Abstract
Candidate vaccine antigens for preventing otitis media caused by nontypeable Haemophilus influenzae (NTHI) should possess one or more conserved epitopes. We sought to evaluate the candidacy of P1, a surface-expressed outer membrane protein knowing that this antigen is subject to diversifying selection. Therefore, we selected NTHI strains from among >500 phylogenically variant isolates representative of the diversity found in natural populations of H. influenzae. Twenty-three variants of P1 (≤95% similarity) were identified among 42 strains. When chinchillas were immunized with recombinant P1 (rP1) obtained from one of these isolates (BCH-3), all animals developed antibodies specific for rP1. Immunized animals were protected against disease when challenged with BCH-3, but not with an ompP1 mutant of BCH-3 or a strain (BCH-2) possessing a heterologous P1 (91% identity). We conclude that (i) while P1 induces protection against NTHI-mediated otitis media, development of a polyvalent vaccine reflecting the variability of P1 would be necessary to construct an efficacious vaccine and (ii) use of a phylogenically characterized collection of representative isolates in concert with gene sequencing, cloning, gene inactivation, and animal testing offers an efficient, rational, and rigorous strategy for evaluating the potential problems associated with variability of vaccine targets and specificity of related immune responses.
Nontypeable (unencapsulated) Haemophilus influenzae (NTHI) colonizes ∼85% of humans (43, 44). NTHI is usually associated with noninvasive diseases, such as sinusitis, otitis media, and pneumonia (33) as well as persistent respiratory infection in patients with chronic bronchitis and cystic fibrosis (27, 64, 74). On occasion, NTHI may become invasive, causing bacteremia and sometimes crossing the blood-brain barrier to cause meningitis (33, 54). With the exception of the H. influenzae type b capsule, the multiple host and microbial factors differentiating commensal from pathogenic behavior are unknown for both typeable H. influenzae and NTHI (43, 44).
Unencapsulated NTHI strains having genes with no homology to capsule biosynthetic genes are found, while others have a partial or complete deletion of the IS1016-flanked capsule biosynthetic locus (35–37). While previous phylogenic studies suggest serotype-specific clustering of H. influenzae capsular serotypes a to f (31, 39, 51–53, 60), the precise evolutionary relationship between types a to f and NTHI remained obscure (50) until recently. Using a phylogenically organized collection of >500 H. influenzae and NTHI isolates (13; unpublished data), we examined the 329 NTHI strains using restriction fragment length polymorphism (RFLP) analysis to identify the serotype correlated with any strains encoding a capsule locus remnant (35–37). For each NTHI isolate in which an interpretable remnant was found, the RFLP profile correlated with the capsule type of the evolutionary lineage to which it was most closely related in the phylogenically organized collection (13).
Effective passive immunization in animals provides evidence that circulating antibodies can prevent both invasive and mucosal diseases caused by typeable H. influenzae (41) and NTHI (6). Further studies with both categories of H. influenzae demonstrated that antibodies specific to outer membrane proteins (OMPs) of homologous strains are associated with protection in animals (32, 69) and humans (7) and that the majority of bactericidal antibody activity present in serum from either a patient recovering from NTHI disease or from normal adult sera is directed to OMPs (24). However, virtually every major antigen on the surface of NTHI is known to be either variable or absent from some isolates (10, 18, 23, 24, 26, 38, 49, 62, 73), a likely consequence of diversifying selection in the absence of an outer capsule. This is the initial problem to be overcome when considering an OMP-based vaccine. The immunogenicity of one of these OMPs, P1, is the subject of the studies described in this report.
OMP P1 (47 kDa) accounts for ∼10% of H. influenzae OMP content. Passive immunization with P1 induces protection against bacteremia in the infant rat model (48). The associated gene, ompP1, has been cloned and sequenced from H. influenzae serotype b (46) and d (19) strains. OMP P1 has eight potentially surface-exposed loops (14), four of which are immunogenic (57, 61, 62). The availability of several ompP1 sequences was particularly relevant, as it offered the information needed for a broader survey of the sequence conservation of the ompP1 gene across a phylogenically classified collection of >500 typeable H. influenzae and NTHI isolates representing the natural population structure of the species (13; unpublished data). Based on this knowledge, we could evaluate the efficacy of P1 immunization in protecting against NTHI isolates with P1 antigens homologous and heterologous to the immunizing antigen. Doing so allowed us to test the proposition that such a phylogenic approach is a viable strategy for the rational development of vaccines by directly addressing the inherent problem of antigenic variation within a microbial species. For this purpose, infectious challenge was carried out with NTHI isolates known to express either a significantly variant OMP P1 on the one hand or an OMP P1 nearly identical to that of the P1 immunogen on the other, in order to determine whether a priori knowledge was predictive before actual animal testing. The ompP1 DNA sequence information also allowed for (i) the cloning, expression, and purification of P1 immunogen and (ii) the construction of an otherwise isogenic ompP1 mutant control strain used in these studies to directly test the specificity of the immunogen.
Results of these studies have direct implications in considering further development of OMP P1 as a vaccine candidate to protect against both NTHI-associated otitis media and other respiratory infections, while also providing basic information about the role of P1 in the virulence and viability of NTHI. Equally important, as utilized, the phylogenic strategy for vaccine development based on foreknowledge of the range of immunogen allele sequence variability within the natural population structure of H. influenzae is broadly applicable to other microbial pathogens.
MATERIALS AND METHODS
Bacterial strains.
NTHI BCH-1 is a β-lactamase-producing H. influenzae strain isolated from the transtracheal aspirate of an adult with pneumonia (32). NTHI strain BCH-2 was isolated from the middle ear of a young child with acute otitis media (middle-ear infection) (32). NTHI strain BCH-3 is a non-β-lactamase-producing isolate from the nasopharyngeal culture of a young child. BCH-1, -2, and -3 each express a phylogenically variant wild-type (wt) ompP1 allele characterized in this study. BCH-3 ompP1 Kmr (kanamycin resistant) is an otherwise isogenic ompP1 mutant of BCH-3, constructed as described below by insertional inactivation with a Kmr cassette. The subset of 42 H. influenzae isolates used for ompP1 DNA sequence analysis represents the evolutionary diversity of a phylogenically organized collection of more than 500 H. influenzae and NTHI isolates (13; unpublished data) and are described in Table 1. Escherichia coli strains JM107 (79) and INVαF′ One Shot (Invitrogen) (28) cells were used for DNA cloning. E. coli strain BLR (Novagen) was used to express recombinant P1 antigen. H. influenzae and NTHI isolates were grown as previously described (24, 32) as were E. coli isolates (2–4).
TABLE 1.
Alignment of four variable regions of OMP P1 from 42 phylogenically diverse typeable H. influenzae and NTHI isolatesa
| P1 typeb | Strain | Sequence of variable regionc:
|
Sd | Loce | |||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||
| 85 107 | 198 232 | 281296 | 427440 | ||||
| 1 | BCH-1 | DSYAIISDS---IKVTNDGSASA | LIADTVKDN-QVKNTLTVQQEPLKFIDKYLPSKDT | LEANVIKASKTGDLTL | KTIGDKRSLALSTT | NT | USA |
| BCH-3 | DSYAIISDS---MKVTNDGSASA | LIADTVKDN-QVKNTLTVQQEPLKFIDKYLPSKDT | LEANVIKASKTGDLTL | KTIGDKRSLALSTT | NT | USA | |
| 3-H-1552 | DSYAIISDS---IKVTNDGSASA | LIADTVKDN-QVKNTLTVQQEPLKYINKYLPSKDT | LEANVINASKTGDLTL | KTIGDKRSLALSTT | NT | USA | |
| 17-H-783 | DSYAIISNS---IKVTNDGSASA | LIADTVKDS-QVKTTLIVQQEPLKSIDKYLPSKDT | LEAEAIKAGKKGXLTL | KTIGDARSLALNTT | NT | USA | |
| 21-H-1328 | NSYAIISNS---IKVTNDGSASA | LIADTVKDS-QVKTTLIVQQEPLKSIDKYLPSKDT | LEAEAIKAGKKGDLTL | KTIGDARSLALNTT | NT | USA | |
| 27-H-1433 | DSYAIISNS---IKVTNDGSASA | LIADTVKDS-QVKNALIVQQEPLKSIDKYLPSKDT | LEAXAIKAGKKGDLTL | KTIGDARSLALNTT | NT | USA | |
| 2 | 200 | NSDAIITSTSSGVKTIKHGSASQ | IIVDSLKDN-QVQTALSVQKEPLKYLHKYLPSKDT | VEADVIKAGKKGDLTL | KTIGDERSLALNTT | NT | Finland |
| 218 | NSDAIITSTSSGVKTIKHGSASQ | IIVDSLKDN-QVQTALSVQKEPLKYLHKYLPSKDT | VEADVIKAGKKGDLTL | KTIGDERSLALNTT | NT | Finland | |
| 667 | NSDAIITSTNSGVKTIKHGSASQ | IIVDSLKDN-QVQTALSVQKEPLKYLHKYLPSKDT | VEADVIKAGKKGDLTL | KTIGDERSLALNTT | NT | Finland | |
| 199 | NSDAIITSTSNGVKTIKHGSASQ | IIVDSLKDN-QVQTALSVQKEPLKYLHKYLPSKDT | VEADVIKAGKKGDLTL | KTIGDERSLALNTT | NT | Finland | |
| 3 | 7416 | DSSAIISNM---IKATKDGSASE | IIADSVKDN-QVKTALTVQQEPLKFLDKYLPSKDT | VEANVIKAGKKGDLTL | KTIGDERSLTLNTT | a | Kenya |
