Abstract
Introduction
Chronic cough affects ∼10% of the population and adversely impacts quality of life. This retrospective observational cohort study aimed to identify the demographics, clinical characteristics and quality of life of the chronic cough population in a Dutch chronic cough clinic, at baseline and following treatment at 6 months. Patients were categorised based on the underlying phenotype and response to treatment.
Methods
Retrospective data on 2397 patients who were diagnosed according to standard guidelines of the American College of Chest Physicians were analysed. Quality of life was captured via the Leicester Cough Questionnaire, the Cough Numeric Rating Scale and the Hospital Anxiety and Depression Scale.
Results
Mean patient age was 59 years; 62.5% of the patients were female; and 69.1% had at least one underlying phenotype associated with chronic cough. Of the latter, 52.1% had bronchial hyperresponsiveness/airflow limitation, 33.3% had airway reflux and 20.1% had upper airway cough syndrome. 46% of patients with a phenotype, and 51% without, experienced no improvement in their quality of life or still had significant cough remaining after 6 months. Of patients with available quality-of-life data, 37.5% were categorised as having refractory chronic cough, and 9.5% were categorised as unexplained chronic cough.
Discussion
This study highlights the poor quality-of-life outcomes in patients with chronic cough, despite interventions to treat underlying conditions, and indicates a need to manage chronic cough irrespective of phenotype.
Short abstract
Of patients attending a specialist chronic cough clinic, ∼70% had an underlying chronic cough phenotype and a significant proportion exhibited poor health-related quality of life outcomes despite interventions to treat underlying conditions and the cough https://bit.ly/3rgy5yV
Introduction
Cough is commonly defined as chronic when it persists for ≥8 weeks, whereas <3 weeks is considered acute and 3–8 weeks is considered subacute [1]. The global prevalence of chronic cough is estimated to be 9.6%, and is thought to be higher in Europe at ∼12.7% [2]. However, there is some degree of variability in Europe, with some countries reporting a prevalence as low as 4% [3].
There are many different risk factors for developing chronic cough; smoking, and the existence of an underlying comorbid condition, are the most common [1]. A worldwide survey in 2014 found that chronic cough has a preponderance for females and higher age groups; two out of three patients of chronic cough clinics were found to be female, and the modal age range for presentation was 60–69 years [4]. A more recent observational study supports these findings [5]. Patients with chronic cough typically present signs of cough reflex hypersensitivity, causing a reaction to low levels of thermal, chemical or mechanical stimulation [1]. To further categorise cough hypersensitivity, different phenotypes are used dependent on the location and type of inflammation. The European Respiratory Society (ERS) 2020 guidelines outline the main phenotypes of chronic cough as asthmatic cough/eosinophilic bronchitis, reflux cough and iatrogenic cough [1].
Historically, patients presenting with suspected chronic cough underwent expensive or invasive procedures [6]. Since the introduction of ERS guidelines, chest radiography, spirometry and looking for eosinophilia are now the recommended actions to determine the phenotype. Once a phenotype has been identified, treating the specific phenotype often leads to a resolution or improvement of chronic cough [7, 8]. However, in a minority of patients, chronic cough cannot be resolved through treatment of the phenotype; these patients are considered to have refractory chronic cough (RCC) [9]. Following extensive investigation, there are some chronic cough patients who still elude diagnosis; such patients are considered to have unexplained chronic cough (UCC) [9].
Many patients with chronic cough report adverse impact on their quality of life, frequently reporting musculoskeletal chest pains, sleep disturbance, gastro-oesophageal reflux, heartburn and regurgitation [10–12]. As well as these symptoms, females with chronic cough commonly report urinary incontinence [13]. Patients with chronic cough report quality-of-life impairments comparable to those with other chronic respiratory diseases, such as COPD [11]. The quality of life impact experienced by those with UCC and RCC has yet to be characterised. This presents a significant data gap, as recent studies indicate that between 42% and 66% of chronic cough patients have no underlying phenotype or do not respond to treatment for an identifiable underlying condition, although these estimates vary depending on the study setting [5, 14–16]. Filling this data gap will allow the treatment needs of these patients to be better understood.
This study retrospectively investigated the demographic, clinical characteristics and quality of life of the chronic cough patients attending the Isala Cough Clinic in the Netherlands at baseline and after intervention at 6-month follow-up.
