Abstract
The adrenal is a small, anatomically unimposing structure that escaped scientific notice until 1564 and whose existence was doubted by many until the 18th century. Adrenal functions were inferred from the adrenal insufficiency syndrome described by Addison and from the obesity and virilization that accompanied many adrenal malignancies, but early physiologists sometimes confused the roles of the cortex and medulla. Medullary epinephrine was the first hormone to be isolated (in 1901), and numerous cortical steroids were isolated between 1930 and 1949. The treatment of arthritis, Addison’s disease, and congenital adrenal hyperplasia (CAH) with cortisone in the 1950s revolutionized clinical endocrinology and steroid research. Cases of CAH had been reported in the 19th century, but a defect in 21-hydroxylation in CAH was not identified until 1957. Other forms of CAH, including deficiencies of 3β-hydroxysteroid dehydrogenase, 11β-hydroxylase, and 17α-hydroxylase were defined hormonally in the 1960s. Cytochrome P450 enzymes were described in 1962-1964, and steroid 21-hydroxylation was the first biosynthetic activity associated with a P450. Understanding of the genetic and biochemical bases of these disorders advanced rapidly from 1984 to 2004. The cloning of genes for steroidogenic enzymes and related factors revealed many mutations causing known diseases and facilitated the discovery of new disorders. Genetics and cell biology have replaced steroid chemistry as the key disciplines for understanding and teaching steroidogenesis and its disorders.
Keywords: Addison disease, adrenal hyperplasia, cortisone, cytochrome P450, genetic disease, steroid
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
The functions of the adrenal were inferred from the adrenal insufficiency syndrome described by Addison and from the Cushingoid obesity and virilization that accompanied many adrenal malignancies.
Medullary epinephrine was the first hormone to be isolated (1901), and numerous cortical steroids were isolated between 1930 and 1949.
Cases of congenital adrenal hyperplasia (CAH) were reported in the 19th century, but the causative biosynthetic defects were defined hormonally in the 1950s and 1960s.
The treatment of arthritis, Addison’s disease, and CAH with cortisone in 1950 revolutionized clinical endocrinology and steroid research.
Great advances in the understanding of steroidogenesis and the various forms of CAH were achieved through steroid chemistry.
Understanding the genetic and biochemical basis of adrenal disorders advanced very rapidly from 1984 to 2004 with the cloning of the genes for steroidogenic enzymes and related factors, revealing a vast array of mutations in a comparatively small number of genes and facilitating detailed understanding of known diseases and discovery of new disorders.
Genetics and cell biology have replaced steroid chemistry as the key disciplines for understanding and teaching steroidogenesis and its disorders.
The history of the adrenal closely parallels the history of Western medical and biological science and vividly illustrates how investigators of different eras formulated their questions and sought answers. The philosophical ruminations of Aristotle and Galen gave way to Renaissance anatomy and then to clinical observation, but scientific understanding of the adrenal required a marriage of clinical medicine with basic science: the chemistry of steroid hormones (1920s-1950s) and the revolution in molecular genetics (1980s-2000s). Hundreds of investigators writing thousands of papers have provided today’s detailed but still incomplete knowledge of the adrenal. Herein we trace this history, concentrating on the adrenal cortex, emphasizing how incisive observation and the application of the newest available scientific techniques have advanced knowledge.
Antiquity to 1800: Finding the Adrenal
The early history of adrenal discovery and research has been reviewed previously (1-6) and is summarized briefly here. It appears that ancient observers did not identify the adrenal as a structure distinct from perinephric fat. In Homer’s Iliad, Book 21, Achilles slew Asteropaios with a single sword stroke: “Near the navel he slashed his belly; all his bowels dropped out uncoiling to the ground” (line 210). Achilles then cast the body into the river Xanthos (Skamander): “… to lie in the sand, where the dark water lapped at it. Then eels and fish attended to the body, picking and nibbling kidney fat away” (lines 236-238) (7). The familiar King James version of the Bible contains 2 verbatim identical passages (Leviticus 3:4 and 4:9) referring to animal sacrifice “… the two kidneys, and the fat that is on them, which is by the flanks . . . .” The great physician of second-century Rome Galen of Pergamon (circa 130-201) may have been the first to mention the adrenal: he described “loose flesh” atop the left kidney and described the vein going to the “capsule of the right kidney.” “For when the vein first appears outside the liver, before reaching the loin, being still high, at its own right side, it sends to the capsule of the right kidney and the bodies around this sometimes spiderweb-like, sometimes hairlike, and sometimes thicker contributions” (8).
The Renaissance brought the scientific study of anatomy. Unpublished anatomical sketches by Leonardo da Vinci in 1485-1490, in the Royal Collection Trust and exhibited in Scotland in 2013 (9), show supra-renal structures that might have been adrenals or perinephric fat (Fig. 1), but Leonardo did not discuss what he was drawing, and he made no effort to inform the world of his observation. Viselius’ famous (and well-illustrated) “De Humanis Corporis Fabrica” (1543) shows the kidneys without adjacent adrenals. The first published description of the adrenal gland was provided by Bartolomeo Eustachio (circa 1520-1574), who described the adrenals as “glandulae quae renibus incumbent” (glands lying on the kidney) in his book Opuscola Anatomica, published in 1564. Eustachio states the adrenals were “diligently overlooked by other anatomists” (perhaps referring to Vesalius) and describes them clearly: “Both kidneys are capped on the extremity towards the cava by a gland. Both are connected with a fold of the peritoneum in such a way that one, if he is not very attentive, does really overlook them, as if they were not present. Their shape resembles that of the kidneys … sometimes one is bigger, sometimes another … early anatomists and those who write ample treatises on this art in our days failed to detect them” (2, 3). Eustachio had planned an anatomical “magnum opus” that was to rival that of Vesalius’ and, with Roman artist Pier Matteo Pini, had prepared 47 engraved copper plates to illustrate the text, but Eustacchio died before completing this project. The first 8 plates were published in the preliminary Opuscula Anatomica, including a clear illustration of the adrenals sitting atop the kidneys (Fig. 2a). Pini’s engraved plates found their way to the papal library, where they remained for about 150 years, until Pope Clement XI gave them to his personal physician, Giovanni M. Lancisi, who published all 47 plates, plus his own notes, in 1714 as Tabulae Anatomicae Clarissimi viri Bartholomaei Eustacci (Fig. 2b). Eustachio’s book was not circulated widely, and most anatomists first encountered his work through Lancisi’s book. Although Lancisi credits Eustachio’s discovery of the adrenal, some authors have reported that this was the first publication of Pini’s engravings (10). D. Lynn Loriaux, who was president of the Endocrine Society in 1995, published his personal account of viewing Eustachio’s book at the Vatican in 1977 or 1978 (11).
Figure 1.
A sketch by DaVinci ca. 1485-1490 of the kidney with an apparent adrenal in the correct anatomic location (RCIN 912597, in the public domain at https://commons.wikimedia.org/wiki/File:Leonardo_da_Vinci_-_RCIN_912597,_The_major_organs_and_vessels_c.1485-90.jpg).
Figure 2.
Left, Plate 2 from Eustacchio’s “Opuscola Anatomica,” as reproduced by JM Lancisi in 1714 as “Tabulae Anatomicae” (image from page 64 of Lancisi’s volume, in the public domain at https://search.lib.virginia.edu/sources/uva_library/items/u3606395?idx=0&page=64. This image was also published as Fireu. 2 in Endocrine Reviews 41(1):1-46, 2020). Right, Faceplate of Lancisi’s book (image captured online at https://en.wikipedia.org/wiki/Bartolomeo_Eustachi).
Eustachio’s work was not widely known; in 1627, Giulio Cesare Casseri (1552-1616) published an illustration of the adrenals atop the kidneys, which may have been the first widely seen illustration of the correct anatomy (Fig. 3) (12). Between 1650 and 1750, multiple anatomists described the adrenals as hollow, fluid-filled organs. The first of these may have been Danish anatomist Caspar Bartholin the Elder (1585-1629), who described the adrenals as filled with “black bile,” probably referring to the medulla undergoing postmortem autolysis (13). His son, Thomas Bartholin, who followed his father as professor of anatomy at the University of Copenhagen, described different anatomic appearances of the adrenal (14), including the hollow adrenal filled with “black bile” (Fig. 4). Bartholin the Elder was the grandfather of Caspar Bartholin the Younger (1655-1738), who described Bartholin’s glands. Others also described an adrenal filled with “black bile,” including Johann Vesling (15), Thomas Wharton (16), and Antonio Molinetti (17); these and other anatomists offered fanciful theories concerning adrenal function, but without evidence (1). There also were “adrenal deniers”: Piccolomini (1586) claimed that the adrenals were rare renal “excrescences” (outgrowths), similar to supernumerary digits (2), and Andre du Laurens, physician to Henry IV of France, wrote in 1640 that “Eustachius claims to find a gland above the kidneys. Sometimes we saw that too; often, however, we stated that there was no such gland” (3) (deniers of science have always been with us). In 1805 Georges Cuvier, professor of animal anatomy at the Museum of Natural History in Paris, established that the adrenal is a solid organ (lacking a hollow cavity filled with “black bile”) and distinguished the cortex from the medulla but provided no insight into their functions (18). In 1831, Friedrich Arnold, professor of anatomy in Heidelberg, showed that the fetal adrenal developed from the embryonic Wolffian ducts (19). Based on histologic studies, the terms “cortex” and “medulla” were introduced in 1836 (20) but without functional insight.
Figure 3.
Left, Giulio Cesare Casseri, from the frontispiece of his book Tabulae Anatomica (Deuchinus, 1627) (in the public domain at https://en.wikipedia.org/wiki/Giulio_Cesare_Casseri#/media/File:Casserius.png). Right, Casseri’s illustration of the kidneys, featuring the adrenals, indicated by “G” and labeled “corpuscula reni incumbentia sive Renes succenturiati” (renal corpuscles lying on or above the kidney) (images from page 174 of Casseri’s volume in the public domain at https://archive.org/details/tabulaeanatomica00cass/page/174/mode/2up).
Figure 4.
Thomas Bartholin’s varied adrenal anatomy. The top figure shows hollow, ovoid adrenals, supporting the notion that the adrenals were filled with “black bile”; a variant of this illustration was also published by Johann Vesling (1647) [from (14)] (in the public domain at http://mateo.uni-mannheim.de/camenaref/bartholin/bartholin1/jpg/s123.html).
1697-1889: Discovering Adrenal Functions
The discovery of the adrenals offered no clues to their function, and as detailed by Shumacker, between the time of Eustaccio and Casseri until the time of Addison, at least 50 publications offered fanciful theories, without evidence (1). In 1716 the Academy of Sciences of Bordeaux sponsored an essay competition to discover “Quel est l’usage des glandes surrenales?” (“What is the use of the suprarenal glands?”). The Baron Montesquieu judged the competition but decided none of the entries merited the prize (21). As described by Schäfer, Montesquieu concluded “Perhaps chance may some day effect what all these careful labours have been unable to perform” (22).
Disease often leads to functional insights, and adrenal tumors suggested the adrenal influenced multiple systems. In 1697, Henry Sampson described the clinical course, death, and autopsy of a 6-year-old girl who died in 1688 (23); a 21st-century reading indicates she was virilized, Cushingoid, and died from metastatic adrenocortical carcinoma and a probable renal vein thrombosis (24). Sampson apparently did not know about the adrenal, although other 17th-century writers had mentioned it. In 1809 Cooke reported a similar case in another 6-year-old obese, virilized girl, having a tumor “intimately blended” into the left kidney, but made no mention of the adrenals (25); Cooke did not cite Sampson or any other reports. In 1865, JW Ogle reported the death and autopsy of a 3-year-old girl with obesity and poor growth who had a 22/16 pound left adrenal mass with at least one hepatic metastasis (26). TC Fox reported a similar course in a 2-year-old girl in 1885; he identified a 1½ pound left adrenal tumor with “secondary growths” in the mesenteric lymph nodes and cited reports of 5 other cases (27). Many of these early cases also had venous thromboses. Bulloch and Sequira reviewed the pathology literature concerning adrenal tumors in 1905, compiling 12 cases (28). These authors notably included early cases of probable CAH, clearly noting that the adrenal could profoundly influence the sexual phenotype. Thus, by the beginning of the 20th century, it was apparent that the adrenal, presumably through some form of hyperactivity, could influence body mass, growth, and virilization, but how this happened was unclear.
The role of adrenal insufficiency was first studied in the mid-18th century. The first reports that the adrenals were physiologically important came from Thomas Addison. In describing probable pernicious anemia, he noted that 3 deceased patients “had a diseased condition of the suprarenal capsules”; one had malignancy, one atrophy and one had ‘struma’ (probable tuberculosis) (29). He followed this with a more detailed description of 11 patients, of whom 6 had adrenal tuberculosis; some others may have had autoimmune adrenalitis (Fig. 5). His clinical description of adrenal insufficiency remains clear and lucid today: “The discoloration pervades the whole surface of the body, but is commonly most strongly manifested on the face, neck, superior extremities, penis, scrotum, and in the flexures of the axillae and around the navel … The leading and characteristic features of the morbid state … are, anaemia, general languor and debility, remarkable feebleness of the heart’s action, irritability of the stomach, and a peculiar change of the colour in the skin, occurring in connection with a diseased condition of the suprarenal capsules” (30).
Figure 5.
Left, Thomas Addison (photographer unknown); in the public domain at (https://commons.wikimedia.org/w/index.php?curid=35152865). Right, Reproduction of Plate 11, showing a postmortem “sketch” of “Mr. S” from Thomas Addison’s 1855 monograph (30) (https://wellcomecollection.org/works/xsmzqpdw). Most of the patients described by Addison had tuberculous adrenalitis, but the patchy vitiligo and scant pubic hair illustrated here suggests autoimmune polyglandular syndrome type 2f.
Aware of Addison’s work, in 1856 the peripatetic Charles-Edouard Brown-Sequard (1817-1894) wrote 2 papers showing that adrenalectomy caused death (in dogs), inferring that this was due to lack of an adrenal secretion (31). Brown-Sequard wrote 3 additional papers describing the lethal effects of adrenalectomy in 1857 and 1858 (32-34) but others questioned his results [see (35)]; it was not until 1908 that the medical world generally agreed that the adrenals were necessary for life (22). Most famously (or infamously), in 1889 Brown-Sequard reported “rejuvenated sexual prowess” after injecting himself with testicular extracts (36). This was certainly a placebo effect, as recapitulation of Brown-Sequard’s procedures yield a fluid with insignificant amounts of steroids [reviewed in (37)]. Brown-Sequard’s “organotherapy” may have been quackery, but it inspired other research work. In 1891 George Murray injected an extract of sheep thyroid into a woman with myxedema (38), and hypothyroidism was successfully treated with oral desiccated thyroid into the 1960s. Many investigators worked on the isolation of sex steroids; for example, TF Gallagher and Fred Conrad Koch (for whom the Endocrine Society’s most prestigious Laureate Award is named) were early leaders in developing the cock’s comb assay for androgens (39) and in isolating “the testicular hormone” (40). The history of testosterone is reviewed elsewhere (41-43).
Nineteenth-Century Reports of CAH
Because it was a relatively common disease, there must have been individuals who survived with virilizing non-salt-losing CAH throughout history, but the earliest probable case may be the one reported anonymously in The Lancet in 1833, which reiterates a case initially reported in Journal Hebdomadaire by M. (monsieur) Bouillaud: “A person named Valmont, aet. 62, a widower, of small stature, was admitted on the 6th of April into the cholera wards of La Pitie, and died next morning. As Valmont appeared to possess all the attributes of the male sex when received into the wards, we were not a little astonished, when the abdominal organs were opened, at finding in the cavity of the pelvis a perfectly-formed womb!” The anatomic examination was detailed by a M. Manec, who observed “a penis, perfectly formed, of middling size” with hypospadias (Fig. 6). He found no testes but “two ovaries, of the same size as in a girl of 16, and two Fallopian-tubes, with their extremities opening into the uterus, as in a perfect female.” There is a detailed description of the uterus, vagina, and the urethra opening “surrounded by a perfect prostate gland” into the vagina; the adrenals were not mentioned. The anonymous British author in The Lancet concludes that “hermaphrodism can no longer be disputed” and that “[i]n many countries, modifying clauses must be, consequently, introduced into the laws relative to the civil state, the succession of property, and the administration of criminal justice, in order to regulate the position, attributes, and social duties of these intermediate beings” (44).
Figure 6.
Autopsy of genitourinary structures of a virilized female patient with congenital adrenal hyperplasia in 1833 (44). The larger illustration, labeled “Fig. 1” is accompanied by a listing of 15 numbered structures; notable structures include (1) the urethra immediately inferior to the glans penis; (6 and 7) bladder and ureters, respectively; (8) vagina; (10) uterus; (11,12) ovaries; (11) 1 of the Fallopian tubes. The smaller illustration, labeled “Fig. 2” is accompanied by a listing of 7 structures labeled a though g, showing the urethra, Cowper’s glands, corpora cavernosa, and accompanying musculature (in the public domain at https://www.sciencedirect.com/science/article/abs/pii/S0140673602937700).
The best-described early case was reported by Luigi de Crecchio (1832-1894), a forensic pathologist (“professor of legal medicine”) in Naples, who reported the postmortem anatomical findings in Giuseppe Marzo, showing that Marzo had a uterus, Fallopian tubes, and ovaries (45) (Fig. 7). De Crecchio investigated Marzo’s life, including interviews with family and associates. Marzo’s sex assignment at birth was female (with the given name Maria Giuseppa) but was changed at age 4 when a surgeon determined Marzo was a boy with undescended testes. Marzo lived as a man, was twice treated for gonorrhea, never married, and died in an apparent Addisonian crisis at age 44. De Crecchio noted that Marzo had very large adrenals but thought Marzo’s findings indicated a disorder of puberty, and he did not discuss a possible role for the adrenal hyperplasia. A complete translation and exegesis of De Crecchio’s paper was published in 2015 (46).
Figure 7.
Plate 3 from De Crecchio’s paper (45), containing 3 drawings. Fig. 1: longitudinal view of the pelvic contents. Some relevant structures are: 5. Urethra (opened). 10. Vagina. 14. Uterus (opened). 15. Cervix. 17. Right broad ligament, containing the right ovary and Fallopian tube 19. Left Fallopian tube and fimbriae. 20. Left ovary. 23. Right corpus cavernosum (cut). 24. Urethral meatus. Fig. 2: connection of the vagina to the prostatic urethra; numbers as in Fig. 1; for details see (46). Fig. 3: drawing of a Graafian follicle from Marzo, as seen microscopically at 620× (in the public domain; from the University of Michigan Library, http://hdl.handle.net/2027/mdp.39015013772457).
Possibly the earliest report of familial CAH was in 1886 from John Phillips, “Physician to the British Lying-In Hospital; Senior Assistant Physician, Chelsea Hospital for Women.” He reported the delivery of a woman’s ninth child; 4 of the previous children had “spurious hermaphroditism” (a term that preceded “pseudohermaphroditism,” both referring to individuals whose external genital anatomy did not correspond to the physician’s expectations based on the gonadal anatomy). The 4 affected children “gradually wasted” and died at 24, 59, 40, and 19 days of age. The last of these children is described in detail, with clitoromegaly, a urogenital sinus, and a palpable uterus. At the postmortem examination “the pelvic organs were found to be entirely female” “with the kidneys and suprarenal capsules (which were very large) …” (47). This history strongly suggests salt-wasting CAH in these 4 infants, but the hereditary basis of CAH was vigorously debated into the 1950s. The proximity of the adrenal and kidney and the resemblance of renal cell cancer cells to adrenocortical cancer cells has led to confusion, as renal-cell cancers were once believed to originate from aberrant adrenal tissue. In 1883 Grawitz described renal-cell cancers and termed them “struma lipomatodes aberrata renis” (48). His theory that these renal tumors arise from adrenal tissue led to the common term “hypernephroma,” a misleading term that persisted into the mid-20th century.
These early reports of CAH contributed to the understanding that the adrenals can influence sexual phenotypes. In 1912 Ernest Glynn, a pathologist in Liverpool, reviewed the literature concerning adrenal tumors that influenced the patient’s sexual phenotype (49). His Table I lists 17 cases of “Sex Abnormalities in Children associated with Adrenal Hypernephroma verified post mortem” and 6 cases of “Adrenal Hypernephroma in Young Adult Females associated with Changes in Sex Characters”; his Table II lists 6 cases of “Male Sex Characters in Adult Females Associated with Adrenal Hypernephroma.” Glynn’s Tables I and II list virilizing adrenal tumors, although his case #22 (on page 166) was most likely a case of CAH. Under the heading of “Pseudo-hermaphroditism” he says, “The occurrence of hyperplasia of the adrenal gland, or of accessory adrenals with some cases of pseudo-hermaphroditism, is another example of the association between the adrenal and sex”; he then summarizes 9 cases, several of whom (including 2 siblings) certainly had CAH. Glynn’s Table III lists 13 cases “of the Association of Pseudo-hermaphroditism, particularly Feminine, with Bilateral Hyperplasia of the Adrenal Gland”; most of these individuals clearly had CAH. Glynn correctly concluded “The adrenal cortex and medulla have a different development and different functions; the former is especially connected with growth and sex characters, the latter with blood pressure” and “In pseudo-hermaphroditism the sex abnormalities are mainly congenital, and the adrenal lesions, if any, are bilateral hyperplasia or cortical rests” (49).
In 1912 Alfred Gallais presented his doctoral thesis to the Faculté de Medécine de Paris (Sorbonne), in which he describes “le Syndrome Génito-Surrénal” (50). His work summarizes a hodge-podge of clinical reports of people with adrenal tumors and Cushing disease, and some with probable CAH, and does not attempt to categorize the cases as does Glynn, but it is remembered as the origin of the term “adrenogenital syndrome.” Gallais was widely cited in the early 20th century, and the term persisted into the late 20th century. For example, in 1940, Marks et al reported “adrenal obesity” in a profoundly Cushingoid 10-month-old girl (Fig. 8) with adrenogenital syndrome caused by an adrenal adenoma found at autopsy (51). Their accompanying review of 24 cases of “obesity in children with tumors of the adrenal cortex” found a 21:3 preponderance of girls and a 14:10 preponderance of carcinomas; of the 6 who underwent surgical removal of the tumor, only 2 survived. A case report and literature review in 1946 described 54 children with adrenogenital syndrome caused by virilizing adrenal carcinomas (52), and a 1953 review of adrenogenital syndrome stated the term should only be applied to adult patients with adrenal hyperplasia or adrenal tumors, although some had ovarian tumors (possibly adrenal rests) or apparent polycystic ovary syndrome (PCOS) (53). Fortunately, the term “adrenogenital syndrome” has been abandoned by contemporary endocrinology.
Figure 8.
10½-month-old girl with “adrenogenital syndrome” and “adrenocortical obesity” caused by a 3 cm diameter, 12 g right adrenal adenoma (51) (in the public domain at https://jamanetwork.com/journals/jamapediatrics/fullarticle/1178993).
Finding the First Adrenal Hormone—Epinephrine
The first adrenal hormone to be isolated, purified, characterized chemically, and synthesized in vitro was epinephrine. Early adrenal investigators had difficulties in differentiating the effects of the medulla and cortex. This important chapter in adrenal history has been reviewed in detail elsewhere (54, 55) and is summarized here.
In 1856 in Paris, Alfred Vulpian (1826-1896) discovered the 2 colorimetric reactions of the adrenal medulla that ultimately identified epinephrine (ferric chloride turns medullary extracts green, whereas iodine turns it “rose-carmine”). He noted this reaction with the adrenals of multiple mammals and birds and that blood from the adrenal vein, but not other veins, gave the same reactions, from which he inferred that the responsible substance is secreted into the circulation (56). Vulpian became dean of the faculty of medicine at the University of Paris and a member of the French Académie des Sciences; a statue of him adorns the Rue de l’École de Médicine in Paris. The reaction of medullary extracts with chromic acid was reported by Bartholdus Werner in 1847 and described in detail by Jacob Henle in 1865 (57); this led to the terms “chromaffin reaction” and medullary “chromaffin cells” introduced by Alfred Kohn in 1902 (58). In 1885, Carl Krukenberg noted that the color reaction of pyrocatechol resembled that the color reaction of adrenal extracts (59), eventually leading to the term “catecholamines.”
In 1894, George Oliver and Edward S. Schäfer reported that intravenous administration of a glycerin extract of calf adrenals caused vasoconstriction and hypertension in dogs (60, 61). In 1908, Schafer said, “Perhaps within the next few years a successor of mine in this lectureship will be able to put before you as much positive knowledge regarding the cortex as we now possess regarding the medulla, the function of which seemed, no more than fifteen years ago, as obscure as that of the cortex appears at present. And with the hope that this obscurity may speedily be removed, I cannot do better than terminate my lecture.” (22). Shortly thereafter, William Horatio Bates, an ophthalmologist in New York, reported that a sheep adrenal extract whitened the conjunctiva of the eye, indicating a vasoconstrictor effect (62) and which others subsequently used as a bioassay for epinephrine. In Philadelphia, Solomon Solis-Cohen suggested that adrenal extracts might be useful therapeutic agents for hay fever and asthma (63, 64), accelerating pharmaceutical interest in the adrenal.
The 3 leading investigators aiming to purify the adrenal hypertensive agent were Benjamin Moore in London, John Jacob Abel at Johns Hopkins, and Otto von Fürth in Strassburg (then in Germany) (65). Abel reacted adrenal extracts with benzyl chloride to prepare an agent that raises blood pressure; he termed this “epinephrin” (no “e”) (66). He proposed the empirical formula C17H15NO4 for “the active principle of the suprarenal capsule” (ie, “epinephrin”) (67). Unfortunately for Abel this was incorrect, as his formula included the benzyl group added in the purification, and benzoylation yielded inactive material. These investigators opposed one another vigorously, preparing different extracts, performing different derivatization procedures, proposing different chemical compositions, and claiming each other’s results were incorrect.
On July 21, 1900, working in the laboratory of Jokishi Takamine on Manhattan’s 109th Street West under a contract with Parke, Davis & Co., Keizo Uenaka (Wooyenaka), Takamine’s lab technician, first purified “adrenalin” (no “e”) from aqueous adrenal extracts furnished by Parke-Davis. Uenaka used procedures similar to those he had used for ephedrine with Nagayoshi Nagai in Tokyo and made the key observation that he had to avoid oxidation (68). When administered to the eye of an unfortunate mouse that had been caught in the lab, the conjunctiva lost its color, indicating vasoconstriction, much as Bates had described. Uenaka then obtained “adrenalin” crystals; he scaled up his procedures, extracting 10 g of crystals from 10 kg of bovine adrenals. On Oct 13, 1900, Uenaka applied a drop of 1:1000 dilution to his own eye and observed vasoconstriction (0.1% epinephrine remains in use today). On Nov 5, 1900, Takamine accepted the suggestion of Dr. Norton Wilson to call the material “adrenalin” (no “e”) and applied for a patent. Takamine published this work in December 1901 (69, 70), estimating the empirical formula to be C10H15NO3. Working at Parke-Davis independently of Takamine, Thomas B. Aldrich, who had worked with Abel, determined the correct formula of C9H13NO3, but says in his paper that his material is the same as Takamine’s (71). Takamine registered the trade name “adrenalin,” and Parke-Davis began sales of “Adrenalin Chloride Solution,” both in 1901. Adrenalin was advertised for “bloodless surgery”, and commercial success was immediate.
In the late 19th century America was awash with “patent medicines” (often termed “snake oil”) that were of dubious value; patents were pursued by charlatans and quacks, not by “real doctors” or “legitimate” pharmaceutical companies. At this time, existing precedent has already drawn the line at patenting natural objects or laws of nature. Takamine had previously obtained US Patent 525 823 for a “Process for making diastatic enzyme,” a yeast preparation that he had devised for the brewing industry but eventually sold as a digestive aid, first marketed in 1895 as “Taka-Diastase” (55). Takamine had licensed this financially successful patent to Parke-Davis. He similarly applied for a US Patent for “adrenalin” in 1901 and was granted US Patent no. 730 175 “Process of obtaining products from suprarenal glands” on June 2, 1903 (72). Adrenaline rapidly became a commercial success, and others marketed equivalent products: Takamine’s patents were challenged in court by HK Mulford Company. In a key ruling by Judge Learned Hand in the US Circuit (District) Court for the Southern District of New York, Takamine’s patents were upheld (73). Judge Hand, who had been on the bench for only 24 months, wrote, “But, even if it were merely an extracted product without change, there is no rule that such products are not patentable. Takamine was the first to make it available for any use by removing it from the other gland-tissue in which it was found, and, while it is of course possible logically to call this a purification of the principle, it became for every practical purpose a new thing commercially and therapeutically.” This foundational (and controversial) precedent affects virtually all pharmaceutical and biotechnology patents to the present day, primarily because “Takamine’s product was patentable as an isolated and purified substance only because purification delivered a transformative difference in utility between the new product and its natural precursor” (74); in other words, the new material was substantially more useful than the natural product, hence it was patentable.
Many early investigators used the name “adrenaline”, as used by Takamine, but this term was copyrighted as a trademark of Parke-Davis, so that “epinephrine” was eventually adopted as the official generic name, with substantial acrimony. Much of the early disagreement among Abel, von Fürth, and Takamine derived from the fact, learned only later, that Uenaka’s crystals of “adrenalin” were contaminated with norepinephrine. In 1906, Ernst Josef Friedmann, who had worked with von Fürth, published the correct chemical structure of epinephrine (75). Hans Meyer and Otto Loewi synthesized “adrenalin” in 1905 but found the pressor effect of the synthetic material to be less than that of the natural product (76); this resulted from the synthesis yielding a racemic mixture of d- and l- optical isomers, whereas the active hormone is only l-epinephrine (77). The epinephrine biosynthetic pathway was proposed by Hermann Blaschko in 1939 (78) and confirmed by others. Epinephrine was distinguished from norepinephrine in the 1940s; the original Takamine “adrenaline” contained about 36% norepinephrine (79).
Finding Steroid Hormones: Early Studies of Adrenal Physiology
Takamine believed he had identified the active principle of the suprarenal gland, and many other early-20th-century investigators also thought the adrenal secreted a single hormone, confounding physiologic work examining the effects of adrenalectomy. In describing the “fight-or-flight” response, Walter Cannon emphasized the sympathetic nervous system and catecholamines but apparently was unaware that adrenocortical hormones also participated (80). Carl and Gerty Cori received a Nobel Prize for describing glycogenolysis (the Cori cycle), which is driven by medullary catecholamines, and noted that adrenalectomy decreased hepatic glycogen (81), but they did not appreciate the role of adrenocortical steroids on carbohydrate metabolism. Glynn had distinguished the functions of the medulla from those of the cortex in 1912 (49), but confusion persisted. Baumann and Kurland may have been first to provide clear evidence for a mineralocorticoid effect by documenting that adrenalectomy (in cats) resulted in hyponatremia and hyperkalemia (82). Rogoff and Stewart showed that adrenalectomized dogs could be kept alive with adrenal cortical extracts (83, 84).
Early efforts to isolate biologically active hormonal substances using extraction procedures with water, saline, or alcohol had been successful with epinephrine (69, 71), thyroxine (85), insulin (86-88) and parathyroid hormone (89) but, as shown by the failure of Brown-Sequard’s testicular extract, had failed with steroids. Frank A. Hartman et al at the University of Buffalo used mild acetic acid and “salting-out” with NaCl to prepare an extract from bovine adrenals that modestly prolonged the life of adrenalectomized cats (90). They concluded: “The hormone of the adrenal cortex has been salted out with NaCl. Cortin is proposed as the name for this substance. Completely adrenalectomized cats injected twice daily with this substance have survived an average of 27.4 days or longer as compared with 5 to 6 days for controls” (90). Philip E. Smith then devised a rat pharyngeal hypophysectomy procedure that spared the pars tuberalis and hypothalamus: the animals stopped growing; the liver, spleen, and kidneys shrunk; prepubertal animals remained prepubertal; and the adrenals, thyroid, and gonads atrophied (91, 92). Smith’s work provided definitive evidence connecting the pituitary to the adrenal and provided important evidence for the role of the hypothalamus (93). In 1930, Swingle and Pfiffner at Princeton (94) and Hartman and Brownell at Buffalo (95, 96) published preliminary reports of the use of lipoidal extraction procedures to prepare adrenocortical material that could sustain the life of adrenalectomized animals and relieve the symptoms of Addison disease; the Swingle-Pfiffner preparation was the first to be used to save an adrenally insufficient, moribund patient at the Mayo Clinic (97). Swingle and Pfiffner improved their preparation somewhat (98, 99), and this preparation was then manufactured and distributed by Parke-Davis under the brand name Eschatin. A few papers describe successful treatment of Addison disease (100) and possible CAH (101) with this preparation, but it was of unclear composition and low potency in vivo (102) and in vitro (103).
Noting that “the sodium content of the blood of patients suffering from Addison’s disease is decreased, as it is in adrenalectomized cats,” Robert F. Loeb at Columbia University showed that oral administration of saline alone extended the life of a patient with Addison’s disease (104), but the basis of this effect remained unclear until aldosterone was isolated 20 years later. George Harrop at Johns Hopkins Hospital similarly noted high potassium and low sodium in animals and patients with adrenal insufficiency. At the Mayo Clinic, patients with Addison’s disease were given a high-sodium, low-potassium diet; some patients were stabilized for months on this diet.
Harvey Cushing (105) described pituitary tumors associated with what we now call Cushing syndrome and noted that many of the clinical features observed resembled those seen in patients with adrenal tumors, and some of the autopsy reports mentioned adrenal enlargement, but the pituitary-adrenal linkage was unclear. His concluding paragraph clearly centers on the pituitary: “While there is every reason to concede, therefore, that a disorder of somewhat similar aspect may occur in association with pineal, with gonadal, or with adrenal tumors, the fact that the peculiar polyglandular syndrome, which pains have been taken herein conservatively to describe, may accompany a basophil adenoma in the absence of any apparent alteration in the adrenal cortex other than a possible secondary hyperplasia, will give pathologists reason in the future more carefully to scrutinize the anterior-pituitary for lesions of similar composition.” It was apparent that the pituitary did not control all adrenal function, because adrenalectomy killed animals more rapidly than hypophysectomy (106), but our contemporary understanding of the hypothalamic-pituitary-adrenal axis developed much later [reviewed in (107)]. Menkin observed an anti-inflammatory effect of adrenal extracts in 1940 but thought this reflected a reduction in capillary permeability (108). In Montreal, Hans Selye reported that rats receiving “nocuous agents” (exposure to cold, surgery, spinal transection, sublethal doses of drugs or formalin) exhibited a 3-phase stress response that included adrenal hyperplasia and involution of the thymus—the discovery of glucocorticoid action (109). He integrated these and other findings into the conceptualization of the hypothalamic-pituitary-adrenal axis, defined what we now know as “the stress response,” coined the terms “mineralocorticoid” and “glucocorticoid,” and emphasized that both classes of steroids were needed for survival (110). Thus, chemical identification of adrenocortical factor(s) was needed.
Finding Steroid Hormones: Early Studies of Adrenal Steroid Chemistry
Heinrich Otto Wieland and Adolf Otto Heinrich Windaus will always be remembered together. These German chemists were personal friends and colleagues and won sequential Nobel Prizes in Chemistry in 1927 and 1928 (both awarded in 1928) for work with sterols (111, 112). Both also openly resisted the Nazi regime and refused to sign the Nazi loyalty oath in 1933 [“Bekenntnis der Professoren an den Universitäten und Hochschulen zu Adolf Hitler und dem nationalsozialistischen Staat” (the “Vow of allegiance of the Professors of the German Universities and High-Schools to Adolf Hitler and the National Socialistic State”)] but were shielded from Nazi persecution by their status as Nobel laureates (113). Wieland was a professor in Munich and in Freiburg who received the Nobel “for his investigations of the constitution of the bile acids and related substances” (111). Windaus, a professor in Göttingen, received his Nobel “for the services rendered through his research into the constitution of the sterols and their connection with the vitamins” (specifically, vitamin D) (112). Illustrating the difficulty of determining complex chemical structures with the tools available in the 1920s, Wieland and Windaus published structures for cholesterol, but both got it wrong (Fig. 9). Rosenheim and King reported the correct structure in 1932 (114).
Figure 9.