| Rd | TSHATIITSSSGIKAIEGGSASA | IIADSVKDN-QVKTALTVQQEPLKFLDKYLPSKDT | VEANVIKAGKKGDLTL | KTIGDERSLTLNTT | d | USA | |
| 4 | 7004 | T--ASIATTK--MNSAKYGSASE | IIANSVNDT-QVKTALSVLAPPLKGLDQNLPSKDK | VEANVIKEGKKGNLTL | KTIGDKRTLTLNTT | b | Holland |
| 6094 | T--ASIATTK--MNSAKYGSASE | IIANSVNDT-QVKTALSVLAPPLKGLDQNLPSKDK | VEANVIKEGKKGNLTL | KTIGDKRTLTLNTT | b | UK | |
| 6107 | T--ASIATTK--MNSAKYGSASE | IIANSVNDT-QVKTALSVLAPPLKGLDQNLPSKDK | VEANVIKEGKKGNLTL | KTIGDKRTLTLNTT | b | UK | |
| 7109 | T--ASIATTK--MNSAKYGSASE | IIANSVNDT-QVKTALSVLAPPLKGLDQNLPSKDK | VEANVIKEGKKGNLTL | KTIGDKRTLTLNTT | NT | Sweden | |
| ATCC9795 | T--ASIATTK--MNSAKYGSASE | IIANSVNDT-QVKTALSVLAPPLRGLDQNLPSKDK | VEANVIKEGKKGNLTL | KTIGDKRTLTLNTT | b | ||
| 161 | T--ASIATTR--MNSAKYGSASE | IIANSVNDT-QVQTALSVLAPPLKGLDQNLPSKDK | VEANVIKEGKKGNLTL | KTIGDKRALTLNTT | NT | Finland | |
| OT-9 | T--ASIATTK--MNSAKYGSASE | IIANSVKDT-QVKTALSVLAPPRTALPQNLPSKDK | VEANVIKEGKKGNLTL | KTIGDKRALTLNTT | NT | Canada | |
| 5 | 1363 | NSYATITSASSGVRTIKHGSASQ | IIVETI-DN-QVNTALSALQEPFRDLKKHLPSKDK | LEANVIKAGKKGDLTF | KTIGEPRALTFNTT | NT | Finland |
| OT-81 | NSYATITSASSGVRTIKHGSASQ | IIVETI-DN-QVNTALSALQEPFRDLKKHLPSKDK | LEANVIKAGKKGDLTF | KTIGEPRALTFNTT | NT | Canada | |
| 6 | 9-H-1194 | TSHATIVTSSSGVRAIKDGSASA | ILAESVNDDDQVKGALLTLSEPFKNLNTHLPSKDK | LEANVIKAGKKADLTF | KTIGEQRSLTFDTT | NT | Canada |
| 7 | MinnA | TSYAQIITNQIGMKAIKDGSASQ | LIADSVKDN-QITSALSTQQEPFRDLKKYLPSKDK | LEANVIKEGKKGNLTF | KTIGDKRTLTLNTT | b | USA |
| 8 | 1071 | ASSVIISNM---VKATQDGSASA | TISDSVNDS-QLTGVLSTLAKPFKELNKHLPSKDK | LEAGVIGAGKTGNLTL | KTIADKSGLPLTST | NT | Finland |
| 9 | BCH-2 | ASSAIVNSA---MKATKEGSASQ | IIVDTVNDD-QVKRGLTVLQPPYKTLDKYLTSKDK | LEAGVIGAGKTGNLTF | KTIGEKSGLPVTAT | NT | USA |
| 10 | LO-127 | PSSVTTNST---MKTTMDGSASE | VIVDIVNDT-QIKXALAALSEPYKDFPNYLTSKDK | VEANVIKAGKKGDLTL | NTIGKESGLPLTTT | NT | Canada |
| 11 | 5 | TSSA--TPANSVMTAIRYGSASA | IIEGTVNDD-QIKRALTTQPKPYKDFPQHLSSKDK | LEAGVIGAGKKGDLTL | KAIGEKRSLTFDTT | NT | USA |
| 12 | 1512A | SVSASITGAS--VKITRNGSASQ | LIADTVNDN-QIKQALSTQQTTLRDLPKHLTSKDK | LEAEAIKAGKKGDLTL | KAISQA-TLTLDTT | NT | USA |
| 49 | DVRASITGVS--VKTTKNGSASQ | IIANTVNDN-QVKLPLSTQPETLRDLHKHLPSKDK | LEAEAIKAGKKGDLTL | KAITKS-VLTLDTT | NT | USA | |
| 13 | 1-H-1085 | KCYASITGIS--VKTTKNGSASA | LIVDSV-EIARNGQAFTVLGEPLKTIGQYLTSKDK | LGAGVIKAGKKGDLTL | ETISQA-QLTLNTT | NT | USA |
| 14 | 6181 | E--AFIATLK--MKTTKNGSASE | IIADTV-KISQG--AFTVGSQEDKAIPKYLTSKDK | LEAGAIGAGKKGDLTL | ATIG--KSVILNTT | e | UK |
| 15 | 22-H-1154 | DSYAIISNS---IKVTNDGSASA | IIKESV-ELAKT--AFTVAQDDEKAIPTYLTSKNT | LEAEAIKAGKKGDLTL | KTIGDARSLALNTT | NT | USA |
| 16 | 4-H-1094 | K--SSTKNLQ--MLAAKYGSASQ | IIKESV-ELAKT--AFTVAQDDEKAIPTYLTSKNT | LEAGVIGAGKKGDLTL | KTTG--KTVILNAT | NT | USA |
| 17 | 6255 | D--SSITATT--MRTTKYGSASA | IIAESVKI-AQN--AIKTVNPKDK-ATDYLTSKDK | LEAEVIEAGKKGNLTL | QKAVGGF---ITTT | NT | UK |
| ATCC9833 | D--SSITATT--MRTTKYGSASA | IIAESVKI-AQN--AIKTVNPKDK-ATDYLTSKDK | LEAEVIEAGKKGNLTL | QKAVGGF---ITTT | f | ||
| C-105 | N--SSITATT--MRTTKYGSASA | IIAESVKI-AQN--AIKTVNPKDK-ATDYLTSKDK | LEAEVIEAGKKGNLTL | QKAVGGF---IXTT | NT | Canada | |
| 18 | 2-H-1038 | A--ASITGSS--IKVTNDDVASE | IIADSVKD-PQAKRALETVVPGTK-IPDYLTSKDK | LGKKGIEAGKTGNLTF | QKVADDF---ITAN | NT | USA |
| 19 | 658 | L--SSITSAS--MKTTKYGSASE | IIVRSVKD-SQAQTALKTVVPGTL-IPDLLTSKDK | LGQKSIEAGKTDNLTL | QKVADGL---ITTN | NT | Finland |
| 20 | 13-H-1157 | D--AYLQSGS--MKFTKYGSASQ | IIADSVQD-SQVKPALQAVDPQNK-ATGYLTSKDK | LEAGAIKAGKKGDLTL | QKAAGGH---IITT | NT | USA |
| 21 | 9 | N--ASIKAT---MNMTKYGSASQ | LIADTVQD-QQIQKALKAVDSKTV-IPDYVTSKDK | LEAGVIGAGKKGDLTL | QQV-GGH---IITN | NT | USA |
| 22 | 7424 | A--ASIKATA--MGRTKYGSASA | IIADSIQD-NQIQQALKAVDPQTK-LHNYLTSKDK | LGAKDIVAGKTGDLTF | QQA-GGH---IITT | NT | Kenya |
| 23 | 4-H-1093 | D--ASIKATA--MARTKYGSASQ | IIADSIQD-NQIQQALKAVDPQTK-IHEYLTSKDK | LGKKDIVAGKTGDLTL | KTI-GAH---IITN | NT | USA |
Alignment is based on data from references 47 and 61 and this study. Putative surface-exposed loops of OMP P1 correlating with variable regions I to IV are loops 2, 4, 5, and 8, respectively (13, 14, 47).
Isolates with the same P1 type have OMP P1 amino acid sequences of ≥95% similarity.
Numbers indicate amino acid positions at the beginning and end of a variable region. Dashes, gaps inserted to optimize alignment.
S, H. influenzae serotype. NT, nontypeable.
Loc, country of strain isolation. USA, United States; UK, United Kingdom.
Chromosomal DNA extraction.
Ten milliliters of an overnight culture of H. influenzae was centrifuged at 4,300 × g for 10 min at 4°C, resuspended in 3 ml of 50 mM Tris–20 mM EDTA at pH 8.0, and recentrifuged. The cell pellet was resuspended in 4 ml of 50 mM Tris–2 mM EDTA (pH 8.0) containing 1 mg of lysozyme and incubated for 30 min at 4°C. Addition of 1 mg of proteinase K and 0.3 ml of 10% sodium dodecyl sulfate was followed by incubation of the lysate with agitation for 4 h at 56°C after which 1 ml of 10% lauroylsarcosine was also added. Chromosomal DNA was then purified and isolated by CsCl2 equilibrium density gradient centrifugation (78) as previously described (2–4).
Plasmid DNA extraction.
Plasmid DNA was extracted with an alkaline lysis procedure (65).
DNA sequencing and analysis.
Forty H. influenzae ompP1 DNA sequences were amplified by PCR from purified chromosomal DNA using primers P1-5 (5′-GCTCCTGCTAACTAGTCGTA-3′) and P1-6 (5′-AATCAAAAGCCGTCCGACTG-3′), derived from reported sequences flanking the ompP1 open reading frame (109 bases upstream and 59 bases downstream, respectively) of type b H. influenzae and strain Rd (19, 46, 47). Primers P1-3 (5′-CAGGTGGCGTTTATATTGATTCTAG-3′), P1-4B (5′-TGTGTCATTACAAGATAGAGCCGCT-3′), and P1-7 (5′-CTTCATTAAATTGATACATT-3′) were chosen based on nested sequences of the ompP1 gene and used in combination with P1-5 and P1-6 to sequence the amplified products (see below). Parameters for PCR amplification involved initial denaturation at 94°C for 7 min, followed by 25 cycles of denaturation at 94°C for 30 s, annealing at 45°C for 30 s, and primer extension at 72°C for 2 min, followed by a final primer extension at 72°C for 4 min. PCR products were purified by electrophoresis through a 0.8% Tris-acetate-EDTA low-melting-point agarose gel and extracted with the phenol-chloroform method (65). PCR products were sequenced with an ABI 377 DNA sequencer using 0.25× Big Dye Terminator reaction mixtures.
Primers P1-5 and P1-6 were also used for PCR-based characterization of the putative, insertionally inactivated ompP1 mutant of NTHI strain BCH-3 as described below.
Amino acid sequence alignment was performed with LASERGENE MegAlign software (DNASTAR) using the CLUSTAL multiple-alignment algorithm (30). In addition to the 40 OMP P1 sequences (this report), two reference OMP P1 sequences from strains Rd (19) and MinnA (46) (GenBank accession no. AAC22060 and AAA24990, respectively) were included in the OMP P1 protein alignment.
Nomenclature.
In the genomically sequenced H. influenzae strain Rd (ATCC 51907) (19), the OMP P1-encoding gene (locus identifier, HI0401) was designated fadL for long-chain fatty acid transport protein (GenBank accession no. AAC22060; protein sequence identifier g1573372), based on the similarity of its product to that of E. coli strain K-12 fadL (GenBank accession no. M60607 and Y00552; SwissProt accession no. P10384; protein sequence identifiers 145910 and 41372). In H. influenzae Rd, fadL is transcribed from the plus DNA strand (coordinates 422144 to 423520), spanning 1,377 nucleotides. Its translated product is 459 amino acids (aa) in length with a predicted 49,477.13-Da molecular mass. This protein's cellular role is as a transport binding protein (carbohydrates, organic alcohols, and acids) as described in the H. influenzae genome database (http://www.tigr.org/tdb/CMR/ghi/htmls/SplashPage.html).
Marker-exchange construction of an ompP1 mutant of NTHI strain BCH-3.
Plasmid vector pFRG100 carries the 969-bp PstI/HindIII ompP1 DNA fragment of H. influenzae serotype b strain DL41 (25). PstI/HindIII digestion allowed for isolation and subsequent ligation of ompP1 into pUC18 (77). The resulting construct, pBJG1, was transformed (described below) into E. coli strain JM107 and plated onto Luria-Bertani (LB) broth (65) agar plates containing 50 μg of ampicillin/ml. Transformed cells were grown in 10 ml of LB broth with 50 μg of ampicillin/ml and incubated at 37°C overnight. In order to insertionally inactivate ompP1 of pBJG1, plasmid pBJG1 was extracted and linearized through BglII digestion, disrupting ompP1 640 bp upstream from the PstI site. A 1,264-bp kanamycin resistance cassette (Kmr) was then removed from pUC4K (56, 77) by digestion with BamHI and ligated into the compatible, BglII-linearized pBJG1. The new Kmr construct, pBJG1-A, was transformed into E. coli JM107 cells, plated onto LB broth agar plates containing 65 μg of kanamycin/ml, and incubated overnight at 37°C. In order to carry out the marker exchange (20, 66), Kmr pBJG1-A, a circular plasmid incapable of replication in H. influenzae, was extracted and transformed into Kms (kanamycin-sensitive) NTHI strain BCH-3. Transformed BCH-3 cells were selected by plating onto chocolate agar plates containing 65 μg of kanamycin/ml and incubated overnight in a candle extinction jar at 37°C.