Methods
This retrospective observational cohort study was performed at the Isala Cough Clinic, an outpatient clinic in the department of pulmonology of Isala Hospital in Zwolle, the Netherlands. This study analysed data that were collected from patients who were first referred to the clinic between 6 January 2010 and 26 April 2019. Subjects had to be aged ⩾18 years upon first encounter at the Isala Cough Clinic and present with chronic cough, defined as cough persisting for ⩾8 weeks. Patients who presented abnormalities in chest radiography were excluded from the study, with no further exclusion criteria applied. Patients were followed-up at 6 months following initial presentation to the clinic, to capture a real-world clinical practice treatment duration of ∼4 months. Data included results of diagnostic tests performed to standard hospital procedure, patient-reported quality of life and demographic characteristics of the patients. All data were anonymised; patients provided signed informed consent on entering the hospital and were free to opt out at any time; however, no patients were excluded due to this reason. The Isala Hospital daily board of the medical ethics committee had no objections to the research proposal and no additional consent was required (medical ethics committee number 190107). The patient inclusion flow chart is outlined in supplementary figure S1.
Objectives
This study aimed to characterise the demographic and clinical characteristics and quality of life of patients with chronic cough, RCC and UCC at first presentation to the clinic, then again after 6 months.
Diagnostic tests
Prior to presentation at the clinic, a battery of diagnostic tests was performed on the chronic cough patients as per the 2006 American College of Chest Physicians guidelines [17], as follows. Lab: complete blood count, total eosinophils, IgE, Phadiatop allergy screen; lung function: spirometry, bronchoprovocation challenge, exhaled nitric oxide fraction (FENO); radiology: chest radiography, sinus imaging. Additionally, the Hull Airway Reflux Questionnaire (HARQ) was administered upon presentation to the clinic. For this study, three phenotypic categories were formed based on diagnostic tests undertaken in the time period the patients were seen in the clinic and taking into account the ERS chronic cough phenotypes: 1) bronchial hyperresponsiveness (BHR)/airflow limitation (AL); 2) upper airway cough syndrome (UACS); and 3) airway reflux (including both acid airway reflux and non-acid airway reflux) [1]. At time of diagnosis, the ERS guidelines had not been published, so direct use of the ERS phenotypes in this study was not possible. BHR was diagnosed by a methacholine challenge result of provocative dose causing a 20% fall in forced expiratory volume in 1 s (FEV1) <490 µg [17] or FEV1/vital capacity (VC) <0.70 and methacholine challenge was negative [17]. UACS was diagnosed by abnormal sinus radiography exclusively. Airway reflux was diagnosed based on reflux symptoms and/or HARQ ≥14 if no other diagnosis was made [17]. On analysis of patient data, diagnoses were inferred from the presence of the described diagnostic tests and their results.
Health-related quality of life
Health-related quality of life (HRQoL) assessments used in the Isala Cough Clinic were the Leicester Cough Questionnaire (LCQ) and the Hospital Anxiety and Depression scale (HADS), to capture physiological and psychological domains of patient wellbeing. The Cough Numeric Rating Scale (NRS-cough), a global 11-point rating of change scale, was also used as a measure of cough severity [18]. These tools were selected as the optimal measures that may be used, in lieu of an objective cough count device commonly used in clinical trials, but not practical in the real-world setting. These assessments were conducted at first presentation to the clinic and at a 6 month follow-up. In this study, the minimal clinically important difference (MCID) for LCQ and NRS-cough were defined as +1.3 and −2.0, respectively [17, 19, 20]. It should be noted that a NRS-cough scale MCID has not yet been determined; therefore, to ensure clinical applicability, the MCID of similar global rating of change Likert scale was applied [21]. The MCID for HADS was −1.7 regarding anxiety and −1.5 regarding depression [22].
RCC and UCC categorisation
Patients were stratified into RCC and UCC subgroups based on response to HRQoL questionnaires, diagnostic tests and presence of any underlying treatable conditions. Patients with RCC were defined as those with one or more treatable condition where treatment was ineffective. Patients with UCC were defined as those with no identified treatable underlying conditions and treatment failure. Ineffective treatment and treatment failure were defined as any of the following: 1) failure to reach MCID on the LCQ after 6 months; 2) failure to reach MCID on the NRS-cough after 6 months; 3) reaching MCID criteria on the LCQ after 6 months, but having significant cough remaining measured by a LCQ score <13; or 4) reaching MCID criteria on the NRS-cough after 6 months, but having significant cough remaining measured by a NRS-cough >5.