Structures proposed for cholesterol. Left, structure according to Heinrich Wieland (111) (in the public domain at https://www.nobelprize.org/uploads/2018/06/wieland-lecture.pdf). Center, structure as proposed by Adolf Windaus (112) (in the public domain at https://www.nobelprize.org/uploads/2018/06/windaus-lecture.pdf). Right, structure as proposed by Rosenheim and King (114).
In 1933, Carl von Ossietzky, the German author, pacifist, and publisher of the political journal The World Stage was arrested by the Nazis and imprisoned at Esterwegen prison and then at the Sonnenburg concentration camp (where he died of tuberculosis in 1938) for documenting German rearmament in violation of the Treaty of Versailles, beginning during the Weimar Republic (115). In response, Hitler ordered all Nazis to refuse Nobel Prizes, much to the dismay of many scientists who viewed themselves as candidates (and had signed the Nazi loyalty oath). In 1939, Windaus’s former student Adolf Freidrich Johann Butenandt (Nazi Party member #3716562) and Leopold Ruzika (a Swiss/Croatian) were awarded the Nobel Prize in Chemistry for isolating estrone, androstenedione, and progesterone. Butenandt apparently lacked his former mentor’s ethics. On orders from the Reich, Butenandt declined the prize and instead was given the infamous “War Merit Cross” by Hitler in 1942 (among the approximately 100 other Nazis so recognized were Adolf Eichmann, Josef Mengele, and Albert Speer). Ruzika delivered his Nobel lecture in 1945, after World War II (116). Butenandt appealed to the Nobel Committee and was officially listed as a Nobel laureate in 1949 and received the diploma and medal but no prize money, and he did not deliver a Nobel lecture. Butenandt directed the Kaiser Wilhelm Institute for Biochemistry from 1937 to 1945, directing biochemical research for Germany’s war effort (115). After the war, Butenandt returned to science and attempted to rehabilitate his reputation: with Karlson he isolated and determined the structure of the insect steroid hormone ecdysone (117).
Major advances in steroid chemistry were made by Tadeus Reichstein, whose family escaped the pogroms in Russian-occupied Poland and eventually settled in Switzerland. Reichstein became a Swiss citizen and received his doctorate from the Swiss Federal Institute of Technology (ETH) in Zürich in 1922, working under Hermann Staudinger (1953 Nobel Prize in Chemistry) and later under Leopold Ruzicka (1939 Nobel Prize in Chemistry) (Figs. 10, 11). Much of Reichstein’s biography has been recorded by Miriam Rothschild, with whom he later isolated insect cardioglycosides (118). Working with his lifelong assistant Joseph von Euw, Reichstein first achieved prominence in organic chemistry by isolating aromatic components of coffee in 1931 (119) and then by devising what is now known as “the Reichstein Process” for synthesizing vitamin C (first isolated in 1928 by Albert Szent-Gyorgyi) (114, 120). His work with steroid hormones at ETH was well underway (121, 122) supported by the Dutch pharmaceutical company NV Organon, but Ruzicka, the director at ETH, had a contract with CIBA that required that patent rights concerning steroid research had to be assigned to CIBA; Reichstein’s contract with Organon excluded this, so Reichstein left in 1938 to become chair of the Department of Pharmaceutical Chemistry in Basel. From 1938 to 1950, Reichstein published hundreds of papers describing the isolation, chemical characterization, and synthesis of over 2 dozen steroids (123). Prior work to identify, purify, and isolate hormones, such as epinephrine, thyroxine, insulin, and parathyroid hormone, had been facilitated by their abundant intracellular storage, but this is not the case for steroids. Steroidogenic cells do not produce steroids and store them for later release; the rate of steroid release is determined by steroid synthesis so that the amount of steroid in an adrenal cortex is surprisingly low (as described later, this ultimately led to the discovery of the steroidogenic acute regulatory protein in the 1990s). Starting with ~500 kg adrenals from 10 000 cattle (provided by NV Organon, whose main business was extracting bovine insulin), Reichstein isolated and determined the structures of 28 adrenal “corticoids,” only 5 of which had biological activity. In 1953, 3 years after winning the Nobel, in collaboration with Sylvia Simpson and James Tait, he isolated aldosterone (124, 125).
Figure 10.
Structures of the 28 steroids that were chemically characterized by Reichstein between 1938 and 1949 (123) (in the public domain at https://www.nobelprize.org/uploads/2018/06/reichstein-lecture.pdf).
Figure 11.
Left, Tadeus Reichstein (1897-1996) as a well-dressed graduate student at the Swiss Federal Institute of Technology in Zürich, 1923. From (118); reproduced courtesy of Schweitzerische Stiftung für die Photographie. Right, The Kendall lab, 1948, with the principal investigators of the use of cortisone for rheumatoid arthritis. Left to right: Charles H. Slocumb, Howard F. Polley, Edward C. Kendall, and Philip S. Hench [from (126), reprinted with permission].
The preparation of “cortin” by Swingle and Pfiffner (94, 98, 99) spurred great interest in adrenal steroids in the United States. Most notably and contemporaneously with Reichstein, Edward C. Kendall at Mayo Clinic was trying to isolate adrenal steroids in the 1930s. Kendall was an established figure: he had purified thyroxine in 1915 (127) and had isolated glutathione and determined its structure (128), but his attempts to determine thyroxine’s structure failed. Harington and Barger at University College London published the correct structure in 1927 (129). In 1929, Albert Szent-Gyogyi, who had isolated vitamin C, was a visiting professor at Mayo for 8 months, where he prepared vitamin C from bovine adrenals. It was at this time that the Swingle-Pfiffner adrenocortical extract (“Eschatin”) had been used by Leonard G. Rowntree, chair of medicine at Mayo, to help save an adrenally insufficient, moribund patient (97). As related by Ingle in his obituary for Kendall for the National Academy of Sciences (130), Rowntree then urged Kendall to pursue the preparation of adrenal extracts, rather than just making cortin, Kendall decided to pursue the isolation of its active principle. Kendall made an arrangement with Parke-Davis to receive about 900 pounds of bovine adrenal glands each week, Kendall would separate crude epinephrine for Parke-Davis and retain the “left-over” cortical material to prepare the cortin that was used to treat Mayo’s Addisonian patients who had failed treatment with their high-sodium, low-potassium diet; this was also the starting material for his efforts to purify the cortical hormone. Like others in the field, Kendall initially thought there was only 1 adrenocortical hormone. Many adrenal steroids will cocrystalize and appear to represent a single compound, but the work of Reichstein in the 1930s showed this was not the case; by about 1934, most investigators acknowledged that the adrenal secreted multiple biologically active steroids. By 1935, 4 groups were trying to isolate adrenal compounds: Kendall at the Mayo Clinic, JJ Pfiffner who had moved from Princeton to join Oskar Wintersteiner at Columbia University (131), Cartland and Kuizenga working at Upjohn, Inc. (132), and Reichstein in Zurich. By then it was known that adrenocortical secretions influenced carbohydrate metabolism, virilization, and salt and water balance; it was logical to presume that different steroids exerted different effects, but only small amounts of the individual steroids could be prepared, and the animal bioassays then available were insufficiently sensitive. Although there was rivalry among the groups, they also shared samples and information. Reichstein and Wintersteiner were superior chemists, but they lacked assays for biological activity.
In 1934, Dwight J. Ingle (president of the Endocrine Society, 1959-1960) finished his doctorate (in psychology) at the University of Minnesota after joining Kendall’s lab in 1932. Ingle had developed an assay to measure muscular work in anesthetized rats: a 100-g weight was affixed to the gastrocnemius muscle and was stimulated electrically to lift the weight 3 times per second. Normal rats could do this for >14 days, but the work capacity of an adrenalectomized rat fell below that of sham-operated controls within 2 hours, and muscular responsiveness was lost within a day (133). Ingle developed a modified 24-h assay of adrenal cortical extract that was sensitive, fast, and reliable. Replacement with epinephrine was of no avail, but Kendall’s cortical extract permitted nearly complete recovery, in a dose-response fashion: there was now a viable laboratory bioassay for glucocorticoid action (134). Although Kendall’s first crystalline adrenocortical preparation (announced with much fanfare in 1934) (135) was inactive, they were on track. Kendall’s “compound E” (later identified as cortisone) was active in the muscle work test in late 1935 (136); Wintersteiner and Pfiffner (131) and Reichstein (122) had also isolated compound E but were unaware of its biological activity. Kendall then converted compound E to a diketone, and Fred Conrad Koch demonstrated this derivative had androgenic activity that “was one-sixth to one-fourth as active as androsterone in stimulating comb-growth in the capon” (136). Thus, independently and by different tactics, the different groups now knew that compound E was a steroid. Ingle left Mayo for the University of Pennsylvania, where he used his assay to measure and compare the work capacity of multiple, structurally characterized steroids (137).
Steiger and Reichstein prepared 11-desoxy-corticosterone (DOC) in 1937 (138), and several groups showed it could sustain the life of adrenalectomized animals or patients with Addison’s disease (139-142). Kendall’s other compounds, termed “A,” “B,” “E,” and “F,” were 11-oxygenated; Hans Selye called the 11-hydroxy steroids “glucocorticoids” and the 11-deoxy steroids “mineralocorticoids” (110); like many oversimplifications, this one contained a nub of truth. Kendall’s operational alphabetical names were applied before the structures were known; “A” was 11-dehydrocorticosterone, “B” was corticosterone, “E” was 17-hydroxy-11-dehydrocorticosterone (cortisone), and “F” was 17-hydroxy corticosterone (cortisol). Wintersteiner and Pfiffner found that noncrystalline adrenocortical extracts were much more potent than Kendall’s compound E in sustaining the life of adrenalectomized animals (131), and only miniscule amounts of DOC were found in adrenal extracts, suggesting that DOC was not the life-maintaining (salt-retaining) adrenocortical hormone. Much of that activity remained in what Kendall called “the amorphous fraction,” which was later shown to contain aldosterone (124, 125).
With the synthesis of DOC, much of the fire went out of the competition to isolate a clinically important adrenocortical hormone; many major players, including Pfiffner and Wintersteiner and Cartland and Kuizenga, left the field. Kendall remained committed. World War II essentially ended expensive animal experimentation but also stimulated adrenal research. It was rumored that Germany was procuring beef adrenals in Argentina and preparing an adrenocortical extract that permitted Luftwaffe pilots to fly to higher altitudes (130). The rumors about Argentine adrenals were false, but the US military considered that adrenocortical extracts might be useful in treating traumatic shock and surgical shock and might improve soldiers’ responses to stressors. The medical research division of the Office of Scientific Research and Development gave high priority to the synthesis of Kendall’s compound A as a precursor to making the more active compound F (cortisol). Several pharmaceutical groups were involved, and communications with Reichstein were maintained; by the end of the war, Merck and Co. had prepared ~100 g of compound A, but animal studies failed to show utility in hypoxia or surgical or traumatic shock, and clinical studies showed little value in Addison’s disease. Most of the chemists involved quit the project (130). Nevertheless, Kendall convinced Merck to continue work toward compound E, and, by 1948, they had prepared ~9 g of compound E; by this time Merck had invested over $14 million on the project. With no proven medical use, Merck was ready to “pull the plug” on compound E.
Philip Showalter Hench was the Mayo Clinic’s first rheumatologist; he had been recruited by Leonard G. Rountree and was appointed head of the new Division of Rheumatology in 1926 at age 30. In 1929 he began to take interest in how the symptoms and clinical courses of arthritis differed in patients who also had other conditions. Hench was a brilliant clinical observer who could see patterns in disease that others had not. He first reported symptomatic improvement in 16 patients with chronic rheumatoid arthritis who also had hepatitis with jaundice (most caused by the hepatotoxicity of the analgesic drug cinchophen) (143). In 1938, he reported 31 more cases; he noted that the degree of symptomatic remission correlated with the degree of jaundice rather than with the cause of the liver disease and that administering bile or bile salts did not reproduce the analgesic effect (144). He also noted that arthritis and allergies improved in pregnant patients and that the benefits persisted long after parturition or the remission of the liver disease, suggesting that the salutary effects were not mediated by metabolites of bilirubin. Hench was a student of medical history, noting dozens of earlier reports about spontaneous temporary remission of arthritis associated with hepatic injury or pregnancy, but none of the earlier reports pursued the observation, considered similarities and differences among the reports, or tried systematic interventions (145). He postulated that the effects were due to an unknown, endogenous “substance x” that was made in both men and women (144).
Kendall and Hench began collaborating in about 1938; in 1941 they agreed to try compound E for arthritis when sufficient material became available. They tried “cortin” in 3 patients, with negative results (146). Hench’s military service in World War II and the high cost and difficult preparation of compound E delayed the planned trial. In September of 1948, compound E became available from Merck and was injected into a severely affected arthritic patient, yielding a dramatic improvement. The clinical study was expanded; almost all arthritic patients so treated went into a remission that lasted so long as the treatment was continued. Intramuscular adrenocorticotropic hormone (ACTH) yielded similar results. This produced great excitement at Mayo, but caution initially prevailed, and the results remained confidential for several months. There were concerns that the doses used to suppress the symptoms of arthritis were large, and it was suspected that prolonged high-dose treatment would cause Cushing’s syndrome, cause adrenal atrophy, or inhibit the pituitary’s ability to control adrenal secretions normally (147). Nevertheless, the results became known to the press, and great excitement ensued about a “cure” for arthritis. Hench, Kendall, and their collaborators (Fig. 11) described their basic work and clinical results in great detail, cautioning that compound E was experimental, that it was not a cure, and that overdose effects were likely (146), but the press and the public didn’t understand or care. A movie clip of a wheelchair-bound arthritic patient arising and walking after 3 days of intramuscular cortisone (100 mg/d) was seen widely, and there was tremendous pressure on the Mayo Clinic and Merck to provide compound E. Kendall, Reichstein, and Hench equally shared the 1950 Nobel Prize for Medicine or Physiology (123). In Hench’s speech at the Nobel banquet, he acknowledged Kendall and Reichstein by saying “Perhaps the ratio of one physician to two chemists is symbolic, since medicine is so firmly linked to chemistry by a double bond.” Many chemists received Nobels for work with sterols (Wieland, Windaus, Butenandt, Ruzika, Kendall, Reichstein), but Hench’s unique contributions vaulted steroids from an esoteric branch of organic chemistry to the very center of medicine and pharmacology.
With the advent of the clinical use of cortisone and the identification of the structures of the “alphabet soup” of compounds identified by Reichstein and Kendall, the golden age of steroid chemistry was nearly over. Only 1 major question remained—the nature of the life-sustaining component (sometimes called “electrocortin”) that powerfully influenced electrolyte balance presumably an 11-desoxy-steroid in Kendall’s “amorphous fraction” of adrenal extracts. Kendall did not pursue this question, but Reichstein and his colleagues did. Simpson and Tait developed a sensitive bioassay that permitted the crystallization of 21 mg of electrocortin from 500 kg of bovine adrenals (124) and, with the subsequent determination of its structure, the last major biologically active steroid, aldosterone, had been discovered [reviewed in (148)], greatly aided by the new technique of paper chromatography (149).
History of CAH Due to 21-Hydroxylase Deficiency
To most endocrinologists, the term “congenital adrenal hyperplasia” means “steroid 21-hydroxylase deficiency” (21OHD), but in fact there are multiple forms of CAH; these are attributable to mutations in different genes and to variations in severity and clinical phenotypes of mutations within single genes. We consider the history of each form separately. Lipoid CAH is discussed in the section titled “The Role of Corticotrophin (ACTH) in Regulating Adrenal Steroidogenesis.”
Clinical Investigation
Understanding of CAH as a distinct entity developed fairly rapidly in the 1930s; several cases were reported, and Marrian and Butler established that the urinary steroid pregnane-3,17,20-triol (pregnanetriol) was elevated in all cases of adrenal virilism (both CAH and virilizing adrenocortical carcinomas) (150-152), while others, notably Russell E. Marker, identified multiple steroids in the urine of pregnant women (153). Marker subsequently achieved fame and fortune by inventing the “Marker Degradation” for preparing progesterone from diosgenin extracted from Mexican Dioscorea root, the origins of the oral contraceptive industry (which will not be covered here). Fifteen years later, Bongiovanni et al showed that urinary pregnanetriol was the hepatic metabolite of 17-hydroxyprogesterone (17OHP) (154). However, the early measurements urinary pregnanetriol required huge volumes of urine (>1 week’s excretion) and was technically demanding, hence its use as a marker for CAH had to await technical advances.
The 1940s saw the first scientific clinical investigations of CAH, reported by 2 of the founding fathers of pediatric endocrinology, Lawson Wilkins (1894-1963) at Johns Hopkins University and Nathan B. Talbot (1909-1994) at Massachusetts General Hospital. Talbot was 15 years younger than Wilkins, but their research careers with CAH began contemporaneously in the late 1930s. By this time, it was generally understood that the adrenal made several hormones that did different things: DOC acted on electrolyte balance, corticosterone (and later compound E) raised blood sugar via gluconeogenesis, and as-yet-undescribed hormones could result in virilization. Working under Allan M. Butler (chief of the Children’s Medical Service and staff physician in charge of the Chemical Laboratories at Massachusetts General Hospital), Talbot co-authored a case report of probable CAH, published in December 1939 (101); that report mentions Wilkins’ oral presentation of a similar case, published in March 1940 (155). The case reported by Butler and Talbot was a male infant followed from age 2 weeks to 20 months with skin pigmentation, enlarged genitalia (but small testes), a propensity to “collapse” with associated hyponatremia and hyperkalemia if fluids or salt were withheld, urinary excretion of large amounts of “estrogens and androgens,” and successful maintenance with added salt and bicarbonate (101). Treatment with the Parke-Davis “Eschatin” adrenal extract was ineffective, but the addition of 5 g of sodium chloride and a charcoal adsorbate of adrenal cortical extract containing “3 rat units” daily resulted in weight gain without edema and normalization of the serum Na and K, but the adrenal extract without added sodium was ineffective (the “rat units” probably refer to Ingle’s muscle-work bioassay, described earlier). The patient could be maintained with parenteral cortical extract from Upjohn “providing 4 rat units daily.” A diet supplemented with sodium chloride and sodium bicarbonate yielded moderate weight gain and improved levels of sodium and potassium.
Lawson Wilkins had been in the private practice of pediatrics in Baltimore for 14 years before establishing the world’s first pediatric endocrine clinic at Johns Hopkins in 1935. Personal biographies of Wilkins have been published by his daughter (156) and by his former trainee and colleague Claude Migeon (157); a summary of his career is contained in Delbert Fisher’s history of pediatric endocrinology (158). Wilkins was at the forefront of efforts to link anatomical adrenal hyperplasia with functional adrenal insufficiency and androgen excess. Wilkins encountered a 31/2-year-old boy with “marked development of his secondary sex organs” who had developmental delay, mental retardation, low serum sodium, and a profound craving for salt, a clinical point he emphasized in his first report of this child (155). Wilkins’ more detailed report of this early case of CAH combined astute clinical observation with then-new bioassays of steroids (159). In addition to the salt craving, Wilkins reported polyuria, polydipsia, and a deceased 5-week-old “female pseudohermaphrodite” sibling. His “parents noticed at birth that his penis and scrotum were unusually large”; he developed acne at 5 months, pubic hair at 15 months, and asymmetric testicular enlargement “several months later.” He was thin, tall, muscular, generally pigmented (including the gums), and had asymmetric pubertal-sized testes; blood pressure was 90/50 (Fig. 12). Laboratory data showed normal glucose, calcium, and phosphorus; hemoglobin of 16.3 g/100 ml; white count of 19 000; and elevated nonprotein nitrogen. He died unexpectedly on day 6 of hospitalization. “Immediately after his death blood was taken by cardiac puncture for chemical studies, which showed serum sodium 111 m.eq. per liter.” Autopsy showed grossly enlarged adrenals (total weight 27 g) with “accumulation of large cells” “characteristic of the androgenic type.” The testes contained masses containing cells “similar to those described in the adrenals” but without Leydig cells (ie, probable testicular adrenal rest tumors). A castrate rat bioassay for urinary androgens showed 30 IU per day, the same as that of a normal adult man. Alcohol extracts of 6.3 g of testicular tissue yielded 5 IU of androgen, whereas 31 g of normal adult testicular tissue yielded no detectable androgen. Using the cock’s comb bioassay for androgens initially described in 1849 by Arnold Berthold (160), an extract of 3.5 g of adrenal tissue “failed to show measurable androgen,” but extract from 2 g of adrenal tissue “caused growth when rubbed into the comb of a 11-day-old-chick over a period of 7 days.” Wilkins’ discussion proposes that “female pseudohermaphrodites” have hyperplasia of the “prenatal zone” of the adrenal and that virilizing tumors contained the same tissue, indicating that this “androgenic zone” is distinguished from “the electrolyte-controlling portions of the cortex, the ‘interrenal cells.’”
Figure 12.

Patient with congenital adrenal hyperplasia reported by Wilkins et al (159), with scrotal enlargement suggesting the presence of testicular adrenal rest tumors.
A friendly rivalry arose between Boston and Baltimore. Boston had all the advantages—a larger clinical base, outstanding biochemistry, and the world’s best endocrinologist: Fuller Albright. Talbot described 12 additional patients with CAH, focusing on 17-ketosteroid (17KS) excretion and contrasting them with patients with virilizing adrenocortical carcinomas (161). Talbot’s 1943 paper about a patient with Addison’s disease (probably due to autoimmune polyglandular syndrome type 1) also reported increases in urinary 17KS in CAH and showed that unilateral adrenalectomy was not valuable in CAH (162). Albright distinguished adrenogenital syndrome (CAH) from Cushing syndrome in 1943 (163). Debate concerning the roles of the pituitary and adrenal in Cushing syndrome persisted (164) until about 1950, when Bauer concluded that both the pituitary and the adrenal can separately cause “the so-called Cushing’s syndrome” and that the term “Cushing’s disease” be reserved for the pituitary form (165).
Talbot’s principal interests were in practical steroid chemistry: he worked with George Langstroth at MIT to devise apparatus for determining urinary androgens in clinical laboratories (166) and refined existing colorimetric assays for 17KS (161, 167, 168) and corticoids (168). However, Talbot’s 1950 paper describing the long-term follow-up of the patient he had described with Butler et al in 1939 added no new clinical, physiological, or chemical insights (169). And despite their shared institutional affiliation and interests, Talbot and Albright collaborated only once, correlating urinary “11-oxocorticosteroid-like substances” (17-hydroxycorticosteroids) and the apparent activity of what they called the “S-hormone” (designating the “sugar-active” adrenal hormone(s), cortisol) (170). Fuller Albright only became involved in CAH in 1950.
In 1949, Zuelzer and Blum proposed that “the combination of cortical insufficiency, masculinization, and diffuse adrenal hyperplasia forms a distinct anatomic-physiologic entity” (171). Their literature survey found 9 papers published from 1937 to 1945 reporting 17 cases, including those reported by Wilkins (155) and by Butler (101) cited earlier. They presumed the disorder to be fairly common as they found 4 cases among 1068 autopsies of children 0 to 13 years old, with most cases in infancy. The genital virilization in the females was typical of the congenital form of the adrenogenital syndrome “in which excessive production of androgens by the fetal adrenals is thought to inhibit differentiation of female structures and to stimulate growth of the sex organs along male lines of development.”
The state of understanding of adrenal pathophysiology just before CAH was first treated with cortisone is vividly shown by the presentations by Talbot and by Wilkins at the 1949 meeting of the American Academy of Pediatrics and the following question and answer dialogue (172). Talbot reviewed steroid structure-function relationships and their physiologic actions. He described DOC as the principal “Na-K hormone,” 11-oxo-corticosteroids as the “sugar-fat-nitrogen” hormone(s) active in carbohydrate metabolism, and an “androgenic, or protein anabolic ‘N’ hormone” that is metabolized to 17KS. He speculated about their importance in normal physiology but did not address disease states. Wilkins discussed (i) “adrenogenital syndromes,” distinguishing congenital (CAH) from “postnatal” (adrenal tumors), and (ii) “Cushing’s syndrome,” which he described as of hypothalamic, pituitary, or primary adrenal origin, and correctly listed the apparent steroidal abnormalities in each. His description of the anatomy of girls with CAH is quite contemporary; he erred in saying that CAH is more common in girls than boys but correctly suggested that boys may be under-ascertained because of infant death and lack of distinct early anatomic findings. Notably, he distinguished CAH from precocious puberty based on testicular enlargement (an important point of the physical exam that many physicians still overlook today). He illustrated infants treated successfully with DOC or “adrenal cortical extract” and discussed the differential diagnosis of the various forms of hyperandrogenism. This paper is remarkable in showing Wilkins’ early understanding of adrenarche, presumed adrenal insufficiency in infants, and the proper management of “pseudohermaphroditism” in CAH, issues that remain under investigation today. At about the same time he showed that adrenalectomy had salutary effects in CAH but that its efficacy was severely limited by then-inadequate adrenal replacement therapy (173).
The isolation and synthesis of cortisone and its success in treating Addison’s disease and arthritis quickly suggested use in CAH. Wilkins saw the potential of Kendall’s compound E, but Talbot had become involved in nonsteroidal areas of endocrinology and was not involved in the revolutionary treatment of CAH with cortisone. By 1949, Wilkins was clearly the leading authority on CAH, but the Boston-Baltimore rivalry remained, as Fuller Albright and his fellow Frederic C. Bartter at Massachusetts General Hospital entered this academic contest. Fuller Albright had an unmatched career of clinical and basic endocrine investigation in the 1930s and 1940s, mostly in bone metabolism, but his progressive Parkinson’s disease impaired him severely in the 1940s. Wilkins and others had successfully treated salt loss with deoxycorticosterone but failed in treating CAH with ACTH or testosterone (174). Wilkins then published a brief “Preliminary Report” in the Bulletin of the Johns Hopkins Hospital describing suppression of urinary 17KS in 1 CAH patient treated with 100 mg cortisone daily (175); the paper was received on Feb 13, 1950, and published in April; the Bulletin did not use external review. Wilkins begins: “Unsuccessful attempts have been made by us (unpublished) to suppress the secretion of 17-ketosteroids in patients with this disorder by the administration of steroids, such as 17-ethyl testosterone, 17-vinyl testosterone, 17-methyl androstenediol and 17-methyl androstanediol, which have a chemical structure similar to that of androgens but possess relatively little androgenic activity” and concludes that cortisone “suppressed the secretion of adrenal hormones responsible for the urinary excretion of 17KS” (175). Wilkins did not mention the pituitary or ACTH. At the same time, Bartter presented an abstract on May 1, 1950, at the annual meeting of the American Society for Clinical Investigation describing his and Albright’s unsuccessful treatment of CAH with ACTH but successful treatment with cortisone. They concluded: “These findings suggest that the adrenogenital syndrome results, not from an abnormal pituitary stimulation of the adrenal, but from an abnormal adrenal response to a normal pituitary” (176). Both groups followed their preliminary reports with full papers. Wilkins’ paper was received for publication on Nov 21, 1950, and was published in Jan 1951 (177); Bartter’s paper was received on Nov 2, 1950, and was published in Feb 1951 (178). Each group cited the other’s preliminary report. Wilkins began by describing unsuccessful treatments with steroids “which have a chemical structure similar to that of androgens but possess relatively little androgenic activity” before trying cortisone. Wilkins correctly concluded: “We suggest that the action of cortisone is to suppress the output of pituitary adrenocorticotropin, thereby causing marked diminution of the secretion of the pathologic adrenals” (177). Bartter and Albright approached the problem by considering the similarities and differences between CAH and Cushing’s syndrome and concluded with a diagram of the correct physiology (Fig. 13) (178). Thus Wilkins and Bartter/Albright deserve equal credit for proposing the treatment of CAH with cortisone. Following the reports of successful treatment of CAH with cortisone, Wilkins exerted tremendous effort to optimize its use and investigate the effects of cortisone on sexual development, growth, electrolyte metabolism, hypertension, and testicular development (179-183). These detailed metabolic balance studies established the therapeutic approach to CAH that largely remains in effect today (184, 185). Talbot also made important further contributions, showing that both cortisone (186) and diethyl stilbesterol (187) could cause growth arrest. Albright was rendered vegetative by unsuccessful chemopallidectomy for his Parkinson’s in 1956, and Bartter went on to describe syndrome of inappropriate antidiuretic hormone ADH release but did not follow up on the work with CAH.
Figure 13.
The physiology of congenital adrenal hyperplasia, described by Bartter et al in 1951 (178) (in the public domain at https://www.jci.org/articles/view/102438/pdf). In these diagrams, “S” does not refer to Reichstein’s compound S (11-deoxycortisol) but to the then-unknown steroid active in sugar metabolism (now known to be cortisol); “N” refers to the then-unknown adrenal androgen that influences nitrogen retention (now known to be 11-keto-testosterone). In the right-hand panel, “E” refers to Kendall’s compound E (cortisone).
What Is Disordered in CAH?
Both Wilkins and Bartter/Albright assessed responses of patients with CAH to treatment with cortisone by measuring urinary 17KS, but they did not know they were dealing with steroid 21OHD. Before the biochemical lesion could be identified, a general knowledge of the adrenal steroidogenic pathways was needed. The 30 steroids identified by Reichstein and others could, hypothetically, be arranged into several different potential steroidogenic pathways. Oscar Hechter and Gregory Pincus at the Worcester Foundation for Experimental Biology in Shrewsbury, Massachusetts, devised the novel procedure of isolating bovine adrenals with their arteries and veins intact, then infusing a potential precursor into the adrenal artery and measuring the steroids in the venous effluent (188, 189). With the new availability of radiolabeled steroids, the specificity of the conversions was assured (190). When adrenals were perfused with 14C-labeled acetate, cholesterol, or progesterone, ACTH stimulated incorporation of 14C into steroid products only when cholesterol was the precursor; ACTH had no effect on conversion of acetate to cholesterol (191). This study established that ACTH acts on the conversion of cholesterol to progesterone. However, their data with 14C-acetate suggested the existence of steroidogenic pathways that do not involve cholesterol; later studies showed that this is not the case. With this powerful new technique, Hechter and Pincus proposed the first steroidogenic pathways (192, 193) (Fig. 14). These and other studies were often inconsistent and difficult to apply to measurements from human patients, as early investigators did not anticipate the significant differences in the enzymes and pathways of steroidogenesis among various mammals, especially human beings.
Figure 14.
The first steroidogenic pathway, as proposed by Hechter and Pincus in 1954 (193); reprinted with permission. The pathway is largely correct, as acetate is converted to cholesterol through a complex, 30-step pathway, represented by “x.” However, none of the intermediates in that pathway is converted to progesterone or other steroids without first being converted to cholesterol.
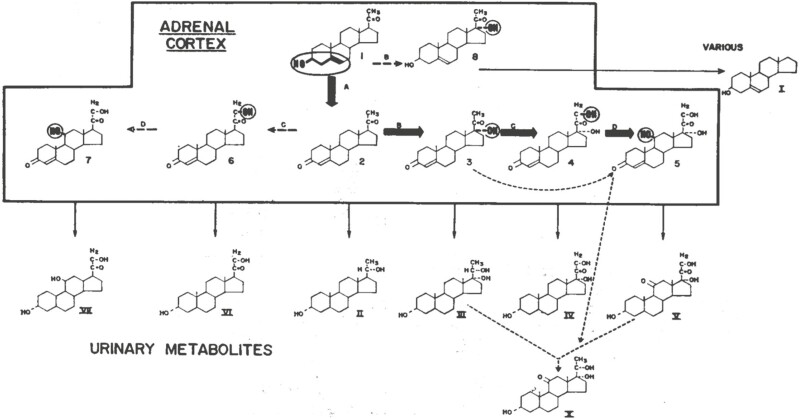
Alfred M. Bongiovanni (1921-1986) trained in steroid chemistry with William Eisenmenger at Rockefeller (194, 195) and clinically with Wilkins at Hopkins, where he became an assistant professor and worked independently, establishing the steroid assay lab at Johns Hopkins (196, 197). In 1955 he showed that different patients with CAH had different steroidogenic lesions: most accumulated pregnanetriol in their urine, indicating an inability to convert 17OHP to compound F(ie, 21OHD), but 1 patient had hypertension and “large quantities of Reichstein’s compound S (11-deoxycortisol) and its metabolites” in blood and urine. This was the first steroidal evidence for a variant form of CAH [11-hydroxylase deficiency (11OHD)] and permitted the drawing of the first truly modern steroidogenic pathway (Fig. 15) (198, 199). In a footnote he adds, correctly, “The hypertension described as a complication of the adrenogenital syndrome may be due to the secretion of desoxycorticosterone.” Bongiovanni followed up on this work with even more sophisticated steroidal analyses showing that (i) CAH is due to a lack of “21-hydroxylase,” (ii) that normal and 21OHD adrenals have an “11-hydroxylase,” and (iii) 21-hydroxylation precedes 11-hydroxylation in the human adrenal (200).
Figure 15.
Bongiovanni’s steroidogenic pathway from 1963 (199). Cortisol is (5) and corticosterone is (7). The various enzymes, A (3 β-hydroxysteroid dehydrogenase), B (17-hydroxylase), C (21-hydroxylase), and D (11-hydroxylase) are illustrated, and their sites of action are shown by the encircled heavy type. The most common form of congenital adrenal hyperplasia is characterized by a lack of 21-hydroxylase (C) and hence an accumulation of 17-hydroxyprogesterone (3) with the excretion of large quantities of the latter’s reduced metabolite, pregnanetriol (III) (reprinted with permission; ©1963 Massachusetts Medical Society. All rights reserved).
Although Bongiovanni had distinguished CAH caused by 21OHD from CAH caused by 11OHD in 1955 (see “11OHD”) (198), this was not immediately accepted. In a study with Joseph Jailer, the prominent steroid chemist Seymour Lieberman (1916-2012; president of the Endocrine Society 1974-1975) concluded: “The evidence accumulated to date does not appear to indicate that congenital adrenal hyperplasia is characterized by a single enzymatic block” (201). Definitive evidence for a defect in 21-hydroxylation in most patients with CAH came from 2 groups in 1957. Fukushima and Gallagher concluded: “Thus this patient exhibited a serious deficiency of 21-hydroxylation with an excessive amount both of steroids intermediate in hydrocortisone production and ‘adrenal androgens,’ defined by the metabolites androsterone and etiocholanolone” (202). Similarly, Bongiovanni and Elder found that “These data thus confirm earlier indirect work pointing to a lack of ‘21-hydroxylase’ and the presence of ‘11-hydroxylase’ in the adrenal glands of patients with the normotensive form of this disorder. The results also suggest that 21-hydroxylation occurs prior to 11-hydroxylation in man” (200). The “block” at 21-hydroxylation was reconfirmed by other work (200, 203). Whether 21-hydroxylation precedes or follows 11-hydroxylation was unclear; if 21-hydroxylation occurred first, 17OHP would accumulate in 21OHD, but if 11-hydroxylatione occurred first, 21-deoxycortisol would accumulate. Actually, both pathways are active. Jailer was the first to report that both 21-deoxycortisol and 17OHP were elevated in CAH (204), and he studied the roles of both steroids in CAH, but he focused on 17OHP because it was thought to be an androgenic steroid (201). Thus the lack of apparent hormonal activity for 21-deoxycortisol, and the close correlations among urinary 17KS, urinary pregnanetriol, and serum 17OHP, resulted in 17OHP becoming the analyte of choice in 21OHD. Although 17OHP is the steroid typically studied in CAH, there are advantages to measuring 21-deoxycortisol, especially in infancy (205).
Description of Nonclassic CAH
By the late 1970s, several groups had reported individuals in whom signs of androgen excess—such as premature adrenarche, menstrual irregularity, hirsutism, or severe acne—developed postnatally, without evidence for adrenal insufficiency but with biochemical abnormalities, suggesting a mild form of 21OHD (206-208). Based on human leukocyte antigen (HLA) associations (see later), it appeared that this disorder—variously termed “late onset,” “acquired,” or “nonclassic” CAH—likely represented an allelic variant of “classic” CAH, and that some affected individuals ascertained in family studies were entirely asymptomatic and were said to have “cryptic” CAH (209). Lenore Levine and Maria New at Cornell developed data relating baseline and ACTH-stimulated blood levels of 17-hydroxyprogesterone, showing that individuals with nonclassic and classic CAH could be readily distinguished but that obligate heterozygous CAH carriers could not be reliably distinguished from unaffected individuals (210). The development of immunoassays for 17OHP in dried blood spots (211) made it possible to develop newborn screening for CAH with the aim of averting potentially fatal salt-wasting crises in neonates (212). This approach has proven successful and has been widely implemented [reviewed in (185, 213)].