In order to screen for absence of OMP P1, Kmr transformants were picked from the kanamycin-containing chocolate agar plates, suspended in 10 μl of Gey's balanced salt solution (Life Technologies), and dot blotted onto Immobilon P membranes (Millipore). wt BCH-3 that expressed P1 was used as a positive control. Membranes were washed twice in 100 ml of 0.1 M phosphate-buffered saline (PBS) containing 1% (wt/vol) nonfat dry milk powder for 10 min with agitation at room temperature, placed in 50 ml of monoclonal antibody (MAb) AD4 (described below) hybridoma cell supernatant (diluted 1:5 in 0.1 M PBS containing 1% bovine serum albumin plus 0.5 M NaCl), and incubated with agitation for 2 h at 20°C. The membranes were washed again as described above and incubated with agitation in 50 ml of 2-μg/ml anti-mouse immunoglobulin G conjugated to alkaline phosphatase (in 0.1 M PBS containing 1% bovine serum albumin) for 2 h at 20°C. A final wash was carried out as described above. The color reaction was developed for approximately 10 min at room temperature (12) and stopped by rinsing the membranes in 200 ml of double-distilled H2O for 5 min.
Western blot analysis was also carried out to screen for the absence of OMP P1. Outer membrane preparations from wt BCH-1, wt BCH-3, and the putative, insertionally inactivated Kmr ompP1 BCH-3 transformed cells were prepared as previously described (32). MAb AD4 (diluted 1:5 in 0.1 M PBS containing 1% bovine serum albumin plus 0.5 M NaCl) was used to probe the outer membranes to confirm the presence or absence of OMP P1. This was followed by incubation in buffer with 2 μg of staphylococcal protein A/ml conjugated to alkaline phosphatase (Sigma) in 0.1 M PBS with 1% bovine serum albumin. The color reaction was developed as described above.
Direct confirmation of the Kmr inactivated ompP1 construct, described in Results, involved analysis of genomic DNA from both the putative Kmr ompP1 mutant of BCH-3 and the wt BCH-3 strain by PCR amplification of ompP1. PCR products were then probed with an ompP1 fragment isolated from pFRG100 and the Kmr cassette from pUC4K to confirm their identities.
Cloning and purification of recombinant P1.
The gene encoding OMP P1 of BCH-3 (ompP1BCH-3) was amplified by PCR using forward primer P1-1B (5′-CGGGATCCGGCAGCGTTTCAATTGGCG-3′) and reverse primer P1-2B (5′-GGAATTCTTAGAAACTATAATTTAAATTCAAACC-3′). The inclusion of the BamHI and EcoRI sites (underlined) in P1-1B and P1-2B, respectively, was intended to ensure proper orientation within the open reading frame of the IPTG (isopropylthio-β-d-galactoside)-inducible expression vector pTrcHisB (Invitrogen) (17). In order for the OMP P1BCH-3 translation product to be isolated from cytoplasmic inclusion bodies, the primers involved in amplifying ompP1BCH-3 did not include the leader sequence of the gene. pTrcHisB was designed by the manufacturer such that expression from it of an insert such as ompP1BCH-3 generates a translated fusion product with 30 additional amino acids at the N terminus of recombinant P1BCH-3 (rP1BCH-3) that can be cleaved based on an enterokinase recognition sequence (Invitrogen). However, as described in the Results, this was not cleaved because these amino acids contain (i) six tandem histidine residues exploited for subsequent purification purposes, as they bind specifically to Invitrogen nickel-coated Pro-bond resin, and (ii) the epitope AspLeuTyrAspAspAspAspLys, recognized by the Invitrogen anti-Xpress MAb that was exploited for confirmatory purposes with rP1BCH-3.
The PCR mixture and parameters used for amplification of ompP1BCH-3 were similar to those described above for ompP1 sequencing. PCR products were purified as described above. The purified DNA was cloned into pCR2.1 (original TA cloning kit; Invitrogen). The cloned ompP1 DNA was removed from pCR2.1 using BamHI and EcoRI. The ompP1 DNA fragment was then inserted into the multicloning site of the E. coli expression vector pTrcHisB and transformed into E. coli strain BLR to form the new construct pBJG2. Transformed E. coli cells were grown overnight at 37°C on LB broth agar plates with 50 μg of ampicillin/ml. Expression of rP1BCH-3 in a 500-ml culture was induced with 5 ml of 100 mM IPTG. The inclusion bodies that contained rP1BCH-3 were harvested from the cells (65) and purified using Pro-bond resin under denaturing conditions as specified by the manufacturer (Invitrogen). The final sample volume was brought to 1.5 ml using a Centricon-30 concentrator (Amicon), and the protein concentration was estimated using Peterson's modification of the Lowry method (59). As described in the Results, the preparation was analyzed by Western blotting using MAbs AD4 and anti-Xpress (Invitrogen) (55), polyclonal chinchilla serum that contained antibodies to P1BCH-1, and normal chinchilla serum. All antisera were diluted in 0.1 M PBS containing 1% bovine serum albumin plus 0.5 M NaCl.
Development of competence, and transformation of H. influenzae.
Competence was achieved using the method of Barcak et al. (5). One-milliliter competent H. influenzae cell aliquots were added to 1 μg of DNA to be transformed and incubated for 30 min at 37°C. Five milliliters of brain heart infusion (BHI) broth supplemented with NADH and defibrinated horse blood (32) was added to the cells, and the mixture was incubated at 37°C for 90 min. Transformed cells were spread onto chocolate agar plates containing 65 μg of kanamycin/ml and incubated overnight at 37°C in a candle extinction jar.
Development of competence and transformation of E. coli.
Competence was achieved using a modification of the CaCl2 procedure (67). Aliquots (100 μl) of overnight cultures (E. coli strains JM107 and BLR, used for cloning and for P1 expression, respectively) were added to 100 ml of fresh LB broth, and the mixtures were incubated with agitation at 37°C until the E. coli reached mid-exponential phase. Cells were collected by centrifugation at 4,068 × g for 10 min at 4°C. The cell pellets were suspended in 20 ml of an ice-cold PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid] buffer (10 mM PIPES, 6.67% [vol/vol] glycerol, 60 mM CaCl2) and incubated on ice for 30 min. Cells were harvested by centrifugation at 1,912 × g for 5 min at 4°C and gently resuspended in 4 ml of ice-cold PIPES buffer. One microgram of plasmid DNA (pBJG1 or pBJG1-A; described above) was added to 100 μl of competent E. coli JM107 cells, and the mixture was incubated for 20 min on ice. Similarly, 1 μg of pTrcHisB (17) containing ompP1 DNA from NTHI strain BCH-3 (pBJG2) was added to 100 μl of competent E. coli strain BLR. Following the addition of 0.5 ml of LB broth, the cells were incubated at 37°C for 1 h. Aliquots transformed with pBJG1 or pBJG2 were spread onto LB broth agar plates with 50 μg of ampicillin/ml. Similarly, cells transformed with pBJG1-A were spread onto LB broth agar plates that contained 65 μg of kanamycin/ml. All plates were incubated for 18 h at 37°C.
P1-specific MAb AD4.
MAb AD4, specific to OMP P1 of NTHI strain BCH-1, was produced using the method of Tsung et al. (75) as follows. BALB/cJ mice were immunized intraperitoneally once a week for 5 weeks with 12.5 μg of BCH-1 OMPs (24). Four days after the last immunization, the spleen cells were fused with P3-NS1/1-AG4-1 myeloma cells (34), with polyethylene glycol as the fusion agent (21). The fused-cell mixture was distributed into 96-well plates in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and hypoxanthine aminopterine thymidine (40). Supernatants (used at 1:5 dilution) from these actively growing cells were initially subjected to an enzyme-linked immunosorbent assay (ELISA) for antibody activity to outer membranes prepared from wt BCH-1 (24); positive supernatants were further screened by Western blotting using OMPs extracted from BCH-1 (24). One P1-specific MAb, AD4, was selected for additional characterization by demonstrating the binding specificity of AD4 for a P1-enriched preparation (46) and recombinant P1 (see above).
ELISA for BCH-3 P1 and rP1.
Immulon I “U”-bottom microtiter plate (Dynatech Laboratories) wells were coated with 0.1 ml of a 5-mg/ml P1BCH-1-enriched preparation or with rP1BCH-3. The wells were washed and subsequently incubated with 0.1 ml of diluted chinchilla serum, 0.1 ml of a 2-mg/ml solution of staphylococcal protein A conjugated to alkaline phosphatase, and 0.1 ml of a 1-mg/ml alkaline phosphatase substrate (Sigma; 104 phosphatase). We have previously described these methods to measure antibodies against bacterial outer membrane antigens (13, 63).
Recombinant BCH-3 P1 immunization of chinchillas.
rP1BCH-3 was emulsified in RIBI adjuvant R-730 emulsion (RIBI Immunochem Research) to a final rP1BCH-3 concentration of 125 μg/ml. Twenty-eight chinchillas were immunized with two 0.1-ml intramuscular (i.m.) injections in the hindquarters on days 0, 35, 57, 79, 99, 120, and 141. Eight control chinchillas received two 0.1-ml i.m. injections of RIBI adjuvant only on the same days. All chinchillas were bled by cardiac puncture using a 23-gauge 3-ml syringe on days 0 (before receiving the first immunization) and 148 (prior to challenge). Blood specimens were allowed to coagulate for 30 min at room temperature before centrifugation at 2,000 × g for 10 min at 4°C. The sera were used in ELISAs (to detect production of antibodies to rP1BCH-3) and Western blots (to detect antibody specificity to rP1BCH-3).
Transbullar middle-ear challenge and induction of otitis media.
Seven days after the final immunization, four nonimmunized chinchillas were added to the experimental group as controls. All animals were examined by otoscopy and tympanometry (70) to document healthy, normal middle ears.
Loopfuls of wt strain BCH-3 and the isogenic BCH-3 ompP1 mutant were inoculated separately into 1 ml of supplemented brain heart infusion medium and incubated without agitation for 16 to 18 h at 37°C. Overnight cultures were diluted by 2 × 10−3 in Gey's balanced salt solution (Sigma) (32). The chinchillas were divided into two groups. One group was challenged with wt BCH-3, and the other was challenged with the BCH-3 ompP1 mutant by injecting 0.1 ml of the diluted cultures containing 50 to 60 CFU directly into the right middle-ear cavity through the superior bulla with a tuberculin syringe. Chinchillas were reexamined by otoscopy and tympanometry on days 2, 4, 6, 8, 10, 14, and 18. On these days, the middle-ear cavities were also examined through a small (4 mm in diameter) incision in the superior bulla, leaving the tympanic membrane intact. The right middle-ear cavities were cultured by swabbing the cavity with a calcium alginate swab (Calgiswab type 1; Hardwood Products Company LP) and streaking onto chocolate agar plates. The plates were incubated overnight at 37°C in a candle extinction jar. The outcome of challenge in animals that were immunized was compared with that in nonimmunized animals using Fisher's exact test (80).