Statistical analysis
Demographic and clinical characteristics, and HRQoL were summarised and presented as measures of central tendency and proportions, respectively. The paired t-test or Wilcoxon signed-rank test was performed for inferential analysis of continuous data, whereas dichotomous variables were assessed using the McNemar test. The size of the population that attends the Isala Cough Clinic was calculated as a percentage of the population of the Isala hospital catchment area.
Results
Baseline characteristics
Patient characteristics are presented in table 1. The study population had a mean±sd age of 59±13.9 years; 62.5% (n=1497) of the cohort were female; and 6.8% (n=147) of patients included were current smokers. Half of the patients tested positive on the methacholine test for airway reactivity, supporting a diagnosis of BHR. However, spirometry was normal in the majority of the patients (FEV1/VC ≥70%, 73.8%, n=1574), as was FENO (median (range) 18 (12–27) ppb). The vast majority of patients (91.5%, n=1033) had a HARQ score ≥14 (beyond the upper limit of normal of 13), reflecting a high burden of airway reflux and high probability of cough hypersensitivity in the included patients. In 30.9% of patients no tentative underlying cause was identified (table 2). A diagnosis of BHR/AL was the modal phenotype, presenting in 60.9% (1117 out of 1835) of patients, followed by airway reflux in 33.3% (297 out of 891) and UACS in 20.1% (459 out of 2282). In 10.5% (248 out of 2352) of patients, at least two underlying phenotypes were identified, whereas 58.5% (1377 out of 2352) presented with only one. Patients presenting with co-existing phenotypes were treated sequentially, addressing one cause prior to targeting subsequent causes.
TABLE 1.
Study cohort baseline characteristics
| Patients | Summary statistics | |
| Demographics | ||
| Age (years) | 2397 | 59.4±13.9 |
| Female | 2397 | 1497 (62.5) |
| Smoking status | 2161 | |
| Nonsmoker | 1169 (54.1) | |
| Ex-smoker | 845 (39.1) | |
| Current smoker | 147 (6.8) | |
| Diagnostics | ||
| Radiography, thorax | 2318 | |
| Abnormal, not contributing to cough | 349 (15.1) | |
| UACS | ||
| Radiography, sinus | 2282 | |
| Abnormal | 459 (20.1) | |
| BHR/AL | ||
| Methacholine test (PC20/PD20) | 1835 | |
| Abnormal | 995 (54.2) | |
| FEV1 (L) | 2243 | 2.9±0.9 |
| FEV1 (%) | 2217 | 96±18.4 |
| VC (L) | 2187 | 3.8±1.1 |
| VC (%) | 2179 | 102.2±17.1 |
| FEV1/VC (%) | 2134 | 75.4 (69.5–80.4) |
| <70% | 560 (26.2) | |
| ≥70% | 1574 (73.8) | |
| Methacholine test result normal and FEV1/VC <70% | 2049 | 122 (6.0) |
| FENO (ppb) | 1882 | 18 (12–27) |
| Airway reflux | ||
| HARQ-total | 1129 | 32.7±13.3 |
| HARQ-total ≥14 | 1129 | 1033 (91.5) |
| Other | ||
| Inhalation allergy, positive | 2292 | 577 (25.2) |
| IgE (kU⋅L−1) | 2234 | 111.3±314.6 |
| IgE >120 kU⋅L−1 | 2234 | 422 (18.9) |
| Eosinophilic leukocytes (×109 cells⋅L−1) | 2330 | 0.2±0.2 |
| Eosinophilic leukocytes >0.4 ×109 cells⋅L−1 | 2330 | 149 (6.4) |
Data are presented as n, n (%), mean±sd or median (range). n=2397. UACS: upper airway cough syndrome; BHR: bronchial hyperresponsiveness; AL: airway limitation; PC20: provocative concentration causing a 20% fall in forced expiratory volume in 1 s (FEV1); PD20: provocative dose causing a 20% fall in FEV1; VC: vital capacity; FENO: exhaled nitric oxide fraction; HARQ: Hull Airway Reflux Questionnaire.
TABLE 2.
Phenotypic characterisation of patients presenting with chronic cough
| Patients | Summary statistics | |
| BHR/AL | 1835 | 1117 (60.9) |
| UACS | 2282 | 459 (20.1) |
| Airway reflux | 891# | 297 (33.3) |
| Phenotype number | 2352 | |
| 0 | 727 (30.9) | |
| 1 | 1377 (58.5) | |
| ≥2 | 248 (10.5) | |
| Missing | 0 |
Data are presented as n or n (%). BHR: bronchial hyperresponsiveness; AL: airway limitation; UACS: upper airway cough syndrome. #: those patients with 1) a valid Hull Airway Reflux Questionnaire; 2) a valid methacholine test result; and 3) a valid sinus radiography result.
