guide to the methods of technology appraisal 2013, 2013. Available: https://www.nice.org.uk/process/pmg9/chapter/foreword [PubMed]
- 20.Groenvold M, Klee MC, Sprangers MA, et al. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol 1997;50:441–50. 10.1016/S0895-4356(96)00428-3 [DOI] [PubMed] [Google Scholar]
- 21.Whistance RN, Conroy T, Chie W, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer 2009;45:3017–26. 10.1016/j.ejca.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 22.Measures M. Measure yourself medical outcomes profile, 2021. Available: https://www.meaningfulmeasures.co.uk/mymop
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.