Nucleotide sequence accession numbers.
Forty ompP1 nucleotide sequences have been deposited in the GenBank database under accession no. AF260337 through AF260376.
RESULTS
Analysis of H. influenzae ompP1.
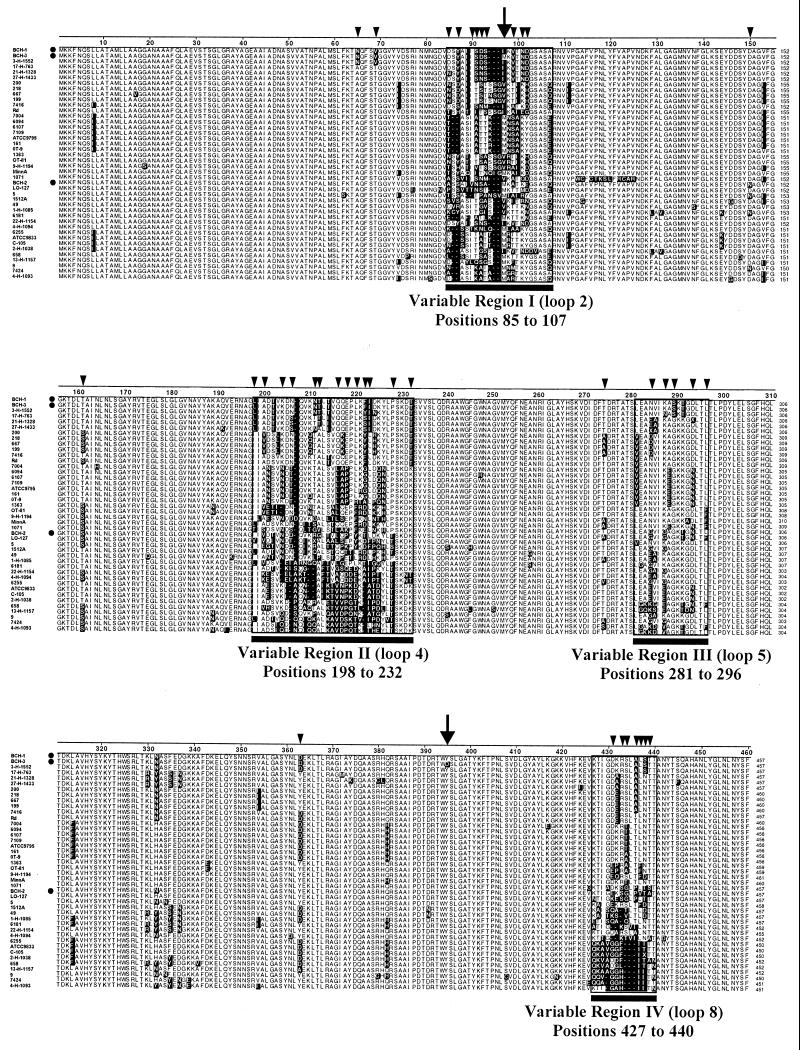
From the natural population structure of H. influenzae depicted in a phylogenically organized collection of >500 H. influenzae and NTHI (13; unpublished data) isolates we selected 42 phylogenically variant typeable H. influenzae and NTHI isolates representing the diversity of this population structure and performed PCR amplification and sequence analysis of ompP1. Alignment of the 42 ompP1 open reading frames (not shown) and deduced amino acid sequences confirmed the presence of three variable regions described by Munson et al. (47) and identified an additional variable region (variable region III, loop 5) (Fig. 1 and Table 1). Each region is located within a putative surface-exposed loop (loops 2, 4, 5, and 8) (13). Regions I (loop 2), II (loop 4), and IV (loop 8) from the deduced P1 sequences vary in residue number, suggesting possible insertions or deletions at the DNA level within encoding genes. A comparison of the percent similarities among P1 sequences identified 23 variants (P1 type) with amino acid sequences of ≤95% similarity (Table 1).
FIG. 1.
Alignment of deduced amino acid sequences of ompP1 alleles carried by the 42 phylogenically variant typeable H. influenzae and NTHI clinical isolates, listed as in Table 1. Dotted rows, isolates BCH-1, BCH-2, and BCH-3 used in the animal challenges; shaded residues, those that differ from the majority consensus sequence (not shown); black bars, variable regions. Numbers to the right of sequences indicate amino acid residue positions. Arrows above residues 97 (variable region I) and 395 (nonvariable region) identify the only two positions at which strains BCH-1 and BCH-3 differ. Smaller arrowheads indicate positions at which phylogenically variant strain BCH-2 differs from BCH-1, BCH-3, or both. Amino acid sequence alignment was performed with the LASERGENE software (DNASTAR), using the CLUSTAL multiple-alignment algorithm (30).
NTHI strains BCH-1, BCH-2, and BCH-3 were included in the analysis and subsequently used to challenge chinchillas immunized with recombinant P1 prepared from BCH-3, i.e., rP1BCH-3. Overall percentages of amino acid identity between P1BCH-3 and P1BCH-1 and between P1BCH-3 and P1BCH-2 are 99.6 and 91%, respectively. Table 1 displays the translated amino acid sequence alignment of the four potentially surface-exposed variable regions for the 42 ompP1 gene sequences. Relevant to the P1 immunogen protection studies described below is the fact that P1BCH-1 and P1BCH-3 differ by only one of the 20 putative surface-exposed amino acid residues located in variable region I, while sequences of P1BCH-2 and P1BCH-3 differ considerably within all four variable regions (Fig. 1). Percentages of identity between individual variable regions in the two sequences are 45 (region I), 38 (region II), 31 (region III), and 50% (region IV).
Construction of an otherwise isogenic ompP1 mutant of NTHI strain BCH-3.
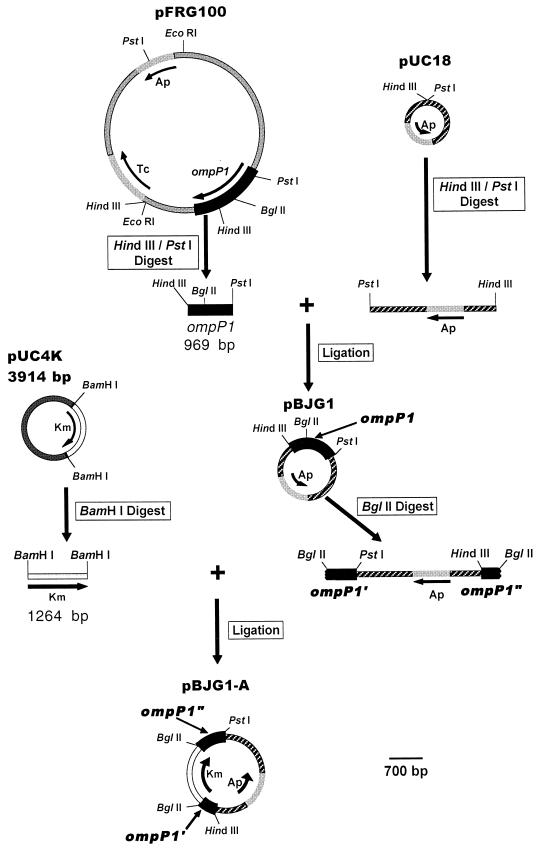
A BCH-3 ompP1-deficient NTHI mutant was constructed to evaluate the specificity of immunization with rP1BCH-3 against experimental otitis media (see Materials and Methods) (Fig. 2). Plasmid pFRG100 (25), carrying a 6.4-kb EcoRI DNA fragment that includes the ompP1 gene of H. influenzae serotype b strain DL41, was digested with HindIII and PstI. The isolated 969-bp PstI/HindIII ompP1 fragment was ligated into pUC18 and subsequently disrupted by insertion of a kanamycin resistance cassette within a unique BglII site. Unable to replicate in H. influenzae, the resultant Kmr suicide vector construct, pBJG1-A, was used to transform NTHI strain BCH-3. This was followed by growth in the presence of kanamycin to select for the desired homologous recombination-based marker-exchange event substituting the Kmr inactivated ompP1 gene in place of the wt chromosomal allele.
FIG. 2.
Construction of pBJG1-A, a suicide plasmid vector carrying a 969-bp fragment of ompP1 inactivated by insertional mutagenesis with a Kmr cassette. All depicted plasmids and constructs are to the scale noted at bottom right. Ap, ampicillin resistance; Tc, tetracycline resistance; Km, kanamycin resistance; ompP1′ and ompP1", ompP1 fragments of the larger 969-bp ompP1 fragment insertionally inactivated with a Kmr cassette at the indicated, unique BglII site. Note that products of two of the plasmid digests shown appear as base pair size markers in Fig. 4A, lanes 1 and 2. This includes (i) the HindIII/PstI digest of pFRG100 yielding five fragments among which is the 969-bp ompP1 fragment and (ii) the BamHI digest of pUC4K yielding the 1,264-bp Kmr cassette as well as the 2,650-bp plasmid backbone.
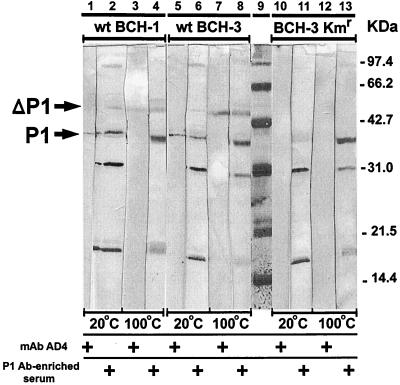
To screen for absence of OMP P1, 46 Kmr BCH-3 transformants were assayed by dot blot analysis using P1 MAb AD4 as a probe. One transformant that failed to bind to AD4 was further characterized by Western blotting (Fig. 3). Outer membrane preparations from NTHI strains wt BCH-1 and wt BCH-3 and the dot blot-negative BCH-3 Kmr transformant were electrophoresed and probed with MAb AD4 and also with serum from a chinchilla immunized with a native P1BCH-1-enriched preparation. Western blots detected both the heat-modified (ΔP1) and nonmodified forms of P1 (76) in the outer membranes prepared from the P1+ strains wt BCH-1 and wt BCH-3 (lanes 1 to 8). In contrast, P1 was not detected by the AD4 MAb (lanes 10 and 12) or by the immune chinchilla serum (lanes 11 and 13) in the outer membrane preparations from the Kmr BCH-3 transformant. Immune chinchilla serum did, however, detect other H. influenzae OMPs (lanes 11 and 13) with intensities similar to those found for wt BCH-1 and wt BCH-3 (lanes 2, 4, 6, and 8) when these outer membrane preparations were used.
FIG. 3.
Western blot analysis of outer membrane preparations from the ompP1+ strains wt BCH-1 (lanes 1 to 4) and wt BCH-3 (lanes 5 to 8), and the putative mutant ompP1 BCH-3 Kmr transformant (lanes 10 to 13). AD4 MAb and serum from a chinchilla immunized with a P1BCH-1-enriched preparation were used to probe unheated (20°C) and heated (100°C) outer membrane preparations. P1 and ΔP1, non-heat-modified and heat-modified forms of OMP P1, respectively (25). Molecular mass standards appear in lane 9.