Health-related quality of life
Results of HRQoL and cough severity measures are presented in table 3. At baseline, more than half of the respondents were experiencing significant cough-related HRQoL impairments, evidenced by an LCQ <13 (52.8% of respondents) or an NRS-cough >5 (55.2% of respondents) on presentation. For patients who responded to the questionnaire at the 6-month follow-up, the mean total LCQ score increased by 2.5 from 12.8 to 15.3 (p<0.001), surpassing the MCID, and the mean NRS-cough score decreased by 1.7 from 5.7 to 4.0 (p<0.001), which was statistically significant. The MCID on the LCQ was reached in 64.4% (n=519) of respondents and the MCID on the NRS-cough was reached in 50.7% (n=880) (table 4). In addition, the percentage of patients with low LCQ <13 decreased, from 52.8% at baseline to 21.5% at 6-month follow-up (p<0.001), reflecting a notable improvement in the HRQoL for these patients over the 6-month period (table 3). The median HADS total score decreased by 2.0 from 8.0 to 6.0 (p<0.001), surpassing the MCID for HADS.
TABLE 3.
Health-related quality of life and cough severity descriptive statistics at baseline and 6-month follow-up
| Baseline (initial) | 6 months (follow-up) | Difference (change) | |||||
| Patients | Summary statistics | Patients | Summary statistics | Patients | Summary statistics | p-value | |
| Time between LCQ at baseline and follow-up (months) | NA | NA | 693 | 6.9 (6.4–7.7) | NA | ||
| Time between NRS-cough at baseline and follow-up (months) | NA | NA | 1565 | 6.6 (6.2–7.5) | NA | ||
| Discrete/continuous variables | |||||||
| LCQ total | 1144 | 12.8±3.0 | 971 | 15.3±3.3 | 806 | 2.5±2.9 | <0.001 |
| LCQ physical | 1194 | 4.3±0.9 | 1008 | 5.0±1.0 | 870 | 0.7±0.9 | <0.001 |
| LCQ psychological | 1228 | 4.5±1.0 | 1034 | 5.2±1.0 | 900 | 0.7±1.0 | <0.001 |
| LCQ social | 1250 | 3.9±1.5 | 1039 | 5.1±1.5 | 929 | 1.2±1.4 | <0.001 |
| NRS-cough 24 h | 2235 | 5.7±2.5 | 1837 | 4.0±2.7 | 1735 | −1.7±3.0 | <0.001 |
| HADS total | 1207 | 8.0 (4–14) | 1024 | 6.0 (2–12) | 886 | −2.0 (−23–29) | <0.001 |
| HADS anxiety | 1229 | 5.0 (2–8) | 1040 | 4.0 (1–6) | 917 | −1 (−3–1) | <0.001 |
| HADS depression | 1239 | 4 (1–7) | 1043 | 2 (1–6) | 922 | −2 (−2–3) | <0.001 |
| Binary categorical variables | |||||||
| LCQ total | 1144 | 971 | 806 | <0.001 | |||
| ≥13 | 540 (47.2) | 762 (78.5) | |||||
| <13 (unfavourable) | 604 (52.8) | 209 (21.5) | |||||
| NRS-cough 24 h | 2235 | 1837 | 1734 | <0.001 | |||
| ≤5 | 1001 (44.8) | 1253 (68.2) | |||||
| >5 (unfavourable) | 1234 (55.2) | 584 (31.8) | |||||
| Cough severity (LCQ and NRS-cough) | 1071 | 909 | 712 | <0.001 | |||
| LCQ ≥13 and NRS-cough ≤5 | 326 (30.4) | 554 (60.9) | |||||
| LCQ ≥13 and NRS-cough >5 | 185 (17.3) | 160 (17.6) | |||||
| LCQ <13 and NRS-cough ≤5 | 131 (12.2) | 49 (5.4) | |||||
| LCQ <13 and NRS-cough >5 | 429 (40.1) | 146 (16.1) | |||||
| HADS total >11 | 1207 | 430 (35.6) | 1024 | 288 (28.1) | 886 | <0.001 | |
| HADS anxiety >11 | 1299 | 99 (8.1) | 1040 | 68 (6.5) | 917 | 0.016 | |
| HADS depression >11 | 1239 | 94 (7.6) | 1043 | 69 (6.6) | 922 | 0.801 | |
Data are presented as n, median (range), mean±sd or n (%), unless otherwise stated. LCQ: Leicester Cough Questionnaire; NRS-cough: Cough Numeric Rating Scale; HADS: Hospital Anxiety and Depression Scale; NA: not applicable.