Identifying the 21-Hydroxylase Enzyme
The determination that CAH was caused by a lesion in 21-hydroxylation led to efforts to isolate and characterize the responsible 21-hydroxylase enzyme. Ryan and Engel showed that steroid 21-hydroxylation by bovine adrenal microsomes was inhibited by carbon monoxide and that the reaction was reversible by light (214). Working on hepatic drug metabolism, Julius Axelrod (Nobel Prize in Medicine 1970) and Bernard Brodie had recognized the existence of an enzyme system in the endoplasmic reticulum that could oxidize xenobiotics, noting that the system was unusual in that it required both oxygen and reduced nicotinamide adenine dinucleotide phosphate (NADPH; then called TPNH) (215). They speculated that “these systems are not essential to the normal economy of the body, but operate primarily against the toxic influences of foreign compounds that gain access to the body”; this turned out to be incorrect, as P450 enzymes are essential to normal physiology, but it did lead to the discovery of cytochrome P450. In 1958, Martin Klingenberg and David Garfinkel (independently) identified a “carbon monoxide binding pigment” in liver microsomes with an absorbance at 450 nm (216, 217). Different personal views of the history of the discovery of P450 have been written by major players in this field (218-220).
Tsuneo Omura and Ryo Sato showed that this hepatic system involved a heme-protein that they named ‘‘cytochrome P450” (221), and they developed the classic procedure of P450 difference spectra to quantitate P450 (222, 223). Upon joining Ronald Estabrook’s lab in Philadelphia in 1963, Omura demonstrated this CO-binding protein (or proteins) was also found in the adrenal cortex. David Cooper, Ronald Estabrook, and Otto Rosenthal developed the method and apparatus that showed that a cytochrome P450 was catalyzing 21-hydrozylation, as light at 450 nm reversed the CO inhibition (224, 225). (Figs. 16 and 17). The Cooper method established that 21-hydroxylase was a cytochrome P450. It took several more years to show that the P450 moiety actually was the catalytic component and not a generic cofactor (hence the outdated and incorrect term “P450-dependent enzyme”), but the discovery of P450-mediated 21-hydroxylation initiated the isolation of steroidogenic enzymes. The initial work with P450 concerned “microsomal” enzymes, but Hechter’s lab showed that the cholesterol side-chain cleavage reaction occurred in mitochondria (226), leading to the demonstration of mitochondrial P450 by Simpson and Boyd (227, 228). Thus cytochrome P450 enzymes could be either microsomal or mitochondrial. It is now known that the human genome encodes 57 distinct P450 enzymes (229). As all of these enzymes are membrane-associated, purification of active enzymes depended on solubilization and stabilization with judiciously selected detergents. Although cytochrome P450 enzymes require auxiliary proteins for activity, these were not required to purify P450s, because P450 enzymes shift their absorbance spectra from a “low-spin” to a “high-spin” state upon binding the substrate, so that the presence of the enzyme during purification could be monitored by assessing the intensity of the difference spectrum in the various fractions.
Figure 16.
Left, Spectrum for the light-mediated reversal of the inhibition of bovine adrenal microsomal 21-hydroxylase by carbon monoxide [from (224)]. Right, Diagram of home-made apparatus built by Cooper et al to measure the reversal of CO-induced P450 spectrum and 21-hydroxylase activity of bovine adrenal microsomes [from (225); reprinted with permission].
Figure 17.
Cooper lab, 1964. Left to right: Levine, Slade, Narasimhulu, Omura, Foroff, Rosenthal, Cooper, and their homemade apparatus for measuring the photochemical action spectra of adrenal microsomal cytochrome P450 [from (230); reprinted with permission].
Kominami, Takemori, and colleagues at Hiroshima University purified cytochrome P450c21 (now termed “CYP21A2”) from bovine adrenal cortex microsomes by hydrophobic chromatography using an ω-amino-n-octyl Sepharose column in the presence of Emulgen 913, a nonionic detergent (231). The cytochrome P450 was immunochemically different from the mitochondrial cytochrome P450s catalyzing the side-chain cleavage of cholesterol and the 11β and 18 hydroxylation of 11-deoxycorticosterone (see later). The purified cytochrome P450 catalyzed C-21 hydroxylation of 17α-hydroxyprogesterone and progesterone when mixed with NADPH-cytochrome P450 reductase.
One of us (PCW) used this purification procedure while working in Bo Dupont’s laboratory at Sloan-Kettering Institute and then generated a rabbit antiserum that he used to screen a bovine complimentary DNA (cDNA) library constructed in a primitive bacterial expression vector, thus isolating a partial cDNA for CYP21A2 (232). Full-length human cDNA (233, 234) was isolated subsequently.
Genetics of 21OHD
A major step in unraveling the genetic basis of 21OHD came from a collaboration between Bo Dupont and Lenore Levine, who found that siblings with 21OHD were almost invariably identical for HLA transplantation antigens. In the initial paper reporting 6 affected kindreds (235), 1 genetic recombination further localized the affected gene near the HLA-B locus. Additional papers from this collaboration confirmed HLA linkage and identified linkage disequilibrium (allelic associations at linked loci) between various forms of 21OHD and different HLA haplotypes (236). In particular, a small proportion of patients with classic CAH carried an unusual HLA haplotype, Bw47;DR7, whereas many patients with mild, nonclassic (also termed “late onset” or “cryptic”) CAH carried HLA-B14;DR1 (237). Moreover, the HLA-Bw47;DR7 haplotype carried a null allele for 1 of the 2 C4 genes encoding the fourth component of serum complement, which were known to be located in the HLA complex between HLA-B and HLA-DR. This made it extremely likely that this haplotype represented a contiguous gene syndrome, with a single deletion encompassing one of the C4 genes and the as-yet-uncharacterized 21-hydroxylase gene (238). The Dupont lab isolated lymphocytes from a CAH patient who was homozygous for HLA-Bw47;DR7 (and thus presumably homozygous for a deletion of the 21-hydroxylase gene) and immortalized them with Epstein-Barr virus to have an unlimited source of DNA for further studies.
PCW and colleagues compared DNA from this cell line with normal DNA by Southern blot hybridization using their recently isolated partial length bovine cDNA clone. Hybridizing normal DNA digested with any of several restriction enzymes produced 2 bands, whereas only 1 band was obtained using DNA from the CAH cell line, strongly suggesting that there were in fact two 21-hydroxylase genes and deletion of 1 of them could cause 21OHD (239). This was confirmed by isolating clones for the entire genomic region, which was greatly facilitated by the knowledge that the 21-hydroxylase genes were near the C4 genes, and overlapping human (240, 241) and mouse (242, 243) genomic clones for that region were already available. It quickly became apparent that there was a tandem duplication in both humans and mice [in humans, this duplication encompassed 30 kilobasepairs (kb) of DNA], with 2 genes for C4 alternating with 2 21-hydroxylase genes. One of us (WLM) showed that the bovine genome also contained 2 21-hydroxylase genes (244). In humans, the restriction endonuclease sites of the 21-hydroxylase gene adjacent to the C4B gene corresponded to the missing band in DNA from the patient with CAH. DNA from a cell line that was homozygous for a deletion of C4A, obtained from an otherwise normal individual, was indeed missing the bands corresponding to the 21-hydroxylase gene adjacent to C4A. These findings suggested that the 21-hydroxylase B gene (now termed CYP21A2) was the normally active gene but that the 21-hydroxylase A gene (CYP21A1P) was not functional. This was confirmed when sequences of the genes were obtained (234, 245), demonstrating that the genes were ~98% identical, but the 21-hydroxylase A gene was nonfunctional by virtue of at least 9 deleterious mutations.
Although the polymerase chain reaction was proposed in 1985 (246), initially it could not be used to analyze the 21-hydroxylase genes because of their high degree of sequence similarity, which made it impossible to locate gene-specific primers close enough together to be practical with early polymerase chain reaction reagents. Thus the first point mutations were identified by screening genomic libraries constructed from DNA from patients with various forms of 21OHD. Surprisingly, all mutations identified in early studies (247-250) were also found in the 21-hydroxylase A pseudogene and were therefore presumably generated by gene conversion events. Expression of mutant enzymes in cultured cells (251) and comparison of the frequencies of different mutations in patients with different levels of disease severity (252, 253) demonstrated excellent genotype-phenotype correlations and established the salt-wasting, simple virilizing, and nonclassic forms of the disease as allelic variants. Based on genetic analysis of thousands of patients [summarized in (185)], 90% of alleles represent intergenic recombinations between the CYP21A1P pseudogene and CYP21A2. Approximately 20% are deletions of 30 kb generated by unequal crossing-over during meiosis (254), with the remainder representing gene conversions that could occur during meiosis or mitosis.
In San Francisco, WLM characterized several additional transcripts originating from the region of the 21-hydroxylase genes. Among these were 2 that were antisense to the 21-hydroxylase genes; one of these, originally termed XB (now termed TNXB), partially overlaps the CYP21A2 gene for 21-hydroxylase and encodes tenascin-X, an extracellular matrix protein (255). Mutations of TNXB may occur without 21OHD and cause a severe, autosomal recessive form of Ehlers-Danlos syndrome (256), or partial deletions that also involve CYP21A2 may cause a contiguous gene syndrome (257), now termed CAH-X, including both CAH and a milder form of Ehlers-Danlos syndrome (258). The history of Tenascin-X has been reviewed recently (259).
Other Forms of CAH
11β-hydroxylase deficiency (11OHD)
The existence of an 11β-hydroxylase had first been demonstrated by Hechter and Pincus (189), and a “hypertensive form” of CAH was first noted by Wilkins in his classic 1951 paper describing the first use of cortisone (177). Two patients had hypertension. A 31/2-year-old boy (“case 2”) with virilization (“macrogenitosomia praecox”), enlarged adrenals seen at surgery, and testicular adrenal rest tumors had baseline blood pressure of 150/100, but his response to cortisone therapy could not be monitored because he developed mania and therapy was stopped. An 18½-year-old girl with “pseudohermaphroditism” (case 7) had progressive virilization, adrenal hyperplasia seen at surgery, and blood pressure of ~150/100 on most occasions. Treatment with 50 mg cortisol, initially daily but later on alternate days, reduced the pressure to 104/78. Later that year, Shepard and Claussen reported a 2½-year-old virilized “infant Hercules” with surgically proven adrenal hyperplasia and hypertension averaging 160/100; a 20-month-old sibling appeared to be similarly affected (260). Treatment with cortisone, initially at 40 mg/d and tapered to 10 mg on alternate days, normalized the blood pressure; further studies were not done. In his third follow-up paper on treatment of CAH, Wilkins described 3 patients with hypertensive CAH whose blood pressures normalized with cortisone (181), but studies of the basis of hypertension in these cases, as opposed to salt loss in other patients, were not pursued. As noted earlier, Bongiovanni had distinguished CAH caused by 21OHD from CAH caused by 11OHD in 1955, principally aiming to distinguish 21OHD from a variant form of CAH in which large amounts of tetrahydro-S (the metabolic product of 11-deoxycortisol or Reichstein’s compound S) were excreted in the urine (215). They followed up that clinical report with detailed studies of the plasma and urinary steroids in this “hypertensive form of CAH” in which paper chromatography confirmed the presence of compound S in the blood and its metabolites in the urine (261). They concluded “No 11-oxygenated C21 or Cl9 steroids were detected in either the blood or urine. The abnormal steroid metabolites disappeared from the urine during administration of hydrocortisone. These findings suggest an essentially complete deficiency of adrenal ‘11β-hydroxylase’ in the hypertensive form of congenital adrenal hyperplasia.”
In the mid-1970s, teams at the University of California Irvine led by Peter Hall (262, 263) and at Kanazawa University led by Masayuki Katagiri and Shigeki Takemori (who later moved to Hiroshima University) (264) purified 2 distinct P450 cytochromes, termed “P450scc” and “P450c11,” from bovine adrenocortical mitochondria. P450scc catalyzed the formation of pregnenolone from cholesterol, whereas P450c11 catalyzed the hydroxylation of deoxycorticosterone at 11β- and 18-positions. Partial length bovine P450c11 cDNA clones were isolated by Waterman’s group (265) and used by White and colleagues to obtain more complete bovine and human cDNA sequences (266); bovine cDNA was independently isolated by Morohashi et al at Kyushu University in Fukuoka (267).
Attempts to isolate the human genomic gene for P450c11 revealed that there were, in fact, 2 genes: 1 (CYP11B1) expressed at high levels and the other (CYP11B2) at much lower levels (268). The second gene was expressed at much higher levels in RNA from an aldosterone-secreting adenoma. Expression studies (269) demonstrated that the enzyme encoded by CYP11B1 had 11β-hydroxylase activity, whereas the enzyme encoded by CYP11B2 also had strong 18-hydroxylase activity and was able to synthesize aldosterone starting from deoxycorticosterone. This latter enzyme was therefore termed “aldosterone synthase” and often referred to as P450aldo or P450c11AS. Similar findings were obtained in cattle (270).
Studies of deficiency states were facilitated by the fact that they occurred in specific populations. In Jews of Moroccan origin, 11OHD occurs relatively frequently: ~1/7000, greater than the frequency of 21OHD in most populations. All such patients carry the same mutation in CYP11B1, Arg-448 to His (R448H), which is located in a conserved peptide, which also contains the cysteine residue that represents the fifth ligand of the catalytic heme group (271).
Aldosterone Synthase Deficiency
The most frequent defect of aldosterone biosynthesis is 21OHD, but rare patients have aldosterone deficiency in the context of entirely normal cortisol and sex steroid synthesis. A detailed review of early reports of this disorder was published in 1981 (272). In 1961, Royer et al (273) at the Hopital des Enfants-Malades (Paris, France) described a brother and sister with profound salt wasting and severe aldosterone deficiency but no other clinical or biochemical features of CAH. Stanley Ulick in New York studied a similar patient in 1964, finding a low aldosterone secretory rate by isotopic dilution (measuring the radioactivity of urinary metabolites after injecting a known amount of radiolabeled aldosterone) but increased secretion of 18-hydroxycorticosterone (274). Similar findings were obtained by Visser and Cost in a study of 3 patients in the Netherlands (275): urinary aldosterone concentrations were undetectable, despite increased excretion of corticosterone, dehydrocorticosterone, and 11-deoxycorticosterone. The excessive production of aldosterone precursors was suppressed by treatment with exogenous mineralocorticoid, which also permitted resumption of normal physical growth and development. Additional studies (276, 277) revealed that different patients could have either increased or decreased secretion of 18-hydroxycorticosterone. This steroid was considered to be a biosynthetic intermediate resulting from the hydroxylation of the methyl group (C18) of corticosterone; the hydroxymethyl group would then be further oxidized to yield aldosterone. Ulick proposed (278) that these successive reactions be termed “corticosterone methyl oxidase (CMO) I and II,” and that isolated aldosterone deficiency could be classified as either “CMO I” or “CMO II” deficiency, depending on whether 18-hydroxycorticosterone was decreased or increased, respectively. Although these data were consistent with 2 distinct enzymes acting in succession on corticosterone, Ulick observed (278, 279) that 18-hydroxycorticosterone was converted to aldosterone much less well than corticosterone when added to adrenal slices, suggesting (correctly) the possibility that corticosterone is converted to aldosterone by a single enzyme through an enzyme-bound intermediate.
A number of kindreds affected with CMO II deficiency were ascertained in a highly endogamous group of Jews originating from Isfahan, Iran, confirming an autosomal recessive mode of inheritance (280, 281). Levels of 18-hydroxycorticosterone were confirmed as being a reliable biochemical marker of the disease, particularly in affected adults, who often had normal electrolytes even without treatment. The disease in these kindreds was closely linked to polymorphisms in the CYP11B gene(s) (282) and eventually shown to result from homozygosity for 2 mutations in CYP11B2, Arg-181 to Trp (R181W) and Val-286 to Ala (V386A), with homozygosity for both mutations required to cause the disease (283).
Glucocorticoid-remediable Aldosteronism
In the mid-1960s, Laidlaw and colleagues in Toronto (284) and New and Petersen in New York (285) described similar patients with moderately severe hypertension from a young age, hypersecretion of aldosterone, suppressed plasma renin, and rapid reversal of these abnormalities after administration of glucocorticoids. In each case there was a similarly affected parent, strongly suggesting autosomal dominant inheritance. The lack of reliable biochemical markers initially made this condition—termed “dexamethasone-suppressible hyperaldosteronism” or “glucocorticoid-remediable aldosteronism”—difficult to ascertain, but by the mid-1980s markedly elevated levels of 18-hydroxycortisol and particularly 18-oxocortisol—to 20 to 30 times normal—were documented in affected patients (286-288). 18-Hydroxycortisol and 18-oxocortisol are 17α-hydroxylated analogs of 18-hydroxycorticosterone and aldosterone, respectively. Because 17α-hydroxylase is not expressed in the zona glomerulosa, the presence of large amounts of a 17α-hydroxy, 18-oxo-steroid, suggested that an enzyme with 18-oxidase activity (ie, aldosterone synthase, CYP11B2) was being abnormally expressed in the zona fasciculata, or alternatively that there was hyperplasia of a population of “transitional cells” that expressed all of the necessary biosynthetic enzymes (286). When the CYP11B1 and CYP11B2 genes were cloned, their patterns of expression identified, and the activities of the encoded enzymes determined, it was apparent that intergenic recombinations analogous to those causing 21OHD could create a chimeric gene that encoded a CYP11B2-like enzyme expressed like CYP11B1 (269). Indeed, it was quickly shown by Lifton and colleagues (289, 290) (first studying an especially large affected kindred (291)) and by PCW and colleagues (292) that all patients with glucocorticoid-remediable aldosteronism have the same type of mutation, a chromosome that carries 3 CYP11B genes instead of the normal 2 (Fig. 18). The middle gene on this chromosome is a chimera with 5’ and 3’ ends corresponding to CYP11B1 and CYP11B2, respectively. The chimeric gene is flanked by presumably normal CYP11B2 and CYP11B1 genes. The invariable presence of a chimeric gene in patients with this disorder suggests that this gene is regulated like CYP11B1 (expressed at high levels in the zona fasciculata and regulated primarily by ACTH) but with enzymatic activity like the aldosterone synthase enzyme encoded by CYP11B2.
Figure 18.
Unequal crossing-over between CYP11B1 and CYP11B2. CYP11B2 is on the left and pairs with the 95% similar CYP11B1. The direction of transcription is from left to right. A and C and B and D are homologous regions 5’ and 3’, respectively, of the CYP11B genes. A crossover between these paired genes during meiosis results in 1 gamete with duplicated genetic material, containing a hybrid gene with 5’ sequences from CYP11B1 and 3’ sequences from CYP11B2. The presence of a single copy of such an allele is sufficient to explain the features of glucocorticoid-remediable aldosteronism. The second gamete is deficient, containing only the complementary hybrid gene [from (292); figure provided by Prof. Perrin C. White].
3β-Hydroxysteroid Dehydrogenase (3β-HSD) Deficiency
Leo T. Samuels, at the University of Utah, noted that all biologically active steroids are in the Δ 4 arrangement, with a double bond in the A-ring between carbons 4 and 5, and that “Cholesterol and Δ 5-3-ol intermediates are presumed to be precursors of the active hormones,” and that hence “Various tissues were investigated for an enzyme that would convert the latter type of structure to the α,βunsaturated ketone” (293). He examined bovine ovarian follicles, corpora lutea and adrenal cortex, uterus, placenta, and liver and “three types of tumors of mice,” finding that “only those tissues that normally form hormones with the α,βunsaturated ketonic structure contained the enzymes” could convert Δ 5-pregnenolone to an α,βunsaturated ketone as evidenced by the appearance of a strong absorption band at 238 to 240 mμ,“in the presence of DPN as a hydrogen acceptor.” This was the first description of 3β-hydroxysteroid dehydrogenase (3β-HSD) activity. In 1956, Beyer and Samuels separated bovine adrenal homogenates into crude nuclear, mitochondrial, microsomal, and soluble fractions; activity was initially found in all fractions, with the highest activity per mg of protein in the microsomes, but after additional fractionation and washing procedures, they concluded that 3β-HSD activity was confined to the microsomes (294). This remained a dominant view, although multiple studies identified various subcellular locations [reviewed in (295)] until the use of immuno-electron microscopy showed that 3β-HSD was found in both microsomes and mitochondria (296).
Alfred M Bongiovanni, who had moved from Johns Hopkins to the University of Pennsylvania in 1954, identified the first patients with 3β-HSD deficiency. He published a preliminary “Letter to the Editor” describing 3 infants who died who had elevated urinary Δ 5 steroids (including 17KS) and unmeasurable urinary Δ 4 steroids; treatment with hydrocortisone (cortisol) normalized the excretion of urinary Δ 5 steroids (297). His follow-up paper reported 6 cases with both clinical and steroidal data and with citation to Samuels’ 1951 Science paper (298). Three girls (2-7 weeks old) had minimal virilization (labial fusion) and salt loss; 2 boys (ages 8 weeks and 3 months) had hypospadias and salt loss, but the 6-year-old boy did not have salt loss. Because it is now known that genetically proven 3β-HSD deficiency is very rare (see later), Bongiovanni’s accumulation of so many apparent cases of true 3β-HSD deficiency would seem to be incompatible with the very low incidence of this disorder. Recent work has identified a cluster of genetically homogeneous cases of 3β-HSD deficiency among the Old-order Amish population (299), which is centered in Pennsylvania’s Lancaster and York counties, near Philadelphia; unfortunately, Bongiovanni’s reports do not include family histories or ethnic information that might suggest whether this was the source of his cases.
Robust extra-adrenal 3β-HSD activity was reported in the early 1980s (300, 301); in retrospect this suggested that there must be at least two 3β-HSD enzymes, but that was not appreciated at the time. Enzymatic studies of enzymes purified from rat adrenals and testes (302) and human placenta (303) showed that a single polypeptide catalyzed both the hydroxysteroid dehydrogenase and isomerase activities and was found in both the microsomes and mitochondria (303, 304). Human placental 3β-HSD (later designated type I) cDNA was isolated and characterized by Fernand Labrie’s group in Quebec (305) by expression screening using a polyclonal antibody to the purified enzyme (303) and independently by Ian Mason and colleagues (306). The second human 3β-HSD isoenzyme, chronologically designated as type II, was isolated from a human adrenal cDNA library (307). Transient expression of human 3β-HSD isoenzymes demonstrated that the 3β-HSD and Δ 5-Δ 4-isomerase activities reside within a single protein (306, 307).
Both 3β-HSD isozymes could convert the Δ 5-steroids [pregnenolone, 17-hydroxypregnenolone, dehydroepiandrosterone (DHEA), and androstenediol] to the corresponding Δ 4-steroids (progesterone, 17OHP, androstenedione, testosterone), but the placental/hepatic HSD3B1 had a low Michaelis-Menten constant (Km), permitting it to act on low concentrations of steroids in the circulation, whereas the Km for the adrenal/gonadal HSD3B2 was 10-fold higher, acting only on locally produced, intraglandular steroids. There are 2 human HSD3B genes: HSD3B1 encodes the isozyme found in the placenta, brain, liver, and elsewhere, and HSD3B2 encodes the enzyme found in the adrenals and gonads (306, 308, 309). These are clustered on chromosome 1p13 along with 5 pseudogenes (310). Mutations in HSD3B2 cause CAH due to 3β-HSD deficiency (311). Mutations in HSD3B1 have not been reported. Genetically proven 3β-HSD deficiency is very rare, and many individuals are seen in mid-childhood with premature adrenarche who appear hormonally to be have mild 3β-HSD deficiency but in whom no genetic lesion can be found [reviewed in (295)].
17α-Hydroxylase and 17,20-Lyase Deficiencies
Edward G. Biglieri (1925-2005), a gifted steroid chemist, established the UCSF General Clinical Research Center at San Francisco General Hospital in 1963 (312). He soon encountered a “prismatic case”: a 35-year-old woman with hypertension and hypokalemia who had been sickly in childhood and adolescence, who also had primary amenorrhea and sexual infantilism. Her urine lacked metabolites of cortisol, but the 17KS assay yielded a muddy brown color instead of the expected clear lavender (313). Biglieri developed an assay for urinary tetrahydro-DOC and found it was very high, accounting for the unexpected color in the 17KS assay; the secretory rate of corticosterone was also high. These data, coupled with the sexual infantilism and absent assayable sex steroids, indicated 17-hydroxylase deficiency (314).
Converting C21 steroids to the C19 precursors of sex steroids requires 17,20-lyase activity. Biglieri’s patients with 17-hydroxylase deficiency did not produce sex steroids, but it was not clear if this was because a single enzyme catalyzed both reactions or reflected an absence of precursor 17OH steroids. In 1972, a paper reported apparent isolated 17,20-lyase deficiency (315), appearing to demonstrate that 17-hydroxylase and 17,20-lyase were separate enzymes. Clinical studies in healthy children suggested that adrenal 17-hydroxylase activity (as indicated by serum cortisol) is fairly constant throughout life, whereas adrenal 17,20-lyase activity [indicated by DHEA and dehydroepiandrosterone sulfate (DHEAS)] is low in early childhood, rising rapidly during adrenarche at 8 to 10 years of age (316), supporting the belief that 17-hydroxylase activity and 17,20-lyase activity were catalyzed by separate enzymes. However, Peter Hall’s laboratory, which had moved to the Worcester Foundation for Experimental Biology, showed that both activities were found in a single microsomal P450 enzyme isolated from pig testes, the first demonstration that distinct steroid biosynthetic steps could be catalyzed by a single enzyme (317, 318). Nevertheless, this concept remained controversial. The first cDNA for the P450 catalyzing 17-hydroxylase activity (P450c17, later termed CYP17A1) was isolated by Michael Waterman’s laboratory in Dallas by screening a bovine adrenal cDNA library with probes made from size-fractionated mRNA from ACTH-treated bovine adrenocortical cells; the correct size fraction was identified by hybrid-selected translation and immunoprecipitation using a specific CYP17A1 antiserum, and a negative control was prepared from cells that had not been treated with ACTH (319). This group then transfected nonsteroidogenic mammalian COS-1 cells with a vector expressing this cDNA, showing that the cells acquired both 17-hydroxylase and 17,20-lyase activities, thus establishing that this 1 enzyme catalyzes both activities (320).
Contemporaneously, WLM collaborated with Peter Hall and with John Shively’s laboratory at Beckman Research Institute of the City of Hope in Duarte, California; they obtained an 83% complete amino acid sequence of porcine CYP17A1 and used this amino acid sequence data to construct degenerate oligonucleotide probes used to screen a porcine adrenal cDNA library, yielding a short partial sequence. The porcine cDNA was then used to screen their human adrenal cDNA library and a commercially obtained human testicular cDNA library, yielding identical CYP17A1 sequences from both sources, demonstrating that the same gene is expressed in both tissues (321). Waterman and colleagues also isolated human cDNA and expressed it in COS-1 cells, demonstrating that it had similar activity to the bovine enzyme (322). The full-length gene, now termed CYP17A1, was then cloned and sequenced, showing it is most closely related to the CYP21A2 gene encoding steroid 21-hydroxylase (323).
The first mutation in CYP17A1, in a patient with both 17-hydroxylase and 17,20-lyase deficiencies, was reported in 1988 (324). Genetically proven cases of isolated 17,20-lyase deficiency appeared later: some patients had mutations in CYP17A1 (325, 326), others had mutations in cytochrome b5 (325, 326), and 1 had a mutation in P450 oxidoreductase (327). These reports show that 17,20-lyase deficiency is a syndrome, not a single disease (328). The patients originally reported in 1972 (319) were eventually found to have mutations in the AKR1C2 and AKR1C4 enzymes in the alternative “backdoor pathway” of androgen synthesis, thus they did not have 17,20-lyase deficiency (329).
P450 Oxidoreductase
P450 oxidoreductase (POR), the enzyme that transfers electrons from NADPH to microsomal cytochrome P450 enzymes, also transfers electrons to many other recipients, hence it was discovered independently in multiple contexts. Early studies identified it as an NADPH-dependent (then called TPNH-dependent) cytochrome c reductase (330, 331) and other studies showed it was required for microsomal P450-mediated hydroxylation reactions (215, 332, 333). POR was then localized to the endoplasmic reticulum (334, 335) and linked to the activities of microsomal P450 enzymes (336), but it was not until 1969 that it was proven that P450 and POR were distinct membrane-bound proteins that interact with one another (337). All of these early biochemical studies assayed POR activity by its capacity to reduce cytochrome c. However, cytochrome c is a mitochondrial protein that does not encounter microsomal POR within a cell; thus, this simple, time-honored assay was convenient but not physiologic, and turned out to be unreliable for assessing the residual activity of disease-causing POR mutants and other human POR variants (338). Analysis of the activities of multiple P450 enzymes supported by different POR variants has shown that no single assay describes POR activity; its activity must be assayed with the specific form of P450 (or other electron recipient) that is being studied (338, 339).
In 1985, Peterson et al described a patient with genital ambiguity and an abnormal urinary steroid profile suggesting partial combined deficiencies of 17α-hydroxylase, 17,20-lyase, and 21-hydroxylase (340). Knowing that these activities were catalyzed by microsomal cytochrome P450 enzymes that must receive electrons from P450 POR, WLM suggested that this patient had a disorder in POR (341) but was unable to obtain any patient samples. Others sought mutations in the CYP17A1 gene (342) and CYP21A2 gene (343), but none were found. Investigators addressing the genetics of drug metabolism created POR knockout mice (344, 345). These knockouts were lethal; the animals probably died due to disruption of extrahepatic P450 enzymes, as liver-specific knockout of POR produced phenotypically normal, reproductively competent mice that had markedly impaired hepatic drug metabolism (346, 347). Similarly, globally hypomorphic POR mice also had impaired hepatic drug metabolism (348). The hypothesis that a POR mutation might explain Peterson’s findings was thought to be incompatible with this embryonic lethality of POR-knockout mice. Thus the POR hypothesis lay dormant until DNA became available to WLM’s lab from such patients, resulting in the first reported cases of POR deficiency (349). This initial report included 3 patients who also had the Antley-Bixler skeletal malformation syndrome (ABS) (characterized by craniosynostosis, midface hypoplasia, radiohumeral or radioulnar synostosis, arachnodactyly, and bowing of the femora), and 1 who had PCOS without ABS. Fibroblast growth factor receptor 2 (FGFR2) mutations cause ABS without defective steroidogenesis (343). Peterson’s original patient from 1985 also had no reported skeletal anomalies; a large study of a heterogeneous population demonstrated the independent segregation of POR and FGFR2 mutations. The steroidogenic disorder, with or without ABS, was caused by recessive POR mutations, whereas ABS without disordered steroidogenesis was caused by dominant FGFR2 mutations (350). POR deficiency is now a well-understood pediatric endocrine disorder that has expanded understanding of POR (351).
3’-Phosphoadenosine 5’-Phosphosulfate Synthase 2 Deficiency
Besides POR deficiency (Antley-Bixler syndrome), another example of discovering an adrenal defect hiding in a skeletal dysplasia syndrome concerns 3’-phosphoadenosine 5’-phosphosulfate synthase 2 (PAPSS2) deficiency. Many tissues make DHEA, but only the adrenal converts DHEA to DHEAS, catalyzed by SULT2A1, a sulfotransferase (352). SULT2A1 uses 3’-phosphoadenine-5’-phosphosulfate (PAPS) as its sulfate donor, which in turn is synthesized by PAPSS2 in the adrenal and cartilage (353). PAPSS2 deficiency was first reported in a Pakistani family with the rare skeletal defect “spondyloepimetaphyseal dysplasia/brachyolmia type 4” (MIM #603005) (354), but steroidal assays were not included in this or other early reports. In 2009, a 6-year-old girl evaluated for clinical hyperandrogenism had elevated circulating DHEA, androstenedione, and testosterone but no DHEAS, suggesting a defect in SULT2A1. No mutations were found in the SULT2A1 gene, but the patient had compound heterozygous PAPSS2 mutations; she also had minimal vertebral anomalies only seen radiographically (355). SULT2A1 cannot function in the absence of PAPSS2, resulting in overproduction of unconjugated C19 androgen precursors and consequent virilization (356).
Acute and Chronic Regulation of Adrenal Steroidogenesis
In the history of hormone purification, epinephrine (69, 70) and thyroxine (85) came first, followed by a succession of polypeptide hormones, including insulin (86), parathyroid hormone (89), and others; these successes were primarily due to the abundant storage of these hormones in their tissues of synthesis. Isolation of steroid hormones, as described in “Finding Steroid Hormones: Early Studies of Adrenal Steroid Chemistry,” was substantially more difficult because of the very small amounts of hormone present in steroidogenic glands. This dichotomy suggested that the regulation of steroid synthesis and release differed fundamentally from the mechanisms regulating these processes for polypeptide hormones. Histologic studies identified eosinophilic granules (secretory vesicles) in cells producing polypeptide hormones, suggesting that these cells stored previously synthesized protein hormones for rapid release, but no such granules were found in steroidogenic cells. Thus a major question became how steroid hormone synthesis and release were regulated. In 1943, Marthe Vogt at the University of London showed that, in comparison to the amounts extractable from tissues, adrenal glands produce large amounts of corticoids, calculating that, in 1 day, a 10 kg dog secretes the amount of corticosteroid found in 17.3 kg of adrenal tissue (357). This indicated ongoing steroid synthesis and presaged the realization that steroid synthesis and release are directly linked, with little intraglandular storage. The infusion of steroidal precursors into the artery of isolated bovine adrenals and measurement of steroids in the venous effluent yielded the first pathways of corticosteroid biosynthesis [reviewed by (193)] and showed that ACTH stimulated steroid biosynthesis (191).
Chronic Effects of ACTH
ACTH affects the adrenal at 3 distinct physiologic levels. The first action to be identified was the slowest: the very-long term promotion of adrenal growth, as hypophysectomy in dogs profoundly reduced adrenal weight (358). The role of the pituitary in maintaining adrenal weight was confirmed by Philip E. Smith (91, 92), and, in 1930, James B. Collip, famous for devising the successful purification procedure for insulin used in 1923, reported preliminary purification of an “adrenotropic hormone” that stimulated rat adrenal growth (359). In the absence of a simple, rapid assay, efforts to purify ACTH languished until Stone and Hechter showed that ACTH promoted conversion of cholesterol to pregnenolone—the birth of the field of “steroidogenesis” (191).
The second, temporally intermediate, action of ACTH [or luteinizing hormone (LH)] is to stimulate [via 3′,5′-cyclic adenosine 5′-monophosphate (cAMP)] the transcription of genes encoding the steroidogenic enzymes, especially the rate-limiting cholesterol side-chain cleavage enzyme, P450scc (now termed CYP11A1). In cultured bovine adrenal cells, ACTH induced accumulation of CYP11A1 mRNA within 8 hours (360), and the kinetics of the increase in CYP11A1 mRNA were paralleled by the increased steroid secretion by human granulosa cells cultured with hCG (361). These 2 actions, on adrenal growth and adrenal gene transcription, constitute the “chronic response.”
ACTH preparations were quickly employed clinically to assess adrenal function. An initial assay for primary adrenal insufficiency measured the fall in the eosinophil count after an injection of ACTH (362), but this test lacked specificity, given that epinephrine produced the same effect. By the early 1950s, urinary cortisol metabolites (17-hydroxy corticoids) could be measured readily. In a 1953 editorial in the Journal of Clinical Endocrinology & Metabolism, George Thorn at Harvard suggested a procedure comprising a baseline 24-hour urine collection, followed by an 8-hour continuous intravenous infusion of 20 to 25 units of ACTH (this is equivalent to 250 mcg, similar to the modern test), followed by a second urine collection for 24 hours (including the 8-hour infusion) and analyzed for 17-hydroxycorticoids. He suggested that secondary adrenal insufficiency—including iatrogenic adrenal suppression from hydrocortisone treatment—could be distinguished by a subnormal response to a single intravenous infusion but with a gradual improvement in response to infusions repeated over several days. Long-acting ACTH preparations were developed to stimulate the chronic response; “Acthar Gel” was developed by Armour & Company, a meatpacking company, and approved by the Food and Drug Administration in 1952 for multiple inflammatory diseases as a long-acting substitute for oral steroids; American Cyanamid Corp. established the complete 39 amino acid sequence of pig ACTH and showed that residues 1 to 24 were sufficient for full biological activity, providing the basis for the synthetic cosyntropin used today (363).