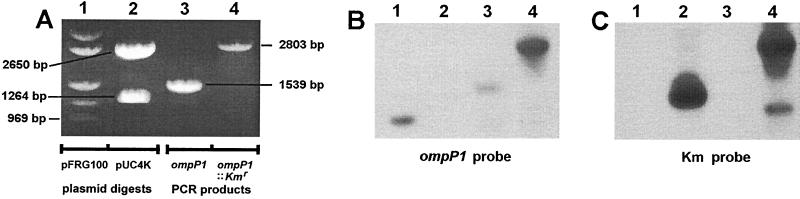
Confirmation of the insertionally inactivated ompP1BCH-3 gene was accomplished by PCR amplification of ompP1 carried by the Kmr BCH-3 transformant. This yielded a DNA fragment that was 1,264 bp larger than the wt allele of strain BCH-3, precisely the size predicted for the Kmr gene cassette insertion (Fig. 4A, lanes 4 and 3, respectively). Southern blot hybridization analysis of this PCR product confirmed that the increase in size was due to the insertion of the Kmr cassette (Fig. 4B, lanes 3 and 4, and Fig. 4C, lanes 3 and 4). These results also indicate that a double-crossover event occurred to generate the insertionally inactivated ompP1BCH-3 mutant by simple allelic exchange rather than cointegration of pBJG1-A.
FIG. 4.
Characterization of the putative P1-deficient isogenic mutant of NTHI BCH-3 by Southern blot analysis. (A) Agarose gel electrophoretic separation of digests and PCR products, stained with ethidium bromide. Lane 1, HindIII/PstI double digest of the ompP1-carrying vector plasmid pFRG100; the bottom band is the predicted 969-bp ompP1 fragment (see Fig. 2); lane 2, BamHI digest of pUC4K; the bottom band is the predicted 1,264-bp Kmr cassette, and the upper band is the predicted 2,650-bp backbone of pUC4K (see Fig. 2); lane 3, PCR amplification of the ompP1 gene (1,371 bp) plus flanks (109 bp upstream and 59 bp downstream) from wt BCH-3 (13; unpublished data) using flanking primers P1-5 and P1-6 (see Materials and Methods) to generate a 1,539-bp product; lane 4, PCR amplification of ompP1 from the putative, insertionally inactivated ompP1 BCH-3 Kmr transformant using flanking primers P1-5 and P1-6 (Materials and Methods); when this product is compared with the ompP1 PCR product in the adjacent lane 3, the size increase is found to correlate with that predicted for insertion of the 1,264-bp Kmr cassette as indicated in Fig. 2. (B) Southern blot transfer of the panel A gel electrophoretic separation to a Zeta Probe nylon membrane (Bio-Rad), followed by hybridization with a 32P-labeled (2) ompP1 969-bp fragment probe (the bottom HindIII/PstI fragment shown in panel A, lane 1, as isolated from an equivalent digest and electrophoretic separation). (C) Duplicate electrophoretic separation of the identical digests and PCR products in panel A, transferred by Southern blotting to a Zeta Probe nylon membrane and hybridized with a 32P-labeled (2) Kmr cassette probe (the smaller, 1,264-bp BamHI fragment shown in panel A, lane 2, as isolated from an equivalent digest and electrophoretic separation). The lower band in lane 4 of panel C is likely a PCR breakdown product.
As described below, this Kmr ompP1 mutant of BCH-3 was used to evaluate the specificity of protection against otitis media following immunization with rP1BCH-3.
Expression and purification of rP1BCH-3.
As described in detail in Materials and Methods, the complete ompP1 DNA sequence of BCH-3, minus its leader sequence, was amplified and inserted into TA cloning vector pCR2.1 (Invitrogen). It was then subcloned into the inducible E. coli expression vector pTrcHisB and transformed into E. coli strain BLR to generate the new construct pBJG2. The induced rP1BCH-3 protein formed insoluble cytoplasmic inclusion bodies that were harvested, purified, and concentrated.
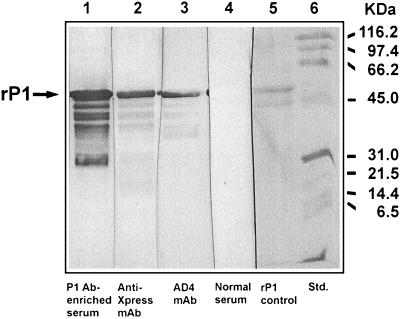
Purified rP1BCH-3 was analyzed by Western blotting with serum antibodies obtained from a chinchilla immunized with a native P1BCH-1-enriched preparation. AD4 MAb and Invitrogen anti-Xpress MAbs (55) were also used as probes (Fig. 5). The anti-Xpress MAb is specific for the epitope AspLeuTyrAspAspAspAspLys, which had been fused to the N-terminal end of rP1BCH-3 expressed from pBJG2 (lane 2). The AD4 MAb, specific to P1, recognized the major rP1 band with the appropriate molecular mass (lane 3). This MAb also bound to lower-molecular-mass bands thought to be breakdown products of rP1. The immune chinchilla serum, included in the analysis as a source of polyclonal antibodies, also recognized the major band and lower-molecular-mass bands (lane 1), similar to those recognized by the two MAbs.
FIG. 5.
Western blot analysis of recombinant H. influenzae OMP P1BCH-3 (rP1) purified from cytoplasmic inclusion bodies of an E. coli cloning host. The rP1 was probed with serum from a chinchilla immunized with a P1BCH-1-enriched preparation (lane 1), the Invitrogen anti-Xpress MAb (lane 2), the AD4 MAb (lane 3), and normal preimmune chinchilla serum (lane 4). Coomassie blue-stained rP1 and molecular mass standards are shown in lane 5 and lane 6, respectively. As described in Materials and Methods, the rP1 generated from expression vector pTrcHisB is a translated fusion product with 30 additional amino acids at the peptide N terminus containing the epitope AspLeuTyrAspAspAspAspLys recognized by the Invitrogen anti-Xpress MAb (lane 2).
Serum antibody response to rP1BCH-3.
Twenty-eight chinchillas received seven doses of 25 μg of BCH-3 rP1 (i.m.) in combination with RIBI adjuvant on days 0, 35, 57, 79, 99, 120, and 141. Simultaneously, eight chinchillas were immunized with RIBI adjuvant only, serving as controls. Preimmune (collected on day 0) and postimmune (collected prior to BCH-3 challenge on day 148) sera were used to measure individual antibody responses to rP1BCH-3 by ELISA. Mean values for the change in optical density per minute ± standard errors are shown in Table 2 for sera diluted 1:1,000.
TABLE 2.
Serum antibody levels against rP1BCH-3
| Immunogen | na | Mean antibody level (ΔOD/min) ± SE inb:
|
|
|---|---|---|---|
| Preimmune sera | Postimmune sera | ||
| rP1/RIBI | 28 | 0.002 ± 0.001 | 0.281 ± 0.053 |
| RIBI | 8 | 0.001 ± 0.001 | 0.002 ± 0.001 |
n, number of animals immunized.
Antibody levels against rP1BCH-3 at a 1:1,000 serum dilution. OD, optical density.
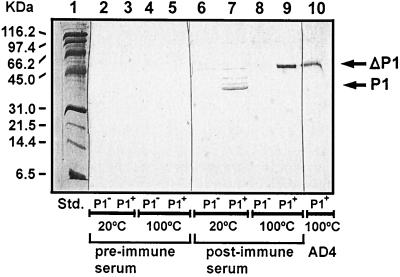
The same pre- and postimmune sera were used for Western blots to analyze outer membrane preparations from wt BCH-3 and the ompP1BCH-3 mutant (Fig. 6). Antibodies present in the postimmune serum reacted only with the normal and heat-modified (Δ) forms of wt P1BCH-3 (lanes 7 and 9, respectively). All animals lacked preimmune antibodies to P1 (lanes 2 to 5), indicating that the immune response to rP1 was highly specific. Specificity was confirmed in lanes 6 and 8, where postimmune antibodies did not bind to the outer membrane preparations from the ompP1BCH-3 mutant.
FIG. 6.
Western blot analysis of outer membrane preparations with rP1BCH-3 immune serum. Outer membrane preparations from NTHI wt BCH-3 (P1+) and the otherwise isogenic BCH-3 ompP1 mutant (P1−) were probed with preimmune chinchilla serum (lanes 2 to 5) and post-rP1BCH-3-immune chinchilla serum (lanes 6 to 9). The wt BCH-3 outer membrane was also probed with the P1-specific AD4 MAb as a control (lane 10). Lane 1, molecular mass standards; P1 and ΔP1, non-heat-modified and heat-modified forms of OMP P1, respectively (25).
Transbullar challenge with NTHI strain wt BCH-3 and the BCH-3 ompP1 isogenic mutant.
Seven days after the final immunization (day 148), 36 chinchillas immunized with either rP1-RIBI or with RIBI alone and 4 nonimmunized animals were challenged with an inoculum containing 50 to 60 CFU of wt BCH-3 or the BCH-3 ompP1 isogenic mutant by direct inoculation into the right middle-ear cavity. Each animal was evaluated for the development of otitis media by otoscopy, tympanometry, and middle-ear cultures on days 2, 4, 6, 8, 10, 14, and 18 postchallenge. The numbers and percentages of animals with culture-positive middle ears versus the total number of animals challenged are shown in Table 3. The peak number of culture-positive animals previously immunized with rP1BCH-3 and homologously challenged with wt BCH-3 occurred on day 4 (8 out of 18 animals infected). By day 6, the number of animals infected had been reduced to 7 of 18 (39%), and by day 18 only 2 of 16 (12%) animals had culture-positive middle-ear disease. The difference between the proportion of rP1BCH-3-immunized animals with culture-positive disease and that of animals immunized solely with RIBI adjuvant was significant (P < 0.05) at all time points except on day 4 when 8 of 18 animals were infected (P = 0.067). All animals challenged with the BCH-3 ompP1 isogenic mutant as well as all unimmunized animals (with the exception of one animal immunized with adjuvant alone that never developed infection) were culture positive by day 2. By day 18, four of six rP1-immunized animals and two of three animals given RIBI adjuvant alone remained culture positive for the BCH-3 ompP1 mutant challenge strain.
TABLE 3.
Transbullar homologous challenge with wt NTHI strain BCH-3 and an otherwise isogenic BCH-3 ompP1 mutant
| Challenge strain | Immunogen | No. of animals with culture-positive middle-ear fluid/no. challenged (%), Pa, on day:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 14 | 18 | ||
| wt BCH-3 | rP1BCH-3 + adjuvant | 1/20 (5) | 8/18 (44) | 7/18 (39) | 5/16 (31) | 5/16 (31) | 2/16 (12) | 2/16 (12) |
| Adjuvant alone | 4/4 (100), 0.001 | 4/4 (100), 0.067 | 4/4 (100), 0.045 | 4/4 (100), 0.026 | 4/4 (100), 0.026 | 3/4 (75), 0.032 | 2/2 (100), 0.039 | |
| Nonimmunized | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) | |
| BCH-3 ompP1 mutant | rP1BCH-3 + adjuvant | 8/8 (100) | 8/8 (100) | 8/8 (100) | 8/8 (100) | 8/8 (100) | 6/7 (86) | 4/6 (67) |
| Adjuvant alone | 3/4 (75), 0.333 | 3/4 (75), 0.333 | 3/4 (75), 0.333 | 3/4 (75), 0.333 | 2/3 (67), 0.273 | 2/3 (67), 0.533 | 2/3 (67), 0.774 | |
| Nonimmunized | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 1/2 (50) | |
P values are based on a comparison of data for chinchillas immunized with rP1 plus adjuvant with those for chinchillas immunized with adjuvant alone, as determined by the Fisher exact test (80). Note that the incidence of culture-positive effusions from chinchillas immunized with rP1 plus RIBI adjuvant and challenged with wt BCH-3 was statistically significant. The incidence of culture-positive effusions from the contralateral unchallenged ears (bilateral effusion) was 5% in the group challenged with wt BCH-3 and 62% in the group challenged with the isogenic BCH-3 ompP1 mutant on days 6 and 8 (P = 0.0045).