TABLE 4.
Patient health-related quality of life 6 months after presentation to the clinic
| Patients | Summary statistics | |
| MCIDs reached | ||
| MCID LCQ +1.3 reached | 806 | |
| No | 287 (35.6) | |
| Yes | 519 (64.4) | |
| MCID NRS-cough −2.0 reached | 1734 | |
| No | 854 (49.3) | |
| Yes | 880 (50.7) | |
| MCID LCQ and/or NRS-cough reached | 712# | |
| LCQ not reached and NRS-cough not reached | 192 (27.0) | |
| LCQ reached and NRS-cough not reached | 146 (20.5) | |
| LCQ not reached and NRS-cough reached | 68 (9.6) | |
| LCQ reached and NRS-cough reached | 306 (43.0) | |
| Any MCID reached | 712# | |
| No | 192 (27.0) | |
| Yes | 520 (73.0) | |
| Residual burden of cough | ||
| LCQ <13 and/or NRS-cough >5 | 909 | |
| No | 554 (60.9) | |
| Yes | 355 (39.1) | |
| Effectiveness of treatment | ||
| Treatment effectiveness | 712# | |
| No MCID and R-cough | 126 (17.7) | |
| No MCID and no R-cough | 66 (9.3) | |
| Any MCID and R-cough | 144 (20.2) | |
| Any MCID and no R-cough | 376 (52.8) | |
| Treatment effectiveness | 712# | |
| Not effective | 336 (47.2) | |
| Effective | 376 (52.8) |
Data are presented as n or n (%). MCID: minimal clinically important difference; LCQ: Leicester Cough Questionnaire; NRS-cough: Cough Numeric Rating Scale; R-cough: remaining cough. #: all patients included in the trial, regardless of available diagnosis data.
Upon evaluation, of patients that had responded to both the LCQ and NRS-cough questionnaires, 27.0% failed to reach a MCID on both scores and the cough remained troublesome in 65.6% (126 out of 192) of these patients (table 4).
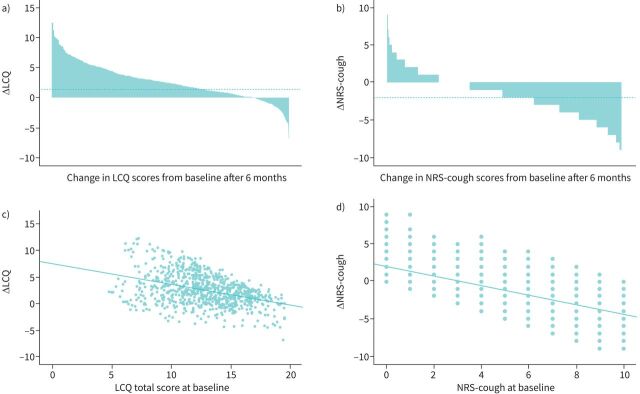
Figure 1 highlights the correlation between patient improvement and severity of initial LCQ and NRS-cough scores. Analysis showed that patients with the lowest scores for LCQ (figure 1a and c) and highest scores on the NRS-cough scale (figure 1b and d) improved the most.
FIGURE 1.
Correlation between patient improvement and Leicester Cough Questionnaire (LCQ) and the Cough Numeric Rating Scale (NRS-cough). a) Change (Δ) LCQ (n=806), mean±sd 2.6±2.9, median (range) 2.4 (0.6–0.4); b) Δ NRS-cough (n=1734), mean±sd −1.7±2.3, median (range) −2.0 (−4.0–0.0); c) the correlation of baseline LCQ with Δ LCQ; d) the correlation of baseline NRS-cough with Δ NRS-cough.