Acute Effects of ACTH
The third, and most rapid, response of steroidogenic cells to tropic stimulation is the mobilization of cholesterol from cellular stores to move to the outer mitochondrial membrane and its transfer to the inner mitochondrial membrane. This constitutes the “acute steroidogenic response.” In the 1960s Ferguson (364) and Garren (365, 366) used inhibitors of protein synthesis (cycloheximide or puromycin) to show that the action of ACTH on the adrenal was not direct but required the synthesis of 1 or more additional protein(s). Garren et al also showed that this action of ACTH was rapid and that the cycloheximide-sensitive protein(s) had a short half-life (365). In the late 1960s, Simpson and Boyd had shown that all steroidogenesis begins with the conversion of cholesterol to pregnenolone by the mitochondrial cholesterol side-chain cleavage enzyme P450scc (CYP11A1) (227, 228). That enzyme system is very slow and is the enzymatic rate-limiting step (367). However, because cholesterol is insoluble in water, other factors are needed for it to reach the mitochondria to initiate steroidogenesis; this constitutes the rate-limiting step in steroidogenesis (368). These and other studies, elegantly reviewed in detail by Stocco and Clark (369), led to the model that the acute regulation of steroidogenesis required a hormonally stimulated, rapidly synthesized, labile protein that appeared to mediate the transfer of cholesterol across the 2 mitochondrial membranes to reach CYP11A1. Several candidates were proposed to function as this acute regulator of steroid biosynthesis, but essential roles for all of these proteins were eventually ruled out [reviewed in (370)].
The responsible protein was independently identified by Nanette R. Orme-Johnson and colleagues at Boston University and Douglas M. Stocco at Texas Tech University, using 2-dimensional gel electrophoresis of newly synthesized proteins labeled with radioactive phosphorus. Orme-Johnson identified a 30 kDa ACTH-induced mitochondrial phosphoprotein in mouse adrenocortical cells, and a similar LH-induced protein in rat corpus luteum cells and mouse Leydig cells (371-374), the appearance of which was temporally related to the induction of steroid hormone biosynthesis. Stocco and colleagues identified a similar candidate mitochondrial protein in mouse MA-10 Leydig cells (375, 376). When cDNA encoding this protein was isolated and constitutively expressed in MA-10 cells, it induced steroid hormone synthesis even in the absence of LH stimulation; Stocco termed this protein the “steroidogenic acute regulatory” protein (StAR) (377). The essential role of StAR in steroidogenesis was proven when WLM, collaborating with Stocco and others, showed that its coexpression with the CYP11A1 system in nonsteroidogenic cells induced steroidogenesis and that StAR mutations cause congenital lipoid adrenal hyperplasia (378).
Lipoid CAH
The first reports of lipoid CAH preceded the studies that led to the discovery of StAR. In Zurich in 1955, Andrea Prader (1919-2001) described a 6-week-old phenotypic female who died in an Addisonian crisis; her autopsy showed huge adrenals; no uterus, tubes, or ovaries; and no Barr bodies. Unfortunately, no steroid data were reported (379). Also in 1955, AT Sandison, a pathologist in Glasgow, reported a 3-month-old infant who also died in apparent Addisonian crisis; she had a normal uterus, tubes, and ovaries and lipid deposits confined to the massively enlarged adrenals (Fig. 19); again, no steroid data were reported (380). Prader described a second case successfully treated with glucocorticoids who succumbed to infection; based on the histologic appearance of the autopsied adrenals, he termed the disorder “lipoid CAH.” He discussed the clinical manifestations in genetic males and females but suggested his patients had defective conversion of Δ 5 to Δ 4 steroids (381). Other early clinical reports (382, 383) added no mechanistic insight.
Figure 19.
A sectioned adrenal gland from a patient with congenital lipoid adrenal hyperplasia, termed “lipidosis of the adrenal.” [from Sandison (380); reprinted with permission].
Shimizu et al had shown that conversion of cholesterol to pregnenolone proceeded via 20α-ΟΗand 22-ΟΗ intermediates, suggesting there were 3 enzymes: 20α-hydroxylase, 22-hydroxylase, and 20,22-desmolase (384). Conversion of cholesterol to pregnenolone required mitochondrial P450scc (CYP11A1) (227). Camacho et al concluded that lipoid CAH involved defective conversion of cholesterol to pregnenolone but did not study how this might happen (385). In 1972, Degenhart et al showed that normal adrenal mitochondria converted cholesterol to pregnenolone but that mitochondria from an autopsied patient with lipoid CAH did not; however, they also showed that the lipoid CAH mitochondria could convert 20α-hydroxycholesterol to pregnenolone, suggesting defective 20α-hydroxylation (386). This conclusion was logical but incorrect, because soluble hydroxysterols such as 20α-hydroxycholesterol (and unlike cholesterol) can freely diffuse into the mitochondria, where CYP11A1 resides. In 1977, Koizumi et al reported that mitochondria from another patient with lipoid CAH failed to convert cholesterol to pregnenolone but had normal 11β- and 18-hydroxylase activities; they suggested that the lipoid CAH mitochondria specifically lacked CYP11A1 (387). This study, in conjunction with Camacho’s 1968 clinical report (385), led to lipoid CAH becoming regarded as a defect in the conversion of cholesterol to pregnenolone, and the disorder was incorrection termed “20,22 desmolase deficiency.”
The enzyme termed “20,22 desmolase” was, in fact, mitochondrial P450scc (CYP11A1) (227). In 1973, Peter Hall and colleagues, then at the University of Calironia Irvine, isolated CYP11A1 from bovine adrenocortical mitochondria (262, 388). Partial clones for the corresponding bovine cDNA were isolated by Michael Waterman’s lab in Dallas (360); the first full-length bovine clone was obtained by Morohashi et al in Omura’s lab in Fukuoka (389). WLM used very long, 72-base synthetic oligonucleotides corresponding to segments of the bovine cDNA to show that the corresponding gene was not deleted in patients with lipoid CAH and to clone a partial-length (818 bp) human cDNA (390), which was then used to clone the full-length human cDNA (391). To elucidate the basis of lipoid CAH, WLM sought mutations in CYP11A1, its electron-donating cofactors, or candidate factors thought to facilitate cholesterol entry into mitochondria, but all were normal (392); thus, lipoid CAH was not “20,22 desmolase” deficiency. That study pointed out that infants with lipoid CAH reached term normally, indicating that placental synthesis of progesterone was unaffected, concluding: “Thus, a molecular model of lipoid CAH should implicate a gene involved in adrenal and gonadal, but not placental steroidogenesis.” Following the cloning of mouse StAR (377), Douglas Stocco, Jerome F. Strauss III in Philadelphia, and WLM collaborated on cloning human StAR and showed that it is expressed in the adrenals and gonads but not placenta (393); before that paper was in print, this team identified StAR’s role in lipoid CAH (378). The complex physiology of the disease was explained by the “2-hit” model (394), subsequently confirmed in clinical studies (395, 396) and in knockout mice (397). A milder, “nonclassic” form of lipoid CAH, without phenotypic sex reversal in 46,XY patients but with compensated glucocorticoid deficiency, mild hypergonadotrophic hypogonadism, and occasionally mildly disordered mineralocorticoid secretion was described in 2006 (398).
CYP11A1 (P450scc) Deficiency
Discovering that lipoid CAH was due to StAR mutations did not prove that a similar syndrome was not caused by defects in CYP11A1 or its electron transfer proteins. However, in 1991 Lin et al (392) pointed out that lipoid CAH patients reached term normally and that term gestation requires placentally produced progesterone (to suppress uterine contractility), indicating that the CYP11A1 system functioned normally in the placentas of lipoid CAH fetuses. This suggested that no one would have CYP11A1 deficiency. That logical supposition was falsified when Toshehiro Tajima and Kenji Fujieda at Hokkaido University encountered a patient with the hormonal features of lipoid CAH but with normal StAR genes on both alleles; further genetic sleuthing revealed a heterozygous CYP11A1 mutation (399). The unexpected mutation of CYP11A1 was soon confirmed in other patients, but the manifesting heterozygosity in the Tajima/Fujieda patient remains unexplained (400). A milder, nonclassic form of CYP11A1 deficiency has been reported that is similar to nonclassic lipoid CAH (401).
Familial Glucocorticoid Deficiency
Familial unresponsiveness to ACTH
In 1959 Shepard and colleagues at the University of Washington (402) described 2 sisters who, in their second year of life, developed hyperpigmentation, weakness, and later adreno convulsions. Both children were large for their age. The first child died at 30 months, and autopsy revealed marked adreno cortical atrophy with some clusters of cells remaining only in the zona glomerulosa. The pituitary gland was largely composed of small cells with pale cytoplasm—a finding similar to what was seen in patients dying of Addison’s disease. Her sister presented at 28 months with a blood glucose of 10 mg/dl, but electrolytes were normal. She was unresponsive to intramuscular ACTH but responded well to intramuscular cortisone with resolution of hyperpigmentation and muscle weakness. Aldosterone secretion was normal. The authors considered and ruled out CAH, autoimmune Addison’s disease, panhypopituitarism, and adrenal insufficiency associated with hypoparathyroidism and moniliasis (ie, APS-1)—a remarkably complete differential for 1959. In 1968 Claude Migeon and colleagues described 6 such patients in 5 kindreds; 3 patients each had at least 1 sibling who had died in infancy or early childhood (403). These patients all responded clinically to cortisone but not to ACTH. An adrenal biopsy was attempted in 1 patient that became a total unilateral adrenalectomy. Histology showed preservation of the zona glomerulosa but absence of the zona fasciculata. Incubation of tissue slices with ACTH produced no response, whereas incubation with cAMP produced a marked increase in corticosterone (but not cortisol) production, suggesting that the lack of ACTH responsiveness was not a consequence of defective cAMP signaling. These authors named this syndrome “congenital adrenocortical unresponsiveness to ACTH,” thus specifying a mechanism. Very high levels of ACTH were subsequently documented in similar patients (404). These facts made it likely that at least some cases were the result of mutations in an as-yet-unidentified cellular receptor for ACTH. Unfortunately, these latter authors used the term “familial glucocorticoid deficiency,” which did not specify a mechanism and permitted the application of this term to several unrelated disorders.
In 1992, Roger Cone and colleagues at Oregon Health Sciences University cloned 2 melanocortin receptors by polymerase chain reaction-amplifying cDNA fragments from a human melanoma cell line that contained a high level of binding sites for melanocyte-stimulating hormone (MSH), using relatively nonspecific primers recognizing conserved portions of G-protein coupled receptors (405). After determining by Northern blotting that their clones bound to RNA from melanocytes and the adrenal cortex, they isolated genomic clones for 2 related but distinct genes. Expression of the corresponding cDNAs in cultured cells demonstrated that cells transfected with 1 of them increased cAMP levels in response to α-MSH, whereas the other induced responsiveness to ACTH. Within 6 months, Adrian Clark and colleagues in the United Kingdom identified a mutation in the ACTH receptor gene (now termed “MC2R”) in a patient with familial glucocorticoid deficiency (406) and subsequently demonstrated that the receptor was impaired functionally (407); thus these patients truly had unresponsiveness to ACTH. Chrousos and colleagues at the National Institutes of Health identified mutations in another patient shortly thereafter (408), but it quickly became clear that the term “familial glucocorticoid deficiency” was being applied to patients with a wide array of disorders not linked to MC2R (409). Some of these patients carried mutations in the MRAP gene encoding the melanocortin 2 receptor accessory protein, which interacts with MC2R and may be required for tracking of MC2R from the endoplasmic reticulum to the cell surface (410).
Other disorders have been misdiagnosed as familial glucocorticoid deficiency, such as nonclassic lipoid CAH (411). The study of genetic defects of aldosterone biosynthesis such as 21OHD (see “Genetics of 21OHD”) and aldosterone synthase deficiency (see “Aldosterone Synthase Deficiency”) has shown that a very small amount of aldosterone biosynthetic capacity is sufficient to prevent clinical aldosterone deficiency. Thus some patients with putative selective glucocorticoid deficiency may, in fact, have more generalized adrenal insufficiency that is not quite severe enough to cause aldosterone deficiency.
Addison-achalasia-alacrima syndrome
In 1974, West and Counahan at the Great Ormond Street Hospital in London reported a boy with isolated glucocorticoid deficiency with normal mineralocorticoid function, alacrima, and dystrophic changes in the skin of the fingertips, with alacrima in a younger brother (412). Four years later, Allgrove and colleagues at the same institution (413) reported that the younger brother now also had adrenal insufficiency and that both brothers had also developed achalasia of the gastric cardia. A biopsy obtained during myotomy of the lower esophageal sphincter to relieve dysphagia showed that the muscle lacked nerve fibers. The authors reported the identical combination of findings in a brother and sister from a separate family. On inquiring of colleagues at other institutions, they learned that a previously reported patient at the University of California San Francisco with familial unresponsiveness to ACTH (414) had subsequently developed achalasia. They concluded that this combination of findings represented a familial disorder of unknown etiology. In the first year after diagnosis, cortisol secretion in 1 patient was unresponsive to ACTH but did increase in response to theophylline, a phosphodiesterase inhibitor, suggesting a defect in ACTH signaling (415). This syndrome is termed the achalasia-ACTH insensitivity-alacrima (triple A) syndrome (416) or, eponymously, Allgrove syndrome (417). The affected gene, AAAS, was identified by positional cloning techniques (418). The gene product, aladin, contains WD repeats and is a nuclear pore protein. It interacts with ferritin heavy chain; mutations in AAAS interfere with nuclear importation of ferritin, rendering the nucleus susceptible to oxidative damage (419).
Adrenocortical Cell Lines and the Elucidation of Trophic Signaling Pathways
Two cell lines have played major roles in mechanistic studies of the regulation of adrenal steroidogenesis [reviewed by (420)]. The first is the mouse Y1 cell line, which was derived from a tumor that arose in an adult C57L × A/HeJ male mouse following exposure of the mouse to an atomic blast. The tumor produced corticosterone and responded to ACTH with increased rates of steroidogenesis (421). It was propagated by intramuscular transplantation. Gordon Sato and colleagues (422) adapted this transplantable tumor to grow ex vivo, and the Y1 cell line was cloned from this culture-adapted tumor (423) and deposited with the American Type Culture Collection. The cloned cells can no longer synthesize corticosterone due to loss of 21-hydroxylase activity; they synthesize 20α-dihydroxyprogesterone and 11β,20α-dihydroxyprogesterone (424, 425). However, the cells remain responsive to ACTH, which causes a 4- to 10-fold increase in steroid production over baseline (426). Y1 cells were particularly valuable in identifying transcription factors important for adrenal-specific gene expression.
Y1 cells stop growing, change shape, and detach from the substratum when treated with agents that raise the intracellular levels of cAMP. These effects drastically reduce the plating efficiency of cAMP responsive cells and permit the recovery of rare drug-resistant mutants. Several such mutant lines carry dominant-negative mutations in the type 1 regulatory subunit of protein kinase A (427, 428). These mutants have been used to demonstrate that cAMP and protein kinase A are essential components for ACTH-mediated upregulation of steroidogenesis and gene expression.
The second is the human NCI-H295 cell line and its derivatives, particularly H295R. The original NCI-H295 cell line was derived in 1980 from a large adrenocortical carcinoma in a 48-year-old woman who presented with weight loss, acne, facial hirsutism, edema, diarrhea, and a recent cessation of menses (429). Based on secreted steroids, these cells apparently contained all major adrenocortical enzyme systems including CYP11A1, HSD3B2, CYP11B1, CYP21A2, CYP17A1, CYP11B2, 3β-hydroxysteroid sulfotransferase, and low levels of aromatase (CYP19). Studies of these cells, which grew in suspension, confirmed the expression of all the principal adrenal steroidogenic enzymes and that the mRNAs for these enzymes were responsive to cAMP (430). The H295R substrain was derived from the parental line using alternative growth conditions to encourage substrate attachment and shorter cell cycle times.
Although H295 and H295R are not ACTH responsive, H295R cells do respond to activation of the cAMP-dependent pathway with either forskolin (to activate adenylyl cyclase) or cAMP analogues (430, 431). Moreover, they respond to angiotensin II and have proven invaluable for studying the regulation of aldosterone biosynthesis. H295R cells express the AT1 receptor for angiotensin II (432); this is coupled to phosphoinositidase C and increases the production of inositol phosphates and cytoplasmic free calcium. The other major physiologic regulator of adrenal aldosterone production is extracellular K+ levels. K+ increases intracellular calcium levels in H295R cells by triggering a voltage-activated calcium channel (433).
Developmental Stages
The Fetal Adrenal
Unlike most mammals, human fetuses have huge adrenals. This strange fact has fascinated and perplexed endocrinologists for over a century. The fetal adrenal was first described in 1910 by Stella Starkel and Leslaw Wegrzynowski (434), medical students at the University of Lwów in Poland, who later married (435). Their survey of about 100 adrenals from 6 months gestation through 5 years of age reported the rapid involution of the fetal adrenal following birth. The following year, 3 similar reports appeared describing “involution” or “degeneration” of the fetal zone (436-438); other anatomists confirmed and expanded this observation, pointing out that the combined adrenal weight at birth was about equal to the combined weight of adult adrenals (8-9 g) (438, 439) but soon involuted to a total weight of about 2 g (440-442), accompanied by a parallel diminution in the synthesis and secretion of DHEA and DHEAS (440-442). The rapid, postnatal involution of the fetal adrenal was described as “necrosis” by Benner (443), but De Sa reported this was a postmortem artifact (444), leading to the view that the fetal zone was “remodeled” into ZF. In 1955, Beatty and Hawes concluded: “During the first month of life, degenerative changes take place in the cortex which are apparently physiologic. This degeneration of the fetal cortex appears to be initiated by the birth processes and is unrelated to the gestational age of the infant. These changes, which reduce the adrenal cortex to near adult proportions, are not seen in the ordinary laboratory animal” (445). A more modern view is that fetal adrenal involution may be mediated by apoptosis (446).
The role of the fetal adrenal in human physiology has been unclear and controversial. The huge fetal adrenal size and tremendous output of DHEA and DHEAS suggested a major role, leading to the concept of the “feto-placental unit,” wherein the fetal adrenal provides the C19 steroid precursors for placental conversion to estrogens (447). However, these phenomena are unusual among mammals and are only seen in a few primates, questioning whether they were essential. It was soon shown that fetuses that cannot synthesize C19 steroids because of steroidogenic disorders or adrenal hypoplasia congenita developed normally and reached term, as did fetuses that lack estrogen receptors. Thus an essential role for feto-placental estrogens and the fetal adrenal has not been established.
Most 20th-century studies of fetal adrenal involution relied on postmortem histology; newer studies showed that DHEAS declined by 50% within a week of parturition, and adrenal ultrasonography showed more rapid adrenal involution, within 2 weeks (448). As suggested previously (445), these authors concluded that the trigger to fetal adrenal involution was parturition itself and suggested that the mechanism was withdrawal of a placental hormone or factor (448). This hypothesis may be true but remains unproven, as no such placental factor has been identified. WLM has presented data for an alternative hypothesis: that the perinatal transition from fetal to “adult” circulation, which increases adrenal oxygenation by a factor of 10, triggers multiple transcriptional events that lead to adrenocellular apoptosis. This oxygenation hypothesis would not exclude a contribution from withdrawal of a placental factor (449).
Adrenarche
In a 1942 paper describing 11 patients with probable Turner syndrome, Albright noted that despite evidence of absent ovarian sex steroid production, these patients had pubic and axillary hair, suggesting the adrenal secretion of androgenic hormones (450). Five years later, in a brilliantly illustrated review of the pathophysiology of osteoporosis, he introduced the term “adrenarche” (451). Albright described the roles of 3 types of steroids on bone mineralization: estrogens, androgens, and the unknown adrenal “N-hormone” (that induced nitrogen retention), which he described having “a somatotrophic action somewhat similar to the male gonadal hormone, testosterone, and the production of which in the female governs the growth of axillary and public hair and the excretion of 17KS in the urine” (ie, adrenal androgens). In outlining the timing of the various adrenal hormones, he noted: “The age of entrance of adrenal cortical ‘N’ hormone on the scene is termed the ‘adrenarche’; the age of exit, the ‘adrenopause.’” In 1943, Talbot had documented the increase in 17KS excretion beginning at about age 8 to 10 and suggested that these 17KS derive from the adrenal because there is little difference between boys and girls, but he did not identify this as a specific adrenal event (162). Urinary 17KS were increased in patients with virilizing adrenocortical carcinomas and in normal men compared to normal women and were essentially absent in women without adrenals; hence it was apparent that 17KS came from the adrenals of both sexes and, in part, represented “adrenal androgens.” Many investigators studied the components of (neutral) urinary 17KS; a clear early exposition is that of Harold Mason and William Engstrom in 1950, who list 18 then-known urinary 17KS compounds (452). These authors notably state that androsterone has one-tenth the androgenic activity of testosterone, and DHEA (then termed “dehydroisoandrosterone”) has one-third the androgenic activity of androsterone (they also list androstenedione but do not ascribe a level of androgenic activity to it; this androgenic activity may have been assessed by the cock’s comb bioassay, but that is not stated). Thus, by 1950, DHEA was thought to be a weak androgen associated with adrenarche.
Wilkins confounded the terminology by describing children who had sexual hair before the age of 8 as having “premature pubarche”; nevertheless, he correctly concluded: “At puberty there is usually a simultaneous increase in secretion of adrenal androgens and sex hormones of the gonads. The latter are controlled by pituitary gonadotropins. It is not known what causes increased production of androgen by the adrenals at adolescence” (453). Early studies utilized large volumes of urine, but Migeon and Plager isolated DHEA from human blood, indicating it was a secreted steroid rather than a metabolite (454). Studies using isotopic dilution of radiolabeled steroids indicated that both the adrenal and testis made DHEA but that only the adrenal made DHEAS (455). By 1972 Migeon listed the so-called adrenal androgens as androstenedione, 11-oxy-androstenedione, DHEA, and DHEAS; he said: “These steroids have limited biologic activity. They can be metabolized to a small extent into potent androgens which contribute greatly to the physiologic level of androgenic activity in women but which are of little significance in men” (456). However, endocrinologists soon equated the terms “adrenal androgen” and “DHEA and DHEAS” (457), even though these steroids lacked inherent androgenic action and needed to be metabolized to active androgens in peripheral tissues (458). It would take another 40 years to identify the true human adrenal androgen as 11-keto-testosterone (see later discussion).
Studies of most endocrine phenomena are illuminated by work with animals; however, this is not the case with adrenarche. In 1978, Gordon Cutler and D. Lynn Loriaux reported that DHEA and DHEAS levels in the rat, guinea pig, hamster, rabbit, dog, sheep, pig, goat, horse, cow, or chicken were an order of magnitude less than human values and did not rise at the time of pubertal development. Similarly, DHEA, DHEAS, and androstenedione did not rise with age in rhesus macaques but did rise dramatically with age in chimpanzees; this rise in chimpanzees preceded the rise in gonadal sex steroids, similarly to human adrenarche (459). Thus only the chimpanzee appeared to model human adrenal C19 steroid physiology. Several subsequent studies have confirmed the proposition that only the chimpanzee (and possibly the gorilla) have an adrenarche that is similar to human adrenarche (460-462).
The question of how adrenarche might be regulated stimulated much research. Beginning in 1978, Melvin Grumbach and colleagues hypothesized the existence of a “cortical androgen stimulating hormone” (CASH), based on clinical inferences from hormonal measurements in various disease states but did no direct experimentation to test this hypothesis (463, 464). In 1979, Lawrence Parker and William Odell reported studies aimed at identifying an “adrenal androgen stimulating hormone” (AASH). Given the species specificity of adrenarche presented earlier, it is clear in retrospect that these studies would be stillborn: the source of material to be tested was bovine pituitaries (even though cattle do not have adrenarche), and the assay system was production of DHEA by castrated, dexamethasone-treated dogs (which also lack adrenarche) (465). Shortly thereafter, David C. Anderson in Salford, United Kingdom, listed several reasons to doubt the existence of such a hormone, arguing that adrenarche was solely due to intra-adrenal events (466). In 1989, Parker reported that CASH/AASH was a specific peptide derived from pro-opiomelanocortin; however, shortly thereafter 3 groups showed that Parker’s peptide lacked adrenal androgen stimulating activity (467-469). Thus “AASH” and “CASH” became “MASH” —mythical androgen stimulating hormone.
Consistent with the suggestion of Anderson in 1980, more recent work supports a wholly intra-adrenal mechanism for adrenarche, including, but not limited to (i) morphologic changes within the zona reticularis (ZR); (ii) decreased expression of HSD3B2 in the ZR; and (iii) increased expression of cytochrome b5 in the ZR, thus enhancing the 17,20-lyase activity of CYP17A1 (470, 471). While these changes remain under investigation, a dramatic recent event is the identification of the principal human adrenal androgen as 11-keto-testosterone (11KT) (472). The human adrenal ZR expresses CYP11B1, which catalyzes the synthesis of 11βOH-androstenedione, which, through the sequential action of HSD11B2 and AKR1C3 (HSD17B5), produces 11KT. Both 11KT and its 5α-reduced derivative, 11KDHT, activate the androgen receptor, are higher in adrenal venous effluent than in peripheral blood, and appear to be the dominant circulating bioactive androgens in adrenarche (472, 473). Their likely roles in CAH, PCOS, and other hyperandrogenic disorders are under active investigation.
Regulation of Adrenal Development and Gene Expression
Steroidogenic Factor 1
A collaboration that led to a major advance in the understanding of adrenal gene regulation indirectly had its beginning at the first Adrenal Cortex Conference in Buffalo in 1984, when PCW was approached by Bernard Schimmer. PCW had recently cloned the CYP21 gene encoding steroid 21-hydroxylase, which is mutated in most cases of CAH and had localized the mouse Cyp21 genes on cosmid clones provided by Jon Seidman at Harvard (242). Schimmer suggested a collaboration to explore the regulation of this gene—and steroidogenesis in general—using mouse Y1 adrenocortical cells, which lack Cyp21 expression (see “Adrenocortical Cell Lines and the Elucidation of Trophic Signaling Pathways”). PCW, who had a small lab at the time, referred him to Seidman who in turn assigned the project to Keith L. Parker, then a postdoctoral fellow in his laboratory.
Parker’s studies demonstrated that Y1 cells stably transfected with the mouse Cyp21 cosmid recovered hormonally regulated expression of 21-hydroxylase (474). Sequences essential for expression were localized to the proximal 330 bp of 5’-flanking DNA (475, 476). When genes encoding other steroid hydroxylases were isolated and their 5’ flanking regions compared, shared AGGTCA promoter elements were identified in several of these genes that interacted with the same DNA-binding protein in steroidogenic cell lines. Parker and colleagues reasoned that the AGGTCA DNA recognition motif represented a binding site for an atypical member of the nuclear hormone receptor family and cloned mouse SF-1 cDNA from a Y1 cell cDNA library using a hybridization probe comprising the DNA-binding region of retinoid X receptor (477). Independently, Ken-ichiro Morohashi and colleagues used an oligonucleotide affinity column to purify the protein (which they termed “Ad4BP”) from bovine adrenal glands, ultimately allowing them to obtain amino acid sequence and clone a bovine cDNA with an oligonucleotide probe (478). Subsequent studies established that the mouse and bovine cDNAs encoded orthologs of a protein that transactivated the steroid hydroxylase promoters in steroidogenic and nonsteroidogenic cells. The sequences of these cDNAs confirmed that SF-1 belonged to the nuclear hormone receptor family; the SF-1 gene is now officially termed NR5A1. Early work on SF-1 structure and function has been reviewed elsewhere (479).
Consistent with the hypothesis that SF-1 is required for adrenal and gonadal steroidogenesis, SF-1 knockout mice die shortly after birth from adrenocortical insufficiency and exhibit male-to-female sex reversal of the external genitalia. However, these abnormalities are not due to poor expression of steroidogenic enzymes but instead to complete absence of the adrenal glands and gonads (Fig. 20) (480, 481). Moreover, knockout mice lack the ventromedial hypothalamic nucleus, a hypothalamic region linked to feeding and appetite regulation and female reproductive behavior (482).
Figure 20.
Newborn SF-1 (NR5A1) null mice lack adrenal glands and gonads. Abbreviations: A, SF-1 null female. B, wild-type female. k, kidney; a, adrenal; o, ovary; od, oviduct [from (480) reprinted with permission].
In contrast to homozygous SF-1 null mice, which can be kept alive with glucocorticoid replacement, no patient has been identified who is homozygous for null mutations in the NR5A1 gene encoding SF-1. The first 2 SF-1-defective patients identified were heterozygous for mutations that abolished the ability of SF-1 to bind DNA, thus rendering the protein transcriptionally inactive without creating a dominant negative effect. Both affected patients had adrenal insufficiency; 1 was a phenotypic female XY infant and the other was an apparently normal XX female who was not diagnosed with adrenal insufficiency until 14 months of age (483, 484). However, heterozygous SF-1 mutations subsequently were identified mainly among undervirilized 46,XY individuals (ie, XY disorders of sexual development) who do not have adrenal insufficiency (485).
Adrenal Hypoplasia Congenita and the Role of DAX1
Whereas CAH had been extensively characterized by the early 1940s, the study of adrenal hypoplasia was impeded by the lack of secretion of abnormal steroids and also by confusion between cases caused by pituitary agenesis or hypoplasia and those that were the presumed result of a primary adrenal defect. Brothers with isolated adrenal hypoplasia were reported in 1959 by Mitchell and Rhaney in Scotland (486). The first boy died at 7 weeks of age; autopsy showed adrenal hypoplasia as the only abnormality, and the authors noted an absence of normal zonation of the adrenal cortex. The younger sibling was successfully treated with fludrocortisone, sodium chloride, and cortisone acetate. Subsequent authors noted a male preponderance of such patients (487). Although CAH was initially thought to be an autosomal recessive condition, in 1970 Weiss and Mellinger (488) summarized data on 3 kindreds, each with at least 2 affected boys with a common mother and different fathers, strongly suggesting X-linked inheritance.
Hypogonadotropic hypogonadism occurred frequently in such patients. Moreover, patients were eventually found who had a contiguous gene syndrome consisting of adrenal hypoplasia and—in its fullest extent—glycerol kinase deficiency and Duchenne muscular dystrophy (489). These patients all had deletions in the Xp21 region, with a minimum overlapping region of 250 to 500 kb. In contrast, 46XY phenotypic female patients without adrenal insufficiency had a duplication of the same region, suggesting the presence of a dosage-sensitive sex reversal gene (490). Eventually a multinational group of investigators isolated yeast artificial chromosomes encompassing the deleted region and found a 1.6 kb fragment enriched in CpG dinucleotides, considered a marker for expressed genes. This fragment was used to screen adult testis and fetal adrenal cDNA libraries, from which a single cDNA was isolated and termed DAX-1 (DSS-AHC critical region on the X chromosome, gene 1; later termed “NR0B1”) (491). The C-terminal half of the encoded protein resembled the ligand-binding domain of nuclear hormone receptors. The N-terminal half did not resemble the DNA-binding domains of other nuclear hormone receptors or any other known protein but instead contained 31/2 repeats of a 65-67 amino acid motif. DAX-1 bound DNA and functioned as a corepressor for several nuclear hormone receptors. Deletions or point mutations in DAX-1 were observed in the vast majority of patients with adrenal hypoplasia congenita, including patients who also had hypogonadotropic hypogonadism (491, 492). Subsequent work has shown that more than half of boys with adrenal hypoplasia carry DAX-1 mutations, whereas SF-1 mutations are rare (493).
Autoimmune Addison’s Disease
Although Addison’s first report described a patient with adrenal tuberculosis (29), his follow-up report of 11 patients included only 6 with adrenal tuberculosis; at least 1 of these, whom he showed in an illustration (Fig. 5), was of a young man with vitiligo, which is now known to be an autoimmune condition (30). Thus noninfectious forms of what we now term “Addison’s disease” were known from the beginning.
Autoimmunity was identified as a major cause of Addison’s disease through several parallel lines of investigation. The first was the recognition of the frequent coexistence of adrenal insufficiency and hypothyroidism without hypopituitarism, first by Schmidt (494). Autopsies of patients with Addison disease demonstrated that many had lymphocytic thyroiditis (495). Although it initially seemed possible that the thyroid disease could be a direct consequence of adrenal insufficiency, coexistent diabetes mellitus occurred in many such patients (496). These combinations of endocrinopathies—often eponymously termed “Schmidt and Carpenter syndromes,” respectively—are now collectively referred to as autoimmune polyglandular syndrome (APS) type 2.
Studies in the 1950s [eg, (497)] showed that lymphocytic thyroiditis was associated with the presence of autoantibodies against thyroid antigens, and both thyroid and adrenal disease could be recreated in experimental animals by injecting the respective homogenized tissue in immunogenic Freund’s adjuvant [discussed in (498)]. These findings prompted a search for adrenal autoantibodies, which were indeed detected in cases of Addison disease with (499, 500) or without (500) thyroid disease. However, it was suspected that the antibodies represented a marker for a more generalized process involving cellular autoimmunity (501).
Although Schmidt and Carpenter syndromes occur with little if any familial clustering, familial cases of polyglandular endocrinopathies including Addison’s disease were reported by the 1950s, often in association with hypoparathyroidism, superficial moniliasis, and occasionally pernicious anemia (500, 502, 503). Adrenal autoantibodies were detected in many of these cases. This syndrome, now termed “APS type 1,” was distinct in its strong Mendelian autosomal recessive pattern of inheritance (504). It is now known to be caused by mutations in the autoimmune regulator gene [reviewed in (505)].
The most characteristic autoantigen in idiopathic Addison’s disease is, in fact, the 21-hydroxylase enzyme, CYP21A2; of note, the 2 papers reporting this finding used antibodies against CYP21A2 developed in the laboratories of PCW (506) and WLM (507), respectively. In contrast, APS1 and APS2 have more diverse repertoires of autoantibodies, including those directed against 2 other steroidogenic enzymes, CYP11A1 and CYP17A1 (508, 509) [reviewed in (510)].
Other Forms of Adrenal Insufficiency
Adrenoleudodystrophy
The history of adrenoleukodystrophy (ALD) has been reviewed by Hugo Moser (511, 512). In 1912, Paul Schilder, a Viennese psychiatrist and medical researcher who was a member of the Vienna Psychoanalytic Society founded by Sigmund Freud, described a progressive demyelinating cerebral sclerosis occurring in childhood (513). Whereas Schilder did not report symptoms of adrenal insufficiency, in 1923 Siemerling and Creutzfeldt reported a boy who had become deeply pigmented by 3 to 4 years of age and then developed behavioral alterations and progressive neurological impairment until his death at age 7 (514). The adrenal glands were atrophic. The cerebral hemispheres showed large confluent areas of demyelination associated with a perivascular inflammatory response. In 1963, Fanconi reviewed 9 cases, all of whom were male, and proposed an X-linked recessive mode of inheritance (515).
Much of the early pathological characterization was reported by Herbert Schaumburg, James Powers, and colleagues at Albert Einstein College of Medicine, who studied 9 male patients in whom Schilder’s disease and adrenal pathological findings occurred together (516). They noted that “cells of the zona reticularis and fasciculata were ballooned and often contained crystalline aggregates. The X-linked recessive inheritance and this derangement of the adrenal cortex in males with Schilder’s disease strongly indicate the presence of a metabolic process.” The crystalline inclusions were eventually found to contain cholesterol esterified with saturated very long chain fatty acids (VLCFA) (517). The name “adrenoleukodystrophy” was in the peer-reviewed literature by 1973 (518). A breakthrough came in 1981 with the finding by Hugo Moser at Johns Hopkins’ Kennedy-Krieger Institute that plasma levels of VLCFA were elevated and could be used to diagnose the disease (519). ALD is now estimated to affect approximately 1:17 000 males in the United States, similar to the frequency of CAH among males.