In addition to the finding that a reduced number of animals developed culture-positive acute otitis media, it was found that rP1BCH-3-immunized animals experienced delayed onset of infection and milder disease due to wt BCH-3, as evidenced by tympanometry, otoscopy, and direct examination of the middle-ear cavity. All immunized animals had normal (type A) tympanograms (22) on day 2 after challenge with wt BCH-3, and 16 (80%) had normal otoscopic examinations. As the course of disease progressed, their tympanograms resembled that of either normal pressure with reduced compliance or negative pressure (type C) but were never flat (type B), indicative of unmodified otitis media in this model. Three of eight (37.5%) animals immunized with rP1BCH-3 and challenged with the BCH-3 ompP1 mutant had normal tympanograms on day 2, but all eight had abnormal otoscopic examinations. The tympanograms observed in these animals became flat (type B) as disease progressed. Experimental otitis media in the chinchilla due to NTHI initially develops with a translucent serous middle-ear effusion, which is followed by a turbid, low-viscosity, white fluid (16). In the partially protected rP1-immunized animals, middle-ear fluid remained serous throughout the course. Both the control unimmunized animals and those challenged with the BCH-3 ompP1 mutant strain developed turbid, purulent middle-ear fluid typical of first infection in pristine animals. The incidence of culture-positive effusions from the contralateral unchallenged ears (bilateral effusion) was 5% (1 of 18 animals) in the group challenged with wt BCH-3 and 62% (5 of 8 animals) in the group challenged with the otherwise isogenic BCH-3 ompP1 mutant on days 6 and 8 (P = 0.0045). These observations indicate that immunization with rP1BCH-3 modifies both the proportion and duration of culture-positive disease as well as the otomicroscopic and tympanometric indicators of inflammation and the likelihood of developing infection in the contralateral (unchallenged) middle ears.
Transbullar heterologous challenge with NTHI strains BCH-1 and BCH-2.
Ten of 20 chinchillas immunized with rP1BCH-3 that had not developed culture-positive otitis media by day 14 were rechallenged (in the previously unchallenged left middle ear) with either wt BCH-1 or wt BCH-2 expressing variant ompP1 alleles, as described above. All animals were evaluated for the development of otitis media using otoscopy, tympanometry, and middle-ear cultures on days 2, 4, and 6.
Four of the five animals rechallenged with BCH-1 had negative middle-ear cultures on day 2 (Table 4), while all five animals rechallenged with BCH-2 became culture positive. Although all the animals had been completely protected against initial BCH-3 challenge, only one animal rechallenged with BCH-1 remained culture negative through day 6. However, all of the animals challenged with BCH-1 developed clear, serous middle-ear fluid and had tympanograms of normal pressure and reduced compliance (type As) (22), suggesting modified disease. In contrast, animals challenged with BCH-2 demonstrated middle-ear effusions typical of unmodified, natural infection in pristine animals. Although these results are limited by the small number of animals and are not statistically significant, the observations nevertheless suggest that immunization with rP1BCH-3 elicits a partially protective immune response against NTHI isolates with nearly identical (99.6%) amino acid sequences, such as BCH-1, but not against isolates with variability as great as that seen with BCH-2 (91%).
TABLE 4.
Transbullar heterologous OMP P1 challenge with NTHI wt strains BCH-1 and BCH-2
| Challenge strain | No. of culture-positive animals/ no. challenged (P) on day:
|
||
|---|---|---|---|
| 2 | 4 | 6 | |
| BCH-1 | 1/5 | 3/5 | 4/5 |
| BCH-2 | 5/5 (0.024) | 5/5 (0.222) | 5/5 |
DISCUSSION
Our study provides conclusive evidence that immunization with rP1BCH-3 elicits protection against mucosal surface infection with NTHI in a homologous-challenge experimental model. Specificity of the protection was demonstrated by (i) the use of an otherwise isogenic ompP1 mutant capable of producing unmodified disease in animals immunized with rP1 from the wild-type strain and (ii) heterologous challenge with two NTHI strains, one with a single amino acid change in variable region I of OMP P1 and a second that has significant amino acid changes in four of the surface-exposed regions of OMP P1 (Fig. 1). These strains produced modified and full disease, respectively, in immunized chinchillas. When considered together, the findings involving the specificity of the rP1 immune response, the variability of P1 epitopes, and the virulence of the ompP1 mutant that we constructed have fundamental implications in the development of OMP P1 as a vaccine candidate against NTHI.
Diseases associated with NTHI are primarily mucosal infections of the respiratory tract. They occur most commonly as otitis media in young children and infectious complications in adults with chronic pulmonary disease; both are characterized by frequent recurrences. Disease in infants typically begins after maternal antibodies have dissipated and before their own immune systems are fully matured (42). While the conjugate H. influenzae serotype b vaccines that have evolved from the prototype developed in the 1980s by Anderson (1; P. Anderson, Letter, J. Infect. Dis. 149:1034–1035, 1984) and Claesson et al. (15) are efficacious, unencapsulated NTHI remains responsible for ∼30% of the >45 million episodes of acute bacterial otitis media yearly in North America and Europe (43, 45, 58, 68) and is implicated in as many as ∼20% of the 4 million fatal acute respiratory infections per annum worldwide occurring mostly in children (9). Studies of these infections demonstrate that NTHI usually elicits bactericidal antibodies (71, 72). However, protection appears to be strain specific, with subsequent infections due to antigenically variant, unrelated strains (11).
A number of studies have suggested that OMPs of H. influenzae might be efficacious vaccine candidates. Increases in specific antibodies to NTHI in sera and middle-ear fluids from children recovering from otitis media have been reported (71, 72). These results are paralleled by our study of an adult patient indicating that the majority of serum bactericidal antibody activity after recovery from NTHI disease was directed to OMPs (24). Furthermore, passive immunoprophylaxis in the chinchilla model has provided evidence that humoral antibodies are sufficient to prevent experimental otitis media due to NTHI. Protection in these studies was a result of antibodies directed mainly to OMPs and less so to lipooligosaccharide antigens (6, 32).
Several reports have characterized aspects of OMP P1 as an experimental vaccine candidate. Polyclonal antibodies and a MAb specific for P1, when used for passive immunization, protected against H. influenzae serotype b bacteremia in the infant rat model (25, 29, 48). Active immunization with P1 was also found to induce partial protection against bacteremia in this model (41). We took a different approach to the question of P1 as an immunogen by analyzing the variability of P1-encoding genes (Table 1 and Fig. 1) found among a phylogenically diverse collection of typeable H. influenzae and NTHI isolates (13; unpublished data). We also examined the capacity of OMP P1 to prevent experimental otitis media due to NTHI in chinchillas actively immunized with a recombinant form of P1 (rP1).
Our preliminary studies with OMP P1 had shown that a P1BCH-1-enriched protein preparation from NTHI protected against experimental disease following homologous challenge but that the antibody response lacked specificity (data not shown). To overcome this problem and clarify the results, we subsequently cloned ompP1 from the clinical NTHI strain BCH-3 and expressed P1 in the cytoplasm of E. coli, thus avoiding contamination with other H. influenzae OMPs. As summarized in Tables 2 and 3, this rP1BCH-3 preparation induced antibodies in all actively immunized chinchillas and protected them against otitis media due to the wt NTHI isolate BCH-3. Fifty-six percent (10 of 18) of the chinchillas immunized with rP1BCH-3 demonstrated complete protection against disease. The remaining 44% (8 of 18) demonstrated modified disease, expressed as delayed onset and milder infection defined by standardized tympanometric criteria (22) to supplement microbiological evidence of infection. The absence of protection in rP1BCH-3-immunized animals challenged with an otherwise isogenic BCH-3 ompP1 mutant confirmed the specificity of the protective response (Table 3).
The isogenic BCH-3 ompP1 mutant produced disease in the P1BCH-3 immune chinchilla model similar to that produced by wt BCH-3 in nonimmunized animals (Table 3). Although the pathogenicity of an H. influenzae serotype b ompP1 mutant in the infant rat model for invasive disease had been described (29), the virulence of an ompP1 mutant in a chinchilla model for otitis media mucosal infection has not been reported previously. The viability and unimpeded virulence of these ompP1 mutants should elicit concern regarding the use of P1 as an immunogen because of the possibility for inadvertent selection of virulent ompP1 escape mutants. Evaluation of BCH-3 isolates recovered from the animals immunized with rP1 that demonstrated modified disease (delayed onset and less purulent effusion) will provide initial insight as to whether selection for P1 variants accounts for the dichotomous results (complete protection versus modified disease). An example of such modified disease was found in the case of immunization with rP1BCH-3 followed by experimental challenge and infection with NTHI BCH-1, a strain differing from BCH-3 by only 1 aa in immunogenic loop 2 (aa 97) and 1 aa in an otherwise conserved region (aa 395) of OMP P1 (Fig. 1). In contrast, while 8 of 18 animals immunized with rP1BCH-3 and infected with BCH-3 eventually displayed such modified disease, none showed full disease similar to that found following challenge with the isogenic BCH-3 ompP1 mutant with an outer membrane devoid of OMP P1.
The 10 animals that were fully protected from disease due to wt NTHI strain BCH-3 were rechallenged in the opposite middle ear cavity with either wt NTHI strain BCH-1 or BCH-2 to determine the effect of variable ompP1 alleles and consequently P1 antigen expression (Table 4). P1BCH-1 and P1BCH-2 display 99.6 and 91% amino acid identity to P1BCH-3, respectively (Table 1 and Fig. 1). P1s of BCH-1 and BCH-3 differ by only 1 of the 20 putative surface-exposed amino acid residues located within variable region I, suggesting that cross-protection against BCH-1 might occur in chinchillas immunized with rP1BCH-3. In contrast, the four identified variable regions of P1BCH-2 differ considerably from those of P1BCH-3, with the percent identities between their individual variable regions being 45 (region I), 38 (region II), 31 (region III), and 50% (region IV). BCH-2 was included in the rechallenge to determine if this considerable degree of variation circumvented protection induced by rP1BCH-3 and also to ensure that a vaccine-independent positive control was present in the rechallenge experiments. While infection with BCH-1 resulted in a mild disease with delayed onset, BCH-2 caused infection and disease typical of first infection in pristine animals. It is possible that BCH-3 challenge may have stimulated immunity in these animals even though no infection was produced. However, we have previously shown that heterologous antibodies against BCH-2 OMPs were not raised in two-thirds (four of six) of the animals challenged and infected with BCH-1 (32). Therefore we have reason to believe that cross-reactive antibodies were not likely to have been raised against BCH-1 and BCH-2 when BCH-3 was used as a challenge, particularly because an infection with BCH-3 did not ensue. Also, in unpublished studies we have found that no cross-protection between BCH-1 and BCH-2 exists when animals initially infected with one strain are challenged with the other. These results suggest that immunity generated by rP1BCH-3 is not broadly protective. This indicates the necessity for studies that further define protective epitopes within the four variable regions and that identify functional aspects of OMP P1 and the anti-P1 immune response. Likewise, the development of an extensive NTHI ompP1 allele-specific typing system applied to a phylogenically diverse population of strains would be necessary for a more complete understanding of the potential of OMP P1 as a vaccine candidate.