RCC and UCC categorisation
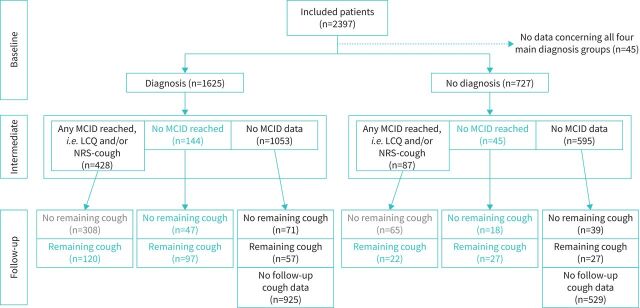
Figure 2 shows the categorisation of patients into RCC or UCC according to phenotype and effect of interventions made prior to the 6-month follow-up, measured by responses to the LCQ and the NRS-cough questionnaire. If we consider only patients with available MCID data, 37.5% (264 out of 704) of the study population were categorised as RCC, and 9.5% (67 out of 704) were categorised as UCC. Supplementary table S1 shows the characteristics of the treatment response groups, which were found to be similar regardless of treatment response and underlying phenotype. Considering patients with MCID data (figure 2), the success rate (defined by patients meeting HRQoL MCID and having no remaining cough) was 54% (308 out of 572) in patients with a phenotype and 49% (65 out of 132) in patients without a phenotype.
FIGURE 2.
Flow chart showing the categorisation of patients as refractory chronic cough (RCC) or unexplained chronic cough (UCC). MCID: minimal clinically important difference; LCQ: Leicester Cough Questionnaire; NRS-cough: Cough Numeric Rating Scale.
Discussion
To the authors’ knowledge, this is the first real-world study capturing patient characteristics and HRQoL of patients with RCC and UCC. Our study found that 37.5% of chronic cough patients had RCC and 9.5% UCC after 6 months. Despite systematic investigation, no phenotype could be identified in 30% of patients, meaning they could not receive targeted therapy for any specific chronic cough phenotype. The percentages of the various phenotypes are comparable to another recent epidemiological study whereby the prevalence of chronic cough according to comorbidities was 33% for asthma and 28.6% for COPD [23], compared to 60.9% for BHR and/or AL in this study. Baseline characteristics, including cough-related quality of life and cough severity, have also been identified as important predictors of outcomes in patients with chronic cough [24]. The findings of the HARQ taken at baseline indicate that 91.5% of patients were positive, suggesting that airway reflux is a major cause of cough hypersensitivity and may speculatively underlie the high incidence of nonasthmatic BHR seen in our study. In line with existing literature, this study found that baseline LCQ and NRS-cough values were positively correlated with change in LCQ and NRS-cough. Irrespective of phenotype or reaching an MCID in one or more measures, 39.1% (figure 2) of respondents still had significant cough remaining after the 6-month follow-up period. Therefore, the observation that improvement was not seen at 6 months in a significant proportion of patients either with or without a phenotype, could imply that over time, cough hypersensitivity may become more dominant in lieu of an underlying phenotype.
Limited attempts have been made to characterise RCC or UCC within a chronic cough population. Estimates vary markedly depending on the study setting and subgroup definition which, in the real-world setting, can vary depending on the diagnosing physician. Two separate UK studies estimate the proportion of patients within the chronic cough population without a phenotype to be 42% and 66%, with the highest prevalence reported in a community setting rather than a specialist cough clinic [15, 16]. The study by Everett et al. [15], conducted in a general UK population, captured only the proportion of chronic persistent cough patients without a co-existing respiratory diagnosis, which may explain the notably higher results at 66%. The study by Haque et al. [16], which took place at the Royal Brompton Hospital Chronic Cough Clinic (London, UK), defined a subgroup with “chronic idiopathic cough” where diagnosis could not be made even after thorough systematic investigation, and found a prevalence of 42% within their chronic cough population, similar to the 30% identified in this study.
The results show that at baseline more than half of respondents were experiencing significant cough-related HRQoL impairments. Given that HRQoL impairment defines categorisation as RCC or UCC in this study, and nearly half of our study population were categorised as such; clearly these patients are in need of effective symptom management. Additionally, more effective diagnosis of RCC and UCC in particular would reduce the economic burden associated with diagnosis. These patients are only diagnosed as end-points to an arduous and expensive diagnostic process. Furthermore, to the authors’ knowledge there have been no attempts to characterise the patient journey of chronic cough patients. A clearer understanding of the patient journey may allow for the streamlining of the current, unstructured approach to diagnosing and managing chronic cough.