It was originally assumed that the defect lay in a fatty acid CoA-ligase specific for VLCFA. The ALD gene, now termed ABCD1, was mapped to Xq28 in 1981 (520). A putative gene was identified by positional cloning techniques in 1993 (521), with the surprising finding that it had a high degree of sequence similarity to a previously identified peroxisomal membrane protein and, not to fatty acid CoA ligases. The ALD gene product, ALDP, is a member of the ATP-binding cassette transporter family (522). Dietary treatment with a mixture of oleic and erucic acids (Lorenzo’s Oil) was popularized in a film with the same name but is, in fact, of limited benefit (523).
New Forms of Adrenal Disease
The availability of modern sequencing techniques has led to the elucidation of several novel syndromes that include adrenal dysfunction. Aside from CAH and autoimmune adrenalitis, a genetic cause can now be identified in more than two-thirds of patients with primary adrenal insufficiency presenting in childhood (524). These can be grouped into several categories. We have already addressed ACTH unresponsiveness, adrenal hypoplasia congenita due to mutations in orphan nuclear hormone receptors, and defects in lipid metabolism, particularly adrenoleukodystrophy. More recently defined syndromes fall into 2 groups: those associated with intrauterine growth restriction, adrenal hypoplasia, and disorders of sexual development, such as IMAGe and MIRAGE syndromes, and deficiencies of mitochondrial reactive oxygen species detoxification.
Multisystem Syndromes Associated With Growth Restriction
MCM4 deficiency
Mutations in genes that affect DNA replication may be associated with primordial dwarfism, immune deficiency, and adrenal insufficiency. Minichromosome maintenance-deficient 4 homolog (MCM4) is a component of a MCM2-7 complex that is a replicative helicase essential for normal DNA replication and genome stability in all eukaryotes. As identified by whole exome sequencing, homozygous deficiency of MCM4 causes a primary immunodeficiency syndrome (occurring with a frequency of 1 in 2506 in the Irish Traveler population) characterized by severe intra- and extrauterine growth retardation, microcephaly, decreased numbers of natural killer cells, recurrent viral infections, and adrenal insufficiency (525, 526).
MIRAGE syndrome
MIRAGE syndrome (MIRAGE) is a form of syndromic adrenal hypoplasia including myelodysplasia, infections, restriction of growth, adrenal hypoplasia, genital abnormalities, and enteropathy. It was first characterized by Narumi, Hasegawa, and colleagues at Keio University in Japan by whole-exome sequencing of 15 patients with adrenal hypoplasia but no mutations in known causative genes (527). It is caused by mutations in the sterile alpha motif domain-containing protein 9 (SAMD9) gene on chromosome 7q21, which encodes a protein that inhibits cell proliferation. Disease-causing mutations are heterozygous and almost invariably arise de novo. When expressed in cultured cells, the mutant proteins more strongly inhibit cell proliferation (ie, gain of function mutations). There is a risk that cells will attempt to escape growth inhibition caused by the mutant protein by selectively losing the chromosome 7 carrying the mutation, thus causing haploinsufficiency of SAMD9. This can cause myelodysplastic syndrome.
IMAGE syndrome
IMAGE syndrome consists of intrauterine growth retardation, metaphyseal dysplasia, adrenal hypoplasia, and genital anomalies, first characterized in 1999 by Vilain and colleagues at the University of California Los Angeles (528). Two forms have been identified. Vilain and colleagues used positional cloning to find that an autosomal dominant form was caused by mutations in the cyclin-dependent kinase inhibitor-1C (CDKN1C) gene (529). An autosomal recessive form was identified by whole-genome sequencing of 14 unrelated kindreds as being caused by mutations in the POLE gene encoding the catalytic subunit of DNA polymerase epsilon (530). Adrenal insufficiency in cases associated with CDKN1C mutations tends to present in early infancy, whereas in cases caused by POLE mutations, it is usually manifested during early childhood. The recessive form is also associated with immunodeficiency and is therefore sometimes termed “IMAGEI syndrome.”
The CDKN1C gene product, also called p57(KIP2), binds G1 cyclin/CDK complexes and thus inhibits cell proliferation. It is located in the imprinted region of chromosome 11p15 and is preferentially expressed from the maternal allele. Inactivating mutations in this gene cause some cases of Beckwith-Wiedemann syndrome, which is associated with macrosomia and tumor risk. Mutations that interfere with binding of CDKN1C to DNA polymerase delta auxiliary protein enhance the growth inhibitory effect of CDKN1C (gain of function mutations) and cause IMAGE syndrome when maternally inherited.
Deficiency of Mitochondrial Reactive Oxygen Species Detoxification
Nicotinamide nucleotide transhydrogenase (NNT) is a mitochondrial protein that catalyzes transfer of a hydride ion from nicotinamide adenine dinucleotide, NADH, to oxidized nicotinamide dinucleotide phosphate, NADP+. Its deficiency generally occurs in infancy or early childhood, and most patients have both mineralocorticoid and glucocorticoid deficiencies (531). A mutation in TXNRD2, encoding a mitochondrial selenoprotein, thioredoxin reductase 2, was identified by whole-exome sequencing of a single consanguineous Pakistani kindred with glucocorticoid deficiency (532). This protein, not to be confused with adrenodoxin (or ferredoxin) reductase, helps maintain mitochondrial redox homeostasis; inactivating it in cultured cells decreases the oxidized to reduced glutathione ratio and increases levels of mitochondrial reactive oxygen species.
Conclusion
A recurring theme in this review is how the history of adrenal research parallels the broader history of biomedical discovery. We expect that to continue. WLM has discussed a series of unanswered questions in steroidogenesis (especially adrenal steroidogenesis) (533), including the elusive roles of the fetal adrenal and of DHEA/DHEAS; these are not reiterated here. Considering some more recent advances that have not yet affected adrenal research suggests we may see some of the following in the future.
First, the 21st-century discoveries of many new deficiency diseases suggests that there are still other adrenal diseases to be discovered. A prime candidate is the mitochondrial flavoprotein, ferredoxin reductase (FdxR) (also known as adrenodoxin reductase). Mitochondrial forms of cytochrome P450, such as the cholesterol side-chain cleavage enzyme (CYP11A1), 11-hydroxylase (CYP11B1), and aldosterone synthase (CYP11B2) must receive electrons from NADPH via the intermediacy of FdxR and ferredoxin (Fdx), an iron-sulfur protein. Both Fdx and FdxR also participate in the biosynthesis of iron-sulfur clusters; defects in this process are associated with Friedrich’s ataxia and with Parkinson disease. Beginning in 2017, mutations in FdxR have been reported in 45 patients (in 33 families) with optic atrophy, neuropathic hearing loss, developmental delay, and other neurodevelopmental problems, reflecting a global mitochondrial disorder (534-539). Studies of potential disorders in steroidogenesis have not yet been reported, nor have human genetic disorders of Fdx been reported. One of us (WLM) has reviewed this topic, speculating that FdxR patients will have mild defects in steroidogenesis analogous to patients with nonclassic forms of lipoid CAH or CYP11A1 deficiency (540).
Second, whereas genetic studies of adrenal diseases in the 20th century concentrated on forms of CAH, the recurrent theme in the 21st century is on diseases that cause cell death by affecting DNA replication and repair (IMAGE types 1 and 2, MCM4 deficiency), impairing the ability to withstand oxidative stress (NNT and TXNRD2 deficiencies and perhaps Allgrove syndrome) and inhibiting cell proliferation (MIRAGE syndrome). Whereas these syndromes variously affect many other physiologic processes, it remains unclear why primary adrenal insufficiency is a consequence of all of them. Further insights into the unique metabolic vulnerabilities of the adrenal cortex would be of great interest.
Third, it is already apparent that some disorders not generally regarded as adrenal disorders do, in fact, involve the adrenal; the prime example of this is PCOS, in which there is hyperandrogenism of both ovarian and adrenal origin (541). Whether this logical link between the adrenal and ovary also applies to testicular disorders remains unclear.
Fourth, contemporary advances in gene therapy and gene editing offer promise for eventual improved treatment or even cure for genetic disorders of steroidogenesis. Nevertheless, as hormonal replacement therapy is so effective, we believe that such technologies will be appropriate for CAH and other adrenal disorders only after they have been proven safe and effective in many other, more lethal disorders. Among adrenal disease, the first candidate should certainly be adrenoleukodystrophy because of its devastating neurological consequences, and indeed, such therapy is in an advanced stage of development (542).
Fifth, newer molecular imaging technologies, notably cryoelectron microscopy, are revolutionizing the ability to image both individual macromolecules and larger macromolecular motors. Complex steroidogenic actions such as interactions of StAR with multiple mitochondrial proteins may be amenable to this approach, perhaps eventually revealing how StAR works.
Finally, we close with a reminder from the great American philosopher Yogi Berra: “It’s tough to make predictions; especially about the future.”
Acknowledgments
The authors thank Dr. Gary Hammer (University of Michigan) for drawing our attention to the Da Vinci drawing in Figure 1, innumerable colleagues who have suggested interesting historical points, and Drs. Robert H. Lustig and Stephen M. Rosenthal (both at the University of California San Francisco) for presubmission reviews and commentary.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- APS
autoimmune polyglandular syndrome
- 3β-HSD
3β-hydroxysteroid dehydrogenase
- CAH
congenital adrenal hyperplasia
- cAMP
3′,5′-cyclic adenosine 5′-monophosphate
- DAX-1
DSS-AHC critical region on the X chromosome, gene 1 (also termed NR0B1)
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- DOC
11-deoxycorticosterone
- 17KS
17-ketosteroids
- 17OHP
17-hydroxyprogesterone
- 11OHD
11-hydroxylase deficiency
- 21OHD
21-hydroxylase deficiency
- HLA
human leukocyte antigen
- Kb
kilo base pairs
- PAPSS2
3’-phosphoadenosine 5’-phosphosulfate synthase 2
- POR
P450 oxidoreductase
- P450
cytochrome P450
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- SF-1
steroidogenic factor 1 (also termed NR5A1 and Ad4BP)
- StAR
steroidogenic acute regulatory protein
- WLM
Walter L. Miller
- PCW
Perrin C. White
- VLCFA
very long chain fatty acids
Contributor Information
Walter L Miller, Department of Pediatrics, Center for Reproductive Sciences, and Institute for Human Genetics, University of California, San Francisco, CA, USA.
Perrin C White, Division of Pediatric Endocrinology, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Financial Support
WLM: none. PCW: Grant U01 HD083493 from the National Institutes of Health.
Disclosures
WLM: none. PCW: Receives sponsored research support from Neurocrine Pharmaceuticals.
References
- 1. Shumacker HB. The early history of the adrenal glands: with particular reference to theories of function. Bull Inst History Med 1936;4(1):39-56. https://www.jstor.org/stable/44433654. [Google Scholar]
- 2. Lenard A. The history of research on the adrenals; 1563-1900. J Hist Med Allied Sci. 1951;6(4):496-505. doi: 10.1093/jhmas/vi.autumn.496. [DOI] [PubMed] [Google Scholar]
- 3. Carmichael SW. The history of the adrenal medulla. Rev Neurosci. 1989;2(2):83-100. doi: 10.1515/REVNEURO.1989.2.2.83. [DOI] [PubMed] [Google Scholar]
- 4. Leoutsakos B, Leoutsakos A. The adrenal glands: a brief historical perspective. Hormones (Athens) 2008;7(4):334-336. doi: 10.14310/horm.2002.1216. [DOI] [PubMed] [Google Scholar]
- 5. Miller WL. A brief history of adrenal research: Steroidogenesis - the soul of the adrenal. Mol Cell Endocrinol. 2013;371(1-2):5-14. doi: 10.1016/j.mce.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 6. Papadakis M, Manios A, Schoretsanitis G, Trompoukis C. Landmarks in the history of adrenal surgery. Hormones (Athens) 2016;15(1):136-141. doi: 10.14310/horm.2002.1612. [DOI] [PubMed] [Google Scholar]
- 7. Homer. The Iliad (R. Fitzgerald, translator). New York: Anchor Press/Doubleday, 1975. [Google Scholar]
- 8. Goss CM. On anatomy of veins and arteries by Galen of Pergamos. Anat Rec. 1961;141:355-366. doi: 10.1002/ar.1091410409. [DOI] [PubMed] [Google Scholar]
- 9. Leonardo da Vinci. The Mechanics of Man. The Queen’s Gallery, Palace of Holyroodhouse, Edinburgh, Scotland. Vol 20222013. [Google Scholar]
- 10. Loriaux DL. Bartolomeo Eustachi (Eustachius) (1520–1574). Endocrinologist 2007;17(4):195. doi: 10.1097/ten.0b013e318141f6f4. [DOI] [Google Scholar]
- 11. Loriaux DL. Frontispiece to A Biographical History of Endocrinology. Ames, Iowa: John Wiley & Sons; 2016. DOI: 10.1002/9781119205791 [DOI] [Google Scholar]
- 12. Casseri GC. Tabulae anatomicae LXXVIII. Venice: Deuchinus, 1627. [Google Scholar]
- 13. Bartholin C. Anatomicae Institutiones Corporis Humani, Utriusque Sexus Historiam et Declarationem Exhibentes. Germany, Conradus Scher, 1626 [Google Scholar]
- 14. Bartholin T. Anatomia. ex Caspari Bartholini parentis Institutionibus, omniumque recentiorum & propriis observationibus: tertiùm ad sanguinis circulationem reformata cum iconibus novis accuratissimis. Leiden: Lgvd. Batav. Apud Franciscvm Hackivm, 1651. (Book 1, Chapter 18 De Capsulis Atrabilariis, pp120-125. Table (figure) 20, p123. [Google Scholar]
- 15. Vesling J. Syntagma anatomicum, publicis dissectionibus in auditorium usum diligenter aptatum. Padua: Typis Pauli Frambotti bibliopolae, 1641. http://books.google.com/books?id=p3pheBYqujYC&hl=&source=gbs_api [Google Scholar]
- 16. Wharton T. Adenographia: sive glandularum totius corporis descriptio. Amsterdam: Sumptibus Johannis Ravesteinii; 1659. https://iiif.library.utoronto.ca/presentation/v2/anatomia:RBAI018/manifest [Google Scholar]
- 17. Molinetti A. Dissertationes anatomico-pathologicae: quibus humani corporis partes accuratissime describuntur morbique singulas divexantes explicantur. Vol 6. Venice: apud Paulum Balleonium 1675:302-303. URL: https://www.jstor.org/stable/44433654. [Google Scholar]
- 18. Cuvier G. Lecons d’Anatomie Comparée, Tome III. Paris: Recueillies et Publiés par L Duvernoy; 1805. ISBN-13. 9782012986596 [Google Scholar]
- 19. Arnold F. Der Kopftheil des vegetativen Nervensystems beim Menschen in anatomischer und physiologischer Hinsicht. Heidelberg und Leipzig: Neue akademische Buchhandlung von Karl Gross 1831:301. https://archive.org/details/b21301402. [Google Scholar]
- 20. Nagel N. Über die Struktur der Nebennieren. Arch Anat Phys Wiss Med 1836:365-383. https://www.biodiversitylibrary.org/item/49865#page/610/mode/1up. [Google Scholar]
- 21. Rostand J. Montesquieu (1689-1755) et la biologie. Revue d’Histoire des Sciences et de Leurs Applications 1955;8(2):129-136. doi: 10.3406/rhs.1955.3511. [DOI] [Google Scholar]
- 22. Schäfer EA. Oliver-Sharpey Lectures: On The Present Condition Of Our Knowledge Regarding The Functions Of The Suprarenal Capsules: Delivered before the Royal College of Physicians of London on April 7th and 9th, 1908. Br Med J 1908;1(2474):1277-1281. https://www.jstor.org/stable/25277786. [PMC free article] [PubMed] [Google Scholar]
- 23. Sampson H. A relation of one Hannah Taylor, a very extraodinary child of about six years of age, who in face, & c. Was as large as a full grown woman; and of what appeared on the dissection of her body. Phil Trans Royal Soc London 1695;19(217):80-82. doi: 10.1098/rstl.1695.0015. [DOI] [Google Scholar]
- 24. Else T, Auchus RJ, Miller WL. Adrenocortical carcinoma in a 17th-century girl. J Steroid Biochem Mol Biol. 2017;165(Pt A):109-113. doi: 10.1016/j.jsbmb.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 25. Cooke W. A case of Hydrocephalus Internus. Medico-Chirurgical Trans (London) 1811;2:17-23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2128842/pdf/medcht00072-0027.pdf. [PMC free article] [PubMed] [Google Scholar]
- 26. Ogle JW. Unusually large mass of carcinomatous deposit in one of the suprarenal capsules of a child. Tr Path Soc London 1865;16(250):1864-1868. [Google Scholar]
- 27. Fox TC. A case of primary sarcoma of the left suprarenal capsule with extensive thrombosis of the vena cava inferior in a child. Tr Path Soc London 1885;36:460-463. https://www.google.com/books/edition/Transactions_of_the_Pathological_Society/XX13gv08pxMC?hl=en. [Google Scholar]
- 28. Bullock W, Sequeira JH. On the relation of the suprarenal capsules to the sexual organs. Tr Path Soc London 1905;56:189-208. https://www.google.com/books/edition/Transactions_of_the_Pathological_Society/usBXAAAAMAAJ?hl=en&gbpv=1. [Google Scholar]
- 29. Addison T. Anaemia—disease of the supra-renal capsules. Lond Med Gaz518 1849;8:517. Hilton J. Discussion of Dr. Addison’s paper on anaemia, &c. ibid pp. 562-563. https://archive.org/details/londonmedicalgaz43londuoft/page/516/mode/2up. [Google Scholar]
- 30. Addison T. On the Constitutional and Local Effects of Disease of the Supra-Renal Capsules. London: Samuel Highley, 1855. https://wellcomecollection.org/works/xsmzqpdw [Google Scholar]
- 31. Brown-Séquard C-E. Recherches expérimentales sur la physiologie et la pathologie des capsules surrénales. C R Hebd Seances Acad Sci. 1856;43(10):542-546. https://wellcomecollection.org/works/x29u839u. [Google Scholar]
- 32. Brown-Séquard C. Nouvelles recherches sur l’importance des fonctions des capsules surrénales. C R Hebd Seances Acad Sci. 1857;44(6):246-248. [Google Scholar]
- 33. Brown-Séquard C-E. Nouvelles recherches sur l’importance des fonctions des capsules surrénales. C R Hebd Seances Acad Sci. 1857;45(25):1036-1039. [Google Scholar]
- 34. Brown-Séquard C-E. Nouvelles recherches sur l’importance des functions des capsules surrénales. J Physiol Homme Animaux 1858;1:160-173. [Google Scholar]
- 35. Aminoff MJ. Brown-Séquard: An Improbable Genius who Transformed Medicine. Oxford: Oxford University Press, 2011. ISBN-13: 978-0199742639 [Google Scholar]
- 36. Brown-Séquard C. Note on the effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet 1889;134(3438):105-107. doi: 10.1016/S0140-6736(00)64118-1. [DOI] [Google Scholar]
- 37. Hansen B. New images of a new medicine: visual evidence for the widespread popularity of therapeutic discoveries in America after 1885. Bull Hist Med. 1999;73(4):629-678. doi: 10.1353/bhm.1999.0166. [DOI] [PubMed] [Google Scholar]
- 38. Murray GR. Note on the treatment of myxoedema by hypodermic injections of an extract of the thyroid gland of a sheep. Br Med J 1891;2(1606):796-797. doi: 10.1136/bmj.2.1606.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moore CR, Gallagher TF, Koch FC. The effects of extracts of testis in correcting the castrated condition in the fowl and in the mammal. Endocrinology 1929;13(4):367-374. doi: 10.1210/endo-13-4-367. [DOI] [Google Scholar]
- 40. Gallagher T, Koch FC. The testicular hormone. J Biol Chem. 1929;84(2):495-500. doi: 10.1016/s0021-9258(18)77008-7. [DOI] [Google Scholar]
- 41. Freeman ER, Bloom DA, McGuire EJ. A brief history of testosterone. J Urol. 2001;165(2):371-373. doi: 10.1097/00005392-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 42. Dotson JL, Brown RT. The history of the development of anabolic-androgenic steroids. Pediatr Clin North Am. 2007;54(4):761-9, xi, doi: 10.1016/j.pcl.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 43. Nieschlag E, Nieschlag S. The history of discovery, synthesis and development of testosterone for clinical use. Eur J Endocrinol. 2019;180(6):R201-R212. doi: 10.1530/EJE-19-0071. [DOI] [PubMed] [Google Scholar]
- 44. Anonymous. Extraordinary case of hemaphrodism. Lancet 1833;20(501):60-62. doi: 10.1016/S0140-6736(02)93770-0. [DOI] [Google Scholar]
- 45. De Crecchio L. Sopra un caso di apparenze virili in una donna. Morgagni 1865;7:151-189. [Google Scholar]
- 46. Delle Piane L, Rinaudo PF, Miller WL. 150 years of congenital adrenal hyperplasia: translation and commentary of De Crecchio’s classic paper from 1865. Endocrinology 2015;156(4):1210-1217. doi: 10.1210/en.2014-1879. [DOI] [PubMed] [Google Scholar]
- 47. Phillips J. Four cases of spurious hermaphroditism in one family. Trans Obstet Soc Lond 1887;28:158-168. https://books.googleusercontent.com/books/content?req=AKW5QacX98X59n6XYilwP0eOjLNhlH3eanAlBK3-8UYQ8orEGyQDGUOG5xrKS7FgnDJLpgdSje0I7C2TFsJHyiW8EA6NBSYMf34fEdacRNHphXvya0N1I9su7Djpuds3ZWEq-Or-ds7_3Rx40ry1NCzEmHsdltoW15ake0WvmjsegaeRzus70UDfsbKmMm_K37hgkyhtAvuea78R0J8_btR5sGemQC2LytHvxucdb6UDTOSwffef-BUWRvK1h-OLYVaWrgvmtqTjnBgmIuIC2XbkmtpnC3LhSR5DBfmdz6HcmA0Jsc1ITJs. [Google Scholar]
- 48. Grawitz P, II. Virchow’s Arch Pathol Anat Physiol klin Med. 1883;93:39-63. doi: 10.1007/BF01929242. [DOI] [Google Scholar]
- 49. Glynn EE. The adrenal cortex, its rests and tumours; its relation to other ductless glands, and especially to sex. Quart J Med 1912;5(2):157-193. doi: 10.1093/oxfordjournals.qjmed.a069304. [DOI] [Google Scholar]
- 50. Gallais A. Le syndrome génito-surrénal. These de Paris No. 2251912. https://archive.org/details/BIUSante_epo1110 [Google Scholar]
- 51. Marks T, Thomas J, Warkany J. Adrenocortical obesity in children. Am J Dis Child 1940;60(4):923-942. doi: 10.1001/archpedi.1940.02000040142013. [DOI] [Google Scholar]
- 52. Goldstein AE, Rubin SW, Askin JA. Carcinoma of adrenal cortex with adrenogenital syndrome in children; complete review of the literature and report of a case with recovery in a child 8 months of age. Am J Dis Child 1946;72(5):563-603. doi: 10.1001/archpedi.1946.02020340064008. [DOI] [PubMed] [Google Scholar]
- 53. Simpson SL. The adreno-genital syndrome. Postgrad Med J. 1953;29(330):184-191. doi: 10.1136/pgmj.29.330.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamashima T. Adrenaline/Epinephrine Hunters: Past, Present and Future at 1900. Emerg Med Inves 2017;4(4):EMIG-145. doi: 10.29011/2475-5605.000045. [DOI] [Google Scholar]
- 55. Ishida M. Hormone Hunters: The Discovery of Adrenaline. Kyoto University Press, 2018.1-203. 10.14989/234214 [DOI] [Google Scholar]
- 56. Vulpian EFA. Note sur quelques réactions propres à la substance des capsules surrénales. C R Hebd Seances Acad Sci. 1856;43(13):663-665. [Google Scholar]
- 57. Henle FGJ. Über das Gewebe der Nebenniere und der Hypophyse. Z rational Med 1865;24(3):143-152. [Google Scholar]
- 58. Kohn A. Das chromaffine Gewebe. Ergebn Anat Entwicklungsgesch. 1902;12:253-348. [Google Scholar]
- 59. Krukenberg CFW. Die farbigen Derivate der Nebennierenchromogene. Virchow’s Arch Pathol Anat Physiol klin Med 1885;101(3):542-571. doi: 10.1007/BF01994785. [DOI] [Google Scholar]
- 60. Oliver G, Schäfer EA. On the physiological action of extract of the suprarenal capsules. J Physiol (London). 1894;16(3-4):i-iv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oliver G, Schäfer EA. The physiological effects of extracts of the suprarenal capsules. J Physiol (London). 1895;18(3):230-276. doi: 10.1113/jphysiol.1895.sp000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bates WH. The use of extract of suprarenal capsule in the eye. New York Med J., 1896:647-650. https://www.central-fixation.com/bates-medical-articles/use-of-extract-of-suprarenal-capsule.php [Google Scholar]
- 63. Solis-Cohen S. A preliminary note on treatment of hay-fever with suprarenal substance-with a report of personal experience. Philadelphia Med J. 1898;II(7):341-343 [Google Scholar]
- 64. Solis-Cohen S. The use of adrenal substance in the treatment of asthma. 1900. J Am Med Assn 1900;34(6):1164-1166. doi: 10.3109/02770909009073358. [DOI] [PubMed] [Google Scholar]
- 65. Davenport HW. Epinephrin(e). Physiologist 1982;25(2):76-82. [PubMed] [Google Scholar]
- 66. Abel JJ, Crawford AC. On the blood-pressure-raising constituent of the suprarenal capsule. Johns Hopkins Hosp Bull 1897;8:151-157. [Google Scholar]
- 67. Abel JJ. Über den blutdruckerregenden Bestandtheil der Nebenniere, das Epinephrin. Hoppe-Seylerʼs Z physiol Chem 1899;28:318-362. doi: 10.1515/bchm2.1899.28.3-4.318. [DOI] [Google Scholar]
- 68. Mieda R, Aso C, Hiroki T, et al. Comparison of four documents describing adrenaline purification, and the work of three important scientists, Keizo Uenaka, Nagai Nagayoshi and Jokichi Takamine. J Anesthesia History 2020;6(2):42-48. doi: 10.1016/j.janh.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 69. Takamine J. Adrenalin the active principle of the suprarenal glands and its mode of preparation. Am J Pharmacy 1901;73 (Nov):523-531. https://www.proquest.com/openview/51821a4c324fb0c1a137e5155f1d82a3/1?pq-origsite=gscholar&cbl=41445 [Google Scholar]
- 70. Takamine J. The isolation of the active principle of the suprarenal gland. (Proc Physiol Soc Dec 14, 1901) J Physiol (London). 1902. (1 Dec);27(Suppl):xxix-xxx. 10.1113/jphysiol.1902.sp000893 [DOI] [Google Scholar]
- 71. Aldrich TB. Adrenalin, the active principle of the suprarenal glands. J Am Chem Soc. 1905;27(9):1074-1091. doi: 10.1021/ja01987a006. [DOI] [Google Scholar]
- 72. Takamine J. US Patent No. 730,175, “Process of obtaining products from suprarenal glands“. 1903. https://patents.google.com/patent/US730175A/en
- 73. Parke-Davis & Co. v. H. K. Mulford Co. United States Circuit Court for the Southern District of New York. 1911;189 F:195. https://cite.case.law/f/189/95/. [Google Scholar]
- 74. Beauchamp C. Patenting nature: a problem of history. Stan Technol Law Rev 2013;16:257-312. https://law.stanford.edu/publications/patenting-nature-a-problem-of-history/. [Google Scholar]
- 75. Friedmann E. Die Konstitution des Adrenalins. Beit Z Chem Phys Path 1906;8:95-120. [Google Scholar]
- 76. Loewi O, Meyer H. Über die Wirkung synthetischer, dem Adrenalin verwandter Stoffe. Archiv f. experiment. Pathol. u. Pharmakol 1905;53(3-4):213-226. doi: 10.1007/bf01876981. [DOI] [Google Scholar]
- 77. Flächer F. Über die Spaltung des synthetischen dl-Suprarenins in seine optisch aktiven Komponenten. Hoppe-Seyler’s Z physiol Chem. 1909. (1 Jan);58:189-194. 10.1515/bchm2.1909.58.3.189 [DOI] [Google Scholar]
- 78. Blaschko H. The activity of l-dopa decarboxylase. J Physiol (London) 1942;101(3):337-349. doi: 10.1113/jphysiol.1942.sp003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. von Euler US. A specific sympathomimetic ergone in adrenergic nerve fibres (sympathin) and its relations to adrenaline and nor‐adrenaline. Acta Physiol Scand. 1946;12(15):73-97. doi: 10.1111/j.1748-1716.1946.tb00368.x. [DOI] [Google Scholar]
- 80. Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage. An Account of Researches into the Function of Emotional Excitement (1915). New York: D. Appleton and Co. 10.1037/10013-000 [DOI] [Google Scholar]
- 81. Cori CF, Cori GT. The fate of sugar in the animal body VII. The carbohydrate metabolism of adrenalectomized rats and mice. J Biol Chem. 1927;74(3):473-494. doi: 10.1016/s0021-9258(20)74040-8. [DOI] [Google Scholar]
- 82. Baumann EJ, Kurland S. Changes in the inorganic constituents of blood in suprarenalectomized cats and rabbits. J Biol Chem. 1927;71(2):281-302. doi: 10.1016/s0021-9258(18)84417-9. [DOI] [Google Scholar]
- 83. Rogoff JM, Stewart GN. The influence of adrenal extracts on the survival period of adrenalectomized dogs. Science 1927;66(1710):327-328. doi: 10.1126/science.66.1710.327. [DOI] [PubMed] [Google Scholar]
- 84. Rogoff JM, Stewart GN. Studies on adrenal insufficiency in dogs: V. The influence of adrenal extracts on the survival period of adrenalectomized dogs. Am J Physiol. 1928;84(3):660-674. doi: 10.1152/ajplegacy.1928.84.3.649. [DOI] [Google Scholar]
- 85. Kendall EC. The isolation in crystalline form of the compound containing iodin, which occurs in the thyroid: its chemical nature and physiologic activity. J Am Med Assn 1915;64(25):2042-2043. doi: 10.1001/jama.1915.02570510018005. [DOI] [PubMed] [Google Scholar]
- 86. Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus. Canad Med Assoc J 1922;12(3):141-146. [PMC free article] [PubMed] [Google Scholar]
- 87. Bliss M. Rewriting medical history: Charles Best and the Banting and Best myth. J Hist Med Allied Sci. 1993;48(3):253-274. doi: 10.1093/jhmas/48.3.253. [DOI] [PubMed] [Google Scholar]
- 88. Roth J, Qureshi S, Whitford I, et al. Insulin’s discovery: new insights on its ninetieth birthday. Diabetes Metab Res Rev. 2012;28(4):293-304. doi: 10.1002/dmrr.2300. [DOI] [PubMed] [Google Scholar]
- 89. Collip JB. The extraction of a parathyroid hormone that will prevent or control parathyroid tetany and which regulates the blood level of calcium. J Biol Chem. 1925;63(2):395-438. doi: 10.1016/s0021-9258(18)85007-4. [DOI] [Google Scholar]
- 90. Hartman FA, Brownell KA, Hartman WE, Dean GA, MacArthur CG. The hormone of the adrenal cortex. Am J Physiol. 1928;86(2):353-359. doi: 10.1152/ajplegacy.1928.86.2.353. [DOI] [Google Scholar]
- 91. Smith PE. The disabilities caused by hypophysectomy and their repair.The tuberal (hypothalamic) syndrome in the rat. J Am Med Assn 1927;88(3):158-161. doi: 10.1001/jama.1927.02680290020005. [DOI] [Google Scholar]
- 92. Smith PE. Hypophysectomy and a replacement therapy in the rat. Am J Anat. 1930;45(2):205-273. doi: 10.1002/aja.1000450203. [DOI] [Google Scholar]
- 93. Christy NP. Philip Edward Smith PhD (1884-1970). Endocrinology 1972;90(6):1415-1416. doi: 10.1210/endo-90-6-1415. [DOI] [PubMed] [Google Scholar]
- 94. Swingle WW, Pfiffner JJ. The revival of comatose adrenalectomized cats with an extract of the suprarenal cortex. Science 1930;72(1855):75-76. doi: 10.1126/science.72.1855.75. [DOI] [PubMed] [Google Scholar]
- 95. Hartman FA, Brownell KA. The hormone of the adrenal cortex. Science 1930;72(1855):7676-76776. doi: 10.1126/science.72.1855.76. [DOI] [PubMed] [Google Scholar]
- 96. Hartman FA, Brownell KA, Hartman WE. A further study of the hormone of the adrenal cortex. Am J Physiol. 1930;95(3):670-680. doi: 10.1152/ajplegacy.1930.95.3.670. [DOI] [Google Scholar]
- 97. Rowntree LG, Greene CH, Swingle WW, Pfiffner JJ. The treatment of patients with Addison’s disease with the “cortical hormone” of Swingle and Pfiffner. Science 1930;72(1871):482-483. doi: 10.1126/science.72.1871.482. [DOI] [PubMed] [Google Scholar]
- 98. Swingle WW, Pfiffner JJ. Studies on the adrenal cortex: IV. Further observations on the preparation and chemical properties of the cortical hormone. Am J Physiol. 1931;98:144-152. doi: 10.1152/ajplegacy.1931.98.1.144. [DOI] [Google Scholar]
- 99. Swingle WW, Pfiffner JJ, Vars HM, Bott PA, Parkins WM. The function of the adrenal cortical hormone and the cause of death from adrenal insufficiency. Science 1933;77(1985):58-64. doi: 10.1126/science.77.1985.58. [DOI] [PubMed] [Google Scholar]
- 100. Cheney WF. Addison’s disease: Report of case treated by Eschatin. Cal West Med. 1933;39(4):262-3. https://pubmed.ncbi.nlm.nih.gov/18742654/ [PMC free article] [PubMed] [Google Scholar]
- 101. Butler AM, Ross RA, Talbot NB. Probable adrenal insufficiency in an infant: report of a case. J Pediatr. 1939;15(6):831-835. doi: 10.1016/s0022-3476(39)80083-x. [DOI] [Google Scholar]
- 102. Rogoff JM. The adrenal cortical hormone: Experiments with a commercial adrenal extract (Eschatin). J Am Med Assn 1934;103(23):1764-1767. doi: 10.1001/jama.1934.02750490020005. [DOI] [Google Scholar]
- 103. Chiu CY, Needham DM. The effect of adrenal cortical preparations added in vitro upon the carbohydrate metabolism of liver slices. 1. The effect of adrenal cortical extract (eschatin) upon synthesis of glycogen and of total carbohydrate. Biochem J. 1950;46(1):114-120. doi: 10.1042/bj0460114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Loeb RF. Effect of sodium chloride in treatment of a patient with Addison’s disease. Proc Soc Exp Biol Med. 1933;30(6):808-812. doi: 10.3181/00379727-30-6686. [DOI] [Google Scholar]
- 105. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp. 1932:137-195 [reprinted in Obes Res 1994; vol 1992:1486-1508. doi: 10.1002/j.1550-8528.1994.tb00097.x]. [DOI] [PubMed] [Google Scholar]
- 106. Ingle DJ, Kendall EC. Atrophy of the adrenal cortex of the rat produced by the administration of large amounts of cortin. Science 1937;86(2228):245-246. doi: 10.1126/science.86.2228.245. [DOI] [PubMed] [Google Scholar]
- 107. Miller WL. The hypothalamic-pituitary-adrenal axis: A brief history. Horm Res Paediatr 2018;89(4):212-223. doi: 10.1159/000487755. [DOI] [PubMed] [Google Scholar]
- 108. Menkin V. Effect of adrenal cortex extract on capillary permeability. Am J Physiol. 1940;129(3):691-697. doi: 10.1152/ajplegacy.1940.129.3.691. [DOI] [Google Scholar]