A high degree of conservation throughout the natural population structure of the pathogenic species is a critical property of any vaccine candidate. Previous phenotypic studies have shown that H. influenzae serotype b P1 varies both antigenically and in molecular weight, with size differences having been exploited as a rudimentary subtyping scheme for H. influenzae serotype b isolates (8). Our DNA sequence-based analysis directly examined the precise degree of conservation of ompP1 among 42 phylogenically variant H. influenzae isolates (13). The calculated percent identities for the DNA sequences and their corresponding amino acid sequences range from 87.8 to 100% and from 82.5 to 100% (Fig. 1), respectively. We identified 23 P1 variants by grouping the amino acid sequences based on their percentages of similarity, using 95% similarity as the cutoff point (Table 1). Additional ompP1 sequences from our phylogenically organized H. influenzae collection will be needed to test whether a limited number of such variants might provide a reasonable degree of protection when considered as possible polyvalent immunogens.
The study described in this report involved a multifaceted approach to vaccine development. This included a broad phylogenic perspective integrated with DNA sequencing, cloning, mutational inactivation, and animal modeling. Cloning and expression of the immunogen allowed for its isolation in the absence of all other antigens of the pathogenic species and its subsequent use in studies that tested an OMP as a protective immunogen. The otherwise isogenic mutant strain allowed for several insights regarding the immunogen of interest, providing the means to directly test its specificity while also revealing whether its encoding gene is necessary for cell viability and virulence. The phylogeny-based DNA-sequencing survey proved equally revealing, making possible unique heterologous challenge studies involving isolates with known minor and major differences in surface-exposed amino acid residues of the native antigen. This provided the means to test the range of specificity of the isolated immunogen. Furthermore, identifying a range of immunogen-encoding alleles with significant variability unambiguously demonstrates that phylogenic screening of the immunogen-encoding gene for any vaccine candidate is a rational prerequisite, particularly for OMPs that are subject to diversifying antigenic selection. This novel strategy based on microbial population structure represents the means to determine a priori a critical aspect of the likely efficacy of any immunogen candidate, thus saving time and expense involved with animal testing until the decisive degree of immunogen conservation has been unambiguously established. As such, we refer to this previously untested approach as the phylogenic strategy for vaccine development.
Based on this rational new approach we have constructed a relatively large-scale model of the natural population structure of typeable H. influenzae and NTHI involving more than 500 phylogenically characterized isolates from different patient populations and geographical locations, isolated over a 30-year span (13; unpublished data). This is currently being used to carry out an initial screen of potential immunogen vaccine candidates for the essential nature of their encoding genes and for their natural conservation. Those immunogens encoded by genes demonstrated to be essential for both viability and virulence, while also exhibiting a relatively high degree of conservation across the natural population structure, are then to be used for animal testing thus eliminating a major factor, i.e., antigenic variability, that has typically confounded vaccine development.
ACKNOWLEDGMENTS
We acknowledge the following colleagues for generously providing typeable H. influenzae and NTHI clinical isolates, laboratory strains, and/or constructs: P. Anderson (Pediatric Infectious Disease Unit, University of Rochester Medical Center), S. Barenkamp (Pediatric Research Institute, Children's Hospital, St. Louis), M. Bergeron (Laboratoire et Service d'Infectiologie, Centre Hôspitalier de L'Université Laval), G. Doern (Microbiology Department, University of Massachusetts Medical Center), J. Eskola (and members of the Finnish Otitis Media Study Group, National Public Health Institute, Finland), E. J. Hansen (Microbiology Department, University of Texas Southwestern Medical Center), E. R. Moxon, D. Hood, D. Crook, and M. Deadman (Department of Paediatrics, The John Radcliffe Hospital and Institute for Molecular Medicine, and The National H. influenzae Reference Laboratory, Oxford University) and A. Thomson (Department of Paediatrics, and CF Centre, The John Radcliffe Hospital, Oxford University). K. Tsung of Boston University Medical Center is acknowledged for generating the AD4 MAb. We are grateful to Richard Moxon, Derek Hood, Alison Holmes, Floyd Dewhirst, Suzanne Steinbach, Anne Thomson, and Heather Huot for discussion of this work, to R. Beall, A. Chobanian, and J. Klein for encouragement to initiate these studies, and D. Gilbert, K. Ward, and T. Loring of Boston Medical Center and Dorian Watson of The John Radcliffe Hospital (Oxford) Paediatrics Department for support.
The described studies were funded in part by research grant awards to R.G. from the NIH (DK-50838), the CF Foundation, and The Trustees of Health and Hospitals of the City of Boston (Biomedical Support Program) and by a prior NIH research grant jointly to S.I.P. and P.A.R. (NS 21914).
REFERENCES
- 1.Anderson P. Antibody responses to Haemophilus influenzae type b in diphtheria toxin by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM 197. Infect Immun. 1983;39:233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur M, Arbeit R, Kim C, Beltran P, Crowe H, Steinbach S, Campanelli C, Wilson R, Selander R, Goldstein R. Restriction fragment length polymorphisms among uropathogenic Escherichia coli isolates: pap-related sequences compared with rrn operons. Infect Immun. 1990;58:471–479. doi: 10.1128/iai.58.2.471-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur M, Campanelli C, Arbeit R, Kim C, Steinbach S, Johnson C, Rubin R, Goldstein R. Structure and copy number of gene clusters related to the pap P-adhesin operon of uropathogenic Escherichia coli. Infect Immun. 1989;57:314–321. doi: 10.1128/iai.57.2.314-321.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur M, Johnson C, Rubin R, Arbeit R, Campanelli C, Kim C, Steinbach S, Agarwal M, Wilkinson R, Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989;57:303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barcak G J, Chandler M S, Redfield R J, Tomb J F. Genetic systems in Haemophilus influenzae. Methods Enzymol. 1991;204:321–342. doi: 10.1016/0076-6879(91)04016-h. [DOI] [PubMed] [Google Scholar]
- 6.Barenkamp S J. Protection by serum antibodies in experimental nontypeable Haemophilus influenzae otitis media. Infect Immun. 1986;52:572–578. doi: 10.1128/iai.52.2.572-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barenkamp S J, Granoff D M, Munson R S., Jr Outer-membrane protein subtypes of Haemophilus influenzae type b and spread of disease in day-care centers. J Infect Dis. 1981;144:210–217. doi: 10.1093/infdis/144.3.210. [DOI] [PubMed] [Google Scholar]
- 8.Barenkamp S J, Munson R S, Jr, Granoff D M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981;143:668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- 9.Barker J, Gratten M, Riley I, Lehmann D, Montgomery J, Kajoi M, Gratten H, Smith D, Marshall T F, Alpers M P. Pneumonia in children in the eastern highlands of Papua New Guinea: a bacteriologic study of patients selected by standard clinical criteria. J Infect Dis. 1989;159:348–352. doi: 10.1093/infdis/159.2.348. [DOI] [PubMed] [Google Scholar]
- 10.Bell J, Grass S, Jeanteur D, Munson R S., Jr Diversity of the P2 protein among nontypeable Haemophilus influenzae isolates. Infect Immun. 1994;62:2639–2643. doi: 10.1128/iai.62.6.2639-2643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein J M, Faden H S, Loos B G, Murphy T F, Ogra P L. Recurrent otitis media with non-typeable Haemophilus influenzae: the role of serum bactericidal antibody. Int J Pediatr Otorhinolaryngol. 1992;23:1–13. doi: 10.1016/0165-5876(92)90074-y. [DOI] [PubMed] [Google Scholar]
- 12.Blake M S, Johnston K H, Russell-Jones G J, Gotschlich E C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 13.Bolduc G. Combining phylogeny and selective DNA sequencing to examine a vaccine candidate. Ph.D. thesis. Boston, Mass: Boston University School of Medicine; 1999. [Google Scholar]
- 14.Chong P, Yang Y P, Persaud D, Haer M, Tripet B, Tam E, Sia C, Klein M. Immunogenicity of synthetic peptides of Haemophilus influenzae type b outer membrane protein P1. Infect Immun. 1995;63:3751–3758. doi: 10.1128/iai.63.10.3751-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claesson B A, Schneerson R, Lagergard T, Trollfors B, Taranger J, Johansson J, Bryla D, Robbins J B. Persistence of serum antibodies elicited by Haemophilus influenzae type b-tetanus toxoid conjugate vaccine in infants vaccinated at 3, 5 and 12 months of age. Pediatr Infect Dis J. 1991;10:560–564. doi: 10.1097/00006454-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 16.DeMaria T F. Animal models for nontypeable Haemophilus influenzae otitis media. Pediatr Infect Dis J. 1989;8(Suppl.):S40–S42. doi: 10.1097/00006454-198901001-00015. [DOI] [PubMed] [Google Scholar]
- 17.Domiati-Saad R, Attrep J F, Brezinschek H P, Cherrie A H, Karp D R, Lipsky P E. Staphylococcal enterotoxin D functions as a human B cell superantigen by rescuing VH4-expressing B cells from apoptosis. J Immunol. 1996;156:3608–3620. [PubMed] [Google Scholar]
- 18.El-Adhami W, Kyd J M, Bastin D A, Cripps A W. Characterization of the gene encoding a 26-kilodalton protein (OMP26) from nontypeable Haemophilus influenzae and immune responses to the recombinant protein. Infect Immun. 1999;67:1935–1942. doi: 10.1128/iai.67.4.1935-1942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 20.Franklin M, Ohman D. Identification of algF in the alginate biosynthetic gene cluster of Pseudomonas aeruginosa which is required for alginate acetylation. J Bacteriol. 1993;175:5057–5065. doi: 10.1128/jb.175.16.5057-5065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galfre G, Howe S C, Milstein C, Butcher G W, Howard J C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977;266:550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- 22.Giebink G S, Heller K A, Harford E R. Tympanometric configurations and middle ear findings in experimental otitis media. Ann Otol Rhinol Laryngol. 1982;91:20–24. doi: 10.1177/000348948209100106. [DOI] [PubMed] [Google Scholar]
- 23.Gilsdorf J R, McCrea K W, Marrs C F. Role of pili in Haemophilus influenzae adherence and colonization. Infect Immun. 1997;65:2997–3002. doi: 10.1128/iai.65.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnehm H E, Pelton S I, Gulati S, Rice P A. Characterization of antigens from nontypeable Haemophilus influenzae recognized by human bactericidal antibodies. Role of Haemophilus outer membrane proteins. J Clin Investig. 1985;75:1645–1658. doi: 10.1172/JCI111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzales F R, Leachman S, Norgard M V, Radolf J D, McCracken G H, Jr, Evans C, Hansen E J. Cloning and expression in Escherichia coli of the gene encoding the heat-modifiable major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1987;55:2993–3000. doi: 10.1128/iai.55.12.2993-3000.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green B A, Farley J E, Quinn-Dey T, Deich R A, Zlotnick G W. The e (P4) outer membrane protein of Haemophilus influenzae: biologic activity of anti-e serum and cloning and sequencing of the structural gene. Infect Immun. 1991;59:3191–3198. doi: 10.1128/iai.59.9.3191-3198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg D P, Stutman H R. Cystic fibrosis. Infection and immunity to Staphylococcus aureus and Haemophilus influenzae. Clin Rev Allergy. 1991;9:75–86. doi: 10.1007/978-1-4612-0475-6_5. [DOI] [PubMed] [Google Scholar]