There are several limitations of this retrospective study. Despite the large sample size, some analyses were limited due to invalid diagnostic tests and/or due to the retrospective study design. Patient examination was conducted prior to the publication of the ERS chronic cough guidelines. Phenotype grouping was done prospectively so direct use of the ERS phenotypes was not possible. Further, although commonly used in chronic cough studies, reliance on subjective patient reported outcome measures such as the LCQ could potentially lead to underreporting of symptom severity. Additionally, 64.8% (1053 out of 1625) of patients with an underlying phenotype and 81.8% (595 out of 727) of patients without phenotype did not respond to the HRQoL questionnaires, so the results may be incomplete. Despite some lack of response, it was possible to evaluate the HRQoL of many patients (table 3); however, the authors recognise the smaller numbers of responses in relation to establishing MCID of NRS-cough and LCQ results. A further limitation exists with the lack of standardisation of classification of RCC and UCC populations, which may limit comparability to other studies. The use of disease-specific, patient-reported quality-of-life measures in the definition of ineffective treatment and treatment failure means that patients can still be considered RCC or UCC even if cough resolves. Additionally, the study sample is a biased representation of chronic cough patients, as only patients with severe cough are referred to the Isala Cough Clinic and generalisation of these results to a general population should be made with caution.
Our analyses highlighted the difficulty in predicting whether a patient will respond to intervention for specific phenotypes, as treatment response was similar across all subgroups of chronic cough patients, as were distributions of patient characteristics. Our study found that 47% of the chronic cough population may be labelled as RCC or UCC. Of the patients attending the Isala Chronic Cough Clinic, ∼70% belonged to a phenotype associated with chronic cough and ∼30% did not. In patients with an underlying diagnosis, 46% of patients showed improvement in HRQoL and had no significant cough remaining after 6 months and 51% of patients did not. The presence or absence of a phenotype had no relationship with HRQoL impact severity, and whether a patient failed to improve in HRQoL or still had significant cough remaining. As such, there is a major unmet clinical need to manage chronic cough irrespective of phenotype. Future studies should aim to characterise the economic and social burden of RCC and UCC so we can better understand their impact and investigate what dictates whether a chronic cough patient will respond to intervention. The similarity in treatment response between patient groups also indicates that development of additional therapeutic options for symptom management in both RCC and UCC would be clinically and economically valuable.
Acknowledgements
The authors would like to acknowledge Adelphi Values PROVE (Bollington, UK) for providing medical writing support.
Provenance: Submitted article, peer reviewed.
This article has an editorial commentary: https://doi.org/10.1183/23120541.00519-2022
Data availability: The datasets supporting the conclusions of this article are included within the article or supplementary material. The full datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Author contributions: All authors attest that they meet the International Committee of Medical Journal Editors criteria for authorship. J.W.K. van den Berg contributed to study design, data acquisition, analysis and interpretation. C.A. Baxter was involved in study design and data interpretation. M.A. Edens, A. Weijerse and H. van der Velden contributed to data analysis and/or interpretation. K.W. Patberg was involved in data acquisition and S. Salomonsson contributed to study design. All authors were involved in the drafting and/or critical revision of the manuscript. The authors have given final approval of the manuscript for publication; and agreement of their full accountability for all aspects of the work.
Conflict of interest: J.W.K. van den Berg participates on a data safety monitoring board or advisory board at GlaxoSmithKline PLC, Chiesi Ltd, Novartis AG and Merck & Co., Inc., Rahway, NJ, USA.
Conflict of interest: C.A. Baxter is a full-time employee of MSD (UK) Limited, London, UK, and shareholder of Merck & Co., Inc., Rahway, NJ, USA.
Conflict of interest: M.A. Edens has nothing to disclose.
Conflict of interest: K.W. Patberg has nothing to disclose.
Conflict of interest: H. van der Velden is a full-time employee of MSD BV, The Netherlands.
Conflict of interest: A. Weijerse is a full-time employee of MSD BV, The Netherlands, and shareholder of Merck & Co., Inc., Rahway, NJ, USA.
Conflict of interest: S. Salomonsson is a full-time employee of MSD Sweden, and shareholder of Merck & Co., Inc., Rahway, NJ, USA.