- 109. Selye H. A syndrome produced by diverse nocuous agents. Nature 3479;1936(138):32. doi: 10.1038/138032a0. [DOI] [PubMed] [Google Scholar]
- 110. Selye H. The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol Metab. 1946;6(2):117-230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- 111. Wieland HO. The chemistry of the bile acids. Nobel Lecture (on line) 1927. https://www.nobelprize.org/prizes/chemistry/1927/summary/. [Google Scholar]
- 112. Windaus A. Constitution of sterols and their connection with other substances occurring in nature. Nobel Lectures. Chemistry 1928;1941:105-121. https://www.nobelprize.org/prizes/chemistry/1928/summary/. [Google Scholar]
- 113. Witkop B. Remembering Heinrich Wieland (1877-1957). Portrait of an organic chemist and founder of modern biochemistry. Med Res Rev. 1992;12(3):195-274. doi: 10.1002/med.2610120303. [DOI] [PubMed] [Google Scholar]
- 114. Rosenheim O, King H. The chemistry of the sterols, bile acids, and other cyclic constituents of natural fats and oils. Annu Rev Biochem. 1934;3(1):87-110. doi: 10.1146/annurev.bi.03.070134.000511. [DOI] [Google Scholar]
- 115. Trunk A. Biochemistry in wartime: the life and lessons of Adolf Butenandt, 1936–1946. Minerva 2006;44(3):285-306. doi: 10.1007/s11024-006-9002-2. [DOI] [Google Scholar]
- 116. Ruzicka L. Multimembered rings, higher terpene compounds and male sex hormones. Nobel Lecture 1945:2-5. https://www.nobelprize.org/uploads/2018/06/ruzicka-lecture.pdf https://www.nobelprize.org/prizes/chemistry/1939/ruzicka/lecture/. [Google Scholar]
- 117. Butenandt A, Karlson P. Über die Isolierung eines Metamorphose-Hormons der Insekten kristallisierter form. Z Naturforschung B 1954;9(6):389-391. doi: 10.1515/znb-1954-0601. [DOI] [Google Scholar]
- 118. Rothschild M. Tadeus Reichstein: 20 July 1897-1 August 1996. Biogr Mem Fellows R Soc 1999;45:451-467. doi: 10.1098/rsbm.1999.0030. [DOI] [PubMed] [Google Scholar]
- 119. Mülhaupt R. Hermann Staudinger and the origin of macromolecular chemistry. Angew Chem Int Ed. 2004;43(9):1054-1063. doi: 10.1002/anie.200330070. [DOI] [PubMed] [Google Scholar]
- 120. Reichstein T, Grüssner A, Oppenauer R. Synthesis of d-and l-ascorbic acid (vitamin C). Nature 1933;132(3329):280280-2802280. doi: 10.1038/132280b0. [DOI] [Google Scholar]
- 121. Reichstein T. “Adrenosteron”. Über die Bestandteile der Nebennierenrinde II (vorläufige Mitteilung). Helv Chim Acta. 1936;19(1):223-225. doi: 10.1002/hlca.19360190135. [DOI] [Google Scholar]
- 122. Reichstein T. Über die Bestandteile der Nebennierenrinde IV. Helv Chim Acta. 1936;19(1):402-412. doi: 10.1002/hlca.19360190165. [DOI] [PubMed] [Google Scholar]
- 123. Reichstein T. Chemistry of the adrenal cortex hormones. Nobel Lecture. 1950;291-308. (https://www.nobelprize.org/prizes/medicine/1950/reichstein/lecture/). [Google Scholar]
- 124. Simpson SA, Tait JF, Wettstein A, Neher R, Von Euw J, Reichstein T. Isolierung eines neuen kristallisierten Hormons aus Nebennerien mit besonders hoher Wirksamkeit auf den Mineralsoffwechsel. Experientia 1953;9(9):333-335. doi: 10.1007/BF02155834. [DOI] [PubMed] [Google Scholar]
- 125. Simpson SA, Tait JF, Wettsein A, et al. Aldosteronisolierung und Eigenschaften über Bestandteile de Nebennierenrinde und verwandte Stoffe. Helv Chim Acta. 1954;37(4):1163-1200. doi: 10.1002/HLCA.19540370423. [DOI] [Google Scholar]
- 126. Glyn JH. The discovery of cortisone: a personal memory. BMJ 1998;317(7161):822-822. doi: 10.1136/bmj.317.7161.822a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kendall EC. A method of decomposition of the protein of the thyroid with a description of certain constituents. J Biol Chem. 1915;20:500-509. doi: 10.1016/S0021-9258(18)88214-X. [DOI] [Google Scholar]
- 128. Kendall EC, McKenzie BF, Mason HL. A study of glutathione I. Its preparation in crystalline form and its identification. J Biol Chem. 1929;84(2):657-674. doi: 10.1016/s0021-9258(18)77022-1. [DOI] [Google Scholar]
- 129. Harington CR, Barger G. Chemistry of thyroxine: Constitution and synthesis of thyroxine. Biochem J. 1927;21(1):169-183. doi: 10.1042/bj0210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ingle DJ. Edward C. Kendall: March 8, 1886-May 4, 1972. National Academies Biographical Memoirs. Vol 47. Washington DC: National Academies Press; 1975:248-291. http://www.nasonline.org/publications/biographical-memoirs/memoir-pdfs/kendall-edward.pdf [Google Scholar]
- 131. Wintersteiner O, Pfiffner J. Chemical Studies on the Adrenal Cortex: III. Isolation of Two New Physiologically Inactive Compounds. J Biol Chem. 1936;116(1):291-305. doi: 10.1016/s0021-9258(18)74684-x. [DOI] [Google Scholar]
- 132. Cartland GF, Kuizenga MH. The preparation of extracts containing the adrenal cortical hormone. J Biol Chem. 1936;116(1):57-64. doi: 10.1016/s0021-9258(18)74662-0. [DOI] [Google Scholar]
- 133. Heron WT, Hales WM, Ingle DJ. Capacity of skeletal muscle in rats to maintain work output. Am J Physiol. 1934;110(2):357-361. doi: 10.1152/ajplegacy.1934.110.2.357. [DOI] [Google Scholar]
- 134. Ingle DJ. Work capacity of the adrenalectomized rat treated with cortin. Am J Physiol. 1936;116(3):622-625. doi: 10.1152/ajplegacy.1936.116.3.622. [DOI] [Google Scholar]
- 135. Kendall EC. Isolation in crystalline form of the hormone essential to life from the suprarenal cortex: its chemical nature and physiologic properties. Trans Assoc Am Physicians 1934;48:147-152. [Google Scholar]
- 136. Mason HL, Myers CS, Kendall EC. Chemical studies of the suprarenal cortex II. The identification of a substance which possesses the qualitative action of cortin; Its conversion into a diketone closely related to androstenedione. J Biol Chem. 1936;116(1):267-276. doi: 10.1016/s0021-9258(18)74681-4. [DOI] [Google Scholar]
- 137. Ingle DJ. The work performance of adrenalectomized rats treated with corticosterone and chemically related compounds. Endocrinology 1940;26(3):472-477. doi: 10.1210/endo-26-3-472. [DOI] [Google Scholar]
- 138. Steiger M, Reichstein T. Desoxy‐cortico‐steron (21‐Oxy‐progesteron) aus Δ 5‐3‐Oxy‐ätio‐cholensäure. Helv Chim Acta. 1937;20(1):1164-1179. doi: 10.1002/hlca.193702001158. [DOI] [Google Scholar]
- 139. Thorn GW, Howard RP, Emerson K. Treatment of Addison’s disease with desoxy-corticosterone acetate, a synthetic adrenal cortical hormone (preliminary report). J Clin Invest. 1939;18(4):449-467. doi: 10.1172/JCI101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cleghorn RA, Fowler JLA, Wenzel JS. The treatment of Addison’s disease by a synthetic adrenal cortical hormone (desoxycorticosterone acetate). Canad Med Assoc J 1939;41(3):226-231. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC537457/pdf/canmedaj00208-0004.pdf. [PMC free article] [PubMed] [Google Scholar]
- 141. Ferrebee JW, Ragan C, Atchley DW, Loeb RF. Desoxycorticosterone esters: certain effects in the treatment of Addison’s disease. J Am Med Assn 1939;113(19):1725-1731. doi: 10.1001/jama.1939.02800440029009. [DOI] [Google Scholar]
- 142. Levy-Simpson S, Wilkinson JF, Himsworth HP, Jones A. Discussion on recent developments in the treatment of Addison’s disease. Proc Roy Soc Med 1939;32(6):685-706. https://journals.sagepub.com/doi/pdf/10.1177/003591573903200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hench PS. The analgesic effect of hepatitis and jaundice in chronic arthritis, fibrositis and sciatic pain. Annals Intern Med 1934;7(10):1278-1294. doi: 10.7326/0003-4819-7-10-1278. [DOI] [Google Scholar]
- 144. Hench PS. Effect of jaundice on chronic infectious (atrophic) arthritis and on primary fibrositis: further observations; attempts to reproduce the phenomenon. Arch Intern Med. 1938;61(3):451-480. doi: 10.1001/archinte.1938.00020030081006. [DOI] [Google Scholar]
- 145. Hench PS. The advantages of hepatic injury and jaundice in certain conditions, notably the rheumatic diseases. Med Clin North America. 1940;24(4):1209-1237. doi: 10.1016/S0025-7125(16)36696-2. [DOI] [Google Scholar]
- 146. Hench PS, Kendall EC, Slocumb CH, Polley HF. Effects of cortisone acetate and pituitary ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions. Arch Intern Med. 1950;85(4):545-666. doi: 10.1001/archinte.1950.00230100002001. [DOI] [PubMed] [Google Scholar]
- 147. Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocortical hormone in arthritis: preliminary report. Ann Rheum Dis. 1949;8(2):97-104. doi: 10.1136/ard.8.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Tait SA, Tait JF, Coghlan JP. The discovery, isolation and identification of aldosterone: reflections on emerging regulation and function. Mol Cell Endocrinol. 2004;217(1-2):1-21. doi: 10.1016/j.mce.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 149. Bush IE. Methods of paper chromatography of steroids applicable to the study of steroids in mammalian blood and tissues. Biochem J. 1952;50(3):370-378. doi: 10.1042/bj0500370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Butler GC, Marrian GF. The isolation of pregnane-3,17,20-triol from the urine of women showing the adrenogenital syndrome. J Biol Chem. 1937;119(2):565-572. doi: 10.1016/s0021-9258(18)74402-5. [DOI] [Google Scholar]
- 151. Butler GC, Marrian GF. Chemical studies on the adreno-genital syndrome: I.The isolation of 3(α)-hydroxyetiocholane-17-one, 3(β)-hydroxyetioallocholane-17-one (isoandrosterone), and a new triol from the urine of a woman with an adrenal tumor. J Biol Chem. 1938;124(1):237-247. doi: 10.1016/S0021-9258(18)74091-X. [DOI] [Google Scholar]
- 152. Marrian GF. Some aspects of the intermediary metabolism of the steroid hormones: Harvey Lecture, October 20, 1938. Bull New York Acad Med 1939;15(1):27-42. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1911341/pdf/bullnyacadmed00586-0029.pdf. [PMC free article] [PubMed] [Google Scholar]
- 153. Marker RE, Sterols XL. The origin and interrelationships of the steroidal hormones. J Am Chem Soc. 1938;60(8):1725-1736. doi: 10.1021/ja01275a007. [DOI] [Google Scholar]
- 154. Bongiovanni AM, Eberlein WR, Cara J. Studies on the metabolism of adrenal steroids in the adrenogenital syndrome. J Clin Endocrinol Metab. 1954;14(4):409-422. doi: 10.1210/jcem-14-4-409. [DOI] [PubMed] [Google Scholar]
- 155. Wilkins L, Richter CP. A great craving for salt by a child with cortico-adrenal insufficiency. J Am Med Assn 1940;114(10):866-868. doi: 10.1001/jama.1940.62810100001011. [DOI] [Google Scholar]
- 156. McMaster EW. Lawson Wilkins: recollections by his daughter. Int J Pediatr Endocr. 2014;2014(1):1-23. doi: 10.1186/1687-9856-2014-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Migeon CJ. Lawson Wilkins and my life: part 2. Int J Pediatr Endocr. 2014;2014(1):1-23. doi: 10.1186/1687-9856-2014-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Fisher DA. A short history of pediatric endocrinology in North America. Pediatr Res. 2004;55(4):716-726. doi: 10.1203/01.PDR.0000113824.18487.9B. [DOI] [PubMed] [Google Scholar]
- 159. Wilkins L, Fleischmann W, Howard J. Macrogenitosomia precox associated with hyperplasia of the androgenic tissue of the adrenal and death from corticoadrenal insufficiency case report. Endocrinology 1940;26(3):385-395. doi: 10.1210/endo-26-3-385. [DOI] [Google Scholar]
- 160. Berthold AA. Transplantation der hoden. Arch Anat Physiol. 1849:42-46. (translation by DP Quiring available at Bull History Med 1944; 16(4):399-401. www.jstor.org/44442835) [Google Scholar]
- 161. Talbot NB, Butler AM, Berman RA. Adrenal cortical hyperplasia with virilism: diagnosis, course and treatment. J Clin Invest. 1942;21(5):559-570. doi: 10.1172/JCI101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Talbot NB, Butler AM, Maclachlan EA. The effect of testosterone and allied compounds on the mineral, nitrogen, and carbohydrate metabolism of a girl with Addison’s disease. J Clin Invest. 1943;22(4):583-593. doi: 10.1172/JCI101430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Albright F. Cushing’s syndrome: its pathology and physiology, its relationship to the adreno-genital syndrome, and its connection with the problem of the reaction of the body to injurious agents (alarm reaction of Selye). Harvey Lectures Series 1942;38:123-186. [Google Scholar]
- 164. Kepler EJ. Cushing’s disease; a primary disorder of the adrenal cortices? Ann N Y Acad Sci. 1949;50(Art. 6):657-678. doi: 10.1111/j.1749-6632.1949.tb39874.x. [DOI] [PubMed] [Google Scholar]
- 165. Bauer J. The so‐called Cushing’s syndrome, its history, terminology and differential diagnosis. Acta Med Scand 1950;137(6):411-416. doi: 10.1111/j.0954-6820.1950.tb12132.x. [DOI] [PubMed] [Google Scholar]
- 166. Talbot NB, Langstroth GO. A rapid extractor for urinary androgens factors to be considered in the preparation of extracts for colorimetric assay. Endocrinology 1939;25(5):729-736. doi: 10.1210/endo-25-5-729. [DOI] [Google Scholar]
- 167. Talbot NB, Butler AM. Urinary 17-ketosteroid assays in clinical medicine. J Clin Endocrinol 1942;2(12):724-729. doi: 10.1210/jcem-2-12-724. [DOI] [Google Scholar]
- 168. Talbot NB, Saltzman AH, Wixom RL, Wolfe JK. The colorimetric assay of urinary corticosteroid-like substances. J Biol Chem. 1945;160(2):535-546. doi: 10.1016/s0021-9258(18)51064-4. [DOI] [PubMed] [Google Scholar]
- 169. Gardner LJ, Sniffen RC, Zygmuntowicz AS, Talbot NB. Follow-up studies in a boy with mixed adrenal cortical disease. Pediatrics 1950;5(5):808-823. doi: 10.1542/peds.5.5.808. [DOI] [PubMed] [Google Scholar]
- 170. Talbot NB, Albright F, Saltzman AH, Zygmuntowicz AS, Wixom RL. The excretion of 11-oxycorticosteroid-like substances by normal and abnormal subjects. J Clin Endocrinol Metab. 1947;7(5):331-350. doi: 10.1210/jcem-7-5-331. [DOI] [PubMed] [Google Scholar]
- 171. Zuelzer WW, Blum A Jr. Adrenocortical insufficiency in infants with the adrenogenital syndrome: A clinical and pathologic study of four cases. J Pediatr. 1949;35(3):344-361. doi: 10.1016/s0022-3476(49)80007-2. [DOI] [PubMed] [Google Scholar]
- 172. Jacobsen AW, Koepf GF, Talbot NB, Wilkins L. The adrenal gland in health and disease. Pediatrics 1949;3(4):515-548. doi: 10.1542/peds.3.4.515. [DOI] [PubMed] [Google Scholar]
- 173. Lewis RA, Klein R, Wilkins L. Congenital adrenal hyperplasia with pseudohermaphrodism and symptoms of Addison’s disease; clinical course following bilateral total adrenalectomy, with metabolic studies, pathologic findings and discussion of etiology. J Clin Endocrinol Metab. 1950;10(7):703-715. doi: 10.1210/jcem-10-7-703. [DOI] [PubMed] [Google Scholar]
- 174. Lewis RA, Wilkins L. The effect of adrenocorticotrophic hormone in congenital adrenal hyperplasia with virilism and in Cushing’s syndrome treated with methyl testosterone. J Clin Invest. 1949;28(2):394-400. doi: 10.1172/JCI102082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wilkins L, Lewis RA, Klein R, Rosemberg E. The suppression of androgen secretion by cortisone in a case of congenital adrenal hyperplasia. Bull Johns Hopkins Hosp 1950;86(4):249-252. [PubMed] [Google Scholar]
- 176. Bartter FC, Forbes AP, Leaf A. Congenital adrenal hyperplasia associated with the adrenogenital syndrome: an attempt to correct its disordered hormonal pattern. J Clin Invest. 1950;29(6):797. [PubMed] [Google Scholar]
- 177. Wilkins L, Lewis RA, Klein R, et al. Treatment of congenital adrenal hyperplasia with cortisone. J Clin Endocrinol Metab. 1951;11(1):1-25. doi: 10.1210/jcem-11-1-1. [DOI] [PubMed] [Google Scholar]
- 178. Bartter FC, Albright F, Forbes AP, Leaf A, Dempsey E, Carroll E. The effects of adrenocorticotropic hormone and cortisone in the adrenogenital syndrome associated with congenital adrenal hyperplasia: an attempt to explain and correct its disordered hormonal pattern. J Clin Invest. 1951;30(3):237-251. doi: 10.1172/JCI102438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wilkins L, Gardner LI, Crigler JF Jr, Silverman SH, Migeon CJ. Further studies on the treatment of congenital adrenal hyperplasia with cortisone. I. Comparison of oral and intramuscular administration of cortisone, with a note on the suppressive action of compounds F and B on the adrenal. J Clin Endocrinol Metab. 1952;12(3):257-276. doi: 10.1210/jcem-12-3-257. [DOI] [PubMed] [Google Scholar]
- 180. Wilkins L, Crigler JF Jr, Silverman SH, Gardner LI, Migeon CJ. Further studies on the treatment of congenital adrenal hyperplasia with cortisone. II. The effects of cortisone on sexual and somatic development, with an hypothesis concerning the mechanism of feminization. J Clin Endocrinol Metab. 1952;12(3):277-295. doi: 10.1210/jcem-12-3-277. [DOI] [PubMed] [Google Scholar]
- 181. Wilkins L, Crigler JF Jr, Silverman SH, Gardner LI, Migeon CJ. Further studies on the treatment of congenital adrenal hyperplasia with cortisone. III. The control of hypertension with cortisone, with a discussion of variations in the type of congenital adrenal hyperplasia and report of a case with probable defect of carbohydrate-regulating hormones. J Clin Endocrinol Metab. 1952;12(8):1015-1030. doi: 10.1210/jcem-12-8-1015. [DOI] [PubMed] [Google Scholar]
- 182. Crigler JF Jr, Silverman SH, Wilkins L. Further studies on the treatment of congenital adrenal hyperplasia with cortisone. IV. The effect of cortisone and compound B in infants with disturbed electrolyte metabolism. Pediatrics 1952;10(4):397-413. doi: 10.1542/peds.102.S1.215. [DOI] [PubMed] [Google Scholar]
- 183. Wilkins L, Cara J. Further studies on the treatment of congenital adrenal hyperplasia with cortisone. V. Effects of cortisone therapy on testicular development. J Clin Endocrinol Metab. 1954;14(3):287-296. doi: 10.1210/jcem-14-3-287. [DOI] [PubMed] [Google Scholar]
- 184. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043-4088. doi: 10.1210/jc.2018-01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, et al. Congenital adrenal hyperplasia - current insights in pathophysiology, diagnostics and management. Endocr Rev. 2022;43(1):91-159. doi: 10.1210/endrev/bnab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Blodgett FM, Burgin L, Iezzoni D, Gribetz D, Talbot NB. Effects of prolonged cortisone therapy on the statural growth, skeletal maturation and metabolic status of children. N Engl J Med. 1956;254(14):636-641. doi: 10.1056/NEJM195604052541402. [DOI] [PubMed] [Google Scholar]
- 187. Sobel EH, Raymond CS, Quinn KV, Talbot NB. The use of methyltestosterone to stimulate growth: relative influence on skeletal maturation and linear growth. J Clin Endocrinol Metab. 1956;16(2):241-248. doi: 10.1210/jcem-16-2-241. [DOI] [PubMed] [Google Scholar]
- 188. Hechter O. Lymphocyte discharge from the isolated rabbit spleen by adrenal cortical extract. Endocrinology 1948;42(4):285-306. doi: 10.1210/endo-42-4-285. [DOI] [PubMed] [Google Scholar]
- 189. Hechter O, Jacobsen RP, Jeanloz RW, Levy H, Marshall CW, Pincus G. The bio-oxygenation of steroids at C-11. Arch Biochem 1950;25(2):457-460. [PubMed] [Google Scholar]
- 190. Zaffaroni A, Hechter O, Pincus G. Adrenal conversion of C14 labeled cholesterol and acetate to adrenal cortical hormones. J Am Chem Soc. 1951;73(3):1390-1391. doi: 10.1021/ja01147a550. [DOI] [Google Scholar]
- 191. Stone D, Hechter O. Studies on ACTH action in perfused bovine adrenals: the site of action of ACTH in corticosteroidogenesis. Arch Biochem Biophys. 1954;51(2):457-469. doi: 10.1016/0003-9861(54)90501-9. [DOI] [PubMed] [Google Scholar]
- 192. Levy H, Jeanloz RW, Jacobsen RP, Hechter O, Schenker V, Pincus G. Chemical transformations of steroids by adrenal perfusion; progesterone, 17α-hydroxyprogesterone, and pregn-5-en-3β-ol-20-one. J Biol Chem. 1954;211(2):867-881. doi: 10.1016/S0021-9258(18)71175-7. [DOI] [PubMed] [Google Scholar]
- 193. Hechter O, Pincus G. Genesis of the adrenocortical secretion. Physiol Rev. 1954;34(3):459-496. doi: 10.1152/physrev.1954.34.3.459. [DOI] [PubMed] [Google Scholar]
- 194. Eisenmenger WJ, Blondheim SH, Bongiovanni AM, Kunkel HG. Electrolyte studies on patients with cirrhosis of the liver. J Clin Invest. 1950;29(11):1491-1499. doi: 10.1172/JCI102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Bongiovanni AM, Eisenmenger WJ. Adrenal cortical metabolism in chronic liver disease. J Clin Endocrinol Metab. 1951;11(2):152-172. doi: 10.1210/jcem-11-2-152. [DOI] [PubMed] [Google Scholar]
- 196. Bongiovanni AM. The detection of pregnandiol and pregnantriol in the urine of patients with adrenal hyperplasia, suppression with cortisone; preliminary report. Bull Johns Hopkins Hosp 1953;92(3):244-251. [PubMed] [Google Scholar]
- 197. Bongiovanni AM, Clayton GW Jr. A simplified method for the routine determination of pregnanediol and pregnanetriol in urine. Bull Johns Hopkins Hosp 1954;94(4):180-186. [PubMed] [Google Scholar]
- 198. Bongiovanni AM, Eberlein WR. Clinical and metabolic variations in the adrenogenital syndrome. Pediatrics 1955;16(5):628-636. doi: 10.1542/peds.16.5.628. [DOI] [PubMed] [Google Scholar]
- 199. Bongiovanni AM, Root AM. The adrenogenital syndrome. N Engl J Med. 1963;268(24):1283-1289. doi: 10.1056/NEJM196306132682406. [DOI] [PubMed] [Google Scholar]
- 200. Bongiovanni AM. In vitro hydroxylation of steroids by whole adrenal homogenates of beef, normal man, and patients with the adrenogenital syndrome. J Clin Invest. 1958;37(10):1342-1347. doi: 10.1172/JCI103723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Jailer JW, Gold JJ, Wiele RV, Lieberman S. 17α-hydroxyprogesterone and 21-desoxyhydrocortisone; their metabolism and possible role in congenital adrenal virilism. J Clin Invest. 1955;34(11):1639-1646. doi: 10.1172/JCI103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Fukushima DK, Gallagher TF. Steroid isolation studies in congenital adrenal hyperplasia. J Biol Chem. 1957;229(1):85-92. doi: 10.1016/S0021-9258(18)70596-6. [DOI] [PubMed] [Google Scholar]
- 203. Frantz AG, Holub DA, Jailer JW. Further evidence of a relative lack of C-21 hydroxylation in congenital adrenal hyperplasia. J Clin Invest. 1960;39(6):904-908. doi: 10.1172/JCI104110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Jailer JW. Virilism. Bull N Y Acad Med. 1953;29(5):377-394. [PMC free article] [PubMed] [Google Scholar]
- 205. Miller WL. Congenital adrenal hyperplasia: Time to replace 17OHP with 21-deoxycortisol. Horm Res Paediatr 2019;91(6):416-420. doi: 10.1159/000501396. [DOI] [PubMed] [Google Scholar]
- 206. Decourt J, Jayle MF, Baulieu EE. Virilisme cliniquement tardif avec excretion de pregnanetriol et insuffisance de la production du cortisol. Ann Endocrinol (Paris). 1957;18(3):416-422. [PubMed] [Google Scholar]
- 207. New MI, Lorenzen F, Pang S, Gunczler P, Dupont B, Levine LS. “Acquired” adrenal hyperplasia with 21-hydroxylase deficiency is not the same genetic disorders as congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1979;48:356-359. doi: 10.1210/jcem-48-2-356. [DOI] [PubMed] [Google Scholar]
- 208. Rosenwaks Z, Lee PA, Jones GS, Migeon CJ, Wentz AC. An attenuated form of congenital virilizing adrenal hyperplasia. J Clin Endocrinol Metab. 1979;49(3):335-339. doi: 10.1210/jcem-49-3-335. [DOI] [PubMed] [Google Scholar]
- 209. Levine LS, Dupont B, Lorenzen F, et al. Cryptic 21-hydroxylase deficiency in families of patients with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1980;51(6):1316-1324. doi: 10.1210/jcem-51-6-1316. [DOI] [PubMed] [Google Scholar]
- 210. New MI, Lorenzen F, Lerner AJ, et al. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J Clin Endocrinol Metab. 1983;57(2):320-326. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- 211. Pang S, Hotchkiss J, Drash AL, Levine LS, New MI. Microfilter paper method for 17-hydroxyprogesterone radioimmunoassay: its application for rapid screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab 1977;45(5):1003-1008. doi: 10.1210/jcem-45-5-1003. [DOI] [PubMed] [Google Scholar]
- 212. Pang S, Murphey W, Levine LS, et al. A pilot newborn screening for congenital adrenal hyperplasia in Alaska. J Clin Endocrinol Metab. 1982;55(3):413-420. doi: 10.1210/jcem-55-3-413. [DOI] [PubMed] [Google Scholar]
- 213. White PC. Neonatal screening for congenital adrenal hyperplasia. Nat Rev Endocrinol. 2009;5(9):490-498. doi: 10.1038/nrendo.2009.148. [DOI] [PubMed] [Google Scholar]
- 214. Ryan KJ, Engel LL. Hydroxylation of steroids at carbon 21. J Biol Chem. 1957;225(1):103-114. doi: 10.1016/S0021-9258(18)64913-0. [DOI] [PubMed] [Google Scholar]
- 215. Brodie BB, Axelrod J, Cooper JR, et al. Detoxication of drugs and other foreign compounds by liver microsomes. Science 1955;121(3147):603-604. doi: 10.1126/science.121.3147.603. [DOI] [PubMed] [Google Scholar]
- 216. Klingenberg M. Pigments of rat liver microsomes. Arch Biochem Biophys. 1958;75(2):376-386. doi: 10.1016/0003-9861(58)90436-3. [DOI] [PubMed] [Google Scholar]
- 217. Garfinkel D. Studies on pig liver microsomes. I. Enzymic and pigment composition of different microsomal fractions. Arch Biochem Biophys. 1958;77(2):493-509. doi: 10.1016/0003-9861(58)90095-x. [DOI] [PubMed] [Google Scholar]
- 218. Cooper DY. Discovery of the function of the heme protein P-450: a systematic approach to scientific research. Life Sci. 1973;13(9):1151-1161. doi: 10.1016/0024-3205(73)90001-5. [DOI] [Google Scholar]
- 219. Estabrook RW. A passion for P450s (rememberances of the early history of research on cytochrome P450). Drug Metab Dispos. 2003;31(12):1461-1473. doi: 10.1124/dmd.31.12.1461. [DOI] [PubMed] [Google Scholar]
- 220. Omura T. Recollection of the early years of the research on cytochrome P450. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(10):617-640. doi: 10.2183/pjab.87.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Omura T, Sato R. A new cytochrome in liver microsomes. J Biol Chem. 1962;237(4):PC1375-PC1376. doi: 10.1016/s0021-9258(18)60338-2. [DOI] [PubMed] [Google Scholar]
- 222. Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239(7):2370-2378. doi: 10.1016/s0021-9258(20)82244-3. [DOI] [PubMed] [Google Scholar]
- 223. Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J Biol Chem. 1964;239:2379-2385. doi: 10.1016/S0021-9258(20)82244-3. [DOI] [PubMed] [Google Scholar]
- 224. Estabrook RW, Cooper DY, Rosenthal O. The light reversible carbon monoxide inhibition of the steroid C21-hydroxylase system of the adrenal cortex. Biochem Z 1963;338:741-755. [PubMed] [Google Scholar]
- 225. Cooper DY, Levin S, Narasimhulu S, Rosenthal O. Photochemical action spectrum of the terminal oxidase of mixed function oxidase systems. Science 1965;147(3656):400-402. doi: 10.1126/science.147.3656.400. [DOI] [PubMed] [Google Scholar]
- 226. Halkerston ID, Eichhorn J, Hechter O. A requirement for reduced triphosphopyridine nucleotide for cholesterol side-chain cleavage by mitochondrial fractions of bovine adrenal cortex. J Biol Chem. 1961;236(2):374-380. doi: 10.1016/S0021-9258(18)64370-4. [DOI] [PubMed] [Google Scholar]
- 227. Simpson ER, Boyd GS. The cholesterol side-chain cleavage system of bovine adrenal cortex. Eur J Biochem. 1967;2(3):275-285. doi: 10.1111/j.1432-1033.1967.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 228. Simpson ER, Boyd GS. The cholesterol side-chain cleavage system of the adrenal cortex: a mixed-function oxidase. Biochem Biophys Res Commun. 1966;24(1):10-17. doi: 10.1016/0006-291x(66)90402-5. [DOI] [PubMed] [Google Scholar]
- 229. Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci 2013;368(1612):20120431. doi: 10.1098/rstb.2012.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Estabrook RW. Steroid hydroxylations: a paradigm for cytochrome P450 catalyzed mammalian monooxygenation reactions. Biochem Biophys Res Commun. 2005;338(1):290-298. doi: 10.1016/j.bbrc.2005.08.168. [DOI] [PubMed] [Google Scholar]
- 231. Kominami S, Ochi H, Kobayashi Y, Takemori S. Studies on the steroid hydroxylation system in adrenal cortex microsomes. Purification and characterization of cytochrome P-450 specific for steroid C-21 hydroxylation. J Biol Chem. 1980;255(8):3386-3394. https://www.jbc.org/article/S0021-9258(19)85711-3/pdf. [PubMed] [Google Scholar]
- 232. White PC, New MI, Dupont B. Cloning and expression of cDNA encoding a bovine adrenal cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci USA. 1984;81(7):1986-1990. doi: 10.1073/pnas.81.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Yoshioka H, Morohashi K, Sogawa K, et al. Structural analysis of cloned cDNA for mRNA of microsomal cytochrome P-450(C21) which catalyzes steroid 21-hydroxylation in bovine adrenal cortex. J Biol Chem. 1986;261(9):4106-4109. https://www.jbc.org/article/S0021-9258(17)35630-2/pdf. [PubMed] [Google Scholar]
- 234. White PC, New MI, Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci USA. 1986;83(14):5111-5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Dupont B, Oberfield SE, Smithwick EM, Lee TD, Levine LS. Close genetic linkage between HLA and congenital adrenal hyperplasia (21-hydroxylase deficiency). Lancet 1977;2(8052-8053):1309-1312. doi: 10.1016/s0140-6736(77)90362-2. [DOI] [PubMed] [Google Scholar]
- 236. O’Neill GJ, Pollack MS, Yang SY, Levine LS, New MI, Dupont B. Gene frequencies and genetic linkage disequilibrium for the HLA- linked genes Bf, C2, C4S, C4F, 21-hydroxylase deficiency, and glyoxalase I. Transplant Proc. 1979;11(4):1713-1715. [PubMed] [Google Scholar]
- 237. Kohn B, Levine LS, Pollack MS, et al. Late-onset steroid 21-hydroxylase deficiency: a variant of classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1982;55(5):817-827. doi: 10.1210/jcem-55-5-817. [DOI] [PubMed] [Google Scholar]
- 238. O’Neill GJ, Dupont B, Pollack MS, Levine LS, New MI. Complement C4 allotypes in congenital adrenal hyperplasia due to 21-hydroxylase deficiency: further evidence for different allelic variants at the 21-hydroxylase locus. Clin Immunol Immunopathol 1982;23(2):312-322. doi: 10.1016/0090-1229(82)90117-9. [DOI] [PubMed] [Google Scholar]
- 239. White PC, New MI, Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci USA. 1984;81(23):7505-7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. White PC, Grossberger D, Onufer BJ, et al. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci USA. 1985;82(4):1089-1093. doi: 10.1073/pnas.82.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Carroll MC, Campbell RD, Porter RR. Mapping of steroid 21-hydroxylase genes adjacent to complement component C4 genes in HLA, the major histocompatibility complex in man. Proc Natl Acad Sci USA. 1985;82(2):521-525. doi: 10.1073/pnas.82.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. White PC, Chaplin DD, Weis JH, Dupont B, New MI, Seidman JG. Two steroid 21-hydroxylase genes are located in the murine S region. Nature 1984;312(5993):465-467. doi: 10.1038/312465a0. [DOI] [PubMed] [Google Scholar]
- 243. Amor M, Tosi M, Duponchel C, Steinmetz M, Meo T. Liver mRNA probes disclose two cytochrome P-450 genes duplicated in tandem with the complement C4 loci of the mouse H-2S region. Proc Natl Acad Sci USA. 1985;82(13):4453-4457. doi: 10.1073/pnas.82.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Chung BC, Matteson KJ, Miller WL. Cloning and characterization of the bovine gene for steroid 21- hydroxylase (P-450c21). DNA 1985;4(3):211-219. doi: 10.1089/dna.1985.4.211. [DOI] [PubMed] [Google Scholar]
- 245. Higashi Y, Yoshioka H, Yamane M, Gotoh O, Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci USA. 1986;83(9):2841-2845. doi: 10.1073/pnas.83.9.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Saiki RK, Scharf S, Faloona F, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985;230(4732):1350-1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 247. Amor M, Parker KL, Globerman H, New MI, White PC. Mutation in the CYP21B gene (Ile-172----Asn) causes steroid 21- hydroxylase deficiency. Proc Natl Acad Sci USA. 1988;85(5):1600-1604. doi: 10.1073/pnas.85.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Globerman H, Amor M, Parker KL, New MI, White PC. Nonsense mutation causing steroid 21-hydroxylase deficiency. J Clin Invest. 1988;82(1):139-144. doi: 10.1172/JCI113562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Speiser PW, New MI, White PC. Molecular genetic analysis of nonclassic steroid 21-hydroxylase deficiency associated with HLA-B14,DR1. N Engl J Med. 1988;319(1):19-23. doi: 10.1056/NEJM198807073190104. [DOI] [PubMed] [Google Scholar]
- 250. Higashi Y, Tanae A, Inoue H, Hiromasa T, Fujii-Kuriyama Y. Aberrant splicing and missense mutations cause steroid 21- hydroxylase [P-450(C21)] deficiency in humans: possible gene conversion products. Proc Natl Acad Sci USA. 1988;85(20):7486-7490. doi: 10.1073/pnas.85.20.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Tusie-Luna MT, Traktman P, White PC. Determination of functional effects of mutations in the steroid 21-hydroxylase gene (CYP21) using recombinant vaccinia virus. J Biol Chem. 1990;265(34):20916-20922. doi: 10.1016/s0021-9258(17)45304-x. [DOI] [PubMed] [Google Scholar]