- 28.Hajishengallis G, Hollingshead S K, Koga T, Russell M W. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–4332. [PubMed] [Google Scholar]
- 29.Hanson M S, Cope L D, Hansen E J. Expression of the heat-modifiable major outer membrane protein of Haemophilus influenzae type b is unrelated to virulence. Infect Immun. 1989;57:1639–1646. doi: 10.1128/iai.57.6.1639-1646.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 31.Jordens J Z, Leaves N I. Source of variation detected in ribotyping patterns of Haemophilus influenzae: comparison of traditional ribotyping, PCR-ribotyping and rDNA restriction analysis. J Med Microbiol. 1997;46:763–772. doi: 10.1099/00222615-46-9-763. [DOI] [PubMed] [Google Scholar]
- 32.Karasic R B, Trumpp C E, Gnehm H E, Rice P A, Pelton S I. Modification of otitis media in chinchillas rechallenged with nontypeable Haemophilus influenzae and serological response to outer membrane antigens. J Infect Dis. 1985;151:273–279. doi: 10.1093/infdis/151.2.273. [DOI] [PubMed] [Google Scholar]
- 33.Klein J O. Role of nontypeable Haemophilus influenzae in pediatric respiratory tract infections. Pediatr Infect Dis J. 1997;16(Suppl.):S5–S8. doi: 10.1097/00006454-199702001-00002. [DOI] [PubMed] [Google Scholar]
- 34.Kohler G, Howe S C, Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976;6:292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- 35.Kroll J S, Ely S, Moxon E R. Capsular typing of Haemophilus influenzae with a DNA probe. Mol Cell Probes. 1991;5:375–379. doi: 10.1016/s0890-8508(06)80009-5. [DOI] [PubMed] [Google Scholar]
- 36.Kroll J S, Hopkins I, Moxon E R. Capsule loss in H. influenzae type b occurs by recombination-mediated disruption of a gene essential for polysaccharide export. Cell. 1988;53:347–356. doi: 10.1016/0092-8674(88)90155-9. [DOI] [PubMed] [Google Scholar]
- 37.Kroll J S, Loynds B M, Moxon E R. The Haemophilus influenzae capsulation gene cluster: a compound transposon. Mol Microbiol. 1991;5:1549–1560. doi: 10.1111/j.1365-2958.1991.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 38.Kyd J M, Dunkley M L, Cripps A W. Enhanced respiratory clearance of nontypeable Haemophilus influenzae following mucosal immunization with P6 in a rat model. Infect Immun. 1995;63:2931–2940. doi: 10.1128/iai.63.8.2931-2940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leaves N I, Jordens J Z. Development of a ribotyping scheme for Haemophilus influenzae type b. Eur J Clin Microbiol Infect Dis. 1994;13:1038–1045. doi: 10.1007/BF02111824. [DOI] [PubMed] [Google Scholar]
- 40.Littlefield J W. Selection of hybrids from matings of fibroblasts in vitro and their presumed recombinants. Science. 1964;145:709. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- 41.Loeb M R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987;55:2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makela P H, Takala A K, Peltola H, Eskola J. Epidemiology of invasive Haemophilus influenzae type b disease. J Infect Dis. 1992;165(Suppl.):S2–S6. doi: 10.1093/infdis/165.supplement_1-s2. [DOI] [PubMed] [Google Scholar]
- 43.Moxon E R. Haemophilus influenzae. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. Vol. 2. New York, N.Y: Churchill Livingstone Inc.; 1995. pp. 2039–2045. [Google Scholar]
- 44.Moxon E R. Haemophilus influenzae. In: Weatherall D J, Ledingham J G G, Warrell D A, editors. Oxford textbook of medicine. Vol. 1. Oxford, United Kingdom: Oxford University Press; 1996. pp. 580–584. [Google Scholar]
- 45.Moxon E R, Wilson R. The role of Haemophilus influenzae in the pathogenesis of pneumonia. Rev Infect Dis. 1991;13:S518–S527. doi: 10.1093/clinids/13.supplement_6.s518. [DOI] [PubMed] [Google Scholar]
- 46.Munson R, Jr, Grass S. Purification, cloning, and sequence of outer membrane protein P1 of Haemophilus influenzae type b. Infect Immun. 1988;56:2235–2242. doi: 10.1128/iai.56.9.2235-2242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munson R, Jr, Grass S, Einhorn M, Bailey C, Newell C. Comparative analysis of the structures of the outer membrane protein P1 genes from major clones of Haemophilus influenzae type b. Infect Immun. 1989;57:3300–3305. doi: 10.1128/iai.57.11.3300-3305.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munson R, Jr, Hunt A. Isolation and characterization of a mutant of Haemophilus influenzae type b deficient in outer membrane protein P1. Infect Immun. 1989;57:1002–1004. doi: 10.1128/iai.57.3.1002-1004.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy T F, Kirkham C, Sikkema D J. Neonatal, urogenital isolates of biotype 4 nontypeable Haemophilus influenzae express a variant P6 outer membrane protein molecule. Infect Immun. 1992;60:2016–2022. doi: 10.1128/iai.60.5.2016-2022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musser J M, Barenkamp S J, Granoff D M, Selander R K. Genetic relationships of serologically nontypeable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986;52:183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musser J M, Granoff D M, Pattison P E, Selander R K. A population genetic framework for the study of invasive diseases caused by serotype b strains of Haemophilus influenzae. Proc Natl Acad Sci USA. 1985;82:5078–5082. doi: 10.1073/pnas.82.15.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musser J M, Kroll J S, Moxon E R, Selander R K. Clonal population structure of encapsulated Haemophilus influenzae. Infect Immun. 1988;56:1837–1845. doi: 10.1128/iai.56.8.1837-1845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musser J M, Kroll J S, Moxon E R, Selander R K. Evolutionary genetics of the encapsulated strains of Haemophilus influenzae. Proc Natl Acad Sci USA. 1988;85:7758–7762. doi: 10.1073/pnas.85.20.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nizet V, Colina K F, Almquist J R, Rubens C E, Smith A L. A virulent nonencapsulated Haemophilus influenzae. J Infect Dis. 1996;173:180–186. doi: 10.1093/infdis/173.1.180. [DOI] [PubMed] [Google Scholar]
- 55.O'Connell M A, Gerber A, Keller W. Purification of human double-stranded RNA-specific editase 1 (hRED1) involved in editing of brain glutamate receptor B pre-mRNA. J Biol Chem. 1997;272:473–478. doi: 10.1074/jbc.272.1.473. [DOI] [PubMed] [Google Scholar]
- 56.Oka A, Sugisaki H, Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981;147:217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- 57.Panezutti H, James O, Hansen E J, Choi Y, Harkness R E, Klein M H, Chong P. Identification of surface-exposed B-cell epitopes recognized by Haemophilus influenzae type b P1-specific monoclonal antibodies. Infect Immun. 1993;61:1867–1872. doi: 10.1128/iai.61.5.1867-1872.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelton S. Challenges in the management of otitis media. Am J Managed Care. 1999;5(Suppl.):S662–S669. [Google Scholar]
- 59.Peterson G L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 60.Porras O, Caugant D A, Lagergard T, Svanborg-Eden C. Application of multilocus enzyme gel electrophoresis to Haemophilus influenzae. Infect Immun. 1986;53:71–78. doi: 10.1128/iai.53.1.71-78.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Proulx C, Hamel J, Chong P, Martin D, Brodeur B R. Epitope analysis of an immunodominant domain on the P1 protein of Haemophilus influenzae type b using synthetic peptides and anti-idiotypic antibodies. Microb Pathog. 1992;12:433–442. doi: 10.1016/0882-4010(92)90006-a. [DOI] [PubMed] [Google Scholar]
- 62.Proulx C, Munson R S, Jr, Grass S, Hamel J, Martin D, Brodeur B R. Identification of a surface-exposed immunodominant epitope on outer membrane protein P1 of Haemophilus influenzae type b. Infect Immun. 1991;59:963–970. doi: 10.1128/iai.59.3.963-970.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rice P A, Vayo H E, Tam M R, Blake M S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986;164:1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenfeld M, Ramsey B. Evolution of airway microbiology in the infant with cystic fibrosis: role of nonpseudomonal and pseudomonal pathogens. Semin Respir Infect. 1992;7:158–167. [PubMed] [Google Scholar]
- 65.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 66.Schiller N, Monday S, Boyd C, Keen N, Ohman D. Characterization of Pseudomonas aeruginosa alginate lyase gene (algL): cloning, sequencing, and expression in Escherichia coli. J Bacteriol. 1993;175:4780–4789. doi: 10.1128/jb.175.15.4780-4789.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seidman C E, Struhl K, Sheen J, Jessen T. Introduction of plasmid DNA into cells. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1997. pp. 1.8.1–1.8.10. [Google Scholar]
- 68.Shappert S M. Office visits for otitis media: United States, 1975–90. Advance data no. 214. National Center for Health Statistics, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 1992. [Google Scholar]
- 69.Shenep J L, Munson R S, Jr, Barenkamp S J, Granoff D M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983;42:257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shurin P A, Pelton S I, Finkelstein J. Tympanometry in the diagnosis of middle-ear effusion. N Engl J Med. 1977;296:412–417. doi: 10.1056/NEJM197702242960802. [DOI] [PubMed] [Google Scholar]
- 71.Shurin P A, Pelton S I, Tager I B, Kasper D L. Bactericidal antibody and susceptibility to otitis media caused by nontypeable strains of Haemophilus influenzae. J Pediatr. 1980;97:364–369. doi: 10.1016/s0022-3476(80)80182-x. [DOI] [PubMed] [Google Scholar]
- 72.Sloyer J L, Jr, Cate C C, Howie V M, Ploussard J H, Johnston R B., Jr The immune response to acute otitis media in children. II. Serum and middle ear fluid antibody in otitis media due to Haemophilus influenzae. J Infect Dis. 1975;132:685–688. doi: 10.1093/infdis/132.6.685. [DOI] [PubMed] [Google Scholar]
- 73.St. Geme J W, Kumar V V, Cutter D, Barenkamp S J. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect Immun. 1998;66:364–368. doi: 10.1128/iai.66.1.364-368.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strausbaugh L J. Haemophilus influenzae infections in adults: a pathogen in search of respect. Postgrad Med. 1997;101:191–200. doi: 10.3810/pgm.1997.02.165. [DOI] [PubMed] [Google Scholar]
- 75.Tsung Y K, Milunsky A, Alpert E. Secretion by a hybridoma of antibodies against human alpha-fetoprotein. N Engl J Med. 1980;302:180. doi: 10.1056/NEJM198001173020320. [DOI] [PubMed] [Google Scholar]
- 76.van Alphen L, Riemens T, Poolman J, Zanen H C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983;155:878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 78.Vinograd J, Hearst J E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;XX:372–422. [PubMed] [Google Scholar]
- 79.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 80.Zar J H. Biostatistical analysis. 2nd ed. Englewood Cliffs, N.J: Prentice-Hall, Inc.; 1984. p. 390. [Google Scholar]