Support statement: This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Funding information for this article has been deposited with the Crossref Funder Registry.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00232-2022.supplement (190.1KB, pdf)
References
- 1.Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020; 55: 1901136. doi: 10.1183/13993003.01136-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song W-J, Chang Y-S, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015; 45: 1479–1481. doi: 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 3.Çolak Y, Nordestgaard BG, Laursen LC, et al. Risk factors for chronic cough among 14,669 individuals from the general population. Chest 2017; 152: 563–573. doi: 10.1016/j.chest.2017.05.038 [DOI] [PubMed] [Google Scholar]
- 4.Morice AH, Jakes AD, Faruqi S, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J 2014; 44: 1149–1155. doi: 10.1183/09031936.00217813 [DOI] [PubMed] [Google Scholar]
- 5.Good JT Jr, Rollins DR, Kolakowski CA, et al. New insights in the diagnosis of chronic refractory cough. Respir Med 2018; 141: 103–110. doi: 10.1016/j.rmed.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 6.Ponsioen B, Hop W, Vermue N, et al. Efficacy of fluticasone on cough: a randomised controlled trial. Eur Respir J 2005; 25: 147–152. doi: 10.1183/09031936.04.00053604 [DOI] [PubMed] [Google Scholar]
- 7.Mathur A, Liu-Shiu-Cheong P, Currie G. The management of chronic cough. QJM 2019; 112: 651–656. doi: 10.1093/qjmed/hcy259 [DOI] [PubMed] [Google Scholar]
- 8.Morice A, Fontana GA, Sovijarvi AR, et al. The diagnosis and management of chronic cough. Eur Respir J 2004; 24: 481–492. doi: 10.1183/09031936.04.00027804 [DOI] [PubMed] [Google Scholar]
- 9.Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest 2016; 149: 27–44. doi: 10.1378/chest.15-1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms among patients with chronic cough. Chest 2006; 130: 1839–1843. doi: 10.1378/chest.130.6.1839 [DOI] [PubMed] [Google Scholar]
- 11.French CL, Irwin RS, Curley FJ, et al. Impact of chronic cough on quality of life. Arch Intern Med 1998; 158: 1657–1661. doi: 10.1001/archinte.158.15.1657 [DOI] [PubMed] [Google Scholar]
- 12.Polley L, Yaman N, Heaney L, et al. Impact of cough across different chronic respiratory diseases: comparison of two cough-specific health-related quality of life questionnaires. Chest 2008; 134: 295–302. doi: 10.1378/chest.07-0141 [DOI] [PubMed] [Google Scholar]
- 13.Zoglmann R, Nguyen T, Engberts M, et al. Do patients with stress incontinence cough or do cough patients suffer from urinary incontinence? Eur Respir J 2015; 46: Suppl. 59, PA713. doi: 10.1183/13993003.congress-2015.PA713 [DOI] [Google Scholar]
- 14.Al-Sheklly B, Satia I, Badri H, et al. P5 Prevalence of refractory chronic cough in a tertiary cough clinic. Thorax 2018; 73: Suppl. 4, A98. doi: 10.1136/thorax-2018-212555.163 [DOI] [Google Scholar]
- 15.Everett CF, Kastelik JA, Thompson RH, et al. Chronic persistent cough in the community: a questionnaire survey. Cough 2007; 3: 5. doi: 10.1186/1745-9974-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest 2005; 127: 1710–1713. doi: 10.1378/chest.127.5.1710 [DOI] [PubMed] [Google Scholar]
- 17.Brown KK. Chronic cough due to nonbronchiectatic suppurative airway disease (bronchiolitis): ACCP evidence-based clinical practice guidelines. Chest 2006; 129: Suppl. 1, 132S–137S. doi: 10.1378/chest.129.1_suppl.132S [DOI] [PubMed] [Google Scholar]
- 18.Koo H-K, Jeong I, Kim J-H, et al. Development and validation of the COugh Assessment Test (COAT). Respirology 2019; 24: 551–557. DOI: 10.1111/resp.13462 [DOI] [PubMed] [Google Scholar]
- 19.Rebelo P, Oliveira A, Paixão C, et al. Minimal clinically important differences for patient-reported outcome measures of cough and sputum in patients with COPD. Int J Chron Obstruct Pulmon Dis 2020; 15: 201–212. doi: 10.2147/COPD.S219480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spinou A, Birring SS. An update on measurement and monitoring of cough: what are the important study endpoints? J Thorac Dis 2014; 6: Suppl. 7, S728–S734. doi: 10.3978/j.issn.2072-1439.2014.10.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther 2009; 17: 163–170. DOI: 10.1179/jmt.2009.17.3.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 23.Arinze JT, de Roos EW, Karimi L, et al. Prevalence and incidence of, and risk factors for chronic cough in the adult population: the Rotterdam Study. ERJ Open Res 2020; 6: 00300-2019. doi: 10.1183/23120541.00300-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koskela HO, Lätti AM, Purokivi MK. Long-term prognosis of chronic cough: a prospective, observational cohort study. BMC Pulm Med 2017; 17: 146. doi: 10.1186/s12890-017-0496-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00232-2022.supplement (190.1KB, pdf)