- 252. Higashi Y, Hiromasa T, Tanae A, et al. Effects of individual mutations in the P-450(C21) pseudogene on the P-450(C21) activity and their distribution in the patient genomes of congenital steroid 21-hydroxylase deficiency. J Biochem (Tokyo). 1991;109:638-644. doi: 10.1093/oxfordjournals.jbchem.a123433. [DOI] [PubMed] [Google Scholar]
- 253. Speiser PW, Dupont J, Zhu D, et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest. 1992;90(2):584-595. doi: 10.1172/jci115897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Tusie-Luna MT, White PC. Gene conversions and unequal crossovers between CYP21 (steroid 21-hydroxylase gene) and CYP21P involve different mechanisms. Proc Natl Acad Sci USA. 1995;92(23):10796-10800. doi: 10.1073/pnas.92.23.10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Bristow J, Tee MK, Gitelman SE, Mellon SH, Miller WL. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol. 1993;122(1):265-278. doi: 10.1083/jcb.122.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Schalkwijk J, Zweers MC, Steijlen PM, et al. A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. N Engl J Med. 2001;345(16):1167-1175. doi: 10.1056/NEJMoa002939. [DOI] [PubMed] [Google Scholar]
- 257. Burch GH, Gong Y, Liu W, et al. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet. 1997;17(1):104-108. doi: 10.1038/ng0997-104. [DOI] [PubMed] [Google Scholar]
- 258. Miller WL, Merke DP. Tenascin-X, congenital adrenal hyperplasia, and the CAH-X syndrome. Horm Res Paediatr 2018;89(5):352-361. doi: 10.1159/000481911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Miller WL. Tenascin-X-Discovery and early research. Front Immunol. 2021;11:612497. doi: 10.3389/fimmu.2020.612497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Shepard TH, Clausen SW. Case of adrenogenital syndrome with hypertension treated with cortisone. Pediatrics 1951;8(6):805-811. doi: 10.1542/peds.8.6.805. [DOI] [PubMed] [Google Scholar]
- 261. Eberlein WR, Bongiovanni AM. Plasma and urinary corticosteroids in the hypertensive form of congenital adrenal hyperplasia. J Biol Chem. 1956;223(1):85-94. doi: 10.1016/s0021-9258(18)65119-1. [DOI] [PubMed] [Google Scholar]
- 262. Shikita M, Hall PF. Cytochrome P-450 from bovine adrenocortical mitochondria: an enzyme for the side chain cleavage of cholesterol. I. Purification and properties. J Biol Chem. 1973;248(16):5598-5604. doi: 10.1016/S0021-9258(19)43546-1. [DOI] [PubMed] [Google Scholar]
- 263. Watanuki M, Granger GA, Hall PF. Cytochrome P-450 from bovine adrenocortical mitochondria. Immunochemical properties and purity. J Biol Chem. 1978;253(9):2927-2931. doi: 10.1016/s0021-9258(17)40784-8. [DOI] [PubMed] [Google Scholar]
- 264. Suhara K, Gomi T, Sato H, Itagaki E, Takemori S, Katagiri M. Purification and immunochemical characterization of the two adrenal cortex mitochondrial cytochrome P-450-proteins. Arch Biochem Biophys. 1978;190(1):290-299. doi: 10.1016/0003-9861(78)90278-3. [DOI] [PubMed] [Google Scholar]
- 265. John ME, John MC, Simpson ER, Waterman MR. Regulation of cytochrome P-45011β gene expression by adrenocorticotropin. J Biol Chem. 1985;260(9):5760-5767. doi: 10.1016/S0021-9258(18)89087-1. [DOI] [PubMed] [Google Scholar]
- 266. Chua SC, Szabo P, Vitek A, Grzeschik KH, John M, White PC. Cloning of cDNA encoding steroid 11-hydroxylase (P450c11). Proc Natl Acad Sci USA. 1987;84(20):7193-7197. doi: 10.1073/pnas.84.20.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Morohashi K, Yoshioka H, Gotoh O, et al. Molecular cloning and nucleotide sequence of DNA of mitochondrial cytochrome P-450(11β) of bovine adrenal cortex. J Biochem. 1987;102(3):559-568. doi: 10.1093/oxfordjournals.jbchem.a122089. [DOI] [PubMed] [Google Scholar]
- 268. Mornet E, Dupont J, Vitek A, White PC. Characterization of two genes encoding human steroid 11β-hydroxylase (P-45011β). J Biol Chem. 1989;264(35):20961-20967. doi: 10.1016/S0021-9258(19)30030-4. [DOI] [PubMed] [Google Scholar]
- 269. Curnow KM, Tusie-Luna MT, Pascoe L, et al. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol. 1991;5(10):1513-1522. doi: 10.1210/mend-5-10-1513. [DOI] [PubMed] [Google Scholar]
- 270. Morohashi K, Nonaka Y, Kirita S, et al. Enzymatic activities of P-450(11)s expressed by two cDNAs in COS-7 cells. βJ Biochem (Tokyo) 1990;107(4):635-640. doi: 10.1093/oxfordjournals.jbchem.a123099. [DOI] [PubMed] [Google Scholar]
- 271. White PC, Dupont J, New MI, Leiberman E, Hochberg Z, Rosler A. A mutation in CYP11B1 (Arg-448His) associated with steroid 11-hydroxylase deficiency in Jews of Moroccan origin. J Clin Invest 1991;87(5):1664-1667. doi: 10.1172/JCI115182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Veldhuis JD, Melby JC. Isolated aldosterone deficiency in man: acquired and inborn errors in the biosynthesis or action of aldosterone. Endocr Rev. 1981;2(4):495-517. doi: 10.1210/edrv-2-4-495. [DOI] [PubMed] [Google Scholar]
- 273. Royer P, Lestradet H, de Menibus C, Vermiel G. Hypoaldosteronisme familial chronique a debut neo-natal. [Chronic familial hypoaldosteronism of neonatal onset]. Ann Pediat 1961;8:133-138. [Google Scholar]
- 274. Ulick S, Gautier E, Vetter KK, Markello JR, Yaffe S, Lowe CU. An aldosterone biosynthetic defect in a salt-losing disorder. J Clin Endocrinol Metab. 1964;24:669-672. doi: 10.1210/jcem-24-7-669. [DOI] [PubMed] [Google Scholar]
- 275. Visser HKA, Cost WS. A new hereditary defect in the biosynthesis of aldosterone: Urinary C21-corticosterond pattern in three related patients with a salt-losing syndrome, suggesting an 18-oxidation defect. Acta Endocrinol (Copenh) 1964;47(4):589-612. doi: 10.1530/acta.0.0470589. [DOI] [PubMed] [Google Scholar]
- 276. Degenhart HJ, Frankena L, Visser HKA, Cost WS, Van Setters AP. Further investigation of a new hereditary defect in the biosynthesis of aldosterone: Evidence for a defect in the 18-hydroxylation of corticosterone. Acta Physiol Pharmacol Neerl 1966;14:88-89. [Google Scholar]
- 277. David R, Golan S, Drucker W. Familial aldosterone deficiency: enzyme defect, diagnosis, and clinical course. Pediatrics 1968;41(2):403-412. doi: 10.1542/peds.41.2.403. [DOI] [PubMed] [Google Scholar]
- 278. Ulick S. Diagnosis and nomenclature of the disorders of the terminal portion of the aldosterone biosynthetic pathway. J Clin Endocrinol Metab. 1976;43(1):92-96. doi: 10.1210/jcem-43-1-92. [DOI] [PubMed] [Google Scholar]
- 279. Nicolis GL, Ulick S. Role of 18-hydroxylation in the biosynthesis of aldosterone. Endocrinology 1965;76:514-521. doi: 10.1210/endo-76-3-514. [DOI] [PubMed] [Google Scholar]
- 280. Cohen T, Theodor R, Rosler A. Selective hypoaldosteronism in Iranian Jews: An autosomal recessive trait. Clin Genet. 1977;11(1):25-30. doi: 10.1111/j.1399-0004.1977.tb01273.x. [DOI] [PubMed] [Google Scholar]
- 281. Rosler A, Rabinowitz D, Theodor R, Ramirez LC, Ulick S. The nature of the defect in a salt-wasting disorder in Jews of Iran. J Clin Endocrinol Metab. 1977;44(2):279-291. doi: 10.1210/jcem-44-2-279. [DOI] [PubMed] [Google Scholar]
- 282. Globerman H, Rosler A, Theodor R, New MI, White PC. An inherited defect in aldosterone biosynthesis caused by a mutation in or near the gene for steroid 11-hydroxylase. N Engl J Med. 1988;319(18):1193-1197. doi: 10.1056/NEJM198811033191804. [DOI] [PubMed] [Google Scholar]
- 283. Pascoe L, Curnow KM, Slutsker L, Rosler A, White PC. Mutations in the human CYP11B2 (aldosterone synthase) gene causing corticosterone methyloxidase II deficiency. Proc Natl Acad Sci USA. 1992;89(11):4996-5000. doi: 10.1073/pnas.89.11.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Sutherland DJ, Ruse JL, Laidlaw JC. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Canad Med Assoc J 1966;95(22):1109-1119. [PMC free article] [PubMed] [Google Scholar]
- 285. New MI, Peterson RE. A new form of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1967;27(2):300-305. doi: 10.1210/jcem-27-2-300. [DOI] [PubMed] [Google Scholar]
- 286. Connell JM, Kenyon CJ, Corrie JE, Fraser R, Watt R, Lever AF. Dexamethasone-suppressible hyperaldosteronism. Adrenal transition cell hyperplasia? Hypertension 1986;8(8):669-676. doi: 10.1161/01.hyp.8.8.669. [DOI] [PubMed] [Google Scholar]
- 287. Stockigt JR, Scoggins BA. Long term evolution of glucocorticoid-suppressible hyperaldosteronism. J Clin Endocrinol Metab. 1987;64(1):22-26. doi: 10.1210/jcem-64-1-22. [DOI] [PubMed] [Google Scholar]
- 288. Gomez-Sanchez CE, Gill JR Jr, Ganguly A, Gordon RD. Glucocorticoid-suppressible aldosteronism: a disorder of the adrenal transitional zone. J Clin Endocrinol Metab. 1988;67(3):444-448. doi: 10.1210/jcem-67-3-444. [DOI] [PubMed] [Google Scholar]
- 289. Lifton RP, Dluhy RG, Powers M, et al. A chimaeric 11-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 1992;355(6357):262-265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 290. Lifton RP, Dluhy RG, Powers M, et al. Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase. Nat Genet. 1992;2(1):66-74. doi: 10.1038/ng0992-66. [DOI] [PubMed] [Google Scholar]
- 291. Rich GM, Ulick S, Cook S, Wang JZ, Lifton RP, Dluhy RG. Glucocorticoid-remediable aldosteronism in a large kindred: clinical spectrum and diagnosis using a characteristic biochemical phenotype. Ann Intern Med. 1992;116(10):813-820. doi: 10.7326/0003-4819-116-10-813. [DOI] [PubMed] [Google Scholar]
- 292. Pascoe L, Curnow KM, Slutsker L, et al. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci USA. 1992;89(17):8327-8331. doi: 10.1073/pnas.89.17.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Samuels LT, Helmreich ML, Lasater MB, Reich H. An enzyme in endocrine tissues which oxidizes Δ5-3β hydroxy steroids to Δ4-3 unsaturated ketones. Science 1951;113(2939):490-491. doi: 10.1126/science.113.2939.490. [DOI] [PubMed] [Google Scholar]
- 294. Beyer KF, Samuels LT. Distribution of steroid-3β-ol-dehydrogenase in cellular structures of the adrenal gland. J Biol Chem. 1956;219(1):69-76. doi: 10.1016/S0021-9258(18)65769-2. [DOI] [PubMed] [Google Scholar]
- 295. Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase gene family. Endocr Rev. 2005;26(4):525-582. doi: 10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- 296. Cherradi N, Rossier MF, Vallotton MB, et al. Submitochondrial distribution of three key steroidogenic proteins (steroidogenic acute regulatory protein and cytochrome P450scc and 3β-hydroxysteroid dehydrogenase isomerase enzymes) upon stimulation by intracellular calcium in adrenal glomerulosa cells. J Biol Chem. 1997;272(12):7899-7907. doi: 10.1074/jbc.272.12.7899. [DOI] [PubMed] [Google Scholar]
- 297. Bongiovanni AM. Unusual steroid pattern in congenital adrenal hyperplasia; deficiency of 3β-hydroxy dehydrogenase. J Clin Endocrinol Metab. 1961;21(7):860-862. doi: 10.1210/jcem-21-7-860. [DOI] [Google Scholar]
- 298. Bongiovanni AM. The adrenogenital syndrome with deficiency of 3β-hydroxysteroid dehydrogenase. J Clin Invest. 1962;41(11):2086-2092. doi: 10.1172/JCI104666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Benkert AR, Young M, Robinson D, Hendrickson C, Lee PA, Strauss KA. Severe salt-losing 3β-hydroxysteroid dehydrogenase deficiency: treatment and outcomes of HSD3B2 c.35G>A homozygotes. J Clin Endocrinol Metab. 2015;100(8):E1105-E1115. doi: 10.1210/jc.2015-2098. [DOI] [PubMed] [Google Scholar]
- 300. Pang S, Levine LS, Stoner E, et al. Nonsalt-losing congenital adrenal hyperplasia due to 3β-hydroxysteroid dehydrogenase deficiency with normal glomerulosa function. J Clin Endocrinol Metab. 1983;56(4):808-818. doi: 10.1210/jcem-56-4-808. [DOI] [PubMed] [Google Scholar]
- 301. Cara JF, Moshang T, Bongiovanni AM, Marx BS. Elevated 17-hydroxyprogesterone and testosterone in a newborn with 3β-hydroxysteroid dehydrogenase deficiency. N Engl J Med. 1985;313(10):618-621. doi: 10.1056/NEJM198509053131007. [DOI] [PubMed] [Google Scholar]
- 302. Ishii-Ohba H, Inano H, Tamaoki B. Testicular and adrenal 3β-hydroxy-5-ene-steroid dehydrogenase and 5-ene-4-ene isomerase. J Steroid Biochem. 1987;27(4-6):775-779. doi: 10.1016/0022-4731(87)90149-x. [DOI] [PubMed] [Google Scholar]
- 303. Thomas JL, Myers RP, Strickler RC. Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 54-ene-isomerase: purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J Steroid Biochem. 1989;33(2):209-217. doi: 10.1016/0022-4731(89)90296-3. [DOI] [PubMed] [Google Scholar]
- 304. Ishii-Ohba H, Inano H, Tamaoki B. Purification and properties of testicular 3-hydroxy-5-ene-steroid dehydrogenase and 5-ene-4-ene isomerase. J Steroid Biochem. 1986;25(4):555-560. doi: 10.1016/0022-4731(86)90402-4. [DOI] [PubMed] [Google Scholar]
- 305. The VL, Lachance Y, Labrie C, et al. Full length cDNA structure and deduced amino acid sequence of human 3-hydroxy-5-ene steroid dehydrogenase. Mol Endocrinol. 1989;3(8):1310-1312. doi: 10.1210/mend-3-8-1310. [DOI] [PubMed] [Google Scholar]
- 306. Lorence MC, Murry BA, Trant JM, Mason JI. Human 3β-hydroxysteroid dehydrogenase/ Δ 5-Δ 4-isomerase from placenta: expression in nonsteroidogenic cells of a protein that catalyzes the dehydrogenation/isomerization of C21 and C19 steroids. Endocrinology 1990;126(5):2493-2498. doi: 10.1210/endo-126-5-2493. [DOI] [PubMed] [Google Scholar]
- 307. Rheaume E, Lachance Y, Zhao HF, et al. Structure and expression of a new cDNA encoding the almost exclusive 3β-hydroxysteroid dehydrogenase/Δ 5-Δ 4 isomerase in human adrenals and gonads. Mol Endocrinol. 1991;5(8):1147-1157. doi: 10.1210/mend-5-8-1147. [DOI] [PubMed] [Google Scholar]
- 308. Lachance Y, Luu-The V, Labrie C, et al. Characterization of human 3 β-hydroxysteroid dehydrogenase/Δ 5–>Δ 4-isomerase gene and its expression in mammalian cells. J Biol Chem. 1990;265(33):20469-20475. https://www.jbc.org/article/S0021-9258(17)30528-8/pdf. [PubMed] [Google Scholar]
- 309. Lachance Y, Luu-The V, Verreault H, et al. Structure of the human type II 3β-hydroxysteroid dehydrogenase/Δ 5-Δ 4 isomerase (3β-HSD) gene: adrenal and gonadal specificity. DNA Cell Biol. 1991;10(10):701-711. doi: 10.1089/dna.1991.10.701. [DOI] [PubMed] [Google Scholar]
- 310. McBride MW, McVie AJ, Burridge SM, et al. Cloning, expression, and physical mapping of the 3β-hydroxysteroid dehydrogenase gene cluster (HSD3BP1-HSD3BP5) in human. Genomics 1999;61(3):277-284. doi: 10.1006/geno.1999.5459. [DOI] [PubMed] [Google Scholar]
- 311. Rheaume E, Simard J, Morel Y, et al. Congenital adrenal hyperplasia due to point mutations in the type II 3β-hydroxysteroid dehydrogenase gene. Nat Genet. 1992;1(4):239-245. doi: 10.1038/ng0792-239. [DOI] [PubMed] [Google Scholar]
- 312. Biglieri EG. My engagement with steroids. A review. Steroids 1995;60(1):52-58. doi: 10.1016/0039-128x(94)00008-z. [DOI] [PubMed] [Google Scholar]
- 313. Biglieri EG. 17α-Hydroxylase deficiency: 1963-1966. J Clin Endocrinol Metab. 1997;82(1):48-50. doi: 10.1210/jcem.82.1.3653. [DOI] [PubMed] [Google Scholar]
- 314. Biglieri EG, Herron MA, Brust N. 17-hydroxylation deficiency in man. J Clin Invest. 1966;45(12):1946-1954. doi: 10.1172/JCI105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Zachmann M, Völlmin JA, Hamilton W, Prader A. Steroid 17,20-desmolase deficiency: a new cause of male pseudohermaphroditism. Clin Endocrinol (Oxf) 1972;1(4):369-385. doi: 10.1111/j.1365-2265.1972.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 316. Apter D, Pakarinen A, Hammond GL, Vihko R. Adrenocortical function in puberty. serum ACTH, cortisol and dehydroepiandrosterone in girls and boys. Acta Paediatr Scand 1979;68(4):599-604. doi: 10.1111/j.1651-2227.1979.tb05062.x. [DOI] [PubMed] [Google Scholar]
- 317. Nakajin S, Hall PF. Microsomal cytochrome P-450 from neonatal pig testis. Purification and properties of a C21 steroid side-chain cleavage system (17α-hydroxylase-C17,20 lyase). J Biol Chem. 1981;256(8):3871-3876. doi: 10.1016/S0021-9258(19)69538-4. [DOI] [PubMed] [Google Scholar]
- 318. Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF. C21 steroid side chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17α-hydroxylase/C17,20-lyase cytochrome P-450. J Biol Chem. 1984;259:3971-3976. https://www.jbc.org/article/S0021-9258(17)43191-7/pdf. [PubMed] [Google Scholar]
- 319. Zuber MX, John ME, Okamura T, Simpson ER, Waterman MR. Bovine adrenocortical cytochrome P-450(17α). Regulation of gene expression by ACTH and elucidation of primary sequence. J Biol Chem. 1986;261:2475-2482. https://www.jbc.org/article/S0021-9258(17)35959-8/pdf. [PubMed] [Google Scholar]
- 320. Zuber MX, Simpson ER, Waterman MR. Expression of bovine 17α-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. Science 1986;234:1258-1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
- 321. Chung BC, Picado-Leonard J, Haniu M, et al. Cytochrome P450c17 (steroid 17α-hydroxylase/17,20 lyase). Cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl Acad Sci USA. 1987;84(2):407-411. doi: 10.1073/pnas.84.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Bradshaw KD, Waterman MR, Couch RT, Simpson ER, Zuber MX. Characterization of complementary deoxyribonucleic acid for human adrenocortical 17α-hydroxylase: a probe for analysis of 17α-hydroxylase deficiency. Mol Endocrinol. 1987;1:348-354. doi: 10.1210/mend-1-5-348. [DOI] [PubMed] [Google Scholar]
- 323. Picado-Leonard J, Miller WL. Cloning and sequence of the human gene for P450c17 (steroid 17α-hydroxylase/17,20 lyase): Similarity with the gene for P450c21. DNA 1987;6(5):439-448. doi: 10.1089/dna.1987.6.439. [DOI] [PubMed] [Google Scholar]
- 324. Kagimoto M, Winter JS, Kagimoto K, Simpson ER, Waterman MR. Structural characterization of normal and mutant human steroid 17α-hydroxylase genes: molecular basis of one example of combined 17α-hydroxylase/17,20 lyase deficiency. Mol Endocrinol. 1988;2(6):564-570. doi: 10.1210/mend-2-6-564. [DOI] [PubMed] [Google Scholar]
- 325. Geller DH, Auchus RJ, Mendonça BB, Miller WL. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet. 1997;17(2):201-205. doi: 10.1038/ng1097-201. [DOI] [PubMed] [Google Scholar]
- 326. Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem. 2003;278(49):48563-48569. doi: 10.1074/jbc.M307586200. [DOI] [PubMed] [Google Scholar]
- 327. Hershkovitz E, Parvari R, Wudy SA, et al. Homozygous mutation G539R in the gene for P450 oxidoreductase in a family previously diagnosed as having 17,20-lyase deficiency. J Clin Endocrinol Metab. 2008;93(9):3584-3588. doi: 10.1210/jc.2008-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Miller WL. The syndrome of 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97(1):59-67. doi: 10.1210/jc.2011-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Flück CE, Meyer-Böni M, Pandey AV, et al. Why boys will be boys. Two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet. 2011;89(2):201-218. doi: 10.1016/j.ajhg.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Hogeboom GH. Cytochemical studies of mammalian tissues; the distribution of diphosphopyridine nucleotide-cytochrome c reductase in rat liver fractions. J Biol Chem. 1949;177(2):847-858. doi: 10.1016/S0021-9258(18)57030-7. [DOI] [PubMed] [Google Scholar]
- 331. Horecker B. Triphosphopyridine nucleotidecytochrome c reductase in liver. J Biol Chem. 1950;183(2):593-605. doi: 10.1016/s0021-9258(19)51185-1. [DOI] [Google Scholar]
- 332. Strittmatter P, Velick SF. A microsomal cytochrome reductase specific for diphosphopyridine nucleotide. J Biol Chem. 1956;221(1):277-286. doi: 10.1016/S0021-9258(18)65247-0. [DOI] [PubMed] [Google Scholar]
- 333. Gillette JR, Brodie BB, La Du BN. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957;119(4):532-540. https://jpet.aspetjournals.org/content/119/4/532. [PubMed] [Google Scholar]
- 334. Williams CH Jr, Kamin H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962;237(2):587-595. doi: 10.1016/S0021-9258(18)93967-0. [DOI] [PubMed] [Google Scholar]
- 335. Phillips AH, Langdon RG. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962;237(8):2652-2660. doi: 10.1016/S0021-9258(19)73803-4. [DOI] [PubMed] [Google Scholar]
- 336. Orrenius S, Ericsson JL, Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965;25(3):627-639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Lu AY, Junk KW, Coon MJ. Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem. 1969;244(13):3714-3721. doi: 10.1016/s0021-9258(18)83427-5. [DOI] [PubMed] [Google Scholar]
- 338. Agrawal V, Huang N, Miller WL. Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genomics. 2008;18(7):569-576. doi: 10.1097/FPC.0b013e32830054ac. [DOI] [PubMed] [Google Scholar]
- 339. Agrawal V, Choi JH, Giacomini KM, Miller WL. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase. Pharmacogenet Genomics. 2010;20(10):611-618. doi: 10.1097/FPC.0b013e32833e0cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Peterson RE, Imperato-McGinley J, Gautier T, Shackleton C. Male pseudohermaphroditism due to multiple defects in steroid-biosynthetic microsomal mixed-function oxidases. A new variant of congenital adrenal hyperplasia. N Engl J Med. 1985;313(19):1182-1191. doi: 10.1056/NEJM198511073131903. [DOI] [PubMed] [Google Scholar]
- 341. Miller WL. Congenital adrenal hyperplasia. N Engl J Med. 1986;314(20):1321-1322. doi: 10.1056/NEJM198605153142015. [DOI] [PubMed] [Google Scholar]
- 342. Adachi M, Tachibana K, Asakura Y, Suwa S, Nishimura G. A male patient presenting with major clinical symptoms of glucocorticoid deficiency and skeletal dysplasia, showing a steroid pattern compatible with 17α-hydroxylase/17,20-lyase deficiency, but without obvious CYP17 gene mutations. Endocr J. 1999;46(2):285-292. doi: 10.1507/endocrj.46.285. [DOI] [PubMed] [Google Scholar]
- 343. Reardon W, Smith A, Honour JW, et al. Evidence for digenic inheritance in some cases of Antley-Bixler syndrome? J Med Genet. 2000;37(1):26-32. doi: 10.1136/jmg.37.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Shen AL, O’Leary KA, Kasper CB. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J Biol Chem. 2002;277(8):6536-6541. doi: 10.1074/jbc.M111408200. [DOI] [PubMed] [Google Scholar]
- 345. Otto DM, Henderson CJ, Carrie D, et al. Identification of novel roles of the cytochrome P450 system in early embryogenesis: Effects on vasculogenesis and retinoic acid homeostasis. Mol Cell Biol. 2003;23(17):6103-6116. doi: 10.1128/MCB.23.17.6103-6116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Gu J, Weng Y, Zhang QY, et al. Liver-specific deletion of the NADPH-cytochrome P450 reductase gene. Impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase. J Biol Chem. 2003;278(28):25895-25901. doi: 10.1074/jbc.M303125200. [DOI] [PubMed] [Google Scholar]
- 347. Henderson CJ, Otto DM, Carrie D, et al. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J Biol Chem. 2003;278(15):13480-13486. doi: 10.1074/jbc.M212087200. [DOI] [PubMed] [Google Scholar]
- 348. Wu L, Gu J, Cui H, et al. Transgenic mice with a hypomorphic NADPH-cytochrome P450 reductase gene. Effects on development, reproduction, and microsomal cytochrome P450. J Pharmacol Exp Ther. 2005;312(1):35-43. doi: 10.1124/jpet.104.073353. [DOI] [PubMed] [Google Scholar]
- 349. Flück CE, Tajima T, Pandey AV, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36(3):228-230. doi: 10.1038/ng1300. [DOI] [PubMed] [Google Scholar]
- 350. Huang N, Pandey AV, Agrawal V, et al. Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005;76(5):729-749. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Pandey AV, Flück CE. NADPH P450 oxidoreductase: structure, function, and pathology of diseases. Pharmacol Ther. 2013;138(2):229-254. doi: 10.1016/j.pharmthera.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 352. Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23(5):703-732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- 353. Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA. The regulation of steroid action by sulfation and desulfation. Endocr Rev. 2015;36(5):526-563. doi: 10.1210/er.2015-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Faiyaz ul Haque M, King LM, Krakow D, et al. Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse. Nat Genet. 1998;20(2):157-162. doi: 10.1038/2458. [DOI] [PubMed] [Google Scholar]
- 355. Noordam C, Dhir V, McNelis JC, et al. Inactivating PAPSS2 mutations in a patient with premature pubarche. N Engl J Med. 2009;360(22):2310-2318. doi: 10.1056/NEJMoa0810489. [DOI] [PubMed] [Google Scholar]
- 356. Idkowiak J, Taylor AE, Subtil S, et al. Steroid sulfatase deficiency and androgen activation before and after puberty. J Clin Endocrinol Metab. 2016;101(6):2545-2553. doi: 10.1210/jc.2015-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Vogt M. The output of cortical hormone by the mammalian suprarenal. J Physiol. 1943;102(3):341-356. doi: 10.1113/jphysiol.1943.sp004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Ascoli G, Legnani T. Die folgen der extripation der hypophyse. Münchener Med Wochenschrift 1912;59(2):518-521. [Google Scholar]
- 359. Collip JB, Anderson E, Thomson D. The adrenotropic hormone of the anterior pituitary lobe. Lancet 1933;222(5737):347-348.. doi: 10.1016/S0140-6736(00)44463-6. [DOI] [Google Scholar]
- 360. John ME, John MC, Ashley P, MacDonald RJ, Simpson ER, Waterman MR. Identification and characterization of cDNA clones specific for cholesterol side-chain cleavage cytochrome P-450. Proc Natl Acad Sci USA. 1984;81(18):5628-5632. doi: 10.1073/pnas.81.18.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Voutilainen R, Tapanainen J, Chung BC, Matteson KJ, Miller WL. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17α-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab. 1986;63:202-207. doi: 10.1210/jcem-63-1-202. [DOI] [PubMed] [Google Scholar]
- 362. Thorn GW, Forsham PH, Frawley TF, et al. The clinical usefulness of ACTH and cortisone. N Engl J Med. 1950;242(22):865-872. doi: 10.1056/NEJM195006012422205. [DOI] [PubMed] [Google Scholar]
- 363. Shepherd R, Willson S, Howard K, et al. Studies with corticotropin. III. Determination of the structure of β-corticotropin and its active degradation products. J Am Chem Soc. 1956;78(19):5067-5076. doi: 10.1021/ja01600a067. [DOI] [Google Scholar]
- 364. Ferguson JJ r. Protein synthesis and adrenocorticotropin responsiveness. J Biol Chem. 1963;238(8):2754-2759. https://www.jbc.org/article/S0021-9258(18)67893-7/pdf. [PubMed] [Google Scholar]
- 365. Garren LD, Ney RL, Davis WW. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci USA. 1965;53(6):1443-1450. doi: 10.1073/pnas.53.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Davis WW, Garren LD. On the mechanism of action of adrenocorticotropic hormone: the inhibitory site of cycloheximide in the pathway of steroid biosynthesis. J Biol Chem. 1968;243(19):5153-5157. doi: 10.1016/S0021-9258(18)92004-1. [DOI] [PubMed] [Google Scholar]
- 367. Tuckey RC, Cameron KJ. Catalytic properties of cytochrome P-450scc purified from the human placenta: comparison to bovine cytochrome P-450scc. Biochim Biophys Acta (BBA)-Prot Struct Mol Enzymol. 1993;1163(2):185-194. doi: 10.1016/0167-4838(93)90180-y. [DOI] [PubMed] [Google Scholar]
- 368. Crivello JF, Jefcoate CR. Intracellular movement of cholesterol in rat adrenal cells: kinetics and effects of inhibitors. J Biol Chem. 1980;255(17):8144-8151. https://www.jbc.org/article/S0021-9258(19)70620-6/pdf. [PubMed] [Google Scholar]
- 369. Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17(3):221-244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 370. Stocco DM, Zhao AH, Tu LN, Morohaku K, Selvaraj V. A brief history of the search for the protein(s) involved in the acute regulation of steroidogenesis. Mol Cell Endocrinol. 2017;441:7-16. doi: 10.1016/j.mce.2016.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Krueger R, Orme-Johnson NR. Acute adrenocorticotropic hormone stimulation of adrenal corticosteroidogenesis. Discovery of a rapidly induced protein. J Biol Chem. 1983;258(16):10159-10167. https://www.jbc.org/article/S0021-9258(17)44619-9/pdf. [PubMed] [Google Scholar]
- 372. Pon LA, Hartigan JA, Orme-Johnson NR. Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid accumulation of a phosphoprotein. J Biol Chem. 1986;261(28):13309-13316. doi: 10.1016/S0021-9258(18)69305-6. [DOI] [PubMed] [Google Scholar]
- 373. Alberta JA, Epstein LF, Pon L, Orme-Johnson NR. Mitochondrial localization of a phosphoprotein that rapidly accumulates in adrenal cortex cells exposed to adrenocorticotropic hormone or to cAMP. J Biol Chem. 1989;264(4):2368-2372. doi: 10.1016/S0021-9258(18)94186-4. [DOI] [PubMed] [Google Scholar]
- 374. Epstein L, Orme-Johnson N. Regulation of steroid hormone biosynthesis. Identification of precursors of a phosphoprotein targeted to the mitochondrion in stimulated rat adrenal cortex cells. J Biol Chem. 1991;266(29):19739-19745. doi: 10.1016/s0021-9258(18)55054-7. [DOI] [PubMed] [Google Scholar]
- 375. Stocco DM, Sodeman TC. The 30-kDa mitochondrial proteins induced by hormone stimulation in MA-10 mouse Leydig tumor cells are processed from larger precursors. J Biol Chem. 1991;266(29):19731-19738. doi: 10.1016/S0021-9258(18)55053-5. [DOI] [PubMed] [Google Scholar]
- 376. Stocco DM, Chen W. Presence of identical mitochondrial proteins in unstimulated constitutive steroid-producing R2C rat Leydig tumor and stimulated nonconstitutive steroid-producing MA-10 mouse Leydig tumor cells. Endocrinology 1991;128(4):1918-1926. doi: 10.1210/endo-128-4-1918. [DOI] [PubMed] [Google Scholar]
- 377. Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem. 1994;269(45):28314-28322. doi: 10.1016/S0021-9258(18)46930-X. [DOI] [PubMed] [Google Scholar]
- 378. Lin D, Sugawara T, Strauss JF 3rd, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 1995;267(5205):1828-1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 379. Prader A, Gurtner HP. Das Syndrom des Pseudohermaphroditismus masculinus bei kongenitaler Nebennierenrinden-Hyperplasie ohne Androgenüberproduktion (adrenaler Pseudohermaphroditismus masculinus) [The syndrome of male pseudohermaphrodism in congenital adrenocortical hyperplasia without overproduction of androgens (adrenal male pseudohermaphrodism)]. Helv Paediatr Acta 1955;10(4):397-412. [PubMed] [Google Scholar]
- 380. Sandison AT. A form of lipoidosis of the adrenal cortex in an infant. Arch Dis Child. 1955;30(154):538-541. doi: 10.1136/adc.30.154.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Prader A, Siebenmann RE. Nebenniereninsuffizienz bei kongenitaler Lipoidhyperplasie der Nebennieren [Adrenal insufficiency in congenital lipoid hyperplasia of the adrenals]. Helv Paediatr Acta 1957;12(6):569-595. [PubMed] [Google Scholar]
- 382. Dhom G, Ross W, Widok K. Die Nebennieren des Feten und des Neugeborenen. Eine quantitative und qualitative Analyse [Adrenal glands of the fetus and newborn; a quantitative and qualitative analysis]. Beitr Pathol Anat 1958;119(2):177-216. [PubMed] [Google Scholar]
- 383. O’Doherty NJ. Lipoid adrenal hyperplasia. Guys Hosp Rep 1964;113:368-379. [PubMed] [Google Scholar]
- 384. Shimizu K, Hayano M, Gut M, Dorfman RI. The transformation of 20α-hydroxycholesterol to isocaproic acid and C21 steroids. J Biol Chem. 1961;236(3):695-699. doi: 10.1016/S0021-9258(18)64292-9. [DOI] [Google Scholar]
- 385. Camacho AM, Kowarski A, Migeon CJ, Brough AJ. Congenital adrenal hyperplasia due to a deficiency of one of the enzymes involved in the biosynthesis of pregnenolone. J Clin Endocrinol Metab. 1968;28(2):153-161. doi: 10.1210/jcem-28-2-153. [DOI] [PubMed] [Google Scholar]
- 386. Degenhart HJ, Visser HKA, Boon H, O’Docherty NJ. Evidence for deficiency of 20-cholesterol hydroxylase activity in adrenal tissue of a patient with lipoid adrenal hyperplasia. Acta Endocrinol (Copenh) 1972;71(3):512-518. doi: 10.1530/acta.0.0710512. [DOI] [PubMed] [Google Scholar]
- 387. Koizumi S, Kyoya S, Miyawaki TM, Kidani H, Funabashi T. Cholesterol side-chain cleavage enzyme activity and cytochrome P-450 content in adrenal mitochondria of a patient with congenital lipoid adrenal hyperplasia (Prader disease). Clin Chim Acta. 1977;77(3):301-306. doi: 10.1016/0009-8981(77)90233-9. [DOI] [PubMed] [Google Scholar]
- 388. Shikita M, Hall PF. Cytochrome P-450 from bovine adrenocortical mitochondria: an enzyme for the side chain cleavage of cholesterol. II. Subunit structure. J Biol Chem. 1973;248(16):5605-5609. doi: 10.1016/S0021-9258(19)43547-3. [DOI] [PubMed] [Google Scholar]
- 389. Morohashi K, Fujii-Kuriyama Y, Okada Y, et al. Molecular cloning and nucleotide sequence of cDNA for mRNA of mitochondrial cytochrome P-450(SCC) of bovine adrenal cortex. Proc Natl Acad Sci USA. 1984;81(15):4647-4651. doi: 10.1073/pnas.81.15.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Matteson KJ, Chung BC, Urdea MS, Miller WL. Study of cholesterol side-chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes. Endocrinology 1986;118(4):1296-1305. doi: 10.1210/endo-118-4-1296. [DOI] [PubMed] [Google Scholar]
- 391. Chung BC, Matteson KJ, Voutilainen R, Mohandas TK, Miller WL. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci USA. 1986;83(23):8962-8966. doi: 10.1073/pnas.83.23.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Lin D, Gitelman SE, Saenger P, Miller WL. Normal genes for the cholesterol side chain cleavage enzyme, P450scc, in congenital lipoid adrenal hyperplasia. J Clin Invest. 1991;88(6):1955-1962. doi: 10.1172/JCI115520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Sugawara T, Holt JA, Driscoll D, et al. Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. Proc Natl Acad Sci USA. 1995;92(11):4778-4782. doi: 10.1073/pnas.92.11.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Bose HS, Sugawara T, Strauss JF 3rd, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia N Engl J Med. 1996;335(25):1870-1878. doi: 10.1056/NEJM199612193352503. [DOI] [PubMed] [Google Scholar]
- 395. Bose HS, Pescovitz OH, Miller WL. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia due to a homozygous frameshift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol Metab. 1997;82(5):1511-1515. doi: 10.1210/jcem.82.5.3962. [DOI] [PubMed] [Google Scholar]
- 396. Fujieda K, Tajima T, Nakae J, et al. Spontaneous puberty in 46,XX subjects with congenital lipoid adrenal hyperplasia. Ovarian steroidogenesis is spared to some extent despite inactivating mutations in the steroidogenic acute regulatory protein (StAR) gene. J Clin Invest. 1997;99(6):1265-1271. doi: 10.1172/JCI119284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA. 1997;94(21):11540-11545. doi: 10.1073/pnas.94.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Baker BY, Lin L, Kim CJ, et al. Nonclassic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J Clin Endocrinol Metab. 2006;91(12):4781-4785. doi: 10.1210/jc.2006-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL. Heterozygous mutation in the cholesterol side chain cleavage enzyme (P450scc) gene in a patient with 46, XY sex reversal and adrenal insufficiency. J Clin Endocrinol Metab. 2001;86(8):3820-3825. doi: 10.1210/jcem.86.8.7748. [DOI] [PubMed] [Google Scholar]
- 400. Miller WL. Disorders in the initial steps of steroid hormone synthesis. J Steroid Biochem Mol Biol. 2017;165(Pt A):18-37. doi: 10.1016/j.jsbmb.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 401. Rubtsov P, Karmanov M, Sverdlova P, Spirin P, Tiulpakov A. A novel homozygous mutation in CYP11A1 gene is associated with late-onset adrenal insufficiency and hypospadias in a 46,XY patient. J Clin Endocrinol Metab. 2009;94(3):936-939. doi: 10.1210/jc.2008-1118. [DOI] [PubMed] [Google Scholar]
- 402. Shepard TH, Landing BH, Mason DG. Familial Addison’s disease; case reports of two sisters with corticoid deficiency unassociated with hypoaldosteronism. AMA Am J Dis Child 1959;97(2):154-162. doi: 10.1001/archpedi.1959.02070010156002. [DOI] [PubMed] [Google Scholar]
- 403. Migeon CJ, Kenny EM, Kowarski A, et al. The syndrome of congenital adrenocortical unresponsiveness to ACTH. Report of six cases. Pediatr Res. 1968;2(6):501-513. doi: 10.1203/00006450-196811000-00008. [DOI] [PubMed] [Google Scholar]
- 404. Thistlethwaite D, Darling JA, Fraser R, Mason PA, Rees LH, Harkness RA. Familial glucocorticoid deficiency. Studies of diagnosis and pathogenesis. Arch Dis Child. 1975;50(4):291-297. doi: 10.1136/adc.50.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science 1992;257(5074):1248-1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 406. Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 1993;341(8843):461-462. doi: 10.1016/0140-6736(93)90208-x. [DOI] [PubMed] [Google Scholar]
- 407. Weber A, Kapas S, Hinson J, Grant DB, Grossman A, Clark AJ. Functional characterization of the cloned human ACTH receptor: impaired responsiveness of a mutant receptor in familial glucocorticoid deficiency. Biochem Biophys Res Commun. 1993;197(1):172-178. doi: 10.1006/bbrc.1993.2456. [DOI] [PubMed] [Google Scholar]
- 408. Tsigos C, Arai K, Hung W, Chrousos GP. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J Clin Invest. 1993;92(5):2458-2461. doi: 10.1172/JCI116853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Weber A, Clark AJ. Mutations of the ACTH receptor gene are only one cause of familial glucocorticoid deficiency. Hum Mol Genet. 1994;3(4):585-588. doi: 10.1093/hmg/3.4.585. [DOI] [PubMed] [Google Scholar]
- 410. Metherell LA, Chapple JP, Cooray S, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37(2):166-170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 411. Metherell LA, Naville D, Halaby G, et al. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab. 2009;94(10):3865-3871. doi: 10.1210/jc.2009-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Counahan R, West R. Ocular and fingertip abnormalities in isolated glucocorticoid deficiency. J Pediatr. 1974;85(4):580-581. doi: 10.1016/s0022-3476(74)80481-6. [DOI] [PubMed] [Google Scholar]
- 413. Allgrove J, Clayden GS, Grant DB, Macaulay JC. Familial glucocorticoid deficiency with achalasia of the cardia and deficient tear production. Lancet 1978;1(8077):1284-1286. doi: 10.1016/s0140-6736(78)91268-0. [DOI] [PubMed] [Google Scholar]
- 414. Kelch RP, Kaplan SL, Biglieri EG, Daniels GH, Epstein CJ, Grumbach MM. Hereditary adrenocortical unresponsiveness to adrenocorticotropic hormone. J Pediatr. 1972;81(4):726-736. doi: 10.1016/s0022-3476(72)80093-3. [DOI] [PubMed] [Google Scholar]
- 415. Geffner ME, Lippe BM, Kaplan SA, et al. Selective ACTH insensitivity, achalasia, and alacrima: a multisystem disorder presenting in childhood. Pediatr Res. 1983;17(7):532-536. doi: 10.1203/00006450-198307000-00003. [DOI] [PubMed] [Google Scholar]
- 416. Ambrosino MM, Genieser NB, Bangaru BS, Sklar C, Becker MH. The syndrome of achalasia of the esophagus, ACTH insensitivity and alacrima. Pediatr Radiol. 1986;16(4):328-329. doi: 10.1007/BF02386875. [DOI] [PubMed] [Google Scholar]
- 417. Moore PS, Couch RM, Perry YS, Shuckett EP, Winter JS. Allgrove syndrome: an autosomal recessive syndrome of ACTH insensitivity, achalasia and alacrima. Clin Endocrinol (Oxf) 1991;34(2):107-114. doi: 10.1111/j.1365-2265.1991.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 418. Tullio-Pelet A, Salomon R, Hadj-Rabia S, et al. Mutant WD-repeat protein in triple-A syndrome. Nat Genet. 2000;26(3):332-335. doi: 10.1038/81642. [DOI] [PubMed] [Google Scholar]
- 419. Storr HL, Kind B, Parfitt DA, et al. Deficiency of ferritin heavy-chain nuclear import in triple a syndrome implies nuclear oxidative damage as the primary disease mechanism. Mol Endocrinol. 2009;23(12):2086-2094. doi: 10.1210/me.2009-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Rainey WE, Saner K, Schimmer BP. Adrenocortical cell lines. Mol Cell Endocrinol. 2004;228(1-2):23-38. doi: 10.1016/j.mce.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 421. Cohen AI, Bloch E, Celozzi E. In vitro response of functional experimental adrenal tumors to corticotropin ACTH. Proc Soc Exp Biol Med. 1957;95(2):304-309. doi: 10.3181/00379727-95-23202. [DOI] [PubMed] [Google Scholar]
- 422. Buonassisi V, Sato G, Cohen AI. Hormone-producing cultures of adrenal and pituitary tumor origin. Proc Natl Acad Sci USA. 1962;48(7):1184-1190. doi: 10.1073/pnas.48.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Yasumura Y, Buonassisi V, Sato G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966;26(3):529-535. [PubMed] [Google Scholar]
- 424. Pierson RW Jr. Metabolism of steroid hormones in adrenal cortex tumor cultures. Endocrinology 1967;81(4):693-707. doi: 10.1210/endo-81-4-693. [DOI] [PubMed] [Google Scholar]
- 425. Kowal J, Fiedler R. Arenal cells in tissue culture. I.Assay of steroid products; steroidogenic responses to peptide hormones. Arch Biochem Biophys. 1968;128(2):406-421. doi: 10.1016/0003-9861(68)90047-7. [DOI] [PubMed] [Google Scholar]
- 426. Schimmer BP, Zimmerman AE. Steroidogenesis and extracellular cAMP accumulation in adrenal tumor cell cultures. Mol Cell Endocrinol. 1976;4(4):263-270. doi: 10.1016/0303-7207(76)90060-5. [DOI] [PubMed] [Google Scholar]
- 427. Schimmer BP, Tsao J, Knapp M. Isolation of mutant adrenocortical tumor cells resistant to cyclic nucleotides. Mol Cell Endocrinol. 1977;8(2):135-145. doi: 10.1016/0303-7207(77)90025-9. [DOI] [PubMed] [Google Scholar]
- 428. Rae PA, Gutmann NS, Tsao J, Schimmer BP. Mutations in cyclic AMP-dependent protein kinase and corticotropin (ACTH)-sensitive adenylate cyclase affect adrenal steroidogenesis. Proc Natl Acad Sci USA. 1979;76(4):1896-1900. doi: 10.1073/pnas.76.4.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Gazdar AF, Oie HK, Shackleton CH, et al. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990;50(17):5488-5496. [PubMed] [Google Scholar]
- 430. Staels B, Hum DW, Miller WL. Regulation of steroidogenesis in NCI-H295 cells: a cellular model of the human fetal adrenal. Mol Endocrinol. 1993;7(3):423-433. doi: 10.1210/mend.7.3.8387159. [DOI] [PubMed] [Google Scholar]
- 431. Rainey WE, Bird IM, Sawetawan C, et al. Regulation of human adrenal carcinoma cell (NCI-H295) production of C19 steroids. J Clin Endocrinol Metab. 1993;77(3):731-737. doi: 10.1210/jcem.77.3.8396576. [DOI] [PubMed] [Google Scholar]
- 432. Bird IM, Hanley NA, Word RA, et al. Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology 1993;133(4):1555-1561. doi: 10.1210/endo.133.4.8404594. [DOI] [PubMed] [Google Scholar]
- 433. Rainey WE, Bird IM, Mason JI. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol. 1994;100(1-2):45-50. doi: 10.1016/0303-7207(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 434. Starkel S, Wegrzynowsk L. Beitrag zur Histologie der Nebennieren bei Feten und Kindern. Arch Anat Physiol 1910;8:214-235. [Google Scholar]
- 435. Malendowicz LK. 100th anniversary of the discovery of the human adrenal fetal zone by Stella Starkel and Lesław Węgrzynowski: how far have we come? Folia Histochem Cytobiol. 2010;48(4):491-506. doi: 10.2478/v10042-010-0062-7. [DOI] [PubMed] [Google Scholar]
- 436. Keene MF. Observations on the development of the human suprarenal gland. J Anat. 1927;61(Pt 3):302-324. [PMC free article] [PubMed] [Google Scholar]
- 437. Swinyard CA. Growth of the human suprarenal glands. Anat Rec. 1943;87(2):141-150. doi: 10.1002/ar.1090870205. [DOI] [Google Scholar]
- 438. Lanman JT. The fetal zone of the adrenal gland: its developmental course, comparative anatomy, and possible physiologic functions. Medicine (Baltim). 1953;32(4):389-430. doi: 10.1097/00005792-195312000-00001. [DOI] [PubMed] [Google Scholar]
- 439. McNutt NS, Jones AL. Observations on the ultrastructure of cytodifferentiation in the human fetal adrenal cortex. Lab Invest 1970;22(6):513-527. [PubMed] [Google Scholar]
- 440. Sucheston ME, Cannon MS. Development of zonular patterns in the human adrenal gland. J Morphol. 1968;126(4):477-491. doi: 10.1002/jmor.1051260408. [DOI] [PubMed] [Google Scholar]
- 441. Kojima S, Yanaihara T, Nakayama T. Serum steroid levels in children at birth and in early neonatal period. Am J Obstet Gynecol. 1981;140(8):961-965. doi: 10.1016/0002-9378(81)90092-2. [DOI] [PubMed] [Google Scholar]
- 442. Grueters A, Korth-Schutz S. Longitudinal study of plasma dehydroepiandrosterone sulfate in preterm and fullterm infants. J Clin Endocrinol Metab. 1982;55(2):314-320. doi: 10.1210/jcem-55-2-314. [DOI] [PubMed] [Google Scholar]
- 443. Benner MC. Studies on the involution of the fetal cortex of the adrenal glands. Am J Pathol. 1940;16(6):787-798. [PMC free article] [PubMed] [Google Scholar]
- 444. de Sa DJ. Stress response and its relationship to cystic (pseudofollicular) change in the definitive cortex of the adrenal gland in stillborn infants. Arch Dis Child. 1978;53(10):769-776. doi: 10.1136/adc.53.10.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Beatty EC Jr, Hawes CR. Cytomegaly of the adrenal gland. AMA Am J Dis Child 1955;89(4):463-467. doi: 10.1001/archpedi.1955.02050110553008. [DOI] [PubMed] [Google Scholar]
- 446. Spencer SJ, Mesiano S, Lee JY, Jaffe RB. Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: implications for growth and remodeling. J Clin Endocrinol Metab. 1999;84(3):1110-1115. doi: 10.1210/jcem.84.3.5513. [DOI] [PubMed] [Google Scholar]
- 447. Diczfalusy E. Endocrine functions of the human fetoplacental unit. 1964. Am J Obstet Gynecol. 2005;193(6):2024-2025. doi: 10.1016/j.ajog.2005.02.117. [DOI] [PubMed] [Google Scholar]
- 448. Ben-David S, Zuckerman-Levin N, Epelman M, et al. Parturition itself is the basis for fetal adrenal involution. J Clin Endocrinol Metab. 2007;92(1):93-97. doi: 10.1210/jc.2005-2720. [DOI] [PubMed] [Google Scholar]
- 449. Agrawal V, Tee MK, Qiao J, Muench MO, Miller WL. Potential role of increased oxygenation in altering perinatal adrenal steroidogenesis. Pediatr Res. 2015;77(2):298-309. doi: 10.1038/pr.2014.194. [DOI] [PubMed] [Google Scholar]
- 450. Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature: report of 11 cases with a digression on hormonal control of axillary and pubic hair. Am J Med Sci. 1942;204(5):625-648. doi: 10.1097/00000441-194211000-00001. [DOI] [Google Scholar]
- 451. Albright F. Osteoporosis. Ann Intern Med. 1947;27(6):861-882. doi: 10.7326/0003-4819-27-6-861. [DOI] [PubMed] [Google Scholar]
- 452. Mason HL, Engstrom WW. The 17-ketosteroids: their origin, determination and significance. Physiol Rev. 1950;30(3):321-374. doi: 10.1152/physrev.1950.30.3.321. [DOI] [PubMed] [Google Scholar]
- 453. Silverman SH, Migeon C, Rosemberg E, Wilkins L. Precocious growth of sexual hair without other secondary sexual development. “Premature pubarche,” a constitutional variation of adolescence. Pediatrics 1952;10(4):426-432. doi: 10.1542/peds.10.4.426. [DOI] [PubMed] [Google Scholar]
- 454. Migeon CJ, Plager JE. Identification and isolation of dehydroisoandrosterone from peripheral human plasma. J Biol Chem. 1954;209(2):767-772. doi: 10.1016/S0021-9258(18)65504-8. [DOI] [PubMed] [Google Scholar]
- 455. Chapdelaine A, MacDonald PC, Gonzalez O, Gurpide E, Wiele RLV, Lieberman S. Studies on the secretion and interconversion of the androgens. IV. Quantitative results in a normal man whose gonadal and adrenal function were altered experimentally. J Clin Endocrinol Metab. 1965;25(12):1569-1579. doi: 10.1210/jcem-25-12-1569. [DOI] [PubMed] [Google Scholar]
- 456. Migeon CJ. Adrenal androgens in man. Am J Med. 1972;53(5):606-26. doi: 10.1016/0002-9343(72)90157-x [DOI] [PubMed] [Google Scholar]
- 457. Reiter EO, Fuldauer VG, Root AW. Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr. 1977;90(5):766-770. doi: 10.1016/s0022-3476(77)81244-4. [DOI] [PubMed] [Google Scholar]
- 458. Grover P, Odell D. Correlation of in vivo and in vitro activities of some naturally occurring androgens using a radioreceptor assay for 5α-dihydrotestosterone with rat prostate cytosol receptor protein. J Steroid Biochem. 1975;6(10):1373-1379. doi: 10.1016/0022-4731(75)90072-2. [DOI] [PubMed] [Google Scholar]
- 459. Cutler GB Jr, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology 1978;103(6):2112-2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- 460. Smail PJ, Faiman C, Hobson WC, Fuller GB, Winter JS. Further studies on adrenarche in nonhuman primates. Endocrinology 1982;111(3):844-848. doi: 10.1210/endo-111-3-844. [DOI] [PubMed] [Google Scholar]
- 461. Copeland KC, Eichberg JW, Parker CR Jr, Bartke A. Puberty in the chimpanzee: somatomedin-C and its relationship to somatic growth and steroid hormone concentrations. J Clin Endocrinol Metab. 1985;60(6):1154-1160. doi: 10.1210/jcem-60-6-1154. [DOI] [PubMed] [Google Scholar]
- 462. Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL. Molecular evolution of adrenarche: structural and functional analysis of P450c17 from four primate species. Endocrinology 2002;143(12):4665-4672. doi: 10.1210/en.2002-220456. [DOI] [PubMed] [Google Scholar]
- 463. Grumbach MM, Richards GS, Conte FA, Kaplan SL. Clinical disorders of adrenal function and puberty: an assessment of the role of the adrenal cortex in normal and abnormal puberty in man and evidence for an ACTH-like pituitary adrenal androgen stimulating hormone. In: James VHT, Serio M, Giusti G, eds. Proceedings of the Serono Symposia. Vol 18. London: Academic Press; 1978:583-612. [Google Scholar]
- 464. Sklar CA, Kaplan SL, Grumbach MM. Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab. 1980;51(3):548-556. doi: 10.1210/jcem-51-3-548. [DOI] [PubMed] [Google Scholar]
- 465. Parker L, Odell W. Evidence for existence of cortical androgen-stimulating hormone. Am J Physiol Endocrinol Metab. 1979;236(6):E616. doi: 10.1152/ajpendo.1979.236.6.E616. [DOI] [PubMed] [Google Scholar]
- 466. Anderson D. The adrenal androgen-stimulating hormone does not exist. The Lancet 1980;316(8192):454-456. doi: 10.1016/s0140-6736(80)91889-9. [DOI] [PubMed] [Google Scholar]
- 467. Mellon SH, Shively JE, Miller WL. Human proopiomelanocortin-(79-96), a proposed androgen stimulatory hormone, does not affect steroidogenesis in cultured human fetal adrenal cells. J Clin Endocrinol Metab. 1991;72(1):19-22. doi: 10.1210/jcem-72-1-19. [DOI] [PubMed] [Google Scholar]
- 468. Penhoat A, Sanchez P, Jaillard C, Langlois D, Begeot M, Saez J. Human proopiomelanocortin-(79–96), a proposed cortical androgen-stimulating hormone, does not affect steroidogenesis in cultured human adult adrenal cells. J Clin Endocrinol Metab. 1991;72(1):23-26. doi: 10.1210/jcem-72-1-23. [DOI] [PubMed] [Google Scholar]
- 469. Robinson P, Bateman A, Mulay S, et al. Isolation and characterization of three forms of joining peptide from adult human pituitaries: lack of adrenal androgen-stimulating activity. Endocrinology 1991;129(2):859-867. doi: 10.1210/endo-129-2-859. [DOI] [PubMed] [Google Scholar]
- 470. Miller WL. Androgen synthesis in adrenarche. Rev Endocr Metabc Disord. 2009;10(1):3-17. doi: 10.1007/s11154-008-9102-4. [DOI] [PubMed] [Google Scholar]
- 471. Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. Adrenal changes associated with adrenarche. Rev Endocr Metabc Disord. 2009;10(1):19-26. doi: 10.1007/s11154-008-9092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Rege J, Turcu A, Kasa-Vubu JZ, et al. 11-ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589-4598. doi: 10.1210/jc.2018-00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Barnard L, du Toit T, Swart AC. Back where it belongs: 11β-hydroxyandrostenedione compels the re-assessment of C11-oxy androgens in steroidogenesis. Mol Cell Endocrinol. 2021;525(Suppl):111189. doi: 10.1016/j.mce.2021.111189. [DOI] [PubMed] [Google Scholar]
- 474. Parker KL, Chaplin DD, Wong M, Seidman JG, Smith JA, Schimmer BP. Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells. Proc Natl Acad Sci USA. 1985;82(23):7860-7864. doi: 10.1073/pnas.82.23.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Handler JD, Schimmer BP, Flynn TG, Szyf M, Seidman JG, Parker KL. An enhancer element and a functional cyclic AMP-dpendent protein kinase are required for expression of adrenocortical 21-hydroxylase. J Biol Chem. 1988;263(26):13068-13073. doi: 10.1016/S0021-9258(18)37672-5. [DOI] [PubMed] [Google Scholar]
- 476. Chaplin DD, Galbraith LJ, Seidman JG, White PC, Parker KL. Nucleotide sequence analysis of murine 21-hydroxylase genes: mutations affecting gene expression. Proc Natl Acad Sci USA. 1986;83(24):9601-9605. doi: 10.1073/pnas.83.24.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477. Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6(8):1249-1258. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- 478. Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem. 1993;268(10):7494-7502. doi: 10.1016/S0021-9258(18)53202-6. [DOI] [PubMed] [Google Scholar]
- 479. Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18(3):361-377. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- 480. Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 1994;77(4):481-490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 481. Crawford PA, Sadovsky Y, Woodson K, Lee SL, Milbrandt J. Adrenocortical function and regulation of the steroid 21-hydroxylase gene in NGFI-B-deficient mice. Mol Cell Biol. 1995;15(8):4331-4316. doi: 10.1128/MCB.15.8.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9(4):478-486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- 483. Achermann JC, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22(2):125-126. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- 484. Biason-Lauber A, Schoenle EJ. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet. 2000;67(6):1563-1568. doi: 10.1086/316893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Köhler B, Lin L, Ferraz-de-Souza B, et al. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum Mutat. 2008;29(1):59-64. doi: 10.1002/humu.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486. Mitchell RG, Rhaney K. Congenital adrenal hypoplasia in siblings. Lancet 1959;1(7071):488-492. doi: 10.1016/s0140-6736(59)91020-7. [DOI] [PubMed] [Google Scholar]
- 487. Cox PJN. Congenital adrenal hypoplasia. Proc R Soc Med 1962;55(11):981-982. doi: 10.1177/003591576205501122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Weiss L, Mellinger RC. Congenital adrenal hypoplasia--an X-linked disease. J Med Genet. 1970;7(1):27-32. doi: 10.1136/jmg.7.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489. Renier WO, Nabben FA, Hustinx TW, et al. Congenital adrenal hypoplasia. progressive muscular dystrophy, and severe mental retardation, in association with glycerol kinase deficiency, in male sibs. Clin Genet. 1983;24(4):243-251. doi: 10.1111/j.1399-0004.1983.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 490. Bardoni B, Zanaria E, Guioli S, et al. A dosage sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nat Genet. 1994;7(4):497-501. doi: 10.1038/ng0894-497. [DOI] [PubMed] [Google Scholar]
- 491. Zanaria E, Muscatelli F, Bardoni B, et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 1994;372(6507):635-641. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- 492. Muscatelli F, Strom TM, Walker AP, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 1994;372(6507):672-676. doi: 10.1038/372672a0. [DOI] [PubMed] [Google Scholar]
- 493. Lin L, Gu WX, Ozisik G, et al. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years’ experience. J Clin Endocrinol Metab. 2006;91(8):3048-3054. doi: 10.1210/jc.2006-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Schmidt MB. Eine biglandulare Erkrankung (Nebennieren und Schilddruse bei Morbus Addisonii). Verh Dtsch Ges Pathol. 1926;21:212-221. [Google Scholar]
- 495. Bloodworth JM Jr, Kirkendall WM, Carr TL. Addison’s disease associated with thyroid insufficiency and atrophy (Schmidt syndrome). J Clin Endocrinol Metab. 1954;14(5):540-553. doi: 10.1210/jcem-14-5-540. [DOI] [PubMed] [Google Scholar]
- 496. Carpenter CC, Solomon N, Silverberg SG, et al. Schmidt’s syndrome (thyroid and adrenal insufficiency). A review of the literature and a report of fifteen new cases including ten instances of coexistent diabetes mellitus. Medicine (Baltim). 1964;43:153-180. [PubMed] [Google Scholar]
- 497. Doniach D, Roitt IM. Auto-immunity in Hashimoto’s disease and its implications. J Clin Endocrinol Metab. 1957;17(11):1293-1304. doi: 10.1210/jcem-17-11-1293. [DOI] [PubMed] [Google Scholar]
- 498. Anonymous. Autoimmunity in idiopathic addison’s disease. Lancet 1967;1(7498):1040-1042. doi: 10.1016/S0140-6736(67)91550-4. [DOI] [PubMed] [Google Scholar]
- 499. Mead RK. Autoimmune Addison’s disease. Report of a possible case. N Engl J Med. 1962;266(12):583-586. doi: 10.1056/NEJM196203222661203. [DOI] [PubMed] [Google Scholar]
- 500. Blizzard RM, Kyle M. Studies of the adrenal antigens and antibodies in Addison’s disease. J Clin Invest. 1963;42(10):1653-1660. doi: 10.1172/jci104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Burnet FM. The new approach to immunology. N Engl J Med. 1961;264(1):24-34. doi: 10.1056/nejm196101052640107. [DOI] [PubMed] [Google Scholar]
- 502. Whitaker J, Landing BH, Esselborn VM, Williams RR. The syndrome of familial juvenile hypoadrenocorticism, hypoparathyroidism and superficial moniliasis. J Clin Endocrinol Metab. 1956;16(10):1374-1387. doi: 10.1210/jcem-16-10-1374. [DOI] [PubMed] [Google Scholar]
- 503. Hung W, Migeon CJ, Parrott RH. A possible autoimmune basis for Addison’s disease in three siblings, one with idiopathic hypoparathyroidism, pernicious anemia and superficial moniliasis. N Engl J Med. 1963;269(13):658-663. doi: 10.1056/nejm196309262691303. [DOI] [PubMed] [Google Scholar]
- 504. Spinner MW, Blizzard RM, Childs B. Clinical and genetic heterogeneity in idiopathic Addison’s disease and hypoparathyroidism. J Clin Endocrinol Metab. 1968;28(6):795-804. doi: 10.1210/jcem-28-6-795. [DOI] [PubMed] [Google Scholar]
- 505. Perniola R, Fierabracci A, Falorni A. Autoimmune Addison’s disease as part of the autoimmune polyglandular syndrome type 1: Historical overview and current evidence. Front Immunol. 2021;12:606860. doi: 10.3389/fimmu.2021.606860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Winqvist O, Karlsson FA, Kämpe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison’s disease. Lancet 1992;339(8809):1559-1562. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
- 507. Bednarek J, Furmaniak J, Wedlock N, et al. Steroid 21-hydroxylase is a major autoantigen involved in adult onset autoimmune Addison’s disease. FEBS Lett. 1992;309(1):51-55. doi: 10.1016/0014-5793(92)80737-2. [DOI] [PubMed] [Google Scholar]
- 508. Winqvist O, Gustafsson J, Rorsman F, Karlsson FA, Kämpe O. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison’s disease. J Clin Invest. 1993;92(5):2377-2385. doi: 10.1172/jci116843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. Uibo R, Aavik E, Peterson P, et al. Autoantibodies to cytochrome P450 enzymes P450scc, P450c17, and P450c21 in autoimmune polyglandular disease types I and II and in isolated Addison’s disease. J Clin Endocrinol Metab. 1994;78(2):323-328. doi: 10.1210/jcem.78.2.8106620. [DOI] [PubMed] [Google Scholar]
- 510. Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23(3):327-364. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- 511. Moser HW, Powers JM, Smith KD. Adrenoleukodystrophy: molecular genetics, pathology, and Lorenzo’s oil. Brain Pathol. 1995;5(3):259-266. doi: 10.1111/j.1750-3639.1995.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 512. Moser HW, Mahmood A, Raymond GV. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. 2007;3(3):140-151. doi: 10.1038/ncpneuro0421. [DOI] [PubMed] [Google Scholar]
- 513. Schilder P. Zur Kenntnis der sogenannten diffusen Sklerose. (Über Encephalitis periaxialis diffusa.). Z gesamte Neurol Psych 1912;10:1-60. doi: 10.1007/BF02901445. [DOI] [Google Scholar]
- 514. Siemerling E, Creutzfeldt HG. Bronzekrankheit und sklerosierende Encephalomyelitis. Arch Psychiatr Nervenkr 1923;68:217-244. doi: 10.1007/BF01835678. [DOI] [Google Scholar]
- 515. Fanconi A, Prader A, Isler W, Luethy F, Siebenmann R. Morbus Addison mit Hirnsklerose im Kindersalter. Ein hereditares syndrom mit X-Chromosomaler vererbung? [Addison’s disease with cerebral sclerosis in childhood. A hereditary syndrome transmitted through chromosome X?]. Helv Paediatr Acta 1963;18(6):480-501. [PubMed] [Google Scholar]
- 516. Schaumburg HH, Richardson EP, Johnson PC, Cohen RB, Powers JM, Raine CS. Schilder’s disease. Sex-linked recessive transmission with specific adrenal changes. Arch Neurol. 1972;27(5):458-460. doi: 10.1001/archneur.1972.00490170090014. [DOI] [PubMed] [Google Scholar]
- 517. Igarashi M, Schaumburg HH, Powers J, Kishmoto Y, Kolodny E, Suzuki K. Fatty acid abnormality in adrenoleukodystrophy. J Neurochem. 1976;26(4):851-860. doi: 10.1111/j.1471-4159.1976.tb04462.x. [DOI] [PubMed] [Google Scholar]
- 518. Powers JM, Schaumburg HH. The adrenal cortex in adrenoleukodystrophy. Arch Pathol 1973;96(5):305-310. [PubMed] [Google Scholar]
- 519. Moser HW, Moser AB, Frayer KK, et al. Adrenoleukodystrophy: increased plasma content of saturated very long chain fatty acids. Neurology 1981;31(10):1241-1249. doi: 10.1212/wnl.31.10.1241. [DOI] [PubMed] [Google Scholar]
- 520. Migeon BR, Moser HW, Moser AB, Axelman J, Sillence D, Norum RA. Adrenoleukodystrophy: evidence for X linkage, inactivation, and selection favoring the mutant allele in heterozygous cells. Proc Natl Acad Sci USA. 1981;78(8):5066-5070. doi: 10.1073/pnas.78.8.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Mosser J, Douar AM, Sarde CO, et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 1993;361(6414):726-730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 522. Mosser J, Lutz Y, Stoeckel ME, et al. The gene responsible for adrenoleukodystrophy encodes a peroxisomal membrane protein. Hum Mol Genet. 1994;3(2):265-271. doi: 10.1093/hmg/3.2.265. [DOI] [PubMed] [Google Scholar]
- 523. Aubourg P, Adamsbaum C, Lavallard-Rousseau MC, et al. A two-year trial of oleic and erucic acids (“Lorenzo’s oil”) as treatment for adrenomyeloneuropathy. N Engl J Med. 1993;329(11):745-752. doi: 10.1056/nejm199309093291101. [DOI] [PubMed] [Google Scholar]
- 524. Buonocore F, Maharaj A, Qamar Y, et al. Genetic analysis of pediatric primary adrenal insufficiency of unknown etiology: 25 years’ experience in the UK. J Endocr Soc. 2021;5(8):bvab086. doi: 10.1210/jendso/bvab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Hughes CR, Guasti L, Meimaridou E, et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012;122(3):814-820. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Gineau L, Cognet C, Kara N, et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122(3):821-832. doi: 10.1172/jci61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Narumi S, Amano N, Ishii T, et al. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet. 2016;48(7):792-797. doi: 10.1038/ng.3569. [DOI] [PubMed] [Google Scholar]
- 528. Vilain E, Le Merrer M, Lecointre C, et al. IMAGe, a new clinical association of intrauterine growth retardation, metaphyseal dysplasia, adrenal hypoplasia congenita, and genital anomalies. J Clin Endocrinol Metab. 1999;84(12):4335-4340. doi: 10.1210/jcem.84.12.6186. [DOI] [PubMed] [Google Scholar]
- 529. Arboleda VA, Lee H, Parnaik R, et al. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat Genet. 2012;44(7):788-792. doi: 10.1038/ng.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Logan CV, Murray JE, Parry DA, et al. DNA polymerase epsilon deficiency causes IMAGe syndrome with variable immunodeficiency. Am J Hum Genet. 2018;103(6):1038-1044. doi: 10.1016/j.ajhg.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Meimaridou E, Kowalczyk J, Guasti L, et al. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet. 2012;44(7):740-742. doi: 10.1038/ng.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Prasad R, Chan LF, Hughes CR, et al. Thioredoxin Reductase 2 (TXNRD2) mutation associated with familial glucocorticoid deficiency (FGD). J Clin Endocrinol Metab. 2014;99(8):E1556-E1563. doi: 10.1210/jc.2013-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Miller WL. Steroidogenesis: Unanswered questions. Trends Endocrinol Metab. 2017;28(11):771-793. doi: 10.1016/j.tem.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 534. Paul A, Drecourt A, Petit F, et al. FDXR mutations cause sensorial neuropathies and expand the spectrum of mitochondrial Fe-S-synthesis diseases. Am J Hum Genet. 2017;101(4):630-637. doi: 10.1016/j.ajhg.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Peng Y, Shinde DN, Valencia CA, et al. Biallelic mutations in the ferredoxin reductase gene cause novel mitochondriopathy with optic atrophy. Hum Mol Genet. 2017;26(24):4937-4950. doi: 10.1093/hmg/ddx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Slone JD, Peng Y, Chamberlin A, et al. Biallelic mutations in FDXR cause neurodegeneration associated with inflammation. J Hum Genet. 2018;63(12):1211-1222. doi: 10.1038/s10038-018-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537. Slone JD, Yang L, Peng Y, et al. Integrated analysis of the molecular pathogenesis of FDXR-associated disease. Cell Death Dis. 2020;11(6):1-13. doi: 10.1038/s41419-020-2637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538. Stenton SL, Piekutowska‐Abramczuk D, Kulterer L, et al. Expanding the clinical and genetic spectrum of FDXR deficiency by functional validation of variants of uncertain significance. Hum Mutat. 2021;42(3):310-319. doi: 10.1002/humu.24160. [DOI] [PubMed] [Google Scholar]
- 539. Jurkute N, Shanmugarajah PD, Hadjivassiliou M, et al. Expanding the FDXR-associated disease phenotype: retinal dystrophy is a recurrent ocular feature. Invest Ophthalmol Visual Sci. 2021;62(6):1-13. doi: 10.1167/iovs.62.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Miller WL. Steroidogenic electron-transfer factors and their diseases. Ann Pediatr Endocrinol Metab 2021;26(3):138-148. doi: 10.6065/apem.2142154.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541. Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord. 2007;8(4):331-342. doi: 10.1007/s11154-007-9054-0. [DOI] [PubMed] [Google Scholar]
- 542. Eichler F, Duncan C, Musolino PL, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377(17):1630-1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]